Abstract
The ultimate goal of nanomedicine has always been the generation of translational technologies that can ameliorate current therapies. Cancer disease represented the primary target of nanotechnology applied to medicine, since its clinical management is characterized by very toxic therapeutics. In this effort, nanomedicine showed the potential to improve the targeting of different drugs by improving their pharmacokinetics properties and to provide the means to generate new concept of treatments based on physical treatments and biologics. In this review, we considered different platforms that reached the clinical trial investigation, providing an objective analysis about their physical and chemical properties and the working mechanism at the basis of their tumoritr opic properties. With this review, we aim to help other scientists in the field in conceiving their delivering platforms for clinical translation by providing solid examples of technologies that eventually were tested and sometimes approved for human therapy.
Keywords: nanomedicine, targeted therapies, EPR, SPION, Abraxane, Doxil, micelles, exosomes
1. Introduction
Nanotechnology development for the medical field has always been focused primarily on translational purposes [1]. Nanomedicine was conceived to increase the safety of very toxic drugs, providing the therapeutics a means for targeting the sick tissues [2]. For this reason, nanotherapeutics were tested extensively to improve chemotherapy performances. Most of the investigations, in fact, were dedicated to enhance the antitumor power of drugs that were already approved in clinics, but characterized by severe adverse effects limiting their use [3]. Liposomal formulation of Doxorubicin (DOX) [4] and Daunorubicin [5] were the first FDA approved nanotherapies in 1995 and 1996, respectively. First generation of nanoparticles targeted the cancer lesions by exploiting leaky tumor vasculature and the lack of an efficient tumor lymphatic system, a phenomenon known as enhanced permeability and retention effect (EPR) [6]. In this case, the nanoparticles were designed with high circulation properties to facilitate their accumulation in the tumor tissue, where they could release their therapeutic payload. Here, surface modifications like polyethylene glycol (PEG) could minimize particle sequestration in the elements of the mononuclear phagocytic system (MPS), inhibiting particle opsonization and internalization [7]. Second generation of nanomedicine provided the carriers with more complex surface modifications [8] or particular shapes [9] that alone or in combination with “stealthing” molecules allowed for active targeting. Most of these modifications were based on peptides, antibodies or ligand moieties that could be recognized by surface receptors over-expressed on cancer cells [10]. This strategy allowed for a more stable interaction of the NPs with cancer cells and favored their internalization [10]. In these technologies, cancer lesion targeting still occurs via EPR, even though some surface modification can impart the carriers with trafficking properties that can enhance their active accumulation in the sick tissue. A recent review by Anselmo et al. [11], showed that in the last 3 decades, a little more 30 than nanoformulations were approved and less than half of them were designed for cancer treatment. The translation of nanomedicine to the clinic has been hindered by the formidable ability of our body to recognize foreign bodies [12], tumor organization [13], and concerns derived from systemic toxicity and immune system activation [3]. Further efforts in facing this issue generated more and more complex surface modifications, as well as carriers derived from natural sources like exosomes [14]. Unfortunately, while in preclinical settings these technologies provided promising results, their clinical translation was hampered by their high costs of production and sometimes by issues of generating nanoformulations in large scale [15,16]. However, the research in the field is still very active and supported by the exploration of alternative administration routes other than intravenous [17,18] and by the development of other applications, including diagnosis. The goal of this review is to summarize the nanocarriers that reached clinical trial experimentation and some examples to the readers about the characteristics that nanoformulations should have to treat the patients, including their size and targeting mechanism. In this goal, we selected the clinical trials on clinicaltrials.gov by searching for “cancer disease” and “nanoparticles”. We excluded suspended, terminated and withdrawn trials. An additional filter was applied to exclude clinical trials that did not investigate nanoformulations for their ability to treat cancer conditions. Considering that some of these studies were performed to test the same technology against different cancers or, in combination with different therapeutics, our work will focus on no more than 15–20 different delivery systems.
2. Inorganic NPs
2.1. AGuIX
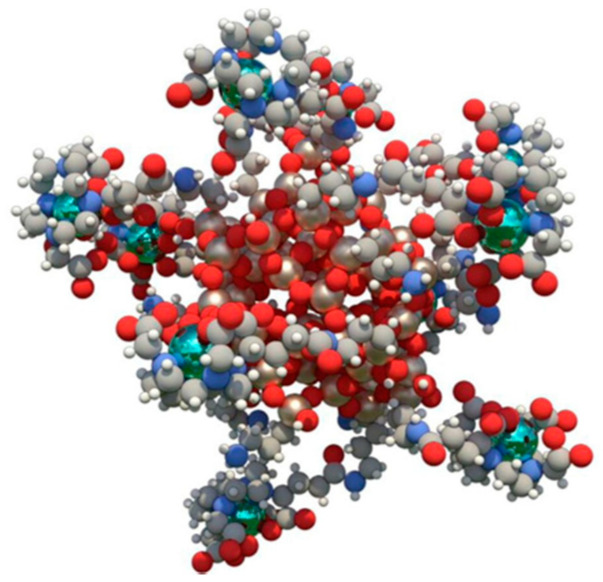
A promising approach to overcome current therapies limitations is the use of new therapeutic agents (molecules or nanoparticles) that sensitize cancer cells to radiotherapy (RT) also known as dose enhancers and radiosensitizers. This enhanced radiation local absorption in combination with the accumulation of high Z-elements in the irradiated cancer tissue results in a larger production of harmful diffused photons, photoelectrons, Auger electrons, Compton electrons, and radical species [19]. AGuIX particles were firstly synthesized in 2011 [20] to increase the radiobiological effect of high-energy radiation in the tumor. This technology is composed of very small (average size 5 nm diameter) polysyloxane particles with chelated cyclic gadolinium covalently grafted into the inorganic matrix [21] (Figure 1). In preclinical experiments, AGuIX NPs showed high radiosensitizing and anti-tumor properties [22] that combined with a solid and reproducible synthesis process favored their evaluation in clinical settings.
Figure 1.
AGuIX structure: Polysiloxane structure of AGuIX chelating gadolinium (green) via dodecane tetraacetic acid ligands. Figure reprinted from Lux et al. [21].
AGuIX biodistribution studies have been performed on healthy animals of different species (rodents and monkeys) and using different administration methods, doses, and time windows of detection biodistribution and PK properties of these particles [21]. After intravenous administration, carriers’ excretion occurred mainly through the kidneys and only less than 0.15% of the administered dose was found in organs other than the kidneys and bladder [20]. These data were confirmed also by another study [23], showing that kidney accumulation already occurred 5 min after the particle administration, with the highest accumulation observed after 4 h, while the gadolinium clearance occurred in a week.
AGuIX have been tested on various central nervous system tumors. These investigations demonstrated that tumor accumulation occurred via EPR, indicated as the mechanism of particle tumor targeting [24], while retention in the sick tissue persisted up to 24 h after intravenous administration [25]. In addition, also intraperitoneal administration was effective in targeting the tumors, without evident accumulation in off-target organs besides the kidneys and bladder. AGuIX showed radiosensitizing efficacy in vitro on various cell lines with typical sensitizer enhancement ratios varying from 1.1 to 2.5 for photon irradiation at different energies ranging from keV to MeV, including the use of clinical irradiators [26,27,28].
The first clinical trial NANO-RAD, investigating the AGuIX benefits [29], was completed in 2019 and aimed at treating multiple brain metastases. This trial was designed to determine the maximum tolerated dose in combination with whole brain radiation therapy (RT). The efficiency of AGuIX in overcoming the blood—brain barrier in brain metastases, was similar to conventional magnetic resonance imaging (MRI) contrast agents. Good tolerability of AGuIX intravenous injection was shown at doses ≤ 100 mg/kg in combination with whole brain RT in patients with multiple brain metastases [30]. Most of the ongoing clinical trials (Phase 1/2) are in the recruitment state and focusing on brain tumor metastases or glioma [31,32,33]. Other clinical trial investigate AGuIX in combination with cisplatin, radiotherapy and brachytherapy in treating advanced cervical cancer [34] in combination with MRI-guided stereotactic body RT in pancreatic and lung tumor treatment [35].
2.2. NBTXR3
NBTXR3 technology is a novel radio sensitizer comprising crystalized hafnium oxide (HfO2) nanoparticles, locally injected into tumor tissue and activated by RT. HfO2 nanoparticles possess excellent x-ray absorption coefficient because of the high electron-density elements composing the particles and acceptable safety. The particles are 50 nm in size and negatively charged thanks to a phosphate coating applied to maintain colloidal stability [36]. NBTXR3 followed by RT could improve the treatment of advanced or borderline-resectable cancers compared to RT alone [36]. Preclinical studies have shown that NBTXR3 working mechanism is mostly physical without targeting specific biological pathways, and its use could be extended to many types of cancer. The system was tested in patients with head and neck squamous cell carcinoma exploring a dose escalation setting [37]. Within 7 weeks after NBTXR3 injection, nanoparticles in the surrounding tissues disappeared, showing that the system was well tolerated. Additionally, this study [37] showed that one intratumor administration of NBTXR3 before radiotherapy could yield remarkable local tolerance, homogeneous dispersion of the particles in the tumor tissue, no leakage, and showed promising signs of anticancer activity in terms of pathological responses. In another study [38] dose optimization and side effects were evaluated for NBTXR3 in association with RT for recurrent and inoperable non-small cell lung cancer patients.
The purpose of NCT04484909 [39] and NCT04615013 [40] trials (phase I) was to determine the recommended phase 2 dose and safety profile of NBTXR3 activated by radiation therapy to treat metastatic, borderline-resectable pancreatic cancers and esophageal adenocarcinoma, respectively.
NBTXR3 nanoparticles have become the subject of many clinical trials to treat solid tumor with metastases to lung and/or liver [41], head and neck cancer [42,43] and soft tissue sarcoma [44]. Here, the patients underwent radiotherapy with or without a previous local injection of the radiosensitizer. The presence NBTXR3 doubled the pathological complete response of the patients with no occurrence of important adverse effect [45]. Promising results were collected also in a Phase 1 trial investigating the safety and efficacy of this technology in elderly patient affected by oropharynx and oral cavity cancer [46].
2.3. Super Magnetic Iron Oxide
Superparamagnetic iron oxide nanoparticles (SPION) found their application in the biomedical field because of their theranostic properties, since they can allow for MRI and thermo-ablation. SPION working mechanism depends on an external alternate magnetic field determining their action only in the sick tissue. The synthesis of these particles is based on the nanomanipulation of magnetite and maghemite [47] and their dispersion is obtained through surface modifications (capping) based on organic molecules [48] and polymeric (i.e., polyethylene glycol) [49] surface functionalization. Their size ranges from 20 to 150 nm [47] and the mechanism of tumor accumulation is based on intratumoral injection [50] or EPR [51] following IV administration. However, in preclinical studies they were object of intense studies to engineering their surface with targeting molecules [52] to increase their residence time and internalization in cancer cells. These manipulations focused also on conjugating therapeutic agents on their surface comprising both small molecules [53] and biologics [50]. Attempts at exploiting polarized magnetic fields to increase their tumor targeting were proposed as well [54]. Finally, they were often used to implement the properties of other delivery platforms in hybrid synthetic settings [55]. Their translational use was deeply investigated mostly for their ability to enhance the MRI resolution, even though their toxicity related to DNA damage and reactive oxygen species formation limited their large application [56]. FDA-approved formulations of SPION are currently intended as iron replacement and they are secondarily used as contrast agents for kidney imaging [57,58]. In this scenario, the only phase 3 and 4 trial focused on this technology aimed at understanding the SPION ability to detect lymphatic metastases in breast [59] and pancreatic cancer [60] after IV infusion. The latter trial showed, in comparison with traditional histology, a matching of the 2 methods higher than the 80%. Ongoing clinical trials aim to investigate the efficacy of locally injected SPION magnetic hyperthermia against brain and prostate tumors. In a phase I clinical trial, the authors showed the effect of thermoablation to treat prostate cancer by local injection of SPION and further thermoablation induction under magnetic field application [61]. Untargeted SPION (Ferumoxytol) are currently evaluated for treatment of primary and metastatic hepatic cancers [62]. The radiotherapy with SPION supported by magnetic resonance imaging guided linear accelerator allowed to detect and maximize avoidance of residual functionally active hepatic parenchyma from over-the-threshold irradiation thus minimizing SBRT liver damages because of stereotactic body radiation therapy in patients with pre-existing hepatic conditions. The safety, efficacy and tolerability of SPION in combination with spinning magnetic field (SMF) and neoadjuvant chemotherapy in osteosarcoma patients is currently evaluated in a Phase I clinical trial [63]. The study comprises intra-tumor injection of SPIONs followed by SMF and conventional neoadjuvant chemotherapy from day 1. The authors declare a synergistic effect of SPIONs/SMF with neoadjuvant chemotherapy in increasing cancer cell killing and improving the ratio of limb retention (amputation).
