Abstract
Intimin is a bacterial outer membrane protein required for intimate attachment of enterohemorrhagic and enteropathogenic Escherichia coli (EHEC and EPEC) to mammalian cells. β1-chain integrins have been proposed as candidate receptors for intimin. We found that binding of mammalian cells to immobilized intimin was not detectable unless mammalian cells were preinfected with EPEC or EHEC. β1-chain integrin antagonists or inactivation of the gene encoding the β1-chain did not affect binding of preinfected mammalian cells to intimin or the actin condensation associated with the attachment of EPEC. The results indicate that β1-chain integrins are not essential for intimin-mediated cell attachment or EPEC-mediated actin polymerization.
Several enteropathogenic bacteria, such as enterohemorrhagic Escherichia coli (EHEC), enteropathogenic E. coli (EPEC), and Citrobacter rodentium, form a specific structure, termed an attaching and effacing (A/E) lesion, on the surface of epithelial cells (6, 15, 18, 26). A/E lesions are thought to promote tight bacterial adherence to intestinal epithelial cells, a critical step during colonization (23, 24). Within these lesions, the actin cytoskeleton is disrupted and the microvilli of the epithelial cell are effaced. A highly organized cytoskeletal structure containing polymerized actin forms beneath the closely attached bacterium (reviewed in reference 4), which is raised on a “pedestal” that may extend several microns above the plane of the cell surface.
A multistep model of the formation of A/E lesions on host cells has been proposed (4). Initial, nonintimate attachment of the bacterium is followed by the injection of bacterial proteins into the host cell (13, 17) via a specialized translocation apparatus, termed a type III secretion system (reviewed in reference 19). This results in cytoskeletal changes and the effacement of microvilli. The last step requires a 94-kDa bacterial outer membrane protein, termed intimin (14), and results in close bacterium-host cell apposition on a pedestal. Intimin is encoded by the eae (E. coli attaching and effacing) gene and is required for A/E lesion formation and for full virulence in animal models and humans (5, 6, 22, 28).
A function for intimin in mammalian cell attachment was suggested by an approximately 30% identity with invasin, a Yersinia pseudotuberculosis outer membrane protein that mediates entry into mammalian cells by binding multiple β1-chain integrins (11, 12, 29). Different candidate molecules, including integrins, have been proposed to function as host cell receptors for intimin. Integrins are a large family of heterodimeric receptors that are involved in a wide variety of cell-cell and cell-extracellular matrix interactions (10). Frankel and coworkers showed that maltose binding protein (MBP) fusions containing the C-terminal 280 amino acids of EPEC intimin bound to the β1-chain integrins α4β1 and α5β1, and antagonists of these integrins inhibited binding of MBP-intimin to human CD4+ T cells (8, 9). More recently, Kenny et al. (16) presented evidence that a bacterial molecule, termed Tir (translocated intimin receptor; also termed EspE [3]), is translocated to the mammalian cell surface after secretion via the type III secretion pathway and acts as a receptor for intimin. Soluble MBP-intimin bound better to HeLa cell monolayers after preinfection with EPEC (25). It has been postulated that perhaps both integrins and Tir play roles in cell binding by intimin (15). In this study, we tested whether β1-chain integrins are essential for intimin-mediated cell attachment and A/E lesion formation.
Efficient mammalian cell attachment to immobilized intimin requires bacterial preinfection.
