Abstract
Cuscuta campestris is a parasitic weed species that inflicts worldwide noxious effects in many broadleaf crops due to its capacity to withdraw nutrients and water directly from the crop vascular system using haustorial connections. Cuscuta campestris control in the majority of crops affected is non-existent, and thus, research for the development of control methods is needed. Hydrocinnamic acid occurs naturally in the rhizosphere, playing regulatory roles in plant–plant and plant–microbe communities. The toxicity of hydrocinnamic acid against C. campestris was recently identified. In the present work, a structure–activity relationship study of 21 hydrocinnamic acid analogues was performed to identify key structural features needed for its allelopathic action against the seedling growth of this parasitic plant. The findings of this study provide the first step for the design of herbicides with enhanced activity for the control of C. campestris infection.
Keywords: field dodder, parasitic weeds, phenylpropanoic acid, allelochemicals, structure–activity relationship, sustainable crop protection
1. Introduction
More than 1% of flowering plants have evolved the capacity to parasitize other plants to obtain all or part of their required nutrients [1]. Among them, the dodders (Cuscuta genus) contain over 170 species distributed across tropical, subtropical, and temperate regions [1,2]. Cuscuta campestris is considered one of the most damaging parasitic weed species, severely affecting the yield of dicotyledonous crops of economic importance [3]. There is no effective Cuscuta control for the most affected crops [4,5]. After Cuscuta germination, a filiform seedling twines around the nearest crop stem producing haustoria, which penetrate into the crop stem and fuse with the crop vascular system to extract nutrients and water [6,7]. Cuscuta plants are root- and leaf-less plants with little or no chlorophyll, and therefore, they are completely dependent on crop-derived nutrition, otherwise they die within 7 to 10 days after germination [8]. The identification of allelochemicals that target the vulnerable pre-attached Cuscuta seedlings and interfere with the necessary host contact for infection is the first step for the design of alternatives that provide efficacy and sustainability to parasitic weed chemical control [7].
During a recent screening to discover new inhibitors of Cuscuta seedling growth, hydrocinnamic acid was identified as a promising compound showing strong inhibitory activity [9]. Hydrocinnamic acid, also known as phenylpropanoic acid, is a carboxylic acid with the formula C9H10O2 belonging to the class of phenylpropanoids that originate from the shikimic acid pathway [10]. Hydrocinnamic acid occurs naturally in the rhizosphere, either originating during the breakdown of crop residues [11] or exuded in intact form from plant roots [12], regulating ecosystems through different roles in plant–plant [13] and plant–microbe interactions [14]. Hydrocinnamic acid has been reported to inhibit seed germination in several species [15,16,17]. Thus, the objective of this work was to study the structure–activity relationships of 21 hydrocinnamic acid analogues in order to identify key structural features needed for its herbicidal action against Cuscuta. This is the first report on the phytotoxic activity of many of the tested compounds.
2. Results and Discussion
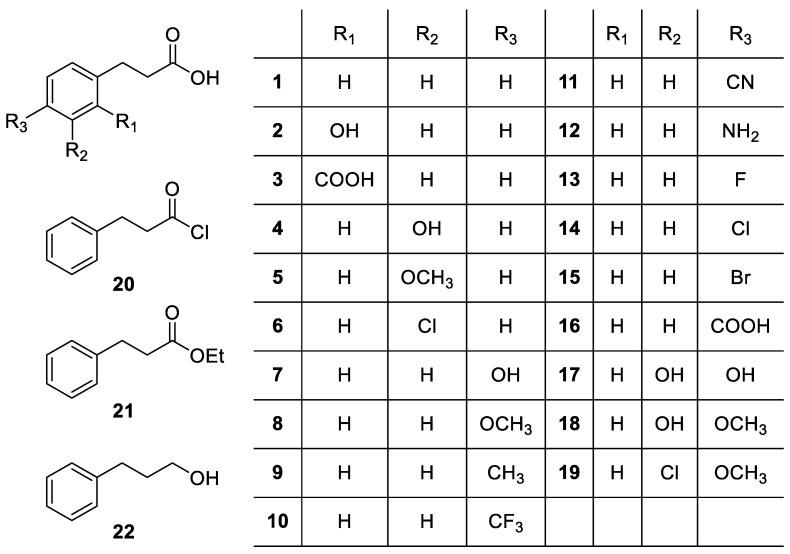
The Cuscuta growth inhibition of hydrocinnamic acid (1, Figure 1) was studied in vitro in comparison with 21 structural analogues (2–22, Figure 1) in a range of concentrations from 0.25 to 1 mM.
Figure 1.
Summary of the structures of compounds 1–22 used in this study.
