Abstract
Leishmaniasis is one of the most neglected tropical diseases that present areal public health problems worldwide. Chemotherapy has several limitations such as toxic side effects, high costs, frequent relapses, the development of resistance, and the requirement for long-term treatment. Effective vaccines or drugs to prevent or cure the disease are not available yet. Therefore, it is important to dissect antileishmanial molecules that present selective efficacy and tolerable safety. Several studies revealed the antileishmanial activity of medicinal plants. Several organic extracts/essential oils and isolated natural compounds have been tested for their antileishmanial activities. Therefore, the aim of this review is to update and summarize the investigations that have been undertaken on the antileishmanial activity of medicinal plants and natural compounds derived, rom plants from January 2015 to December 2021. In this review, 94 plant species distributed in 39 families have been identified with antileishmanial activities. The leaves were the most commonly used plant part (49.5%) followed by stem bark, root, and whole plant (21.9%, 6.6%, and 5.4%, respectively). Other plant parts contributed less (<5%). The activity was reported against amastigotes and/or promastigotes of different species (L. infantum, L. tropica, L. major, L. amazonensis, L. aethiopica, L. donovani, L. braziliensis, L. panamensis, L. guyanensis, and L. mexicana). Most studies (84.2%) were carried out in vitro, and the others (15.8%) were performed in vivo. The IC50 values of 103 plant extracts determined in vitro were in a range of 0.88 µg/mL (polar fraction of dichloromethane extract of Boswellia serrata) to 98 µg/mL (petroleum ether extract of Murraya koenigii). Among the 15 plant extracts studied in vivo, the hydroalcoholic leaf extract of Solanum havanense reduced parasites by 93.6% in cutaneous leishmaniasis. Voacamine extracted from Tabernaemontana divaricata reduced hepatic parasitism by ≈30 times and splenic parasitism by ≈15 times in visceral leishmaniasis. Regarding cytotoxicity, 32.4% of the tested plant extracts against various Leishmania species have a selectivity index higher than 10. For isolated compounds, 49 natural compounds have been reported with anti-Leishmania activities against amastigotes and/or promastigotes of different species (L. infantum, L. major, L. amazonensis, L. donovani and L. braziliensis). The IC50 values were in a range of 0.2 µg/mL (colchicoside against promastigotes of L. major) to 42.4 µg/mL (dehydrodieuginol against promastigotes of L. amazonensis). In conclusion, there are numerous medicinal plants and natural compounds with strong effects (IC50 < 100 µg/mL) against different Leishmania species under in vitro and in vivo conditions with good selectivity indices (SI > 10). These plants and compounds may be promising sources for the development of new drugs against leishmaniasis and should be investigated in randomized clinical trials.
Keywords: Leishmania, medicinal plant, natural product, neglected tropical disease, phytotherapy, pharmacognosy, promastigotes
1. Introduction
Leishmaniasis is a group of diseases caused by protozoa parasites from more than 20 Leishmania species. In 2018, 92 countries and 83 territories were considered endemic for Leishmania species or had previously reported cases of cutaneous and visceral leishmania, respectively. Today, more than 1 billion people live in areas endemic to leishmaniasis and are at risk of infection. An estimated 30,000 new cases of visceral leishmania and more than 1 million new cases of cutaneous leishmania occur annually [1]. The parasite is categorized into two main groups: Old World leishmaniasis, which is endemic in Africa, Asia, the Mediterranean, and the Middle East. Leishmania tropica, L. major, L. aethiopica, and L. donovani are the four common species causing Old World leishmaniasis. New World leishmaniasis is caused by L. mexicana, L. amazonensis, L. braziliensis, L. panamensis, L. peruviana, L. guyanensis, L. pifanoi, L. venezuelensis, L. shawi, and L. lainsoni [2]. There are three clinical forms of leishmaniasis in humans: namely, cutaneous, mucocutaneous and visceral leishmaniasis. Cutaneous leishmaniasis is a less severe form of the disease which usually manifests in self-healing ulcers. Mucocutaneous leishmaniasis results in disfiguring lesions of mucous membranes in the nose, mouth, and throat. Visceral leishmaniasis is the most severe form of the disease which can result in 95% mortality of infected patients if not treated [3].
In 2020, more than 90% of new cases of visceral leishmaniasis reported to the WHO occurred in Bangladesh, Brazil, China, Ethiopia, Eritrea, India, Kenya, Somalia, South Sudan, Sudan, and Yemen [1]. Over 90% of mucocutaneous leishmaniasis occurred in Bolivia, Brazil, Ethiopia, and Peru, and more than 85% of cutaneous leishmaniasis cases appeared in Afghanistan, Algeria, Brazil, Colombia, Iran, Libya, Pakistan, Peru, Syria, and Tunisia [1]. Depending on the stage of its life cycle, the parasite exhibits two morphological forms in its life cycle: The amastigotes in macrophages of the mammalian host and the promastigotes in the gut of the sand fly vectors. The life cycle of the Leishmania parasite starts if a parasitized female sand fly takes a blood meal from a vertebrate host to produce its eggs. As the sand fly feeds, infective promastigotes enter the vertebrate host via the insect’s proboscis. The promastigotes are then phagocytosed by macrophages which they transform into amastigotes and reproduce by binary fission. They increase in number until the cell eventually bursts and then infects other phagocytic cells to continue the cycle [4]. Over the years, a number of drugs have been employed for the treatment of leishmaniasis. A brief account of the mechanism of action and mode of administration of these drugs has been presented in Table 1 [5].
Table 1.
Drugs used for the treatment of leishmaniasis.
| Name of the Drug |
Mode of Action | Mode of Administration |
Adverse Effects |
|---|---|---|---|
| Pentavalent antimonials |
Inhibition of glycolysis and β-oxidation of fatty acids of parasite | Intralesional for CL, Parenteral | Abdominal pain, erythema, nausea, toxicity (hepatic, pancreas, renal, muscular, and skeletal cardiothrombocytopenia or leukopenia) |
| Amphotericin B | Binding to parasite’s membrane sterols and changing its permeability selective to K+ and Mg2+ | Liposomal formulations, Deoxycholate formulations |
Fever, nausea, hypokalemia, anorexia, leukopenia, kidney failure, and heart problems |
| Pentamidine | Interferes with DNA synthesis and modifies the morphology of kinetoplast | Parenteral, Intramuscular administration |
Pain, nausea, vomiting, dizziness, myalgia, hypertension, headache, hypoglycemia, and transient hyperglycemia |
| Miltefosine | Associated with phospholipid biosynthesis and alkyl-lipid metabolism in leishmania | Oral for VL | Nausea, vomiting, diarrhea, and raised creatinine |
| Paromomycin | Inhibition of protein biosynthesis in sensitive organism | Topical for CL Parenteral for VL |
Erythema, pain, edema, and ototoxicity (damage to the internal ear) |
Latest developments in the prevention and treatment regarding a permanent solution for leishmaniasis in terms of successful human vaccination is still a major challenge. However, there are different vaccinations currently being tested in mouse models. One of them uses “killed but metabolically active” parasites to induce host immune system reaction. Using salivary peptides of the sandfly holds the potential to be used as a vaccine component. However, the complex immune response makes it a challenge [6]. Macrophage-targeted drug delivery systems are another novel approach to directly affect Leishmania parasites that live in the macrophages. As getting into macrophages is a challenge, liposomes, microspheres, nanoparticles, and carbon nanotubes are some of the various drug carriers that are studied to target macrophages. In addition, the use of specific receptors expressed by macrophages to actively deliver a drug is also used [7].
The current treatment by chemical drugs has several limitations such as toxic side effects, high costs, frequent relapses, the development of resistance, and the requirement for long-term treatment [8,9]. Thus, investments in novel drug development against this parasitic disease may be a risky affair. Medicinal plants are centuries-old sources in the various traditional herbal medicine systems of the world. For instance, their importance lies in the fact that the WHO concludes that about 80% of the world’s population relies on them for primary health care [10]. Moreover, 25 to 50% of the pharmacopeias worldwide contain plant products and drugs derived from natural products [11]. Therefore, current research approaches for the treatment of leishmaniasis should largely consider medicinal plants as an important area of search.
The aim of this review is to update and summarize the investigations that have been undertaken on the antileishmanial activity of medicinal plants and natural compounds derived from plants from January 2015 to December 2021.
2. Results
As shown in Table 2, 92 plant species distributed in 39 families have been identified with anti-Leishmania activities. The family Fabaceae accounted for the highest percentage (9.7%) followed by Asteraceae (7.6%). Lamiaceae and Solanaceae account for 6.5% each.
Table 2.
