Abstract
Ruthenium red staining of Cryptosporidium parvum oocysts revealed the presence of a carbohydrate matrix on their outer bilayers that is characteristic of a glycocalyx. Surface labeling of intact oocysts identified material of high molecular weight (>106) that reacted positively with sera from cryptosporidium-infected patients and with immunoglobulin A monoclonal antibodies.
Cryptosporidium parvum is a coccidian protozoa which causes severe diarrhea in patients with AIDS (3, 14). In parasitic infections, the surface coat of the parasite, which forms the interface between the parasite and its environment, must facilitate parasite survival in both the extracellular and intracellular stages of the life cycle. The principal components of this surface coat, for example, the glycoproteins in Trypanosoma brucei (9), the lipophosphoglycans (13), and the glycoinositolphospholipids in Leishmania spp. (8), form a dense glycocalyx (GX) which effectively covers the entire surface of the parasite. The GX plays an important role in several organisms by modulating resistance to proteolysis (13), antibody binding (12), and adhesion (4). The present investigation is centered on the morphological, biochemical, and immunological characterization of the surface of the C. parvum oocyst.
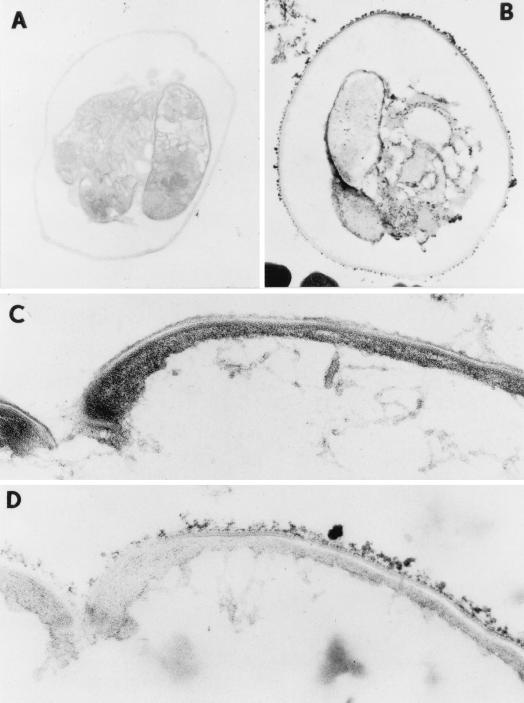
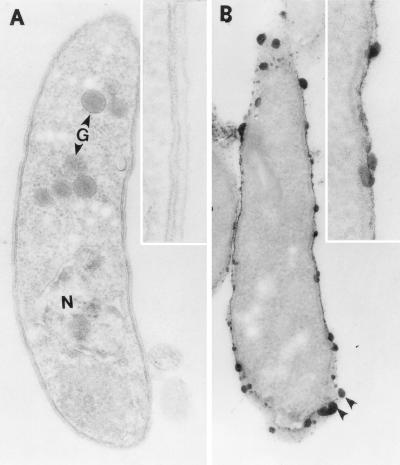
Oocysts collected from stools from AIDS patients diagnosed with active cryptosporidiosis were purified (2), fixed, and stained with ruthenium red to characterize the carbohydrate-rich GX (6, 7). Transmission electron micrographs of an osmium-fixed oocyst show three visible sporozoites parallel to one another with their anterior ends all pointing in the same direction (Fig. 1A). Higher magnification shows that the oocyst is composed of two electron-dense layers (50 nm thick) (Fig. 1C) separated by a thin electron-lucent space. Ruthenium red staining of the oocyst shows a regularly spaced array of dense aggregates (20 to 30 nm thick) (Fig. 1B and D). In addition, some electron-dense stained material was seen inside the oocyst on the surfaces of sporozoites, suggesting that the GX may be present throughout sporozoite development. To confirm this, sporozoites were isolated, fixed, and stained with ruthenium red. Transmission electron micrographs (Fig. 2A) show crescent-shaped sporozoites averaging 4.8 by 1.2 μm in size with prominent nuclei and dense granules. Higher magnification shows that each surface is comprised of a trilaminar membrane (Fig. 2a, inset). The ruthenium red staining pattern was restricted to irregularly spaced 15- to 20-nm electron-dense bodies (Fig. 2b).
FIG. 1.
Visualisation of the surfaces of C. parvum oocysts with ruthenium red staining. (A) Transmission electron micrograph of an unstained oocyst showing three sporozoites. (B) Ruthenium red stain showing a regularly spaced array of dense aggregates. (C and D) Higher magnification (×50,000) of the surface of the oocyst showing two 50-nm-thick electron-dense layers (C) and dense 20- to 30-nm-thick ruthenium red-stained aggregates (D).
FIG. 2.
