Abstract
The gut microbiota is the most abundant and diverse microbiota in the human body and the vagus nerve is the most widely distributed and complex nerve in the body, both of them are essential in maintaining homeostasis. The most important phenomenon is how they coordinate to regulate functions, which has attracted the great attention of scientists. The academic literature on the correlation with a host of intestinal diseases and even systemic diseases has revealed the bidirectional communication between the gut microbiota and the brain, which can be carried out via multiple patterns. In the review, firstly, we have a general overview of the gut microbiota and the gut microbiota-brain axis. Secondly, according to the distribution characteristics of the vagus nerve, we analyzed and summarized its function in the intestinal tract. At the same time, we have summarized the underlying mechanism of some behavior changes such as depressive and anxiety-like behaviors and related neurodegenerative diseases caused by the vagus nerve and intestinal microecological environment disorders, and then we also analyzed inconsistency of the experimental evidence in order to propose novel strategies for the clinical practice.
Keywords: gut microbiota-brain axis, gut microbiota, brain, vagus nerve, behavior, neurodegenerative diseases
Introduction
Gut microbiota is one of the most abundant and diverse microorganismsin the human body, which is associated with many diseases, even systemic diseases.1 Apart from exploring the connection between the gut flora and gut-related diseases, researchers have now found the gut microbiota and the brain are inextricably linked due to substantial evidence. The gut microbiota-brain axis, regarded as a bridge and link between the gut microbiota and the brain, can be achieved through the neuroanatomical pathways such as vagus nerve (VN) or spinal cord, the neuroendocrine system such as the hypothalamic-pituitary-adrenal axis (HPA axis), and cytokines, as well as direct or indirect chemical pathways that may involve microbial metabolites and some specific neurotransmitters.2,3 Within these mechanisms, how the VN, one of the longest and most widely distributed cranial nerves in the body, connects the gastrointestinal tract to the brain and coordinates functions has attracted great attention fromscientists. Therefore, we summarize the structure and functional characteristics of the VN, focusing on how it serves as a bridge linking the gut microbiota to the brain, and the underlying mechanisms through which VN plays a role in behavioral changes and degenerative diseases induced by gut microbes. Finally, but more than that, we also look forward to and comment on the future prospects of clinical practice.
Gut Microbiota-Brain Axis
Gut Microbiota
As the most abundant and diverse microbiota in the human body, the gut is a complex microecosystem inhabited by a variety of microorganisms, such as bacteria, parasites, archaea, fungi, and viruses.1 Among them, anaerobic bacteria such as Bacteroidetes and Firmicutes are the main bacteria, which dominate the gut environment. However, other bacterial phylotypes such as Proteobacteria, Actinomyces, Fusobacterium, and Verrucomicrobia only accounts for a small part of the system.4 As the home of 4 trillion microbes, with more than 1000 species, it is not surprising that 99% of the human genetic composition comes from the genetic material carried by the intestinal microbiota.5 At present, the theory of the origin of intestinal flora has been generally accepted. First of all, before the birth of the first child, the mother’s bacteria are transported to the fetus through the placenta, amniotic fluid, and circulatory system, based on the results that Propionibacterium, Escherichia, Staphylococcus, as well as Enterococcus were detected in the blood of mice which was isolated from the umbilical cord by PCR.5,6 Secondly, in the process of natural childbirth, the gastrointestinal tract will be customized by the microorganisms in the mother’s uterus and vagina.7 Although the gastrointestinal tract has been colonized by a variety of microorganisms at birth, the stability of the microbiota is acquired through development with the host, which requires an interaction of host genetic and environmental factors, including but not limited to region, diet, stress, and use of antibiotics.1 In view of the fact that the existing technology cannot isolate and culture all the gut flora, the effective quantification and characterization of intestinal flora through targeted sequencing and metagenomic sequencing technology will be of great benefit to the study of the gut flora.8
In the past few years, whether in basic research or clinical research related to diseases, the gut microbiota has undoubtedly become a hotspot. According to the functional research, gut microbiota, as a part of the host, participates in the digestion and absorption of nutrients. It is particularly noteworthy that short-chain fatty acids (SCFA) produced or metabolized by intestinal microorganisms during the process of decomposing dietary fiber can be a significant energy source for intestinal mucosa and microorganisms in the gut, play a vital part in modulating immune responses, and is even closely related to the occurrence, development and metastasis of intestinal tumors.9 It is worth mentioning that there is a complicated and bidirectional relationship between intestinal microorganisms and the host’s immune system.8 The gut microbiota can not only protect the human body by forming a “membrane barrier”, but also furnish pathogen-associated molecular patterns (PAMP) as ‘molecular signatures’ and metabolites such as SCFA to accelerate the establishment and development of the immune system.10,11
Novel methodological adoption to the investigation of the gut microbiota drive trend to the contributions in deeper insight on the role of the gut microbiota in gastrointestinal disorders, such as inflammatory bowel disease (IBD), irritable bowel syndrome, non-alcoholic fatty liver disease, and even prognosis of malignant tumor. Take IBD as an example, by using 16S rRNA gene sequencing to detect and compare the intestinal microbial composition of 26 Crohn’s disease (CD) patients and 14 non-IBD controls, Nishino et al showed an evident decline in the abundance of the phyla Firmicutes and Bacteroidetes, along with an obvious elevation in the abundance of the phylum Proteobacteria in CD patients.12 In recent years, with the gradual deepening of research, researchers have expanded their horizons beyond digestive tract diseases and found that there is an inseparable link between the gut microbiota and brain, such as neuropsychiatric ailment of the depression and autism spectrum disorders, and the neurodegenerative diseases of Parkinson’s disease, Alzheimer’s disease as well as amyotrophic lateral sclerosis (ALS).2 Based on experimental evidence and correlation analysis, we have made a detailed summary of the changes in the correlation between intestinal flora and neuro-psychiatric diseases, as shown in Table 1. From the above results, we can see that the occurrence of neuro-psychiatric diseases is closely related to the imbalance of intestinal flora, but their underlying mechanisms still keep uncovered.
Table 1.
Gut Microbiota Changes in Brain Disorders
| Brain Disorders | Subjects | Specimen | Gut Microbiota with Increased Abundance | Gut Microbiota with Decreased Abundance | Refs |
|---|---|---|---|---|---|
| Depression | MDD (n=43) HCs (n=47) |
Fecal samples | Phylum Bacteroidetes Classe Gammaproteobacteria, Bacteroidia Order Bacteroidales |
Phylum Firmicutes Class Clostridia Order Clostridiales Family Ruminococcaceae, Christensenellaceae |
[96] |
| Anxiety | GAD (n=12) HCs (n=17) |
Stool samples |
Bacteroides, Escherichia-Shigella Lactobacillus, Fusobacterium Ruminococcus gnavus |
Faecalibacterium, Eubacterium rectale Roseburia, Subdoligranulum Lachnospira |
[97] |
| Autism spectrum disorders | ASD (n=40) HCs (n=40) |
Stool samples |
Collinsella, Lactobacillus Corynebacterium, Dorea |
Alistipes, Bilophila, Veillonella Dialister, Parabacteroides |
[98] |
| Parkinson’s disease | PD (n=197) controls (n=130) |
Stool sample | Family Bifidobacteriaceae, Lactobacillaceae Tissierellaceae, Christensenellaceae Verrucomicrobiaceae Genus Bifidobacterium, Lactobacillus Akkermansia |
Family Lachnospiraceae, Pasteurellaceae Genus Blautia, Roseburia Faecalibacterium |
[99] |
| Alzheimer’s disease | AD (n=43) HCs (n=43) |
Fecal samples | Phylum Actinobacteria Class Actinobacteria and Bacilli Family Ruminococcaceae, Enterococcaceae Lactobacillaceae |
Phylum Bacteroidetes Class Negativicutes, Bacteroidia Family Lachnospiraceae, Bacteroidaceae Veillonellaceae |
[100] |
| Amyotrophic lateral sclerosis | ALS (n=20) HCs (n=10) |
Fecal samples | Phylum Bacteroidetes Genus Kineothrix, Parabacteroides, Odoribacter Sporobacter, Eisenbergiella, Mannheimia Anaerotruncus unclassified Porphyromonadaceae |
Phylum Firmicutes Genus Megamonas |
[91] |
Abbreviations: HC, healthy controls; MDD, major depressive disorder; GAD, generalized anxiety disorder.