Botanical characteristics of the medicinal herbs in the present study.
| No | Family Name | Scientific Name | Part Used |
|---|---|---|---|
| 1. | Anacardiaceae | Pistacia lentiscus | Leaves |
| Schinus terebinthifolia | Fruits | ||
| Schinus molle | Leaves | ||
| Spondias mombin | Leaves | ||
| 2. | Annonaceae | Annona senegalensis | Stem bark |
| Bocageopsis multiflora | Leaves | ||
| Guatteria latifolia | Branch | ||
| Cleistopholis patens | Stem bark | ||
| 3. | Apiaceae | Ferula communis | Whole plant |
| 4. | Apocynaceae | Tabernaemontana divaricata | Voacamine |
| Mondia whitei | Roots | ||
| Pentalinon andrieuxii | Pentalinon sterol | ||
| 5. | Araliaceae | Oreopanax floribundus | Leaves |
| 6. | Arecaceae | Phoenix dactylifera | Kernel and date fruit |
| 7. | Asteraceae | Acanthospermum hispidum | Whole plant |
| Tessaria integrifolia | Leaves | ||
| Abuta grandifolia | Leaves | ||
| Cynara scolymus | Leaves | ||
| Artemisia absinthium | Leaves | ||
| Artemisia campestris | Leaves | ||
| Artemisia herba-alba | Aerial parts, Leaves | ||
| Bidens pilosa | Whole plant | ||
| Tessaria integrifolia | Whole plant | ||
| 8. | Balanophoracea | Thonningia sanguinea | Whole plant |
| 9. | Bignoniaceae | Handroanthus serratifolius | Lapachol |
| Jacaranda glabra | Bark | ||
| 10. | Burseraceae | Boswellia serrata | Resin |
| 11. | Cannabaceae | Celtis australis | Leaves |
| 12. | Capparaceae | Capparis spinosa | Fruits |
| 13. | Cistaceae | Citrus sinensis | Leaves |
| 14. | Combretaceae | Terminalia ivorensis | Leaves |
| 15. | Cupressaceae | Juniperus excelsa | Leaves, fruits |
| 16. | Ericaceae | Arbutus unedo | Leaves |
| Erica arborea | Flower | ||
| 17. | Euphorbiaceae | Bridelia ferruginea | Leaves |
| Ejije bidu | Leaves | ||
| Croton caudatus | Leaves | ||
| 18. | Fabaceae | Afzelia africana | Stem bark |
| Baphia nitida | Stem bark | ||
| Cassia alata | Leaves | ||
| Cassia gloca | Leaves | ||
| Cassia sieberiana | Roots, leaves | ||
| Prosopis laevigata | Leaves | ||
| Parkia clappertoniana | Stem bark, leaves | ||
| Tamarindus indica | Leaves | ||
| Prosopis juliflora | Leaves | ||
| 19. | Gentianaceae | Anthocleista nobilis | Leaves, stem bark, root |
| Centaurium erythraea | Flowering, stems | ||
| 20. | Lamiaceae | Marrubium vulgare | Leaves |
| Mentha pulegium | Leaves | ||
| Otostegia integrifolia | Whole plant | ||
| Rosmarinus officinalis | Leaves | ||
| Salvia clandestina | Aerial parts | ||
| Vitex fosteri | Stem bark, leaves | ||
| 21. | Lauraceae | Aniba riparia | Fruits |
| Persea ferruginea | Leaves | ||
| Cinnamomum cassia | Bark | ||
| 22. | Loranthaceae | Loranthus europaeus | Aerial part |
| 23. | Malvaceae | Ceiba pentandra | Stem bark |
| Cola acuminata | Stem bark | ||
| Cola cordifolia | Stem bark, leaves | ||
| Glyphaea brevis | Leaves | ||
| 24. | Marantaceae | Thalia geniculata | Roots |
| Iresine diffusa | Flower | ||
| 25. | Meliaceae | Khaya grandifoliola | Stem bark |
| Cedrela spp | Bark | ||
| Azadirachta indica | Leaves | ||
| 26. | Moraceae | Treculia africana | Stem bark |
| Ficus capensis | Stem bark, leaves | ||
| 27. | Myrtaceae | Eugenia uniflora | Leaves, seed |
| 28. | Ochnaceae | Lophira lanceolata | Stem bark, roots |
| 29. | Olacaceae | Ximenia americana | Stem and twigs |
| 30. | Papaveraceae | Argemone mexicana | Aerial parts |
| 31. | Piperaceae | Piper pseudoarboreum | Leaves |
| 32. | Rhamnaceae | Ziziphus spina-christi | Whole plant |
| 33. | Rosaceae | Pyrus communis | Leaves |
| Pyrus pashia | Leaves | ||
| Prunus armeniaca | Leaves | ||
| Eryobotrya japonica | Leaves | ||
| 34. | Rubiaceae | Mitragyna inermis | Stem bark, leaves |
| Psychotria buhitenii | Leaves | ||
| 35. | Rutaceae | Zanthoxylum zanthoxyloides | Roots, stem bark |
| Murraya koenigii | Stem bark | ||
| Clausena anisata | Roots | ||
| 36. | Scrophulariaceae | Scoparia dulcis | Aerial part |
| Licania salicifolia | Leaves | ||
| 37. | Solanaceae | Solanum havanense | Leaves |
| Solanum lycocarpum | Leaves | ||
| Solanum myriacanthum | Leaves | ||
| Solanum nudum | Leaves | ||
| Physalis angulata | Flowers | ||
| Solanum seaforthianum | Leaves | ||
| 38. | Urticaceae | Urtica dioica | Leaves |
| 39. | Verbenaceae | Lantana camara | Leaves |
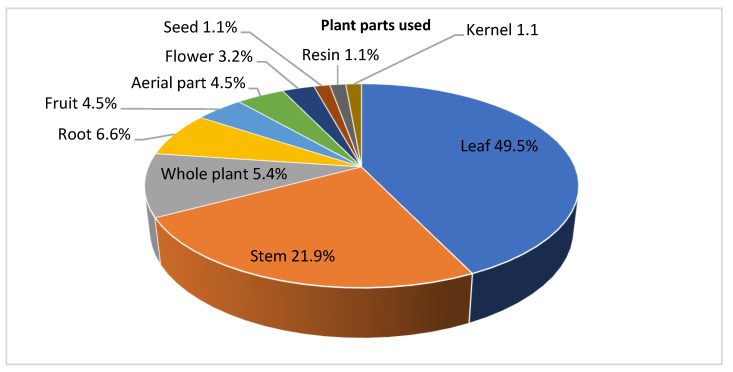
The leaves were the most commonly used plant part as compared to other parts (49.5%) followed by stem bark, roots, and whole plant (21.9%, 6.6%, and 5.4%, respectively). Aerial parts and fruits accounted for 4.5% each. Other plant parts (flowers, seeds, resins, branches, and kernels) contributed less (<4%) (Figure 1).
Figure 1.
Fraction of plant parts used in anti-Leishmania studies.
With respect to the test methods, 84.2% of studies were carried in vitro, while 15.8% of them were performed using in vivo assays (Table 3 and Table 4). For in vitro assay, 80 medicinal plants were screened in vitro for antileishmanial activities against different Leishmania species (L. infantum, L. tropica, L. major, L. amazonensis, L. aethiopica, L. donovani, L. braziliensis, L. panamensis, L. guyanensis, and L. mexicana) and life cycle forms (amastigotes and/or promastigotes). The IC50 value of 103 plant extracts/essential oils determined in vitro was in a range of 0.88 µg/mL (polar fraction of dichloromethane extract of Boswellia serrata) to 98 µg/mL (petroleum ether extract of Murraya koenigii) (Table 3). B. serrata (resins), R. officinalis (leaves), A. riparia (fruits), M. pulegium (leaves) as extracts had strong anti-Leishmania activity (0.88, 1.2, 1.3, and 1.3 µg/mL, respectively).
Table 3.
Anti-Leishmania activity of medicinal plants in vitro.
