Abstract
Simple Summary
Deregulated cellular metabolism is one of the major hallmarks of cancer. Cancer cells orchestrate abnormal metabolic reprogramming to satisfy high energy demands. The review focuses on the mechanics of the major metabolic pathways, significant intermediates, and associated enzymes that are altered by the oncogenic progression. The emphasis is laid on therapeutically targeting clinically relevant metabolic intermediates which are crucial to cancer cell survival, and proliferation. The clinical intervention of metabolic pathways, critical enzymes, and the intermediate, thus offers a distinct niche in cancer therapies.
Abstract
Metabolic reprogramming enables cancer cells to proliferate and produce tumor biomass under a nutrient-deficient microenvironment and the stress of metabolic waste. A cancer cell adeptly undergoes a variety of adaptations in metabolic pathways and differential expression of metabolic enzyme genes. Metabolic adaptation is mainly determined by the physiological demands of the cancer cell of origin and the host tissue. Numerous metabolic regulators that assist cancer cell proliferation include uncontrolled anabolism/catabolism of glucose metabolism, fatty acids, amino acids metabolism, nucleotide metabolism, tumor suppressor genes, microRNAs, and many regulatory enzymes and genes. Using this paradigm, we review the current understanding of metabolic reprogramming in tumors and discuss the new strategies of cancer metabolomics that can be tapped into for cancer therapeutics.
Keywords: cancer metabolism, glycolysis, fatty acid metabolism, amino acid metabolism, Warburg effect, microRNA, oncogenes, tumor suppressor genes, cancer therapeutics
1. Introduction
Due to the rising prevalence of cancer throughout the globe, it is of utmost importance to investigate the underlying processes that contribute to cancer development to combat this looming issue [1]. While a wide range of genetic, molecular, and cancer metabolism research has led to great advancements and landmark discoveries in this field, much remains to be learned about cancer metabolism. Otto Warburg established in the 1920s that cultured tumor tissues absorb glucose and generate lactate at high rates even when oxygen is present (aerobic glycolysis) [2,3]. The three metabolic features that define the Warburg effect are glucose intake, lactate secretion, and oxygen availability. Long before Warburg, Pasteur demonstrated that oxygen inhibits sugar fermentation, recognizing glucose-to-lactate conversion as an expected reaction to hypoxia. If this notion is applied to cancer, tumors may be hypoxic, and hypoxia may boost lactate generation in tumors, as it does everywhere. That, however, is not the Warburg effect. Even when there was adequate oxygen to convert glucose to CO2, which other tissues prefer and which we now know is far more productive in terms of ATP synthesis, the tumor samples took up glucose and converted it to lactate [4,5]. Because tumor cells demand a limitless source of energy, the ATP synthesis rate of glycolysis is significantly higher than that of oxidative phosphorylation, even though the efficiency of ATP production per molecule of glucose is substantially lower via glycolysis [6,7]. In reality, cancer cells can have dysregulated central metabolic pathways [8]. More crucially, new research suggests that cancer cells can inhibit anti-tumor immune responses by competing for and depleting vital resources, or by otherwise lowering the metabolic fitness of tumor-infiltrating immune cells [9,10]. Thus, this cancer hallmark provides a means for malignant cells to evade the body’s immune system, allowing cancerous cells to proliferate and cancer to progress to later stages [11,12].
The recent use of sophisticated technologies such as metabolomics, metabolic flux studies, and functional genomics has revealed that cancer cells and tumors exhibit diverse metabolic tendencies and dependencies. Metabolic phenotypes in tumors are both varied and flexible, resulting from the interplay of many distinct elements. These elements are often inherent to the cancer cell (e.g., cell lineage; differentiation state; somatically acquired mutations) while others are imposed by the microenvironment (e.g., nutrient milieu; interactions with extracellular matrix and stromal cells).
Furthermore, aberrant lipid metabolism, amino acid metabolism, mitochondrial biogenesis, and other cellular metabolism pathways have been associated with metabolic remodeling in cancer cells. Therefore, understanding how the metabolic phenotypes originate and evolve will necessitate an understanding of the effect of these components, as well as their proportional impact on tumor progression [13].
Physiological stress, such as the shortage of O2, is thought to be one of the key causes of the metabolic transition in tumor cells. Early tumor cells become hypoxic as they proliferate, but still, require blood and nutrients for continuous growth. Because of the decreased reliance on aerobic respiration, tumor metabolism is oriented toward glycolysis, balancing O2 demand with O2 supply. One key player during this adaptive response to low oxygen is hypoxia-inducible factor-1 (HIF-1), a transcription factor that accumulates and delivers O2 to tumor cells during hypoxia via enhancing angiogenesis, erythropoiesis, and rapid glycolysis, allowing cancer cells to thrive even under physiological stress [14,15,16].
Most tumor cells also exhibit significantly enhanced anabolism pathways, including aerobic glycolysis, glutaminolysis, fatty acid synthesis (FAS), and pentose phosphate pathway (PPP), compared to normal cells, which depend exclusively on oxidative phosphorylation (OXPHOS) to produce the energy needed for cellular homeostasis.
Additionally, glutamine is converted to glutamate by glutaminase and subsequently converted into alpha-ketoglutarate (α-KG) which replenishes and maintains the tricarboxylic acid (TCA) cyclic pathway [17].
Furthermore, in addition to producing phosphopeptides and ribonucleotides, the PPP is a key source of NADPH, a critical antioxidant for cellular redox adaptation [18,19,20].
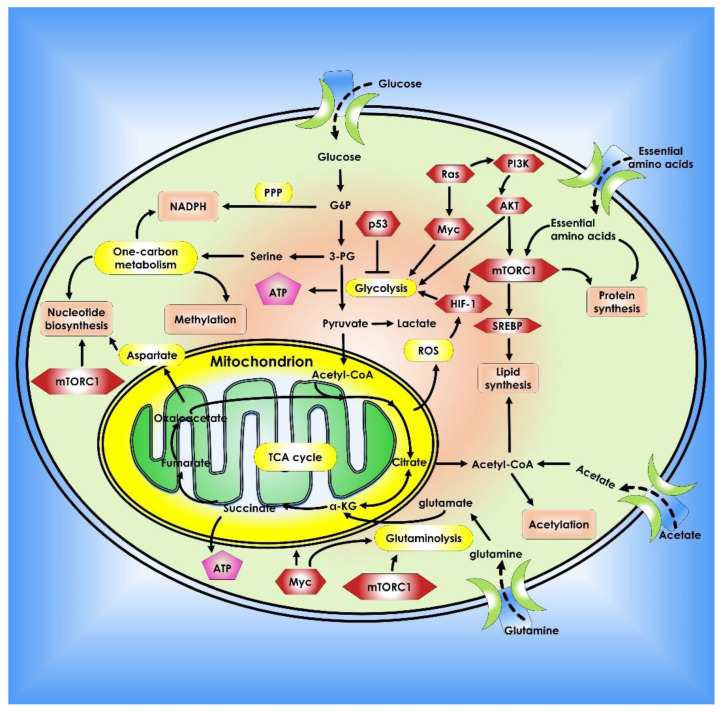
The reasons for cancer cells’ metabolic alterations are many and can be linked to both internal and external sources. Oncogenic and tumor suppressor signaling pathways, such as HIF-1, p53, and Myc genes, all contribute to the regulation of cancer cell metabolism [21]. Changes in oncogenes such as Myc, Ras, and BRAF have been linked to metabolic reprogramming [22,23,24]. Myc transcriptionally controls several metabolic enzymes involved in DNA synthesis and glycolysis, such as thymidylate kinase and lactate dehydrogenase A [25,26]. Myc is also implicated in the metabolic reprogramming of fatty acids, glutamine, proline, and nucleic acids, either directly or indirectly via various microRNAs (miRNAs) [26,27,28,29]. Overall, altered or dysregulated cellular metabolism is an extensive process that requires the use of several different pathways and factors that contribute to cancer development. Thus, investigating the reprogramming of energy metabolism might reveal underlying molecular processes in cancer and aid in the development of new methods for early detection and treatment (Figure 1).
Figure 1.
Metabolic pathways that control cancer’s ability to grow and spread. Nucleotide, protein, and lipid synthesis are induced by abnormal activation of mTORC1 in tumor cells. Further anabolism is promoted when tumor suppressors like p53 are lost, or oncogenes are activated. Regulation of ROS, acetylation, and methylation by metabolism is one way that metabolic signaling is kept under control.
2. Glucose Metabolism in Cancer Cells
Major metabolic pathways, including the glycolysis pathway, the PPP, and the serine synthesis pipeline (SSP) in the cytoplasmic compartment, as well as the TCA cycle in the mitochondria, are all fundamental to glucose metabolism. Glycolysis is an important pathway for glucose metabolism that results in the synthesis of pyruvate, which is converted into lactate for extracellular secretion or enters the TCA cycle in the mitochondria. The glycolysis is also linked to the PPP and the serine production process via its metabolic intermediate. Furthermore, the hexosamine biosynthetic pathway is branched from the glucose metabolism pathway via fructose-6-phosphate and contributes to protein modification by producing Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as a byproduct. In addition, it also regulates growth factor-induced glucose and glutamine metabolism. Recent findings indicate that these pathways have been transformed or reconfigured in cancer cells [30].
Cancer cells favor aerobic glycolysis for ATP synthesis while keeping OXPHOS activity for the following reasons:
(i) Glycolysis is more conducive to cancer development. Because cancer cells proliferate faster than normal tissues, they require not just energy but also metabolic intermediates for macromolecule production. Many intermediates from glycolysis and the shortened TCA cycle can be utilized to synthesize macromolecules necessary for cancer development and proliferation, including nucleic acids, lipids, and proteins [31,32].
(ii) Overly efficient ATP products may be harmful to cancer cells. ADP is turned into ATP when cancer cells consume high-efficiency glucose. The high quantity of ATP inhibits phosphofructokinase 1 (PFK1), the rate-limiting p53 mutations enzyme in glycolysis, as well as pyruvate kinase 1 (PK1), resulting in glycolysis inhibition [33].
Although glycolysis produces less ATP than OXPHOS, the rate of ATP synthesis in the former is faster, making it suitable for the energy demands of rapidly proliferating cells such as cancer and embryonic tissues [32]. In general, fast proliferation tissues rely heavily on glycolysis for ATP synthesis, whereas differentiated tissues rely more on OXPHOS [13,34].
(iii) Hypoxia is commonly seen in cancer tissues, and glycolysis gives malignancies an edge in this hypoxic environment [35,36]. Lactate is produced by glycolysis and is released into the extracellular space. An acidic microenvironment gives cancer tissues a growth advantage over normal tissues and promotes cancer cell invasion and metastasis [37,38]. Furthermore, lactic acidosis limits glycolysis and promotes aerobic respiration as a source of energy [38,39,40].
(iv) As mitochondrial OXPHOS decreases, there is less production of reactive oxygen species (ROS), which are deadly to cancer cells [39,41].
Although cancer cells may preserve OXPHOS activity, this does not imply that they do not have mitochondrial respiration abnormalities. Enhanced glycolysis in certain cancers is caused by mitochondrial dysfunction [42,43], including decreased expression of mitochondrial oxidative enzymes and transporters. A truncated TCA cycle, a decrease in the number of mitochondria per cell and a defective respiratory chain, an increase in natural inhibitors of mitochondrial ATP synthase, and a higher sensitivity of mtDNA to oxidative stress [44,45].
Furthermore, glycolysis increase is not just for ATP production but also biomass synthesis, including ribonucleotides [35] and amino acids, for proliferation and growth in a constantly shifting microenvironment with many material constraints, such as oxygen and nutrient deficiencies. Enhanced glycolysis might potentially be explored as a technique to lower ROS and hence oxidative stress in cancer cells [46,47].
Cancer cells promote the primary step in glucose metabolism by boosting glucose absorption and generating high-level expression of hexokinase 2 (HK2), which is already expressed in normal cells [35,48]. HK1 is the most common isoform found across most adult tissues, whereas HK2 is exclusively present in skeletal muscle, heart, and adipose tissues, however, it is also detected in embryonic tissues. HK2 expression, on the other hand, is elevated in cancer cells to boost glucose flow into multiple metabolic pathways [49,50]. PFK1 catalyzes the second committed phase of glycolysis, whereas pyruvate kinases catalyze the third committed step. F6P is converted into fructose-1,6-bisphosphate (F1,6BP) by PFK1, while phosphoenolpyruvate (PEP) is converted into pyruvate by pyruvate kinases. The first two committed actions use ATP, while the third committed step creates ATP. Except for the third and final committed phase (which creates ATP and pyruvate), cancer cells employ multiple strategies to enhance the flow of glucose in glycolysis. The last committed step is mitigated in part by employing the low-affinity pyruvate kinase M2 (PKM2) isoform to catalyze this reaction [51].
Modulation of the final dedicated step of glycolysis disperses metabolites into bifurcation routes such as the PPP and the SSP. It generates enough metabolic intermediates and augments the anabolic processes necessary for cell growth and proliferation. Although the final dedicated step (the conversion of PEP to pyruvate) is reduced, the following conversion of pyruvate to lactate is significantly enhanced, with the majority of the lactate being released. The two most abundant isozymes, lactate dehydrogenase A (LDH-A) and lactate dehydrogenase B (LDH-B) can form homotetramers or heterotetramers. Because LDH-A has a larger affinity for pyruvate and LDH-B has a higher affinity for lactate, LDH-A prefers the forward reaction while LDH-B prefers the reverse reaction. In cancer cells, LDH-A is the most abundant LDH [52,53]. Lactate can be taken up by neighboring stromal cells and utilized as an energy source to maintain development or to produce pyruvate, which is subsequently released by the stroma and taken up by cancer cells [54,55]. Cancer cells can employ extracellular lactate or pyruvate to maintain the TCA cycle and supply citrate and acetyl-CoA for fatty acid synthesis when glucose is scarce.
LDH-A catalyzes the enhanced pyruvate to lactate flow in cancer cells, generating NAD+ from NADH. In glycolysis, NAD+ is transformed into NADH, which is necessary to sustain a high flux of glucose metabolism.
