Abstract
RNA interference (RNAi) is one of the most widely used techniques to study gene functions. There is still a lack of RNAi techniques that can be applied in Phytoseiidae conveniently and efficiently. Star Polycation is a new nanomaterial commonly used as a carrier of dsRNA in RNAi. Five genes of P. persimilis (PpATPb, PpATPd, PpRpL11, PpRpS2, and Pptra-2) were selected to verify whether SPc promotes the delivery of dsRNA into P. persimilis through soaking. When each of the five genes were interfered using SPc-mediated dsRNA, the total number of success offspring produced per female in six days decreased by ca. 92%, 92%, 91%, 96%, and 64%. When PpATPb, PpATPd, PpRpL11, or PpRpS2 was interfered, both the fecundity and egg hatching rate decreased. In contrast, when Pptra-2 was interfered, reduction in the reproductive capability was mainly the result of the decreased egg hatching rate. Correspondingly, when the target gene was interfered, P. persimilis expression of PpRpL11 reduced by 63.95%, while that of the other four genes reduced by at least 80%. Our studies showed that nanomaterials, such as SPc, have the potential to be used in RNA interference of phytoseiid mites.
Keywords: Phytoseiidae, nanomaterial, soaking, reproduction
1. Introduction
Phytoseiid mites (Chelicerata: Arachnida: Acari) play important roles in agroecosystems, as highly effective biocontrol agents of many small pests, including spider mites, whiteflies, and thrips, etc. [1,2,3]. Studies on phytoseiids have mainly focused on their biology, and many interesting phenomena were observed and reported. For example, they have a reproductive pattern called “paternal genome elimination”, referring to males that develop from fertilized eggs but become haploid with their paternal genomes lost at early embryonic stages [4,5]. However, the mechanisms behind such a special pattern are still unclear. Molecular biological research on Phytoseiidae is overall retarded, especially research on gene function, regulation mechanism and pathway are limited [6,7,8,9,10]. One major reason is that their highly ossified small bodies became barriers, thus blocking successful applications of many molecular biological techniques.
RNAi is one of the most widely used techniques to study gene function, regulation, and interaction [11,12]. The first step of RNAi is delivering exogenous dsRNA into the target organism. Commonly used methods include microinjection, oral feeding, and soaking. Overall, microinjection is the most commonly used method in dsRNA delivering. However, microinjection on phytoseiid mites generally cause a high level of physical injury and mortality [13,14]. Oral feeding was the only RNAi method that was relatively successfully applied in Phytoseiidae, with an efficiency ranging from 40% to 80% [15,16]. Since starvation is required for the oral delivery of dsRNA in phytoseiids, this method can only be used when expression of the target gene will not be affected by starvation. In addition, there are high risks that dsRNA may degrade due to an unsuitable pH environment or be shared by nucleases that exist in their alimentary canals [17]. Among the three methods, soaking is the easiest to perform and appears to be less harmful. However, poor interference efficiency was observed in phytoseiid, possibly due to the low permeability of its dense body surface [18].
Nanomaterials are natural, incidental, or manufactured materials with sizes ranging from 1 to 100 nm [19]. Some nanomaterials can bind dsRNA and protect it from degradation due to their chemical stability, minimal cytotoxicity, and biocompatibility, therefore they were used as carriers to transfer exogenous dsRNA into cells efficiently and rapidly [20,21,22,23,24,25]. Star polycation (SPc) is a new nanomaterial synthesized by Li et al. The molecular weight is 19,440 (GPC) and the pdi is 1.07. It is a cationic dendrimer that consists of four peripheral amino acid functionalized arms, with outer shells positively charged (+20.9 mV) to bind the negative charge double strand RNA (dsRNA). This combination could decrease the zeta potential to +4.0 mV by binding dsRNA. Meanwhile, the size of SPc is 100.5 nm and increases to 260 nm when bound with dsRNA [24]. Showing improved delivery efficiency of dsRNA in interfering key genes required for life activities by aphids and black cutworms [26,27,28,29], SPc showed its high potential in pest management [30,31,32,33,34]. It might also be expected to enhance RNAi efficiency through soaking in Phytoseiidae.
Phytoseiiulus persimilis is probably one of the most well studied species in Phytoseiidae, but still only a small number of its genes have been studied. Our previous study showed that, when dsRNA was mediated with nanocarrier SPc, it might be delivered into the P. persimilis body successfully through soaking [35]. In the present study, five more genes were selected to verify the capability of this nanomaterial in enhancing RNAi efficiency through soaking in P. persimilis. Reproductive capabilities of interfered females were evaluated and the relative expression of the target genes were measured. The aim of the present study is to establish a nanocarrier SPc-mediated dsRNA delivery system in P. persimilis and provide a potential tool in studying gene functions of phytoseiids.
2. Methods and Materials
2.1. Mite Colony
Phytoseiulus persimilis used in this study were obtained from the colony maintained in the Lab of the Predatory Mite, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. Tetranychus urticae Koch (Acari: Tetranychidae), reared on two-week-old soybean seedlings, were used as the prey of P. persimilis.
For experimental purposes, P. persimilis were reared individually using small units, as described by Zhang et al. [36]. The major piece to make a rearing unit arena is a transparent acrylic board (30 × 20 × 3 mm3) with a 10 mm diameter hole in the center. Two pieces of rectangular glass were used to seal the hole, each on one side, creating a 10 (dia.) × 3 mm3 arena for P. persimilis. A piece of bean leaf was used as the floor, placed between one rectangular glass and the central board. The four layers were clipped together on both ends to avoid mites escaping. Individuals were all reared under 25 ± 1 °C, 60 ± 5% RH, and L:D = 16:8.