| No. | Scientific Name | Organism | Stage | Part Used | Most Active Extract/ Essential Oil |
IC50 (µg/mL) | Bioactive Compounds |
Data Analysis (Activity) |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. |
Abuta
grandifolia |
L. amazonensis | Promastigotes | Leaves | Ethanol | 38.1 | Alkaloids, triterpenes, saponins | Moderate | [12] |
| L. braziliensis | 31.1 | Moderate | |||||||
| 2. | Acanthospermum hispidum | L. donovani | Promastigotes | Whole plant | 50% aqueous ethanol | 32.10 | Essential oil, alkaloids | Moderate | [13] |
| 3. | Afzelia africana | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 77.10 | Alkaloids, tannins, flavonoids, saponins |
Weak | [13] |
| 4. | Aniba riparia |
L. amazonensis
|
Amastigotes | Fruits | 50% aqueous ethanol | 1.30 | Riparin E | High | [14] |
| Promastigotes | 4.70 | High | |||||||
| 5. |
Annona
senegalensis |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 10.80 | Alkaloids, tannins, flavonoids, saponins, terpenoids, glycosides |
Moderate | [13] |
| Stem bark | 27.80 | Moderate | |||||||
| 6. |
Anthocleista
nobilis |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 41.50 | Glycosides, saponins, steroids | Moderate | [13] |
| Root | 79.0 | Anthocleistol | Weak | ||||||
| 7. | Arbutus unedo | L. infantum | Promastigotes | Leaves | n-Hexane | 64.05 | Phenolics, flavonoids | Weak | [15] |
| L. tropica | 79.57 | Weak | |||||||
| 8. |
Argemone
mexicana |
L. donovani | Promastigotes | Aerial part | Petroleum ether | 50.0 | - | Moderate | [16] |
| 9. |
Artemisia
absinthium |
L. major | Promastigotes | Leaves | Hydrodistillation | 1.49 | Essential oil | High | [17] |
| 10. |
Artemisia
campestris |
L. major | Promastigotes | Leaves | Hydrodistillation | 2.20 | Essential oil | High | [17] |
| 11. | Artemisia herba-alba | L. major | Promastigotes | Leaves | Hydrodistillation | 1.20 | Essential oil | High | [17] |
| 12. | Artemisia herba-alba | L. infantum. | Amastigote | Aerial part | Methanol extract | 68.25 | - |
Weak | [18] |
| L. major | 37.87 | Moderate | |||||||
| L. infantum | Promastigotes | 77.97 | Weak | ||||||
| L. major | 55.21 | Weak | |||||||
| 13. |
Azadirachta
indica |
L. infantum | Amastigotes | Leaves | Oil | 15.3 | Phenolics, flavonoids |
Moderate | [19] |
| L. tropica | 17.6 | Moderate | |||||||
| 14. | Baphia nitida | L. donovani | Promastigotes | Stem-bark | 50% aqueous ethanol | 34.40 | Tannins, flavonoids, saponins, glycosides | Moderate | [13] |
| 15. | Bidens pilosa | L. donovani | Promastigotes | Whole plant | 50% aqueous ethanol | 28.90 | Essential oil, flavonoids, alkaloids, saponins, triterpenes | Moderate | [13] |
| 16. | Bocageopsis multiflora | L. amazonensis | Promastigotes |
Leaves | Ethanol | 37.9 | Essential oil, alkaloids | Moderate | [12] |
| L. braziliensis | 19.1 | Moderate | |||||||
| 17. |
Boswellia
serrata |
L. donovani | Amastigotes | Resin | Polar fractions of dichloromethane |
0.88 | Boswellic acids | High | [20] |
| 18. |
Bridelia
ferruginea |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 16.50 | Flavonoids, tannins, triterpenoids | Moderate | [13] |
| 19. |
Capparis
spinosa |
L. tropica | Promastigotes | Fruits | Methanol | 44.6 | Tannins, alkaloids, saponins, terpenoids, glycosides |
Moderate | [21] |
| Aqueous | 28.5 | Moderate | |||||||
| 20. | Cassia alata | L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 10.10 | Flavonoids, glycosides | Moderate | [22] |
| 21. | Cassia gloca | L. tropica | Promastigotes | Leaves | Methanol | 9.62 | Flavonoids | High | [22] |
| 22. |
Cassia
sieberiana |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 62.90 | Flavonoids, alkaloids | Weak | [23] |
| 23. | Cedrela spp. | L. amazonensis | Promastigotes | Bark | Ethanol | 36.8 | Sesquiterpenes, triterpenes |
Moderate | [22] |
| L. braziliensis | 18.2 | Moderate | |||||||
| 24. | Ceiba pentandra | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 31.10 | Isoflavones, sesquiterpenoids |
Moderate | [13] |
| 25. |
Centaurium
erythraea |
L. tropica | Promastigotes | Flowering stems | n-Hexane | 37.20 | Phenolics, flavonoids | Moderate | [23] |
| L. major | 64.52 | Weak | |||||||
| 26. | Celtis australis | L. tropica | Promastigotes | Leaves | Methanol | 69.13 | Flavonoids | Weak | [22] |
| 27. | Cistus crispus | L. major | Promastigotes | Leaves | Methanol | 84.29 | Phenolics, flavonoids | Weak | [15] |
| L. infantum | n-Hexane | 82.39 | Weak | ||||||
| L. tropica | 96.82 | Weak | |||||||
| L. major | 47.29 | Moderate | |||||||
| 28. | Citrus sinensis | L. tropica | Promastigotes | Leaves | Methanol | 12.27 | Flavonoids | Moderate | [22] |
| 29. | Cola acuminata | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 47.80 | Purine alkaloids, catechins, (tannins) |
Moderate | [13] |
| 30. | Cola cordifolia | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 25.10 | Tannins, phenolics | Moderate | [13] |
| Leaves | 18.20 | Moderate | |||||||
| 31. |
Clausena
anisata |
L. donovani | Promastigotes | Roots | 50% aqueous ethanol | 12.10 | Essential oil, indole alkaloids, coumarins |
Moderate | [13] |
| 32. | Cleistopholis patens | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 60.20 | Flavonoids, saponins, alkaloids | Weak | [13] |
| 33. |
Croton
caudatus |
L. donovani | Promastigotes | Leaves | Ethyl acetate –hexane (9:1) | 10.0 | Terpenoids | High | [23] |
| Amastigote | 2.5 | High | |||||||
| 34. |
Cynara
scolymus |
L. tropica | Promastigotes | Stem leaf | Ethanol | 80.0 | - | Weak | [24] |
| 35. | Ejije bidu | L. amazonensis | Promastigotes | Leaves | Ethanol | 17.8 | - | Moderate | [12] |
| L. braziliensis | 13.3 | Moderate | |||||||
| 36. | Erica arborea | L. major | Promastigotes | Flower | Methanol | 43.98 | - | Moderate | [18] |
| L. infantum. | 61.27 | Weak | |||||||
| L. major | Amastigotes | 36 | Moderate | ||||||
| L. infantum. | 53.93 | Weak | |||||||
| 37. |
Eryobotrya
japonica |
L. tropica | Promastigotes | Leaves | Methanol | 10.59 | Flavonoids | Moderate | [22] |
| 38. |
Eugenia
uniflora |
L. amazonensis | Amastigotes | Leaves | n-Hexane | 9.20 | Sesquiterpenes, flavonoids |
High | [25] |
| L. donovani | Promastigotes | Seeds | 50% aqueous ethanol | 26.60 | Essential oil, flavonoids, tannins | Moderate | [13] | ||
| 39. |
Ferula
communis |
L. aethiopica | Promastigotes | Whole parts | 80% methanol | 11.38 | Phenolics, flavonoids | Moderate | [26] |
| L. donovani | 23.41 | Moderate | |||||||
| L. aethiopica | Amastigotes | 14.32 | Moderate | ||||||
| L. donovani | 31.12 | Moderate | |||||||
| 40. | Ficus capensis | L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 37.0 | Alkaloids, phenolics, flavonoids | Moderate | [13] |
| Leaves | 88.90 | Weak | |||||||
| 41. | Glyphaea brevis | L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 43.40 | Tannins, alkaloids, flavonoids |
Moderate | [13] |
| 42. |
Guatteria
Latifolia |
L. amazonensis | Promastigote | Branch | n-hexane fraction of ethanol | 51.7 | Alkaloids | Weak | [27] |
| 43. | Iresine diffusa | L. amazonensis | Promastigotes |
Flower | Ethanol | 30.5 | Sesquiterpenes, triterpenes |
Moderate | [12] |
| L. braziliensis | 11.1 | Moderate | |||||||
| 44. |
Jacaranda
Glabra |
L. amazonensis | Promastigotes | Bark | Ethanol | 29.8 | - | Moderate | [12] |
| L. braziliensis | 17.4 | Moderate | |||||||
| 45. |
Khaya
grandifolia |
L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 43.20 | Alkaloids, saponins, tannins | Moderate | [13] |
| 46. | Lantana camara | L. amazonensis | Amastigotes | Leaves | Dichloromethane | 21.8 | Terpenoids | Moderate | [28] |
| 47. |
Licania
Salicifolia |
L. panamensis | Amastigotes | Leaves | Ethyl acetate | 9.8 | Triterpenes, flavonoids | High | [29] |
| 48. |
Lophira
lanceolata |
L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 68.60 | Flavonoids, saponins, alkaloids | Weak | [13] |
| Roots | 66.0 | Alkaloids | Weak | ||||||
| 49. | Marrubium vulgare | L. infantum | Amastigotes | Leaves | Methanol | 18.64 | - | Moderate | [18] |
| L. major | 32.15 | Moderate | |||||||
| L. infantum | Promastigotes | 35.63 | Moderate | ||||||
| L. major | 45.84 | Moderate | |||||||
| 50. | Mentha pulegium | L. infantum | Promastigotes | Leaves | Essential oil | 2.0 | Menthone, pulegone | High | [30] |
| L. tropica | 2.2 | High | |||||||
| L. major | 1.30 | High | |||||||
| 51. |
Mitragyna
Inermis |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 21.90 | Indole alkaloids, triterpenoids | Moderate | [13] |
| Stem bark | 28.0 | Moderate | |||||||
| 52. | Mondia whitei | L. donovani | Promastigotes | Roots | 50% aqueous ethanol | 31.0 | Glycosides | Moderate | [13] |
| 53. |
Murraya
koenigii |
L. donovani | Promastigotes | Stem | Petroleum ether | 98.0 | - | Weak | [16] |
| 54. |
Oreopanax
floribundus |
L. panamensis | Amastigotes | Leaves | Dichloromethane | 24.6 | Triterpenes | Moderate | [29] |
| Ethyl acetate | 23.7 | Triterpenes, flavonoids | Moderate | ||||||
| 55. |
Otostegia
integrifolia |
L. aethiopica | Promastigotes Amastigotes |
Whole parts | 80% methanol | 13.03 | Phenolics, flavonoids | Moderate | [31] |
| L. donovani | 17.24 | Moderate | |||||||
| L. aethiopica | 16.84 | Moderate | |||||||
| L. donovani | 14.55 | Moderate | |||||||
| 56. |
Parkia