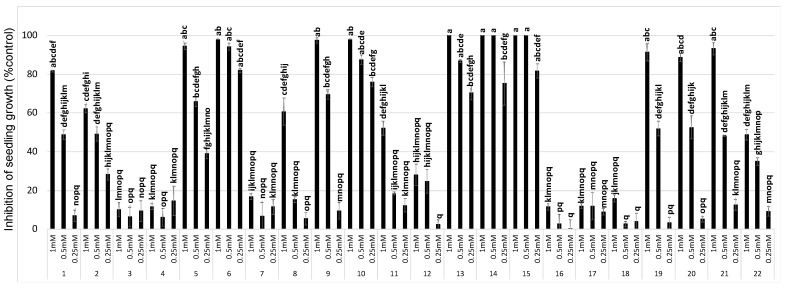
Five days after treatment, Cuscuta growth was significantly affected by the compound treatment (ANOVA, p < 0.001) by the concentration applied (ANOVA, p < 0.001), and by the interaction of compound x concentration (ANOVA, p < 0.001). Figure 2 shows different levels of activity among hydrocinnamic acid (1) and its analogues (2–22), which allowed the classification of compounds with (i) enhanced activity, (ii) similar activity, and (iii) decreased activity in comparison with the parent compound (1).
Figure 2.
In vitro assessment of the Cuscuta growth inhibition induced by compounds 1–22 at concentrations of 1, 0.5, and 0.25 mM. Bars with different letters are significantly different (Tukey test at p < 0.05). Error bars represent the standard error of the mean.
2.1. Study of Structure–Activity Relationship on the Growth Inhibition Induced by Hydrocinnamic Acid
The growth inhibition results shown in Figure 2 were used to calculate the IC50 values in order to compare the effect of the substitution on the bioactivity, and CLogp values were calculated to correlate the activity level with the lipophilicity. These parameters are shown in Table 1.
Table 1.
IC50 and CLogp values of compounds 1–22, ~1000, inhibition was close to 50% at the highest tested concentration; >1000, compound was far from 50% inhibition at the highest concentration tested but activity was significant; <250, IC50 was lower than the lowest tested concentration.
| Compound | CLogp | IC50 (μM) | R2 | Compound | CLogp | IC50 (μM) | R2 |
|---|---|---|---|---|---|---|---|
| 1 | 1.903 | 518 | 0.9961 | 12 | 0.676 | >1000 | - |
| 2 | 1.186 | 572 | 0.9978 | 13 | 2.046 | <250 | - |
| 3 | 0.746 | >1000 | - | 14 | 2.616 | <250 | - |
| 4 | 1.236 | >1000 | - | 15 | 2.766 | <250 | - |
| 5 | 1.822 | 334 | 0.9950 | 16 | 1.646 | >1000 | - |
| 6 | 2.616 | <250 | - | 17 | 0.639 | >1000 | - |
| 7 | 1.236 | >1000 | - | 18 | 1.085 | >1000 | - |
| 8 | 1.822 | 879 | 0.9990 | 19 | 2.445 | 488 | 0.9987 |
| 9 | 2.402 | 415 | 1.000 | 20 | 2.228 | 484 | 0.9979 |
| 10 | 2.786 | <250 | - | 21 | 2.808 | 511 | 0.9981 |
| 11 | 1.336 | ~1000 | - | 22 | 1.712 | ~1000 | - |
The strongest bioactivity was found for compounds 6, 10, and 13–15, with IC50 values less than 250 µM. All of them achieved 100% (or close) inhibition at the highest concentration (1 mM), with compounds 6, 14, and 15 maintaining this level of inhibition at 0.5 mM. The key success of these compounds (6, 10, and 13–15) was also their ability to cause inhibition values higher than 75% at the lowest concentration tested (0.25 mM). In this regard, it should be noted that there is a structural correlation between compound 14 and 2,4-dichlorophenoxyacetic acid, one of the most frequently used herbicides due to its efficacy, selectivity, and broad spectrum of weed control [18].
On the other hand, the lowest bioactivity was found for compounds 3, 4, 7, 12, and 16–18. The bioactivity of these compounds was statistically significant, but below the 50% baseline for the highest concentration tested. This result for compound 7 is in agreement with previous studies that showed moderate or weak phytotoxicity for this compound [19,20], though it should be noted that higher phytotoxicity was previously found on the root growth of tomato and radish [21].
Regarding the correlation between the activity level and the lipophilicity, it was observed that compounds with CLogp values lower than 1.712 (Table 1) were found to be the least active. On the other hand, compounds with CLogp values higher than 2.046 were found to be the most active. However, no clear direct correlation was found between the linear evolution of CLogp and the bioactivity. The relationship of high CLogp with bioactivity could be due to the fact that the compounds are preferentially distributed in hydrophobic environments, such as the lipid bilayer of the membrane. This is related to studies where it has been reported that cinnamic acid and derivatives produce changes in the permeability of the cell membrane [22] and reduce H+ ATPase activity [23].