2.2. Acquisition of the Five Genes and the SPc Mediated dsRNA Complexes
Gene Selection
Five genes were selected for the present study. Three of the selected genes, ribosomal protein L11 (PpRpL11), ribosomal protein S2 (PpRpS2), and transformer-2 (Pptra-2), were believed to be involved in P. persimilis reproduction regulation [15,16]. Two V-ATPase genes (VATPb, VATPd) were also selected. V-ATPases are important enzymes in arthropods, and are essential for multiple secretory pathways, from the synthesis and modification of biomolecules to the intracellular transport, secretion, and degradation. ATPases consists of variant subunits determined by different genes [37,38]. These genes were often used as RNAi candidate genes because remarkable changes in biological performances of target organisms were often expected after interference [14,23].
Sequences of VATPb and VATPd were blasted from the transcriptome (submitted to NCBI) of P. persimilis, referring to orthologs in Aphis gossypii (NCBI Accession XP_027836958.1) and Drosophila melanogaster (NCBI Accession NP_001287401.1). These two sequences were termed as PpVATPb and PpVATPd. Sequences of PpRpL11, PpRpS2 and Pptra-2 were identical, as reported by Bi et al. [16].
2.3. Gene Cloning and cDNA Synthesis
Approximately 2 μg RNA was extracted from P. persimilis (ca. 100 individuals of mixed stages), using MicroElute Total RNA Kit (Omega bio-tek, lnc. R6831-01, Norcross, GA, USA), according to the manufacturer’s recommended protocols. Quality control of extracted RNA were evaluated with spectroscopic quantitation using nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was reverse-transcribed using 1 μg RNA following the instructions of UnionScript First-strand cDNA Synthesis Mix for qPCR (Genesand, Beijing, China).
2.4. dsRNA Synthesis
All sequences (Supplementary File) were cloned using PCR. The reaction mixture (50 μL) for PCR contained: 25 μL 2 × GS Taq PCR Mix (Genesand, Beijing, China), 1 μL cDNA template, 0.5 μmol forward and reverse primers and nuclear-free water. Conditions of PCR reaction were: 95 °C for 3 min, 35 cycles of 94 °C for 25 s, 58 °C for 25 s and 72 °C for 30 s. PCR products were purified and cloned into pTOPO-Blunt Simple Vector following the instructions of Zero Background pTOPO-Blunt Simple Cloning Kit (Aidlab, Beijing, China). The vectors were transformed into DH5α strain of Escherichia coli (Vazyme, Nanjing, China) and the positive single colony were selected for Sanger sequencing (Tsingke, Tianjin, China). Five cloned full sequences were aligned with the transcriptome of P. persimilis to make sure the accuracy of nucleotide. Primers used in current study were designed in DANMAN 6.0 and synthesized by Sangon bio-teck (Shanghai, China) (Table 1).
Table 1.
All primers used for dsRNA synthesis and RT-qPCR.
| Primers | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| dsRNA synthesis | ||
| dsGFP | TAATACGACTCACTATAGGG TGAGCAAGGGCGAGGAG |
TAATACGACTCACTATAGGG CGGCGGTCACGAACTCCAG |
| dsPpATPb | TAATACGACTCACTATAGGG CCCACTCACTGTAGCCAAT |
TAATACGACTCACTATAGGG TGTCGTTTACGGAACTCGG |
| dsPpATPd | TAATACGACTCACTATAGGG ACTGGGTGAAGTTGGCTGA |
TAATACGACTCACTATAGGG ATTGCTGAGTCTCGTGGTC |
| dsPpRpL11 | TAATACGACTCACTATAGGG CCGGCAGAGTTCAGAAAGAC |
TAATACGACTCACTATAGGG CTACGGTGAGGCACGTTGTA |
| dsPpRpS2 | TAATACGACTCACTATAGGG GACGCTTTTCTTGGAACGAC |
TAATACGACTCACTATAGGG CCACAAGTCCGGAGTCAGAT |
| dsPptra-2 | TAATACGACTCACTATAGGG GGAGACGAAGGAAAACGTCA |
TAATACGACTCACTATAGGG CGAGTATATCTCCGGCTTCG |
| RT-qPCR | ||
| PpATPb | GAGGATGGGCTTCATACCT | ACGGCAACTCCTGAGAAGA |
| PpATPd | GGTTCGGAAAGAGGAAATG | TCGGCAAGTTTGGGATTC |
| PpRpl11 | CGGGAATACGAACTACGC | TCTGCTGGAACCATTTGAT |
| PpRpS20 | CAAGGAAGGCGAGAAGG | TGACACCGAGACCAACG |
| Pptra-2 | AGATCGGCGTAGCAGGAGT | TCTGGGCATCGTAGACAACC |
| actin | TGGTCGGTATGGGTCAGA | TGGCAGGAGTGTTGAAGGTC |
The T7 promoter sequence 5′ TAATACGACTCACTATAGGG 3′ was added in the front of the forward and reverse PCR primers to synthesize the subsequent dsRNA (Table 1). Then, 50 μL of the reaction mixture was made, as described in the PCR reaction. Finally, the dsGFP, dsPpATPb, dsPpATPd, dsPpRpL11, dsPpRpS2, and dsPptra-2 fragments were synthesized according to the instruction of T7 RNAi Transcription Kit (TR102) produced by Vazyme.