Gut Microbiota–Brain Axis is a Complicated Network
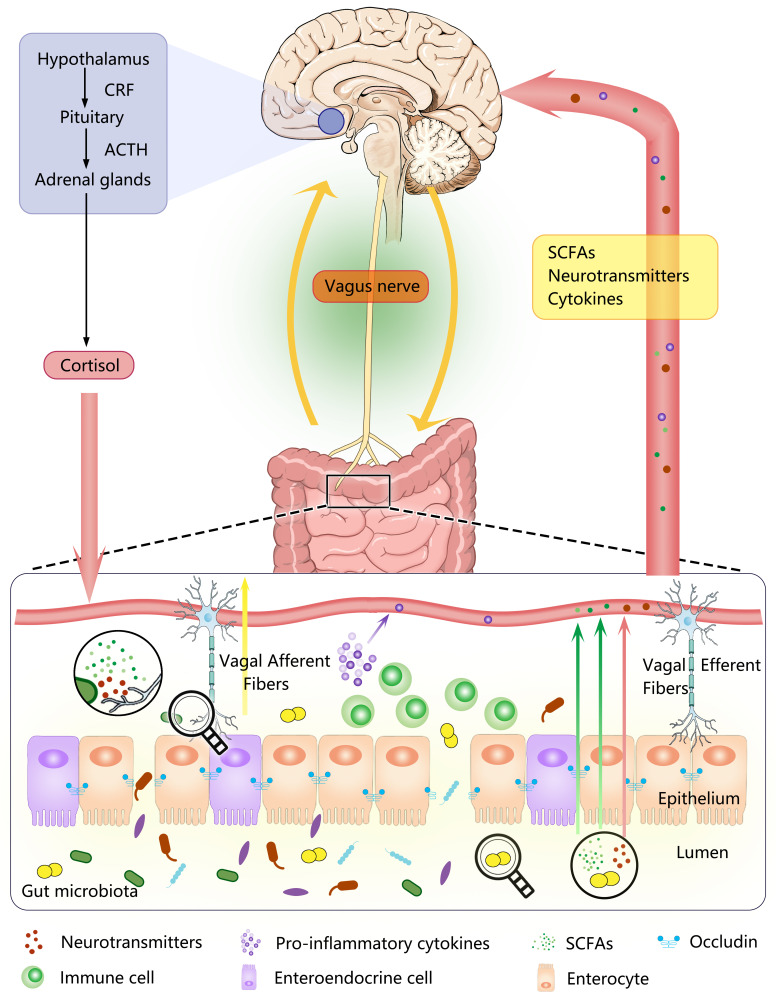
The gut microbiota-brain axis is always regarded as “bridge” or a kind of bidirectional communication medium in other words between the gut microbiota and the brain, which is based on complex biological systems and involved in multiple mechanisms to preserve the homeostasis of the gastrointestinal tract, central nervous system (CNS), and microbial system.2 Although the specific mechanisms of this bidirectional communication between the intestinal microbiota and the brain remain unclear, current studies suggest that this communication can be carried out directly and indirectly (depicted in Figure 1).2,3 The first is the direct exchange of information between the gut and the brain through neuroanatomical approach, exchanging information directly between the intestinal tract and brain by the VN, the pelvic splanchnic nerve and some branches of the sympathetic nerve in the spinal cord, and also through the neuroendocrine system, such as the HPA-axis based on the evidence that plasma ACTH and corticosterone concentrations in germ-free mice were significantly increased in response to stress inhibition compared with specific pathogen-free mice.13 In addition, the gut microbes can communicate with the brain directly or indirectly through chemical signals. For instance, SCFA produced by the gut microbes, which can involve in the regulation of the neuroplasticity, epigenetics, and gene expression, thereby affecting the functional condition of the CNS.14 Within the indirect chemical pathway, it contributes to the synthesis and release of γ-aminobutyric acid (GABA), serotonin, dopamine by microbes, and at present, bacterial enzymes that are involved in the case can also produce neurotoxin products such as D-lactic acid and ammonia.4 Specifically, enteroendocrine cells (EECs) release the neurotransmitter serotonin when stimulated by microbial metabolites including SCFAs, indole, and tyramine.15 Of course, some microorganisms, including Bifidobacterium, Bacteroides, Parabacteroides, as well as Escherichia spp. also have the ability to produce and release neurotransmitters GABA.16 Another possible underlying pathway is through the immune system, based on the evidence that the abnormal brain immune signals and exacerbated peripheral inflammation reactions have been detected in many neuropsychiatric disorders. Cytokines produced by the gut microbes, such as IL-1α, IL-1β, IL-6 and TNF-α,17,18 can eventually enter the CNS through the blood-brain barrier (BBB). Despite the multiple mechanisms, the VN route is probably the fastest and the most direct way for the interplay between the gut microbes and the brain. Therefore, in the next section, we will focus on summarizing the role of the VN in the gut microbiota-brain axis.
Figure 1.
A schematic diagram of the Gut Microbiota-Brain Axis and the signal transduction Pathway. As a bidirectional communication linking the gut microbiota to the brain, the gut microbiota-brain axis can be achieved through the neuroendocrine system (the hypothalamic-pituitary-adrenal axis), the neuroanatomical pathways (vagus nerve), cytokines, as well as direct or indirect chemical pathways that may involve microbial metabolites (SCFA) and some specific neurotransmitters.
Figure 3.
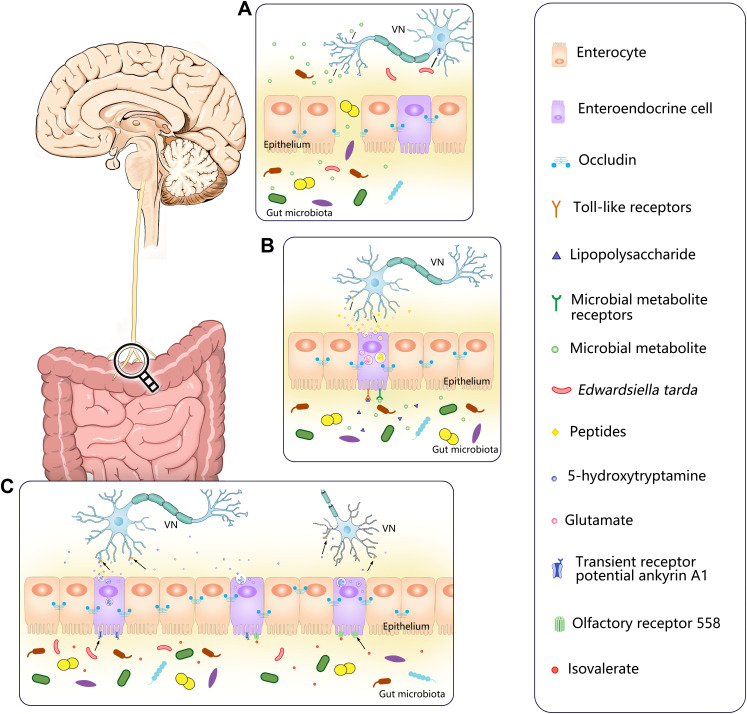
Pathways of vagus nerve collection of multiple signals from the gut microbiota. (A) When the intestinal epithelium is destroyed, the gut microbiota and their metabolites have the opportunity to invade and directly contact with VN, activating the vagal afferent fibers through specific receptors or more direct ways. Edwardsiella tarda can bind to the receptor transient receptor potential ankyrin A1 (TRPA1) which are widely distributed on nodose ganglia (NG) to directly stimulate NG. (B) Enteroendocrine cells can recognize bacterial products in the intestinal lumen, such as LPS, via toll-like receptors (TLR) on its surface or use other receptors to recognize microbial metabolites, such as SCFA, then transmitted intestinal signals to vagal afferents through synaptic connections using glutamate as a neurotransmitter or release more than 30 known peptides to stimulate vagal afferents. (C) Edwardsiella tarda can activity enteroendocrine cells via the receptor transient receptor potential ankyrin A1 (TRPA1) to directly stimulate vagal sensory ganglia by releasing the neurotransmitter 5-hydroxytryptamine (5-HT). Olfactory receptor 558 of enteroendocrine cells can act as a sensory receptor for microbial metabolites, accepting isovalerate stimulation. Then enteroendocrine cells release 5-HT which binds to the 5-HT receptors on vagal afferents so as to realize the transmission of intestinal information. The arrows in the figure indicate neurotransmitters, bacteria or bacterial products bound to the corresponding receptors.
