Abstract
In recent years, increasing evidence has shown that the gut microbiota and their metabolites play a pivotal role in human health and diseases, especially the cardiovascular diseases (CVDs). Intestinal flora imbalance (changes in the composition and function of intestinal flora) accelerates the progression of CVDs. The intestinal flora breaks down the food ingested by the host into a series of metabolically active products, including trimethylamine N-Oxide (TMAO), short-chain fatty acids (SCFAs), primary and secondary bile acids, tryptophan and indole derivatives, phenylacetylglutamine (PAGln) and branched chain amino acids (BCAA). These metabolites participate in the occurrence and development of CVDs via abnormally activating these signaling pathways more swiftly when the gut barrier integrity is broken down. This review focuses on the production and metabolism of TMAO and SCFAs. At the same time, we summarize the roles of intestinal flora metabolites in the occurrence and development of coronary heart disease and hypertension, pulmonary hypertension and other CVDs. The theories of “gut-lung axis” and “gut-heart axis” are provided, aiming to explore the potential targets for the treatment of CVDs based on the roles of the intestinal flora in the CVDs.
Keywords: cardiovascular diseases, intestinal microecology, trimethylamine oxide, short-chain fatty acids, gut-heart axis
Introduction
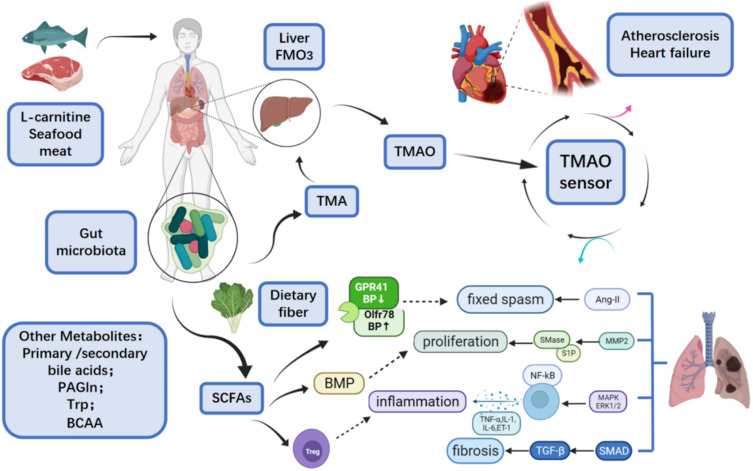
Cardiovascular diseases (CVD) refer to the manifestations of systemic vascular diseases in the heart, and they are a group of diseases and disorders that usually manifest as heart attack and stroke,1 including atherosclerosis, hypertension, heart failure, atrial fibrillation and myocardial fibrosis. Among these diseases, atherosclerosis is a major disease, and its common symptoms include cyanosis, dyspnea, chest tightness, chest pain, palpitations, edema and syncope.2 The morbidity and mortality of CVDs are increasing worldwide in recent years. According to the statistics of World Health Organization (WHO), among the top ten causes of death worldwide, CVDs have been the leading cause of death. In 2015, the WHO estimated that the number of deaths caused by CVD is more than 17.7 million, accounting for 31% of deaths worldwide.3 “The Cardiovascular Disease Report of China (2017)” shows that the prevalence of CVDs in China is still rising, and the estimated number of CVDs patients is 290 million in China. Deaths caused by CVDs account for more than 40% of disease-related deaths in China, which is higher than that caused by tumors and other diseases.4 In recent years, the homeostasis of the intestinal flora and their metabolites have been considered to provide opportunistic pathogenic conditions for the occurrence and outcome of CVDs and may affect the onset of chronic diseases. TMAO, SCFAs, bile acids, BCAA, tryptophan and indole derivatives are the main metabolites of intestinal flora and play an important role in the pathogenesis of systemic diseases related to intestinal bacteria. Bacterial gene sequencing, bioinformatics analysis and metabolomics can help researchers better study the structure and function of the intestinal flora, as well as the possible signaling pathways related to the occurrence and development of certain diseases.5 In this review, we summarize the roles of intestinal flora and its main metabolites in the occurrence and development of CVDs and introduce the possible mechanisms (Figure 1).
Figure 1.
Overview of intestinal flora metabolites and cardiovascular diseases.84
Intestinal Microecology
In 2007, the National Institutes of Health launched the Human Microbiome Project. It believes that there are more than millions of pairs of microbiome genes encoded by more than 1000 symbiotic microorganisms that colonize the human intestines, mouth, skin, vagina and other organs after birth in addition to about 25,000 pairs of genetic genes. Both genomes coordinate with each other to maintain the body in a steady state.6 Gut microbiome refers to thousands of intestinal bacteria and the synthesis, secretion and absorption of their metabolites in the human intestine.7 There are 100 trillion microorganisms in the human intestine, and more than 99% of microorganisms are bacteria, which is higher than that at any other part of the human body. The diversity of dominant flora and its quantitative ratio determine the stability of the flora and metabolites.8 Through metagenomic analysis, Eckburg et al found that the intestinal microbial community consisted of five phyla, namely Firmicutes, Bacteroides, Proteobacteria, Actinomycetes and Verrucomicrobia, most of which were anaerobic organisms.9 Firmicutes and Bacteroides are the dominant flora of healthy hosts, accounting for more than 90% of the total flora.10 Different from the composition of intestinal flora, intestinal microbes vary greatly at different regions, with the ascending colon occupying the most, followed by the distal ileum, and the proximal ileum and jejunum occupying the least.11 As a health indicator of intestinal microbiotathe, Firmicutes/Bacteroidetes ratio (F/B ratio) is not the same in individuals. Several experiments proved that F/B ratio is higher in hypertension patients and is related to other CVDs.12–14 In a study, the composition of viral microorganisms and intestinal flora were investigated in the intestine of infants, and the results revealed that the similarity of composition was much higher in the twins than in unrelated infants, and the phage viruses in infant enteroviruses were the most abundant. With the increase of age, the viruses and bacteria in their intestines gradually expanded, and the diversity of intestinal bacteria also gradually increased when the host was continuously stimulated by the outside environment, but at the same time, the proportion of bacteriophages continued to reduce, finally reaching a dynamic balance of microecology in the intestine.5,15,16 Researchers have proposed the predator–prey relationship to explain the dynamics between viruses and bacteria.15 When the intestine is infected, the dietary habit changes, stress from life events is present, antibiotics are abused, the symbiosis of intestinal flora becomes abnormal, the proportion of dominant flora changes, the epigenetics is altered, and the metabolites become disordered, the immunity and metabolism will change, which has been proven to cause diseases such as diabetes, obesity, inflammatory bowel disease (IBD), cancer, autism, ankylosing spondylitis, uveitis and other diseases.17 The food with a specific structure after ingestion may be metabolized by the bacteria in the intestine, which therefore improves the biochemical pathways of the host. This has brought new strategy for the prevention and treatment of various diseases. The addition of probiotics to optimize the intestinal microbiota has been widely used in clinical practice, and the fecal microbiota transplantation (FMT) has provided the possibility of eradicating intestinal flora-related disorders18 (Figure 1).