2.5. SPc Synthesis and RNA Interference
In the present study, SPc was synthesized, as described by Li et al. [24], and provided by the Department of Entomology and MOA Key Lab of Pest Monitoring and Green Management, College of Plant Protection, Chinese Agricultural University. For each gene, SPc and dsRNA were 1:1 mixed to make a complex, with 0.1% tween 20 added. Concentration of each complex was 500 ng/μL. Stability of the complexes were checked using 1% agarose gel electrophoresis, with corresponding unbound dsRNA as the control. The complexes were expected to be blocked with no band presented on the gel.
Approximately 600 newly emerged females were used for interference. According to our previous attempts, ca. 15–20 mites can be soaked in 5 μL formulation [35]. Each soaking lasts for 7 min. For each target gene, ca. 100 P. persimilis were interfered in total. Another ca. 100 individuals were interfered using GFP as the control. Five groups were designed. In each group, 15–20 mites were conducted in RNAi on all five target genes simultaneously, with GFP as the control.
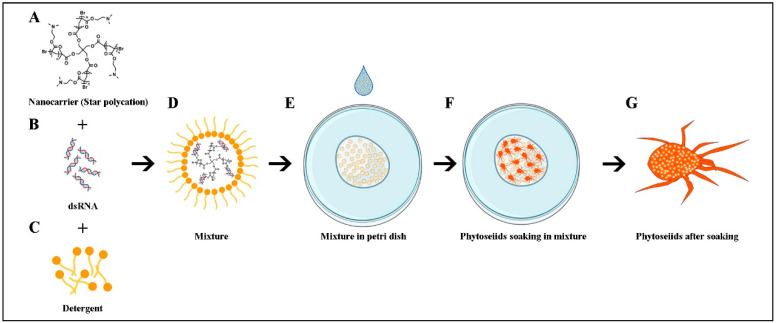
After soaking, the complex solution was dried using a piece of filter paper. The mites were left at room temperature until they could move normally. Then, they were placed in rearing arenas, and were reared individually (Figure 1). All individuals were used to evaluate biological performances and measure the relative expression of the target gene.
Figure 1.
Process of nanocarrier SPc mediated dsRNA delivering through soaking. (A) Nanocarrier SPc; (B) dsRNA; (C) detergent tween 20; (D) formulation of SPc, dsRNA and detergent; and (E–G) soaking process.
2.6. Change in Reproductive Capabilities of P. persimilis as Affected by RNAi
Each interfered female was provided with a newly emerged male as a mate. Pairs that mated for at least 2 h were reared for 6 days for observing the females’ reproductive capability. Daily fecundity and the hatching rate of eggs were recorded. For each individual, the effective fecundity was estimated as the total number eggs produced in 6 days that hatched successfully. Daily fecundity, hatching rate, and total effective fecundity were compared among the five genes and the control, using one-way ANOVA, with multiple comparisons performed using Tukey’s HSD. For each gene interfered, fecundity and hatching rates were also compared across the 6 days using one-way ANOVA. All statistics were performed using IBM-SPSS 22.0, with diagrams made with GraphPad Prism 8.0. Egg morphology were also observed, photographed using the M205 FCA (Leica Microsystems Ltd., Hessian, Germany).
2.7. Relative Expression of the Five Genes in P. persimilis When Interfered
After biological performance observation, all individuals were collected to detect relative expression of the target gene. Our preliminary experiments showed that most changes in the P. persimilis reproductive capability due to RNAi could have been observed in 6 days. For each treatment, ca. 15 females were mixed as a replicate, and 4 biological replicates were created for expression analysis. Total RNAs were extracted and the first strand was synthesized using the same method, as described above. CT values of each gene were obtained by real time quantitative PCR. Reaction mixture (20 μL) contained: 10 μL 2 × GS AntiQ qPCR SYBR Green Master Mix, 0.4 μL ROX Dye, 1 μL cDNA template, 0.4 μmol forward and reverse primers, and RNase-free water. Reactions were conducted as follows: 95 °C for 1 min, 40 cycles of 95 °C for 20 s, 58 °C for 20 s and 72 °C for 30 s. β-actin gene was used as an internal control. Relative expression of five genes were normalized using 2−ΔΔCT according to the method [39]. For each biological replicate, its expression of the target gene was estimated as the mean of 4 technique replicates. For each gene, relative expression when interfered were compared with the GFP control using the t-test (IBM-SPSS 22.0).
3. Results
3.1. Acquisition of the Five Genes and the SPc Mediated dsRNA Complexes
Sequences of PpVATPb and PpVATPd were obtained from the transcriptome of P. persimilis. The length of these two sequences was 585 and 525 bp, respectively, and encoded 194 and 174 amino acids. All five target sequences were successfully cloned and were identical as blasted sequences in transcripts.
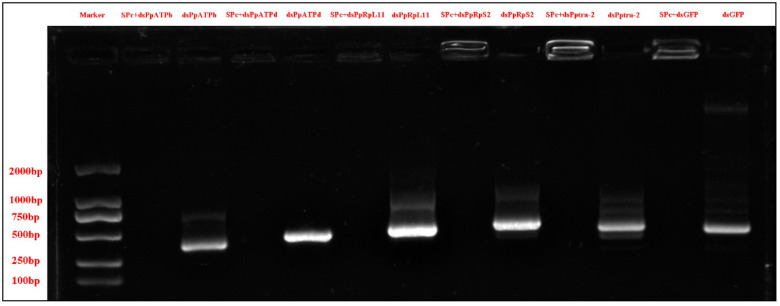
The lengths of all five dsRNAs ranged from 400 to 500 bp. When each dsRNA alone was added into the gel, there was a clear band that showed the same length with the fragments. When combined with the SPc, the SPc mediated dsRNA complexes were blocked in the gel with no band observed (Figure 2). The results were consistent with those reported by Li et al. [24], indicating that SPc can bind dsRNA successfully and stably.