Multiplicity of Vagus Nerve Action
As we all know, the VN is the longest and most widely distributed autonomic nerve that originates in the brain stem and extends down through the neck and into the chest and abdomen. It possesses both motor and sensory information and supplies innervation to the multiple systems, which is involved in critical aspects of human physiology, including the heart rate, blood pressure, sweating, digestion, and even speaking.19 However, the issue of the VN innervation has always been controversial, a previous study of 29 rats injected with cholera toxin-horseradish peroxide into the colon has indicated that VN can dominate all the gastrointestinal tract except the rectum.20 But in human, previous theories have suggested that the vagus nerve innervates the human colon before it reaches ahead of spleen bends of the colon, while another study reported that VN can innervate the entire digestive tract in humans, as it does in rats.19 It has been accepted for a century and furthermore improved to better utilize observed evidence that the VN is a parasympathetic nerve containing afferent fibers, 80% of which are responsible for transmitting taste, visceral and somatic sensations, as well as efferent fibers, 20% of which are involved in the regulation of that gastrointestinal, heart and lungs functions,21 presenting a likely bridge act as a signal bridge in the maintenance of communication between CNS and multiple systems. Therefore, in the next section, we mainly hit the high role of vagus nerve in the regulation of digestive system immunity and intestinal flora, in order to find novel hot spots and provide new valuable clues for the future clinical treatment of related diseases (depicted in Figure 2).
Figure 2.
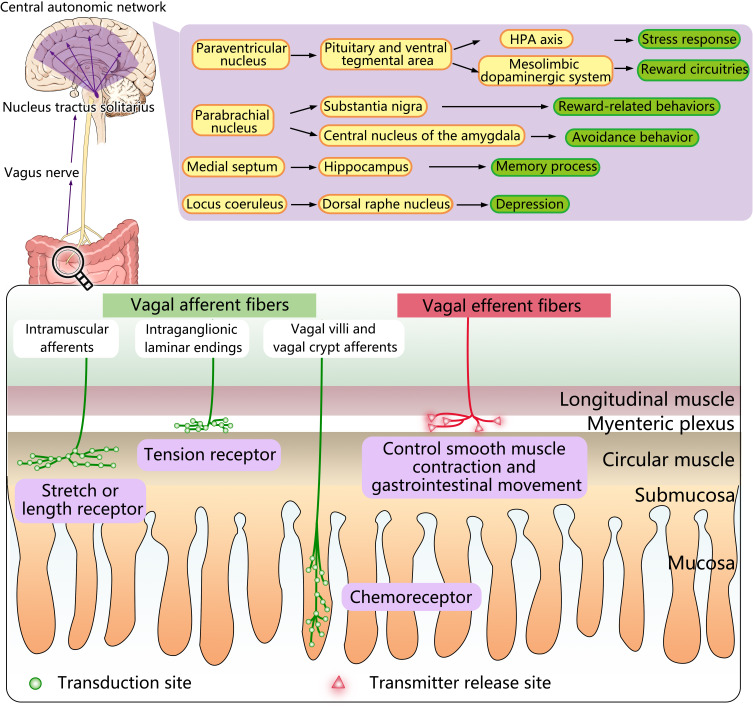
Diagram of the vagus nerve anatomy and mode of action. As a mixed nerve, the vagus nerve is composed of 80% afferent fibers and 20% efferent fibers. There are three types of afferent endings: the intraganglionic laminar endings which are located in the myenteric plexus, serve as tension receptors; the intramuscular afferents which are also located in the muscle layer, are characterized as a stretch or length receptor; vagal villi, and vagal crypt afferents terminate at the top of the villus near the epithelial layer or surround the luminal end of intestinal crypts and is regarded as chemoreceptors. The vagus nerve can be activated by the gut microbiota, transmitting information from the gastrointestinal tract to the NTS which can transmit information further into the central autonomic network through a variety of neuronal pathways and lead to specific effects. The efferent vagus nerve, which extends into the myenteric plexus, releases different neurotransmitters to regulate smooth muscle contraction and gastrointestinal movement.
Vagal Afferent Fibers
The afferent fibers of the vagus nerve originate from endings located in the different layers of the intestinal wall, such as in the mucosal lamina propria, the external muscle layers, as well as myenteric plexus, from which the cell bodies are located in the nodose ganglia (NG), pass through the VN and terminate in the nucleus tractus solitarius (NTS) based on a viscerotropic distribution.22 According to the morphological structure and distribution characteristics, the endings of vagal afferent fibers can be categorized as three types: (1) the intraganglionic laminar endings (IGLEs), located in the intestinal myenteric plexus, between the longitudinal and circular muscle layers, which might act as muscle tone receptors in response to muscle tension caused by both passive stretch and active contraction of the muscle layers in the gastrointestinal tract;22 (2) the intramuscular arrays (IMAs), as a stretch or length receptor and equivalent to the muscle spindle afferents, which are also located in the muscle layer and travel in the same direction as the smooth muscle fibers;23 (3) the mucosal endings, terminating mostly at the top of the villus, or surrounding the end of intestinal crypt lumen.1 Although these vagal afferents have long been widely known as a free nerve terminals, Kaelberer et al used a mouse model to corroborate that EECs and vagal afferents in the luminal part of the crypt of the distal colon can transmit intestinal signals through synaptic connections by glutamate.24
The afferents endings of the VN are involved in the formation of chemoreceptors, thermoreceptors, osmoreceptors, mechanoreceptors.25 Accumulating evidence suggests that gut hormones and regulatory peptides such as ghrelin, cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY) can bind to specific chemoreceptors, thereby have an impact on the regulation of food intake and energy balance.26 Regardless of the type of vagal afferent information, vagal afferent fibers activate ionic and metabolic receptors corresponding to NTS with glutamate as the main neurotransmitter.27 The NTS is located in the medulla, specifically dorsolateral to the dorsal motor nucleus of the vagus (DMNV), where the efferent fibers of the VN with the nucleus ambiguous originate.21 Therefore, there is no reason not to doubt whether there is a strong connection between the NTS and the DMNV. In fact, by injecting cholera toxin-HRP conjugate (CT-HRP) into the dorsal and ventral muscle tissue of the stomach wall and using tetramethylbenzidine or diaminobenzidine histochemistry to visualize centrally transported tracer, one study corroborated the presence of retrogradely labeled gastric DMNV neurons and observed a connection between their dendrites and vagus afferent endings at the NTS, where the vago-vagal reflex loop begin.28 VN afferent fibers that transmit information to the central autonomic network (CAN) for analysis and integration. In this process, the NTS acts as a relay, projecting vagal afferent signaling information to CAN such as the parabrachial nucleus (PBN), the locus coeruleus (LC), the hypothalamus, especially the paraventricular nucleus of the hypothalamus (PVH), as well as the limbic system including the thalamus, amygdala, hippocampus (HPC), and the prefrontal cortex and the insula of the brain.29 The CAN can modulate the functioning of the HPA axis response and the ANS. Moreover, the VN is also partly involved in the interoceptive awareness and a perturbation of this interoception, which may be associated with psychiatric disorders, neurodegenerative and neurological disorders.21 The above conclusions give us a great hint that the disorder of nerve function dominated by VN may be closely related to the nervous system diseases, which provides an important insight for future research and clinical practice.