TMAO
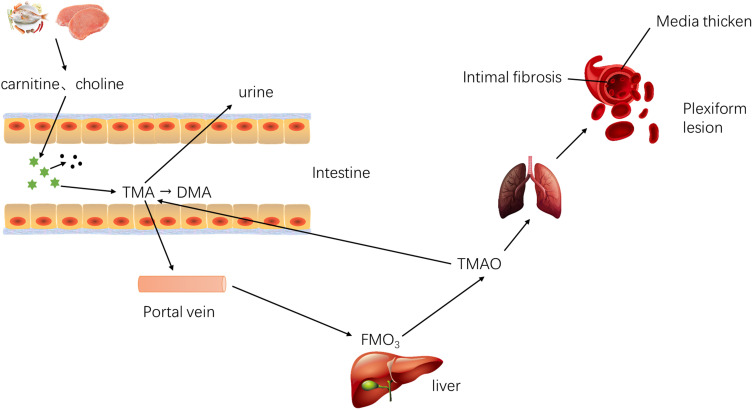
Trimethylamine N-Oxide (TMAO) is a metabolite synthesized by two steps, the gut metabolites metabolize dietary phosphatidylcholine (PC) and l-carnitine into TMA,19 which is then carried to the liver and oxidized by hepatic flavin monooxygenase 3 (FMO3), resulting in the production of TMAO, respectively,20 and after ingestion of foods containing choline, carnitine and phosphatidylcholine and others containing trimethylamines or derived from seafood directly. Studies have shown that TMAO plays an important role in the development of CVDs.
Metabolic Processes
Bacteria degrade the methylamines in the fish, meat, eggs, nuts and other foods into TMA (a precursor of TMAO), which is then absorbed into the blood via the small intestine.15 TMA enters the liver through the portal vein and is oxidized by FMO family (especially FMO3), to TMAO, which subsequently enters the systemic circulation. After treatment with broad-spectrum antibiotics, the blood TMAO significantly reduces.21 In the small intestine, a part of the TMA is metabolized by probiotics to CH4. At the same time, the excessive TMAO in the small intestine may be subjected to N-demethylation, resulting in the formation of dimethylamine (DMA) and formaldehyde in the colon. In the end, the majority of TMAO and a small amount of TMA and DMA are excreted through the kidneys, and a small amount of TMAO is excreted through the sweat glands and respiratory tract, instead of excretion through feces.22 In addition, intestinal bacteria still have a “metabolic reversal” mechanism. That is, TMAO is reduced to TMA in the intestine, part of which is excreted by the kidney, and the rest is re-oxidized into the TMAO. The “metabolic reversal” depends on the intestinal flora, especially Enterobacteriaceae, such as Bifidobacterium, Lactobacillus, Escherichia coli and others. Interestingly, E. coli in different parts of the intestine has different metabolic capabilities to TMAO. The contents of TMA and co-metabolites produced by E. coli in the cecum and proximal colon are significantly higher than those in the colon.23 In case of high TMAO level, the production of lactic acid by the Lactobacillus increases (Figure 2).
Figure 2.
Metabolic process of TMAO in vivo. Metabolism of TMAO: (I) TMA is absorbed into blood and oxidized to TMAO; (II) Excretion pathway of TMAO and other metabolites; (III) Metabolic reversal.15,21–23
Molecular Mechanisms
Intestinal bacteria directly bind to human pattern recognition receptors (PRRs) via the microbial-associated molecular patterns (MAMPs), contributing to regulate host metabolism, promote inflammation, and increase the risk of CVDs. Their metabolites are transported to the heart and lungs through the systemic circulation. Under this condition, the molecular mechanisms of metabolites have been studied extensively.24
TMAO Activates MAPK Pathway
In studies on atherosclerosis (AS), LDLR(-/-) rats were fed with diet containing high-dose choline or physiological dose TMAO, and results showed the activation of MAPK, ERK and NF-κB in both groups. The same results were obtained in studies on human arterial endothelial cells and smooth muscle cells. Therefore, these findings indicate that TMAO may activate the MAPK/ERK/NF-κB pathway to promote the inflammation-induced plaque formation in the AS.25 In the presence of TMAO, the addition of nobiletin may inhibit vascular oxidative stress and suppress the expression and phosphorylation of ERK/NF-κB, which increases Bcl-2 mRNA expression, decrease Bax mRNA expression, and reduce downstream inflammatory factors, therefore inhibiting inflammation.26 The direct intake of food rich in fish oil, choline food or TMAO may directly increase the inflammatory factors in the body, which supports the theory that TMAO activates MAPK or non-MAPK signaling pathways to induce inflammation.27
In studies on chronic kidney disease (CKD), the increased TMAO level elevated the expression of TGF-β/SMAD3, which was considered to play a key role in the pathogenesis of renal tubular fibrosis, and it has become one of the diagnostic markers of CKD.28,29 The TGF-β binds to its receptor to activate fibroblasts via the second messenger SMAD to induce the secretion of type I collagen, which mediates cardiac and pulmonary fibrosis.30 In the model of right ventricular fibroblast hypoxia, the expression of molecules in the TGF-β/p38MAPK/SMAD pathway increases, suggesting that it functions in the pathogenesis of right ventricular fibrosis.29,30
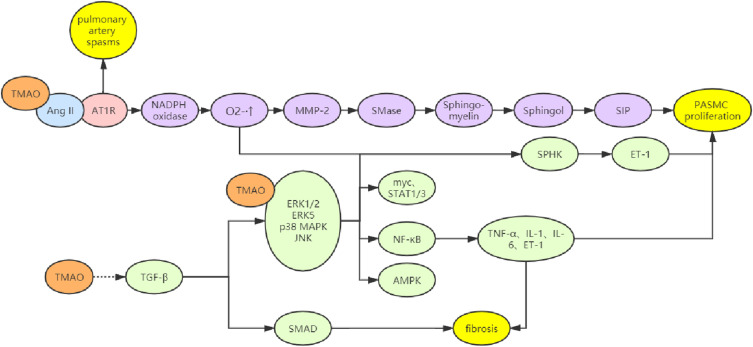
TMAO Activates MMP-2 and MAPK Pathways via Ang-II
TMAO cannot directly cause vasoconstriction but forms a ligand that can bind to the renin-angiotensin-aldosterone system (RAAS). Thus, it may bind to the receptor and angiotensin-II (Ang-II) to prolong the pressure elevation of Ang-II, leading to the persistent arterial spasmodic hypertension.16 Chowdhury et al found that the addition of Ang-II induced the proliferation of PASMCs in vitro, which was most obvious when the concentration of Ang-II was 100 nM; however, in the presence of pre-treatment with AT1 receptor blockers, the proliferation of these cells significantly slowed down. Further studies have shown that Ang-II can promote the MMP-2 expression, ERK1/2 phosphorylation and NADPH oxidase activation, which accelerates the expression of sphingomyelinase (SMase) and S1P (sphingosine-1- Phosphate), leading to the abnormal cell proliferation. In cells, ET-1 may increase the SPHK activity to elevate the phosphorylation of ERK1/2, which in turn increases cell proliferation. In case of NADPH oxidase inhibition, the effects of Ang-II on the MMP-2 expression and ERK1/2 phosphorylation are abolished.31,32 In addition, studies have also confirmed that Ang-II may activate the p38 MAPK, JNK and ERK5 pathways to induce the proliferation and migration of vascular smooth muscle cells, thereby causing hypertension33 (Figure 3).