2.4. Gold Nanoparticles
Besides their use for increasing the sensitivity of current diagnostic and prognostic tests [64], NU-0129 gold nanoparticles were tested in a Phase 0 clinical trial against gliosarcoma and glioblastoma [65,66]. The particles were modified covalently with a spherical RNAi corona targeting BCL2L12 messenger. The author could detect the accumulation of the particles in the tumor and a decrease in the BCL2L12 expression. However, the authors of this work could not find more information of this technology in terms of size and surface charge, speculating that the ability of this technology to overcome the blood–brain barrier is probably due to their small size, as demonstrated in other pre-clinical studies [67]. On the other hand, the overcoming of the blood–brain barrier could be favored by the nucleic acid coating of the particles since spherical RNAi could be trafficked via transcytosis, with the gold core fundamental to avoid the fast clearance of the system [66].
2.5. ELU001 (Folic-Acid Functionalized C’Dot-Drug-Conjugate)
The folate receptor alpha (FRα) represents a promising target in oncology because of its over-expression in tumors (i.e., ovarian, breast and lung cancers), low and restricted distribution in normal tissues [68], emerging insights about its tumor promoting functions, and association with patient prognosis. ELU001 is a new molecular C’Dot Drug Conjugate (CDC). ELU001 comprises a very small silica core (6 nm) functionalized with ~12 folic acid targeting moieties and ~22 exatecan topoisomerase-1 inhibitor payloads linked to via Cathepsin-B cleavable linkers covalently bound to the surface of the nanoparticles. Because of their small size, ELU001 are characterized by tumor penetration ability via receptor-mediated endocytosis and are rapidly eliminated by the kidneys. ELU001 high avidity is believed to promote internalization into FRα over-expressing cancer cells, selectively delivering its therapeutic payload. The first Phase I/II clinical trial [69] dedicated to ELU-FRα-1 is under recruiting phase for advanced, recurrent or refractory FRα over-expressing tumors, considered being topoisomerase 1 inhibitor-sensitive [70] and with no other therapeutic options available. The study will focus on dose escalation and safety to determine the recommended Phase 2 dose and on the expansion of the patient cohort, where specific cancer types will be evaluated for efficacy and safety of this technology.
3. Polymeric Particles
3.1. CALAA-01
CALAA-01 is considered the first targeted polymeric carrier designed for delivering siRNA tested in human [71]. A positively charged cyclodextrin core allows the loading the negative nucleic acid payload. Polydispersity, stability, circulation, and targeting properties depend on surface PEGylation and transferrin modification (Figure 2) [72].
Figure 2.
CALAA-01 structure: The system is composed by a core of cyclodestrin encapsulating the siRNA and functionalized with PEG bearing adamantine (AD-PEG) and transferrin (AD-PEG-TF). Figure from Kurreck et al. [72].
The use of transferrin as targeting agent is very common in nanomedicine applied to cancer disease, since many tumors over-express the receptor for this molecule [73]. When fully assembled, the carriers have an average size of 50–70 nm and can be trafficked in the endosomal compartment of the cells after internalization favored by the interaction with transferrin receptor, even though EPR is still fundamental for the particles to reach cancer cells [71]. After sequestration in the endosomal compartment, the system can disassemble and eventually escape from these vesicles, probably thanks to the positive charge of the cyclodextrin structure. The system was enriched with imidazole groups to favor the buffering properties of the system and induce particle endosomal escape via proton sponge effect [71]. In a Phase 1 clinical trial [74], the system was tested for its efficacy and safety against solid tumors (i.e., melanoma) by delivering siRNA against the M2 subunit of ribonucleotide reductase. The trial was terminated before being completed, perhaps because of toxicity issues probably related to the carrier [75], and the results were not reported. However, a work on this trial was published showing the ability of this technology to induce tumor regression and decreasing the expression of functional ribonucleotide reductase [76].
3.2. Micelles
Polymeric micelles are nanocarriers composed of a core–shell structure that can be generated via self-assembly of amphiphilic block copolymers [77]. Because of their self-assembly and amphiphilic nature, micelles are relatively easy to synthesize compared to other technologies and for this reason they are often studied as drug delivery vehicles for poor water-soluble compounds [78]. Hydrophilic polymers including (but not limited to) PEG, polyoxazolines, chitosan, dextran, and hyaluronic acids can wrap their hydrophobic core, while the therapeutic payloads can be also chemically conjugated to these structures [79]. Micelle surface can be easily modified in function of the number of monomers used in their fabrication and conjugation of tumor-specific ligands is easy and reproducible [79,80]. Regarding their clinical translation, one of the major limitations of micelles is represented by their low mechanical properties and re-assembly when their amount in aqueous solution is below the so-called critical micellar concentration [77]. Currently, there are several micellar-based nanoformulations approved for improving cancer treatment, and others are in advanced clinical trials. The chemotherapeutic drug Paclitaxel (PTX) that has a very low solubility in water (less than 0.1 µg/mL) is often used in micellar-based systems to avoid Cremophor-EL and ethanol formulations, resulting in adverse reactions like dyspnea, hypotension, angioedema, and generalized hives (2–4% of patients). Genexol-PM, a monomethoxy-poly (ethylene glycol)-block-poly(D,L-lactide) with a mean size of 20–50 nm was approved in clinics in several Asian countries (South Korea, Philippines, India, and Vietnam) for breast cancer, lung cancer, and ovarian cancer [78]. In Genexol-PM, PTX is physically incorporated into the inner core of the micelles that target the tumors via EPR effect. Currently, Genexol-PM in combination with carboplatin is tested for its safety as an adjuvant treatment in patients with newly diagnosed ovarian cancer that underwent cytoreductive surgery [81]. Other polymeric micelles represent a promising vehicle for PTX delivery, and they show similarity with Genexol-PM including a core–shell structure with physical entrapment of PTX, PEG coating, small size, and passive targeting through the EPR mechanism. Despite there are limited evidence of superior efficacy of polymeric-PTX compared to Cremophor-PTX, micelles allow administration of an increased PTX dose and offer improved patient safety. Similar platforms worth to mention are Apalea/Paclical (mean size 20–30 nm) and pm-Pac (mean size 20 nm) that target the tumor via EPR [82,83]. Apalea/Paclical contains retinoic acid to solubilize PTX and is approved in different countries (Russian Federation, Kazakhstan, and European Union) against platinum-sensitive ovarian, peritoneal, and fallopian tube cancer [84,85]. Finally, pm-Pac (mean size 20 nm) successfully passed a Phase III study as first-line treatment in combination with cisplatin for advanced non-small cell lung cancer (NSCLC) [86,87]. Trials (Phase 1–3) dedicated to investigate the benefits of micellar-based technologies are currently ongoing in China [88] and Japan [89] where they showed similar therapeutic benefits compared to PTX, but less toxicity in treating metastatic or recurrent breast cancer.
Docetaxel (DTX) is another taxane with solubility issues. An analog of Genexol-PM, called Nanoxel-PM micelles loaded with DTX [90] is currently under clinical trial for efficacy evaluation against different cancers [91]. Additionally, it is tested as neoadjuvant in patients with breast cancer in combination with DOX and cyclophosphamide [92] and against salivary duct carcinoma in combination with anti-HER2 monoclonal antibody [93]. Other versions of this therapeutic formulation are tested in trials as well [93]. Micelle-based technologies are under clinical trial also for evaluating their ability to deliver cisplatin. NC-6004 micelles have an average size of 30 nm and are composed by PEG and poly-glutamic acid copolymers (PGlu). NC-6004 combination with gemcitabine (GEM) has been studied in NSCLC patients, biliary tract, and bladder cancer patients [94] resulting in long-lasting antitumor activity and favorable safety profile. Similar data were registered in combination with Pembrolizumab in the treatment of head and neck cancer [95] and in combination with GEM against advanced solid tumors [96]. Similar formulations are widely investigated [97] including the NK012 where the payload SN-38 is covalently attached to the PGlu structure. Here, the efficacy of NK012 was tested in patients with not-resectable colon cancer, but more data are necessary to evaluate its benefits in comparison with the common treatment irinotecan [98,99]. A novel epirubicin drug conjugated polymeric micelle (NC-6300; 40–80 nm in diameter) was developed by conjugating the payload to PEG polyaspartate block copolymer through a pH-sensitive linker which enables the selective epirubicin release in tumor. This technology exploits tumor pH as targeting, representing a perfect example of smart technology in clinics in the treatment of cutaneous and not cutaneous angiosarcoma [100] and advanced, metastatic, or unresectable solid tumors, including soft-tissue sarcomas [101].
3.3. EP0057
EP0057 (formerly known as CRLX101) is a formulation of camptothecin (CPT) conjugated with a cyclodextrin polymer backbone and is currently being evaluated clinically in multiple refractory solid tumors [102,103,104,105,106]. The micelles have a size of approximately 30–40 nm and significantly increase CPT (topoisomerase I inhibitor) solubility while preserving its active lactone form [107]. EP0057 also exhibits better patient tolerance than other CPT analogs. The nanoparticles-drug conjugate is administered via intravenous infusions, and nanoparticles preferentially accumulate in the tumors through EPR [107]. EP0057 has been shown to inhibit significantly also hypoxia-inducible factor-1 alpha (HIF-1α) and therefore serving as a radiosensitizer with the potential to improve the efficacy of chemoradiation therapy [108,109,110].
Prior studies showed EP0057 efficacy in recurrent or persistent, epithelial ovarian, fallopian tube or colorectal, peritoneal, and gastroesophageal cancer [111,112], where it showed promising results [112]. Ongoing clinical trials are designed to evaluate the efficacy and safety (Phase 1/2) of this therapeutic in lung [102], gastric [113] and ovarian [103] cancer in combination with Olaparib, as well as to evaluate its pharmacokinetics properties (PK) [102,114] using a population model. From the data obtained from 27 patients enrolled on two-Phase II clinical trials, the release of CPT was characterized by an initial rapid clearance, which decreased via first-order decay to the steady-state value by 4 h after the infusion. A second Phase I/IIa clinical study involved 22 efficacy-evaluable patients with metastatic renal cell carcinoma, who received increasing doses of EP0057 combined with bevacizumab [106]. Partial response or stable disease was observed in 86% of the patients, with a median progression free survival (mPFS) of 9.9 months. Most patients achieved a reduction of tumor and increased the progression-free survival compared to their previous therapy [115]. In addition, a Phase Ib/II study of EP0057 combined with PTX in women with recurrent epithelial ovarian cancer reported a 31.6% overall response rate, including one complete response with a 5.4 month median progression-free survival [105]. However, the analysis of trials including data highlights the need of more investigation to evaluate the clinical benefits of this therapeutic also because of the onset of considerable side effects [116,117,118,119].