In general, by comparison with hydrocinnamic acid (1), whose IC50 value was 518 μM, the bioactivity was influenced by the position and the type of functional groups it contained. It was observed that depending on the position at the aromatic ring, the effect of the functional group would improve or decrease the bioactivity of hydrocinnamic acid (1). Therefore, halogens at para or meta positions would significantly increase the bioactivity (13–15, and 6), a methoxy group at meta (5) increases the activity of compound 1 while at para (8) it would decrease it. In addition, the hydroxy group at ortho (2) maintained similar level of activity than compound 1, while at meta (4) and para (7), they drastically decreased the bioactivity, even when a second functionalization was included in the form of an extra hydroxy (17) or methoxy group (18). This low activity observed for compound 17 supports the moderate or low inhibitory activity reported in other studies on the growth of Triticum aestivum, Lactuca sativa, and Setaria viridis [19,24,25].
The detailed analysis of the effects of each functional group on the bioactivity is reported below.
Halogenated substituents increased the inhibitory activity of the core molecule, hydrocinnamic acid, regardless of the position. The compound possessing the largest and least electronegative Br group (15) had the strongest inhibitory activity. There appeared to be no significant difference between a substituent Cl at para and meta (14 and 6, respectively). Among the halogenated derivatives, the lowest bioactivity was shown by that containing the smallest halogen atom (F, compound 13). Inclusion of a halogen atom even increased the bioactivity of the weakly active compound 8 (containing a methoxy group), almost doubling it in the case of compound 19. To contextualize these results regarding the halogenation effects on the bioactivity, it can be highlighted that the phytotoxic activity of several halogenated derivatives of natural compounds has been known for some time. Halogenated derivatives of benzoic acid (weakly phytotoxic) with improved phytotoxicity were reported [26,27], as well as the finding of chlorinated and fluorinated derivatives of benzoxazinones with potent phytotoxicity [28,29]. These last studies described how the position of the halogen atom plays a key role in the level of phytotoxicity as a consequence of the marked electronic transformation of the tested compounds. A study with different chlorinated derivatives of benzophenones also showed that the position of the Cl atom in the aromatic ring is relevant for growth-inhibiting or chlorosis-inducing activities, suggesting the impact of unknown factors, involving steric effects [30]. Given that studies such as those already mentioned have found different levels of activity regarding the position of the Cl atom, it is worth highlighting the results herein reported for hydrocinnamic acid, in which compounds 6 and 14, differing in the ortho or para positions of the Cl atom, showed similar inhibition on Cuscuta growth.
Hydroxyl groups had a negative effect on the bioactivity. There were differences in activity depending on where the alcohol was substituted, being found to induce an improved activity for the ortho derivative (2, IC50 = 572 μM) greater than the meta (4) or para (7) derivatives (IC50 > 1000 μM). The activity for the para derivative is similar to the activity of the related compound, p-coumaric acid, found in a previous study on Cuscuta [9]. The increased activity of the hydroxylated derivative at the ortho position (2) was also found in a previous study on Cuscuta [9], which may be due to its ability to cyclize and form coumarins [31]. Indeed, compound 2 also generates growth inhibition on radish seedlings [21]. In a previous study, scopoletin and umbelliferone were found to have low but significant activity on Cuscuta [9]. The cyclization in scopoletin and umbelliferone occurs from cinnamic acid, but an ortho alcohol is necessary for cyclization, and could be also relevant for the increased activity found in hydroxyl at the ortho position.
Methoxy groups had a positive effect on the bioactivity at the meta (5) but negative at the para position (8). Nevertheless, in both cases, methylation of the corresponding hydroxylated compounds at these positions increased the bioactivity (4 and 7). This improvement regarding the hydrocinnamic acids was also previously described for the family of flavones, for which it has been reported that the most active are generally methoxy-substituted [32].
Carboxy groups on the aromatic ring both at para (16) and ortho (3) positions reduced the activity to negligible levels. Methyl and trifluoromethyl groups at the para position both increased the bioactivity, especially in the case of the CF3 group (10), which could be related to the similarity in size to hydrogen but the different electronegativity of F. Cyano and amino groups at the para position, on the other hand, substantially decreased the bioactivity of compound 1, which was more apparent in the case of compound 12 with an amino group.
Carboxylic acid derivatives. Three molecules derived from the carboxylic acid of hydrocinnamic acid were studied: acyl chloride (20), ethyl ester (21), and alcohol (22). Derivatization of hydrocinnamic acid (1) to form the ethyl ester (21) had little effect on the bioactivity, with very similar EC50 (518 and 511 μM, respectively), while the CLogp was very different (1.903 and 2.808, respectively). Thus, neither the lipophilicity or the acidity of the compounds appeared to be affecting the bioactivity. This finding might be a key factor, since the control of the solubility and acidity of the bioactive compound by esterification may ease the future formulation of the bioactive compounds. Stronger activity was found for the acyl chloride derivative of 1 (20), demonstrating again the beneficial effect of halogens on the bioactivity. In the case of compound 22, where the acid group has been reduced to an alcohol, the bioactivity was almost lost, demonstrating the importance of the acid group for the growth inhibition activity.