Figure 3.
Multiple mechanisms of TMAO involved in CVD. (I) Delaying the action time of Ang - II, leading to continuous arterial spasm and activating MMP-2 pathway and ET-1; (II) Increase the expression of downstream inflammatory factors and ET-1 through MAPK pathway; (III) Activate TGF- β/ Smad pathway mediates fibrosis; (IV) Affect platelet function, increase blood lipid and blood viscosity.25–33
Physiological Functions
Zhu et al found that the elevation of plasma TMAO level may increase blood viscosity, which is positively correlated with the risk of adverse cardiovascular events.34 High blood viscosity triggers the development of atherosclerosis and cardiovascular events, and platelets play an essential role in it. Plasma levels of TMAO can induce the hyper-activity in human blood platelets as observed in diabetes and hyperlipidemic conditions.35 TMAO produces IP3 by decomposing platelet membrane phospholipids, thus triggering intracellular Ca2+ release from internal storage and activating platelets36 and altering cholesterol metabolism in human body.37 TMAO enhanced platelet reactivity to collagen by promoting the phosphorylation levels of extracellular signal-regulated kinase 1/2 (ERK1/2) and Jun N-terminal kinase (JNK) in platelets.38 TMAO may also reduce the endoplasmic reticulum (ER) stress and lipogenesis of adipocytes through its pseudo-osmotic protective effect and blocking the neurotoxicity of glutamate and inhibit the reverse transport of cholesterol, increasing the blood lipids and blood viscosity.39,40 Zhao’s results offer novel insight into the mechanisms that TMAO may inhibit intestinal cholesterol absorption and ameliorate hepatic ER stress under cholesterol overload, thereby attenuating steatohepatitis.41 However, in alternative studies including the brain, TMAO is observed to induce ER stress. Govindarajulu et al found that TMAO may induce deficits in synaptic plasticity through the ER stress-mediated PERK-EIF2α-ER stress signaling pathway, resulting in cognitive deficits.42 Thus, the role of TMAO in ER stress remains either positive or negative.
Intestinal flora and metabolites have regulatory effects on the number and activity of regulatory T cells (Treg). Treatment with amoxicillin and clavulanic acid for 2 weeks can attenuate brain injury secondary to middle cerebral artery occlusion (MCAO) in mice by regulating the intestinal flora. This is ascribed to the alteration of dendritic cell activity and increase of Tregs number because Tregs can inhibit the inflammatory response in the ischemic brain, exerting neuroprotective effects.43 The increase of TMAO may inhibit the proliferation and activity of Tregs, resulting in immune dysfunction, which then accelerates inflammation progression and antagonizes SCFAs. Huang et al supposed TMAO in vitro, significantly increased the expression of Kng1, Cxcl1, Cxcl2, Cxcl6, and Il6 in bone-marrow-derived macrophage; moreover, TMAO-treated conditioned medium from macrophage increased the proliferation and migration of pulmonary artery smooth muscle cells. Therefore, their study concludes that TMAO will increase in pulmonary arterial hypertension (PAH) and the reduction of TMAO can alleviate PAH through restraining the macrophage production of chemokines and cytokines, thus suppressing pulmonary vascular muscularization.44
Treatments
For lesions caused by elevated TMAO, the fundamental and foremost treatment is to reduce the concentration of TMAO. Reducing intake is crucial for the treatment. A previous study indicated that there are clinical and mechanistic links between atherosclerosis and the microbial metabolism of certain dietary nutrients producing intestinal TMAO, and traditional treatment for CVDs has focused on fecal transplantation, the antibacterial effect of antibiotics, probiotics and prebiotics.45 Besides, the intake of meat food should be strictly controlled, and the consumption of seafood products and other fitness supplements containing L-carnitine should also be strictly prohibited. When vegetarians take carnitine supplements daily, the ability of intestinal flora to convert carnitine into TMAO is significantly lower than that of meatarians in the first 30 days. Therefore, vegetarian diet is recommended. FMT, a radical treatment for the intestinal microecological disorders, can re-establish the homeostasis of intestinal flora and adjust the TMAO concentration to a physiological level. 3,3-Dimethyl-1-butanol (DMB) is a structural analogue of choline. It can inhibit TMA synthesis by inhibiting the unique microbial TMA lyase in the body, which can reduce TMAO level in mice fed with high choline or L-carnitine diet, which inhibits the formation of endogenous macrophage-derived foam cells and atherosclerotic plaques. In addition, it can also reduce the proportion of flora-related plasma trimethylammonium chloride, TMAO and extent of aortic plaques. DMB produces less selective pressure for resistance.46 FMO3 blocker can reduce the amount of TMAO synthesized in the liver, helping to maintain the homeostasis of glucose and lipids, promote the reverse transport of cholesterol and facilitate the absorption of cholesterol in the intestine.47 Probiotics (such as bifidobacteria) can enhance metabolic reversal, reduce bacterial translocation and alter the composition of intestinal flora, which significantly speeds up the conversion of TMAO back to TMA, therefore reducing the concentration of TMAO. The colonization of Escherichia coli in the small intestine can significantly enhance the metabolism of TMAO. Theoretically, the addition of non-enterotoxin-producing Escherichia coli into the small intestine may promote the reversal TMAO metabolism, which may become a potential therapeutic target.