Figure 2.
Gel electrophoresis results of SPc mediated dsRNA complexes and corresponding unbound dsRNAs. For each gene, when dsRNA was added, a clear band was observed on the gel. In contrast, no band was observed with dsRNA was mixed with SPc, suggesting that the complex was blocked.
3.2. Change in Reproductive Capabilities of P. persimilis as Affected by RNAi
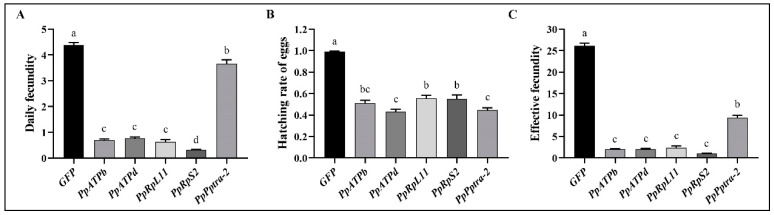
Decrease in reproductive capability were observed in all treatments. When each of the four genes interfered, daily fecundity ranged between 1.9 and 26.3, while the hatching rate ranged between 0.43 to 0.99 (Figure 3A,B). Overall, total effective fecundity of P. persimilis in six days decreased by 92%, 92%, 91%, 96%, and 64% when PpATPb, PpATPd, PpRpL11, PpRpS2, and Pptra-2 were interfered, respectively (Figure 3C).
Figure 3.
Reproductive capabilities of P. persimilis when the five genes were interfered. (A). Daily fecundity per female. (B) Egg hatching rate. (C) Effective fecundity per female. Letters above each bar (Means ± SEM) indicate significant differences across each treatment (SNK tests: p < 0.05).
The greatest reduction in reproductive capability was observed when PpRpS2 was interfered, with 10.2% of individuals becoming completely sterile, and almost all the others stopped laying eggs on the second day. When PpATPb, PpATPd, and PpRpL11 were interfered, 44%, 75%, and 70% of individuals stopped laying eggs on the second day, while the proportion of females who stopped laying eggs increased continuously (Table 2). Almost no egg laid after the third day hatched (Table 2). In contrast, when Pptra-2 was interfered, only ca. 5.3% females stopped laying eggs on the second day. Daily fecundity was only 30% lower than the control, but the egg hatching rate decreased continuously since the third day (Table 3).
Table 2.
Daily fecundity of Phytoseiulus persimilis when five genes were interfered.
| Gene | Daily Fecundity (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| GFP | 4.03 ± 0.16 a (n = 79) |
5.04 ± 0.13 a (n = 79) |
4.98 ± 0.17 a (n = 79) |
4.36 ± 0.15 a (n = 77) |
3.92 ± 0.15 a (n = 77) |
3.89 ± 0.24 a (n = 77) |
| PpATPb | 3.32 ± 0.16 a (n = 79) |
0.79 ± 0.11 b (n = 77) |
0.05 ± 0.05 b (n = 74) |
0 b (n = 74) |
0 b (n = 74) |
0.10 ± 0.07 b (n = 71) |
| PpATPd | 3.49 ± 0.14 a (n = 73) |
0.48 ± 0.10 b (n = 73) |
0.38 ± 0.08 b (n = 73) |
0.11 ± 0.06 b (n = 73) |
0.06 ± 0.03 b (n = 73) |
0.11 ± 0.04 b (n = 72) |
| PpRpL11 | 2.25 ± 0.11 a (n = 92) |
0.61 ± 0.14 b (n = 92) |
0.26 ± 0.09 bc (n = 92) |
0.21 ± 0.10 bc (n = 91) |
0.16 ± 0.09 bc (n = 92) |
0.29 ± 0.13 c (n = 91) |
| PpRpS2 | 1.89 ± 0.10 a (n = 88) |
0.02 ± 0.02 b (n = 88) |
0.01± 0.01 b (n = 88) |
0 b (n = 88) |
0 b (n = 88) |
0 b (n = 88) |
| Pptra-2 | 3.60 ± 0.18 a (n = 79) |
3.52 ± 0.15 a (n = 79) |
2.98 ± 0.20 a (n = 79) |
4.32 ± 0.27 a (n = 79) |
3.51 ± 0.22 a (n = 79) |
4.01 ± 0.27 a (n = 77) |
Means within a row followed by different letters are significant differences across each day (SNK tests: p < 0.05).
Table 3.