clappertoniana |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 17.0 | Saponins, flavonoids, Tannins | Moderate | [13] |
| Stem bark | 17.60 | Saponins, steroids, triterpenes | Moderate | ||||||
| 57. | Persea ferruginea | L. panamensis | Amastigotes | Leaves | Ethyl acetate | 25.5 | Triterpenes, leucoanthocyanidins, coumarins | Moderate | [29] |
| 58. |
Phoenix
dactylifera |
L. major | Promastigotes | kernel | Methanol | 23.0 | Gallic acid | Moderate | [32] |
| 59. |
Physalis
angulata |
L. amazonensis | Promastigotes | Flower | Ethanol | 17.6 | Terpenes, phenolic acids, flavonoids |
Moderate | [12] |
| L. braziliensis | 43.5 | Moderate | |||||||
| 60. |
Piper
pseudoarboreum |
L. amazonensis | Promastigotes |
Leaves | Ethanol | 31.4 | Alkamides | Moderate | [33] |
| L. braziliensis | 21.3 | Moderate | |||||||
| L. guyanesis | 41.3 | Moderate | |||||||
| L. infantum | 32.3 | Moderate | |||||||
| 61. |
Pistacia
lentiscus |
L. infantum | Promastigotes | Leaves | Essential oil | 11.28 | Myrcene, α-pinene | Moderate | [23] |
| L. tropica | 23.50 | Moderate | |||||||
| L. major | 17.52 | Moderate | |||||||
| L. infantum | Fruits | Essential oil | 8.0 | Limonene α-pinene |
High | ||||
| L. tropica | 26.20 | Moderate | |||||||
| L. major | 21.42 | Moderate | |||||||
| 62. |
Rosmarinus
officinalis |
L. infantum | Promastigotes | Leaves | Essential oil | 1.20 | α-Pinene, 1,8-cineole, borneol | High | [23] |
| L. tropica | 3.50 | High | |||||||
| L. major | 2.60 | High | |||||||
| 63. |
Prosopis
juliflora |
L. donovani | Promastigotes | Leaves | Methanol | 3.12 | Saponins, tannins, flavonoids, alkaloids |
High | [34] |
| 64. |
Prosopis
laevigata |
L. amazonensis | Amastigotes | Leaves | Aqueous | 35.2 | Alkaloids, anthraquinones |
Moderate | [28] |
| 65. |
Prunus
armeniaca |
L. tropica | Promastigotes | Leaves | Ethanol | 16.18 | Alkaloids, phenolics, tannins, flavonoids, terpenoids, coumarins |
Moderate | [35] |
| 66. | Psychotria buhitenii | L. panamensis | Amastigotes | Leaves | Dichloromethane | 21.5 | Triterpenes, flavonoids | Moderate | [29] |
| Ethyl acetate | 14.1 | Triterpenes, saponins, Coumarins, anthocyanins | Moderate | ||||||
| Ethanol | 29.4 | Saponins, phenolics, tannins, coumarins, anthocyanins |
Moderate | ||||||
| 67. |
Pyrus
communis |
L. tropica | Promastigotes | Leaves | Ethanol | 56.68 | Alkaloids, phenolics, tannins, flavonoids, terpenoids, quinones, saponins |
Weak | [35] |
| 68. | Pyrus pashia | L. tropica | Promastigotes | Leaves | Ethanol | 60.95 | Alkaloids, phenolics, tannins, flavonoids, terpenoids, quinones, saponins |
Weak | [35] |
| 69. |
Salvia
clandestina |
L. infantum | Promastigotes | Aerial part | n-Hexane | 14.11 | - | Moderate | [36] |
| L. infantum | Dichloromethane | 31.57 | Moderate | ||||||
| L. tropica | 33.77 | Moderate | |||||||
| L. major | 24.56 | Moderate | |||||||
| 70. | Schinus molle | L. amazonensis | Amastigotes | Leaves | Dichloromethane | 25.9 | Terpenoids | Moderate | [28] |
| Dichloromethane: Methanol (1:1) | 21.8 | Terpenoids, phenolics | Moderate | ||||||
| 71. |
Schinus
terebinthifolia |
L. amazonensis | Promastigotes | Fruits | n-Hexane | 13.90 | Triterpenes | Moderate | [29] |
| 72. | Scoparia dulcis | L. amazonensis | Promastigotes | Aerial part | Ethanol | 23.9 | Diterpenes, triterpenes, flavonoids | Moderate | [12] |
| L. braziliensis | 25.1 | Moderate | |||||||
| 73. | Spondias mombin | L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 81.50 | - | Weak | [13] |
| 74. |
Tamarindus
indica |
L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 58.12 | Phenolics, flavonoids | Weak | [13] |
| 75. | Terminalia ivorensis | L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 24.90 | Terminolic acid, quercetin, β-glycyrrhetinic acid |
Moderate | [13] |
| 76. |
Tessaria
integrifolia |
L. amazonensis | Promastigotes | Leaves | Ethanol | 54.20 | Sesquiterpenes, flavonoids |
Weak | [12] |
| L. braziliensis | 31.60 | Moderate | |||||||
| 77. |
Thalia
geniculata |
L. amazonensis | Promastigotes | Roots | Ethanol | 29.8 | Phytosterols | Moderate | [12] |
| L. braziliensis | 17.4 | Moderate | |||||||
| 78. | Thonningia sanguinea | L. donovani | Promastigotes | Whole plant | 50% aqueous ethanol | 18.60 | Alkaloids, tannins, flavonoids |
Moderate | [13] |
| 79. |
Treculia
africana |
L. donovani | Promastigotes | Stem bark | 50% aqueous ethanol | 44.80 | Catechin, cyanidin glycosides |
Moderate | [13] |
| 80. | Vitex fosteri | L. donovani | Promastigotes | Leaves | 50% aqueous ethanol | 72.40 | Essential oil, flavonoids | Weak | [13] |
| Stem bark | 49.80 | Moderate | |||||||
| 81. |
Ximenia
americana |
L. donovani | Promastigotes | Stem and twigs | 50% aqueous ethanol | 36.10 | Tannins, flavonoids, alkaloids |
Moderate | [13] |
| 82. | Zanthoxylum zanthoxyloides | L. donovani | Promastigotes | Roots | 50% aqueous ethanol | 13.50 | Alkaloids, tannins, flavonoids, essential oil |
Moderate | [13] |
| Stem bark | 45.20 | Moderate | |||||||
| 83. |
Ziziphus
spina-christi |
L. major | Amastigotes | Leaves | Methanol | 54.6 | Tannins, flavonoids, Glycosides, alkaloids, terpenoids | Moderate | [37] |
Table 4.
Anti-Leishmania activity of medicinal plants in vivo.
| No. | Plant Species | Leishmania Species | Route, Dose, and Scheme of Treatment | Efficacy | Bioactive Compounds | Reference |
|---|---|---|---|---|---|---|
| 1. | Cinnamomum cassia | Visceral leishmaniasis (L. donovani) | Oral: 100 mg/kg/d for 10 days | Reduction of hepatic parasitism by 80.9% and splenic parasitism by 82.9% | Cinnamaldehyde and its derivatives | [38] |
| 2. | Croton caudatus | Visceral leishmaniasis (L. donovani) | Oral: 5 mg/kg/d five consecutive days |
Reduction of hepatic parasitism by 65% and splenic parasitism by 69.1% | Terpenoids | [23] |
| 3. | Handroanthus serratifolius | Cutaneous leishmaniasis (L. amazonensis) |
Oral: 25 mg/kg/d for 10 days | 24.5-fold reduction of parasite number | Lapachol | [39] |
| Visceral leishmaniasis (L. infantum) | Reduction parasite number in spleen (4.6-fold) and liver (5.3-fold) | |||||
| 4. |
Loranthus
europaeus |
Cutaneous leishmaniasis (unspecific) | Topical: ointment (40%) once daily at bedtime for 6 h under occlusion for maximal 6 weeks | 79.0% cure rate without side effects | Flavonoids, alkaloids, glycosides, triterpenes, phenolic acids | [40] |
| 5. |
Pentalinon
andrieuxii |
Visceral leishmaniasis (L. donovani) | 2.5 mg/kg i.v. | Reduction of 64, 83, and 57% of parasites in the liver, spleen, and bone marrow. | Pentalinonsterol | [41] |
| 6. |
Piper
pseudoarboreum |
Cutaneous leishmaniasis (L. amazonensis) |
Intralesional: 25 mg/kg/d for 4 days | Reduction of skin lesions by 40% and visceralization by 55%. | (E)-Piplartine | [33] |
| 7. |
Prosopis
juliflora |
Visceral leishmaniasis (L. donovani) | Oral: 100 mg/kg/d for 21 days | 85.1% reduction of parasite number in spleen | Saponins, tannins, flavonoids, alkaloids |
[34] |
| 8. | Solanum havanense | Cutaneous leishmaniasis (L. amazonensis) |
Intralesional: 30 mg/kg every 4 days, 5 doses | 93.6% reduction of parasite number | Steroidal alkaloids, saponins, phenolics, triterpenes, coumarins |
[42] |
| 9. |
Solanum
lycocarpum |
Cutaneous leishmaniasis (L. mexicana) |
Topical: 10 μg/d for 6 weeks | 71.4% reduction of parasite number |
Alkaloids (solamargine, solasonine) | [43] |
| 10. |
Solanum
myriacanthum |
Cutaneous leishmaniasis (L. amazonensis). |
Intralesional: 30 mg/kg every 4 days, 5 doses | 56.8% reduction of parasite number | Steroidal alkaloids, saponins, phenolics, triterpenes, coumarins |
[42] |
| 11. |
Solanum
nudum |
Cutaneous leishmaniasis (L. amazonensis) |
Intralesional: 30 mg/kg every 4 days, 5 doses | 80% reduction of parasite number | Steroidal alkaloids, saponins, phenolics, triterpenes, coumarins |
[42] |
| 12. | Solanum seaforthianum | Cutaneous leishmaniasis (L. amazonensis) |
Intralesional: 30 mg/kg every 4 days, 5 doses | 49.9% reduction of parasites in treated animals | Steroidal alkaloids, saponins, phenolics, triterpenes, coumarins |
[42] |
| 13. | Tabernaemontana divaricata | Visceral leishmaniasis (L. donovani) | Intraperitoneal: 5 mg/kg twice a week for 3 weeks | Decreased the hepatic parasitism by ≈30 times and splenic parasitism by ≈15 times | Voacamine | [35] |
| 14. | Urtica dioica | Cutaneous leishmaniasis (L. major) |
Intramuscular and intralesional: 250 mg/kg for 10 weeks | Intralesional treatment reduced lesions more than amphotericin B (control) | - | [44] |
| 15. | Ziziphus spina-christi | Cutaneous leishmaniasis (L. major) | Topical: 100 and 200 mg/kg/d for 4 weeks | Reduction of lesion size by 6.4- and 8.6-fold | Tannins, flavonoids, glycosides, alkaloids, terpenoids | [37] |
For in vivo assay, among the 15 medicinal plants studied in vivo, the highest activity against cutaneous leishmaniasis was exhibited by the hydroalcoholic leaf extract of Solanum havanense, which reduced parasites by 93.6%, and the highest activity against visceral leishmaniasis was shown by the voacamine compound extracted from Tabernaemontana divaricata, which reduced the hepatic and splenic parasitism by ≈30 times and ≈15 times, respectively (Table 4). For cytotoxic activity, 32.4% of tested plant extracts have good cytotoxic activity with a selectivity index of SI > 10. (Table 5).