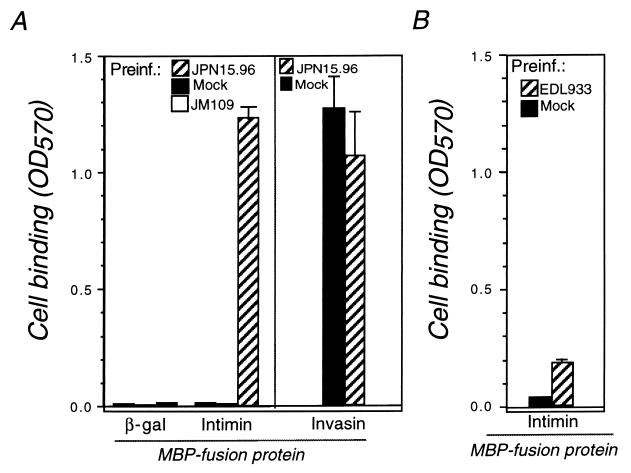
To develop an assay that could be used to easily detect a role for integrins in intimin-mediated cell binding, we tested whether preinfection of HEp-2 cells with EPEC or EHEC could promote cell attachment to immobilized intimin. Given the clinical significance of EHEC in the United States (23), we examined cell binding to EHEC intimin, which is 83% identical to EPEC intimin overall (29). The 3′ 395 codons of the EHEC EDL933 eaeA gene were amplified and inserted into the 3′ end of the malE gene of pMAL-c2 (New England Biolabs, Beverly, Mass.), and the resultant intimin fusion protein, MBP-Int395, was purified as previously described (20). Because EPEC generates more pronounced A/E lesions on cultured cells than EHEC (2), we initially tested the effect on intimin binding of preincubation of mammalian cells with EPEC strain JPN15.96/pMAR7, an eae mutant of EPEC that retains a functional type III protein translocation system (14). Bacteria grown overnight in Luria broth were added to HEp-2 (epithelial) cells at a multiplicity of infection (MOI) of approximately 200, centrifuged onto monolayers at 196 × g for 10 min, and incubated for 3 h at 37°C. Monolayers were washed three times in phosphate-buffered saline (PBS), incubated for 20 min in RHFM (RPMI 1640 supplemented with 20 mM HEPES [pH 7.0], 2% fetal bovine serum, and 0.5% d-mannose) containing 100 μg of gentamicin/ml to kill bacteria, and again washed three times with PBS. Cells were dispersed with EDTA as described previously (20) and were resuspended at 106/ml in RHFM. Next, 0.1 ml of this suspension was added to microtiter wells coated with 50 μl of 5-μg/ml MBP-Int395 or the equivalent MBP-invasin fusion protein, MBP-Inv497, which contains the C-terminal 497 amino acids of invasin (21). Bound cells were quantitated by crystal violet staining (20). Mock-infected or E. coli K-12-infected cells did not bind MBP-Int395 above background levels, but HEp-2 cells that had been preinfected with JPN15.96/pMAR7 efficiently recognized MBP-Int395 (Fig. 1A). Preinfected cells did not bind to a control MBP–β-galactosidase protein, and both mock-infected and preinfected cells bound to MBP-Inv497. HEp-2 cells preinfected with EHEC strain EDL933 prior to binding MBP-Int395 also bound to intimin, albeit less efficiently than cells preinfected with EPEC strain JPN15.96/pMAR7 (Fig. 1B).
FIG. 1.
Preinfection of HEp-2 cells with EPEC or EHEC stimulates binding to immobilized MBP-intimin. HEp-2 cells were mock infected or infected with E. coli K-12 JM109, EPEC strain JPN15.96/pMAR7 (eae mutant) (A), or EHEC strain EDL933 (B). Bacteria were killed with gentamicin, and the HEp-2 cells were dispersed and added to microtiter wells coated with a control MBP fusion protein (β-gal), MBP-Int395 (Intimin) or MBP-Inv497 (Invasin). Bound cells were quantitated as previously described (20). Shown are representative results of two to six separate experiments. Binding to invasin was performed in a separate experiment in which JM109 preinfection (Preinf.) was omitted. Error bars indicate the standard deviations of quadruplicate samples. OD570, optical density at 570 nm.
β1-Chain integrins are not required for intimin binding by mammalian cells.