Daily hatching rate of eggs produced by Phytoseiulus persimilis when five genes were interfered.
| Gene | Hatching Rate (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| GFP | 0.99 ± 0.01 a (n = 77) |
0.99 ± 0.01 a (n = 79) |
0.99 ± 0.01 a (n = 78) |
1.00 ± 0.00 a (n = 74) |
0.98 ± 0.01 a (n = 74) |
0.99 ± 0.003 a (n = 72) |
| PpATPb | 0.62 ± 0.03 a (n = 74) |
0.10 ± 0.04 b (n = 41) |
0 b (n = 1) |
0 b (n = 5) |
- | 0 b (n = 1) |
| PpATPd | 0.56 ± 0.03 a (n = 71) |
0.04 ± 0.02 b (n = 46) |
0 b (n = 16) |
- | 0 b (n = 3) |
0 b (n = 1) |
| PpRpL11 | 0.67 ± 0.03 a (n = 83) |
0.18 ± 0.07 b (n = 28) |
0.08 ± 0.13 b (n = 10) |
0 b (n = 5) |
0 b (n = 4) |
0 b (n = 5) |
| PpRpS2 | 0.56 ± 0.04 a (n = 79) |
0 b (n = 1) |
0 b (n = 1) |
- | - | - |
| Pptra-2 | 0.96 ± 0.02 a (n = 69) |
0.87 ± 0.03 a (n = 71) |
0.40 ± 0.05 b (n = 71) |
0.19 ± 0.0 c (n = 63) |
0.03 ± 0.02 d (n = 63) |
0.07 ± 0.03 d (n = 60) |
Means within a row followed by different letters are differences across each day (SNK tests: p < 0.05).
3.3. Relative Expression of the Five Genes in P. persimilis When Interfered
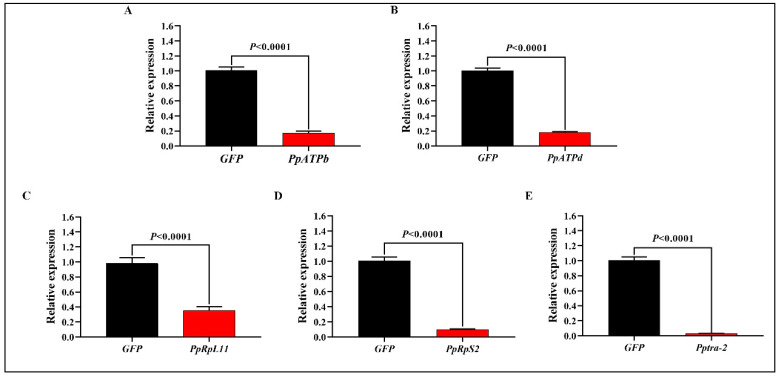
Relative expression of all five target genes decreased significantly when interfered (Figure 4A–E). Among which, relative expression of PpRpL11 reduced by 64% (Figure 4C), while that of the other four genes decreased by at least 80%. The maximum decrease in expression (97%) was observed when Pptra-2 was interfered (Figure 4E).
Figure 4.
Relative expression of five genes (A–E) in Phytoseiulus persimilis when interfered or not. Total RNAs were extracted from 10–15 P. persimilis individuals. Four biological replicates were set for each gene. Differences of relative expression between target gene and GFP were analyzed with a t-test using IBM-SPSS 22.0 and all graphs were created using GraphPad Prism 8.0.
4. Discussion
After being soaked in the SPc mediated dsRNA complex, both the reproductive capability of P. persimilis and relative expression of the target genes decreased. These results suggested that SPc mediated dsRNA entered P. persimilis body successfully through soaking, which became a RNAi technique that was very convenient to perform. In the present study, expression of PpRpL11, PpRpS2 and Pptra-2 when interfered were 6%, 70%, and 55% lower, respectively, than those reported by Bi et al. When interferences were performed through oral delivery, suggesting the soaking method is also highly effective [16]. In addition, relative expression of the target genes was measured six days after interference, suggesting the long-term effect of this method.
Previous studies showed that PpRpL11, PpRpS2 and Pptra-2 are involved in reproduction regulation in phytoseiids [15,16]. Generally, VATPases were not considered as directly related to reproduction. However, PpATPb and PpATPd were expected to be tightly involved in energy metabolism. Arthropod females often had their energetic requirement increase significantly after mating for reproductive purposes [40,41,42]. For example, in wolf spider, the content of Glucose in fertilized female is twice that in virgin individuals [42]. Consumption rates of P. persimilis increased by ca. seven times after mating, suggesting there is also high energetic requirement for egg production [43]. When ATP genes were interfered, many small arthropods had fecundity reduced due to disturbed energy metabolic [44,45]. It is reasonable to expect similar patterns in P. persimilis.