Table 5.
Cytotoxic activity and selectivity index of medicinal plants in the present study (p = promastigote; a = amastigote).
| No. | Plant Species |
Leishmania Species |
Part Used | Bioactive Extract/ Compounds |
Cytotoxicity (CC50 µg/mL) |
Selectivity Index (CC50/IC50) |
Reference |
|---|---|---|---|---|---|---|---|
| 1. | Abuta grandifolia | L. amazonensis p | Leaves | Ethanol | 15.2 | 0.4 | [12] |
| L. braziliensis p | 15.6 | 0.5 | |||||
| 2. | Acanthospermum hispidum | L. donovani p | Whole plant | 50% aqueous ethanol |
55.5 | 1.73 | [13] |
| 3. | Afzelia africana | L. donovani p | Stem bark | 50% aqueous ethanol |
232.8 | 3.02 | [13] |
| 4. | Aniba riparia | L. amazonensis a | Fruits | 50% aqueous ethanol |
50.6 | 38.9 | [14] |
| 5. |
Annona
senegalensis |
L. donovani p | Leaves | 50% aqueous ethanol |
273.5 | 25.32 | [13] |
| Stem bark | 127.9 | 4.60 | |||||
| 6. |
Anthocleista
nobilis |
L. donovani p | Leaves | 50% aqueous ethanol |
245.7 | 5.92 | [13] |
| Root | 716.5 | 9.07 | |||||
| 7. |
Argemone
mexicana |
L. donovani p | Aerial part | Petroleum ether | 52.1 | 0.95 | [16] |
| 8. |
Artemisia
absinthium |
L. major p | Leaves | Essential oils | 11.22 | 7.5 | [17] |
| 9. |
Artemisia
campestris |
L. major p | Leaves | Essential oils | 21.12 | 9.6 | [17] |
| 10. |
Artemisia
herba-alba |
L. major p | Leaves | Essential oils | 11.24 | 9.4 | [17] |
| 11. |
Artemisia
herba-alba |
L. major p | Aerial part | Methanol | 131.5 | 2.38 | [18] |
| L. infantum p | 131.5 | 1.86 | |||||
| 12. |
Azadirachta
indica |
L. infantum a | Leaves | Oil | 703.8 | 46 | [19] |
| L. tropica a | 721.6 | 41 | |||||
| 13. | Baphia nitida | L. donovani p | Stem bark | 50% aqueous ethanol |
990.7 | 28.8 | [13] |
| 14. | Bidens pilosa | L. donovani p | Whole plant | 50% aqueous ethanol |
192.8 | 6.67 | [13] |
| 15. |
Bridelia
ferruginea |
L. donovani p | Leaves | 50% aqueous ethanol |
392.9 | 23.81 | [13] |
| 16. |
Bocageopsis
multifolia |
L. amazonensis p | Leaves | Ethanol | 26.5 | 0.7 | [12] |
| L. braziliensis p | 26.7 | 1.4 | |||||
| 17. | Boswellia serrata | L. donovani a | Resin | Polar fractions of dichloromethane | 33 | 38 | [20] |
| 18. | Capparis spinosa | L. tropica p | Fruits | Methanol | 44.6 | 9.1 | [21] |
| Aqueous | 28.5 | 8.4 | |||||
| 19. | Cassia gloca | L. tropica p | Leaves | Methanol | 1030 | - | [22] |
| 20. | Cassia alata | L. donovani p | Leaves | 50% aqueous ethanol |
371.5 | 36.78 | [13] |
| 21. | Cassia sieberiana | L. donovani p | Leaves | 50% aqueous ethanol |
62.90 | 0.77 | [13] |
| 22. | Cedrela spp. | L. amazonensis p | Bark | Ethanol | 66.3 | 1.8 | [12] |
| L. braziliensis p | 67.4 | 3.7 | |||||
| 23. | Ceiba pentandra | L. donovani P | Stem bark | 50% aqueous ethanol |
160.7 | 3.32 | [13] |
| 24. | Celtis australis | L. tropica p | Leaves | Methanol | 1209 | - | [22] |
| 25. | Cinnamomum cassia | L. donovani a | Barks | Dichloromethane fraction | No cytotoxicity at 500 µg/mL | - | [38] |
| 26. | Citrus sinensis | L. tropica p | Leaves | Methanol | 1755 | - | [22] |
| 27. | Clausena anisata | L. donovani p | Roots | 50% aqueous ethanol |
29.2 | 24.23 | [13] |
| 28. |
Cleistopholis
patens |
L. donovani p | Stem bark | 50% aqueous ethanol |
214.9 | 3.57 | [13] |
| 29. | Cola cordifolia | L. donovani p | Stem bark | 50% aqueous ethanol |
465.6 | 18.55 | [13] |
| Leaves | 465.6 | 25.58 | |||||
| 30. | Cola acuminata | L. donovani p | Stem bark | 50% aqueous ethanol |
156.8 | 3.28 | [13] |
| 31. | Cynara scolymus | L. tropica p | Stem leaves | Ethanol | 40.0 | 4.96 | [24] |
| 32. | Ejije bidu | L. amazonensis p | Leaves | Ethanol | 133.5 | 7.5 | [12] |
| L. braziliensis p | 133.0 | 10 | |||||
| 33. | Erica arborea | L. major p | Flower | Methanol | 89.6 | 2.04 | [18] |
| L. infantum p | 89.6 | 1.46 | |||||
| 34. |
Eryobotrya
japonica |
L. tropica p | Leaves | Methanol | 1903 | - | [22] |
| 35. | Eugenia uniflora | L. amazonensis a | Leaves | n-Hexane | 50.5 | 3.6 | [25] |
| 36. | Eugenia uniflora | L. donovani p | Seed | 50% aqueous ethanol |
94.4 | 3.55 | [13] |
| 37. | Ferula communis | L. aethiopica a, | Aerial part | 80% methanol | 175.22 | - | [26] |
| L. donovani a, | |||||||
| 38. | Ficus capensis | L. donovani p | Stem bark | 50% aqueous ethanol |
56.6 | 1.53 | [13] |
| Leaves | 257.8 | 2.90 | |||||
| 39. | Glyphaea brevis | L. donovani p | Leaves | 50% aqueous ethanol |
962.2 | 22.17 | [13] |
| 40. | Handroanthus serratifolius | L. amazonensis p | Lapachol | Lapachol | 3405.8 | 42.6 | [39] |
| L. infantum p | 33.0 | ||||||
| 41. | Iresine diffusa | L. amazonensis p | Flower | Ethanol | 39.7 | 1.3 | [12] |
| L. braziliensis p | 11.1 | 1.7 | |||||
| 42. | Jacaranda glabra | L. amazonensis p | Bark | Ethanol | 18.9 | 6.4 | [12] |
| L. braziliensis p | 191.4 | 11 | |||||
| 43. |
Khaya
grandifolia |
L. donovani p | Stem bark | 50% aqueous ethanol |
50.1 | 1.16 | [13] |
| 44. | Lantana camara | L. amazonensis a | Leaves | Aqueous | 125.9 | >9 | [28] |
| 45. | Licania salicifolia | L. panamensis a | Leaves | Ethyl acetate | >200 | >20.4 | [29] |
| 46. |
Lophira
lanceolata |
L. donovani p | Stem bark | 50% aqueous ethanol |
45.962 | 0.67 | [13] |
| Roots | 38.9 | 0.59 | |||||
| 47. |
Marrubium
vulgare |
L. major p | Leaves | Methanol | 107.4 | 2.34 | [18] |
| L. infantum p | 107.2 | 3.01 | |||||
| 48. |
Mitragyna
inermis |
L. donovani p | Leaves | 50% aqueous ethanol |
193.2 | 8.82 | [13] |
| Stem bark | 424.5 | 15.16 | |||||
| 49. | Mondia whitei | L. donovani p | Roots | 50% aqueous ethanol |
434.5 | 13.97 | [13] |
| 50. | Murraya koenigii | L. donovani p | Stem | Petroleum ether | 73.9 | 1.32 | [16] |
| 51. |
Oreopanax
floribundus |
L. panamensis a | Leaves | Dichloromethane | 47.4 | 2.0 | [29] |
| Ethyl acetate | 54.1 | 2.2 | |||||
| 52. |
Otostegia
integrifolia |
L. aethiopica a, | Aerial part | 80% methanol | 144.55 | - | [26] |
| L. donovani a,p | |||||||
| 53. |
Parkia
clappertoniana |
L. donovani p | Leaves | 50% aqueous ethanol |
112.7 | 6.63 | [13] |
| Stem bark | 42.4 | 2.41 | |||||
| 54. | Persea ferruginea | L. panamensis a | Leaves | Ethyl acetate | >200 | >7.8 | [29] |
| 55. |
Physalis
angulata |
L. amazonensis p | Flower | Ethanol | 19.4 | 1.1 | [12] |
| L. braziliensis p | 17.4 | 0.4 | |||||
| 56. |
Piper
pseudoarboreum |
L. amazonensis p | Leaves | Ethanol | 55.0 | 1.8 | [33] |
| L. braziliensis p | 2.6 | ||||||
| L. guyanesis p | 1.3 | ||||||
| L. infantum p | 1.7 | ||||||
| 57. | Prosopis juliflora | L. donovani p | Leaves | Methanol | 0.85 | 0.26 | [34] |
| 58. |
Prosopis
laevigata |
L. amazonensis a | Leaves | Dichloromethane | 57.0 | 7 | [28] |
| 59. |
Prunus
armeniaca |
L. tropica p | Leaves | Ethanol | 1912.31 | - | [44] |
| 60. |
Psychotria
buhitenii |
L. panamensis a | Leaves | Dichloromethane | 76.8 | 3.57 | [29] |
| Ethyl acetate | 109.5 | 7.75 | |||||
| Ethanol | >200 | >6.81 | |||||
| 61. | Pyrus communis | L. tropica p | Leaves | Ethanol | 1411.30 | - | [35] |
| 62. | Pyrus pashia | L. tropica p | Leaves | Ethanol | 1230.66 | - | [35] |
| 63. | Schinus molle | L. amazonensis a | Leaves | Dichloromethane | 69.7 | 5 | [28] |
| Dichloromethane: Methanol (1:1) | 186.8 | 6 | |||||
| 64. |
Schinus
terebinthifolia |
L. amazonensis p | Fruits | n-Hexane | 52.0 | 3.7 | [25] |
| 65. | Scoparia dulcis | L. amazonensis p | Aerial part | Ethanol | 71.7 | 3.0 | [12] |
| L. braziliensis p | 72.8 | 2.9 | |||||
| 66. |
Solanum
lycocarpum |
L. mexicana a | Fruits | Solamargine | 1515.5 | 43.3 | [43] |
| Solasonine | 1397.9 | 38.3 | |||||
| 67. | Spondias mombin | L. donovani p | Leaves | 50% aqueous ethanol |
55.42 | 0.68 | [13] |
| 68. | Tamarindus indica | L. donovani p | Leaves | 50% aqueous ethanol |
77.9 | 1.34 | [13] |
| 69. | Terminalia ivorensis | L. donovani p | Leaves | 50% aqueous ethanol |
939.2 | 37.72 | [13] |
| 70. | Tessaria integrifolia | L. amazonensis p | Leaves | Ethanol | 119.2 | 2.2 | [12] |
| L. braziliensis p | 120.0 | 3.8 | |||||
| 71. | Thalia geniculata | L. amazonensis p | Roots | Ethanol | 50.7 | 1.7 | [12] |