(A) Electron micrograph of an unstained sporozoite showing a prominent nucleus (N) in the posterior third of the body and dense granules (G) in the anterior. The inset shows a higher magnification of the surface of the trilaminar membrane. (B) Ruthenium red stain of the sporozoite showing that the stain is restricted to dense bodies. The inset shows a higher magnification of the stained bodies, which are 15 to 20 nm in size.
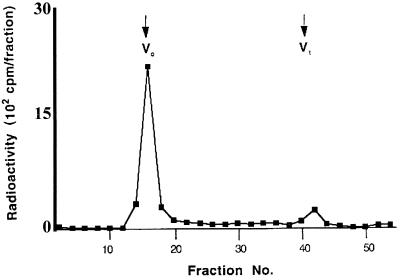
To characterize the ruthenium red-stained material on the surfaces of oocysts, we used a reductive procedure employing NaB3H4 and periodate oxidation, which is known to label only the surface of an organism (11). Labeled oocysts were subjected to 85% phenol to disassociate the GX into its aqueous phase, dialyzed, and chromatographed on Sepharose Cl-6B in the presence of 0.1% sodium dodecyl sulfate (SDS) (1, 10). About 90% of the dialyzed labeled material eluted in the void volume, indicating that it had a molecular mass of >106 Da (Fig. 3). The yields of protein and carbohydrate from 2 × 107 oocysts averaged 8 and 40 μg, respectively, after SDS chromatography. The high-molecular-weight material was highly resistant to proteases (trypsin, proteinase K, pronase, and thermolysin) and remained totally excluded from the running gel in SDS-polyacrylamide gel electrophoresis with or without proteolytic treatments (data not shown). Carbohydrate composition analysis indicated that glucose was the predominant sugar (65%), followed by galactose (12%). Mannose, xylose, and ribose were present in small amounts (4 to 8%). Both an alditol acetate derivative and a trimethylsilyl method showed that GalNAc was the only amino sugar present. In addition, trace amounts of a C18 fatty acid was identified in the preparations by its characteristic fragmentation pattern.
FIG. 3.
Sepharose Cl-6B elution profile of material from oocysts labeled with iodate-NaB3H4. Dialyzed material labeled with iodate-NaB3H4 was fractionated on a Sepharose Cl-6B column in the presence of SDS and calibrated with blue dextran to obtain the void volume (V0) and with cytochrome c to obtain the total (Vt). Ninety percent of the labeled material eluted as a peak at the void volume.
Studies of the antigenic composition of the oocyst wall have shown that carbohydrate moieties comprise a significant proportion of the epitopes that bind to antibodies in the immune response to C. parvum (15). The GX reacted positively on immunoblots with cryptosporidium-infected human sera (1/25 dilution) (Fig. 4B), indicating that it is antigenic. No such reactivity was observed with normal human sera (Fig. 4A). In addition, the purified GX also recognized two monoclonal antibodies (3D8 2B11 and 3F101G3) raised in BALB/c mice by repeated injections of C. parvum oocyst extracts (5) in a standard enzyme-linked immunosorbent assay and this reactivity was found to be specifically of the immunoglobulin A (IgA) type (Table 1). Treatment of GX with periodate at a concentration (10 mM) known to cleave specifically carbohydrate vicinal hydroxyl groups (16) abolished the reactivities of both the antibodies by about 50% (data not shown), suggesting that the epitope is glycosylated. Higher concentrations of periodate did not show any further decrease in the inhibition of binding. Partial inhibition of binding may have been due to incomplete removal of hydroxyl groups due to stearic hindrance. The high molecular mass of the GX distinguishes it from previously reported oocyst surface antigens ranging in molecular mass from 40 to 250 kDa (15).
FIG. 4.
Immunoblot of high-molecular-weight material. The high-molecular-weight material was slot blotted onto nitrocellulose at two different concentrations (0.1 and 1.0 μg of carbohydrate) and blocked with 5% nonfat dry milk. The blot was reacted with normal human sera (A) or sera from human patients with cryptosporidiosis infections (B) (1/25 dilution) and then with peroxidase-conjugated protein A. The blot was developed with 4-chloro-1-napthol as the substrate. All incubations and washings were done at room temperature. The high-molecular-weight material was highly antigenic.
TABLE 1.