3.4. NanoPac
Other attempts to formulate PTX in nanostructure to avoid the use of Cremophore-EL were performed. NanoPac (also known as, Nanotax) are pure PTX nanoparticles generated in supercritical carbon oxide environment in the presence of organic solvents. These particles have a size of 600–800 nm inhibiting their clearance and making their use helpful for topical and local administration (i.e., inhalation) [120], with no targeting mechanism associated. A Phase 2 trial focused on investigating the effects of different concentration of NanoPac against prostate cancer directly injected into the prostate. Interestingly, the lower dose of drug showed higher benefits in terms of tumor reduction compared to higher doses. The drug showed reasonable side effects, also at the highest dose used [121]. In similar experimental settings, other trials measured the ability of intra-cystic injected Nanopac to contrast the progress of pancreatic cancer [122] and of intraperitoneal administration against ovarian cancer [123]. Additionally, in these cases, lower doses of NanoPac showed higher clinical benefits even though the occurrence of side effect was significantly more pronounced.
4. Abraxane and Related Technologies
4.1. Abraxane
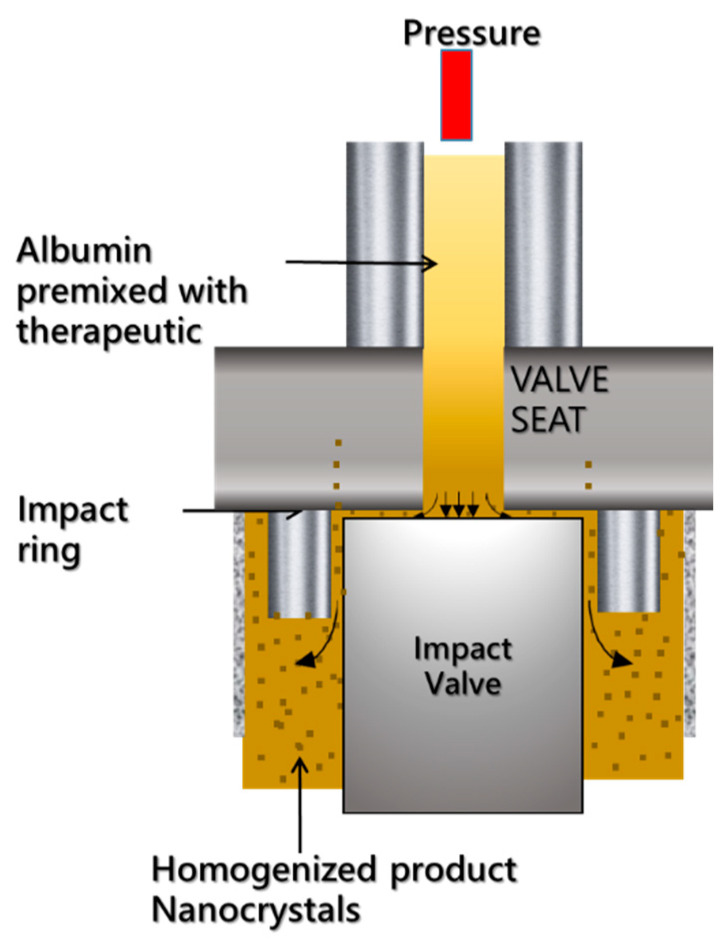
ABI 007, nanoparticle albumin-bound (Nab) PTX, Abraxane are all names used to identify albumin nanocarriers loaded with PTX, to avoid the use of Cremophor-EL [124]. Abraxane allowed for PTX encapsulation, exploiting the natural ability of Albumin to interact with hydrophobic drugs at multiple sites of its structure [124]. The interactions between albumin and drug are solely hydrophobic without formation of covalent bounds, even though some low-degree of crosslinking that can occur between the albumin molecules on the surface of the nanoparticles [124]. The nanocomplexes have an average size diameter of 130 nm and the energy to generate the hydrophobic interactions is provided by the synthetic route, based on high-pressure homogenization where drug and albumin molecules are mixed in an aqueous solution and pushed (under high pressure) in the narrow spaces of the homogenizer (Figure 3) [125].
Figure 3.
Abraxane synthesis: The particles are generated via high-pressure homogenization where PTX and Albumin interactions occur generating a stable complex. Figure reproduced from Parodi et al. [125].
Besides the generation of a safer PTX formulation, Abraxane demonstrated also significant benefits in terms of PK, fast drug distribution and an increased distribution volume. While the size of these particles allowed for exploiting the EPR, it is important to highlight that the nanocomplex degradation in circulation is quick and can lead to the formation of single molecules of albumin bound to PTX. In this scenario, the trafficking of albumin can be also regulated by endothelial receptors like GP60 that favor caveolae-mediated translocation of albumin from the lumen of the blood vessels to the sub-endothelial space. In a trial focusing on NSCLC treatment [126,127], a correlation between the expression of caveolin-1 and the drug efficacy was registered, showing this transporter as one of the mechanism of Abraxane tumor trafficking [126].
Here, some authors show that secreted protein acid rich in cysteine (SPARC), over-expressed on the surface of different cancer cells including pancreatic cancer [128] might eventually favor Abraxane internalization. Additionally, SPARC [129] was object of clinical investigations in pancreatic cancer. These studies confirmed a correlation between Abraxane and SPARC expression [130,131,132]. However, it is worth to mention that Abraxane trafficking was never proved directly and that recent evidence shows other receptors evolved to manage the trafficking of denaturated albumin could be responsible for Abraxane trafficking to the tumor [133]. Abraxane was approved by FDA and EMA for metastatic breast cancer, locally advanced or metastatic NSCLC [124], and as the first-line treatment of metastatic adenocarcinoma of the pancreas [134]. Phase 3 and 4 clinical trials demonstrated Abraxane is more effective when combined with other drugs like atezolizumab [135], GEM and carboplatin in contrasting triple negative breast cancer [136], with GEM against pancreatic cancer [137] and melanoma [138], and with carboplatin against NSCLC [139]. On the other hand, with bevacizumab (and sometimes other agents) the onset of serious side effects was registered [140,141,142,143,144,145], even though slightly beneficial effects on tumor growth were observed like in patients with inoperable melanoma [146,147]. Abraxane was shown also to improve the effect of biological therapy like the immune modulator TLR-7 activator Imiquimod [148,149]. Another clinical trial showed that the best effect of Abraxane as single agent for treating metastatic breast cancer is achieved by prolonging the administration time of reduced doses, after a brief period at normal doses administration [150]. Abraxane showed beneficial effects also on pancreatic cancer as secondary line therapy in patients that progressed on GEM [129] and it was tested also to reduce tumor mass before radiotherapy and surgery in combination with GEM [151], even though its efficacy did not peak significantly higher compared to irinotecan/oxaliplatin/5-fluorouracyl combination [130,131,132]. Finally, it is worth it to mention that Abraxane was tested also against non-Hodgkin’s lymphoma [152]. Here, the system was covalently coated with rituximab, generating a new carrier with the toxicity of Abraxane and the targeting/toxicity of Rituximab against CD20 positive cancer cells [153].
4.2. Abi 009
The same technology at the basis of Abraxane was applied to deliver Rapamycin. Nab-Rapamycin or Abi 009 is a colloidal albumin nanoformulation loaded with this therapeutic that affects cancer viability via mTOR inhibition. Additionally, in this case, encapsulation in albumin complexes resulted very useful to deliver very hydrophobic molecules and it is normal to expect that the same approach could be used to deliver other drugs with the same physical and chemical features as well as targeting mechanism. The only completed trials by far were performed against advanced carcinomas characterized by mTOR mutations [154] and to evaluate the effective not toxic doses (Phase 1/2) of AB009 in the treatment of bladder cancer. Here, the particles were administered directly in the bladder at different concentrations, and retention time in the organ and with and without GEM. All the conditions tested showed good tolerability [155]. Other active or recruiting trials are testing the ability of ABI009 (alone or in combination with other drugs) against sarcoma [156], and different solid pediatric tumors including the central nervous system cancer [157]. In this scenario also albumin nanoparticle loaded with sirolimus (mTOR inhibitor) was tested against glioblastoma [158].
5. Lipid/Proteolipid Technologies
5.1. Doxil
Doxil (also known as Caelix) is a PEGylated liposomal formulation of DOX (average size 70–100 nm), a molecule that intercalates into nucleic acids, inhibiting topoisomerase II enzyme, DNA/RNA synthesis and causing oxidative damage to nucleic acids, proteins and lipids [4]. In this multi-component system, DOX is loaded into the liposome core, providing efficient drug targeting via EPR effect. The system is PEGylated to prolong its circulation time and protect it from MPS sequestration. Interesting, the intellectual property of the system is not based on the liposomal formulation, but on the drug loading mechanism based on a transmembrane ammonium sulfate gradient. Ammonia, continuously produced by tumor cells during glutaminolysis, allows the efficient drug release at the site of the tumor [159]. Doxil was the first nanotherapeutic approved to treat cancer, and it is used for treating ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma [160]. It is also the first nanotherapeutic approved in the generic version under the name of Lipodox [161].
While Doxil has been widely used in the clinic since 1995, its efficacy was variable in different cancers. In some pathologies, such as metastatic breast cancer, Doxil efficacy was not significantly different from free DOX [162]. This controversy was attributed to the limited or insignificant role of EPR in many “cold” human cancers [163], blunting the anti-cancer efficacy of the drug because of liposome-mediated activation of macrophages enhancing tumor growth, and suboptimal release of the drug from liposomes in the tumor bed.
Currently, no new clinical trials of Doxil have been started. The most recent trials include 5 Phase III/Phase IV studies between 2008 and 2019, two of them were completed, and three terminated. These trials include the investigation of Doxil for treatment of newly diagnosed multiple myeloma [164], advanced and/or metastatic breast cancer [165,166], recurrent epithelial ovarian carcinoma [167], and advanced-relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer [168].
In a Phase IV trial to study Doxil as a monotherapy for 25 patients with locally advanced and/or metastatic breast cancer [165], Doxil was administered to elderly women (>65 years old) every 28 days until treatment failure. As a result, 16% of patients had to discontinue treatment because of adverse effects (cardiac events and palmar–plantar-erythrodysestesia), 14% had a partial response, no patients had a complete response, and the rest (60%) had stable disease by the end of the study. Overall survival of patients receiving Doxil was 20.6 months, and time to progression was 5.7 months [163]. Based on these results, the authors concluded Doxil is a safe and effective in elderly breast cancer patients.
The most recent trial (results posted in 2019) analyzed the combination of trabectedin with Doxil for treating recurrent ovarian cancer [168]. The aim of the trial was to compare the overall survival of women with platinum-sensitive, recurrent ovarian cancer treated with Doxil as a monotherapy or in combination with third-line drug trabectedin. It was found that none of the patients reached primary endpoints of overall survival, and the combination therapy did not show any advantage compared to monotherapy in terms of overall survival or safety. However, a subset of patients bearing a BRCA1/2 mutation in the combination therapy group showed a clinically significant reduction in the risk of death (45.8%) [169]. The effect of BRCA1/2 mutations is consistent with previous reports which found that Doxil plus trabectedin therapy prolonged patient overall survival compared to Doxil monotherapy [170]. In patients with advanced soft tissue sarcomas and recurrent ovarian cancer, it was found that trabectedin and Doxil combination showed increased risk of cardiac-related treatment-emergent adverse effects [171], and adverse effects emerged also in other trials [166,167] where Doxil safety was lower than expected (i.e., when used in combination with [164] thalidomide and dexamethasone in patients with newly diagnosed multiple myeloma).
5.2. Other Liposomal Formulations
Onivyde is the nanoformulation of irinotacan. It comprised pegylated liposomes of 110 nm and is approved to treat metastatic adenocarcinoma of the pancreas [172]. The system showed a very high encapsulation yield because of a refined synthetic method based on drug-stabilizing agents like polyphosphate or sucrose octasulfate [173]. Interestingly, it was shown that the interaction between the PEG and the serum protein could increase particle cancer cell internalization, providing a natural-occurring targeting at the level of the cancer lesions, after liposome extravasation via EPR [174]. Besides pancreatic cancer, Onivyde was tested in clinical trial also for lung [175], glioblastoma (administered via convection) [176], oesophagea l [177], and other solid tumors [178].