Vagal Efferent Fibers
Vagal efferent fibers stem from the pre-ganglionic neurons located in the DMNV, go down with the vagal preganglionic fibers to the post-ganglia neuron, which located the intermuscular plexus between the longitudinal and annular muscles of the enteric nervous system (ENS).30 As pelvic parasympathetic pre-ganglia neurons, most of the neurons of DMNV are cholinergic neurons, which synthesize and release acetylcholine (Ach) specifically binding to nicotinic receptors (N-type receptors) located in the post-ganglia neurons, followed by the post-ganglia neuron to regulate gastrointestinal dynamics through two patterns: one of which is synchronization of acetylcholine excitation, which causes smooth muscle contraction and enhances smooth muscle movement. The other is through non-cholinergic pathway to achieve the opposite effect in regulating smooth muscle.31 Cruz et al microinjected baclofen, a GABAB agonist, into the DMNV of anesthetized rats and found that the enhancement of gastric tension and motility was strongly dose-dependent but could be attenuated by ipsilateral vagotomy, and could be offset by atropine pretreatment, suggesting that both pathways stemmed from DMVN, and can be activated simultaneously by neurons from NTS simultaneously, resulting in double effects on gastrointestinal motility regulation by reducing nerve activity.32
The vago-vagal reflex loop is composed of vagal afferents and efferents of VN. It can not only regulate gastrointestinal motility, but also adjust the secretion of glands, mucosal blood flow, and intestinal immunity via various pathways. The first pathway is that the vagal afferent fibers activate the HPA axis, which in turn causes the adrenal glands to release cortisol.33 In addition, Wang et al34 observed that TNF synthesis was inhibited in wild-type mice after electrical stimulation of the VN; however, this phenomenon did not occur in α-7-deficient mice, and the results implied that ACh from vagal efferent fibers could inhibit the secretion of TNF-α and exert anti-inflammatory effects by binding to α-7-nicotinic ACh receptors (α7nAChR) of macrophage.35 The last way is that the VN stimulates the splenic sympathetic nerve, causing the distal splenic nerve to release norepinephrine (NE), which can bind to β2 adrenergic receptors on splenic lymphocytes to release Ach. Eventually, Ach inhibits the release of TNF-α from splenic macrophages through α7nAChR.33 Thus, the role of the VN in the gut is clear, but the pathways and processes are complex. Meanwhile, a growing body of research is also revealing the significant part of the VN in the relationship between the intestinal microbiota and behavior and neurodegenerative diseases, which is what we will further clarify in the following section.
How Does Vagus Nerve Pick Up Multiple Signals from the Gut Microbiota?
As mentioned above, we already know that vagal afferent fibers can sense various intestinal information, whether mechanical or chemical, and then pass into NTS along with the VN, and then project to the CNS, creating different behaviors and effects. Although vagal afferent fibers originate from terminal in the diverse layers of the intestinal wall, it is not possible for vagal afferent fibers to directly access to gut microbiota as vagal afferents have no chance to penetrate the intestinal epithelium except in pathological conditions where the integrity of the gut epithelium is destroyed and create an opportunity for microbial invasion.
The subepithelial compartment may provide a sanctuary for communication between enteral nerves and the gut microbiota.3 A couple of experiments have indicated that the gut microbiota is sensed by IPANs of the ENS.3 By feeding rats with vehicle control or 109 Lactobacillus reuteri (LR) for 9 days and using patch-clamp recordings, Kunze et al detected that the intrinsic excitability of rat colon myenteric IPANs increased in the LR group with a decrease of the slow after hyperpolarization (sAHP) in sensory AH neurons and calcium-dependent potassium channel (IKCa) opening.36 Similarly, another study using probiotics including Lactobacillus rhamnosus (JB-1) further convinced that the sensory responses of IPANs could be recorded within 8 seconds after treatment with JB-1 and excitability promoted within 15 minutes, and the influence of JB-1 on the intrinsic excitability of sAHP and IPANs can be imitated by TRAM-34, a specific IKCa blocker, suggesting that the molecular mechanism which probiotics or its PSA activate IPANs by inhibiting IKCa channel.37 Both the above animal studies and other research have demonstrated the significance of probiotics on the normal excitability of intestinal sensory neurons and may affect gut motility and pain perception.36–38 Additionally, evidence is mounting that the acquisition of gut microbial signals by the VN might require dissemination of microbial metabolites or compounds, or signal transmission by certain cells that reside in the intestinal epithelium, and depends on multiple receptors of the VN.
Lal et al have investigated different mechanisms of the activation of vagal afferent fibers by short-chain fatty acids. When anesthetized male Wistar rats were injected with equal amounts of normal saline, sodium oleate, and sodium butyrate, the characteristic vagal afferent fibers discharge was observed after induction of both fatty acids, which was different from normal saline. However, this reaction was eliminated after subdiaphragmatic vagotomy, while the influence of oleate but not butyrate was eliminated by Devazepide, a CCK-A receptor antagonist, suggesting that oleate belonging to long fatty acids and activates vagal afferent fibers via CCK-mediated mechanism, whereas butyrate, as a short fatty acid, seems to take a relatively more direct approach to stimulate afferent terminals39(Figure 3A).
On the other hand, the VN can also directly recognize signals from gut microbes, because TLR 2, 3, 4, and 7 are expressed on the membrane surface of the VN, as well as in both neurons and glial cells present in the ENS which was confirmed by combining experiments.40 It is generally believed that vagal afferent neurons can be activated by LPS from transcellular or paracellular transport, which in the long run can lead to NG inflammation and impair the transmission of gut satiety signals in the brain, and even result in neurodegenerative diseases.41 Interestingly, subdiaphragmatic vagotomy seems not to restrain the peripheral LPS-induced brain-mediated behavioral and neural effects;42 one study has noted this and the mRNA and protein expression of TLR4 were detected in NG of rats, implying that vegal afferent nerve can be activated by LPS at the NG level.43 In addition to being equipped with TLR4, the strong and diffuse expression of the transient receptor potential ankyrin A1 (TRPA1) has been detected in the NG by immunostaining, where it functions as a sensor of environmental and endogenous chemical irritants, including allyl isothiocyanate, acrolein, and 4-hydroxynonenal, and contributes to cellular mechanisms underlying inflammatory pain.44 In the meantime, Ye et al have also proposed that bacteria can bind to TRPA1 on enteroendocrine cells,45 it is speculated that when the integrity of intestinal epithelium is destroyed, E. tarda may directly act on TRPA1 on NG and then transmit intestinal signals to the brain, which leads to changes in the structure and function of neurons in the central nervous system, thus leading to corresponding diseases. Of course, this phenomenon needs to be confirmed by future experiments (depicted in Figure 3A).
Besides these direct pathways we have discussed above, other potential approaches via a cell-mediated way have also been put forward that are worthy of consideration in the VN receiving intestinal microbial information. Enteroendocrine cells (EECs), located between the enterocytes, are the largest endocrine system in the human body, although they account for less than 1% of the mucosal cells. EECs can sense the presence of intestinal lumen contents and release more than 30 known peptides including motilin, ghrelin, CCK, glucose-dependent insulinotropic polypeptide (GIP), GLP-1 and PYY, which can also activate the relevant receptors of the vagal afferents by using c-fos expression as an indicator of neuronal activation.46 Moreover, one study using immunofluorescence staining and light-sheet confocal imaging has confirmed that EECs subgroup can directly contact with vagal afferent fibers.45 Furthermore, previous studies have shown that fluorescence intensity markedly enhanced in a subsection of vagal afferent neurons after enteric stimulation with live Edwardsiella tarda bacteria and this bacteria can activate EECs via the TRPA1 to directly stimulate vagal sensory ganglia by releasing the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) (Figure 3C). The conclusion is also based on experimental evidence that in zebrafish rather than in EEC ablated zebrafish, the amount of activated pERK+ vagal afferent neurons increased after E. tarda gavage while conversely the number of pERK+ vagal afferent neurons was obviously declined in E. tarda treated Trpa1−/− zebrafish.45 However, another recent experiment in mice corroborated that EECs transmitted intestinal information directly to vagal afferent fibers in the luminal part of the crypt of the distal colon through synaptic connections using glutamate24(Figure 3B). Apart from TRPAI, the olfactory receptor 558 was also found on EECs, which acts as a sensory receptor for microbial metabolites, accepting isovalerate stimulation47 (Figure 3C). Moreover, there are also TLR or other receptors on EECs, which can recognize bacterial products in the intestinal lumen, such as LPS and SCFA, thus effect on vagal afferents.48
Although multiple mechanisms have been proposed for how VN picks up various signals from gut microbiota, including direct recognition of gut microbiota information and EECs mediated indirect pathways, whether these mechanisms interact with each other and have specific associations with other diseases remains to be further discovered.