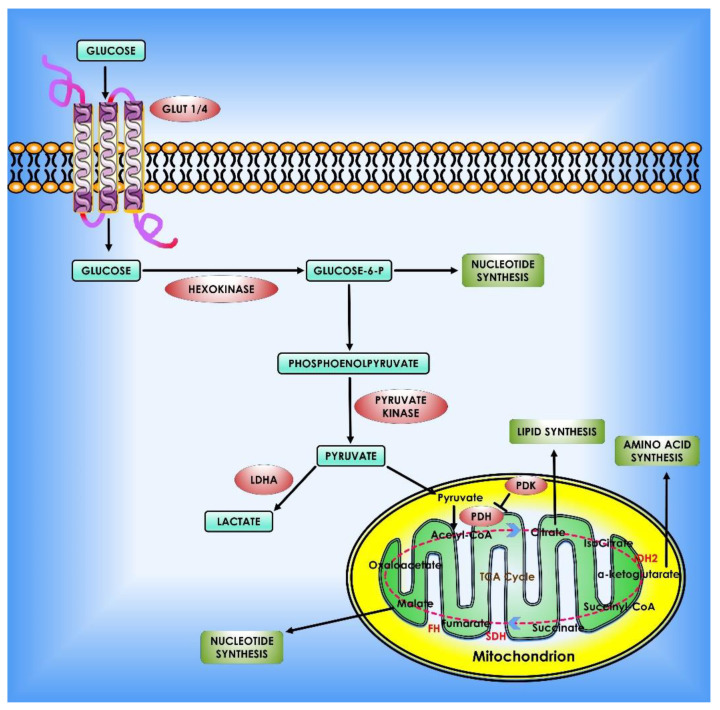
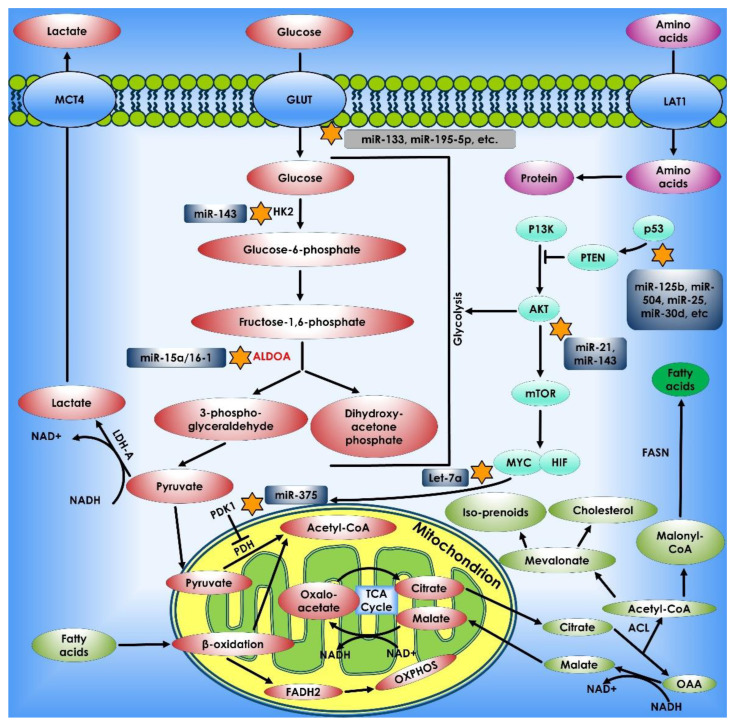
To reiterate, glycolysis is increased in cancer cells to nourish the branching pathways that produce nucleotides, amino acids, and fatty acids for anabolic processes [56]. Notably, numerous glycolytic enzymes’ activities are favorably and negatively controlled to maintain homeostasis. Cancer cells frequently use these regulatory mechanisms to meet their anabolic demands and adapt to different microenvironments (Figure 2).
Figure 2.
Glucose metabolism in cancer cells. When glucose enters the cell via glycolysis, it is phosphorylated by HK to glucose-6 phosphate, which is then converted by glycolysis to pyruvate in the cytosol. Under an aerobic environment, normal cells employ pyruvate dehydrogenase (PDH) to convert the majority of pyruvate to acetyl-CoA. The acetyl-CoA is subsequently oxidized via the TCA cycle, providing sources of ATP production. On the contrary, the molecular mechanisms of glucose consumption in cancer are switched from oxidative phosphorylation to glycolysis. Furthermore, cancer cells require the creation of new macromolecules to proliferate (for example, nucleic acids, lipids, and proteins). Critical enzymes which might be viable targets for cancer treatment are indicated in orange. TCA enzymes iso-citrate dehydrogenase 2 (IDH2), succinate dehydrogenase (SDH), and fumarate hydratase (FH) which are known to be mutated in cancer are depicted in red within the mitochondrion.
3. Role of Oncogenes in Cancer Metabolism
A significant volume of research reveals that metabolic rewiring is caused by changes in oncogenes and tumor suppressors, which are regulated by major metabolic enzyme effectors [57]. The proto-oncogene c-Myc has been shown to cause a metabolic shift to glycolysis in tumor cells and to control the majority of glycolytic enzymes [58]. An initial study found that c-Myc also activates genes involved in mitochondrial structure and function, as well as promoting mitochondrial biogenesis [26]. Another major oncogene, HIF-1 is an important regulator in glucose metabolism in cancer cells in a hypoxic environment a hallmark feature of tumorigenesis. Several enzymes of the glycolytic pathway, including glucose transporter 1 (GLUT1), glucose transporter 3 (GLUT3), HK1, HK2, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase 1 (PGK1), PKM2, LDH-A, and 3-phosphoinositide-dependent kinase 1 (PDK1), are activated by HIF-1A. Moreover, HIF-1 stimulates glucose absorption and lactate production by inhibiting the TCA cycle and oxidative phosphorylation in the mitochondria [59,60] (Figure 3).
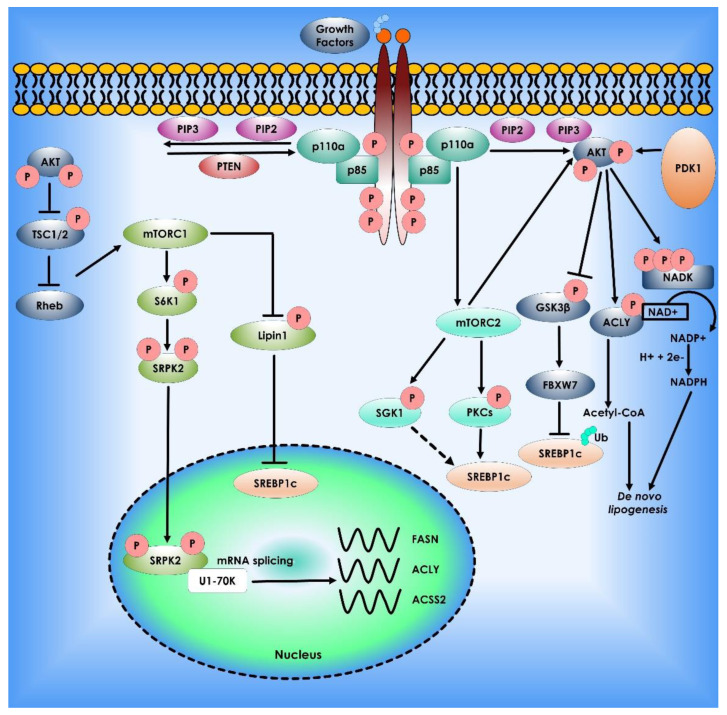
Figure 3.
Akt mTOR signaling Pathway in Lipid Synthesis. The phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling regulate lipid metabolism. The most often dysregulated mechanism in cancer is PI3K signaling, which promotes growth, proliferation, and survival. When receptor tyrosine kinases are activated, PI3K is recruited to the plasma membrane and phosphorylates Phosphatidylinositol 4,5-bisphosphate (PIP2) to Phosphatidylinositol (3,4,5)-trisphosphate (PIP3). AKT binds to PIP3, allowing PDK1 and mTORC2 to activate. AKT stimulates lipogenesis directly by inhibiting Glycogen synthase kinase 3, activating ATP citrate lyase to make acetyl-CoA, and phosphorylating NADK to produce NADP+ for NADPH production. PI3K signaling is also intertwined with mTORC1 and mTORC2. mTORC1 controls lipogenesis by inhibiting lipin-1, a negative regulator of nuclear Sterol regulatory element-binding protein-1c, and activating the splicing factor Serine/threonine-protein kinase (SRPK2), boosting the production of lipogenic enzymes such as ATP citrate lyase (ACLY), Fatty acid synthase (FASN), and acetyl-CoA synthetase 2 (ACSS2). Finally, mTORC2 activation promotes lipogenesis via AKT-dependent and independent processes, with the latter involving phosphorylation of serum- and glucocorticoid-regulated kinase (SGK1) and Protein kinase C (PKCs), followed by sterol regulatory element binding protein-1c (SREBP1c) activation.
4. Role of Tumour Suppressor Genes and miRNAs in Cancer Metabolism
Numerous studies have demonstrated the pivotal role of protein-coding tumor suppressor genes, as well as regulatory noncoding small miRNAs in normal and cancer cell metabolism. The master regulator and tumor-suppressor p53 decrease glucose absorption in cells by downregulating GLUT1 and GLUT4 expression in cancer cells. Hence, it plays a vital role in suppressing glycolysis under normoxic or hypoxic conditions via its transcriptional target genes [59,60]. The DNA Binding Domain (DBD) especially the amino acid residues 98–293, which is located in exons 5, 6, 7, part of exons 4, and 8 of p53, contains the majority of the high-frequency oncogenic mutations, as well as an aggregation-prone region (APR), and has been proven in multiple studies to be a useful model system to emulate the behavior of full-length p53, including aggregation [61,62]. Expression of murine double minute 2 (Mdm2) can also be targeted by the activation of a ROS-ERK2-MDM2 axis in cancer cells by destabilizing mutant p53 gene, the p53 negative regulator, is commonly amplified and/or overexpressed in numerous cancers, resulting in p53 signaling failure [61]. TP53-induced glycolysis and apoptosis regulator (TIGAR) a p53-transcriptional target gene reduces fructose-2,6-bisphosphate levels in cells and slows glycolysis. Interestingly, TIGAR operates through the PPP to reduce intracellular ROS levels [63]. Recent research shows that sirtuin-family deacetylases (SIRT) play key roles in the regulation of metabolism, therefore, affecting cancer development. It has been shown that SIRT1, SIRT2, SIRT3, SIRT4, or SIRT6 deletion causes tumor development in an animal model of cancers [64]. Sirtuins appear to tune glucose metabolism via modulating c-Myc and HIF-1, two important factors of cancer metabolic remodeling. Importantly sirtuins affect the activity of glycolytic enzymes through deacetylation [63,64,65,66]. SIRT3, a mitochondrial deacetylase, enhances mitochondrial metabolism by deacetylating and activating the TCA cycle and fatty acid oxidation enzymes [67] (Figure 4).
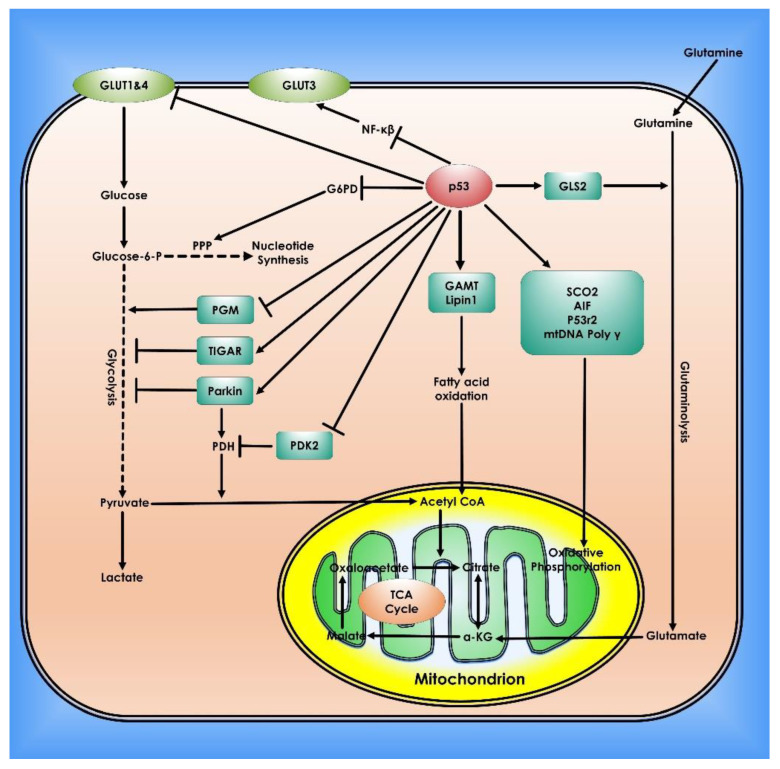
Figure 4.
Role of p53 in Cancer Metabolism. In cells, p53 controls mitochondrial oxidative phosphorylation, glycolysis, glutaminolysis, and fatty acid oxidation. To preserve mitochondrial integrity and increase oxidative phosphorylation, p53 transcriptionally stimulates the synthesis Of Cytochrome C Oxidase 2 (SCO2), Apoptosis-inducing Factor (AIF), and p53-controlled ribonucleotide reductase (p53R2), and physically interacts with mtDNA Poly. p53 inhibits glucose absorption through direct suppression of GLUT 1 and 4 transcription and indirect repression of GLUT 3 expression. p53 inhibits glycolysis via adversely regulating phosphoglycerate mutase (PGM) at the protein level and transcriptionally inducing TIGAR and Parkin. PDH, which converts pyruvate to acetyl-CoA, is favorably regulated by Parkin. p53 suppresses the production of PDK2, which phosphorylates and inhibits the function of PDH. Glutaminase 2 (GLS2) catalyzes the hydrolysis of glutamine to glutamate and is induced by p53. This can then be transformed into α-KG. GLS2 enhances the TCA cycle and oxidative phosphorylation by raising α-KG levels. p53 physically interacts with G6PD to inhibit its activity, hence inhibiting PPP, a crucial mechanism for nucleotide synthesis and NADPH generation. p53 promotes fatty acid oxidation by inducing the expression of Guanidinoacetate methyltransferase deficiency (GAMT) and Lipin1. Fatty acid oxidation helps the maintenance of the TCA cycle and mitochondrial oxidative phosphorylation by generating acetyl-CoA.
Evidence is growing that miRNAs have a role in the control of the Warburg effect, through interactions with oncogenes/tumor suppressors including c-Myc, HIF-1, and p53. Prostate cancer gene expression marker 1 (PCGEM1), a long noncoding RNA (LncRNA), has lately been found to increase glucose absorption for aerobic glycolysis by c-Myc activation [68,69] Many cancers, including lymphoma and colorectal cancer, have high levels of the miR-17-92 cluster miRNAs, which are transcribed as a polycistronic unit. They can enhance the oncogenic action of c-Myc and so act as an oncogene [70,71,72]. In lung and liver tumors, miR-221 and miR-222 are typically overexpressed. Their overexpression has been shown to increase tumorigenicity by inhibiting the tumor suppressors phosphatase and tensin homolog, and tissue inhibitor of metalloproteinase 3 [73]. miR-504 is a new miRNA that can suppress p53 production by binding to two binding sites in the human p53 3′-UTR. The results show that ectopic expression of miR-504 decreases p53 protein levels and inhibits p53 activities, particularly p53-mediated apoptosis and G1 cell cycle arrest in stressed cells [74]. According to one research, miR-125b is another new miRNA that targets p53. miR-125b is a brain-enriched miRNA that functions as a p53-negative regulator in both zebrafish and humans. Overexpression of miR-125b suppresses endogenous p53 protein levels and decreases apoptosis in cells, whereas knockdown of miR-125b enhances p53 protein levels and causes apoptosis in human cells and the zebrafish brain [75] (Figure 5).
Figure 5.
miRNAs and Cancer Metabolism. miRNAs control cell metabolism by targeting critical metabolic enzymes and oncogenic signaling pathways. miRNAs (shown in the dark blue shaded boxes) potentially influence cell metabolism via altering the expression of metabolic transporters (such as GLUT) or enzymes (such as HK2, ALDOA, and PDK1), as well as by acting on the p53, c-Myc, and AKT/mTOR signaling pathways.
5. c-Myc and Cancer Metabolism
The revelation that LDH-A, which converts pyruvate to lactate as part of the glycolytic pathway, was one of twenty potential c-Myc target genes was the first indication that c-Myc played an essential role in glycolysis regulation [26,76,77]. Further research has revealed that c-Myc regulates numerous additional glucose metabolism genes as well [78]. GLUT1, HK2, PFKM, and enolase 1 (ENO1) are among the genes involved [79,80,81]. c-Myc directly contributes to the Warburg effect (aerobic glycolysis) and the capacity of transformed cells to convert glucose to pyruvate even under appropriate oxygen tension by up-regulating these genes. ENO1 has been demonstrated to give rise to another translation initiation product, c-Myc promoter-binding protein-1 (MBP-1), which is a negative regulator of c-Myc expression [82]. This creates a negative feedback effect that is regulated by hypoxia [83].