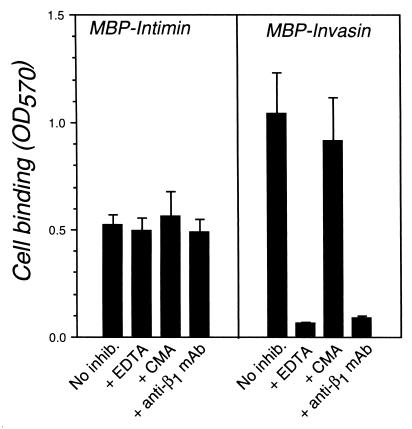
Two integrin antagonists were tested for the ability to block intimin binding to HEp-2 cells. Neither EDTA, which chelates the divalent cations required for integrin function, nor the anti-β1-chain monoclonal antibody (MAb) P4C10 (Gibco-BRL) inhibited intimin binding by EPEC-induced HEp-2 cells (Fig. 2, left). In contrast, both agents blocked invasin binding by these cells (Fig. 2, right).
FIG. 2.
Integrin antagonists do not inhibit mammalian cell binding to intimin. HEp-2 cells were preinfected with EPEC strain JPN15.96/pMAR7 as described in the text, and incubated in buffer alone (No inhib.), 10 mM EDTA (EDTA), control mouse ascites diluted 1:300 (CMA), or ascites containing the anti-β1 MAb P4C10 (Gibco-BRL) diluted 1:300 (anti-β1 mAb) for 30 min. Cells were then added to microtiter wells coated with MBP-Int395 (Intimin) or MBP-Inv497 (Invasin). Bound cells were quantitated. Shown are the means ± standard deviations of quadruplicate samples. OD570, optical density at 570 nm.
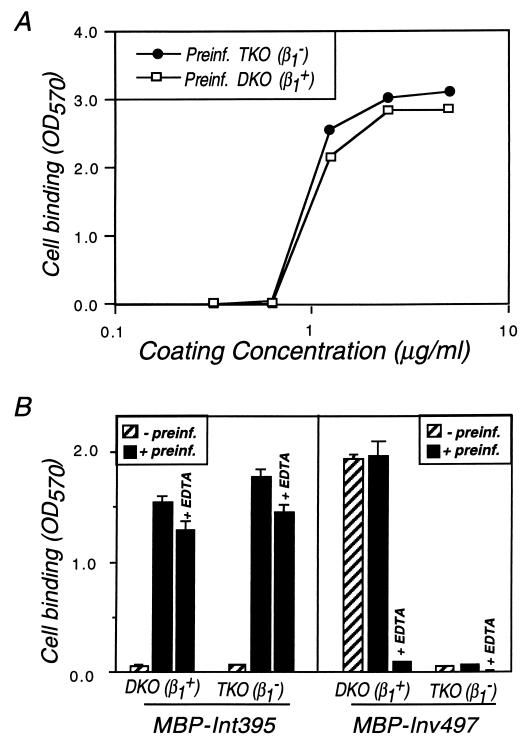
Mammalian cells that are deficient for β1-chain integrin expression were tested for the ability to bind intimin. F9 embryonal carcinoma cells carry three copies of the gene encoding the β1 chain, and TKO (triple knockout) cells fail to express β1-chain integrins due to insertions in each of the three copies (27). DKO (double knockout) F9 cells retain one intact copy of the β1 gene and thus retain expression of β1-integrins (27). The expression of β1-chain integrins in focal plaques of DKO but not TKO cells was confirmed by immunofluorescent staining with an anti-mouse β1-chain MAb (9EG7; Pharmingen, San Diego, Calif.) (data not shown). TKO and DKO cells were preinfected with EPEC JPN15.96/pMAR7 and tested for the ability to bind MBP-Int395. Both cell lines bound intimin after preinfection, and the minimal intimin coating concentration required for cell binding was identical for the two cell lines (Fig. 3A). Preinfection with JPN15.96/pMAR7 was required for binding, and EDTA had little effect on intimin attachment by either cell line (Fig. 3B). As expected, DKO but not TKO cells bound to MBP-Inv497; this binding was independent of JPN15.96/pMAR7 preinfection and was blocked by EDTA.