Myocet is another liposomal formulation of DOX approved to treat breast cancer. Similar to Doxil, the system allowed for increasing DOX efficacy and safety, particularly for what concerns cardiotoxicity. At the basis of the generation of this technology stands a pH gradient that favor DOX encapsulation while drug stabilization in the liposomes is achieved through citrate complexation [179]. This phenomenon is fundamental to avoid particle leakage that occurs preferentially at the cancer lesion because of the presence of high concentrations of phospholipases that induce particle degradation and consequent payload release. The particles have a size of around 150 nm and a loading yield of about 95% [179]. Even though Myocet does not contain any surface modification to prolong its circulation time, the system had enormous benefits in terms of PK [180] increasing the area under the curve of 20 times and targeting the tumor via EPR. Despite clinical trials to contrast metastatic breast cancer, Myocet was tested also against glioma in children [181], lymphomas [182,183], ovarian, fallopian and peritoneal cavity cancer [184,185,186], but to our knowledge the results of these trials were not reported.
Daunoxome is the liposomal formulation of Daunorubicin and it was approved to treat HIV-related Kaposi’s sarcoma [5]. These particles are about 45 nm in size and even though they are not PEGylated as Doxil, they were designed with a neutral surface charge to minimize opsonization and sequestration by the element of the mononuclear phagocytic system [187]. The particles are intravenously administered and accumulate in the tumor via EPR. The benefits of this formulation were shown in preclinical studies showing an increase of tumor targeting of almost 10 times compared to free-administered drug and a controlled released in the cancer lesion up to 36 h [188]. Daunoxom showed also to protect the drug from biotransformation in toxic and inactive derivates [189]. Besides Kaposi’s sarcoma [5,190], Daunoxome (in combination with other drugs like Cytarabine) was extensively clinically tested to treat acute myeloid leukemia, where it showed higher efficacy compared to free-administered drug and acceptable side effects [189,191].
Vyxeos is the liposomal formulation of Daunorubicin and Cytarabin and it is approved for leukemia treatment [192]. The drugs are loaded with a ratio 1:5 (D/C) because previous reports demonstrated the occurrence of synergistic effects in this formulation. In freeze-dried conditions, the particles have a size of 110 [193] and they are not provided with surface targeting properties or stealthing mechanisms [11]. However, drug encapsulation allowed for better PK properties and when interacting with targeted cells in the bone marrow, the delivery of both the therapeutics allowed for significant improvement in their efficacy, compared to free-administered drugs [194]. In clinics, Vyxeos showed to increase the overall survival of elderly patients with acute myeloid leukemia with side effects comparable to standard treatments [195]. In this regard, a specific trial was dedicated to evaluate kidney toxicity [196].
ATU-027 is a liposomal formulation of about 100 nm in size, designed to deliver siRNA. The system is composed of pegylated, cationic, and fusogenic lipids to allow the particles to load siRNA, maintain proper circulation properties, and deliver the payload in the cell cytoplasm [197]. This liposomal formulation was designed to deliver siRNA against PKN3 expression, a key factor in the downstream pathway of PI3K/PTEN [197]. Here, the liposomes are supposed to target the neo-angiogenesis process, and even though with no specific targeting for endothelial cells, they demonstrated in vitro and in vivo the ability to target angiogenesis and lymphoangiogenesis. The system showed good tolerability in clinical trials against solid tumors [198,199] and in particular against pancreatic cancer in combination with GEM [200,201].
Marqibo is a liposomal formulation of vincristine designed to improve the PK properties of this drug and increase its efficacy and safety. The system comprises sphingomyelin/cholesterol liposomes [202] of about 100 nm encapsulated with the drug. The composition of these liposomes is important because it was shown to provide a neutral charge to the particles, decreasing their opsonization and consequent clearance by the MPS. Enhanced PK properties, prolonged circulation and relatively small size can favor tumor targeting via EPR [203]. However, Marqibo was approved to treat Philadelphia chromosome-negative acute lymphoblastic leukemia [204]. The system showed high tolerability also in children receiving adult doses against refractory solid tumors and leukemia [205], while more data are needed to evaluate its efficacy against retinoblastoma [206]. Marqibo showed promising results against metastatic uveal melanoma [207] and B-cell lymphoma in combination with other therapeutics [208].
MEPACT is the liposomal formulation of the immune modulator mifamurtide and it is approved to treat high-grade non-metastatic osteosarcoma in young patients [209]. The liposomes are multilamellar and their size is claimed to be below 100 nm from different sources [172,210], even though other reports claim that this technology is about 3 um in size [211]. Additionally, known as liposomal muramyl tripeptide phosphatidylethanolamine, the system encapsulate a synthetic biologics that can target the macrophages of liver and spleen that in turns can activate other leukocytes against cancer cells [211], decreasing the importance of the size.
5.3. Exosomes
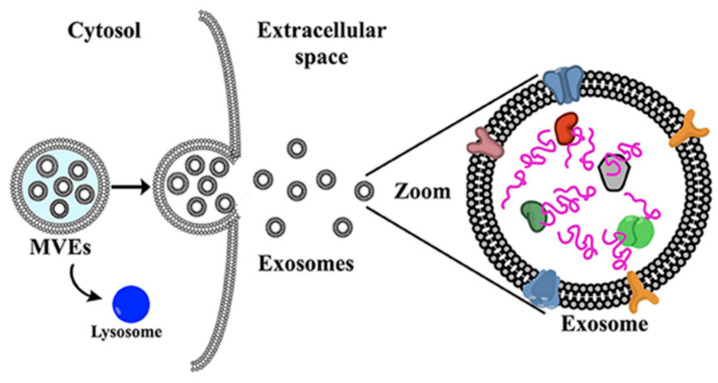
Exosomes are biological extracellular vesicles secreted from different cells with a size of 30–120 nm and a characteristic biological protein identity providing resistance to MPS clearance and tumor tropism (Figure 4) [212]. In the Phase I clinical trial [213] “iExosomes” mesenchymal stem cells (MSC)-derived exosomes loaded with anti-KrasG12D siRNA for treating metastatic pancreatic ductal adenocarcinoma (PDAC) with KrasG12D mutation are tested for the best dose and side effects. Mutations in the GTPase KRAS drive initiation, progression, and metastasis of PDAC. This clinical trial studies the effects of the treatment first described in 2017 by Kamerkar et al. [214] reporting the use of targeted exosomes (iExosomes) loaded with anti-KRAS siRNA and equipped with a CD47 “do not eat me” signal for treating PDAC. iExosomes showed remarkably higher efficiency of siRNA delivery into target pancreatic cells compared to liposomes and non-functionalized exosomes, which was attributed to the functional role of CD47, a signal that helps to evade phagocytosis, in suppressing systemic clearance of nanoparticles. CD47 expression on the surface of iExosomes increased the half-life of nanoparticles in systemic circulation and favored their internalization into pancreatic cells. In preclinical models, iExosomes’ administration resulted in tumor disappearance (an effect that persisted even after 200 days post-treatment). In advanced PDAC models, treated mice responded with partial control of tumor growth, reduced tumor burden, and improved histopathology of the pancreatic tissue. The secondary objectives of this trial include evaluation of iExosomes PK, assessing the disease control rate with the therapy, determining median progression-free survival and overall survival of PDAC patients with the treatment. Overall, this is the first clinical trial on the use of (a) functionalized exosomes; (b) exosomes loaded with siRNA and (c) exosomes with CD47 signal protecting nanoparticles from phagocytosis. In case of success, this study could lay a firm ground for further development of exosome-based therapeutics and exosome nanovehicles for treating cancer and non-cancer diseases.
Figure 4.
Exosome origin: Exosomes derive from the secretion of multivesicular endosome and can be purified and used for drug delivery purposes. Figure reprinted from de la Torre Gomez et al. [212].
In another Phase I clinical trial [215] exosomes were tested to deliver of curcumin against colon cancer. This drug showed anti-inflammatory, antioxidant and antitumor activity, but frustratingly low bioavailability. The strong inhibitory effects of curcumin on many colon cancer cell lines were previously showed [216]. These effects were mainly attributed to signal transduction pathways’ modulation, including β-catenin and NF-κB. This trial attempts to use plant (fruit-derived) exosomes to increase curcumin bioavailability and delivery into colon tumors. In this early clinical trial, the effects of curcumin-loaded exosomes on the immune modulation, phospholipid profile and cellular metabolism of colon cancer and normal colon cells will be analyzed. While the method for loading of curcumin into exosomes is not described, it is most likely related to natural binding of curcumin, as a hydrophobic drug, to these particles. Plant-derived exosomes from different fruits are also well described, as well as their uptake by intestine and immune cells [217].
Grape exosomes [218] containing Lortab drug (acetaminophen and hydrocodone, a non-opioid pain reliever) with Fentanyl patch (opioid analgesic) are studied in a trial to evaluate the ability of powdered grape exosomes as anti-inflammatory agents to reduce the incidence of oral mucositis, a frequent adverse effect during radiation and chemotherapy treatment for head and neck tumors. This powder will be administered daily by mouth for 35 days during chemoradiotherapy along with orally prescribed oral mucositis standard therapy (pain relievers and anti-fungal mouth washes). Efficacy will be estimated by pain caused by oral mucositis and levels of immune biomarkers in blood and mucosal tissue.
Another clinical trial uses dendritic cell-derived exosomes in combination with an immune suppressive drug cyclophosphamide. The study describing this approach was first published in 2016 by Besse et al. [219]. Dendritic cell-derived exosomes (Dex), containing a wide range of antigen presentation, adhesion, costimulatory and docking molecules, were shown to trigger NK and T cell immune responses in Phase I clinical trials [220,221]. In a Phase II trial [222], Dex loaded with MHC class I and class II-restricted cancer antigen showed clinical benefits in patients with inoperable NSCLC including longer progression-free survival in patients with advanced NSCLC. 32% of patients receiving Dex experienced stabilization of cancer growth for over 4 months, but the primary endpoint (to observe 50% of patients with progression-free survival at 4 months after cessation of chemotherapy) was not reached. The authors discussed the obstacles that may have led to this failure, including suboptimal use of cancer-testis antigens loaded into exosomes, the use of IFN-γ during manufacturing of Dex that may upregulate PD-1 ligands on exosomes and potential benefit of using Dex in combination with immune checkpoint blockers in lung cancer. Still, this strategy demonstrated activation of antitumor immunity, feasibility of using dendritic cell-derived exosomes for immunotherapy, and clinical benefits for patients with advanced NSCLC. Using Dex may also be portrayed for other cancers with NKp30-specific functional defects, such as gastrointestinal stromal tumors, neuroblastoma, and chronic lymphocytic leukemia. The different technologies presented in this work are summarized in Table 1.
Table 1.