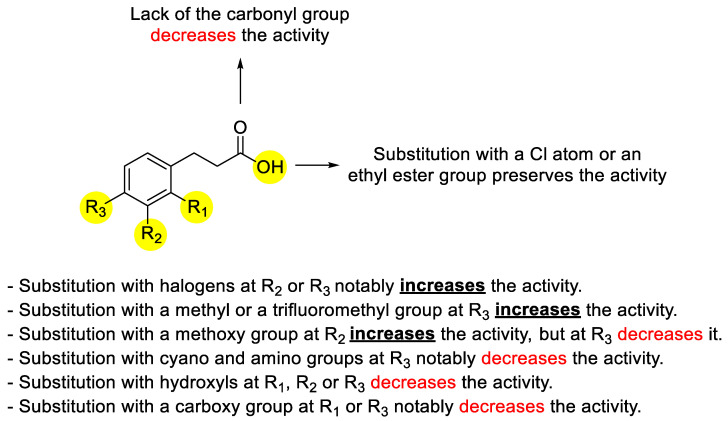
The overall structure–activity relationship discussion described above is summarized in Figure 3.
Figure 3.
Structure–activity relationship of hydrocinnamic acid regarding the inhibitory activity on Cuscuta growth.
2.2. Identification of Inductors of Necrosis in C. campestris Seedlings
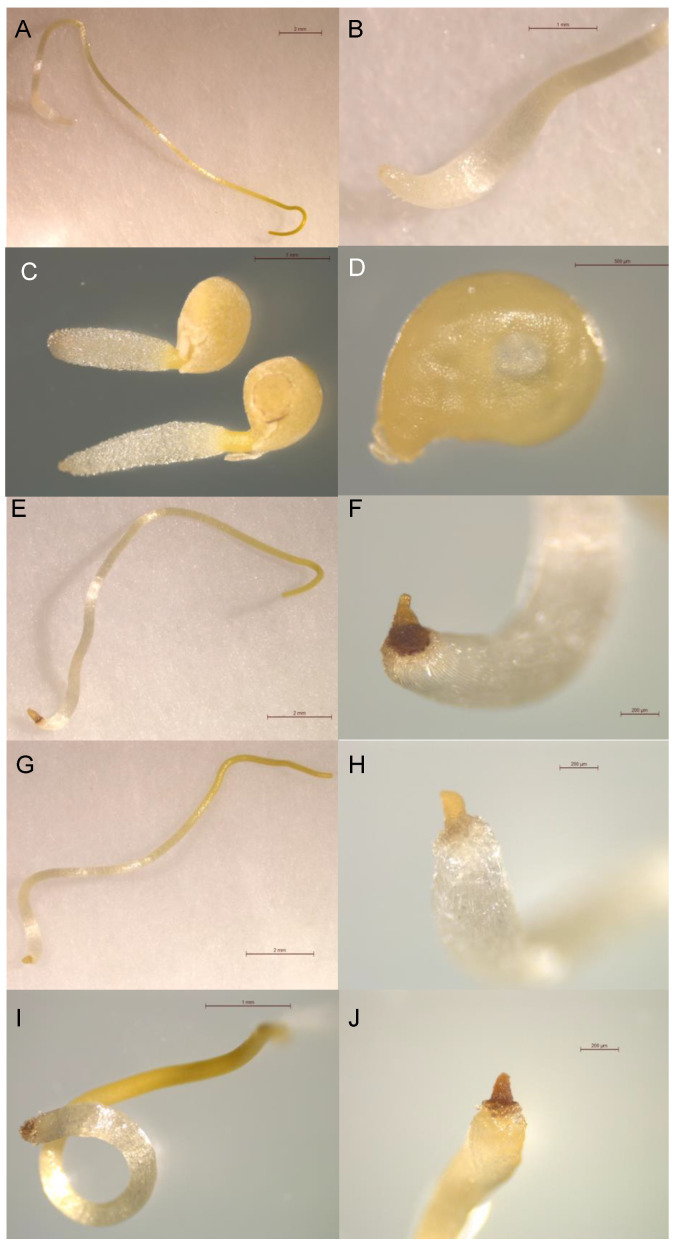
Additional to the modification of inhibitory activity of seedling growth, this SAR study was also used to identify structural features that induced necrosis in Cuscuta seedlings (Figure 4). The induction of necrosis observed in this study was significantly affected by the compound treatment (ANOVA, p < 0.001). No necrosis was observed in Cuscuta seedlings treated with the control or hydrocinnamic acid (Figure 4A–C). Despite not having significant growth-reducing activity, intense necrosis was observed in the root apices of all seedlings treated with compounds 17 and 12 (Figure 4E,F,I,J). Treatments with compound 18 (Figure 4G,H) also induced necrosis, but necrosis was observed to be less intense than the necrosis induced by compounds 12 and 17. Compound 17 is similar to caffeic acid, the only difference being the absence of the double bond between carbons 2 and 3. It has been observed that, as in compound 17, caffeic acid does not induce Cuscuta growth inhibition, but rather the induction of necrosis [9]. Both compounds with the highest necrosis effect (12 and 17) also possess low and similar CLogp values (0.676 and 0.639, respectively), lower than in the case of compound 18 (1.085), indicating that low lipophilicity may play a role in producing the necrotic effect. It is interesting to consider that other studies also reported necrotic activity for compound 7 [33,34].