FIG. 3.
β1-Chain integrins are not required for attachment to intimin. (A) TKO (β1−) or DKO (β1+) cells were preinfected (Preinf.) with EPEC strain JPN15.96/pMAR7 and then dispersed as described in the text. Cells were added to microtiter wells coated with varying concentrations of MBP-Int395. Cell binding was quantitated as described previously (20), and the means of quadruplicate samples are shown. (B) DKO (β1+) or TKO (β1−) cells were mock-infected or infected with EPEC strain JPN15.96/pMAR7 and then added to microtiter wells coated with 5-μg/ml MBP-Int395 or 2-μg/ml MBP-Inv497. EDTA indicates binding in the presence of 10 mM EDTA. Cell binding was quantitated, and the means ± standard deviations of quadruplicate samples are shown. OD570, optical density at 570 nm.
β1-Chain integrins are not required for EPEC-mediated actin condensation.
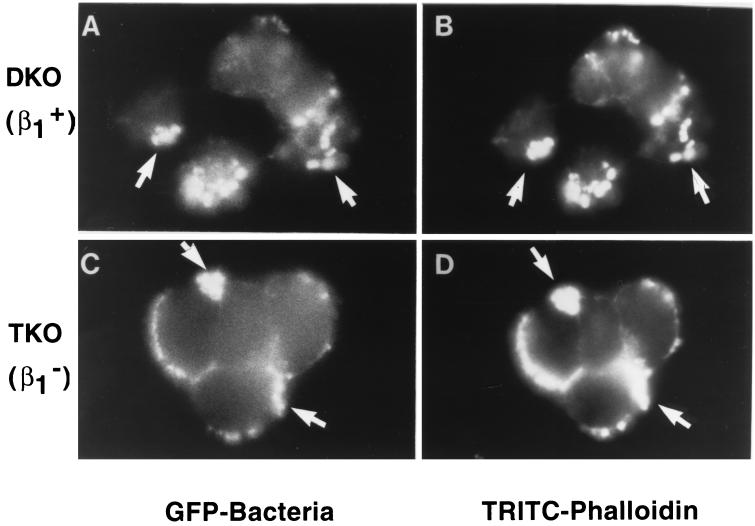
The results described above indicated that integrins are not required for intimin binding after preinfection of mammalian cells with EPEC. To determine whether integrins could be required for some other step in the formation of A/E lesions, we examined the ability of E. coli to induce actin condensation on β1-chain integrin-deficient mammalian cells. Because EPEC generates more pronounced A/E lesions in vitro than does EHEC (2), TKO and DKO cells were infected with EPEC JPN15/pMAR7 (14). Filamentous actin (F-actin) was detected by using fluorochrome-tagged phalloidin (18). A total of 50 microscopic fields containing over 400 bound bacteria were visually scored for colocalization of bacteria and F-actin, and for both TKO and DKO cells, every bound bacterium was associated with F-actin (Fig. 4; data not shown).
FIG. 4.
β1-Chain integrins are not required for EPEC-mediated actin polymerization. DKO (β1+) (A and B) or TKO (β1−) (C and D) cells were infected at an MOI of 20 with EPEC strain JPN15/pMAR7 expressing green fluorescent protein (GFP) for 6 h at 37°C. Monolayers were washed and permeabilized, and microscopic fields were then examined for the presence of GFP-expressing bacteria (A and C) or were stained with tetramethylrhodamine isothiocyanate(TRITC)-labeled phalloidin to visualize F-actin (B and D). Arrows indicate examples of colocalized bacteria and F-actin. Magnification, ×1,000.