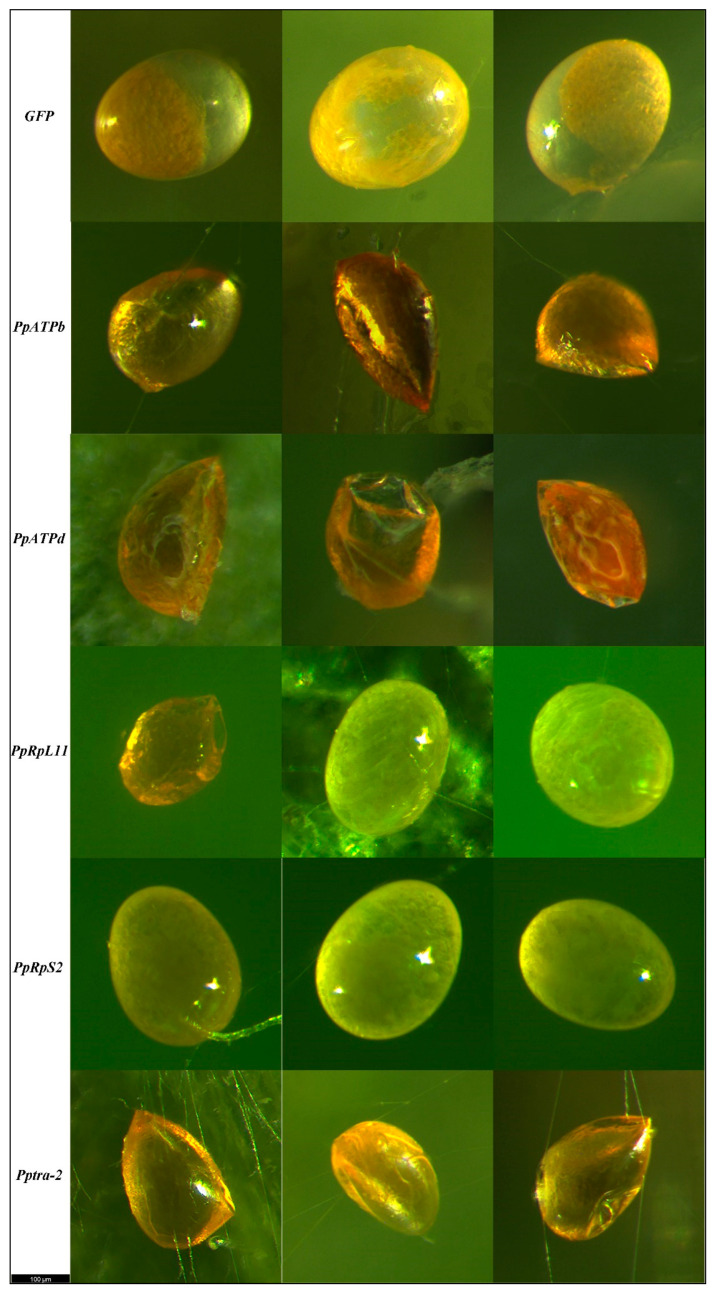
Regulation of reproduction is undoubtedly very complicated. For example, in the model organism D. melanogaster, there were 30,075 distal regulatory elements related to embryonic development by analyzing cis-regulatory dynamics after egg laying. Gene-expression at different timings during embryonic development also differ [46,47]. In the present study, eggs that failed to hatch show different morphological changes when different genes were interfered. According to the terms generally used in insect embryo development [48], the opacite and transparent parts of each egg were termed as yolk and developmental basis, respectively. When PpATPb and PpATPd were interfered, almost all eggs failed to hatch had the developmental basis shriveled. When PpRpL11 and PpRpS2 were interfered, partial eggs had the yolk shriveled, and some eggs just failed to hatch with no obvious morphological change observed. When Pptra-2 was interfered, all eggs that produced from the third to sixth day failed to hatch had the yolk shriveled (Figure 5). However, due to limitations of our knowledge, we are currently not able to link the phenotypes to gene functions theoretically.
Figure 5.
Morphology of normal P. persimilis eggs (laid by females interfered with GFP) and unhatched eggs laid by females had each of the five genes interfered. When the mother had PpATPb and PpATPd interfered, all eggs failed to hatch had the developmental basis shriveled. When the mother had PpRpL11 and PpRpS2 interfered, partial eggs had the yolk shriveled, and some eggs just failed to hatch with no obvious morphological change observed. When the mother had Pptra-2 interfered, all eggs produced from the fifth day failed to hatch had the yolk shriveled. Eggs were photographed using the M205 FCA (Leica Microsystems Ltd., Hessian, Germany).
Studies on molecular mechanism of P. persimilis mainly focused on molecular characteristic, protein structure and expression pattern. Limited molecular biological techniques have been applied to phytoseiids successfully in investigating and verifying gene functions. Our study showed that the nanocarrier SPc-mediated dsRNA delivery system can be conveniently and effectively applied to interfere functional genes in phytoseiids, which is the required first step to study gene functions. With this system developed, we expect more techniques, such as fluorescence in situ hybridization, protein–protein interaction, CRISPR, etc., can and will be applied to these tiny organisms, to explain the mechanisms behind their specific biological features.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12213809/s1. Sequences presented in the current study were listed in supporting information.
Author Contributions
Conceptualization, X.X. and J.L.; Investigation, Z.W., M.L. and Z.K.; Data Curation, Z.W.; Writing–Original Draft Preparation, Z.W.; Writing–Review & Editing, X.X. and J.L.; Supervision, B.Z.; Funding Acquisition, X.X. and E.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data used in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31972337 and 31872028) and Beijing Innovation Consortium of Agriculture Research System, BAIC01-2022.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moraes G.J., Mcmurtry J.A., Denmark H.A., Campos C.B. A revised catalog of the mite family Phytoseiidae. Zootaxa. 2004;434:1–494. doi: 10.11646/zootaxa.434.1.1. [DOI] [Google Scholar]
- 2.McMurtry J.A., Gilberto J., Moraes G., Sourassou N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013;18:297–320. doi: 10.11158/saa.18.4.1. [DOI] [Google Scholar]
- 3.van Lenteren J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol. 2012;57:1–20. doi: 10.1007/s10526-011-9395-1. [DOI] [Google Scholar]
- 4.Helle W., Bolland H.R., Arendonk R.J., Boer R.D., Schulten G.G., Russell V.M. Genetic evidence for biparental males in haplo-diploid predator mites (Acarina: Phytoseiidae) Genetica. 1978;49:165–171. doi: 10.1007/BF00120562. [DOI] [Google Scholar]
- 5.Gardner A., Ross L. Mating ecology explains patterns of genome elimination. Ecol. Lett. 2014;17:1602–1612. doi: 10.1111/ele.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera A.R., Donohue K.V., Khalil S.M., Scholl E., Opperman C., Sonenshine D.E., Roe R.M. New approach for the study of mite reproduction: The first transcriptome analysis of a mite, Phytoseiulus persimilis (Acari: Phytoseiidae) J. Insect Physiol. 2011;57:52–61. doi: 10.1016/j.jinsphys.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y.-L., Li D.-S., Zhang M., Chen W., Zhang G.-R. Food source affects the expression of vitellogenin and fecundity of a biological control agent, Neoseiulus cucumeris. Exp. Appl. Acarol. 2014;63:333–347. doi: 10.1007/s10493-014-9781-3. [DOI] [PubMed] [Google Scholar]