Effects of Vagus Nerve on Behavior
At present, many animal experiments have shed light on that gut microbiota can activate the VN, transmitting messages from the digestive tract to the NTS which can transmit information further into CAN, such as LC, PBN, hypothalamus, especially PVH, and amygdala, thalamus, HPC, insula as well as the prefrontal cortex, providing a pathway to affect emotion and motivation, among which PVH plays a more striking role. By projecting to the pituitary and ventral tegmental area (VTA), PVH is capable of providing the opportunity to directly affect the mesolimbic dopaminergic system and the HPA axis, thus permitting VN to communicate with reward circuitries and the stress response, respectively. Moreover, one study using cell-specific transneuronal tracing further convinced that information from the right vagal afferent ganglion can pass through the glutamatergic neurons of the dorsolateral PBN via NTS and eventually reach substantia nigra, and activation of this pathway will induce reward-related behaviors.49 Conversely, if vagal afferent signals reach the central nucleus of the amygdala (CeA) via PBN, avoidance behavior will be triggered by the activation of the PBN-CeA route. In addition, the connection between NTS and HPC through the medial septum (MS) has been confirmed by monosynaptic and multisynaptic virus-based tracing methods.50 In this pathway, gut information transmitted through the VN can affect the HPC-dependent memory process, based on the evidence that the hippocampal expression of neurotrophic and neurogenic markers was decreased and destroys episodic and spatial memory dependent on HPC in rats after a saporin (SAP)-based lesioning procedure.50 VN can also activate noradrenergic neurons belongs to the LC which may have some correlation with depression, and influence the serotonergic system located in the dorsal raphe nucleus (DRN) in turn, so that the NTS-LC-DRN is likely to be another neuronal pathway. In short, the vagus nerve plays an important role in the signal transmission between microorganisms and the brain, and the projection of the VN to other parts of the brain via NTS produces various behavioral and psychological effects(depicted in Figure 2).
At the beginning of the study on the gut microbiota-brain axis, pathogenic microorganisms were the first to be concerned. Wang et al have corroborated that the expression of c-fos in the PVH and supraoptic nucleus of rats with Salmonella Typhimurium infection was significantly reduced in the model of subdiaphragmatic vagotomy.51 By comparing the mice that received oral normal saline, Goehler et al detected the neurons c-fos expression in bilaterally in the vagal ganglia was significantly increased with activation of visceral sensory nuclei in the brainstem of male CF1 mice 4–12h after oral administration of Campylobacter jejuni. C. jejuni triggered neurons in NTS, brain regions relevant to primary visceral sensory pathways that regulate digestion and feeding behavior, as well as brain regions related to the central autonomic neural network which may bear relevance for anxiety-like behavior or emotional reaction,52 and therewith, the brain regions relevant to anxiety-like behavior were further pinpointed.
In addition to pathogens, the effect of probiotics on behavior has received much attention. Bravo et al have pointed out that adult male BALB/c mice who were orally gavaged with a Lactobacillus rhamnosus strain (JB1) showed neurochemical and behavioral changes mediated by the VN, such as the decline of stress-induced corticosterone levels, reduction in anxiety-like behavior which can be evaluated with the help of an elevated plus-maze and in immobility time during forced swimming tests after the chronic treatment with this bacteria, while these changes disappeared in vagotomized mice.53 Compared with the control group that took the sterile broth orally, oral administration of JB1 can increase the mRNA of the GABAB1b receptors in the prefrontal cortex of JB1-fed mice, which is the exact opposite of what is seen in animal models of depression,54 however, the mRNA of the GABAB1b receptors were reduced in amygdala, HPC and LC in accordance with the pharmacological mechanism of the antidepressant-like effect of GABAB receptor antagonists.55 Moreover, the expression of GABAAα2 receptor mRNA in the amygdala and cortical areas was decreased, whereas there was an enhanced level in the HPC, which is associated with changes caused by benzodiazepines, a well-known sedative.56 In addition, a recent study using optogenetic tools has confirmed that Lactobacillus reuteri could change social behavior in a mouse model of ASD through the NTS-PVN-VTA pathway.57 Specifically, L. reuteri increases PVN oxytocin levels by activating the VN, thereby inducing long-term enhancement of dopamine neurons in the VTA, which leads to the normalization of social behavior.57 Bifidobacterium longum NCC3001, another probiotics, has also been confirmed to normalize anxiety-like behavior induced by infectious colitis in mice,58 and has further been shown to have an anxiolytic effect after subdiaphragmatic vagotomy, but its specific mechanism is still unclear.58
Based on the fact that the gut microbiota is linked to the brain and probiotics may alleviate some psychiatric symptoms and behaviors, researchers have begun to consider whether some microbiota technologies, for example, Fecal Microbiota Transplant (FMT), could be used to prevent and treat certain psychiatric disorders. A number of animal experiments used the tail suspension, open-field, forced swimming, as well as elevated plus-maze tests to assess depressive and anxiety-like behavior in mice after FTM, and these transplanted fecal microbiotas can come from animals or humans.59 All the animal experiments displayed varying degrees of reduction in depressive and anxiety-like behaviors after healthy microbiota transplantation. The evidence that transplanting microbiota from donors with psychiatric disorders to healthy recipients can lead to the spread of depression and anxiety-like symptoms and behaviors hints that the reverse is also true.60 In addition, several clinical studies have reached the same conclusion by transplanting the fecal microbiota of healthy people into humans with depression and quantifying depressive symptoms on specific scales.61 Although these studies have revealed the feasibility of FTM in preventing and treating psychiatric disorders in the future, there are still many problems that hinder this process, such as how to define a “healthy microbiota”. This puts forward more stringent ethical requirements for laboratory animal scholars, and also provides a broad prospect for the clinical application of FTM.
Role of Vagus Nerve in Neurodegenerative Diseases
Multiple neurodegenerative diseases including PD, AD, and ALS are featured by progressive neuron loss, impaired movement function, cognitive deterioration, behavioral changes, and even dementia. Considering the occurrence of diseases is closely related to age, so as the speeding up of the aging population, the incidence of neurodegenerative diseases has to arouse our concerns. Given the important effect of the gut microbiota in brain development and aging, so many researches have naturally linked gut microbiota to degenerative diseases and found that the gut microbiota composition of patients with neurodegenerative diseases was not exactly the same as that of healthy controls, and highlighted the role of VN in neurodegenerative diseases.
Parkinson’s Disease
As the second most frequent neurodegenerative disease in the world, PD is characterized by the best-known movement dysfunction, including bradykinesia, muscle rigidity, and resting tremor. However, some non-motor symptoms, such as gastrointestinal symptoms such as constipation, appear earlier than motor symptoms and promote the disease process, which implies their application value in disease diagnosis.62 Nowadays, the etiology of PD continues to puzzle us, despite years of research suggesting that PD is most likely the consequence of a complex interplay between genetic susceptibility, the aging process, and environmental factors. As research on the gut microbiota-brain axis continues, there is growing evidence of a link between gut microbiota and PD.