Figure 4.
Photographs showing a Cuscuta seedling treated with control (A,B); hydrocinnamic acid (1) 1 mM (C); 3-(4-fluorophenyl)propionic acid (13) (D); 3,4-dihydroxyhydrocinnamic acid (17) (E,F); 3-(3-hydroxy-4-methoxyphenyl)propionic acid (18) (G,H); 3-(4-aminophenyl)propionic acid (12) (I,J).
In our previous study, we reported the cinnamic-acid-derived compounds caffeic acid, ferulic acid, vanillic acid, and naringenin, all having necrosis-inducing effects on Cuscuta seedlings that varied from strong to moderate [9].
By comparing previous findings with the results reported in the present research, some hints about the structural requirements for necrosis-inducing activity can be found. First, the presence of the double bond of cinnamic acid is not mandatory to obtain a strong necrosis-inducing effect, since compound 17 has a similar necrotic effect to caffeic acid. In addition, the presence of two adjacent hydroxy groups in the aromatic ring is beneficial for inducing necrosis, and the methylation of one of these groups decreases the necrotic effect, as observed previously between caffeic and ferulic acid [9], and confirmed in the present work with compound 17 when compared with compound 18.
Moreno-Robles et al. [9] reported that vanillic acid, also containing a methoxy group, shows the negative effect of the methylation on the necrosis, while also hints at the relevance of the phenol fragment for necrosis. On the other hand, compound 12, with an amino group, has a similar effect to the hydroxylated compounds, hinting that polar groups, such as amino and hydroxyl groups, with the possibility to form hydrogen bonds, could have a positive effect on the necrosis-inducing capacity.
3. Materials and Methods
3.1. Plant Material and Chemicals
Seeds of Cuscuta were collected in July 2019 from mature Cuscuta campestris plants parasitizing field pea at the Institute for Sustainable Agriculture (IAS-CSIC), Alameda del Obispo Research Center (Córdoba, southern Spain, coordinates 37.856 N, 4.806 W, datum WGS84). Dry Cuscuta seeds were separated from capsules by sifting with a 0.6 mm mesh sieve followed by winnowing with a fan. Cuscuta seeds were stored dry in the dark at room temperature until use for this work in 2022.
Hydrocinnamic acid and its 21 analogues were purchased from Sigma-Aldrich (St. Louis, MO, USA): hydrocinnamic acid (1, cat. no. 135232), 3-(2-hydroxyphenyl)propionic acid (2, cat. no. 393533), 3-(2-carboxyphenyl)propionic acid (3, cat. no. 406465), 3-(3-hydroxyphenyl)propanoic acid (4, cat. no. PH011597), 3-(3-methoxyphenyl)propionic acid (5, cat. no. 349763), 3-(3-chlorophenyl)propionic acid (6, cat. no. 631302), 3-(4-hydroxyphenyl)propionic acid (7, cat. no. H52406), 3-(4-methoxyphenyl)propanoic acid (8, cat. no. M23527), 3-(p-tolyl)propionic acid (9, cat. no. 118265), 4-(trifluoromethyl)hydrocinnamic acid (10, cat. no. 457035), 3-(4-cyanophenyl)propionic acid (11, cat. no. 746010), 3-(4-aminophenyl)propionic acid (12, cat. no. 560251), 3-(4-fluorophenyl)propionic acid (13, cat. no. 560502), 3-(4-chlorophenyl)propionic acid (14, cat. no. 656151), 3-(4-bromophenyl)propionic acid (15, cat. no. 595438), 3-(4-carboxyphenyl)propionic acid (16, cat. no. 531553), 3,4-dihydroxyhydrocinnamic acid (17, cat. no. 102601), 3-(3-hydroxy-4-methoxyphenyl)propionic acid (18, cat. no. CDS006461), 3-(3-chloro-4-methoxyphenyl)propionic acid (19, cat. no. 638773), hydrocinnamoyl chloride (20, cat. no. 249440), ethyl 3-phenylpropionate (21, cat. no. 284416), 3-phenyl-1-propanol (22, cat. no. 140856).