- 8.Pomerantz A.F., Hoy M.A. Expression analysis of Drosophila doublesex, transformer-2, intersex, fruitless-like, and vitellogenin homologs in the parahaploid predator Metaseiulus occidentalis (Chelicerata: Acari: Phytoseiidae) Exp. Appl. Acarol. 2015;65:1–16. doi: 10.1007/s10493-014-9855-2. [DOI] [PubMed] [Google Scholar]
- 9.Ding L., Chen F., Luo R., Pan Q., Wang C., Yu S., Cong L., Liu H., Li H., Ran C. Gene cloning and difference analysis of vitellogenin in Neoseiulus barkeri (Hughes) Bull. Entomol. Res. 2018;108:141–149. doi: 10.1017/S0007485317000591. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J.-Q., Zhang Y., Ma L., Niu T.-T., Dong T.-T., Sheng R.-R., Li L., Xu Y.-Y., Xi L.-Y., Li G.-T. Molecular characterization of Neoseiulus barkeri vitellogenin genes and vitellogenin receptor during reproductive diapause. Insects. 2020;11:203. doi: 10.3390/insects11040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamore P.D. RNA interference: Listening to the sound of silence. Nat. Struct. Biol. 2001;8:746–750. doi: 10.1038/nsb0901-746. [DOI] [PubMed] [Google Scholar]
- 12.Meister G., Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 13.Zhu K.Y., Palli S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020;65:293–311. doi: 10.1146/annurev-ento-011019-025224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan S., Ren B.-Y., Shen J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021;28:21–34. doi: 10.1111/1744-7917.12822. [DOI] [PubMed] [Google Scholar]
- 15.Pomerantz A.F., Hoy M.A. RNAi-mediated knockdown of transformer-2 in the predatory mite Metaseiulus occidentalis via oral delivery of double-stranded RNA. Exp. Appl. Acarol. 2015;65:17–27. doi: 10.1007/s10493-014-9852-5. [DOI] [PubMed] [Google Scholar]
- 16.Bi S.-J., Lv J.-L., Xu J., Shi D.-Y., Wang E.-D., Li G.-T., Xu X.-N. RNAi mediated knockdown of RpL11, RpS2, and tra-2 led to reduced reproduction of Phytoseiulus persimilis. Exp. Appl. Acarol. 2019;78:505–520. doi: 10.1007/s10493-019-00403-2. [DOI] [PubMed] [Google Scholar]
- 17.Kunte N., McGraw E., Bell S., Held D., Avila L.A. Prospects, challenges, and current status of RNAi through insect feeding. Pest Manag. Sci. 2020;76:26–41. doi: 10.1002/ps.5588. [DOI] [PubMed] [Google Scholar]
- 18.Silver K., Cooper A.M., Zhu K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021;77:2645–2658. doi: 10.1002/ps.6277. [DOI] [PubMed] [Google Scholar]
- 19.Dolez P. Nanoengineering: Global Approaches to Health and Safety Issues. Elsevier; Amsterdam, The Netherlands: 2015. Nanomaterials Definitions, Classifications, and Applications; pp. 3–40. [Google Scholar]
- 20.Miele E., Spinelli G.P., Miele E., Di Fabrizio E., Ferretti E., Tomao S., Gulino A. Nanoparticle-based delivery of small interfering RNA: Challenges for cancer therapy. Int. J. Nanomed. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponnuswamy N., Bastings M.M.C., Nathwani B., Ryu J.H., Chou L.Y.T., Vinther M., Li W.A., Anastassacos F.M., Mooney D.J., Shih W.M. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 2017;8:15654. doi: 10.1038/ncomms15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung H.M., Chan M.S., Liu L.-S., Wong S.W., Lo T.W., Lau C.-H., Tin C., Lo P.K. Dual-function, cationic, peptide-coated nanodiamond systems: Facilitating nuclear-targeting delivery for enhanced gene therapy applications. ACS Sustain. Chem. Eng. 2018;6:9671–9681. doi: 10.1021/acssuschemeng.8b00446. [DOI] [Google Scholar]
- 23.Niu J.-Z., Shen G.-M., Christiaens O., Smagghe G., He L., Wang J.-J. Beyond insects: Current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag. Sci. 2018;74:2680–2687. doi: 10.1002/ps.5071. [DOI] [PubMed] [Google Scholar]
- 24.Li J.-H., Qian J., Xu Y.-Y., Yan S., Shen J., Yin M.-Z. A facile-synthesized star polycation is constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019;7:6316–6322. doi: 10.1021/acssuschemeng.9b00004. [DOI] [Google Scholar]
- 25.Ma Z.-Z., Zheng Y., Chao Z.-J., Chen H.-T., Zhang Y.-H., Yin M.-Z., Shen J., Yan S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022;20:124. doi: 10.1186/s12951-022-01336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan S., Qian J., Cai C., Ma Z.-Z., Li J.-H., Yin M.-Z., Ren B.-Y., Shen J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2019;93:449–459. doi: 10.1007/s10340-019-01157-x. [DOI] [Google Scholar]
- 27.Ma Z.-Z., Zhang Y.-H., Li M.-S., Chao Z.-J., Du X.-G., Yan S., Shen J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae control. J. Pest Sci. 2022;95:1–13. doi: 10.1007/s10340-022-01485-5. [DOI] [Google Scholar]