Transgenic animal studies have validated the immediate effects of c-Myc expression on glycolytic capability [84]. Mice with c-Myc overexpression in the liver have enhanced glycolytic enzyme activity and create more lactic acid. Stably transfected mouse fibroblasts overexpressing LDH-A alone or those transformed by c-Myc, on the other hand, overproduce lactate. This shows that LDH-A, a downstream target of c-Myc, can cause the Warburg effect. By reducing LDH-A expression, the soft agar clonogenicity of Burkitt’s lymphoma cells is significantly reduced [26].
c-Myc is vital in mitochondrial biogenesis in addition to its involvement in regulating cellular metabolism through modulating the expression of genes involved in metabolic pathways. Large-scale gene expression investigations in rat and human systems originally revealed that c-Myc overexpression might activate nuclear-encoded mitochondrial genes [85,86,87]. Furthermore, c-Myc has been found to bind to the promoters of genes encoding mitochondrial proteins [85,88]. It was demonstrated that mitochondrial biogenesis is reliant on c-Myc expression using an inducible c-Myc-dependent human B cell model of cell proliferation [26]. Furthermore, mitochondrial biogenesis genes were among the most strongly elevated c-Myc target genes.
c-Myc appears to improve mitochondrial function in addition to its involvement in mitochondrial synthesis. It has been demonstrated that c-Myc stimulates mitochondrial acetyl-CoA production, which contributes to large increases in histone acetylation and fatty acid biosynthesis in rapidly proliferating cells [89,90]. Mitochondria not only serve as a means for efficient production of ATP in the presence of oxygen, but they also play an essential role in generating substrates for macromolecular synthesis in dividing cells. The ability of c-Myc to induce mitochondrial biogenesis in proliferating cells while impeding mitochondrial respiration is critical. Pyrimidines, whose production is directly related to the electron transport chain, the carbon backbone for amino acids, and citrate, which is converted to acetyl-CoA for lipid biosynthesis are among these components. These roles supplement c-Myc’s stimulation of glucose uptake and metabolism, which provides carbon backbones for essential cellular components such as ribose for nucleotide biosynthesis and NADPH for redox balance via the PPP, triglycerides, and ATP via glycolysis.
Studies have shown that c-Myc expression has a considerable effect on cancer-related changes in glucose and glutamine metabolism [26]. The observation of persistent, c-Myc-dependent hypoxic glutamine metabolism even in the absence of glucose shows that c-Myc expression has a significant impact on this. Overexpression in transformed cells resulted in the simultaneous metabolism of glucose to lactate and the oxidation of glutamine via the TCA cycle [26]. Under hypoxic circumstances with elevated c-Myc, a significant portion of the glucose eaten was converted to expelled lactate, whereas glutamine was utilized by the TCA cycle for cell survival. This study also discovered that under glucose-depleted culture conditions, a glutamine-dependent and glucose-independent TCA cycle may occur under both aerobic and hypoxic circumstances. Furthermore, they discovered an increase in glutamine to glutathione conversion under hypoxia; glutathione is a key reducing agent for limiting the buildup of mitochondrial ROS. They also showed that inhibiting glutaminase kills hypoxic cancer cells in vitro and slows tumor xenograft development.
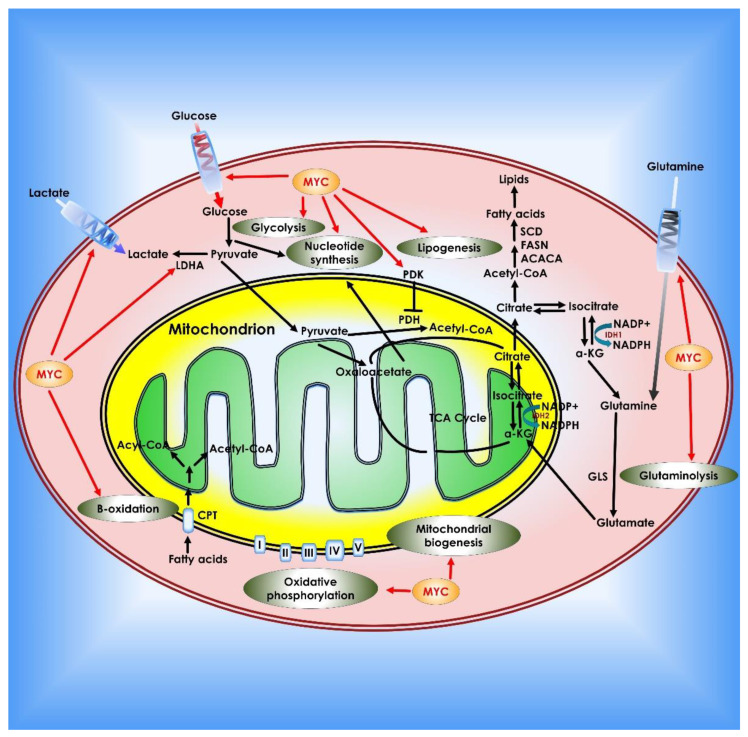
Interestingly, c-Myc appears to influence glutamine metabolism by suppressing the miRNAs miR-23a and miR-23b [26,91]. As a result, the expression of their target protein, mitochondrial glutaminase, increases. This, in turn, causes an increase in glutamine catabolism, resulting in more glutamate, which is then metabolized via the TCA cycle or acts as a substrate for glutathione formation [92]. This surprising gene regulatory system links Myc control of miRNAs, glutamine metabolism, and ROS homeostasis (Figure 6).
Figure 6.
Role of c-Myc in cancer metabolism. Myc contributes to tumor cell metabolic reprogramming on several levels. Myc enhances the Warburg effect by boosting glucose absorption by up-regulation of glucose transporters, increasing glycolysis and lactate generation (via regulation of LDH-A and the lactate transporter MCT1). Myc also regulates pyruvate dehydrogenase, which prevents pyruvate from entering the TCA cycle. Myc promotes glutamine addiction in cancer cells by upregulating glutamine transporters and suppressing the production of miRNA-23a/b, which inhibits GLS1. Myc stimulates fatty acid synthesis by modulating numerous lipogenesis enzymes such as acetyl-CoA carboxylase (ACACA), FASN, and stearoyl-CoA desaturase (SCD). Myc also promotes nucleotide synthesis, mitochondrial biogenesis, fatty acid β-oxidation, and oxidative phosphorylation.
6. Fatty Acid Metabolism in Cancer Cells
Higher fat production has lately been established as another major distortion of metabolism requisite for carcinogenesis while garnering less emphasis than aerobic glycolysis [93]. FA production is generally enhanced in cancer cells to meet the lipid need for membrane and signaling molecule synthesis, and cancer cells typically accumulate more lipids in the form of lipid droplets than normal cells. Citrate is synthesized in the mitochondria via the TCA cycle and then transported across the inner mitochondrial membrane into the cytosol by the transport protein citrate carrier (CIC), where it is used in de novo FA production. CIC activity is necessary for tumor proliferation in vitro and carcinogenesis in vivo, and its levels are raised in human cancer cell lines [94].
In de novo lipogenesis, ACLY functions as a primary rate-limiting enzyme. It links glucose and FA metabolism by converting citrate to oxaloacetate and acetyl-CoA, which is used in FA synthesis in the cytoplasm, by converting glucose-derived citrate into acetyl-CoA. ACLY upregulates histone acetylation in mammalian cells in response to growth factor stimulation [90]. ACLY expression is highly upregulated in colorectal cancer, breast cancer, glioblastoma, and ovarian cancer suggesting its role in the metabolic activities of different cancers. ACLY upregulation enhances tumor cell development, whereas ACLY silencing decreases tumor cell growth [95,96,97,98]. Acetylation of ACLY stabilizes it by preventing ubiquitylation and degradation, and it stimulates de novo lipid synthesis and tumor cell proliferation, whereas deacetylation of ACLY by a deacetylase, such as SIRT2, destabilizes it [99]. ACLY has been shown to influence the cell cycle in addition to its major role in de novo lipogenesis. ACLY inhibits AMP-activated protein kinase (AMPK) activity by directly interacting with the catalytic domain of AMPK, and activation of AMPK in the absence of ACLY may result in p53 activation, ultimately leading to cellular senescence.
Acetyl-CoA carboxylase (ACC) catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, another rate-limiting step in fatty acid synthesis [100]. The human genome has two types of ACC, ACC1, and ACC2. ACC1 is found in the cytosol and is predominantly expressed in lipogenic tissues, whereas ACC2 is found in the mitochondrial membrane and is found largely in oxidative tissues [101]. ACC1 is in charge of the rate-limiting phase of de novo fatty acid synthesis, converting acetyl-CoA to malonyl-CoA. ACC2 is suspected to play a role in the regulation of fatty acid oxidation rather than fatty acid production. AMPK, a critical metabolic sensor that is directly phosphorylated and activated by the tumor suppressor LKB1, significantly reduces fatty acid synthesis by phosphorylating and inactivating ACC1 [99].
Malonyl-CoA decarboxylase (MCD) catalyzes the conversion of malonyl-CoA to acetyl-CoA and carbon dioxide. Malonyl-CoA levels in the cell alter the balance of FA synthesis and oxidation. SIRT4 binds, deacetylates, and inhibits malonyl-CoA decarboxylase as the mitochondrial sirtuin MCD. As a result, SIRT4 inhibits FA oxidation while boosting lipid anabolism [102]. The final stages in the de novo synthesis of FA are catalyzed by FASN. Palmitate was generated from malonyl-CoA and acetyl-CoA substrates by sequential condensation processes in the presence of NADPH and then processed into saturated fatty acids. FASN is overexpressed in many forms of cancer, and increased FASN expression and activity confer a survival advantage in cancer cells [103]. According to research, FASN is a metabolic marker of cell proliferation rather than a hallmark of malignancy in ovarian cancer and its precursor cells. Although the absence of FASN expression in quiescent normal cells indicates that it is a promising target for future therapeutic development [104]. SREBP1, a transcription factor and a member of the basic helix loop helix leucine zipper (bHLHLZ) family, regulates lipogenic processes by activating a varied range of genes involved in fatty acid and triglyceride syntheses, such as ACLY, ACC1, and FASN [102]. SIRT6, an NAD+-dependent protein deacetylase, inhibits SREBP1 expression and activity, as well as transcription of its target genes, resulting in decreased triglyceride levels in the hepatocytes [103]. SIRT1, however, promotes endometrial tumor formation by upregulating SREBP1 expression, consequently enhancing lipogenesis [104]. It was shown that mTORC1 regulates SREBP1 activity and contributes to Akt-dependent lipogenesis and cell proliferation. However, a new study discovered that mTORC2 also serves as a critical signaling hub for FA metabolism, and this is accomplished by activating downstream AGC kinases such as AKT, serum- and glucocorticoid-regulated kinases (SGKs), and PKCs [105,106] (Figure 7).
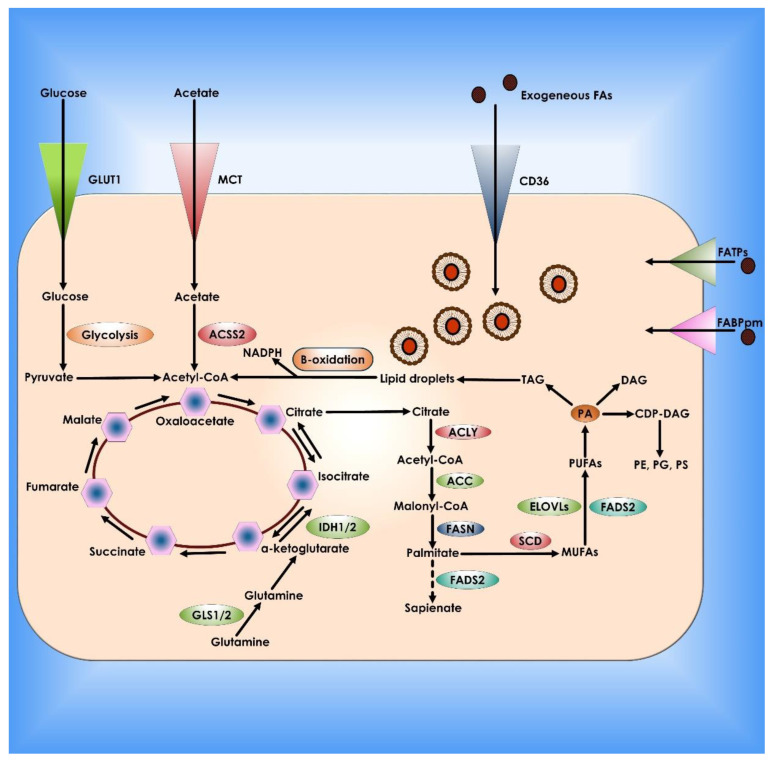
Figure 7.
Lipid metabolism in Cancer Cells. De novo lipogenesis and exogenous absorption provide fatty acids (FAs) to cancer cells. Specialized transporters, including CD36, FATPs, and FABPpm, allow external absorption of FAs from the surrounding milieu. FAs and their synthetic products can then be stored as LDs and utilized to produce NADPH and acetyl-CoA via oxidation. Cancer cells use glucose, glutamine, and acetate to synthesize citrate as carbon sources for de novo lipogenesis. Palmitate is formed from citrate by the enzymatic activities of ACLY, ACC, and FASN and can then be desaturated and extended to create a broad set of lipid species. There is an alternate mechanism for palmitate desaturation that produces sapienate rather than palmitoleate via FADS2.