3.2. In Vitro Experiments for Screening of Allelopathy against Growth of Cuscuta Seedling
A screening of the 21 compounds (2–22) described in Figure 1 was performed to identify allelopathic activity against the growth of Cuscuta seedling. Seeds of C. campestris show physical dormancy induced by a thick seed coat that preserves seedbank viability in agricultural fields over time [7]. To promote Cuscuta germination, the hard coat of Cuscuta seeds was eliminated by scarification with sulfuric acid for 45 min [35], followed by thorough rinses. Then, five scarified Cuscuta seeds were placed using tweezers onto 5 cm diameter filter paper discs inside 5.5 cm diameter Petri dishes. All compounds were dissolved in methanol and then diluted to 1, 0.5, and 0.25 mM in sterilized distilled water. This was conducted for all compounds except for the compound 3-(4-carboxyphenyl)propionic acid, which was dissolved in dimethyl sulfoxide, or the compounds 3-phenyl-1-propanol, ethyl 3-phenylpropionate, and hydrocinnamoyl chloride, which were purchased in liquid formulation and dissolved directly in water. The final concentration of organic solvent in all treatments was 1%, including for the compounds 3-phenyl-1-propanol, ethyl 3-phenylpropionate, and hydrocinnamoyl chloride. Triplicate aliquots of 1 mL of each treatment were applied to filter paper discs containing the scarified Cuscuta seeds. Triplicate aliquots of treatment only containing 1% of solvent and sterile distilled water were used as a control. Treated Cuscuta seeds were incubated in the dark at 23 °C for 5 days. The seedling length was measured in each of the five Cuscuta seedlings for each of the three replicate filter paper discs per treatment. Seedling growth for each treatment was calculated in relation to the seedling growth of the corresponding control. In addition, notes were taken for each Cuscuta seedling regarding whether the root apex had developed necrosis. The percentage of seedlings that developed a necrotic root was calculated in each triplicated disk for each treatment.
3.3. Calculations and Statistical Analysis
Compounds that reached inhibitions of 50% and that were active at more than one concentration were statistically analyzed to determine their IC50 using GraphPad Prism v.5.00 software package (GraphPad Software, Inc., San Diego, CA, USA). The bioactivity data were fitted to a sigmoidal dose–response model with variable slope. Calculation of CLogp was performed using ChemOffice v20.1 (PerkinElmer, Wal-tham, MA, USA) using the appropriate tool in ChemDraw Professional [36]. All bioassays were performed using a completely randomized design. Percentage data were approximated to normal frequency distribution by means of angular transformation. Then, percentage data were subjected to analysis of variance (ANOVA). The significance of mean differences among treatments was evaluated by Tukey test at p < 0.05. Statistical analysis was performed using SPSS software 27 (SPSS Inc., Chicago, IL, USA).
4. Conclusions
The results demonstrate that phytotoxicity is influenced by the position and the type of functional groups present on the substituted hydrocinnamic acid analogues. In particular, the carbonyl group of the propanoid chain seems an important factor for activity. Furthermore, the presence of halogens on the aromatic ring increases the activity, while substitutions with cyano and amino groups, as well as hydroxyl or carboxyl groups, decreases the activity. Interesting data were also obtained with the presence of a methoxy group in the meta position, while its presence in the para position had a negative effect on the bioactivity. Structural features that induced necrosis in Cuscuta seedlings were also identified, and the results suggest that low lipophilicity may play a role in determining the necrotic effect. These results provide interesting and useful information for the design of herbicides for the control of C. campestris, starting from the compounds with increased activity in respect to hydrocinnamic acid. However, future studies are needed to determine the mode of action of the active compounds and their ecotoxicity before realizing formulations for practical application as herbicides.
Acknowledgments
We thank CSIC Interdisciplinary Thematic Platform (PTI) Optimization of Agricultural and Forestry Systems (PTI-AGROFOR), the “Consejería de Transformación Económica, Industria, Conocimiento y Universidades de la Junta de Andalucía, project ID: QUAL21 023 IAS” and “Máster Universitario en Agroalimentación, Córdoba University, Spain)”. A.C.P. expresses his sincere gratitude to the “Plan Propio—UCA 2022–2023” (REF. EST2022-087), the “Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía” and the “Programa Operativo Fondo Social Europeo de Andalucía 2014–2020” for their financial support. J.G.Z. thanks the University of Cadiz for the postdoctoral support with the Margarita Salas fellowship (2021-067/PN/MS-RECUAL/CD), funded by the NextGenerationEU programme of the European Union.