- 28.Zhang Y.-H., Ma Z.-Z., Zhou H., Chao Z.-J., Yan S., Shen J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022;29:669–682. doi: 10.1111/1744-7917.12953. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y., Hu Y.-S., Yan S., Zhou H., Song D.-L., Yin M.-Z., Shen J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019;75:1993–1999. doi: 10.1002/ps.5313. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Yan S., Li M.-J., Wang Y., Shi X.-Y., Liang P., Yin M.-Z., Shen J., Gao X.-W. Nanodelivery System Alters an Insect Growth Regulator’s Action Mode: From Oral Feeding to Topical Application. ACS Appl. Mater. Interfaces. 2022;14:65105–65113. doi: 10.1021/acsami.2c08239. [DOI] [PubMed] [Google Scholar]
- 31.Li M.-S., Ma Z.-Z., Peng M., Li L., Yin M.-Z., Yan S., Shen J. A gene and drug co-delivery application helps to solve the short life disadvantage of RNA drug. Nano Today. 2022;43:101452. doi: 10.1016/j.nantod.2022.101452. [DOI] [Google Scholar]
- 32.Jiang Q.-H., Xie Y.-H., Peng M., Wang Z.-J., Li T.-H., Yin M.-Z., Shen J., Yan S. A nanocarrier pesticide delivery system with promising benefits in the case of dinotefuran: Strikingly enhanced bioactivity and reduced pesticide residue. Environ. Sci. Nano. 2022;9:988–999. doi: 10.1039/D1EN00752A. [DOI] [Google Scholar]
- 33.Dong M., Chen D.-M., Che L., Gu N., Yin M.-Z., Du X.-G., Shen J., Yan S. Biotoxicity evaluation of a cationic star polymer on a predatory ladybird and cooperative pest control by polymer-delivered pesticides and ladybird. ACS Appl. Mater. Interfaces. 2022;14:6083–6092. doi: 10.1021/acsami.1c24077. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S., Nehra M., Dilbaghi N., Marrazza G., Hassan A.A., Kim K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release. 2019;294:131–153. doi: 10.1016/j.jconrel.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z.-H., Cai Q., Yan S., Yang S.-Y., Lu Q., Wang E.-D., Zhang B., Lv J.-L., Xu X.-N. Molecular characterization, expression, and function of Vitellogenin genes in Phytoseiulus persimilis. Exp. Appl. Acarol. 2022;86:343–356. doi: 10.1007/s10493-022-00698-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X.-X., Lv J.-L., Hu Y., Wang B.-M., Chen X., Xu X.-N., Wang E.-D. Prey Preference and Life Table of Amblyseius orientalis on Bemisia tabaci and Tetranychus cinnabarinus. PLoS ONE. 2015;10:e0138820. doi: 10.1371/journal.pone.0138820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasanthakumar T., Rubinstein J.L. Structure and Roles of V-type ATPases. Trends Biochem. Sci. 2020;45:295–307. doi: 10.1016/j.tibs.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Wieczorek H., Beyenbach K.W., Huss M., Vitavska O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009;212:1611–1619. doi: 10.1242/jeb.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Arrese E.L., Soulages J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flatt T. Paying the costs of reproduction. Elife. 2015;4:e09556. doi: 10.7554/eLife.09556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruhland F., Pétillon J., Trabalon M. Physiological costs during the first maternal care in the wolf spider Pardosa saltans (Araneae, Lycosidae) J. Insect Physiol. 2016;95:42–50. doi: 10.1016/j.jinsphys.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Laing J.E. Life history and life table of Phytoseiulus persimilis Athias-Henriot. Acarologia. 1968;10:578–588. [PubMed] [Google Scholar]
- 44.Ibrahim A.B., Monteiro T.R., Cabral G.B., Aragão F.J.L. RNAi-mediated resistance to whitefly (Bemisia tabaci) in genetically engineered lettuce (Lactuca sativa) Transgenic Res. 2017;26:613–624. doi: 10.1007/s11248-017-0035-0. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T., Nunes M.A., España M.U., Namin H.H., Jin P., Bensoussan N., Zhurov V., Rahman T., De Clercq R., Hilson P., et al. RNAi-based reverse genetics in the chelicerate model Tetranychus urticae: A comparative analysis of five methods for gene silencing. PLoS ONE. 2017;12:e0180654. doi: 10.1371/journal.pone.0180654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooper S.D., Boué S., Krause R., Jensen L.J., Mason C.E., Ghanim M., White K.P., Furlong E.E., Bork P. Identification of tightly regulated groups of genes during Drosophila melanogaster embryogenesis. Mol. Syst. Biol. 2007;3:72. doi: 10.1038/msb4100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cusanovich D.A., Reddington J.P., Garfield D.A., Daza R.M., Aghamirzaie D., Marco-Ferreres R., Pliner H.A., Christiansen L., Qiu X., Steemers F.J., et al. The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature. 2018;555:538–542. doi: 10.1038/nature25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donoughe S. Insect egg morphology: Evolution, development, and ecology. Curr. Opin. Insect Sci. 2022;50:100868. doi: 10.1016/j.cois.2021.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available on request from the corresponding authors.