7. Enzyme of the Branched Chain Amino Acid Metabolism in Cancer Cells
Branched-chain amino acids (BCAA) metabolism remodeling is determined by changes in the expression and activity of BCAA transporters and metabolic enzymes involved. Branched-chain amino acid aminotransferase (BCAT) is controlled by oncogenes and tumor suppressors in cancer cells, resulting in carcinogenesis. Several transcriptional regulators, including c-Myc, HIF-1, and SMAD5, have binding sites in BCAT1’s promoter region. HIF-1 has been shown to upregulate both the mRNA and protein levels of BCAT1 in human glioblastoma cell lines and primary glioblastoma spheres in hypoxia circumstances [105,106,107,108]. Although both HIF-1 and HIF-2 may directly bind to the hypoxia response element in the first intron of the BCAT1 gene, only HIF-1 is functional in activating BCAT1 transcription. Similarly, HIF-1 and HIF-2 increase the BCAA transporter large neutral amino acid transporter (LAT1) in human glioblastoma cells under hypoxia, while HIF-2 is responsible for LAT1 expression in ccRCC cells [109]. Hypoxia and HIF did not affect BCAT2 expression in glioblastoma. The metabolic tracing experiment revealed that hypoxia promotes nitrogen transfer from BCAAs to glutamate, which is inhibited by HIF-1/2 deletion, implying that HIF is a critical regulator of BCAA metabolic remodeling in human glioblastoma cells in response to hypoxia [110]. Musashi2 (MSI2) was shown to bind to MSI binding sites in the 3′ untranslated region of BCAT1 in human chronic myeloid leukemia cell lines and positively regulate BCAT1 transcription. MSI2 is inappropriately active in various human malignancies such as glioma and breast cancer. SMAD5 is translocated into the nucleus and interacts with the BCAT1 promoter to stimulate BCAT1 expression in cancer-associated fibroblasts from pancreatic ductal adenocarcinoma (PDAC) tumors when the transforming growth factor is activated [108,111]. BCAT2 transcription is activated in pancreatic cancer cells by the transcription factor sterol regulatory element-binding protein 1 (SREBP1) [112]. Furthermore, the mutant Kirsten rat sarcoma viral oncogene homolog (KRAS) oncogene controls the increase of BCAT2 expression at the post-translational level. In KRAS-mutant PDAC, the spleen tyrosine kinase SYK is downregulated, resulting in lower phosphorylation of tyrosine 228 on BCAT2 protein, limiting Tripartite motif containing-21 mediated ubiquitination and consequent protein degradation of BCAT2 [113]. The expression of genes involved in BCAA metabolism has been demonstrated to be controlled by several nuclear receptors. Through several nuclear receptors, the peroxisome proliferator-activated receptor (PPAR) coactivator 1 (PGC-1) can promote BCAT2 and branched-chain keto acid dehydrogenase E1 subunit alpha (BCKDHA) expression in transgenic mice but not branched-chain ketoacid dehydrogenase kinase (BCKDK) expression [114]. Bioinformatics investigation revealed that transcription factors including PPAR and Krüppel-like factor (KLF) 4 are enriched in BCAT2 and/or BCKDHA genes in PGC-1 transgenic mice. By binding to BCAT2’s promoter, the glucocorticoid receptor-KLF15 axis co-activates its expression in rat muscle cells [115]. In the rat liver, the peroxisome proliferator-activated receptor (PPAR) is thought to activate the BCKDH complex by downregulating BCKDK. The expression of PPAR in muscle, liver, and white adipose tissue was increased in BCAA-fed mice, showing a feedback loop between nuclear receptors and BCAA metabolism [116,117,118]. DNA methylation at BCAT1’s promoter inhibits transcription, which is linked to BCAT1 downregulation in isocitrate dehydrogenase (IDH) mutant anaplastic astrocytoma and glioblastoma. In hepatocellular carcinoma, methylation of the BCAT1 promoter is decreased, resulting in higher BCAT1 expression [119]. G9a and SUV39H1 are histone modifiers that catalyze di- and tri-methylation of histone H3 (H3K9) at the promoter of the BCAT1 gene, resulting in BCAT1 downregulation in lung cancer cells [120]. Similarly, in leukemia, EZH2, the catalytic member of the polycomb repressive complex 2 that promotes H3K27 methylation, inhibits BCAT1 expression [121]. The oncometabolite R-2-hydroxyglutarate (2-HG), which is generated by mutant IDH1/2 in glioma and competes with α-KG for binding, inhibits the transaminase activity of BCAT1 and BCAT2. Oncogenic factors and tumor suppressors work together to fine-tune the expression levels of BCAT and BCKDH, causing BCAA metabolic reprogramming [122] (Figure 8).
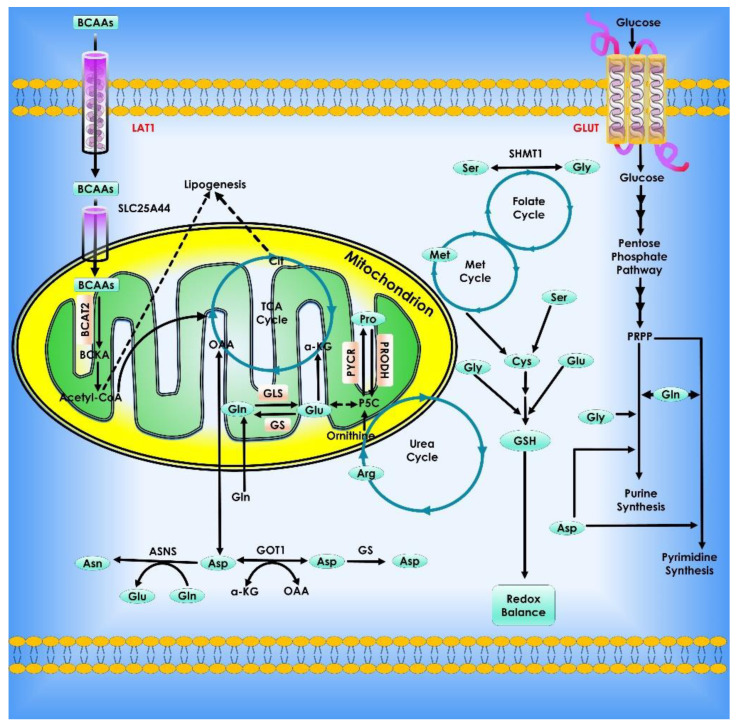
Figure 8.
Amino acid metabolism in cancer cells. Cancer cell growth and proliferation rely heavily on metabolic reprogramming. Both essential and non-essential amino acids (EAAs and NEAAs) contribute to altered metabolism by acting as energy sources, biosynthetic agents, and redox balance mediators. Amino acids provide metabolic intermediates like acetyl-CoA, which fuel energy generation via the citric acid cycle. Amino acids also serve as the foundation for nucleotide synthesis and lipogenesis, both of which are essential for a cell’s capacity to grow and develop. To counteract the consequences of oxidative stress, amino acids can control redox equilibrium by producing glutathione. Furthermore, EAA catabolism contributes to the production of NEAAs by chemical processes such as those mediated by transaminases.
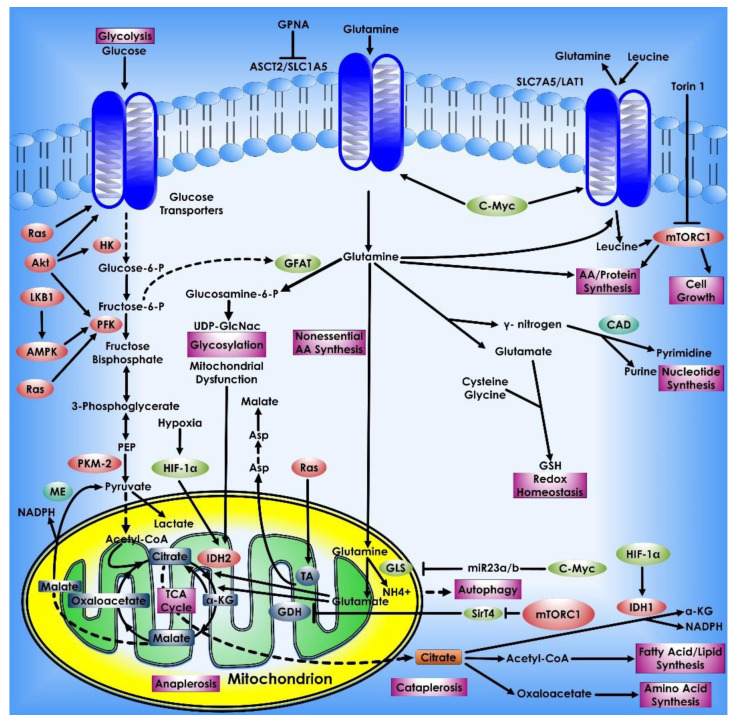
8. Glutamine Metabolism in Cancer Cells
Upregulation of the glutamine metabolism is a signature metabolic alteration in carcinogenesis. Interestingly, glutamine is the second most important nutrient after glucose in multiple cancer types. Glutamate contributes nitrogen and carbon to several activities in cancer cells, including energy generation, macromolecular synthesis, and signal transmission. Transporters (such as SLC1A5 or ASCT2) carry glutamine into the cytoplasm, and glutaminase (GLS) facilitates glutamine catabolism by converting glutamine to glutamate [123,124]. The oncogenic transcription factor c-Myc enhanced glutaminase production and glutamine metabolism in cancer cells [26,125]. Master tumor suppressor p53 directly binds to GLS2 and transcriptionally modulates its expression affecting glutamine metabolism and GSH antioxidant activity and consequently lower levels of intracellular ROS [126]. Glutamate can be carried into mitochondria and converted into α-KG by oxidative deamination by GLUD1. Transamination of glutamate to α-KG can occur in the cytoplasm or mitochondria, resulting in the production of non-essential amino acids (e.g., serine). Following that, the mitochondrion’s α-ketoglutarate is used as a TCA cycle intermediary for energy recycling. To manufacture AcCoA for lipid synthesis, human cells use reductive metabolism of α-KG. Under normal culture circumstances, this IDH1-dependent process is active in most cell lines, whereas cells grown under hypoxia depend nearly entirely on the reductive carboxylation of glutamine-derived α-KG for de novo lipogenesis. Moreover, when glucose is depleted, glutamine-derived fumarate, malate, and citrate were dramatically elevated, suggesting that glutamine drives the glucose-independent TCA cycle. Many tumor cells have increased glutamine usage for mitochondrial-dependent bio-energy generation and cellular biosynthesis. Silencing of a tumor suppressor, which is also a kinase B1, a serine/threonine kinase leads to increased glucose and glutamine consumption in tumor cells. The kinase B1 appears to link bioenergetics processes to cell growth control via the regulation of mTOR activity [127]. Many malignant cells utilize acetyl-CoA, which is mostly synthesized from the glucose metabolism product pyruvate, whereas glucose deprivation triggers a cascade in which acetyl-CoA is produced from glutamine via GDH. Inhibiting mitochondrial pyruvate transport, on the other hand, activates GDH which modulates glutamine metabolism to create oxaloacetate and acetyl-CoA, resulting in lipid synthesis that is glutamine-dependent [128].
In addition to the extracellular absorption, Serine is also produced from the glucose metabolism pathway. Interestingly, Serine and glycine biosynthesis which appears to be connected produces precursors essential for the synthesis of proteins, nucleic acids, and lipids. These macromolecules in turn are essential ingredients for cancer development. In this context, it is important to note that SHMTs genes, which are direct c-Myc transcriptional targets, may catalyze both de novo serine generation from 3-phosphoglycerate and imported serine conversion to glycine [129]. Threonine dehydrogenase and glycine C-acetyltransferase can also convert threonine to glycine. Glycine then offers methyl groups for one-carbon metabolism, which is essential for nucleic acid, protein, and lipid production, as also DNA methylation [130].
Proline is a one-of-a-kind proteinogenic secondary amino acid that is stored in collagen, the body’s most ubiquitous protein [131]. Proline and glutamate are interconvertible, using D1-pyrroline-5-carboxylate (P5C) and glutamic-c-semialdehyde (GSA) as intermediates. Proline Dehydrogenase/Proline Oxidase is a mitochondrial tumor suppressor that catalyzes the conversion of proline to P5C. It is activated by p53 and PPARc but repressed by miR-23b* and c-Myc [123]. In the Urea cycle, GSA generated from glutamate or proline can be converted to ornithine, which is a prerequisite for arginine production [131]. Arginine is an important amino acid, and arginine deficiency caused several types of solid tumor cells to die quickly in culture media [124]. Arginine is involved in several essential cellular metabolic processes, including the urea cycle, nitric oxide biosynthesis, nucleotides, proline, and glutamate biosynthesis [125]. The rate-limiting enzyme for the de novo production of arginine is argininosuccinate synthetase (ASS), which catalyzes the synthesis of argininosuccinate from L-citrulline and aspartic acid. Following that, argininosuccinate lyase (ASL) transforms argininosuccinate into L-Arginine and fumaric acid, the latter of which connects arginine metabolism to glucose-generated energy metabolism via the TCA cycle. Some human malignancies, particularly malignant melanoma and hepatocellular carcinoma, lack ASS and so are vulnerable to arginine deprivation treatment employing arginine-degrading enzymes because they are unable to generate arginine [125]. Recombinant arginine-degrading enzymes were employed to treat this type of tumor since these cancer cells would die if they were exposed to them (arginine deiminase or arginase) (Figure 9).
Figure 9.
Glutamine metabolism in Cancer Cells. Glutamine is a key metabolic fuel that assists rapidly growing cells in meeting the increased need for ATP, biosynthetic precursors, and reducing agents. Glutamine enters the cell via the amino acid transporter ASCT2/SLC1A5 and is transformed into glutamate in the mitochondria via a glutaminase-catalyzed deamination process (GLS). Glutamate is metabolized to the TCA cycle intermediate α-KG by GDH or alanine or aspartate transaminases (TAs), which also create the respective amino acid. α-KG is an important molecule that aids in both ATP generation and the replenishment of TCA cycle intermediates, a process known as anaplerosis. α-KG can be converted to citrate in a reductive carboxylation process mediated by IDH2 during periods of hypoxia or mitochondrial malfunction. The freshly generated citrate leaves the mitochondria and is utilized to manufacture fatty acids and amino acids as well as the reducing agent, NADPH (cataplerosis). In the cytosol, glutamine lends its (amide) nitrogen for the synthesis of nucleotides and hexosamines, resulting in the production of glutamate. Through the synthesis of glutathione, cytosolic glutamate is essential for maintaining redox equilibrium and protecting cells from oxidative damage. Many cancer cells have oncogene-dependent glutamine addictions, and glutamine can increase proliferative signaling. For example, glutamine influx via SLC1A5 is linked to efflux via the SLC7A5/LAT1 transporter, enabling leucine into the cell and activating mTORC1-mediated cell growth. Furthermore, the signaling molecules Akt, Ras, and AMPK stimulate glycolytic enzymes and promote lactate synthesis (Warburg effect), requiring cancer cells to use glutamine metabolism to fulfill higher energy needs. The proto-oncogene c-Myc promotes glutaminolysis by activating the GLS and SLC1A5 genes through transcriptional activation. Proteins that have been glycosylated by glutamine, including growth factor receptors, can be targeted to the cell surface and activated.
9. Targeting Cancer Metabolism for Therapeutic Purposes
Cancer metabolism is an extremely promising and quickly expanding therapeutic avenue in the current landscape of anti-cancer drug research due to its crucial part in tumorigenesis, which is caused by metabolic reprogramming. The selective and effective inhibition of tumor-relevant metabolic enzymes is now possible with a wide variety of new drugs (Table 1). Many factors must be taken into account while deciding on the best metabolic target for cancer therapy. Some metabolic enzymes may be systemically harmful because of their physiological roles in normal tissues [126]. These pathways can be targeted therapeutically if the systemic inhibition of the route is tolerated. As cancer cells alter their metabolism, so do normal proliferating cells, such as immune cells and stem cells [127,128]. The adaptive immune system should be unaffected by metabolic inhibitors. It’s still possible to find outstanding instances of cancer therapies that benefit from the reprogramming of certain circuits. Anti-folates (methotrexate, pemetrexed, and others) have been shown to target tumor cells’ increased nucleotide and DNA synthesis, which is a hallmark of cancer [129]. Cancer treatment regimens are generally effective although these medications might cause toxicity in normal proliferative tissues such as the intestinal epithelium and bone marrow. Because of this, it is important to study the impact on normal cells of metabolic enzyme inhibition. There is a growing body of evidence indicating cancer cells can change their metabolic profile during carcinogenesis and metastasis. As a result, cancer cells may be able to build resistance to a certain metabolic pathway’s blockage by producing alternative protein isoforms or up-regulating compensatory pathways. For this reason, it is important to target numerous metabolic pathways concurrently or to target a specific metabolic system in conjunction with medicines targeting oncogenic or signaling pathways. Some intriguing metabolic targets are highlighted in this section [128].
Table 1.