Author Contributions
Conceptualization, M.F.-A. and S.V.-R.; data acquisition, A.M.-R. and M.F.-A.; data curation, A.M.-R., A.C.P., J.G.Z. and M.F.-A.; writing—original draft preparation, A.C.P., J.G.Z., G.S., M.M. and M.F.-A.; writing—review and editing, A.M.-R., A.C.P., J.G.Z., G.S., M.M., S.V.-R., A.C. and M.F.-A.; Supervision, M.F.-A.; Funding acquisition, M.F.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Agencia Estatal de Investigación/Ministerio de Ciencia Innovación (projects PID2020-114668RB-I00 and RYC-2015-18961) and by a CSIC-ALGOSUR research contract. Authors wish to express gratitude for the Ph.D. grant to Gabriele Soriano funded by INPS (Istituto Nazionale Previdenza Sociale), and for the Galileo grant from Córdoba University-Diputación to Antonio Moreno-Robles.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nickrent D.L. Parasitic angiosperms: How often and how many? Taxon. 2020;69:5–27. doi: 10.1002/tax.12195. [DOI] [Google Scholar]
- 2.Dawson J.H., Musselman L.J., Wolswinkel P., Dörr I. Biology and control of Cuscuta. Rev. Weed Sci. 1994;6:265–317. [Google Scholar]
- 3.Lanini W.T., Kogan M. Biology and management of Cuscuta in crops. Int. J. Agric. Nat. Resour. 2005;32:165–179. doi: 10.7764/rcia.v32i3.317. [DOI] [Google Scholar]
- 4.Goldwasser Y., Miryamchik H., Sibony M., Rubin B. Detection of resistant chickpea (Cicer arietinum) genotypes to Cuscuta campestris (field dodder) Weed Res. 2012;52:122–130. doi: 10.1111/j.1365-3180.2012.00904.x. [DOI] [Google Scholar]
- 5.Córdoba E.M., Fernández-Aparicio M., González-Verdejo C.I., López-Grau C., Muñoz-Muñoz M.V., Nadal S. Search for resistant genotypes to Cuscuta campestris infection in two legume species, Vicia sativa and Vicia ervilia. Plants. 2021;10:738. doi: 10.3390/plants10040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaštier P., Krasylenko Y.A., Martincová M., Panteris E., Šamaj J., Blehová A. Cytoskeleton in the parasitic plant Cuscuta during germination and pre-haustorium formation. Front. Plant Sci. 2018;9:794–810. doi: 10.3389/fpls.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Aparicio M., Delavault P., Timko M.P. Management of infection by parasitic weeds: A review. Plants. 2020;9:1184. doi: 10.3390/plants9091184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Aparicio M., Soriano G., Masi M., Carretero P., Vilariño-Rodríguez S., Cimmino A. (4Z)-Lachnophyllum lactone, an acetylenic furanone from Conyza bonariensis, identified for the first time with allelopathic activity against Cuscuta campestris. Agriculture. 2022;12:790. doi: 10.3390/agriculture12060790. [DOI] [Google Scholar]
- 9.Moreno-Robles A., Cala Peralta A., Soriano G., Zorrilla J.G., Masi M., Vilariño S., Cimmino A., Fernández-Aparicio M. Identification of allelochemicals with differential modes of phytotoxicity on Cuscuta campestris. Agriculture. 2022;12:1746. doi: 10.3390/agriculture12101746. [DOI] [Google Scholar]
- 10.Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; Chichester, UK: 2009. [Google Scholar]
- 11.Tanrisever N., Fronczek F.R., Fischer N.H., Williamson G.B. Ceratiolin and other flavonoids from Ceratiola ericoides. Phytochemistry. 1987;26:175–179. doi: 10.1016/S0031-9422(00)81505-8. [DOI] [Google Scholar]
- 12.Tang C.S., Young C.C. Collection and identification of allelopathic compounds from the undisturbed root system of Bigalta Limpogress (Hernarthria altissima) Plant Physiol. 1982;69:155–160. doi: 10.1104/pp.69.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson G.B., Obee E.M., Weidenhamer J.D. Inhibition of Schizachyrium scoparium (Poaceae) by the allelochemical hydrocinnamic acid. J. Chem. Ecol. 1992;18:2095–2105. doi: 10.1007/BF00981930. [DOI] [PubMed] [Google Scholar]
- 14.Hu A., Hu M., Chen S., Xue Y., Tan X., Zhou J. Five plant natural products are potential type III secretion system inhibitors to effectively control soft-rot disease caused by Dickeya. Front. Microbiol. 2022;13:839025. doi: 10.3389/fmicb.2022.839025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams R.D., Hoagland R.E. The effects of naturally occurring phenolic compounds on seed germination. Weed Sci. 1982;30:206–212. doi: 10.1017/S0043174500062342. [DOI] [Google Scholar]
- 16.Reynolds T. Comparative effects of aromatic compounds on inhibition of lettuce fruit germination. Ann. Bot. 1978;42:419–427. doi: 10.1093/oxfordjournals.aob.a085475. [DOI] [Google Scholar]
- 17.Chung E.J., Park J.H., Park T.S., Ahn J.W., Chung Y.R. Production of a phytotoxic compound, 3-phenylpropionic acid by a bacterial endophyte, Arthrobacter humicola YC6002 isolated from the root of Zoysia japonica. Plant Pathol. J. 2010;26:245–252. doi: 10.5423/PPJ.2010.26.3.245. [DOI] [Google Scholar]