| L. braziliensis p | 50.4 | 2.9 | |||||
| 72. | Thonningia sanguinea | L. donovani p | Whole plant | 50% aqueous ethanol |
286.1 | 15.38 | [13] |
| 73. | Treculia africana | L. donovani p | Stem bark | 50% aqueous ethanol |
172.0 | 3.84 | [13] |
| 74. | Urtica dioica | L. major p | Leaves | Aqueous | 4500 | 4.4 | [44] |
| 75. | Vitex fosteri | L. donovani p | Leaves | 50% aqueous ethanol |
114.4 | 1.58 | [13] |
| Stem bark | 420.3 | 8.44 | |||||
| 76. |
Ximenia
americana |
L. donovani p | Stem and twigs | 50% aqueous ethanol |
42.3 | 1.17 | [13] |
| 77. | Zanthoxylum zanthoxyloides | L. donovani p | Roots | 50% aqueous ethanol | 247.1 | 18.30 | [13] |
| Stem bark | 583.5 | 12.91 | |||||
| 78. |
Ziziphus
spina-christi |
L. major a | Leaves | Methanol | 563.3 | 10.31 | [37] |
For isolated compounds, 49 natural compounds have been identified with anti-Leishmania activities against amastigotes and/or promastigotes of different species (L. infantum, L. major, L. amazonensis, L. donovani and L. braziliensis). The IC50 values were in the range of 0.2 µg/mL (colchicoside against promastigotes of L. major) to 42.4 µg/mL (dehydrodieuginol against promastigotes of L. amazonensis) (Table 6).
Table 6.
Anti-Leishmania activity of isolated natural compounds.
| No. | Compound Name |
Leishmania Species |
Stage | Assay | Values (IC50) |
Data Analysis (Activity) | Authors |
|---|---|---|---|---|---|---|---|
| 1 | 2,3-Dihydrobenzofuran | L. amazonensis | Promastigotes | In vitro | 1.04 µg/mL | High | [45] |
| Amastigotes | 1.4 µg/mL | High | |||||
| 2 | Dehydrodieuginol | L. amazonensis | Promastigotes | In vitro | 42.4 µg/mL | Moderate | [31] |
| 3 | Erytro-manassatin A | L. amazonensis | Promastigotes | In vitro | 35.4 µg/mL | Moderate | [46] |
| Amastigotes | 20.4 µg/mL | Moderate | |||||
| 4 | Threo-manassatin A | L. amazonensis | Promastigotes | In vitro | 17.6 µg/mL | Moderate | [46] |
| Amastigotes | 16.0 µg/mL | Moderate | |||||
| 5 | Epipinoresinol-4-O-β-D-glucopyranoside | L. major | Promastigotes | In vitro | 36.5 µg/mL | Moderate | [47] |
| 6 | Calanolide E1 | L. major | Promastigotes | In vitro | 36.5 µg/mL | Moderate | [48] |
| 7 | Calanolide E2 | L. major | Promastigotes | In vitro | 29.1 µg/mL | Moderate | [48] |
| 8 | Caffeic acid | L. infantum | Promastigotes | In vitro | 12.5 µg/mL | Moderate | [49] |
| Amastigotes | 21.9 µg/mL | Moderate | [50] | ||||
| 10 | Capsaicin | L. infantum | Promastigotes | In vitro | 5.01 µg/mL | High | [51] |
| Amastigotes | 24.2 µg/mL | Moderate | |||||
| 11 | Cassine | L. amazonensis | Promastigotes | In vitro | 25.2 µg/mL | Moderate | [52] |
| 12 | Spectaline | L. amazonensis | Promastigotes | In vitro | 15.8 µg/mL | Moderate | [52] |
| 13 | Berberine | L. donovani | Promastigotes | In vitro | 4.8 µg/mL | High | [53] |
| 14 | Colchicoside | L. major | Promastigotes | In vitro | 0.2 µg/mL | High | [54] |
| Amastigotes | 4.0 µg/mL | High | |||||
| 15 | Bisabolol | L. donovani | Visceral leishmaniasis |
In vivo | 39.4 µM | Moderate | [55] |
| 16 | 2-Demethyl colchicine | L. major | Promastigotes | In vitro | 0.5 µg/mL | High | [54] |
| Amastigotes | 10.2 µg/mL | Moderate | |||||
| 17 | 3-Demethyl colchicine | L. major | Promastigotes | In vitro | 0.4 µg/mL | High | [54] |
| Amastigotes | 11.1 µg/mL | Moderate | |||||
| 18 | Cornigerine | L. major | Promastigotes | In vitro | 0.8 µg/mL | High | [54] |
| Amastigotes | 11.9 µg/mL | Moderate | |||||
| 19 | Piperine | L. infantum | Promastigotes | In vitro | 3.03 µg/mL | High | [51] |
| 20 | Colchicine | L. major | Promastigotes | In vitro | 0.4 µg/mL | High | [54] |
| Amastigotes | 8.7 µg/mL | High | |||||
| 21 | N-deacetyl-N-formyl colchicine |
L. major | Promastigotes | In vitro | 0.5 µg/mL | High | [54] |
| Amastigotes | 10.2 µg/mL | Moderate | |||||
| 22 | Colchifoline | L. major | Promastigotes | In vitro | 0.7 µg/mL | High | [54] |
| Amastigotes | 14.0 µg/mL | Moderate | |||||
| 23 | Demecolcine | L. major | Promastigotes | In vitro | 0.7 µg/mL | High | [54] |
| Amastigotes | 14.8 µg/mL | Moderate | |||||
| 24 | Staurosporine | L. amazonensis | Promastigotes | In vitro | 0.08 µM | High | [56] |
| Amastigotes | 10.0 µM | High | |||||
| L. donovani | Promastigotes | 2.1 µM | High | ||||
| 25 | 7-Oxostaurosporine | L. amazonensis | Promastigotes | In vitro | 3.6 µM | High | [56] |
| Amastigotes | 0.1 µM | High | |||||
| L. donovani | Promastigotes | 0.6 µM | High | ||||
| 26 | 4′-Demethylamine-4′- oxostaurosporine |
L. amazonensis | Promastigotes | In vitro | 17.1 µM | Moderate | [56] |
| Amastigotes | 2.0 µM | High | |||||
| 27 | Streptocarbazole B |
L. amazonensis
|
Promastigotes | In vitro | 10.4 µg/mL | Moderate | [56] |
| Amastigotes | 2.5 µg/mL | High | |||||
| 28 | 3-O-acetylspectaline | L. donovani | Promastigotes | In vitro | 25.9 µg/mL | Moderate | [53] |
| 29 | 3-O-acetylcassine | L. donovani | Promastigotes | In vitro | 30.3 µg/mL | Moderate | [53] |
| 30 | Soranjidiol | L. amazonensis | Promastigotes | In vitro | 16.3 J/cm2 | Moderate | [57] |
| 31 | Epigallocatechin 3-O- gallate |
L. infantum | Visceral leishmaniasis |
In vivo | ED50 = 12.4 mg/kg/day | Moderate | [58] |
| 32 | 5-Chlorosoranjidiol | L. amazonensis | Promastigotes | In vitro | 13.8 J/cm2 | Moderate | [58] |
| 33 | Bisoranjidiol | L. amazonensis | Promastigotes | In vitro | 15.2 J/cm2 | Moderate | [58] |
| 34 | Gallic acid | L. major | Promastigotes | In vitro | 23.0 µg/mL | Moderate | [32] |
| 35 | Calanolides E1 | L. infantum. | Amastigotes | In vitro | 37.1 µM | Moderate | [48] |
| 36 | Calanolides E2 | 29.1 µM | Moderate | ||||
| 37 | Apigenin | L. amazonensis | Promastigotes | In vitro | 23.7 µM | Moderate | [59] |
| Amastigotes | 4.3 µM | High | |||||
| 38 | 2′-hydroxyflavanone | L. amazonensis | Promastigotes | In vitro | 20.5 µM | Moderate | [60] |
| Amastigotes | 3.09 µM | High | |||||
| 39 | 5,7,3′,4′-tetrahydroxy- 6,8-diprenylisoflavone |
L. amazonensis | Promastigotes | In vitro | 2.7 µM | High | [61] |
| Amastigotes | 1.1 µM | High | |||||
| 40 | Brachydin B | L. braziliensis | Promastigotes | In vitro | 7.05 µM | High | [62] |
| 41 | Brachydin C | L. amazonensis | Promastigotes | In vitro | 10.0 µM | High | [62] |
| Amastigotes | 6.25 µM | High | |||||
| L. braziliensis | Promastigotes | 8.8 µM | High | ||||
| 42 | Ursolic acid | L. amazonensis | Promastigotes | In vitro | 6.2 µg/mL | High | [63] |
| L. donovani | Amastigotes | 1.8 µM | High | ||||
| 43 | Aplysulphurin | L. donovani | Amastigotes | In vitro | 3.1 µM | High | [64] |
| 44 | Tetrahydroaplysulphurin-1 | L. donovani | Amastigotes | In vitro | 3.5 µM | High | [64] |
| 45 | Membranolide | L. donovani | Amastigotes | In vitro | 9.7 µM | High | [64] |
| 46 | Apigenin | Cutaneous leishmaniasis | Cutaneous leishmaniasis | In vivo | ED50 = 0.73 mg/kg | High | [65] |
| 47 | Darwinolide | L. donovani | Amastigotes | In vitro | 11.2 µM | Moderate | [63] |
| 48 | Pukalide aldehyde | L. donovani | Amastigotes | In vitro | 1.9 µM | High | [66] |
| 49 | Epigallocatechin 3-O- gallate |
L. infantum | Amastigotes | In vitro | 2.6 µM | High | [58] |
Numerous natural compounds were isolated from different parts of the plants that were used in traditional medicine to treat leishmaniasis [67]. These compounds act against Leishmania by various mechanisms including the disintegration of cytoplasmic membranes, electron flow disturbances, active transport of crucial substances, coagulation of the cell contents, and destabilization of proton motive forces [68]. For example:
Some medicinal plants are enriched with essential oils composed of different hydrophobic molecules which can diffuse easily across cell membranes and consequently gain access to intracellular targets [67,69]. They may also act on ATPases and other proteins located in cytoplasmic membranes that are surrounded by lipid molecules. They can also cause a distortion of lipid–protein interactions in hydrophobic parts of the proteins, or they can interact with the enzymes involved in the synthesis of structural sections.