In addition, blocking the Ang-II/MMP-2 and Ang-II/MAPK signaling pathways related to TMAO is also a potential therapeutic target. The use of ACEI (such as captopril), AT1 blocker (such as losartan), ERK blocker, MMP-2 blocker and other molecularly targeted drugs have also been new strategies for the prevention and treatment of arterial wall remodeling and thickening secondary to intestine derived TMAO.
SCFAs
Metabolic Process
Short-chain fatty acids (SCFAs), mainly including acetate, propionate and butyrate, are produced through the metabolism of indigestible dietary fibers and other foods by the intestinal flora. The acetate and propionate are mainly produced in the small intestine and colon, and butyrate is mainly produced in the colon and cecum. After SCFAs are produced, they can be absorbed by colonic epithelial cells through passive diffusion, SMCT1/Slc5a8 carriers (especially butyrate) or MCT1/Slc16a1 carriers or they directly stimulate G protein-coupled receptors G-protein-coupled receptor 41 (GPR41), GPR43 and GPR109a. GPR41 also called free fatty acid receptor 3 (FFAR3), a Gαi-coupled receptor activated by SCFAs mainly produced from dietary complex carbohydrate fibers by microbiota.48 The short-chain fatty acid receptors FFA2 (GPR43) and FFA3 (GPR41) can be activated by acetate, propionate, and butyrate.49,50
Physiological Functions
SCFAs can directly regulate blood pressure. Acetate and propionate can directly activate FFAR2/3 and Olfr78, which reduce or increase blood pressure, respectively, by affecting renin secretion and regulating vascular tone. The difference is that low concentrations of SCFAs can stimulate FFAR2/3 to lower blood pressure, but SCFAs at high concentrations can stimulate Olfr78 to raise blood pressure, which antagonizes FFAR2/3 and exerts protective effects. Both maintain blood pressure in a dynamic balance through mutual coordination.51 Hsu et al confirmed the vasodilation effects of butyrate, they found treatment with butyrate rescued maternal tryptophan-free diet (TF)-exposed offspring from hypertension, which may be associated with the increasing expression of FFAR3 and GPR109A, and restoration of the renin–angiotensin system (RAS) balance.52
SCFAs influence metabolism and immune function, participate in autoimmune responses and inflammatory processes, and can regulate the function of almost all types of immune cells. It has been shown that SCFAs are able to treat various diseases such as allergic asthma and colitis (IBD) by regulating the immune response of the host.53 SCFAs can affect histone acetylation to induce dendritic cells (DC) to facilitate the differentiation of naive T cells into FoxP3-expressing regulatory T cells (Tregs), Th1 or Th17 cells.54,55 By activating the Gpr43 receptor on T cells, SCFAs enhance the Foxp3 expression in colonic T cells and regulate the size and function of colonic Treg cell pools, which exerts protective effect on the enteritis.56 Gpr43 may secrete IL-18 to promote the translocation of neutrophils to inflammatory areas and also enhance their phagocytic ability. SCFAs may also promote the expression of inflammatory factors (IL-1, IL-6, and TNF-α) via inducing NF-κB expression in the epithelial cells.57 Moreover, FFAR2/3 and Gpr109a/b can also exert their immunoregulatory effects.
Molecular Mechanisms
Treg exerts anti-inflammatory effects and regulates the immune tolerance and autoimmune balance by releasing inflammatory inhibitors such as IL-10, IL-4 and secreted TGF-β. At the cellular level, the expanded Treg cells have significant inhibitory effect on the freshly sorted and expanded natural killer cells (NK cells).58 In addition, Treg cells directly contact antigen-presenting cells (APC), binding to APC via TCR, or competing with costimulatory molecules on APC (such as CD-40 and CD-80/86) to inhibit the function of effector T cells. In in vitro studies, SCFAs (especially butyrate) have been found to down-regulate the levels of TNF-α, IL-12 and IFN-γ by stimulating Foxp3(-) Treg cells, promote the differentiation of Tregs into Tr1 and Th3, and up-regulate IL- 4, IL-10 and TGF-β1 to suppress the immune response. Studies have shown that SCFAs can promote the secretion of anti-inflammatory cytokines at the cytokine level.59
Treatment
Bartolomaeus et al investigated effects of the SCFA propionate in 2 different mouse models of hypertensive cardiovascular damage. Their data emphasizes an immune-modulatory role of SCFAs and their importance for cardiovascular health, including that SCFA propionate can attenuate vascular Inflammation and atherosclerosis, ameliorate cardiac immune cell infiltration and remodeling and reduce susceptibility to ventricular arrhythmias as well, which suggests that lifestyle modifications leading to augmented SCFA production can be a beneficial nonpharmacological preventive strategy for patients with hypertensive cardiovascular disease. In the wake of more oral supplementation with SCFA propionate or its precursors may beneficially prevent high-risk group from damaging to target organs, it is indeed an affordable dietary intervention.60
Bile Acid
Bile acids are synthesized from cholesterol in the liver. In humans, approximately 500 mg/day of cholesterol is converted into bile acids in the liver.61 There are two different pathways, which are involved in the neutral (classic) and the alternative (acidic) pathways, with cholesterol-7α-hydroxylase (CYP7A1) being the rate-limiting enzyme in the former, and for cholesterol metabolism in the latter.62 To be specific, the acidic pathway is that cholesterol is catalyzed by CYP7A1 to produce 7α hydroxycholesterol, which in turn catalyzes the production of primary bile acid as the initial product, namely chenodeoxycholic acid (CDCA) and cholic acid (CA), which then bind to glycine and taurine, followed by concentration and storage in the gallbladder.63 Previous studies have shown that CYP7A1 is regulated by dietary cholesterol,64 circadian rhythm,65 transcription factors,66 and microRNAs.67,68 In the ileum, the conjugated bile acids are reabsorbed and transported to the liver through the portal vein, which is called enterohepatic circulation. Every day, 90–95% of bile acids in the bile acid pool are subjected to the enterohepatic circulation and they circulate 6–10 times between the intestine and the liver, aiming to maintain a constant bile acid pool. In the distal ileum, primary bile acids are hydrolyzed by bile acid hydrolase in the intestine to remove glycine and taurine, and then the products are dehydroxylated into deoxycholic acid (DC) and lithocholic acid (LA), which are called secondary bile acids. Secondary bile acids are hydrophobic and can be excreted with feces, which is the last step of cholesterol circulation. Many studies have shown that intestinal flora can regulate the cholesterol metabolism in liver and affect the pathways related to the bile acid metabolism, and the bile acids can also alter the composition of intestinal microbes through dose-dependent manner, participating in the pathogenesis of many diseases.69