Compounds targeting cancer metabolomics: The table lists the most common and recent small molecules, compounds, anti-metabolites, and cytotoxins.
| Compound | Target | Effect | Tumour Types Targeted |
|---|---|---|---|
| 2-deoxyglucose | Hexokinase | Inhibits glycolysis | Advanced solid tumors (e.g., lung, breast, prostate, and gastric) |
| Lonidamine | Hexokinase | Inhibits glycolysis | Benign prostatic hyperplasia |
| 3-bromopyruvate | Hexokinase | Inhibits glycolysis | N/A |
| TLN-232 | Pyruvate kinase | Inhibits glycolysis | Metastatic melanoma and renal cell carcinoma |
| Dichloroacetate | PDK1 | Reactivates PDH | Metastatic solid tumors, glioma, and GBM |
| Phenylacetate | Glutamine | Reduces plasma glutamine levels | Brain tumors (e.g., glioma, astrocytoma and medulloblastoma) |
| Asparaginase and Pegasparaginase | Asparagine | Reduces plasmaasparagine levels | ALL, TCL, and BCL |
| Arginine deiminase | Arginine | Reduces plasma asparagine levels | Metastatic melanoma and hepatocellular carcinoma |
| Acetazolamide, Indisulam and other sulfonamides | Carbonic anhydrases | pH regulation | Solid tumors (e.g., pancreatic, lung, melanoma and metastatic breast) |
| Cariporide | NHE1 | pH regulation | N/A |
| SB-204990 | ATP-citrate lyase | Inhibits fatty acid synthesis | N/A |
| Orlistat, GSK837149A, and C75 | FASN | Inhibits fatty acid synthesis | N/A |
| Temsirolimus and Everolimus | mTORC1 | Inhibits mTORC1 | Solid tumors (both metastatic and non-metastatic) |
| Other rapalogues | mTORC1 | Inhibits mTORC1 | Solid tumors (e.g., pancreatic, endometrial and glioblastoma) and lymphoma |
| Torin1 and PP242 | mTORC1 and mTORC2 |
Inhibits mTORC1 andmTORC2 | N/A |
| PX-478 | HIF1α | Inhibits HIF signaling | Advanced solid tumors and lymphoma |
| Acriflavine | HIF1α | Inhibits HIF signaling | N/A |
| Tirapazamine and other bioreductivecompounds | Hypoxia | Resensitizes cells to other treatments | Solid tumors (e.g., cervical, SCLC and NSCLC) |
| Bevacizumab and related compounds | Hypoxia, VEGF and VEGFR |
Blocks angiogenesis | Solid tumors (e.g., malignant glioma, NSCLC, ovarian, and colorectal) |
| MK-0646, BIIB022, AVE1642, and others | IGF1R | Blocks IGF signaling | Solid tumors (e.g., NSCLC, pancreatic, hepatocellular carcinoma and metastatic breast) |
| BEZ235, XL765, SF1126, and BGT226 | PI3K and mTOR |
Inhibits signaling from PI3K andmTORC1 andmTORC2 | Advanced solid tumors (e.g., malignant glioma and NSCLC) |
| GDC-0941 and PX866 | PI3K | Inhibits PI3Ksignaling | Advanced solid tumors (metastatic breast and non-Hodgkin’s lymphoma) |
| Perifosine andGSK690693 | AKT | Inhibits AKT | Solid tumors (e.g., renal cancer and NSCLC) and lymphoma |
| Metformin | AMPK andComplex I(mitochondrial) | Activates AMPK | Solid tumors and lymphoma |
| Antimetabolites (e.g., 5-FU, cytarabine andmethotrexate | Nucleotidebiosyntheticpathway | Inhibits cell proliferation | Many tumor types |
One of the initial metabolic targets of Myc was LDH-A, an enzyme that converts pyruvate to lactate, which is a byproduct of glycolysis [26]. Experiments in xenograft models have revealed that LDH-A suppression can reduce Myc-driven malignancies [35,132]. Studies in mice with genetically engineered NSCLC show that suppression of LDH-A causes tumor shrinkage without causing systemic damage [133]. Myeloid leukemia development is also slowed by the genetic ablation of LDH-A [134]. That’s why cancer cells with Myc mutations may be a good target for LDH-A overexpression. HK2 is another glycolytic protein that might be used as a therapeutic target. When HK2 is inhibited in preclinical models of genetically altered NSCLC and breast cancer, tumor growth is delayed [35]. Moreover, systemic HK2 deletion in mice has no negative physiological effects on the organisms involved. LDH-A and HK2 are yet to be studied concerning the adaptive immune system. Immune checkpoint inhibitors may work along with LDH-A inhibition to unleash host inflammatory T lymphocytes that will preferentially assault tumor cells, as lactate has been reported to limit T-cell cytotoxicity [135]. Reprogramming macrophages by lactate can potentially be used to induce cancer [136]. Thus, targeting LDH-A or HK2 in cancers that are highly glycolytic and overexpress these proteins may be beneficial.
PHGDH, an enzyme in the de novo serine synthesis pathway, is another glucose-dependent target. Some human melanoma and breast tumors require PHGDH for development in vitro, which is why high levels of PHGDH have been reported [35,137]. The tumorigenicity of p53-null tumors in mice is reduced by the deprivation of the amino acid serine [138]. In the mitochondria, SHMT2 transforms serine into glycine to produce folate intermediates from de novo or exogenous synthesis [139,140]. SHMT2 expression is high in several forms of cancer and is associated with a poor prognosis. Under hypoxia, the transcription factors Myc and HIF-1 also support survival by inducing SHMT2, another transcription factor [141,142]. Targeting PHGDH, SHMT2, or other enzymes in the one-carbon metabolic pathway has not yet been proven to slow or halt tumor development in genetically modified, patient-derived xenografts (PDX), or syngeneic animal models of cancer. Even while one-carbon metabolism is critical for the anabolic demands of tumor cells, this route is likely required for tumor growth in vivo [143]. As a result of the discovery that metformin, an antidiabetic medicine, is also an anticancer agent, mitochondrial metabolism has emerged as a crucial target for cancer therapy [144]. Diabetic people who use metformin to regulate their blood glucose levels are less likely to acquire cancer and have an increased survival rate if cancer is already present, according to several epidemiological studies [145]. Metformin has also been shown to have anti-cancer properties in laboratory experiments [141,146,147]. Reversible inhibition of mitochondrial complex I by metformin has been discovered by biochemists [148,149,150]. Recent research shows that metformin inhibits mitochondrial ETC complex I, which is linked to cancer [151]. Metformin specifically inhibits the generation of mitochondrial ATP, resulting in the death of cancer cells when glycolytic ATP levels drop due to a lack of glucose. Cancer cells’ ability to synthesize lipids, amino acids, and nucleotides is similarly inhibited by metformin, which is found in the mitochondria [152]. Organs such as the liver and kidneys include organic cation transporters (OCTs) that allow metformin to be absorbed, making it safe for long-term use [153]. Metformin can be absorbed by tumor cells that express OCTs [154]. There is no metformin to block mitochondrial complex I in malignancies that do not have OCTs. It is not apparent if the current antidiabetic dose of metformin used in clinical studies is sufficient to inhibit mitochondrial complex I in malignancies. Metformin at greater dosages than those now used for diabetes may thus be more effective and safe. Phenylbiguanide phenformin, like metformin, has anticancer effects due to its ability to suppress mitochondrial complex I activity [155]. Aside from the fact that it is more easily carried into tumor cells than metformin, Phenformin is no longer prescribed to humans for this same reason. Despite this, phenformin should be considered a viable cancer treatment because lactic acidosis can be managed. Serine deprivation or tumors that have lost p53 or LKB1 can increase the susceptibility of mice to biguanide [141,156,157]. Because of this, biguanides and other mitochondrial complexes I inhibitors may be useful anticancer medicines.
Autophagy and glutaminase inhibitors might also be used to decrease mitochondrial metabolism in some cancers. Short-term suppression of autophagy has been demonstrated to reduce tumor development without causing systemic damage in NSCLC mice models. Autophagy produces amino acids, such as glutamine, that feed the TCA cycle in NSCLC and pancreatic malignancies [141,158]. However, in models where tumors are dependent on glutamine for TCA cycle metabolism in the lack of autophagy, the antitumor efficacy of glutaminase inhibitors has been shown [26,159,160]. Acetate metabolism can be targeted as an alternate strategy. Mitochondria provide the cell with acetyl-CoA, but cancer cells can also utilize acetate during metabolic stress (hypoxia or food restriction) to sustain cell growth and survival [161]. Accumulation of acetyl-CoA is not required for proper development, so ACCS2 is an attractive target for acetate metabolism. ACCS2 knockout mice do not show any obvious signs of disease, yet models of hepatocellular carcinoma with ACCS2 genetic deletion experience reduced tumor burden [162]. Inhibitors of the oxidation of acetate may be effective against human glioblastomas [163]. Autophagy and other mechanisms that supply important metabolic intermediates may thus be effective in specific settings.
Due to the ineffectiveness of single-agent mitochondrial inhibitors, combined treatment is likely the best option. The combination of metformin and current therapeutic PI3K inhibitors, which restrict glucose uptake and glycolysis, is an example of a strategy that might affect both sources of ATP within cells [164]. Although most cancer cells are killed by treatments targeting oncogenes such as KRAS, BRAF, and NOTCH1, resistant cells that are more sensitive to inhibitors that disrupt mitochondrial metabolism are formed [165,166,167]. Increased mitochondrial inhibitor sensitivity in cancer-initiating cells provides more evidence that blocking mitochondrial metabolism may limit tumor recurrence [168,169]. Additionally, cancer cells enhance their antioxidant ability to counteract the increased ROS generation that occurs during growth and metastasis [170]. To combat cancer, one additional treatment option is to inhibit cancer cells’ ability to scavenge antioxidants, hence increasing ROS levels and leading to cancer cell death [141]. Multiple antioxidant defense mechanisms rely on the reducing equivalent NADPH. To generate NADPH in the cytosol, the oxidative PPP, malic enzyme 1, IDH1, and one-carbon metabolism all contribute. IDH2 and one-carbon metabolism govern mitochondrial NADPH production in part. Many of these NADPH-producing mechanisms are essential for cell survival and function. Two NADPH-generating systems, however, might be therapeutic targets in the future. G6PDH, an enzyme in the oxidative PPP, transforms NADP+ to NADPH in 400 million persons globally. This system, however, serves as a key source of NADPH for some malignancies, therefore it may be therapeutic to inhibit this process and produce oxidative stress to reduce tumor development. NADPH-producing mitochondrial one-carbon metabolism protein methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2 (MTHFD2) was shown to be substantially expressed in 19 distinct cancer types, but not in normal adult proliferating cells [143]. In vitro, cancer cells that lack MTHFD2 are more susceptible to oxidant-induced cell death when MTHFD2 is depleted. NADPH depletion and ROS production can be increased by administering large dosages of vitamin C, (ascorbate), sodium-dependent vitamin C transporters absorb vitamin C into cells, while the oxidized form of vitamin C, dehydroascorbate (DHA), is imported by glucose transporters such as GLUT1 into cells [171]. GSH in the cell reduces DHA back to vitamin C, which in turn creates GSSG. GSH is regenerated from GSSG via NADPH-dependent GR thereafter. When vitamin C enters the blood, it is oxidized to DHA, which is then absorbed by cells. By depleting the NADPH and GSH pools and boosting ROS levels, large dosages of vitamin C reduce carcinogenesis in colorectal cancers with oncogenic KRAS mutations, which exhibit high amounts of GLUT1 [172]. When used in combination with standard paclitaxel-carboplatin treatment, substantial dosages of vitamin C given intravenously have been shown to be safe in people [173]. Buthionine sulfoximine, an irreversible inhibitor of -glutamylcysteine synthase, which can be safely delivered to people and is beneficial in preclinical tumor models, is another option for reducing GSH levels [174]. It’s also made up of cysteine, glutamate, and glycine; these three amino acids form the tripeptide glutathione. Thus, reducing glutamate levels with glutaminase inhibitors or blocking extracellular cysteine absorption might similarly boost ROS levels in cancer cells to promote cell death.
As stem cells are sensitive to ROS levels, it’s crucial to stratify patients based on their expression levels of a certain antioxidant protein or pathway. Because cancer cells produce a significant amount of ROS, it is important to identify which antioxidant pathways are likely to be activated. Targeting the NRF2 pathway, which is used by many cancer types to maintain redox equilibrium, might be a promising treatment approach [175]. Additionally, NSCLC cells are overexpressed in SOD1 (superoxide dismutase 1), and inhibition of SOD1 kills human NSCLC cells and reduces tumor burden in mice models of NSCLC [175]. Short-term suppression of NRF2 and SOD1 may be an efficient method of killing cancer cells, as these knockout mice grow properly (Table 1).
Nanotechnology has been progressively employed in medicine over the last few decades, including applications for safer and more effective tumor diagnostics, therapy, and targeting. Many advantages of nanoparticle (NP)-based drug delivery systems in cancer treatment have been demonstrated, including excellent pharmacokinetics, specific targeting metabolic components of tumor cells, reduced side effects, and drug resistance [176,177]. NPs utilized in medication delivery systems are often created or selected depending on their size and pathophysiology of the malignancies. Mechanically, nano-carriers in cancer therapy target tumor cells following absorption via the carrier effect of NPs and the positioning effect of the targeted chemical. The medications are then delivered to tumor cells to destroy them [178].
A new glucose oxidase (GOD)-loaded therapeutic vesicular NRs (theraNR) targeted cancer cells by raising tumor oxidative stress and decreasing cancer cell antioxidative capabilities, ablating tumors while generating low systemic damage [179].
Anti-angiogenesis drugs prevent neovascularization, reducing the availability of nutrients and oxygen to the tumor and eventually starving the cancer cells. A therapeutic regime involving nanocarriers loaded with plasmid DNA expressing a human soluble version of vascular endothelial growth factor receptor-1 was carried out in mice bearing pancreatic adenocarcinoma xenografts with promising results [180,181].
Nanomedicine-mediated cancer starvation therapy, as an appealing technique for cancer treatment, might selectively deny nutrition and oxygen supply by antiangiogenesis treatment, tumor vascular disruption or blockage, direct depletion of intratumoral glucose and oxygen, and other mechanisms. Furthermore, two or more therapeutic agents could be easily integrated into a single formulation by incorporating chemotherapeutic drugs, therapeutic genes, enzymes, metal NPs, hypoxia-activated prodrugs, inorganic NPs, Fenton-reaction catalysts, photosensitizers, or photothermal agents, leading to improved treatment outcomes [182].
Recent research suggests that mitochondria might be a potential therapeutic target in cancer. So far, the FDA has authorized only a handful of mitochondria-targeted medications. The ability of medications to reach the target location, with the regulated release of the proper quantities of drug at the correct time, is essential for targeted drug delivery. Mitochondria-targeted nanoparticle platforms (mitoNANO) are a unique family of agents capable of delivering medications to the mitochondria, where they are most required [183].
Oncoproteins can also be targeted for high selectivity, the efficiency of delivery, and potential in vivo performance. A pH-responsive polymeric micelle system was developed in a study to serve as a nanocarrier for the intracellular delivery of a therapeutic protein targeting c-Myc [184].
10. Conclusions and Challenges
The ability of malignant cells to survive and expand by using conventional metabolic pathways to produce energy, generate biosynthetic precursors, and maintain redox equilibrium is reliant on metabolic reprogramming.
We have witnessed tremendous progress toward understanding the underlying molecular mechanisms and consequences of metabolic reprogramming in cancer. A great deal of data delineates mechanisms of reprogrammed metabolic pathways which are essential for cancer cell growth and proliferation. The current review explains the commonly affected metabolic pathways in cancer and focuses on the crosstalk between metabolic pathways, enzymes, metabolites, and cancer cells and how these can be exploited for potential therapeutic regimes. Chemotherapy and hormone therapy are two commonly used treatments for cancer, but their side effects demonstrate the need for more advanced and specialized techniques. Cancer metabolism is being targeted using a variety of conversion routes, enzymes, and metabolites. Cancer cells have altered metabolic pathways that cause them to proliferate more than normal, which is unusual in differentiated normal cells. c-Myc, HIF-1, Ras, and PI3K/Akt are all prominent oncogenes that cause cancer metabolic changes. Major tumor suppressors, on the other hand, such as p53 and LKB1/AMPK, counteract these alterations and regulate cellular metabolism. As a result, cancerous cells can be killed by aiming at these statues. The tumor cells are encouraged to rely on aerobic glycolysis via altering glycolysis, which results in lactate production instead of pyruvate delivery to the TCA cycle. Even though certain tumors have shown an increase in the OXPHOS pathway in comparison to their normal counterparts, this is a big discovery for cancer cells. Uncontrolled cell growth can be combated by selective targeting of the several enzymes in these pathways. Novel reprogrammed metabolic pathways facilitating cancer cells to tolerate intrinsic and extrinsic stress such as nutrient depletion might also be aided by novel tumorigenic mutations resulting in the development of drug resistance.