The diversity of terpenoids increases their biological activity spectrum, including several Leishmania species [70]. Terpenes can easily penetrate the lipid bilayer of the cell membrane and produce changes in the integrity of cell structure and the mitochondrial membrane of Leishmania parasites [67]. For example, Artemisinin induced apoptosis, depolarization of the mitochondrial membrane potential, and DNA fragmentation [71,72]. Ursolic acid induce programmed cell death independent of caspase 3/7 but dependent on mitochondria. The compound reduced the lesion size and parasite load of cutaneous leishmaniasis in vivo [70]. (−)-α-Bisabolol induced phosphatidylserine externalization and caused cytoplasmic membrane damage, both of which are apoptosis indicators. The compound also decreased ATP levels and disrupted the mitochondrial membrane potential [73].
Plants enriched with antioxidant compounds such as flavonoids may act by initiating morphological changes and causing a loss of cellular integrity, leading to cell cycle arrest in the G1 phase [59]. They also may act by damaging the mitochondria of the parasites [67]. For example, apigenin increased intracellular reactive oxygen species (ROS) and the number of double-membrane vesicles as well as myelin-like membrane inclusions, which are characteristics of the autophagic pathway. Furthermore, the fusion between autophagosome-like structures and parasitophorous vacuoles was observed [65]. Epigallocatechin 3-O-gallate (EGCG) has increased ROS levels, which decreased the mitochondrial membrane potential and the ATP levels [58].
The diversity of structures within the coumarin group enables them to exhibit many biological activities, including anti-Leishmania activity. It represents a promising natural compound that can act on two fronts: as a treatment for leishmaniasis (able to induce mitochondrial membrane damage and changes in ultrastructure [74] and as a tool to control Leishmania vectors (might block the transmission of leishmaniasis since they decrease parasite loads [27].
Many alkaloids have been described as having biological activities against trypanosomatids, such as Leishmania spp. For example, heterocyclic steroids (solamargine and solasonine) induced different immunochemical pathways in macrophages and dendritic cells. Additionally, they were capable of enhancing the expression levels of transcription factors, such as NFκB/AP-1 [43]. In addition, isoquinoline alkaloid (berberine) has leishmanicidal activity through a reduction in the viability of promastigotes and the generation of ROS in these cells. It also increased the levels of mitochondrial superoxide and induced the depolarization of mitochondrial transmembrane potential [53].
3. Methods
3.1. Study Design and Setting
In order to perform this review, the following aspects were addressed: identification and selection of the theme of the research question, establishment of criteria for selection of the sampling, the definition of information to be extracted from selected studies, assessment of the studies included in the integrative review, and final explanation of the results.
3.2. Search Strategies
The databases used for this article were PubMed, Google Scholar, Web of Science, Research Gate, SCOPUS, and Scientific Electronic Library Online (SciELO) using the keywords: neglected tropical disease, Leishmania species, anti-Leishmania activity, natural product, medicinal plants, and promastigote form. We used the search terms separately and in combination with the Boolean operators “OR” or “AND”.
3.3. Inclusion and Exclusion Criteria
The initial total articles (1374) were adjusted for the restriction in the year of publication (from 1 January 2015 to 31 December 2021) (806), duplicates (273), articles that were not available in full (67) and articles in other languages (4). After a review of their titles and abstracts, some articles were discarded, since the anti-leishmanial activity (IC50) values were higher than 100 µg/mL (134), and they tested extract/natural compounds obtained through other natural sources (algae, fungi, etc.) (11). The full texts of the remaining articles were reviewed in detail. However, further articles were discarded after the full text had been reviewed (18) since they did not address much of the required information. Finally, 61 articles were evaluated as valuable to reach the goals of this review. The methodological validity of all 61 studies was proven prior to inclusion in the review by undertaking a critical appraisal using a standardized instrument [75].
3.4. Data Extraction and Analysis
The data extraction protocol included the scientific and family names, parts of the plant used, most active extract/ essential oil employed in the experiment, name of natural compound, Leishmania species and form, IC50 values, potential groups/compounds responsible for activity, clinical form of leishmaniasis, route, the dose of administration and scheme of treatment, the efficacy of the treatments in the experiment, cytotoxic activity, selectivity index, the authors, and year of publication. In the results analysis, an active extract/compound was considered if the IC50 value was less than or equal to 10 µg/mL against the promastigote or amastigote forms. Moderate activity was defined if the IC50 was greater than 10 and less than 50 µg/mL and weakly active if the IC50 value was greater than 50 µg/mL and less than 100 µg/mL.
4. Conclusions and Perspectives
Leishmaniasis threatens about 350 million people around the world and continues to represent a menace on a global scale. Without a doubt, it requires utmost attention due to the lack of vaccines for the prevention and reported resistance against available chemical drugs for treatment. The intolerably high incidence of millions of new cases of leishmaniasis per year worldwide and deficiencies in current treatment point to an urgent need for new medications.
As a means to facilitate the accessibility of information, this review updates and summarizes recent results on medicinal plants and natural compounds against different Leishmania species. The plants presented here have demonstrated a diverse range of activities against different forms of leishmaniasis with some showing high activities that could be reasonable starting points for the further development of effective and affordable novel drugs.
However, it was also evident that the majority of experiments were performed with the promastigote form. We believe that these studies are undoubtedly important because promastigotes are infectious to man and other animals. However, it is urgent that future studies should be conducted to find compounds with anti-amastigote activity too, since the morbimortality associated with Leishmania is caused by this form.
It Is pleasing that more and more investigations report on the anti-Leishmania activity in vivo and more studies are needed in this respect, increasing the number of potential candidate compounds for further drug development. In vitro studies are valuable for the screening of extracts and isolated compounds as well as for investigations of the cellular and molecular modes of action. Since many natural compounds are rapidly metabolized in the human body by liver enzymes and gastrointestinal microflora, animal experiments are indispensable to identify candidates with sufficient half-life times in vivo and anti-Leishmania activities in concentration ranges that are reachable in the human blood. However, in the literature inspected by us, only four plants and two natural compounds have been investigated both in vitro and in vivo, i.e., Prosopis juliflora [34], Ziziphus spina-christi [37], Piper pseudoarboreum [33], and Croton caudatus [76] as well as epigallocatechin 3-O-gallate [58] and apigenin [65], respectively. More investigations are required to allow a direct comparison of in vitro and in vivo data.