The predominant pathological alternations of PD are the dramatic decline of dopaminergic (DA) neurons in the striatum, the degeneration and death of DA neurons in the substantia nigra, as well as the presence of eosinophilic inclusion bodies in the cytoplasm of neurons, known as Lewy bodies, in the central and peripheral nervous systems. Lewy bodies originate from α-synuclein (α-Syn), a protein composed of 140 amino acids. When misfolded, α-Syn spreads between cells in a way similar to prions, leading to agglomeration of α-Syn aggregates and the formation of oligomers, which can further form fibrils and finally form Lewy bodies.63
Braak et al conducted autopsy analysis and found that α-Syn is detected in DMNV and ENS of patients with PD, and pioneered the hypothesis that in PD, misfolded α-Syn is probably retrograde transported from the ENS to the brain, a process mediated by VN.64 Furthermore, the authors noted that an unidentified pathogen that is capable of crossing the gastric epithelium may induce misfolding of α-Syn via a consecutive series of projection neurons.64 Considering that the hypothesis of dynamic transmission was based only on autopsy results, subsequent studies using biopsy detected that aggregated α-Syn was accumulated in the stomach, duodenum, and colon in 7 of 62 patients, which were found eight years before the onset of motor symptoms.65 Recently, Stokholm et al observed phosphorylated α-Syn deposits in the ENS in 22 of 39 patients by analyzing the archived paraffin-embedded tissue blocks from several regions of the gastrointestinal tract that were obtained on average 7 years prior to the onset of motor symptoms.66 These biopsies from the gastrointestinal tract of patients with prodromal PD may support the hypothesis that PD is most likely to originate in the gastrointestinal tract. In addition, many large cohort studies have manifested that compared with the general population, patients who had undergone vagotomy had a reduced risk of PD, thus further confirming the effect of the VN.67 Animal experiments also provide evidence for Braak’s hypothesis. Holmqvist et al injected α-Syn extracted from brain lysates of PD patients and the recombinant α-Syn labeled with Atto-550 was injected into the submucosa layer of the digestive tract of mice and observed that α-Syn was transported to the brain through the VN in a retrograde and time-dependent manner.68
It is worth noting that Braak’s hypothesis also presupposes that the enterocyte should be sufficiently permeable to allow neurotropic pathogens to induce α-Syn misfolding. Therefore, a bevy of related studies came on the heels of Braak’s hypothesis and indeed found increased intestinal permeability in PD patients through sugar probe absorption,69 and changes in the expression and distribution of occludin, a tight-junction protein that forms the gastrointestinal epithelial barrier in PD patients.70 The evidence from gut microbiota in PD patients hint inflammatory responses are likely to increase intestinal permeability, which may also be due to direct or indirect disruption of the intestinal epithelial barrier by gut bacteria-derived LPS that induce pro-inflammatory cytokines.71 Increased intestinal wall permeability provides the probability for microbes along with its metabolites to migrate to the lamina propria, ulteriorly amplifying the inflammatory response that can induce α-Syn misfolding through oxidative stress.72 Interestingly, α-Syn, in turn can further induce oxidative stress, creating a positive feedback circuit that leads to neurodegeneration in the brain.71 Immunostaining of α-Syn in duodenal biopsies from 42 children revealed that the degree of inflammation, whether chronic or acute, was a positive relationship between the degree of inflammation and the intensity and extent of α-Syn staining.73 To further demonstrate the correlation between infection and α-Syn induction, this study also selected 14 children and 2 adults with confirmed norovirus infection, assessed duodenal biopsy specimens taken and revealed strong expression of α-Syn in samples taken during infection.73 Moreover, other possible underlying pathways, such as the functional amyloid produced by a variety of gut bacteria, for instance, Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus mutans, Bacillus subtilis, E. coli, Salmonella enterica, and Salmonella typhimurium, can also act as a trigger to initiate the accumulation of α-Syn.74,75 Chen et al have found that rats fed with curli produced by E. coli showed that the neuronal expression level of α-Syn was enhanced in both the submucosal and myenteric plexus, and is also highly expressed in the brain, which provides evidence supporting this notion.76 Furthermore, the effects of the gut microbiota and their toxins on α-Syn can also be realized through indirect channels, in which EEC is required as a mediator based on the experimental results that researchers could detect the expression of α-Syn in enteroendocrine STC-1 via a variety of detection techniques.77
According to all the above-discussed data, it can, therefore, be summarized that the gut microbes can lead to misfolding and accumulation of α-Syn by producing functional amyloid and inflammatory reactions. Concomitant with this, the inflammatory response also results in enhanced permeability of the gut, which in turn provides an opportunity for the transport of misfolded α-Syn to the brain through the VN and accumulation in the brain. However, the assumption that α-Syn is transported through the VN to the DMNV has not been fully accepted. One study injected the striatum or guts of baboon monkeys with a component enriched in Lewy bodies comprising pathological α-Syn which were purified from the brains of posthumous Parkinson’s patients and found that after two years, in addition, dopaminergic neurons and fibers were impaired in the monkeys injected with Lewy bodies compared with the control group regardless of the injection site, but there was no prominent alteration in the immunoreactivity of S129 phosphorylation of α-Syn in the VN between both monkeys and no increase of the aggregated α-Syn in the VN of monkeys injected with Lewy bodies, implying that the VN perhaps does not participate in the transfer of α-Syn between the gut and the brain.78 Moreover, another study also described an alternative pathway through the celiac ganglion, independent of the VN.79 In conclusion, the α-Syn delivery pathway may depend on animal models, the inoculum, and so on, which requires further researches.
Alzheimer’s Disease
Alzheimer’s disease (AD) is a chronic progressive neurodegenerative disease closely related to age, which is a typical type of dementia.80 Its main clinical manifestations are progressive memory impairment, cognitive dysfunction and deterioration of language ability, which seriously affect the quality of life of patients.80 Despite rising life expectancy, dementia is increasing so rapidly that AD and other forms of dementia (see Box 1 for details) have become the fifth leading cause of death worldwide, with an estimated 50 million people living with dementia, and even more frightening is that the prevalence rate is expected to triple by 2050.80 The main pathological features of AD are extracellular plaques formed by amyloid β (Aβ) deposition, and intracellular neurofibrillary tangles formed by hyperphosphorylation of tau protein, which leads to neuron loss accompanied by glial cell proliferation that causes inflammation.81 Even though the initiation of Aβ in pathogenesis has been proposed for many years, the repeated failure of anti- Aβ therapy in many clinical trials clearly manifests that the amyloid deposition protein hypothesis cannot fully explain the pathogenesis of AD, which suggests that the occurrence of AD is multifactorial and further exploration is needed.
Box 1.
Lewy Body Dementia
| Lewy body dementia and AD are two degenerative diseases that seriously affect mental intelligence, but the symptoms are similar between the two. It is the second most common type of progressive dementia after Alzheimer’s disease. Protein deposits, called Lewy bodies, develop in nerve cells in the brain regions involved in thinking, memory and movement. Lewy body dementia causes a progressive decline in mental abilities always along with the visual hallucinations and changes in alertness and attention. |
A variety of studies have described changes in the gut microorganism in AD patients, which are summarized in Table 1. The abundance of pro-inflammatory microorganisms including Escherichia/Shigella in the gastrointestinal tract of AD patients increases while the amount of anti-inflammatory microorganisms such as Eubacterium rectale decreases, which in turn may lead to gut microbial dysbiosis and then cause the destruction of the intestinal epithelial barrier and the blood-brain barrier (BBB), thereby inducing neuroinflammation and aggravating Aβ plaques formation.82 In addition, LPS and amyloid derived from intestinal flora can exacerbate Aβ fibril formation in AD.83 A recent study transplanted feces from elderly people or aged mice to young mice, which carried far greater numbers of Paenalcaligenes hominis than young groups, and cognitive impairment was detected via Barnes maze tasks, novel object recognition and Y-maze.84 Notably, the cognitive impairment caused by extracellular vesicles but not LPS after oral administration of P. hominis was significantly inhibited by celiac vagotomy (a unilateral truncal vagotomy at the subdiaphragmatic level).84 These phenomena suggest that VN may be participated in the pathogenesis of AD, but the underlying mechanism remains betwixt and between. Given that the product of bacteria-induced α-Syn misfolding in PD patients can be transmitted to specific parts of the brain along the VN, as well as the diffusion mode of α-Syn is similar diffusion pattern to that of Aβ, many scientists have boldly put forward a speculation that VN plays a role in the pathogenesis of AD, similar to that of PD, but this hypothesis needs to be verified by further experiments.