A study from the Baylor College of Medicine in Houston revealed that excessive bile acids can reduce the oxidation of fatty acids in the myocardial cells and cause cardiac dysfunction. This cardiac dysfunction is called bile-derived heart disease. The cause of this cardiac syndrome is the increased bile acid level and the following inhibition of a key factor in the fatty acid metabolism-proliferation-activated receptor-γ co-activator 1α. Overexpression of this factor in the heart cells can inactivate the genes related to the bile acid-mediated fatty acid oxidation.70
On the other hand, CYP7A1, as a key enzyme in the cholesterol metabolism, can emulsify dietary fat to help digestion and promote the absorption of fat-soluble vitamins. It also helps to regulate the energy metabolism, expands the bile acid pool, and increases cholesterol transport. However, the increase in TMAO will inhibit the CYP7A1 expression, causing intracellular accumulation of cholesterol, which ultimately increases the risk of atherosclerotic plaque formation.71 A study of Myerhofer et al indicated that the primary bile acids in the plasma of patients with heart failure reduced, resulting in a higher ratio of secondary bile acids to primary bile acids, suggesting that the intestinal flora can maintain homeostasis by adjusting the ratio of bile acids. If the intestinal flora becomes imbalanced, the secondary bile acids decrease and the primary bile acids increase, thereby increasing the risk of coronary heart disease.72 In addition, bile acids can act as hormones on the nuclear receptors farnesoid X receptor (FXR) and G protein-coupled membrane receptor 5 (TGR5) to reduce triglyceride accumulation and fatty acid oxidation;73 they can also reduce the expression of pro-inflammatory cytokines and chemokines in the aorta.74
Acting as steroid hormones, bile acids also control inflammation and fibrosis through activating nuclear (FXR, VDR, PXR) and membrane G protein-coupled (TGR5, S1PR2) receptors. The result from Comeglio et al represents that applying the TGR5 agonist INT-777 or the dual FXR/TGR5 agonist INT-767 to TGR5 expressed in the lung contributes to the treatment of PAH by remodeling vascular and endothelial dysfunctions. Therefore, bile acid-derived agonist is a potential pharmacological treatment towards PAH.75
Phenylacetylglutamine
Phenylalanine (Phe), as one of the essential amino acids, is a raw material for the synthesis of neurotransmitters and hormones. In both humans and mice, phenylacetylglutamine(PAGln) and phentlacetylglycine(PAGly) are produced in vivo via a metaorganismal pathway.76 The unabsorbed Phe is metabolized to phenylpyruvate (PKU) and phenylacetic acid (PAA), respectively, which are subsequently absorbed by the intestinal microflora. Studies have shown that PAGln is produced through phenylacetic acid and glutamine (Gln) (preferred in humans) in the presence of liver enzymes. In addition, PAA can also bind to glycine (Gly) (preferred in rodents) to form PAGly.77
In 2020, the Stanley Hazen research team from the Cleveland Clinic employed non-targeted metabolomics in their study and they found that Phe was associated with the increased risk of CVD in patients with T2DM. There is a positive correlation between human PAGln and thrombosis, and intestinal flora-related metabolites PAGln and PAGly can significantly affect platelet function. They can enhance the adhesion of platelets to the collagen matrix in a dose-dependent manner, increase the stimulation-dependent intracytoplasmic Ca2+ [Ca2+]i and enhance the aggregation response to agonists. In addition, investigators reveal that PAGln is structurally similar to catecholamines, suggesting that PAGln can regulate adrenergic receptors (ADRs). PAGln can mediate cellular responses through G protein-coupled receptors (including α2A, α2B and β2-ADRs), increase platelet reactivity and thrombosis, while β-blockers (such as carvedilol) can significantly reduce PAGln-related high risk of thrombosis.76
Recent studies demonstrated that PAGln enhanced PLT activation and the possibility of thrombosis in animal models of whole blood, isolated platelets, and arterial injury by triggering adrenergic receptor (ADR) signalling.78 Chen et al confirmed that plasma PAGln levels in coronary artery disease patients with stent stenosis and hyperplasia were significantly higher than those in patients with stent patency. Therefore, the destruction of intestinal microbial structure and function caused by the increase of bacterial PAGln synthase and the increase of circulating microbiota-derived PAGln are related to in-stent stenosis in CAD patients who had undergone percutaneous coronary intervention (PCI).79
Tryptophan and Indole Derivatives
Tryptophan (Trp) is one of the essential amino acids and the only amino acid containing an indole structure. The level of free tryptophan in the body is determined by the amount of tryptophan in the diet and the activity of three Trp metabolic pathways: first, direct metabolism via the intestinal flora: the metabolites include the ligands of aromatic hydrocarbon receptors and play an important role in the barrier function of the gut; second, the kynurenine (Kyn) pathway mediated by indoleamine 2.3-plus dioxygenase 1 (IDO1), this pathway plays a pivotal role in the inflammation and immune response; third, the serotonin (5-HT) production pathway mediated by tryptophan hydroxylase 1 (TpH1). This may stimulate gastrointestinal motility through neurons in the enteric nervous system.80