Characteristics and targeting mechanism of nanocarriers in clinical trials.
| Particle | Material | Size (nm) | Targeting Mechanism | Killing Mechanism |
|---|---|---|---|---|
| AguIX | Polysyloxane | 5 | EPR | radiosensitizer |
| NBTXR3 | Hafnium oxide | 50 | intratumor | radiosensitizer |
| SPION | Iron oxide | 20–150 | EPR, intratumor, polarized magnetic fields | Thermoablation, conjugated drug |
| NU-0129 | Gold | N.A. | EPR, transcytosis | RNAi |
| ELU001 | Silica | 6 | Folic acid | topoisomerase-1 inhibitor |
| CALAA-01 | Cyclodextrin | 50–70 | Transferrin | RNAi |
| Genexol-PM | Polymeric micelles | 20–50 | EPR | PTX |
| Apalea/ Paclical | Polymeric micelles | 20–30 | EPR | PTX |
| Pm-PAC | Polymeric micelles | ~20 | EPR | PTX |
| Nanoxel-PM | Polymeric micelles | 10–50 | EPR | DTX |
| NC-6004 | Polymeric micelles | ~30 | EPR | GEM |
| NK012 | Polymeric micelles | ~20 | EPR | SN-30 |
| NC-6300 | Polymeric micelles | 40–80 | EPR, acidic pH | epirubicin |
| EP0057 | Polymeric micelles | 30–40 | EPR | Camptothecin, radiosensitizer |
| Abraxane | Albumin | 130 | Receptor mediated trafficking | PTX |
| Abi-009 | Albumin | 130 | Receptor mediated trafficking local administration | Rapamycin |
| NanoPac | PTX | 600–800 | Local administration | PTX |
| Doxil | Lipids | 70–100 | EPR | DOX |
| Myocet | Lipids | 150 | EPR | DOX |
| Marqibo | Lipids | 100 | EPR | Vincristine |
| MEPACT | Lipids | >100 | Targeting MPS | Mifamurtide |
| Onyvide | Lipids | 110 | EPR | Irinotecan |
| Daunoxome | Lipids | 45 | EPR | Daunorubicin |
| Vyxeos | Lipids | Daunorubicin/Cytarabine | ||
| ATU-027 | Lipids | 100 | EPR | RNAi |
| Exosomes | Proteo/lipids | 30–120 | Natural tropism | RNAi |
6. Conclusions
In this work, we analyzed different nanotherapeutics that reach clinical trial evaluation excluding nanotherapeutics tested for diagnostic [64,223], improvement of surgical interventions [224,225], pain relievers [226] or cancer vaccines [227,228]. Nanomedicine showed the potential to improve the delivery of small molecules and biologics and to represent a means to develop treatments based on physical methods. Most of these carriers accumulate in the tumor via EPR and a small size (below 150 nm) represents a common factor in the clinical success of nanotherapeutics. Only a few of them were engineered with targeting moieties compatible with high-scale production. In the considered studies emerged that, trafficking complexity could be reached only via biology-inspired carriers like Abraxane and exosomes and, for this reason, more investigations in this direction should be performed [229]. It has also to be noted that most times the nanoformulations that reached clinical trial evaluation were tested for delivering other therapeutics with similar chemical and physical properties, developing novel combinatorial approaches and contrasting the growth of different tumor diseases. In this context, the success obtained against one kind of cancer did not guarantee the same results in other oncological diseases, highlighting the importance to characterize the trafficking properties of each oncological disease.
Author Contributions
Conceptualization and writing A.P.; Inorganic nanoparticle section E.P.K. and T.P.; Polymeric nanoparticle section D.B.T. and R.A.; Protein nanoparticle section, M.V.V. and A.S.F.; Lipid and proteolipid nanoparticle section D.K.; writing—review and editing, A.A.Z.J. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Russian Science Foundation (grant # 21-75-30020).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lammers T., Ferrari M. The success of nanomedicine. Nano Today. 2020;31:100853. doi: 10.1016/j.nantod.2020.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L.-P., Wang D., Li Z. Grand challenges in nanomedicine. Mater. Sci. Eng. C. 2020;106:110302. doi: 10.1016/j.msec.2019.110302. [DOI] [PubMed] [Google Scholar]
- 3.Gabizon A.A., de Rosales R.T., La-Beck N.M. Translational considerations in nanomedicine: The oncology perspective. Adv. Drug Deliv. Rev. 2020;158:140–157. doi: 10.1016/j.addr.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Barenholz Y.C. Handbook of Harnessing Biomaterials in Nanomedicine. Jenny Stanford Publishing; Dubai, United Arab Emirates: 2021. Doxil®—the first FDA-approved nano-drug: From an idea to a product; pp. 463–528. [Google Scholar]
- 5.Petre C.E., Dittmer D.P. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007;2:277. [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y., Van der Meel R., Chen X., Lammers T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Zang F., Wu H., Li J., Xie J., Ma M., Gu N., Zhang Y. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale. 2018;10:1788–1797. doi: 10.1039/C7NR08319J. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal P., Singh R.P., Kumari L., Sharma G., Koch B., Rajesh C.V., Mehata A.K., Singh S., Pandey B.L., Muthu M.S. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C. 2017;74:167–176. doi: 10.1016/j.msec.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Adriani G., de Tullio M.D., Ferrari M., Hussain F., Pascazio G., Liu X., Decuzzi P. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials. 2012;33:5504–5513. doi: 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attia M.F., Anton N., Wallyn J., Omran Z., Vandamme T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019;71:1185–1198. doi: 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- 11.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021;6:e10246. doi: 10.1002/btm2.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evangelopoulos M., Parodi A., Martinez J.O., Tasciotti E. Trends towards biomimicry in theranostics. Nanomaterials. 2018;8:637. doi: 10.3390/nano8090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santagiuliana R., Milosevic M., Milicevic B., Sciumè G., Simic V., Ziemys A., Kojic M., Schrefler B.A. Coupling tumor growth and bio distribution models. Biomed. Microdevices. 2019;21:33. doi: 10.1007/s10544-019-0368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: Biology and translational medicine. Theranostics. 2018;8:237. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita T., Takahashi Y., Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018;41:835–842. doi: 10.1248/bpb.b18-00133. [DOI] [PubMed] [Google Scholar]
- 16.Bosetti R., Jones S.L. Cost–effectiveness of nanomedicine: Estimating the real size of nano-costs. Nanomedicine. 2019;14:1367–1370. doi: 10.2217/nnm-2019-0130. [DOI] [PubMed] [Google Scholar]
- 17.Borodina T., Kostyushev D., Zamyatnin A.A., Jr., Parodi A. Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. Int. J. Transl. Med. 2021;1:306–322. doi: 10.3390/ijtm1030018. [DOI] [Google Scholar]
- 18.Parodi A., Buzaeva P., Nigovora D., Baldin A., Kostyushev D., Chulanov V., Savvateeva L.V., Zamyatnin A.A. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021;19:354. doi: 10.1186/s12951-021-01100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon S.J., Hyland W.B., Muir M.F., Coulter J.A., Jain S., Butterworth K.T., Schettino G., Dickson G.R., Hounsell A.R., O’sullivan J.M. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011;1:18. doi: 10.1038/srep00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lux F., Mignot A., Mowat P., Louis C., Dufort S., Bernhard C., Denat F., Boschetti F., Brunet C., Antoine R. Ultrasmall rigid particles as multimodal probes for medical applications. Angew. Chem. 2011;123:12507–12511. doi: 10.1002/ange.201104104. [DOI] [PubMed] [Google Scholar]
- 21.Lux F., Tran V.L., Thomas E., Dufort S., Rossetti F., Martini M., Truillet C., Doussineau T., Bort G., Denat F. AGuIX® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019;92:20180365. doi: 10.1259/bjr.20180365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancey L., Lux F., Kotb S., Roux S., Dufort S., Bianchi A., Cremillieux Y., Fries P., Coll J.-L., Rodriguez-Lafrasse C. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014;87:20140134. doi: 10.1259/bjr.20140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimenez Y., Busser B., Trichard F., Kulesza A., Laurent J., Zaun V., Lux F., Benoit J.-M., Panczer G., Dugourd P. 3D imaging of nanoparticle distribution in biological tissue by laser-induced breakdown spectroscopy. Sci. Rep. 2016;6:29936. doi: 10.1038/srep29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotb S., Detappe A., Lux F., Appaix F., Barbier E.L., Tran V.-L., Plissonneau M., Gehan H., Lefranc F., Rodriguez-Lafrasse C. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: Proof of concept before phase I trial. Theranostics. 2016;6:418. doi: 10.7150/thno.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Duc G., Roux S., Paruta-Tuarez A., Dufort S., Brauer E., Marais A., Truillet C., Sancey L., Perriat P., Lux F. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014;5:4. doi: 10.1186/s12645-014-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lux F., Detappe A., Dufort S., Sancey L., Louis C., Carme S., Tillement O. Nanoparticules ultrafines en radiothérapie: Le cas des AguIX. Cancer/Radiothérapie. 2015;19:508–514. doi: 10.1016/j.canrad.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Mowat P., Mignot A., Rima W., Lux F., Tillement O., Roulin C., Dutreix M., Bechet D., Huger S., Humbert L. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells. J. Nanosci. Nanotechnol. 2011;11:7833–7839. doi: 10.1166/jnn.2011.4725. [DOI] [PubMed] [Google Scholar]
- 28.Luchette M., Korideck H., Makrigiorgos M., Tillement O., Berbeco R. Radiation dose enhancement of gadolinium-based AguIX nanoparticles on HeLa cells. Nanomed. Nanotechnol. Biol. Med. 2014;10:1751–1755. doi: 10.1016/j.nano.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Radiosensitization of Multiple Brain Metastases Using AguIX Gadolinium Based Nanoparticles. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02820454.
- 30.Verry C., Dufort S., Villa J., Gavard M., Iriart C., Grand S., Charles J., Chovelon B., Cracowski J.-L., Quesada J.-L. Theranostic AguIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial) Radiother. Oncol. 2021;160:159–165. doi: 10.1016/j.radonc.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Radiotherapy of Multiple Brain Metastases Using AguIX®. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02820454.
- 32.AguIX Nanoparticles with Radiotherapy Plus Concomitant Temozolomide in the Treatment of Newly Diagnosed Glioblastoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04881032.
- 33.Stereotactic Brain-directed Radiation with or without Aguix Gadolinium-Based Nanoparticles in Brain Metastases. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04899908.
- 34.AguIX Gadolinium-based Nanoparticles in Combination with Chemoradiation and Brachytherapy. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03308604.
- 35.Nano-SMART: Nanoparticles with MR Guided SBRT in Centrally Located Lung Tumors and Pancreatic Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04789486.
- 36.Marill J., Anesary N.M., Zhang P., Vivet S., Borghi E., Levy L., Pottier A. Hafnium oxide nanoparticles: Toward an in vitropredictive biological effect? Radiat. Oncol. 2014;9:150. doi: 10.1186/1748-717X-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Tourneau C., Moreno V., Salas S., Mirabel X., Calvo E., Doger B., Florescu C., Thariat J., Fijuth J., Rutkowski T. Hafnium oxide nanoparticles NBTXR3 activated by radiotherapy as a new therapeutic option for elderly/frail HNSCC patients. J. Clin. Oncol. 2019;37:6069. doi: 10.1200/JCO.2019.37.15_suppl.6069. [DOI] [Google Scholar]
- 38.Bilynsky C., Millot N., Papa A.L. Radiation nanosensitizers in cancer therapy—From preclinical discoveries to the outcomes of early clinical trials. Bioeng. Transl. Med. 2022;7:e10256. doi: 10.1002/btm2.10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NBTXR3 Activated by Radiation Therapy for the Treatment of Locally Advanced or Borderline-Resectable Pancreatic Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04484909.
- 40.NBTXR3, Chemotherapy, and Radiation Therapy for the Treatment of Esophageal Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04615013.
- 41.NBTXR3, Radiation Therapy, Ipilimumab, and Nivolumab for the Treatment of Lung and/or Liver Metastases from Solid Malignancy. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05039632.
- 42.NBTXR3, Radiation Therapy, and Pembrolizumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04862455.
- 43.NBTXR3 with or without Cetuximab in LA-HNSCC. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04892173.
- 44.NBTXR3 Crystalline Nanoparticles and Radiation Therapy in Treating Randomized Patients in Two Arms with Soft Tissue Sarcoma of the Extremity and Trunk Wall. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01433068.