As a result of targeting cancer-reprogrammed metabolism, a new class of anticancer medications may be developed that may treat a wide range of cancers. Several metabolite analogs are now being explored as possible therapeutic options for tumor metabolism targeting and some have reached a stage of clinical trials as well with promising results. There may be a way to increase the efficacy and reduce the toxicities of potentially strong chemotherapy medications by studying the role of mitochondria in cancer cell metabolism more thoroughly. There is a hope that novel cancer metabolic profiles will lead to the development of a new class of cancer therapies. Because of this, the use of metabolic inhibitors may be a clinically advantageous treatment option.
Acknowledgments
We would like to thank the University of Allahabad for providing all the basic infrastructure and facilities and the Department of Biotechnology, Government of India, for providing financial support under the ‘Research Resources, Service Facilities, and Platforms’ program to carry out the study work. R.K. thanks UGC for the SRF grant. Research in the G.C.S. lab is supported by NIH 1R15CA252997-01A1.
Author Contributions
Conceptualization, R.K., M.K. and G.C.S.; Writing—original draft preparation, R.K.; Writing—review and editing, R.K., M.K., G.C.S., A.M., P.G., Z.F., S.V. and E.M.L.; Supervision, M.K. and G.C.S.; Funding acquisition, G.C.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declared no potential conflict of interest concerning the research, authorship, and/or publication of this article.
Funding Statement
Concepts covered in this review are supported by NIH 1R15CA252997-01A1 to G.C.S.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang W., Zhang S.-L., Hu X., Tam K.Y. Targeting Tumor Metabolism for Cancer Treatment: Is Pyruvate Dehydrogenase Kinases (PDKs) a Viable Anticancer Target? Int. J. Biol. Sci. 2015;11:1390–1400. doi: 10.7150/ijbs.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. The Metabolism of Carcinoma Cells1. J. Cancer Res. 1925;9:148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 3.Warburg O., Wind F., Negelein E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chae Y.C., Kim J.H. Cancer Stem Cell Metabolism: Target for Cancer Therapy. BMB Rep. 2018;51:319–326. doi: 10.5483/BMBRep.2018.51.7.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Outschoorn U.E., Peiris-Pagés M., Pestell R.G., Sotgia F., Lisanti M.P. Cancer Metabolism: A Therapeutic Perspective. Nat. Rev. Clin. Oncol. 2017;14:11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J., Yang J., Lin C., Liu W., Huo Y., Yang M., Jiang S.-H., Sun Y., Hua R. Endoplasmic Reticulum Stress-Dependent Expression of ERO1L Promotes Aerobic Glycolysis in Pancreatic Cancer. Theranostics. 2020;10:8400–8414. doi: 10.7150/thno.45124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domiński A., Krawczyk M., Konieczny T., Kasprów M., Foryś A., Pastuch-Gawołek G., Kurcok P. Biodegradable PH-Responsive Micelles Loaded with 8-Hydroxyquinoline Glycoconjugates for Warburg Effect Based Tumor Targeting. Eur. J. Pharm. Biopharm. 2020;154:317–329. doi: 10.1016/j.ejpb.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Boroughs L.K., DeBerardinis R.J. Metabolic Pathways Promoting Cancer Cell Survival and Growth. Nat. Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley H.J., Dewald H., Rothkopf Z.S., Singh S., Jenkins F., Deb P., De S., Barnes B.J., Fitzgerald-Bocarsly P. Frontline Science: AMPK Regulates Metabolic Reprogramming Necessary for Interferon Production in Human Plasmacytoid Dendritic Cells. J. Leukoc. Biol. 2021;109:299–308. doi: 10.1002/JLB.3HI0220-130. [DOI] [PubMed] [Google Scholar]
- 10.Guerra L., Bonetti L., Brenner D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep. 2020;32:107848. doi: 10.1016/j.celrep.2020.107848. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh A., Deshpande K., Arfuso F., Newsholme P., Dharmarajan A. Cancer Stem Cell Metabolism: A Potential Target for Cancer Therapy. Mol. Cancer. 2016;15:69. doi: 10.1186/s12943-016-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villalba M., Rathore M.G., Lopez-Royuela N., Krzywinska E., Garaude J., Allende-Vega N. From Tumor Cell Metabolism to Tumor Immune Escape. Int. J. Biochem. Cell Biol. 2013;45:106–113. doi: 10.1016/j.biocel.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., DeBerardinis R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019;30:434–446. doi: 10.1016/j.cmet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahimi-Horn M.C., Chiche J., Pouysségur J. Hypoxia Signalling Controls Metabolic Demand. Curr. Opin. Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Casazza A., Di Conza G., Wenes M., Finisguerra V., Deschoemaeker S., Mazzone M. Tumor Stroma: A Complexity Dictated by the Hypoxic Tumor Microenvironment. Oncogene. 2014;33:1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 16.Pouysségur J., Mechta-Grigoriou F. Redox Regulation of the Hypoxia-Inducible Factor. Biol. Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Pavlova N.N., Thompson C.B. Cancer Cell Metabolism: The Essential Role of the Nonessential Amino Acid, Glutamine. EMBO J. 2017;36:1302–1315. doi: 10.15252/embj.201696151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra K.C., Hay N. The Pentose Phosphate Pathway and Cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon S.-M., Chandel N.S., Hay N. AMPK Regulates NADPH Homeostasis to Promote Tumour Cell Survival during Energy Stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfarouk K.O., Ahmed S.B.M., Elliott R.L., Benoit A., Alqahtani S.S., Ibrahim M.E., Bashir A.H.H., Alhoufie S.T.S., Elhassan G.O., Wales C.C., et al. The Pentose Phosphate Pathway Dynamics in Cancer and Its Dependency on Intracellular PH. Metabolites. 2020;10:285. doi: 10.3390/metabo10070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairns R.A., Harris I.S., Mak T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 22.Iurlaro R., León-Annicchiarico C.L., Muñoz-Pinedo C. Regulation of Cancer Metabolism by Oncogenes and Tumor Suppressors. Methods Enzymol. 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan A., Malvi P., Wajapeyee N. Oncogene-Directed Alterations in Cancer Cell Metabolism. Trends Cancer. 2016;2:365–377. doi: 10.1016/j.trecan.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr E.M., Martins C.P. Metabolic Rewiring in Mutant Kras Lung Cancer. FEBS J. 2018;285:28–41. doi: 10.1111/febs.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pusch O., Soucek T., Hengstschläger-Ottnad E., Bernaschek G., Hengstschläger M. Cellular Targets for Activation by C-Myc Include the DNA Metabolism Enzyme Thymidine Kinase. DNA Cell Biol. 1997;16:737–747. doi: 10.1089/dna.1997.16.737. [DOI] [PubMed] [Google Scholar]
- 26.Li F., Wang Y., Zeller K.I., Potter J.J., Wonsey D.R., O’Donnell K.A., Kim J.-W., Yustein J.T., Lee L.A., Dang C.V. Myc Stimulates Nuclearly Encoded Mitochondrial Genes and Mitochondrial Biogenesis. Mol. Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannava S., Grachtchouk V., Wheeler L.J., Im M., Zhuang D., Slavina E.G., Mathews C.K., Shewach D.S., Nikiforov M.A. Direct Role of Nucleotide Metabolism in C-MYC-Dependent Proliferation of Melanoma Cells. Cell Cycle. 2008;7:2392–2400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Le A., Hancock C., Lane A.N., Dang C.V., Fan T.W.-M., Phang J.M. Reprogramming of Proline and Glutamine Metabolism Contributes to the Proliferative and Metabolic Responses Regulated by Oncogenic Transcription Factor C-MYC. Proc. Natl. Acad. Sci. USA. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edmunds L.R., Sharma L., Kang A., Lu J., Vockley J., Basu S., Uppala R., Goetzman E.S., Beck M.E., Scott D., et al. C-Myc Programs Fatty Acid Metabolism and Dictates Acetyl-CoA Abundance and Fate. J. Biol. Chem. 2014;289:25382–25392. doi: 10.1074/jbc.M114.580662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellen K.E., Lu C., Mancuso A., Lemons J.M.S., Ryczko M., Dennis J.W., Rabinowitz J.D., Coller H.A., Thompson C.B. The Hexosamine Biosynthetic Pathway Couples Growth Factor-Induced Glutamine Uptake to Glucose Metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunt S.Y., Vander Heiden M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and Competition in the Evolution of ATP-Producing Pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G., Pouyssegur J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Berridge M.V., Herst P.M., Tan A.S. Metabolic Flexibility and Cell Hierarchy in Metastatic Cancer. Mitochondrion. 2010;10:584–588. doi: 10.1016/j.mito.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Fantin V.R., St-Pierre J., Leder P. Attenuation of LDH-A Expression Uncovers a Link between Glycolysis, Mitochondrial Physiology, and Tumor Maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Gatenby R.A., Gillies R.J. A Microenvironmental Model of Carcinogenesis. Nat. Rev. Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 37.Vaupel P. Metabolic Microenvironment of Tumor Cells: A Key Factor in Malignant Progression. Exp. Oncol. 2010;32:125–127. [PubMed] [Google Scholar]
- 38.Chen J.L.-Y., Lucas J.E., Schroeder T., Mori S., Wu J., Nevins J., Dewhirst M., West M., Chi J.-T. The Genomic Analysis of Lactic Acidosis and Acidosis Response in Human Cancers. PLoS Genet. 2008;4:e1000293. doi: 10.1371/journal.pgen.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denko N.C. Hypoxia, HIF1 and Glucose Metabolism in the Solid Tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 40.Andreucci E., Peppicelli S., Ruzzolini J., Bianchini F., Biagioni A., Papucci L., Magnelli L., Mazzanti B., Stecca B., Calorini L. The Acidic Tumor Microenvironment Drives a Stem-like Phenotype in Melanoma Cells. J. Mol. Med. 2020;98:1431–1446. doi: 10.1007/s00109-020-01959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogueira V., Park Y., Chen C.-C., Xu P.-Z., Chen M.-L., Tonic I., Unterman T., Hay N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra D., Singh K.K. Genetic Insights into OXPHOS Defect and Its Role in Cancer. Biochim. Biophys. Acta. 2011;1807:620–625. doi: 10.1016/j.bbabio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owens K.M., Kulawiec M., Desouki M.M., Vanniarajan A., Singh K.K. Impaired OXPHOS Complex III in Breast Cancer. PLoS ONE. 2011;6:e23846. doi: 10.1371/journal.pone.0023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno-Sánchez R., Rodríguez-Enríquez S., Marín-Hernández A., Saavedra E. Energy Metabolism in Tumor Cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 45.López-Ríos F., Sánchez-Aragó M., García-García E., Ortega A.D., Berrendero J.R., Pozo-Rodríguez F., López-Encuentra A., Ballestín C., Cuezva J.M. Loss of the Mitochondrial Bioenergetic Capacity Underlies the Glucose Avidity of Carcinomas. Cancer Res. 2007;67:9013–9017. doi: 10.1158/0008-5472.CAN-07-1678. [DOI] [PubMed] [Google Scholar]
- 46.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamanaka R.B., Chandel N.S. Cell Biology. Warburg Effect and Redox Balance. Science. 2011;334:1219–1220. doi: 10.1126/science.1215637. [DOI] [PubMed] [Google Scholar]
- 48.Patra K.C., Hay N. Hexokinase 2 as Oncotarget. Oncotarget. 2013;4:1862–1863. doi: 10.18632/oncotarget.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinohara Y., Yamamoto K., Kogure K., Ichihara J., Terada H. Steady State Transcript Levels of the Type II Hexokinase and Type 1 Glucose Transporter in Human Tumor Cell Lines. Cancer Lett. 1994;82:27–32. doi: 10.1016/0304-3835(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 50.Mathupala S.P., Rempel A., Pedersen P.L. Glucose Catabolism in Cancer Cells: Identification and Characterization of a Marked Activation Response of the Type II Hexokinase Gene to Hypoxic Conditions. J. Biol. Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 51.Israelsen W.J., Vander Heiden M.G. Pyruvate Kinase: Function, Regulation and Role in Cancer. Semin. Cell Dev. Biol. 2015;43:43–51. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valvona C.J., Fillmore H.L., Nunn P.B., Pilkington G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2015;26:3–17. doi: 10.1111/bpa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doherty J.R., Cleveland J.L. Targeting Lactate Metabolism for Cancer Therapeutics. J. Clin. Investig. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feron O. Pyruvate into Lactate and Back: From the Warburg Effect to Symbiotic Energy Fuel Exchange in Cancer Cells. Radiother. Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 55.Hirschhaeuser F., Sattler U.G.A., Mueller-Klieser W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 56.Hay N. Reprogramming Glucose Metabolism in Cancer: Can It Be Exploited for Cancer Therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao P., Sun L., He X., Cao Y., Zhang H. MicroRNAs and the Warburg Effect: New Players in an Old Arena. Curr. Gene Ther. 2012;12:285–291. doi: 10.2174/156652312802083620. [DOI] [PubMed] [Google Scholar]
- 58.Mikawa T., LLeonart M.E., Takaori-Kondo A., Inagaki N., Yokode M., Kondoh H. Dysregulated Glycolysis as an Oncogenic Event. Cell Mol. Life Sci. 2015;72:1881–1892. doi: 10.1007/s00018-015-1840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kress S., Stein A., Maurer P., Weber B., Reichert J., Buchmann A., Huppert P., Schwarz M. Expression of Hypoxia-Inducible Genes in Tumor Cells. J. Cancer Res. Clin. Oncol. 1998;124:315–320. doi: 10.1007/s004320050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang D., Li C., Zhang H. Hypoxia and Cancer Cell Metabolism. Acta Biochim. Biophys. Sin. 2014;46:214–219. doi: 10.1093/abbs/gmt148. [DOI] [PubMed] [Google Scholar]
- 61.Du J., Gong H.-J., Xiao H. Somatic TP53 mutations and comparison of different TP53 functional domains in human cancers: Data analysis from the IARC TP53 database and the National Cancer Institute GDC data portal. Med. Data Min. 2021;4:3. [Google Scholar]
- 62.Malhotra L., Singh A., Kaur P., Ethayathulla A.S. Comprehensive Omics Studies of P53 Mutants in Human Cancer. Brief. Funct. Genom. 2022:elac015. doi: 10.1093/bfgp/elac015. [DOI] [PubMed] [Google Scholar]