The aryl hydrocarbon receptor (AHR) is a transcription factor activated by the ligand in the cytoplasm and involved in multiple cellular processes. Liu et al found that tryptophan metabolites may serve as ligands to activate AHR signals in various diseases, and they play important roles in the pathogenesis of CVDs, cancer, aging-related diseases and CKD via inflammation, oxidative stress and other pathways.81 In the Tampere blood vessel study, the IDO expression increased in the macrophage-rich core of human advanced atherosclerotic plaques.82 Song et al found that catabolite 3-hydroxykynurenine (3-HK) of KP mediated Ang II-induced EC cell apoptosis in vivo and subsequent endothelial dysfunction through superoxide anion derived from NAD(P)H oxidase. Moreover, patients with heart failure have higher plasma Kyn level, and Kyn level may be used as an indicator to predict the incidence of myocardial infarction in patients with stable angina.83
Sanchez-Gimenez et al84 have conducted a prospective cohort study, they found positive associations between indole derivatives and major adverse CVDs, while a negative relationship was reported between tryptophan and all-cause mortality. They speculated the influence of tryptophan on mortality might depend on the degradation rate of kynurenine. Meanwhile, indole sulfate is defined as a protein-binding molecule of indole derivatives. Some prospective studies85 presented that the circulating indoxyl sulfate levels occupied a significant part in the vascular dysfunction in CKD patients, with degenerative renal function, indoxyl sulfate could not be removed effectively,86 accumulating in the vessels and leading to epithelial damage caused by elevated vascular calcification and stiffness.87 Huć et al supposed that indole and indoxyl sulfate regulate arterial blood pressure via peripheral and central mechanisms dependent on serotonin signaling.88 In order to elucidate whether the plasma levels of indoxyl sulfate can predict the occurrence of CVDs, Imazu et al measured the plasma indoxyl sulfate levels of 165 patients with chronic heart failure and eventually demonstrated that indoxyl sulfate may become a proper biomarker to predict CVDs.89 Indoleamine 2,3-dioxygenase is an enzyme induced in a variety of immune cells in response to inflammatory stimulation, thereby promoting the degradation of tryptophan along the kynurenine pathway. However, when elevating the activity of this enzyme, it may aggravate CVDs and accelerate vascular inflammation and atherosclerosis.90
Conclusion
In conclusion, we summarize the diversified roles of main metabolites of the intestinal flora (TMAO, SCFAs, bile acids, PAGln Trp and indole derivatives) in the pathogenesis or protection of CVDs and propose the potential mechanisms bridging the relationship between intestinal microbiota and CVDs from the following multiple perspectives. 1. TMAO activates the MAPK signaling pathway, and induces cardiovascular media thickening through downstream ERK/NF-κB-mediated inflammation and promotes endometrial fibrosis remodeling via TGF-β/SMAD pathway; 2. TMAO delays the action of Ang-II to induce persistent vascular spasm and activates the MMP-2 and ERK1/2 pathways to induce the proliferation and migration of vascular smooth muscle cells (VSMCs); 3. TMAO increases blood lipids and activates platelet responses, increasing blood viscosity and in situ thrombosis; 4. TMAO inhibits the proliferation and activity of Tregs, causing immune dysfunction; 5. SCFAs inhibit the proliferation of VSMCs by inducing the BMP expression and regulate blood pressure, metabolism, and immunity, thereby inhibiting the development of CVDs; 6. Primary bile acids inhibit activated receptor-γ co-activator 1α to increase the risk of coronary heart disease, while secondary bile acids reduce the expression of pro-inflammatory cytokines and chemokines in the aorta by acting on FXR and TGR5; 7. PAGln regulates ADRs and mediates cellular responses through G protein-coupled receptors to influence platelet function and thrombosis; 8. Indole derivatives are in positive associations with CVDs, while a negative relationship is between tryptophan and all-cause mortality. In view of this, it deserves to be promising approaches that some new microecological preparations are expected to ameliorate CVDs through improvement of gut microbiota, nowadays the research and development of which are still in the clinical trial stage. There are more works need to do for the potential benefits and limitations of microecological preparation in the treatment of CVDs.
Prospect
The “Gut-Lung Axis” theory was first proposed in 1991. As early as thousands of years ago, Chinese medicine had the view that “the heart and the small intestine share a paired relationship”, which is consistent with the theory of Gut-Heart Axis. In addition, we discuss potential strategies for the prevention and treatment of CVDs through improving gut microbiota. Reducing intake, blocking synthesis, and enhancing metabolism and reversal as well as the introduction of molecularly targeted drugs may significantly benefit patients as the prevention and treatments of CVDs. Probiotics such as “Bifidobacterium containing quadruple viable bacteria tablets” have been widely used. The fecal microbiota transplantation (FMT) provides a new direction in the field of intestinal microecology. In the FMT, the functional bacteria in the intestinal tract of a healthy donor are transplanted into the gastrointestinal tract of a patient to restore the intestinal microbiota to a new balance. This process involves the collection of filtered feces from healthy donors or recipients themselves (autologous FMT) at the time point before the onset of diseases and dysbiosis and transplantation of these bacteria into the intestine of a patient with a specific disease.91 In 2010, the American Academy of Infectious Diseases and the American Academy of Healthcare Epidemiology included FMT as a recommended treatment for human Clostridioides difficile infection (CDI) in their clinical guidelines, and great progresses have been achieved in the application of FMT in weight loss, treatment of severe infection as an alternative to antibiotic treatment, prediction of diseases and clearance of drug-resistant bacteria. However, FMT is currently limited due to its associated risks, including the endotoxin-related complications of the gut, the transfer of infectious pathogens, the overuse of antibiotics and the feasibility of FMT in diseases outside the digestive system.