- 45.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.-P., Agoston P., Hong A., Mervoyer A., Rastrelli M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann C., Calugaru V., Borcoman E., Moreno V., Calvo E., Liem X., Salas S., Doger B., Jouffroy T., Mirabel X. Phase I dose-escalation study of NBTXR3 activated by intensity-modulated radiation therapy in elderly patients with locally advanced squamous cell carcinoma of the oral cavity or oropharynx. Eur. J. Cancer. 2021;146:135–144. doi: 10.1016/j.ejca.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Dulińska-Litewka J., Łazarczyk A., Hałubiec P., Szafrański O., Karnas K., Karewicz A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials. 2019;12:617. doi: 10.3390/ma12040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassenberger A., Scheberl A., Stadlbauer A., Stiglbauer A., Helbich T., Reimhult E. Individually stabilized, superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relaxivities. ACS Appl. Mater. Interfaces. 2017;9:3343–3353. doi: 10.1021/acsami.6b12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cano M., Núñez-Lozano R., Lumbreras R., González-Rodríguez V., Delgado-García A., Jiménez-Hoyuela J.M., de la Cueva-Méndez G. Partial PEGylation of superparamagnetic iron oxide nanoparticles thinly coated with amine-silane as a source of ultrastable tunable nanosystems for biomedical applications. Nanoscale. 2017;9:812–822. doi: 10.1039/C6NR07462F. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z.-Q., Song S.-C. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials. 2017;132:16–27. doi: 10.1016/j.biomaterials.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 51.Talluri S., Malla R.R. Superparamagnetic iron oxide nanoparticles (SPIONs) for diagnosis and treatment of breast, ovarian and cervical Cancers. Curr. Drug Metab. 2019;20:942–945. doi: 10.2174/1389200220666191016124958. [DOI] [PubMed] [Google Scholar]
- 52.Li X., Taratula O., Taratula O., Schumann C., Minko T. LHRH-targeted drug delivery systems for cancer therapy. Mini Rev. Med. Chem. 2017;17:258–267. doi: 10.2174/1389557516666161013111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onbasli K., Erkısa M., Demirci G., Muti A., Ulukaya E., Sennaroglu A., Acar H.Y. The improved killing of both androgen-dependent and independent prostate cancer cells by etoposide loaded SPIONs coupled with NIR irradiation. Biomater. Sci. 2022;10:3951–3962. doi: 10.1039/D2BM00107A. [DOI] [PubMed] [Google Scholar]
- 54.Liu J.F., Lan Z., Ferrari C., Stein J.M., Higbee-Dempsey E., Yan L., Amirshaghaghi A., Cheng Z., Issadore D., Tsourkas A. Use of oppositely polarized external magnets to improve the accumulation and penetration of magnetic nanocarriers into solid tumors. ACS Nano. 2019;14:142–152. doi: 10.1021/acsnano.9b05660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvear-Jiménez A., Zabala Gutierrez I., Shen Y., Villaverde G., Lozano-Chamizo L., Guardia P., Tinoco M., Garcia-Pinel B., Prados J., Melguizo C. Electrospraying as a Technique for the Controlled Synthesis of Biocompatible PLGA@ Ag2S and PLGA@ Ag2S@ SPION Nanocarriers with Drug Release Capability. Pharmaceutics. 2022;14:214. doi: 10.3390/pharmaceutics14010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vakili-Ghartavol R., Momtazi-Borojeni A.A., Vakili-Ghartavol Z., Aiyelabegan H.T., Jaafari M.R., Rezayat S.M., Arbabi Bidgoli S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020;48:443–451. doi: 10.1080/21691401.2019.1709855. [DOI] [PubMed] [Google Scholar]
- 57.Imaging Kidney Transplant Rejection Using Ferumoxytol-Enhanced Magnetic Resonance. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02006108.
- 58.Thakor A.S., Jokerst J.V., Ghanouni P., Campbell J.L., Mittra E., Gambhir S.S. Clinically approved nanoparticle imaging agents. J. Nucl. Med. 2016;57:1833–1837. doi: 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delayed Sentinel Lymph Node Biopsy in Ductal Cancer In Situ. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04722692.
- 60.Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging (SPIO MRI) [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00920023.
- 61.Magnetic Nanoparticle Thermoablation-Retention and Maintenance in the Prostate: A Phase 0 Study in Men. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02033447.
- 62.Radiotherapy with Iron Oxide Nanoparticles (SPION) on MR-Linac for Primary & Metastatic Hepatic Cancers. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04682847.
- 63.A Phase I Clinical Trial of Neoadjuvant Chemotherapy with/without SPIONs/SMF for Patients with Osteosarcoma. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04316091.
- 64.Diagnostic and Prognostic Accuracy of Gold Nanoparticles in Salivary Gland Tumours. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04907422.
- 65.NU-0129 in Treating Patients with Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03020017.
- 66.Kumthekar P., Ko C.H., Paunesku T., Dixit K., Sonabend A.M., Bloch O., Tate M., Schwartz M., Zuckerman L., Lezon R. A first-in-human phase 0 clinical study of RNA interference–based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021;13:eabb3945. doi: 10.1126/scitranslmed.abb3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh P., Mijakovic I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021;21:627–630. doi: 10.1080/14737159.2021.1933447. [DOI] [PubMed] [Google Scholar]
- 68.Boogerd L.S., Boonstra M.C., Beck A.-J., Charehbili A., Hoogstins C.E., Prevoo H.A., Singhal S., Low P.S., van de Velde C.J., Vahrmeijer A.L. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget. 2016;7:17442. doi: 10.18632/oncotarget.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.A Study to Evaluate ELU001 in Patients with Solid Tumors that Overexpress Folate Receptor Alpha (FRα) [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05001282.
- 70.Criscitiello C., Morganti S., Curigliano G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021;14:20. doi: 10.1186/s13045-021-01035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 72.Kurreck J. Proof of RNA interference in humans after systemic delivery of siRNAs. Angew. Chem. Int. Ed. 2010;49:6258–6259. doi: 10.1002/anie.201002867. [DOI] [PubMed] [Google Scholar]
- 73.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Safety Study of CALAA-01 to Treat Solid Tumor Cancers. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00689065.
- 75.Parvani J.G., Jackson M.W. Silencing the roadblocks to effective triple-negative breast cancer treatments by siRNA nanoparticles. Endocr. Relat. Cancer. 2017;24:R81–R97. doi: 10.1530/ERC-16-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis M.E., Zuckerman J.E., Choi C.H.J., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghezzi M., Pescina S., Padula C., Santi P., Del Favero E., Cantù L., Nicoli S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release. 2021;332:312–336. doi: 10.1016/j.jconrel.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 78.Hwang D., Ramsey J.D., Kabanov A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020;156:80–118. doi: 10.1016/j.addr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 80.Cabral H., Miyata K., Osada K., Kataoka K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 81.Safety of Genexol PM and Carboplatin as First-Line Therapy in Ovarian Cancer. [(accessed on 6 October 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05300828.
- 82.Borgå O., Henriksson R., Bjermo H., Lilienberg E., Heldring N., Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: A dose-escalation study. Adv. Ther. 2019;36:1150–1163. doi: 10.1007/s12325-019-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vergote I., Bergfeldt K., Franquet A., Lisyanskaya A., Bjermo H., Heldring N., Buyse M., Brize A. A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecol. Oncol. 2020;156:293–300. doi: 10.1016/j.ygyno.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 84.European Medicines Agency . Assessment Report Apealea. European Medicines Agency; Amsterdam, The Netherlands: 2018. [Google Scholar]
- 85.Apealea®-Elevar Therapeutics. [(accessed on 1 October 2022)]. Available online: https://elevartherapeutics.com/apealea-paclitaxel-micellar-elevar/
- 86.Shi M., Gu A., Tu H., Huang C., Wang H., Yu Z., Wang X., Cao L., Shu Y., Yang R. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: An open-label, randomized, multicenter, phase III trial. Ann. Oncol. 2021;32:85–96. doi: 10.1016/j.annonc.2020.10.479. [DOI] [PubMed] [Google Scholar]
- 87.Lu J., Gu A., Wang W., Huang A., Han B., Zhong H. Polymeric micellar paclitaxel (pm-Pac) prolonged overall survival for NSCLC patients without pleural metastasis. Int. J. Pharm. 2022;623:121961. doi: 10.1016/j.ijpharm.2022.121961. [DOI] [PubMed] [Google Scholar]
- 88.Study of Paclitaxel Micelles for Injection in Chinese Patients with Advanced Solid Tumors. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04778839.
- 89.Fujiwara Y., Mukai H., Saeki T., Ro J., Lin Y.-C., Nagai S.E., Lee K.S., Watanabe J., Ohtani S., Kim S.B. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer. 2019;120:475–480. doi: 10.1038/s41416-019-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Study to Evaluate the Efficacy and Safety of Docetaxel Polymeric Micelle (PM) in Recurrent or Metastatic HNSCC. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02639858.
- 91.Study to Evaluate the Safety of Nanoxel M Inj. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04066335.
- 92.Compare the Efficacy and the Safety of Doxorubicin and Cyclophosphamide Followed by Taxotere versus Doxorubicin and Cyclophosphamide Nanoxel M as Neoadjuvant Chemotherapy in Breast Cancer. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05207514.
- 93.Combination of Nanoxel and Herzuma in Salivary Duct Carcinoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03614364.
- 94.Volovat S.R., Ciuleanu T.-E., Koralewski P., Olson J.E.G., Croitoru A., Koynov K., Stabile S., Cerea G., Osada A., Bobe I. A multicenter, single-arm, basket design, phase II study of NC-6004 plus gemcitabine in patients with advanced unresectable lung, biliary tract, or bladder cancer. Oncotarget. 2020;11:3105. doi: 10.18632/oncotarget.27684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osada A., Mangel L., Fijuth J., Żurawski B., Ursulovic T., Nikolin B., Djan I., Olson J.G. Phase IIa/IIb clinical trial of NC-6004 (Nanoparticle Cisplatin) plus Pembrolizumab in patients with head and neck cancer (HNSCC) who have failed platinum or a platinum-containing regimen. Eur. J. Cancer. 2020;138:S35. doi: 10.1016/S0959-8049(20)31164-3. [DOI] [Google Scholar]
- 96.Subbiah V., Grilley-Olson J.E., Combest A.J., Sharma N., Tran R.H., Bobe I., Osada A., Takahashi K., Balkissoon J., Camp A. Phase Ib/II Trial of NC-6004 (Nanoparticle Cisplatin) Plus Gemcitabine in Patients with Advanced Solid TumorsNC-6004/Gemcitabine in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018;24:43–51. doi: 10.1158/1078-0432.CCR-17-1114. [DOI] [PubMed] [Google Scholar]
- 97.A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Antitumor Efficacy of Cisplatin Micelle Injection (HA132) in Patients with Advanced Malignant Solid Tumors. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05478785.
- 98.Hamaguchi T., Tsuji A., Yamaguchi K., Takeda K., Uetake H., Esaki T., Amagai K., Sakai D., Baba H., Kimura M. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018;82:1021–1029. doi: 10.1007/s00280-018-3693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ri M., Suzuki K., Iida S., Hatake K., Chou T., Taniwaki M., Watanabe N., Tsukamoto T. A phase I/II study for dose-finding, and to investigate the safety, pharmacokinetics and preliminary efficacy of NK012, an SN-38-incorporating macromolecular polymeric micelle, in patients with multiple myeloma. Intern. Med. 2018;57:939–946. doi: 10.2169/internalmedicine.9567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riedel R.F., Chua V.S., Kim T., Dang J., Zheng K., Moradkhani A., Osada A., Chawla S.P. Results of NC-6300 (nanoparticle epirubicin) in an expansion cohort of patients with angiosarcoma. Oncologist. 2022;27:809-e765. doi: 10.1093/oncolo/oyac155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chawla S.P., Goel S., Chow W., Braiteh F., Singh A.S., Olson J.E.G., Osada A., Bobe I., Riedel R.F. A Phase 1b Dose Escalation Trial of NC-6300 (Nanoparticle Epirubicin) in Patients with Advanced Solid Tumors or Advanced, Metastatic, or Unresectable Soft-tissue SarcomaNanoparticle Epirubicin in Solid Tumors and Sarcoma. Clin. Cancer Res. 2020;26:4225–4232. doi: 10.1158/1078-0432.CCR-20-0591. [DOI] [PubMed] [Google Scholar]
- 102.Trial of EP0057, a Nanoparticle Camptothecin with Olaparib in People with Relapsed/Refractory Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02769962.
- 103.EP0057 in Combination with Olaparib in Advanced Ovarian Cancer. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04669002.
- 104.A Study of CRLX101(NLG207) in Combination with Weekly Paclitaxel in Patients with Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02389985.