- 18.Islam F., Wang J., Farooq M.A., Khan M.S., Xu L., Zhu J., Zhao M., Muños S., Li Q.X., Zhou W. Potential impact of the herbicide 2, 4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018;111:332–351. doi: 10.1016/j.envint.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 19.An M., Pratley J.E., Haig T. Phytotoxicity of Vulpia residues: III. Biological activity of identified allelochemicals from Vulpia myuros. J. Chem. Ecol. 2001;27:383–394. doi: 10.1023/A:1005640708047. [DOI] [PubMed] [Google Scholar]
- 20.D’Abrosca B., Della Greca M., Fiorentino A., Monaco P., Zarrelli A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agric. Food Chem. 2004;52:4101–4108. doi: 10.1021/jf049847v. [DOI] [PubMed] [Google Scholar]
- 21.Nicollier G.F., Thompson A.C. Phytotoxic compounds from Melilotus alba (white sweet clover) and isolation and identification of two new flavonoids. J. Agric. Food Chem. 1982;30:760–764. doi: 10.1021/jf00112a034. [DOI] [Google Scholar]
- 22.Baziramakenga R., Leroux G.D., Simard R.R. Effects of benzoic and cinnamic acids on membrane permeability of soybean roots. J. Chem. Ecol. 1995;21:1271–1285. doi: 10.1007/BF02027561. [DOI] [PubMed] [Google Scholar]
- 23.Abenavoli M.R., Lupini A., Oliva S., Sorgonà A. Allelochemical effects on net nitrate uptake and plasma membrane H+-ATPase activity in maize seedlings. Biologia Plantarum. 2010;54:149–153. doi: 10.1007/s10535-010-0024-0. [DOI] [Google Scholar]
- 24.Fraga B.M., González-Coloma A., Alegre-Gómez S., López-Rodríguez M., Amador L.J., Díaz C.E. Bioactive constituents from transformed root cultures of Nepeta teydea. Phytochemistry. 2017;133:59–68. doi: 10.1016/j.phytochem.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Li X.Z., Yan Z.Q., Pan L., Jin H., Yang X.Y., He X.F., Ren X., Xie M., Guon K., Qin B. Caffeic acid derivatives as growth inhibitors of Setaria viridis: Structure-activity relationships and mechanisms. Phytochem. Lett. 2017;20:208–213. doi: 10.1016/j.phytol.2017.04.041. [DOI] [Google Scholar]
- 26.Huffman C.W., Godar E.M., Torgeson D.C. Inhibition of plant growth by halogenated benzoic acids. J. Agric. Food Chem. 1967;15:976–981. doi: 10.1021/jf60154a015. [DOI] [Google Scholar]
- 27.Sparks T.C., Duke S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021;69:8324–8346. doi: 10.1021/acs.jafc.1c02616. [DOI] [PubMed] [Google Scholar]
- 28.Macias F.A., Chinchilla N., Arroyo E., Molinillo J.M., Marin D., Varela R.M. Combined strategy for phytotoxicity enhancement of benzoxazinones. J. Agric. Food Chem. 2010;58:2047–2053. doi: 10.1021/jf903445m. [DOI] [PubMed] [Google Scholar]
- 29.Arroyo E., Chinchilla N., Carrera C., Molinillo J.M., Macías F.A. First approach to enhance the physicochemical properties of 2-amino-3H-phenoxazin-3-one (APO) J. Allelochem. Interac. 2015;1:57–68. [Google Scholar]
- 30.Yamada O., Kurozumi A., Futatsuya F., Ito K., Ishida S., Munakata K. Studies on chlorosis-inducing activities and plant growth inhibition of benzophenone derivatives. Agric. Biol. Chem. 1979;43:1467–1471. doi: 10.1080/00021369.1979.10863666. [DOI] [Google Scholar]
- 31.Adams R., Bockstahler T.E. Preparation and reactions of o-hydroxycinnamic acids and esters. J. Am. Chem. Soc. 1952;74:5346–5348. doi: 10.1021/ja01141a038. [DOI] [Google Scholar]
- 32.De Martino L., Mencherini T., Mancini E., Aquino R.P., De Almeida L.F.R., De Feo V. In vitro phytotoxicity and antioxidant activity of selected flavonoids. Int. J. Mol. Sci. 2012;13:5406–5419. doi: 10.3390/ijms13055406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natsume H., Seto H., Ōtake N. Studies on apple canker disease. The necrotic toxins produced by Valsa ceratosperma. Agric. Biol. Chem. 1982;46:2101–2106. doi: 10.1271/bbb1961.46.2101. [DOI] [Google Scholar]
- 34.Wang C., Li C., Li B., Li G., Dong X., Wang G., Zhang Q. Toxins produced by Valsa mali var. mali and their relationship with pathogenicity. Toxins. 2014;6:1139–1154. doi: 10.3390/toxins6031139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaertner E.E. Studies of seed germination, seed identification, and host relationship in dodders, Cuscuta spp. Mem. Cornell Agric. Exp. Stn. 1950;294:1–56. [Google Scholar]
- 36.Cala A., Zorrilla J.G., Rial C., Molinillo J.M.G., Varela R.M., Macías F.A. Easy access to alkoxy, amino, carbamoyl, hydroxy, and thiol derivatives of sesquiterpene lactones and evaluation of their bioactivity on parasitic weeds. J. Agric. Food Chem. 2019;67:10764–10773. doi: 10.1021/acs.jafc.9b03098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.