The role of VN in the pathogenesis of AD has been explored, with a research team from Mexico finding that long-term exposure to high levels of the fine particulate particles and high concentrations of combustion-derived nanoparticles (CDNPs) showed semblable neuropathological features of AD and PD, such as tau hyperphosphorylation with pre-tangles, Aβ diffuse and mature plaques, and misfolded α-Syn in the olfactory bulb and brainstem accumulation.85 There is also some direct evidence: COX2 and CD14 were upregulated in the VN,85 and CDNPs deposition was also observed in the VN along with the destruction of the tight junction of the enterocyte.86 Therefore, it is generally accepted that inhaling airborne particulate matter could alter the gut flora, induce the immune response, and enhance the intestinal permeability.87 Particularly, some nanoparticles with high and very effective pro-oxidation potential and inflammatory capacity can travel backwards along the VN to the brain and cause neuroinflammation, dopaminergic neuron injury, oxidative stress and neurodegeneration. This is currently considered a dangerous environmental factor for PD and AD.86
It is undeniable that intestinal hormones play an essential role in the regulation of intestinal function under physiological conditions, and mental or neurological dysfunction caused by some disorders of intestinal hormone secretion has attracted wide attention. It is worth noting that many studies have demonstrated that elevated blood glucose is closely related to amnesiac, and people with elevated blood glucose have a significantly increased risk of AD.88 This unexpected result suggests that glucose in the gut can trigger the secretion of glucagon-like peptide-1 (GLP-1) and induces the activation of NG, which in turn can send information to the brain via VN, affecting areas associated with memory, namely the hippocampus and amygdala.89 As previously summarized, changes in the gut microbiota can affect changes in the exocrine secretion of GLP-1, thereby affecting memory signaling and cognitive function. At present, there are few studies on this aspect, but the emergence of this phenomenon provides new research ideas and methods for clinical diagnosis and treatment in the future.
Amyotrophic Lateral Sclerosis
Although the feature of ALS is neurodegeneration and muscle weakness that affects the cerebral cortex, lower brainstem, as well as motor neurons of the spinal cord, it can also exhibit gastrointestinal symptoms such as delayed gastric empty and slow transit in the colon.90 In human ALS, a recent cross-sectional study confirmed dysregulated fecal microbiota of ALS individuals with a lower diversity and a lower Firmicutes/Bacteroidetes (F/B) ratio.91 In addition, a longitudinal research simultaneously evaluated the gut microbe, immunophenotype, and alteration in epigenetic marks in ileum and brain in a mouse model of ALS with familial superoxide dismutase 1 (SOD1G93A) mutation, and the result showed that dysbiosis indicated by F/B ratio in SOD1G93A mice began preceded the onset of disease (37 days) and lasted up to terminal stage (~150 days). Concomitantly, with the progression of the disease, the infiltration range of neutrophils, CD8+ T cells, and leukocytes microglia in the spinal cord of SOD1G93A mice gradually expanded which implied obvious inflammatory changes.92 Furthermore, there is also evidence of membrane integrity degradation of the blood-spinal cord barrier and the BBB in ALS patients.93 Overall, alterations of the gut microbiota which probably induce the inflammatory response and lead to intestinal and CNS barrier damage may constitute a small part of the pathogenesis of ALS.
Unfortunately, although there is no direct evidence that the VN plays a role in this process, the hypothesis that inflammatory factors are transmitted to the brain through the VN after breaking the intestinal barrier deserves further confirmation in future experiments. However, recent studies have found a reduction in the size of VN at the layer of the thyroid gland in ALS patients with medulla oblongata infection via vagal nerve sonography; however, whether there is a certain correlation between the size and the disease process has not been confirmed, so further studies are needed.94,95 This finding leads to extrapolate that gut microbiota causes bulbar inflammation, which may affect the size and function of VN through underlying mechanism. Although there is no clear evidence of the role of VN size changes in the pathogenesis of ALS and its diagnostic value, it also provides a thought-provoking clue for future research.
Conclusion
A growing number of evidence has corroborated a potential link between the gut microbiota and the brain, also named as the gut microbiota-brain axis. In this review, we summarize the role of the vagus nerve in the interplay between the gut microbiota and the brain. As the longest and most widely distributed cranial nerve, the VN afferent fibers can directly or indirectly sense a variety of signals from the intestinal environment through multiple patterns, and transmit to the NTS or further into central autonomic network, which in turn has emerged as a key pathway to affect behavior. Additionally, the signals from NTS can be related to the DMNV, where the vagus efferent fibers originate, to regulate gastrointestinal dysfunction. The vagus nerve is associated with many neurodegenerative diseases including PD, AD and ALS, but the underlying mechanism remains poor understanding. Of course, for the shortcomings of this review, we should also see that due to the complex role of the vagus nerve and its wide distribution, although some studies have shown that there is a relationship in many diseases, its precise mechanism has not yet been elucidated, so in the description of this article, we have made a review based on the existing experimental evidence.
In summary, the interconnection between vagus nerve, gut microbiota, behavioral changes and neurodegenerative diseases is no accident, and more research will establish novel approaches to advance understanding of disease mechanisms, thereby facilitating clinical practice.
Funding Statement
This work was supported by the National Natural Science Foundation of China Grant (No. 82174056, 81673671, JD Xu).
Abbreviations
VN, vagus nerve; HPA axis, hypothalamic-pituitary-adrenal axis; SCFA, short-chain fatty acids; PAMP, pathogen-associated molecular patterns; IBD, inflammatory bowel disease; CD, Crohn’s disease; ALS, amyotrophic lateral sclerosis; CNS, central nervous system; GABA, γ-aminobutyric acid; EECs, enteroendocrine cells; BBB, blood-brain barrier; NG, nodose ganglia; NTS, nucleus tractus solitarius; IGLEs, intraganglionic laminar endings; IMAs, intramuscular arrays; CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; PYY, peptide YY; DMNV, dorsal motor nucleus of the vagus; CAN, central autonomic network; PBN, parabrachial nucleus; LC, locus coeruleus; PVH, paraventricular nucleus of the hypothalamus; HPC, hippocampus; Ach, acetylcholine; α7nAChR, α-7-nicotinic ACh receptors; TRPA1, transient receptor potential ankyrin A1; VTA, ventral tegmental area; CeA, central nucleus of the amygdala; FMT, Fecal Microbiota Transplant; PD, Parkinson’s disease; α-Syn, α-synuclein; AD, Alzheimer’s disease; Aβ, amyloid β; GLP-1, glucagon-like peptide-1.
Data Sharing Statement
The information in this review generated from PubMed.
Author Contributions
All authors discussed the manuscript and approved of the final version. All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no financial and non-financial competing interests.