- 105.Duska L., Krasner C., O’Malley D., Hays J., Modesitt S., Mathews C., Moore K., Thaker P., Miller A., Purdy C. A phase Ib/II and pharmacokinetic study of EP0057 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Gynecol. Oncol. 2021;160:688–695. doi: 10.1016/j.ygyno.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 106.Voss M.H., Hussain A., Vogelzang N., Lee J., Keam B., Rha S., Vaishampayan U., Harris W., Richey S., Randall J. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol. 2017;28:2754–2760. doi: 10.1093/annonc/mdx493. [DOI] [PubMed] [Google Scholar]
- 107.Weiss G.J., Chao J., Neidhart J.D., Ramanathan R.K., Bassett D., Neidhart J.A., Choi C.H.J., Chow W., Chung V., Forman S.J. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs. 2013;31:986–1000. doi: 10.1007/s10637-012-9921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaur S., Chen L., Yen T., Wang Y., Zhou B., Davis M., Yen Y. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomed. Nanotechnol. Biol. Med. 2012;8:721–730. doi: 10.1016/j.nano.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Kemp J.A., Kwon Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021;8:34. doi: 10.1186/s40580-021-00282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian X., Nguyen M., Foote H.P., Caster J.M., Roche K.C., Peters C.G., Wu P., Jayaraman L., Garmey E.G., Tepper J.E. CRLX101, a Nanoparticle–Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1αCRLX101 Improves Cancer Chemoradiotherapy. Cancer Res. 2017;77:112–122. doi: 10.1158/0008-5472.CAN-15-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tian X., Nguyen M., Foote H., Garmey E., Eliasof S., Wang A. CRLX101, an Investigational Nanoparticle Drug Conjugate of Camptothecin, as a Potentially Effective Radiosensitizer in Chemoradiation Treatment of Colorectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:S49. doi: 10.1016/j.ijrobp.2015.07.118. [DOI] [Google Scholar]
- 112.Chao J., Lin J., Frankel P., Clark A.J., Wiley D.T., Garmey E., Fakih M., Lim D., Chung V., Luevanos E. Pilot trial of CRLX101 in patients with advanced, chemotherapy-refractory gastroesophageal cancer. J. Gastrointest. Oncol. 2017;8:962. doi: 10.21037/jgo.2017.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.EP0057 in Combination with Olaparib in Relapsed Advanced Gastric Cancer and Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05411679.
- 114.Schmidt K.T., Huitema A.D., Dorlo T.P., Peer C.J., Cordes L.M., Sciuto L., Wroblewski S., Pommier Y., Madan R.A., Thomas A. Population pharmacokinetic analysis of nanoparticle-bound and free camptothecin after administration of NLG207 in adults with advanced solid tumors. Cancer Chemother. Pharmacol. 2020;86:475–486. doi: 10.1007/s00280-020-04134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keefe S., Hoffman-Censits J., Cohen R., Mamtani R., Heitjan D., Eliasof S., Nixon A., Turnbull B., Garmey E., Gunnarsson O. Efficacy of the nanoparticle–drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I–IIa clinical trial. Ann. Oncol. 2016;27:1579–1585. doi: 10.1093/annonc/mdw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Combining CRLX101, a Nanoparticle Camptothecin, with Enzalutamide in People with Progressive Metastatic Castration Resistant Prostate Cancer Following Prior Enzalutamide Treatment. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03531827.
- 117.A Phase 2 Study of CRLX101(NLG207) in Patients with Advanced Non-Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01380769.
- 118.Neoadjuvant Chemoradiotherapy with CRLX-101 and Capecitabine for Rectal Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02010567.
- 119.Topotecan Hydrochloride or Cyclodextrin-Based Polymer-Camptothecin CRLX101 in Treating Patients with Recurrent Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01803269.
- 120.Verco J., Johnston W., Baltezor M., Kuehl P.J., Gigliotti A., Belinsky S.A., Lopez A., Wolff R., Hylle L., diZerega G. Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac®) in a rodent model. J. Aerosol Med. Pulm. Drug Deliv. 2019;32:99–109. doi: 10.1089/jamp.2018.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trial of NanoPac Focal Therapy for Prostate Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03077659.
- 122.Intracystic Injection of NanoPac® in Subjects with Mucinous Cystic Pancreatic Neoplasms. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03188991.
- 123.Phase II Study of Intraperitoneal NanoPac® in Patients with Ovarian Cancer. [(accessed on 6 October 2022)]; Available online: ://ClinicalTrials.gov/show/NCT03029585.
- 124.Desai N. Albumin in Medicine. Springer; Berlin/Heidelberg, Germany: 2016. Nanoparticle albumin-bound paclitaxel (Abraxane®) pp. 101–119. [Google Scholar]
- 125.Parodi A., Miao J., Soond S.M., Rudzińska M., Zamyatnin A.A., Jr. Albumin nanovectors in cancer therapy and imaging. Biomolecules. 2019;9:218. doi: 10.3390/biom9060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertino E.M., Williams T.M., Nana-Sinkam S.P., Shilo K., Chatterjee M., Mo X., Rahmani M., Phillips G.S., Villalona-Calero M.A., Otterson G.A. Stromal caveolin-1 is associated with response and survival in a phase II trial of nab-paclitaxel with carboplatin for advanced NSCLC patients. Clin. Lung Cancer. 2015;16:466–474.e4. doi: 10.1016/j.cllc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients with Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00729612.
- 128.Hosein P.J., de Lima Lopes G., Jr., Pastorini V.H., Gomez C., Macintyre J., Zayas G., Reis I., Montero A.J., Merchan J.R., Lima C.M.R. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am. J. Clin. Oncol. 2013;36:151–156. doi: 10.1097/COC.0b013e3182436e8c. [DOI] [PubMed] [Google Scholar]
- 129.Abraxane Therapy in Patients with Pancreatic Cancer Who Failed First-Line Gemcitabine Therapy. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00691054.
- 130.S1505: Combination Chemotherapy or Gemcitabine Hydrochloride and Paclitaxel Albumin-Stabilized Nanoparticle Formulation before Surgery in Treating Patients with Pancreatic Cancer that Can be Removed by Surgery. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02562716.
- 131.Sohal D.P., Duong M., Ahmad S.A., Gandhi N.S., Beg M.S., Wang-Gillam A., Wade J.L., Chiorean E.G., Guthrie K.A., Lowy A.M. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2021;7:421–427. doi: 10.1001/jamaoncol.2020.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahmad S.A., Duong M., Sohal D.P., Gandhi N.S., Beg M.S., Wang-Gillam A., Wade J.L., III, Chiorean E.G., Guthrie K.A., Lowy A.M. Surgical outcome results from SWOG S1505: A randomized clinical trial of mFOLFIRINOX vs. gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann. Surg. 2020;272:481. doi: 10.1097/SLA.0000000000004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hama M., Ishima Y., Chuang V.T.G., Ando H., Shimizu T., Ishida T. Evidence for Delivery of Abraxane via a Denatured-Albumin Transport System. ACS Appl. Mater. Interfaces. 2021;13:19736–19744. doi: 10.1021/acsami.1c03065. [DOI] [PubMed] [Google Scholar]
- 134.Saif M.W. US Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP J. Pancreas. 2013;14:686–688. doi: 10.6092/1590-8577/2028. [DOI] [PubMed] [Google Scholar]
- 135.Adams S., Diéras V., Barrios C., Winer E., Schneeweiss A., Iwata H., Loi S., Patel S., Henschel V., Chui S. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann. Oncol. 2020;31:582–589. doi: 10.1016/j.annonc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 136.Yardley D.A., Brufsky A., Coleman R.E., Conte P.F., Cortes J., Glück S., Nabholtz J.-M.A., O’Shaughnessy J., Beck R.M., Ko A. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): Study protocol for a randomized controlled trial. Trials. 2015;16:575. doi: 10.1186/s13063-015-1101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hersh E.M., Del Vecchio M., Brown M., Kefford R., Loquai C., Testori A., Bhatia S., Gutzmer R., Conry R., Haydon A. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann. Oncol. 2015;26:2267–2274. doi: 10.1093/annonc/mdv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.A Study of Atezolizumab in Combination with Carboplatin + Paclitaxel or Carboplatin + Nab-Paclitaxel Compared with Carboplatin + Nab-Paclitaxel in Participants with Stage IV Squamous Non-Small Cell Lung Cancer (NSCLC) IMpower131. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02367794.
- 140.S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim with or without Bevacizumab in Treating Women with Inflammatory or Locally Advanced Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00856492.
- 141.Phase II NCT (Neoadjuvant Chemotherapy) w/Weekly Abraxane in Combination with Carboplatin & Bevacizumab in Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00675259.
- 142.Mrózek E., Layman R., Ramaswamy B., Lustberg M., Vecchione A., Knopp M.V., Shapiro C.L. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin. Breast Cancer. 2014;14:228–234. doi: 10.1016/j.clbc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Phase II Study with Abraxane, Bevacizumab and Carboplatin in Triple Negative Metastatic Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00479674.
- 144.Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients with Locally Recurrent or Metastatic Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00654836.
- 145.Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Gemcitabine, and Bevacizumab in Treating Patients with Metastatic Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00662129.
- 146.Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients with Stage IV Melanoma that Cannot be Removed by Surgery. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02158520.
- 147.Bevacizumab and Temozolomide or Bevacizumab and Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients with Stage IV Malignant Melanoma that Cannot be Removed by Surgery. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00626405.
- 148.Topical Imiquimod and Abraxane in Treating Patients with Advanced Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00821964.
- 149.Salazar L.G., Lu H., Reichow J.L., Childs J.S., Coveler A.L., Higgins D.M., Waisman J., Allison K.H., Dang Y., Disis M.L. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: A phase 2 clinical trial. JAMA Oncol. 2017;3:969–973. doi: 10.1001/jamaoncol.2016.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schedules of Nab-Paclitaxel in Metastatic Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01746225.
- 151.Utembe W., Clewell H., Sanabria N., Doganis P., Gulumian M. Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials. Nanomaterials. 2020;10:1267. doi: 10.3390/nano10071267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nab-Paclitaxel/Rituximab-Coated Nanoparticle AR160 in Treating Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03003546.
- 153.Nevala W.K., Butterfield J.T., Sutor S.L., Knauer D.J., Markovic S.N. Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20+ B-cell lymphoma. Sci. Rep. 2017;7:45682. doi: 10.1038/srep45682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nanoparticle Albumin-Bound Rapamycin in Treating Patients with Advanced Cancer with mTOR Mutations. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02646319.
- 155.Phase 1/2 Study of ABI-009 in Nonmuscle Invasive Bladder Cancer. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02009332.
- 156.Nanoparticle Albumin-Bound Rapamycin and Pazopanib Hydrochloride in Treating Patients with Advanced Nonadipocytic Soft Tissue Sarcomas. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03660930.
- 157.Nanoparticle Albumin-Bound Rapamycin, Temozolomide, and Irinotecan Hydrochloride in Treating Pediatric Patients with Recurrent or Refractory Solid Tumors. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02975882.
- 158.ABI-009 (Nab-Rapamycin) in Recurrent High Grade Glioma and Newly Diagnosed Glioblastoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03463265.
- 159.Haran G., Cohen R., Bar L.K., Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta (BBA)-Biomembr. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 160.Vyas M., Simbo D.A., Mursalin M., Mishra V., Bashary R., Khatik G.L. Drug Delivery Approaches for Doxorubicin in the Management of Cancers. Curr. Cancer Ther. Rev. 2020;16:320–331. doi: 10.2174/1573394716666191216114950. [DOI] [Google Scholar]
- 161.Smith J.A., Mathew L., Burney M., Nyshadham P., Coleman R.L. Equivalency challenge: Evaluation of Lipodox® as the generic equivalent for Doxil® in a human ovarian cancer orthotropic mouse model. Gynecol. Oncol. 2016;141:357–363. doi: 10.1016/j.ygyno.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 162.O’Brien M.E., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R., Kieback D., Tomczak P., Ackland S. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 163.Grodzinski P., Kircher M., Goldberg M., Gabizon A. Integrating nanotechnology into cancer care. ACS Nano. 2019;13:7370–7376. doi: 10.1021/acsnano.9b04266. [DOI] [PubMed] [Google Scholar]
- 164.A Study of Thalidomide plus Dexamethasone (Thal-Dex) versus DOXIL Plus Thalidomide Plus Dexamethasone (DOXIL-Thal-Dex) in Patients with Newly Diagnosed Multiple Myeloma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00097981.