The ability of EPEC to generate F-actin at sites of bacterial attachment on cells that do not express β1-chain integrins suggests that β1-chain integrins do not play an essential role at any step in A/E lesion formation. Rather, the requirement of preinfection for cell attachment to immobilized MBP-intimin is consistent with the model that an intimin receptor is injected during bacterium-host cell contact (16). The MBP-intimin protein was derived from EHEC strain EDL933, so if injected Tir acts as a receptor for intimin, EPEC Tir apparently can recognize EHEC intimin. Consistent with this, DeVinney and Finlay have recently found that EPEC and EHEC Tir recognize EHEC intimin indistinguishably (3a).
Frankel and coworkers showed that antagonists of β1-chain integrins blocked binding of CD4+ T cells to EPEC intimin (9). The most obvious methodological difference between these two studies is the preinfection of mammalian cells by E. coli competent for type III secretion. It is also possible that the apparent discrepancy may be due to differences in the cell lines used or to a fundamental functional difference between EPEC and EHEC intimins, which are only 49% identical in the C-terminal putative cell-binding domain (29).
Other laboratories have also detected binding of soluble intimin to uninduced cells (1, 9a), and the experiments described in this study do not rule out the possibility that intimin also contributes to binding of mammalian cells prior to signaling by bacteria. The interaction of intimin with uninduced cells may be too weak to be detected in the assay described here or may vary with the particular recombinant intimin. A detailed description of the bacterium-host cell interactions critical for binding and pedestal formation will require further studies of the cell-binding activity of intimin.
Acknowledgments
We thank Steve Luperchio, Trudy Morrison, Jon Goguen, and Jenifer Coburn for valuable discussion and careful review of the manuscript; Duane Jenness for assistance with microscopy; and Lisa Gansheroff, Alison O’Brien, Rebekah DeVinney, and Brett Finlay for communication of unpublished results. Ralph Isberg provided invaluable advice and recombinant invasin protein, Mike Alrutz provided technical advice, Gerald Keusch and David Acheson provided strains, and Caroline Damsky provided the TKO and DKO cell lines used in this study. We especially thank David Schauer for strains, encouragement, and invaluable advice.
This work was supported by a grant from the Small Grants Program of the University of Massachusetts Medical Center.
REFERENCES
- 1.An H, Fairbrother J M, Dubreuil J D, Harel J. Cloning and characterization of the eae gene from a dog attaching and effacing Escherichia coli strain 4221. FEMS Microbiol Lett. 1997;148:239–245. doi: 10.1111/j.1574-6968.1997.tb10295.x. [DOI] [PubMed] [Google Scholar]
- 2.Cantey R J, Moseley S L. HeLa cell adherence, actin aggregation, and invasin by nonenteropathogenic Escherichia coli possessing the eae gene. Infect Immun. 1991;59:3924–3929. doi: 10.1128/iai.59.11.3924-3929.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 3a.DeVinney, R., and B. B. Finlay. Unpublished results.
- 4.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Investig. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel G, Candy D C, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel G, Candy D C, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips A D, Dougan G. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–4328. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel G, Lider O, Hershkoviz R, Mould A P, Kachalsky S G, Candy D, Cahalon L, Humphries M J, Dougan G. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 9a.Gansheroff, L., and A. O’Brien. Unpublished data.
- 10.Hynes R O. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–27. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 11.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promoted bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 12.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaper J B. EPEC delivers the goods. Trends Microbiol. 1998;6:169–173. doi: 10.1016/s0966-842x(98)01266-9. [DOI] [PubMed] [Google Scholar]
- 16.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 17.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 20.Leong J M, Fournier R, Isberg R R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong J M, Morrissey P E, Marra A, Isberg R R. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O’Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacteria receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 26.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens L E, Sonne J E, Fitzgerald M L, Damsky C H. Targeted deletion of beta 1 integrins in F9 embryonal carcinoma cells affects morphological differentiation but not tissue-specific gene expression. J Cell Biol. 1993;123:1607–1620. doi: 10.1083/jcb.123.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzipori S, Gunzer F, Donnenberg M S, de Montigny L, Kaper J B, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]