- 63.Zwaans B.M.M., Lombard D.B. Interplay between Sirtuins, MYC and Hypoxia-Inducible Factor in Cancer-Associated Metabolic Reprogramming. Dis. Model. Mech. 2014;7:1023–1032. doi: 10.1242/dmm.016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hallows W.C., Yu W., Denu J.M. Regulation of Glycolytic Enzyme Phosphoglycerate Mutase-1 by Sirt1 Protein-Mediated Deacetylation. J. Biol. Chem. 2012;287:3850–3858. doi: 10.1074/jbc.M111.317404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsusaka T., Guo T., Yagura T., Inoue T., Yokode M., Inagaki N., Kondoh H. Deacetylation of Phosphoglycerate Mutase in Its Distinct Central Region by SIRT2 Down-Regulates Its Enzymatic Activity. Genes Cells. 2014;19:766–777. doi: 10.1111/gtc.12176. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y., Li F., Lv L., Li T., Zhou X., Deng C.-X., Guan K.-L., Lei Q.-Y., Xiong Y. Oxidative Stress Activates SIRT2 to Deacetylate and Stimulate Phosphoglycerate Mutase. Cancer Res. 2014;74:3630–3642. doi: 10.1158/0008-5472.CAN-13-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun L., He X., Cao Y., Gao P., Zhang H. MicroRNAs and Energy Metabolism in Cancer Cells. In: Babashah S., editor. MicroRNAs: Key Regulators of Oncogenesis. Springer International Publishing; Cham, Switzerland: 2014. pp. 83–95. [Google Scholar]
- 69.Hung C.-L., Wang L.-Y., Yu Y.-L., Chen H.-W., Srivastava S., Petrovics G., Kung H.-J. A Long Noncoding RNA Connects C-Myc to Tumor Metabolism. Proc. Natl. Acad. Sci. USA. 2014;111:18697–18702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He L., Thomson J.M., Hemann M.T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S.W., Hannon G.J., et al. A MicroRNA Polycistron as a Potential Human Oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diosdado B., van de Wiel M.A., Terhaar Sive Droste J.S., Mongera S., Postma C., Meijerink W.J.H.J., Carvalho B., Meijer G.A. MiR-17-92 Cluster Is Associated with 13q Gain and c-Myc Expression during Colorectal Adenoma to Adenocarcinoma Progression. Br. J. Cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mu P., Han Y.-C., Betel D., Yao E., Squatrito M., Ogrodowski P., de Stanchina E., D’Andrea A., Sander C., Ventura A. Genetic Dissection of the MiR-17~92 Cluster of MicroRNAs in Myc-Induced B-Cell Lymphomas. Genes Dev. 2009;23:2806–2811. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garofalo M., Di Leva G., Romano G., Nuovo G., Suh S.-S., Ngankeu A., Taccioli C., Pichiorri F., Alder H., Secchiero P., et al. MiR-221&222 Regulate TRAIL Resistance and Enhance Tumorigenicity through PTEN and TIMP3 Downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Hu W., Chan C.S., Wu R., Zhang C., Sun Y., Song J.S., Tang L.H., Levine A.J., Feng Z. Negative Regulation of Tumor Suppressor P53 by MicroRNA MiR-504. Mol. Cell. 2010;38:689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le M.T.N., Teh C., Shyh-Chang N., Xie H., Zhou B., Korzh V., Lodish H.F., Lim B. MicroRNA-125b Is a Novel Negative Regulator of P53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis B.C., Shim H., Li Q., Wu C.S., Lee L.A., Maity A., Dang C.V. Identification of Putative C-Myc-Responsive Genes: Characterization of Rcl, a Novel Growth-Related Gene. Mol. Cell Biol. 1997;17:4967–4978. doi: 10.1128/MCB.17.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramanathan A., Wang C., Schreiber S.L. Perturbational Profiling of a Cell-Line Model of Tumorigenesis by Using Metabolic Measurements. Proc. Natl. Acad. Sci. USA. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dang C.V., Le A., Gao P. MYC-Induced Cancer Cell Energy Metabolism and Therapeutic Opportunities. Clin. Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J., Gao P., Liu Y.-C., Semenza G.L., Dang C.V. Hypoxia-Inducible Factor 1 and Dysregulated c-Myc Cooperatively Induce Vascular Endothelial Growth Factor and Metabolic Switches Hexokinase 2 and Pyruvate Dehydrogenase Kinase 1. Mol. Cell Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osthus R.C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y., Wonsey D., Lee L.A., Dang C.V. Deregulation of Glucose Transporter 1 and Glycolytic Gene Expression by C-Myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 81.Kim J., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Subramanian A., Miller D.M. Structural Analysis of Alpha-Enolase. Mapping the Functional Domains Involved in down-Regulation of the c-Myc Protooncogene. J. Biol. Chem. 2000;275:5958–5965. doi: 10.1074/jbc.275.8.5958. [DOI] [PubMed] [Google Scholar]
- 83.Sedoris K.C., Thomas S.D., Miller D.M. Hypoxia Induces Differential Translation of Enolase/MBP-1. BMC Cancer. 2010;10:157. doi: 10.1186/1471-2407-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valera A., Pujol A., Gregori X., Riu E., Visa J., Bosch F. Evidence from Transgenic Mice That Myc Regulates Hepatic Glycolysis. FASEB J. 1995;9:1067–1078. doi: 10.1096/fasebj.9.11.7649406. [DOI] [PubMed] [Google Scholar]
- 85.Menssen A., Hermeking H. Characterization of the C-MYC-Regulated Transcriptome by SAGE: Identification and Analysis of c-MYC Target Genes. Proc. Natl. Acad. Sci. USA. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coller H.A., Grandori C., Tamayo P., Colbert T., Lander E.S., Eisenman R.N., Golub T.R. Expression Analysis with Oligonucleotide Microarrays Reveals That MYC Regulates Genes Involved in Growth, Cell Cycle, Signaling, and Adhesion. Proc. Natl. Acad. Sci. USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo Q.M., Malek R.L., Kim S., Chiao C., He M., Ruffy M., Sanka K., Lee N.H., Dang C.V., Liu E.T. Identification of C-Myc Responsive Genes Using Rat CDNA Microarray. Cancer Res. 2000;60:5922–5928. [PubMed] [Google Scholar]
- 88.Fernandez P.C., Frank S.R., Wang L., Schroeder M., Liu S., Greene J., Cocito A., Amati B. Genomic Targets of the Human C-Myc Protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morrish F., Noonan J., Perez-Olsen C., Gafken P.R., Fitzgibbon M., Kelleher J., VanGilst M., Hockenbery D. Myc-Dependent Mitochondrial Generation of Acetyl-CoA Contributes to Fatty Acid Biosynthesis and Histone Acetylation during Cell Cycle Entry. J. Biol. Chem. 2010;285:36267–36274. doi: 10.1074/jbc.M110.141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morrish F., Hockenbery D. Myc’s Mastery of Mitochondrial Mischief. Cell Cycle. 2003;2:11–13. doi: 10.4161/cc.2.1.275. [DOI] [PubMed] [Google Scholar]
- 91.O’Donnell K.A., Wentzel E.A., Zeller K.I., Dang C.V., Mendell J.T. C-Myc-Regulated MicroRNAs Modulate E2F1 Expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 92.Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. Deficiency in Glutamine but Not Glucose Induces MYC-Dependent Apoptosis in Human Cells. J. Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swierczynski J., Hebanowska A., Sledzinski T. Role of Abnormal Lipid Metabolism in Development, Progression, Diagnosis and Therapy of Pancreatic Cancer. World J. Gastroenterol. 2014;20:2279–2303. doi: 10.3748/wjg.v20.i9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Catalina-Rodriguez O., Kolukula V.K., Tomita Y., Preet A., Palmieri F., Wellstein A., Byers S., Giaccia A.J., Glasgow E., Albanese C., et al. The Mitochondrial Citrate Transporter, CIC, Is Essential for Mitochondrial Homeostasis. Oncotarget. 2012;3:1220–1235. doi: 10.18632/oncotarget.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y., Bollu L.R., Tozzi F., Ye X., Bhattacharya R., Gao G., Dupre E., Xia L., Lu J., Fan F., et al. ATP Citrate Lyase Mediates Resistance of Colorectal Cancer Cells to SN38. Mol. Cancer Ther. 2013;12:2782–2791. doi: 10.1158/1535-7163.MCT-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szutowicz A., Kwiatkowski J., Angielski S. Lipogenetic and Glycolytic Enzyme Activities in Carcinoma and Nonmalignant Diseases of the Human Breast. Br. J. Cancer. 1979;39:681–687. doi: 10.1038/bjc.1979.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beckner M.E., Fellows-Mayle W., Zhang Z., Agostino N.R., Kant J.A., Day B.W., Pollack I.F. Identification of ATP Citrate Lyase as a Positive Regulator of Glycolytic Function in Glioblastomas. Int. J. Cancer. 2010;126:2282–2295. doi: 10.1002/ijc.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Wang Y., Shen L., Pang Y., Qiao Z., Liu P. Prognostic and Therapeutic Implications of Increased ATP Citrate Lyase Expression in Human Epithelial Ovarian Cancer. Oncol. Rep. 2012;27:1156–1162. doi: 10.3892/or.2012.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin R., Tao R., Gao X., Li T., Zhou X., Guan K.-L., Xiong Y., Lei Q.-Y. Acetylation Stabilizes ATP-Citrate Lyase to Promote Lipid Biosynthesis and Tumor Growth. Mol. Cell. 2013;51:506–518. doi: 10.1016/j.molcel.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J.-H., Jang H., Lee S.-M., Lee J.-E., Choi J., Kim T.W., Cho E.-J., Youn H.-D. ATP-Citrate Lyase Regulates Cellular Senescence via an AMPK- and P53-Dependent Pathway. FEBS J. 2015;282:361–371. doi: 10.1111/febs.13139. [DOI] [PubMed] [Google Scholar]
- 101.Bourbeau M.P., Bartberger M.D. Recent Advances in the Development of Acetyl-CoA Carboxylase (ACC) Inhibitors for the Treatment of Metabolic Disease. J. Med. Chem. 2015;58:525–536. doi: 10.1021/jm500695e. [DOI] [PubMed] [Google Scholar]
- 102.Laurent G., German N.J., Saha A.K., de Boer V.C.J., Davies M., Koves T.R., Dephoure N., Fischer F., Boanca G., Vaitheesvaran B., et al. SIRT4 Coordinates the Balance between Lipid Synthesis and Catabolism by Repressing Malonyl CoA Decarboxylase. Mol. Cell. 2013;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menendez J.A., Lupu R. Fatty Acid Synthase and the Lipogenic Phenotype in Cancer Pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 104.Hopperton K.E., Duncan R.E., Bazinet R.P., Archer M.C. Fatty Acid Synthase Plays a Role in Cancer Metabolism beyond Providing Fatty Acids for Phospholipid Synthesis or Sustaining Elevations in Glycolytic Activity. Exp. Cell Res. 2014;320:302–310. doi: 10.1016/j.yexcr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 105.Zhou W., Feng X., Ren C., Jiang X., Liu W., Huang W., Liu Z., Li Z., Zeng L., Wang L., et al. Over-Expression of BCAT1, a c-Myc Target Gene, Induces Cell Proliferation, Migration and Invasion in Nasopharyngeal Carcinoma. Mol. Cancer. 2013;12:53. doi: 10.1186/1476-4598-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng Y.-H., Hu W.-J., Chen B.-C., Grahn T.-H.-M., Zhao Y.-R., Bao H.-L., Zhu Y.-F., Zhang Q.-Y. BCAT1, a Key Prognostic Predictor of Hepatocellular Carcinoma, Promotes Cell Proliferation and Induces Chemoresistance to Cisplatin. Liver Int. 2016;36:1836–1847. doi: 10.1111/liv.13178. [DOI] [PubMed] [Google Scholar]
- 107.Xu M., Liu Q., Jia Y., Tu K., Yao Y., Liu Q., Guo C. BCAT1 Promotes Tumor Cell Migration and Invasion in Hepatocellular Carcinoma. Oncol. Lett. 2016;12:2648–2656. doi: 10.3892/ol.2016.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Z., Achreja A., Meurs N., Animasahun O., Owen S., Mittal A., Parikh P., Lo T.-W., Franco-Barraza J., Shi J., et al. Tumour-Reprogrammed Stromal BCAT1 Fuels Branched-Chain Ketoacid Dependency in Stromal-Rich PDAC Tumours. Nat. Metab. 2020;2:775–792. doi: 10.1038/s42255-020-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elorza A., Soro-Arnáiz I., Meléndez-Rodríguez F., Rodríguez-Vaello V., Marsboom G., de Cárcer G., Acosta-Iborra B., Albacete-Albacete L., Ordóñez A., Serrano-Oviedo L., et al. HIF2α Acts as an MTORC1 Activator through the Amino Acid Carrier SLC7A5. Mol. Cell. 2012;48:681–691. doi: 10.1016/j.molcel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 110.Zhang B., Chen Y., Shi X., Zhou M., Bao L., Hatanpaa K.J., Patel T., DeBerardinis R.J., Wang Y., Luo W. Regulation of Branched-Chain Amino Acid Metabolism by Hypoxia-Inducible Factor in Glioblastoma. Cell Mol. Life Sci. 2021;78:195–206. doi: 10.1007/s00018-020-03483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hattori A., Tsunoda M., Konuma T., Kobayashi M., Nagy T., Glushka J., Tayyari F., McSkimming D., Kannan N., Tojo A., et al. Cancer Progression by Reprogrammed BCAA Metabolism in Myeloid Leukaemia. Nature. 2017;545:500–504. doi: 10.1038/nature22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dey P., Baddour J., Muller F., Wu C.C., Wang H., Liao W.-T., Lan Z., Chen A., Gutschner T., Kang Y., et al. Genomic Deletion of Malic Enzyme 2 Confers Collateral Lethality in Pancreatic Cancer. Nature. 2017;542:119–123. doi: 10.1038/nature21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J.-T., Yin M., Wang D., Wang J., Lei M.-Z., Zhang Y., Liu Y., Zhang L., Zou S.-W., Hu L.-P., et al. BCAT2-Mediated BCAA Catabolism Is Critical for Development of Pancreatic Ductal Adenocarcinoma. Nat. Cell Biol. 2020;22:167–174. doi: 10.1038/s41556-019-0455-6. [DOI] [PubMed] [Google Scholar]
- 114.Hatazawa Y., Tadaishi M., Nagaike Y., Morita A., Ogawa Y., Ezaki O., Takai-Igarashi T., Kitaura Y., Shimomura Y., Kamei Y., et al. PGC-1α-Mediated Branched-Chain Amino Acid Metabolism in the Skeletal Muscle. PLoS ONE. 2014;9:e91006. doi: 10.1371/journal.pone.0091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu N., Yoshikawa N., Ito N., Maruyama T., Suzuki Y., Takeda S., Nakae J., Tagata Y., Nishitani S., Takehana K., et al. Crosstalk between Glucocorticoid Receptor and Nutritional Sensor MTOR in Skeletal Muscle. Cell Metab. 2011;13:170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 116.Shimomura Y., Murakami T., Nakai N., Nagasaki M., Harris R.A. Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise. J. Nutr. 2004;134:1583S–1587S. doi: 10.1093/jn/134.6.1583S. [DOI] [PubMed] [Google Scholar]
- 117.Terakura D., Shimizu M., Iwasa J., Baba A., Kochi T., Ohno T., Kubota M., Shirakami Y., Shiraki M., Takai K., et al. Preventive Effects of Branched-Chain Amino Acid Supplementation on the Spontaneous Development of Hepatic Preneoplastic Lesions in C57BL/KsJ-Db/Db Obese Mice. Carcinogenesis. 2012;33:2499–2506. doi: 10.1093/carcin/bgs303. [DOI] [PubMed] [Google Scholar]
- 118.Arakawa M., Masaki T., Nishimura J., Seike M., Yoshimatsu H. The Effects of Branched-Chain Amino Acid Granules on the Accumulation of Tissue Triglycerides and Uncoupling Proteins in Diet-Induced Obese Mice. Endocr. J. 2011;58:161–170. doi: 10.1507/endocrj.K10E-221. [DOI] [PubMed] [Google Scholar]