- 165.Pegylated Liposomal Doxorubicin (Caelyx(R)) as Monotherapy in Elderly Patients with Locally Advanced and/or Metastatic Breast Cancer (Study P05059) [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00604968.
- 166.Safety Study of CAELYX in Patients with Metastatic Breast Cancer Previously Treated with Anthracyclines (Study P04057)(TERMINATED) [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00779285.
- 167.A Study to Compare CAELYX with Topotecan HCL in Patients with Recurrent Epithelial Ovarian Carcinoma Following Failure of First-Line, Platinum-Based Chemotherapy. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01840943.
- 168.A Study Comparing the Combination of Trabectedin (YONDELIS) and DOXIL/CAELYX with DOXIL/CAELYX for the Treatment of Advanced-Relapsed Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01846611.
- 169.Monk B.J., Herzog T.J., Wang G., Triantos S., Maul S., Knoblauch R., McGowan T., Shalaby W.S., Coleman R.L. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol. Oncol. 2020;156:535–544. doi: 10.1016/j.ygyno.2019.12.043. [DOI] [PubMed] [Google Scholar]
- 170.Monk B., Ghatage P., Parekh T., Henitz E., Knoblauch R., Matos-Pita A., Nieto A., Park Y., Cheng P., Li W. Effect of BRCA1 and XPG mutations on treatment response to trabectedin and pegylated liposomal doxorubicin in patients with advanced ovarian cancer: Exploratory analysis of the phase 3 OVA-301 study. Ann. Oncol. 2015;26:914–920. doi: 10.1093/annonc/mdv071. [DOI] [PubMed] [Google Scholar]
- 171.Jones R.L., Herzog T.J., Patel S.R., von Mehren M., Schuetze S.M., Van Tine B.A., Coleman R.L., Knoblauch R., Triantos S., Hu P. Cardiac safety of trabectedin monotherapy or in combination with pegylated liposomal doxorubicin in patients with sarcomas and ovarian cancer. Cancer Med. 2021;10:3565–3574. doi: 10.1002/cam4.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: An updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drummond D.C., Noble C.O., Guo Z., Hong K., Park J.W., Kirpotin D.B. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 174.Papi M., Caputo D., Palmieri V., Coppola R., Palchetti S., Bugli F., Martini C., Digiacomo L., Pozzi D., Caracciolo G. Clinically approved PEGylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale. 2017;9:10327–10334. doi: 10.1039/C7NR03042H. [DOI] [PubMed] [Google Scholar]
- 175.Study of Irinotecan Liposome Injection (ONIVYDE®) in Patients with Small Cell Lung Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03088813.
- 176.Study of Convection-Enhanced, Image-Assisted Delivery of Liposomal-Irinotecan in Recurrent High Grade Glioma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02022644.
- 177.Nal-iri/lv5-fu versus Paclitaxel as Second Line Therapy in Patients with Metastatic Oesophageal Squamous Cell Carcinoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03719924.
- 178.Study of Onivyde with Talazoparib or Temozolomide in Children with Recurrent Solid Tumors and Ewing Sarcoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT04901702.
- 179.Batist G., Barton J., Chaikin P., Swenson C., Welles L. Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opin. Pharmacother. 2002;3:1739–1751. doi: 10.1517/14656566.3.12.1739. [DOI] [PubMed] [Google Scholar]
- 180.Mross K., Niemann B., Massing U., Drevs J., Unger C., Bhamra R., Swenson C.E. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004;54:514–524. doi: 10.1007/s00280-004-0825-y. [DOI] [PubMed] [Google Scholar]
- 181.Myocet® in Children with Relapsed or Refractory Non-Brainstem Malignant Glioma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02861222.
- 182.A Multi-Centre Study of MBVD in Elderly and/or Cardiopathic Patients Affected by Hodgkin’s Lymphoma (HL) [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01523847.
- 183.Open Label, Single Arm, Phase II Study Using R-COMP in Elderly Patients with Aggressive NHL. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00244127.
- 184.Liposome-Encapsulated Doxorubicin Citrate and Carboplatin in Treating Patients with Advanced or Metastatic Recurrent Endometrial Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01100359.
- 185.Liposome-Encapsulated Doxorubicin Citrate with or without Gemcitabine Hydrochloride in Treating Patients with Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01100372.
- 186.Addition of Carboplatin to Neoadjuvant Therapy for Triple-Negative and HER2-Positive Early Breast Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01426880.
- 187.Allen T.M., Martin F.J. Seminars in Oncology. Elsevier; Amsterdam, The Netherlands: 2004. Advantages of liposomal delivery systems for anthracyclines; pp. 5–15. [DOI] [PubMed] [Google Scholar]
- 188.Forssen E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997;24:133–150. doi: 10.1016/S0169-409X(96)00453-X. [DOI] [Google Scholar]
- 189.Fassas A., Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma. 2005;46:795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- 190.Liposomal Daunorubicin in Treating Patients with HIV-Related Kaposi’s Sarcoma. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00427414.
- 191.S0106 Cytarabine and Daunorubicin w/ or w/o Gemtuzumab Followed by HD Cytarabine and Either Gemtuzumab or Nothing in De Novo AML. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00085709.
- 192.Alfayez M., Kantarjian H., Kadia T., Ravandi-Kashani F., Daver N. CPX-351 (vyxeos) in AML. Leuk. Lymphoma. 2020;61:288–297. doi: 10.1080/10428194.2019.1660970. [DOI] [PubMed] [Google Scholar]
- 193.Crommelin D.J., van Hoogevest P., Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release. 2020;318:256–263. doi: 10.1016/j.jconrel.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 194.Blair H.A. Daunorubicin/cytarabine liposome: A review in acute myeloid leukaemia. Drugs. 2018;78:1903–1910. doi: 10.1007/s40265-018-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Phase III Study of CPX-351 versus 7 + 3 in Patients 60–75 Years Old with Untreated High Risk (Secondary) Acute Myeloid Leukemia. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01696084.
- 196.A Trial to Evaluate the Potential Impact of Renal Impairment on the Pharmacokinetics and Safety of CPX-351. [(accessed on 6 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03555955.
- 197.Aleku M., Schulz P., Keil O., Santel A., Schaeper U., Dieckhoff B., Janke O., Endruschat J., Durieux B., Röder N. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 198.Schultheis B., Strumberg D., Santel A., Vank C., Gebhardt F., Keil O., Lange C., Giese K., Kaufmann J., Khan M. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 199.Study with Atu027 in Patients with Advanced Solid Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00938574.
- 200.Schultheis B., Strumberg D., Kuhlmann J., Wolf M., Link K., Seufferlein T., Kaufmann J., Feist M., Gebhardt F., Khan M. Safety, efficacy and pharcacokinetics of targeted therapy with the liposomal RNA interference therapeutic Atu027 combined with gemcitabine in patients with pancreatic adenocarcinoma. a randomized phase Ib/IIa study. Cancers. 2020;12:3130. doi: 10.3390/cancers12113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Atu027 Plus Gemcitabine in Advanced or Metastatic Pancreatic Cancer (Atu027-I-02) [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01808638.
- 202.Boman N.L., Cullis P.R., Mayer L.D., Bally M.B., Webb M.S. Long Circulating Liposomes: Old Drugs, New Therapeutics. Springer; Berlin/Heidelberg, Germany: 1998. Liposomal vincristine: The central role of drug retention in defining therapeutically optimized anticancer formulations; pp. 29–49. [Google Scholar]
- 203.Silverman J.A., Deitcher S.R. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Davis T., Farag S.S. Treating relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: Liposome-encapsulated vincristine. Int. J. Nanomed. 2013;8:3479. doi: 10.2147/IJN.S47037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vincristine Sulfate Liposome Injection (Marqibo®) in Combination with UK ALL R3 Induction Chemotherapy for Children, Adolescents, and Young Adults with Relapsed ALL. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02879643.
- 206.Neoadjuvant Carboplatin and Vincristine and Standard Local Ophthalmic Therapy in Treating Patients with Intraocular Retinoblastoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00079417.
- 207.Safety and Efficacy of Marqibo in Metastatic Malignant Uveal Melanoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00495079.
- 208.Ollila T.A., Butera J., Egan P.C., Reagan J.L., Thomas A.G., Yakirevich I., MacKinnon K., Margolis J., Rosati V., McMahon J. Vincristine Sulfate Liposome Injection (VSLI) with Bendamustine and Rituximab As First-Line Therapy for Indolent B-Cell Lymphomas: A Phase 1 Study. Blood. 2020;136:15–16. doi: 10.1182/blood-2020-134054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mifamurtide (L-MTP-PE) for High-Risk Osteosarcoma. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00631631.
- 210.Nieto González N., Obinu A., Rassu G., Giunchedi P., Gavini E. Polymeric and Lipid Nanoparticles: Which Applications in Pediatrics? Pharmaceutics. 2021;13:670. doi: 10.3390/pharmaceutics13050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nardin A., Lefebvre M., Labroquere K., Faure O., Abastado J. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr. Cancer Drug Targets. 2006;6:123–133. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- 212.de la Torre Gomez C., Goreham R.V., Bech Serra J.J., Nann T., Kussmann M. “Exosomics”—A review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front. Genet. 2018;9:92. doi: 10.3389/fgene.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT03608631.
- 214.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01294072.
- 216.Tomeh M.A., Hadianamrei R., Zhao X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019;20:1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cai Y., Zhang L., Zhang Y., Lu R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics. 2022;14:822. doi: 10.3390/pharmaceutics14040822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer. [(accessed on 1 October 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01668849.
- 219.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Escudier B., Dorval T., Chaput N., André F., Caby M.-P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Trial of a Vaccination with Tumor Antigen-Loaded Dendritic Cell-Derived Exosomes. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01159288.
- 223.A Study to Evaluate Diagnostic Performance and Safety of Pegsitacianine, an Intraoperative Fluorescence Imaging Agent for the Detection of Lung Malignancies in Patients Undergoing Routine Surgery. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05048082.
- 224.Clinical Study on the Harvesting Lymph Nodes with Carbon Nanoparticles for Advanced Gastric Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT02123407.
- 225.Carbon Nanoparticles and Indocyanine Green for Sentinel Lymph Node Biopsy in Early Stage Cervical Cancer. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05167149.
- 226.M.D. Anderson Cancer Center Four Quadrants Transverse Abdominus Plane (4Q-TAP) Block with Plain and Liposomal Bupivacaine vs. Thoracic Epidermal Analgesia (TEA) in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) on an Enhanced Recovery Pathway. [(accessed on 1 October 2022)];2017 Available online: https://ClinicalTrials.gov/show/NCT03359811.
- 227.Novel RNA-Nanoparticle Vaccine for the Treatment of Early Melanoma Recurrence Following Adjuvant Anti-PD-1 Antibody Therapy. [(accessed on 1 October 2022)]; Available online: https://ClinicalTrials.gov/show/NCT05264974.
- 228.EMD Serono; Merck KGaA, Darmstadt, Germany. Cancer Vaccine Study for Unresectable Stage III Non-Small Cell Lung Cancer (START) [(accessed on 1 October 2022)];2007 Available online: https://ClinicalTrials.gov/show/NCT00409188.
- 229.Parodi A., Kostyushev D., Brezgin S., Kostyusheva A., Borodina T., Akasov R., Frolova A., Chulanov V., Zamyatnin A.A., Jr. Seminars in Cancer Biology. Academic Press; Cambridge, MA, USA: 2022. Biomimetic approaches for targeting tumor inflammation. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.