Metabolites such as TMAO and SCFAs are only a corner of the intestinal microecology, and the role of more intestinal bacteria or substances in the pathogenesis of CVDs should be deeply explored. In the “gut-lung axis” and “gut-heart axis” theories, the intestinal microecology and its metabolites are related to the occurrence and development of CVDs, which may systematize and logicalize the molecular and pathophysiological mechanisms of CVD-related diseases, forming a network.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nitsa A, Toutouza M, Machairas N, Mariolis A, Philippou A, Koutsilieris M. Vitamin D in cardiovascular disease. In Vivo (Brooklyn). 2018;32(5):977–981. doi: 10.21873/invivo.11338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and National Burden of Cardiovascular Diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018;26(7):563–574. doi: 10.1016/j.tim.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Wampach L, Heintz-Buschart A, Hogan A, et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front Microbiol. 2017;8:738. doi: 10.3389/fmicb.2017.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demarquoy J, Georges B, Rigault C, et al. Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries. Food Chem. 2004;86(1):137–142. doi: 10.1016/j.foodchem.2003.09.023 [DOI] [Google Scholar]
- 8.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 9.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim ES, Zhou Y, Zhao G, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21(10):1228–1234. doi: 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe. 2019;26(3):314–324. doi: 10.1016/j.chom.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robles-Vera I, Toral M, de la Visitación N, et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. 2020;177(9):2006–2023. doi: 10.1111/bph.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robles-Vera I, Toral M, Romero M, et al. Antihypertensive effects of probiotics. Curr Hypertens Rep. 2017;19(4):26. doi: 10.1007/s11906-017-0723-4 [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyles L, Jiménez-Pranteda ML, Chilloux J, et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome. 2018;6(1):73. doi: 10.1186/s40168-018-0461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30(12):1700–1705. doi: 10.1016/j.cjca.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 17.D’Odorico I, Di Bella S, Monticelli J, Giacobbe DR, Boldock E, Luzzati R. Role of fecal microbiota transplantation in inflammatory bowel disease. J Dig Dis. 2018;19(6):322–334. doi: 10.1111/1751-2980.12603 [DOI] [PubMed] [Google Scholar]
- 18.Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. World J Gastroenterol. 2016;22(32):7186–7202. doi: 10.3748/wjg.v22.i32.7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan G, Wang J, Yi T, et al. Baicalin prevents pulmonary arterial remodeling in vivo via the AKT/ERK/NF-κB signaling pathways. Pulm Circ. 2019;9(4):2045894019878599. doi: 10.1177/2045894019878599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear Factor-κB. J Am Heart Assoc. 2016;5(2). doi: 10.1161/JAHA.115.002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Lin CC, Yang Y, et al. Nobiletin prevents trimethylamine oxide-induced vascular inflammation via inhibition of the NF-κB/MAPK pathways. J Agric Food Chem. 2019;67(22):6169–6176. doi: 10.1021/acs.jafc.9b01270 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Wang Y, Antony V, Sun H, Liang G. Metabolism-Associated Molecular Patterns (MAMPs). Trends Endocrinol Metab. 2020;31(10):712–724. doi: 10.1016/j.tem.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Zhang X, Zhao Y, Yang X. High l-Carnitine ingestion impairs liver function by disordering gut bacteria composition in mice. J Agric Food Chem. 2020;68(20):5707–5714. doi: 10.1021/acs.jafc.9b08313 [DOI] [PubMed] [Google Scholar]
- 26.Guignabert C, Humbert M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur Respir J. 2021;57(2):2002341. doi: 10.1183/13993003.02341-2020 [DOI] [PubMed] [Google Scholar]
- 27.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Deeb OS, Atef MM, Hafez YM. The interplay between microbiota-dependent metabolite trimethylamine N-oxide, Transforming growth factor β/SMAD signaling and inflammasome activation in chronic kidney disease patients: a new mechanistic perspective. J Cell Biochem. 2019;120(9):14476–14485. doi: 10.1002/jcb.28707 [DOI] [PubMed] [Google Scholar]
- 29.Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217(3):e20190103. doi: 10.1084/jem.20190103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojonazarov B, Novoyatleva T, Boehm M, et al. p38 MAPK inhibition improves heart function in pressure-loaded right ventricular hypertrophy. Am J Respir Cell Mol Biol. 2017;57(5):603–614. doi: 10.1165/rcmb.2016-0374OC [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury A, Sarkar J, Pramanik PK, Chakraborti T, Chakraborti S. Cross talk between MMP2-Spm-Cer-S1P and ERK1/2 in proliferation of pulmonary artery smooth muscle cells under angiotensin II stimulation. Arch Biochem Biophys. 2016;603:91–101. doi: 10.1016/j.abb.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 32.Sarkar J, Chakraborti T, Chowdhury A, Bhuyan R, Chakraborti S. Protective role of epigallocatechin-3-gallate in NADPH oxidase-MMP2-Spm-Cer-S1P signalling axis mediated ET-1 induced pulmonary artery smooth muscle cell proliferation. J Cell Commun Signal. 2019;13(4):473–489. doi: 10.1007/s12079-018-00501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhang X, Gao L, et al. Cortistatin exerts antiproliferation and antimigration effects in vascular smooth muscle cells stimulated by Ang II through suppressing ERK1/2, p38 MAPK, JNK and ERK5 signaling pathways. Ann Transl Med. 2019;7(20):561. doi: 10.21037/atm.2019.09.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duttaroy AK. Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: a review. Nutrients. 2021;13(1):144. doi: 10.3390/nu13010144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett BJ, De Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Chang M, Guo Y, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018;17(1):286. doi: 10.1186/s12944-018-0939-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang Y, Yan C, Zhao Q, et al. The roles of microbial products in the development of colorectal cancer: a review. Bioengineered. 2021;12(1):720–735. doi: 10.1080/21655979.2021.1889109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumas ME, Rothwell AR, Hoyles L, et al. Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 2017;20(1):136–148. doi: 10.1016/j.celrep.2017.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo S, Luo Y. Brain Foxp3(+) regulatory T cells can be expanded by Interleukin-33 in mouse ischemic stroke. Int Immunopharmacol. 2020;81:106027. doi: 10.1016/j.intimp.2019.106027 [DOI] [PubMed] [Google Scholar]
- 41.Zhao ZH, Xin FZ, Zhou D, et al. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J Gastroenterol. 2019;25(20):2450–2462. doi: 10.3748/wjg.v25.i20.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govindarajulu M, Pinky PD, Steinke I, et al. Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci. 2020;13:138. doi: 10.3389/fnmol.2020.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Lin F, Tang R, et al. Gut microbial metabolite Trimethylamine N-Oxide aggravates pulmonary hypertension. Am J Respir Cell Mol Biol. 2022;66(4):452–460. doi: 10.1165/rcmb.2021-0414OC [DOI] [PubMed] [Google Scholar]