- 119.Zou H., Liao M., Xu W., Yao R., Liao W. Data Mining of the Expression and Regulatory Role of BCAT1 in Hepatocellular Carcinoma. Oncol. Lett. 2019;18:5879–5888. doi: 10.3892/ol.2019.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y., Zhang J., Ren S., Sun D., Huang H.-Y., Wang H., Jin Y., Li F., Zheng C., Yang L., et al. Branched-Chain Amino Acid Metabolic Reprogramming Orchestrates Drug Resistance to EGFR Tyrosine Kinase Inhibitors. Cell Rep. 2019;28:512–525.e6. doi: 10.1016/j.celrep.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 121.Gu Z., Liu Y., Cai F., Patrick M., Zmajkovic J., Cao H., Zhang Y., Tasdogan A., Chen M., Qi L., et al. Loss of EZH2 Reprograms BCAA Metabolism to Drive Leukemic Transformation. Cancer Discov. 2019;9:1228–1247. doi: 10.1158/2159-8290.CD-19-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McBrayer S.K., Mayers J.R., DiNatale G.J., Shi D.D., Khanal J., Chakraborty A.A., Sarosiek K.A., Briggs K.J., Robbins A.K., Sewastianik T., et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell. 2018;175:101–116. doi: 10.1016/j.cell.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phang J.M., Liu W., Hancock C., Christian K.J. The Proline Regulatory Axis and Cancer. Front. Oncol. 2012;2:60. doi: 10.3389/fonc.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scott L., Lamb J., Smith S., Wheatley D.N. Single Amino Acid (Arginine) Deprivation: Rapid and Selective Death of Cultured Transformed and Malignant Cells. Br. J. Cancer. 2000;83:800–810. doi: 10.1054/bjoc.2000.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feun L.G., Kuo M.T., Savaraj N. Arginine Deprivation in Cancer Therapy. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:78–82. doi: 10.1097/MCO.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 126.Erez A., DeBerardinis R.J. Metabolic Dysregulation in Monogenic Disorders and Cancer—Finding Method in Madness. Nat. Rev. Cancer. 2015;15:440–448. doi: 10.1038/nrc3949. [DOI] [PubMed] [Google Scholar]
- 127.Pearce E.L., Poffenberger M.C., Chang C.-H., Jones R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ito K., Suda T. Metabolic Requirements for the Maintenance of Self-Renewing Stem Cells. Nat. Rev. Mol. Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vander Heiden M.G. Targeting Cancer Metabolism: A Therapeutic Window Opens. Nat. Rev. Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 130.Amelio I., Cutruzzolá F., Antonov A., Agostini M., Melino G. Serine and Glycine Metabolism in Cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phang J.M., Liu W., Hancock C.N., Fischer J.W. Proline Metabolism and Cancer: Emerging Links to Glutamine and Collagen. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:71–77. doi: 10.1097/MCO.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of Lactate Dehydrogenase A Induces Oxidative Stress and Inhibits Tumor Progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie H., Hanai J.-I., Ren J.-G., Kats L., Burgess K., Bhargava P., Signoretti S., Billiard J., Duffy K.J., Grant A., et al. Targeting Lactate Dehydrogenase—A Inhibits Tumorigenesis and Tumor Progression in Mouse Models of Lung Cancer and Impacts Tumor-Initiating Cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y.-H., Israelsen W.J., Lee D., Yu V.W.C., Jeanson N.T., Clish C.B., Cantley L.C., Vander Heiden M.G., Scadden D.T. Cell-State-Specific Metabolic Dependency in Hematopoiesis and Leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haas R., Smith J., Rocher-Ros V., Nadkarni S., Montero-Melendez T., D’Acquisto F., Bland E.J., Bombardieri M., Pitzalis C., Perretti M., et al. Lactate Regulates Metabolic and Pro-Inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015;13:e1002202. doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colegio O.R., Chu N.-Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K., Sethumadhavan S., Woo H.-K., Jang H.G., Jha A.K., et al. Functional Genomics Reveal That the Serine Synthesis Pathway Is Essential in Breast Cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maddocks O.D.K., Berkers C.R., Mason S.M., Zheng L., Blyth K., Gottlieb E., Vousden K.H. Serine Starvation Induces Stress and P53-Dependent Metabolic Remodelling in Cancer Cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lewis C.A., Parker S.J., Fiske B.P., McCloskey D., Gui D.Y., Green C.R., Vokes N.I., Feist A.M., Vander Heiden M.G., Metallo C.M. Tracing Compartmentalized NADPH Metabolism in the Cytosol and Mitochondria of Mammalian Cells. Mol. Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Labuschagne C.F., van den Broek N.J.F., Mackay G.M., Vousden K.H., Maddocks O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 141.Das K., Roychoudhury A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 142.Kim D., Fiske B.P., Birsoy K., Freinkman E., Kami K., Possemato R.L., Chudnovsky Y., Pacold M.E., Chen W.W., Cantor J.R., et al. SHMT2 Drives Glioma Cell Survival in Ischaemia but Imposes a Dependence on Glycine Clearance. Nature. 2015;520:363–367. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nilsson R., Jain M., Madhusudhan N., Sheppard N.G., Strittmatter L., Kampf C., Huang J., Asplund A., Mootha V.K. Metabolic Enzyme Expression Highlights a Key Role for MTHFD2 and the Mitochondrial Folate Pathway in Cancer. Nat. Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weinberg S.E., Chandel N.S. Targeting Mitochondria Metabolism for Cancer Therapy. Nat. Chem. Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Evans J.M.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and Reduced Risk of Cancer in Diabetic Patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Memmott R.M., Mercado J.R., Maier C.R., Kawabata S., Fox S.D., Dennis P.A. Metformin Prevents Tobacco Carcinogen-Induced Lung Tumorigenesis. Cancer Prev. Res. 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tomimoto A., Endo H., Sugiyama M., Fujisawa T., Hosono K., Takahashi H., Nakajima N., Nagashima Y., Wada K., Nakagama H., et al. Metformin Suppresses Intestinal Polyp Growth in ApcMin/+ Mice. Cancer Sci. 2008;99:2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bridges H.R., Jones A.J.Y., Pollak M.N., Hirst J. Effects of Metformin and Other Biguanides on Oxidative Phosphorylation in Mitochondria. Biochem. J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 150.Owen M.R., Doran E., Halestrap A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Pt 3Biochem. J. 2000;348:607–614. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., et al. Metformin Inhibits Mitochondrial Complex I of Cancer Cells to Reduce Tumorigenesis. eLife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Griss T., Vincent E.E., Egnatchik R., Chen J., Ma E.H., Faubert B., Viollet B., DeBerardinis R.J., Jones R.G. Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol. 2015;13:e1002309. doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Emami Riedmaier A., Fisel P., Nies A.T., Schaeffeler E., Schwab M. Metformin and Cancer: From the Old Medicine Cabinet to Pharmacological Pitfalls and Prospects. Trends Pharmacol. Sci. 2013;34:126–135. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 154.Pollak M. Overcoming Drug Development Bottlenecks with Repurposing: Repurposing Biguanides to Target Energy Metabolism for Cancer Treatment. Nat. Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- 155.Birsoy K., Possemato R., Lorbeer F.K., Bayraktar E.C., Thiru P., Yucel B., Wang T., Chen W.W., Clish C.B., Sabatini D.M. Metabolic Determinants of Cancer Cell Sensitivity to Glucose Limitation and Biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shackelford D.B., Abt E., Gerken L., Vasquez D.S., Seki A., Leblanc M., Wei L., Fishbein M.C., Czernin J., Mischel P.S., et al. LKB1 Inactivation Dictates Therapeutic Response of Non-Small Cell Lung Cancer to the Metabolism Drug Phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gravel S.-P., Hulea L., Toban N., Birman E., Blouin M.-J., Zakikhani M., Zhao Y., Topisirovic I., St-Pierre J., Pollak M. Serine Deprivation Enhances Antineoplastic Activity of Biguanides. Cancer Res. 2014;74:7521–7533. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- 158.Karsli-Uzunbas G., Guo J.Y., Price S., Teng X., Laddha S.V., Khor S., Kalaany N.Y., Jacks T., Chan C.S., Rabinowitz J.D., et al. Autophagy Is Required for Glucose Homeostasis and Lung Tumor Maintenance. Cancer Discov. 2014;4:914–927. doi: 10.1158/2159-8290.CD-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shroff E.H., Eberlin L.S., Dang V.M., Gouw A.M., Gabay M., Adam S.J., Bellovin D.I., Tran P.T., Philbrick W.M., Garcia-Ocana A., et al. MYC Oncogene Overexpression Drives Renal Cell Carcinoma in a Mouse Model through Glutamine Metabolism. Proc. Natl. Acad. Sci. USA. 2015;112:6539–6544. doi: 10.1073/pnas.1507228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xiang Y., Stine Z.E., Xia J., Lu Y., O’Connor R.S., Altman B.J., Hsieh A.L., Gouw A.M., Thomas A.G., Gao P., et al. Targeted Inhibition of Tumor-Specific Glutaminase Diminishes Cell-Autonomous Tumorigenesis. J. Clin. Investig. 2015;125:2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schug Z.T., Peck B., Jones D.T., Zhang Q., Grosskurth S., Alam I.S., Goodwin L.M., Smethurst E., Mason S., Blyth K., et al. Acetyl-CoA Synthetase 2 Promotes Acetate Utilization and Maintains Cancer Cell Growth under Metabolic Stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Comerford S.A., Huang Z., Du X., Wang Y., Cai L., Witkiewicz A.K., Walters H., Tantawy M.N., Fu A., Manning H.C., et al. Acetate Dependence of Tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mashimo T., Pichumani K., Vemireddy V., Hatanpaa K.J., Singh D.K., Sirasanagandla S., Nannepaga S., Piccirillo S.G., Kovacs Z., Foong C., et al. Acetate Is a Bioenergetic Substrate for Human Glioblastoma and Brain Metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Engelman J.A., Chen L., Tan X., Crosby K., Guimaraes A.R., Upadhyay R., Maira M., McNamara K., Perera S.A., Song Y., et al. Effective Use of PI3K and MEK Inhibitors to Treat Mutant Kras G12D and PIK3CA H1047R Murine Lung Cancers. Nat. Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Herranz D., Ambesi-Impiombato A., Sudderth J., Sánchez-Martín M., Belver L., Tosello V., Xu L., Wendorff A.A., Castillo M., Haydu J.E., et al. Metabolic Reprogramming Induces Resistance to Anti-NOTCH1 Therapies in T Cell Acute Lymphoblastic Leukemia. Nat. Med. 2015;21:1182–1189. doi: 10.1038/nm.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Viale A., Pettazzoni P., Lyssiotis C.A., Ying H., Sánchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V., et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuan P., Ito K., Perez-Lorenzo R., Del Guzzo C., Lee J.H., Shen C.-H., Bosenberg M.W., McMahon M., Cantley L.C., Zheng B. Phenformin Enhances the Therapeutic Benefit of BRAF(V600E) Inhibition in Melanoma. Proc. Natl. Acad. Sci. USA. 2013;110:18226–18231. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roesch A., Vultur A., Bogeski I., Wang H., Zimmermann K.M., Speicher D., Körbel C., Laschke M.W., Gimotty P.A., Philipp S.E., et al. Overcoming Intrinsic Multidrug Resistance in Melanoma by Blocking the Mitochondrial Respiratory Chain of Slow-Cycling JARID1B(High) Cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Janzer A., German N.J., Gonzalez-Herrera K.N., Asara J.M., Haigis M.C., Struhl K. Metformin and Phenformin Deplete Tricarboxylic Acid Cycle and Glycolytic Intermediates during Cell Transformation and NTPs in Cancer Stem Cells. Proc. Natl. Acad. Sci. USA. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chandel N.S., Tuveson D.A. The Promise and Perils of Antioxidants for Cancer Patients. N. Engl. J. Med. 2014;371:177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 171.Yun J., Mullarky E., Lu C., Bosch K.N., Kavalier A., Rivera K., Roper J., Chio I.I.C., Giannopoulou E.G., Rago C., et al. Vitamin C Selectively Kills KRAS and BRAF Mutant Colorectal Cancer Cells by Targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C., Khosh D.B., Drisko J., Levine M. Pharmacologic Doses of Ascorbate Act as a Prooxidant and Decrease Growth of Aggressive Tumor Xenografts in Mice. Proc. Natl. Acad. Sci. USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 174.Tagde A., Singh H., Kang M.H., Reynolds C.P. The Glutathione Synthesis Inhibitor Buthionine Sulfoximine Synergistically Enhanced Melphalan Activity against Preclinical Models of Multiple Myeloma. Blood Cancer J. 2014;4:e229. doi: 10.1038/bcj.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Glasauer A., Sena L.A., Diebold L.P., Mazar A.P., Chandel N.S. Targeting SOD1 Reduces Experimental Non–Small-Cell Lung Cancer. J. Clin. Investig. 2014;124:117–128. doi: 10.1172/JCI71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dadwal A., Baldi A., Kumar Narang R. Nanoparticles as Carriers for Drug Delivery in Cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:295–305. doi: 10.1080/21691401.2018.1457039. [DOI] [PubMed] [Google Scholar]
- 177.Palazzolo S., Bayda S., Hadla M., Caligiuri I., Corona G., Toffoli G., Rizzolio F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018;25:4224–4268. doi: 10.2174/0929867324666170830113755. [DOI] [PubMed] [Google Scholar]
- 178.Chen Y., Gao D.-Y., Huang L. In Vivo Delivery of MiRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li J., Dirisala A., Ge Z., Wang Y., Yin W., Ke W., Toh K., Xie J., Matsumoto Y., Anraku Y., et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. 2017;129:14213–14218. doi: 10.1002/ange.201706964. [DOI] [PubMed] [Google Scholar]
- 180.Dirisala A., Osada K., Chen Q., Tockary T.A., Machitani K., Osawa S., Liu X., Ishii T., Miyata K., Oba M., et al. Optimized Rod Length of Polyplex Micelles for Maximizing Transfection Efficiency and Their Performance in Systemic Gene Therapy against Stroma-Rich Pancreatic Tumors. Biomaterials. 2014;35:5359–5368. doi: 10.1016/j.biomaterials.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 181.Oba M., Vachutinsky Y., Miyata K., Kano M.R., Ikeda S., Nishiyama N., Itaka K., Miyazono K., Koyama H., Kataoka K. Antiangiogenic Gene Therapy of Solid Tumor by Systemic Injection of Polyplex Micelles Loading Plasmid DNA Encoding Soluble Flt-1. Mol. Pharm. 2010;7:501–509. doi: 10.1021/mp9002317. [DOI] [PubMed] [Google Scholar]
- 182.Yu S., Chen Z., Zeng X., Chen X., Gu Z. Advances in Nanomedicine for Cancer Starvation Therapy. Theranostics. 2019;9:8026–8047. doi: 10.7150/thno.38261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tabish T.A., Hamblin M.R. Mitochondria-Targeted Nanoparticles (MitoNANO): An Emerging Therapeutic Shortcut for Cancer. Biomater. Biosyst. 2021;3:100023. doi: 10.1016/j.bbiosy.2021.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen P., Yang W., Hong T., Miyazaki T., Dirisala A., Kataoka K., Cabral H. Nanocarriers Escaping from Hyperacidified Endo/Lysosomes in Cancer Cells Allow Tumor-Targeted Intracellular Delivery of Antibodies to Therapeutically Inhibit c-MYC. Biomaterials. 2022;288:121748. doi: 10.1016/j.biomaterials.2022.121748. [DOI] [PubMed] [Google Scholar]