Monoclonal antibody reactivity with high-molecular-weight material
| Monoclonal antibody | Mean level of reactivity (OD405) ± SD to:
|
||
|---|---|---|---|
| IgM | IgG | IgA | |
| 3D8 2B11 | 0.20 ± 0.08 | 0.19 ± 0.05 | 1.22 ± 0.175 |
| 3F10 1G3 | 0.13 ± 0.042 | 0.14 ± 0.038 | 1.13 ± 0.148 |
| 2F4 2H8 | 0.31 ± 0.02 | 0.31 ± 0.04 | 0.04 ± 0.05 |
In this study, we identified a polysaccharide matrix on the surface of a C. parvum oocyst that meets all the characteristics of a GX and is antigenic. First, ruthenium red-stained preparations of oocysts and sporozoites viewed by electron microscopy revealed uniformly distributed aggregates on the surfaces of C. parvum oocysts and randomly distributed vesicles on the surfaces of C. parvum sporozoites. Second, the GX was labeled by a periodate-NaB3H4 procedure which labels only the surface of a parasite. Third, 90% of the labeled material had an apparent molecular mass of >106 Da in the presence of SDS, indicating that the material is not likely due to aggregation. Fourth, compositional analyses showed that 82% of the total mass was carbohydrate, with glucose being the abundant sugar. The resistance of the GX to proteases may be due to a putative peptide backbone being concealed by the abundance of carbohydrate. Resistance may be of biological importance, as it may impart structural and functional stability under gastrointestinal conditions. However, apart from protein estimation, there is no evidence that the GX includes a peptide backbone. Indeed it is possible that a glycolipid moiety is responsible for anchoring the GX of an oocyst.
Of particular interest, the high-molecular-weight carbohydrate material from C. parvum oocysts reacted positively with sera from cryptosporidium-infected patients and with IgA monoclonal antibodies raised against C. parvum oocyst extracts, demonstrating that it is antigenic. Additionally, partial loss of the antigenicity of the GX after mild periodate oxidation treatment indicated that carbohydrate is the major antigenic determinant. Since GX is the first protein with which the host comes into contact and because of its carbohydrate antigenicity, it may be an important immunological target.
Acknowledgments
This work was supported by a grant from the NIAD, NIH (KO8 AI01085).
REFERENCES
- 1.Caulfield J P, Cianci C M, McDiamid S S, Suyemitsu T, Schmid K. Ultrastructure, carbohydrate, and amino acid analysis of two preparations of the cercarial glycocalyx of Schistosoma mansoni. J Parasitol. 1987;73:514–522. [PubMed] [Google Scholar]
- 2.Flanigan T P, Aji T, Marshall R, Soave R, Aikawa M, Kaetzel C. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect Immun. 1991;59:234–239. doi: 10.1128/iai.59.1.234-239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanigan T P, Soave R. Cryptosporidiosis. Prog Clin Parasitol. 1993;3:1–20. doi: 10.1007/978-1-4612-2732-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Giannasca K T, Giannasca P J, Neutra M R. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect Immun. 1996;64:135–145. doi: 10.1128/iai.64.1.135-145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kipps T J, Herzenberg L A. Schemata for the production of monoclonal antibody-producing hybridomas. Handb Exp Immunol. 1986;4:108–111. [Google Scholar]
- 6.Luft J H. Electron microscopy of cell extraneous coats as revealed by ruthenium red staining. J Cell Biol. 1964;23:54A–55A. [Google Scholar]
- 7.Lumsden R D. Surface ultrastructure and cytochemistry of parasitic helminths. Exp Parasitol. 1975;37:267–339. doi: 10.1016/0014-4894(75)90078-8. . (Review.) [DOI] [PubMed] [Google Scholar]
- 8.McConville M J, Ferguson M A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon A K, Schwarz R T, Mayor S, Cross G A. Cell-free synthesis of glycosyl-phosphatidylinositol precursors for the glycolipid membrane anchor of Trypanosoma brucei variant surface glycoproteins. Structural characterization of putative biosynthetic intermediates. J Biol Chem. 1990;265:9033–9042. [PubMed] [Google Scholar]
- 10.Nanduri J, Dennis J E, Rosenberry T L, Mahmoud A F F, Tartakoff A M. Glycocalyx of bodies versus tails of Schistosoma mansoni cercariae. J Biol Chem. 1991;266:1341–1347. [PubMed] [Google Scholar]
- 11.Samuelson J C, Caulfield J P. The cercarial glycocalyx of Schistosoma mansoni. J Cell Biol. 1985;100:1423–1434. doi: 10.1083/jcb.100.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuelson J C, Caulfield J P. Cercarial glycocalyx of Schistosoma mansoni activates human complement. Infect Immun. 1986;51:181–186. doi: 10.1128/iai.51.1.181-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turco S, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 14.Vakil N, Schwartz S, Buggy B, Brummitt C, Kherellah M, Letzer D, Gilson I, Jones P. Biliary cryptosporidiosis in HIV-infected people after the waterborne outbreak of cryptosporidiosis in Milwaukee. N Engl J Med. 1996;334:19–23. doi: 10.1056/NEJM199601043340104. [DOI] [PubMed] [Google Scholar]
- 15.Ward H, Cevallos A M. Cryptosporidium: molecular basis of host-parasite interaction. Adv Parasitol. 1998;40:151–178. doi: 10.1016/s0065-308x(08)60120-7. [DOI] [PubMed] [Google Scholar]
- 16.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]