- 45.Shen X, Li L, Sun Z, et al. Gut microbiota and atherosclerosis-focusing on the plaque stability. Front Cardiovasc Med. 2021;8:668532. doi: 10.3389/fcvm.2021.668532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31(11–12):1317–1323. doi: 10.1016/j.nut.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 48.Nøhr MK, Egerod KL, Christiansen SH, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 49.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 50.Tang C, Offermanns S. FFA2 and FFA3 in metabolic regulation. Handb Exp Pharmacol. 2017;236:205–220. [DOI] [PubMed] [Google Scholar]
- 51.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1–8. doi: 10.1007/s00535-016-1242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu CN, Yu HR, Lin IC, et al. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J Nutr Biochem. 2022;108:109090. doi: 10.1016/j.jnutbio.2022.109090 [DOI] [PubMed] [Google Scholar]
- 53.Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J. 2015;469(2):267–278. doi: 10.1042/BJ20150242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93. doi: 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol. 2015;6:554. doi: 10.3389/fimmu.2015.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asarat M, Apostolopoulos V, Vasiljevic T, Donkor O. Short-chain fatty acids produced by synbiotic mixtures in skim milk differentially regulate proliferation and cytokine production in peripheral blood mononuclear cells. Int J Food Sci Nutr. 2015;66(7):755–765. doi: 10.3109/09637486.2015.1088935 [DOI] [PubMed] [Google Scholar]
- 58.Yeo Y, Yi ES, Kim JM, et al. FGF12 (Fibroblast Growth Factor 12) Inhibits vascular smooth muscle cell remodeling in pulmonary arterial hypertension. Hypertension. 2020;76(6):1778–1786. doi: 10.1161/HYPERTENSIONAHA.120.15068 [DOI] [PubMed] [Google Scholar]
- 59.Wu PH, Chiu YW, Zou HB, et al. Exploring the Benefit of 2-Methylbutyric acid in patients undergoing hemodialysis using a cardiovascular proteomics approach. Nutrients. 2019;11(12):3033. doi: 10.3390/nu11123033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712 [DOI] [PubMed] [Google Scholar]
- 62.Chambers KF, Day PE, Aboufarrag HT, Kroon PA. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: a review. Nutrients. 2019;11(11):2588. doi: 10.3390/nu11112588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56(6):1085–1099. doi: 10.1194/jlr.R054114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu G, Pan LX, Li H, et al. Dietary cholesterol stimulates CYP7A1 in rats because farnesoid X receptor is not activated. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G730–735. doi: 10.1152/ajpgi.00397.2003 [DOI] [PubMed] [Google Scholar]
- 65.Chen P, Zhang R, Mou L, Li X, Qin Y, Li X. An impaired hepatic clock system effects lipid metabolism in rats with nephropathy. Int J Mol Med. 2018;42(5):2720–2736. doi: 10.3892/ijmm.2018.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taniguchi T, Chen J, Cooper AD. Regulation of cholesterol 7 alpha-hydroxylase gene expression in Hep-G2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J Biol Chem. 1994;269(13):10071–10078. doi: 10.1016/S0021-9258(17)36991-0 [DOI] [PubMed] [Google Scholar]
- 67.Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58(3):1111–1121. doi: 10.1002/hep.26427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song KH, Li T, Owsley E, Chiang JY. A putative role of micro RNA in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J Lipid Res. 2010;51(8):2223–2233. doi: 10.1194/jlr.M004531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokota A, Fukiya S, Islam KB, et al. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 2012;3(5):455–459. doi: 10.4161/gmic.21216 [DOI] [PubMed] [Google Scholar]
- 70.Desai MS, Mathur B, Eblimit Z, et al. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65(1):189–201. doi: 10.1002/hep.28890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8(1):36. doi: 10.1186/s40168-020-00821-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levi M. Role of bile acid-regulated nuclear receptor FXR and G protein-coupled receptor TGR5 in regulation of cardiorenal syndrome (Cardiovascular Disease and Chronic Kidney Disease). Hypertension. 2016;67(6):1080–1084. doi: 10.1161/HYPERTENSIONAHA.115.06417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53(9):1723–1737. doi: 10.1194/jlr.R024794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Comeglio P, Morelli A, Adorini L, Maggi M, Vignozzi L. Beneficial effects of bile acid receptor agonists in pulmonary disease models. Expert Opin Investig Drugs. 2017;26(11):1215–1228. doi: 10.1080/13543784.2017.1385760 [DOI] [PubMed] [Google Scholar]
- 76.Nemet I, Saha PP, Gupta N, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862–877.e822. doi: 10.1016/j.cell.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen W, Zhang S, Wu J, et al. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta. 2020;507:236–241. doi: 10.1016/j.cca.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 78.Huynh K. Novel gut microbiota-derived metabolite promotes platelet thrombosis via adrenergic receptor signalling. Nat Rev Cardiol. 2020;17(5):265. doi: 10.1038/s41569-020-0367-y [DOI] [PubMed] [Google Scholar]
- 79.Fang C, Zuo K, Fu Y, et al. Dysbiosis of gut microbiota and metabolite phenylacetylglutamine in coronary artery disease patients with stent stenosis. Front Cardiovasc Med. 2022;9:832092. doi: 10.3389/fcvm.2022.832092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 81.Liu JR, Miao H, Deng DQ, Vaziri ND, Li P, Zhao YY. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. 2021;78(3):909–922. doi: 10.1007/s00018-020-03645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niinisalo P, Oksala N, Levula M, et al. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med. 2010;42(1):55–63. doi: 10.3109/07853890903321559 [DOI] [PubMed] [Google Scholar]
- 83.Song P, Ramprasath T, Wang H, Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. 2017;74(16):2899–2916. doi: 10.1007/s00018-017-2504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Gimenez R, Ahmed-Khodja W, Molina Y, et al. Gut microbiota-derived metabolites and cardiovascular disease risk: a systematic review of prospective cohort studies. Nutrients. 2022;14(13):2654. doi: 10.3390/nu14132654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. doi: 10.2215/CJN.03980609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koike H, Morita T, Tatebe J, et al. The relationship between serum indoxyl sulfate and the renal function after catheter ablation of atrial fibrillation in patients with mild renal dysfunction. Heart Vessels. 2019;34(4):641–649. doi: 10.1007/s00380-018-1288-0 [DOI] [PubMed] [Google Scholar]
- 87.Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5(6):1302–1308. doi: 10.1111/j.1538-7836.2007.02540.x [DOI] [PubMed] [Google Scholar]
- 88.Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res. 2018;130:172–179. doi: 10.1016/j.phrs.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 89.Imazu M, Fukuda H, Kanzaki H, et al. Plasma indoxyl sulfate levels predict cardiovascular events in patients with mild chronic heart failure. Sci Rep. 2020;10(1):16528. doi: 10.1038/s41598-020-73633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Metghalchi S, Ponnuswamy P, Simon T, et al. Indoleamine 2,3-Dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of Interleukin-10 production. Cell Metab. 2015;22(3):460–471. doi: 10.1016/j.cmet.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 91.Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, et al. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients. 2020;12(3):605. doi: 10.3390/nu12030605 [DOI] [PMC free article] [PubMed] [Google Scholar]