References
- 1.Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002. doi: 10.1016/j.neuron.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 2.Morais LH, HLt S, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- 3.Forsythe P, Kunze W, Bienenstock J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis? BMC Med. 2016;14:58 doi: 10.1186/s12916-016-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HX, Wang YP. Gut microbiota-brain axis. Chin Med J. 2016;129 (19) :2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dicks LMT, Hurn D, Hermanus D. Gut bacteria and neuropsychiatric disorders. Microorganisms. 2021;9 (12) :52583. doi: 10.3390/microorganisms9122583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borre YE, O’Keeffe GW, Clarke G, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20 (9) :509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Kim YK. Understanding the connection between the gut-brain axis and stress/anxiety disorders. Curr Psychiatry Rep. 2021;23 (5) :22. doi: 10.1007/s11920-021-01235-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S, Jiang Y, Xu K, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17 (1) :25. doi: 10.1186/s12974-020-1705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Gallausiaux C, Marinelli L, Blottiere HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80 (1) :37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 10.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341 (6145) :569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrieta MC, Finlay BB. The commensal microbiota drives immune homeostasis. Front Immunol. 2012;3:33. doi: 10.3389/fimmu.2012.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino K, Nishida A, Inoue R, et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J Gastroenterol. 2018;53(1):95–106. doi: 10.1007/s00535-017-1384-4 [DOI] [PubMed] [Google Scholar]
- 13.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16 (8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 15.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216(1):84–98. doi: 10.1242/jeb.073411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735.. doi: 10.1016/j.bbi.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 19.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104(2):502–509. doi: 10.1016/0016-5085(93)90419-D [DOI] [PubMed] [Google Scholar]
- 21.Bonaz B, Sinniger V, Pellissier S. Therapeutic potential of vagus nerve stimulation for inflammatory bowel diseases. Front Neurosci. 2021;15:650971. doi: 10.3389/fnins.2021.650971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powley TL, Jaffey DM, McAdams J, et al. Vagal innervation of the stomach reassessed: brain-gut connectome uses smart terminals. Ann N Y Acad Sci. 2019;1454(1):14–30. doi: 10.1111/nyas.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev. 2000;34(1–2):1–26. doi: 10.1016/s0165-0173(00)00036-9 [DOI] [PubMed] [Google Scholar]
- 24.Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5236. doi: 10.1126/science.aat5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85 (1–3) :1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ. Roles for gut vagal sensory signals in determining energy availability and energy expenditure. Brain Res. 2018;1693 (B) :151–153. doi: 10.1016/j.brainres.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81 (2) :929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 28.Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci. 1989;9 (6) :1985–1996. doi: 10.1523/JNEUROSCI.09-06-01985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46 (1) :17–23. [PubMed] [Google Scholar]
- 30.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 31.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz MT, Dezfuli G, Murphy EC, et al. GABAB receptor signaling in the dorsal motor nucleus of the vagus stimulates gastric motility via a cholinergic pathway. Front Neurosci. 2019;13:967. doi: 10.3389/fnins.2019.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421 (6921) :384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 35.Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594 (20) :5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13 (8B) :2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao YK, Kasper DL, Wang B, et al. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun. 2013;4:1465. doi: 10.1038/ncomms2478. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Mao YK, Diorio C, et al. Lactobacillus reuteri ingestion and IK(Ca) channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol Motil. 2010;22 (1) :98–107. e133. doi: 10.1111/j.1365-2982.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 39.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281 (4) :G907–915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 40.Jamar G, Ribeiro DA, Pisani LP. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr. 2021;61 (5) :836–854. doi: 10.1080/10408398.2020.1747046. [DOI] [PubMed] [Google Scholar]
- 41.de La Serre CB, de Lartigue G, Raybould HE. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav. 2015;139:188–194. doi: 10.1016/j.physbeh.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz GJ, Plata-Salaman CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Am J Physiol. 1997;273:R1193–1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- 43.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci. 2005;120 (1–2) :104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108 (46) :E1184–1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye L, Bae M, Cassilly CD, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29 (2) :179–196. e179. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128 (1) :175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 47.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170 (1) :185–198. e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105 (43) :16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han W, Tellez LA, Perkins MH, et al. A neural circuit for gut-induced reward. Cell. 2018;175 (3) :665–678. e623. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suarez AN, Hsu TM, Liu CM, et al. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun. 2018;9 (1) :2181. doi: 10.1038/s41467-018-04639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Wang BR, Zhang XJ, et al. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8 (3) :540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goehler LE, Gaykema RP, Opitz N, et al. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19 (4) :334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108 (38) :16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26 (1) :36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Evenseth LSM, Gabrielsen M, The SI. GABAB receptor-structure, ligand binding and drug development. Molecules. 2020;25 (13) :234. doi: 10.3390/molecules25133093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng L, Morford KL, Levander XA. Benzodiazepines and related sedatives. Med Clin North Am. 2022;106 (1) :113–129. doi: 10.1016/j.mcna.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101 (2) :246–259. e246. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23 (12) :1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearson-Leary J, Zhao C, Bittinger K, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2020;25 (5) :1068–1079. doi: 10.1038/s41380-019-0380-x. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Guo R, Liu F, et al. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat. 2020;16:859–869. doi: 10.2147/NDT.S243551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry. 2020;20 (1) :299. doi: 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liddle RA. Parkinson’s disease from the gut. Brain Res. 2018;1693 (Pt B) :201–206. doi: 10.1016/j.brainres.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349 (6248) :1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 64.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110 (5) :517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 65.Hilton D, Stephens M, Kirk L, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127 (2) :235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 66.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79 (6) :940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 67.Lin SY, Lin CL, Wang IK, et al. Dementia and vagotomy in Taiwan: a population-based cohort study. BMJ Open. 2018;8 (3) :e019582. doi: 10.1136/bmjopen-2017-019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128 (6) :805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 69.Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012;39 (2) :185–188. doi: 10.1017/s0317167100013202. [DOI] [PubMed] [Google Scholar]
- 70.Clairembault T, Leclair-Visonneau L, Coron E, et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun. 2015;3:12. doi: 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill AE, Wade-Martins R, Burnet PWJ. What is our understanding of the influence of gut microbiota on the pathophysiology of Parkinson’s disease? Front Neurosci. 2021;15:708587. doi: 10.3389/fnins.2021.708587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lema Tome CM, Tyson T, Rey NL, et al. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease--is there a link? Mol Neurobiol. 2013;47 (2) :561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stolzenberg E, Berry D, Yang DE, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9 (5) :456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lionnet A, Leclair-Visonneau L, Neunlist M, et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135 (1) :1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 75.Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A Jr. The Gut and Parkinson’s disease-A bidirectional pathway. Front Neurol. 2019;10:574. doi: 10.3389/fneur.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen SG, Stribinskis V, Rane MJ, et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight. 2017;2 (12) :e92295. doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arotcarena ML, Dovero S, Prigent A, et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain. 2020;143 (5) :1462–1475. doi: 10.1093/brain/awaa096. [DOI] [PubMed] [Google Scholar]
- 79.Van Den Berge N, Ferreira N, Gram H, et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019;138 (4) :535–550. doi: 10.1007/s00401-019-02040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397 (10284) :1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol. 2020;57 (12) :5026–5043. doi: 10.1007/s12035-020-02073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goyal D, Ali SA, Singh RK. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110112. doi: 10.1016/j.pnpbp.2020.110112. [DOI] [PubMed] [Google Scholar]
- 83.Asti A, Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39 (1) :169–179. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- 84.Lee KE, Kim JK, Han SK, et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8 (1) :107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36 (2) :289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 86.Calderon-Garciduenas L, Reynoso-Robles R, Perez-Guille B, Mukherjee PS, Gonzalez-Maciel A. Combustion-derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorylated alpha synuclein and tau in young Mexico City residents. Environ Res. 2017;159:186–201. doi: 10.1016/j.envres.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Kish L, Hotte N, Kaplan GG, et al. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One. 2013;8 (4) :e62220. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yogi-Morren D, Galioto R, Strandjord SE, et al. Duration of type 2 diabetes and very low density lipoprotein levels are associated with cognitive dysfunction in metabolic syndrome. Cardiovasc Psychiatry Neurol. 2014;2014:656341. doi: 10.1155/2014/656341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hossain MS, Oomura Y, Fujino T, Akashi K. Glucose signaling in the brain and periphery to memory. Neurosci Biobehav Rev. 2020;110:100–113. doi: 10.1016/j.neubiorev.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Fang X. Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int J Neurosci. 2016;126 (9) :771–776. doi: 10.3109/00207454.2015.1096271. [DOI] [PubMed] [Google Scholar]
- 91.Zeng Q, Shen J, Chen K, et al. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci Rep. 2020;10 (1) :12998. doi: 10.1038/s41598-020-69845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Figueroa-Romero C, Guo K, Murdock BJ, et al. Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis Model Mech. 2019;13 (2) :32. doi: 10.1242/dmm.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bataveljic D, Milosevic M, Radenovic L, Andjus P. Novel molecular biomarkers at the blood-brain barrier in ALS. Biomed Res Int. 2014;2014:907545. doi: 10.1155/2014/907545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holzapfel K, Naumann M. Ultrasound detection of vagus nerve atrophy in bulbar amyotrophic lateral sclerosis. J Neuroimaging. 2020;30 (6) :762–765. doi: 10.1111/jon.12761. [DOI] [PubMed] [Google Scholar]
- 95.Tawfik EA. Vagus nerve ultrasound in a patient with amyotrophic lateral sclerosis. Muscle Nerve. 2016;54 (5) :978–979. doi: 10.1002/mus.25126. [DOI] [PubMed] [Google Scholar]
- 96.Liu RT, Rowan-Nash AD, Sheehan AE, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Strati F, Cavalieri D, Albanese D, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5 (1) :24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32 (5) :739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuang ZQ, Shen LL, Li WW, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(4):1337–1346. doi: 10.3233/JAD-180176 [DOI] [PubMed] [Google Scholar]