Further down this line of argumentation, standardized extracts and/or isolated phytochemicals need to be tested in randomized clinical trials. Without convincing clinical evidence on safety and efficacy, preparations from traditional medicine will hardly reach considerable recognition in the medical world.
Author Contributions
A.H.A.: wrote the manuscript draft; H.E.K.: manuscript editing, supervision of A.A.H.; A.H.A., M.M.M., W.J.O.: literature collection, manuscript editing; T.E.: supervised whole project and wrote, edited, and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Leishmaniasis Fact-Sheets. WHO; Geneva, Switzerland: 2020. [(accessed on 23 September 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. [Google Scholar]
- 2.Center for Food Security and Public Health . Leishmaniasis (Cutaneous and Visceral) Iowa State of University, College of Veterinary Medicine; Ames, IA, USA: 2009. [Google Scholar]
- 3.Bereket A., Mihiretu A. Leishmaniasis: A review on parasite, vector and reservoir host. Health Sci. J. 2017;11:519. [Google Scholar]
- 4.Roberts L., Janovy J., Schmidt G. Foundations of Parasitology. 8th ed. McGraw-Hill; New York, NY, USA: 2009. [Google Scholar]
- 5.Gagandeep K., Bhawana R. Comparative analysis of the omics technologies used to study antimonial, amphotericin B, and pentamidine resistance in Leishmania. J. Parasitol. Res. 2014;2014:726328. doi: 10.1155/2014/726328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghorbani M., Farhoudi R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Develop. Ther. 2018;12:25–40. doi: 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawed J., Majumdar S. Recent trends in Leishmania research: A therapeutic perspective. J. Infect. Epidemiol. 2018;1:1–4. doi: 10.29245/2689-9981/2018/3.1120. [DOI] [Google Scholar]
- 8.Haldar A., Sen P., Roy S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Mol. Biol. Int. 2011;2011:571242. doi: 10.4061/2011/571242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murugan N., Natarajan D. Bionanomedicine for antimicrobial therapy—A case study from Glycosmis pentaphylla plant-mediated silver nanoparticles for control of multidrug-resistant bacteria. Lett. Appl. NanoBioSci. 2018;8:523–540. [Google Scholar]
- 10.Et-Touys A., Bouyahyal A., Fellah H., Mniouil M., El Bouryl H., Dakka N., Bakri Y. Antileishmanial activity of medicinal plants from Africa: A review. Asian Pac. J. Trop. Dis. 2017;7:826–840. doi: 10.12980/apjtd.7.2017D7-215. [DOI] [Google Scholar]
- 11.Santos D., Coutinho C., Madeira M., Bottino C., Vieira R., Nascimento S., Rodrigues C. Leishmaniasis treatment a challenge that remains: A review. Parasitol. Res. 2008;103:1–10. doi: 10.1007/s00436-008-0943-2. [DOI] [PubMed] [Google Scholar]
- 12.Arévalo-Lopéz D., Nelida N., Ticona J.C., Limachi I., Salamanca E., Udaeta E., Paredes C., Espinoza B., Serato A., Garnica D., et al. Leishmanicidal and cytotoxic activity from plants used in Tacana traditional medicine (Bolivia) J. Ethnopharmacol. 2018;216:120–133. doi: 10.1016/j.jep.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi M., Amoa-Bosompem M., Kwofie K., Agyapong J., Adegle R., Sakyiamah M., Ayertey F., Owusu K., Tuffour I., Atcholog P., et al. In vitro antiprotozoan activity and mechanisms of action of selected Ghanaian medicinal plants against Trypanosoma, Leishmania, and Plasmodium parasites. Phytother. Res. 2018;32:1617–1630. doi: 10.1002/ptr.6093. [DOI] [PubMed] [Google Scholar]
- 14.Costa L., Alves M., Brito L., Abi-Chacra E., Barbosa-Filho J., Gutierrez S., Barreto H., Carvalho F. In vitro antileishmanial and immunomodulatory activities of the synthetic analogue riparin E. Chem. Biol. Interact. 2021;336:109389. doi: 10.1016/j.cbi.2021.109389. [DOI] [PubMed] [Google Scholar]
- 15.Bouyahya A., Et-Touys A., Dakka N., Fellah H., Abrini J., Bakri Y. Antileishmanial potential of medicinal plant extracts from the North-West of Morocco. Beni-Suef Univ. J. Basic Appl. Sci. 2018;7:50–54. doi: 10.1016/j.bjbas.2017.06.003. [DOI] [Google Scholar]
- 16.Sultana S. In vitro antileishmanial activity of three medicinal plants: Argemone mexicana Murraya Koenig and Cinnamomum tamala against miltefosine resistant promastigotes of Leishmania donovani parasites. Int. J. Pharm. Pharmaceut. Sci. 2021;13:27–33. doi: 10.22159/ijpps.2021v13i9.42349. [DOI] [Google Scholar]
- 17.Mathlouthi A., Belkessan M., Sdiri M., Fethi-Diouani M., Souli A., El-Bok S., Ben-Attia M. Chemical composition and anti-leishmania major activity of essential oils from Artemesia spp. grown in central Tunisia. J. Essent. Oil Bear. Plants. 2018;21:1186–1198. doi: 10.1080/0972060X.2018.1526128. [DOI] [Google Scholar]
- 18.Eddaikra N., Boudjelal A., Sbabdji M.A., Eddaikra A., Boudrissa A., Bouhenna M.M., Smain C., Zoubir H. Leishmanicidal and cytotoxic activity of Algerian medicinal plants on Leishmania major and Leishmania infantum. J. Med. Microbiol. Infect. Dis. 2019;7:66–71. doi: 10.29252/JoMMID.7.3.66. [DOI] [Google Scholar]
- 19.Cesa S., Sisto F., Zengin G., Scaccabarozzi D., Kokolakis A., Scaltrito M., Grande R., Locatelli M., Cacciagrano F., Angiolella L., et al. Phytochemical analyses and pharmacological screening of neem oil. S. Afr. J. Bot. 2019;120:331–337. doi: 10.1016/j.sajb.2018.10.019. [DOI] [Google Scholar]
- 20.Greve H., Kaiser M., Mäser P., Schmidt T. Boswellic acids show in vitro activity against Leishmania donovani. Molecules. 2021;26:3651. doi: 10.3390/molecules26123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammad R., Sareh J., Katrin E., Massumeh N., Maryam S., Mehrdad K., Sam K. Cytotoxic and antileishmanial effect of various extracts of Cappris spinose L. Turk. J. Pharm. Sci. 2020;18:146–150. doi: 10.4274/tjps.galenos.2020.87259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah N.A., Khan M.R., Nigussie D. Phytochemical, antioxidant and anti-Leishmania activity of selected Pakistani plants. J. Pharmacol. Clin. Res. 2016;1:53461276. [Google Scholar]
- 23.Bouyahya A., Bakri Y., Belmehdi O., Et-Touys A., Abrini J., Dakka N. Phenolic extracts of Centaurium erythraea with novel antiradical, antibacterial and antileishmanial activities. Asian Pac. J. Trop. Dis. 2019;7:433–439. doi: 10.12980/apjtd.7.2017D6-462. [DOI] [Google Scholar]
- 24.Ahmet Y., Tulay A., Sahra C., Husniye K., Eda T., Cuneyt B. Assessment of in vitro activity of Cynara scolymus extracts against leishmania tropica. Kafkas Univ. Vet. Fak. Derg. 2021;27:381–387. [Google Scholar]
- 25.Beatriz M., Adriana B., Sonia A., Eliana R., Eric U., João H., Márcia D., Susan P., Luiz F. Ethnopharmacology study of plants from Atlantic forest with leishmanicidal activity. Evid. Based Complement. Alternat. Med. 2019;2019:8780914. doi: 10.1155/2019/8780914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nigatu H., Belay A., Ayalew H., Abebe B., Tadesse A., Tewabe Y., Degu A. In vitro antileishmanial activity of some Ethiopian medicinal plants. J. Exp. Pharmacol. 2021;13:15–22. doi: 10.2147/JEP.S285079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira C., Passos C., Soares D., Costa K., Rezende M., Lobao A., Pinto A., Hamerski L., Saraiva E. Leishmanicidal activity of alkaloids-rich fraction from Guatteria latifolia. Exp. Parasitol. 2017;172:51–60. doi: 10.1016/j.exppara.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Ronna D., Lianet M., Abel P., Heike V., Fausto R., Cesar I., Alejandra R. In vitro antileishmanial activity of Mexican medicinal plants. Heliyon. 2017;3:e00394. doi: 10.1016/j.heliyon.2017.e00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson C., Sara R., Fernando A., Andres F., Cristian H., Ivan D., Juan C., Isabel V. Antileishmanial and cytotoxic activities of four Andean plant extracts from Colombia. Vet. World. 2020;13:2178–2182. doi: 10.14202/vetworld.2020.2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouyahya A., Et-Touys A., Bakri Y., Talbaui A., Fellah H., Abrini J., Dakka N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017;111:41–49. doi: 10.1016/j.micpath.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues L., Barbosa-Filho J., de Oliveira M., do Nascimento N., Borges F., Mioso R. Synthesis and antileishmanial activity of natural dehydrodieugenol and its mono- and dimethyl ethers. Chem. Biodivers. 2016;13:870–874. doi: 10.1002/cbdv.201500280. [DOI] [PubMed] [Google Scholar]
- 32.Albakhit S., Khademvatan S., Doudi M., Forutan-Rad M. Antileishmanial activity of date (Phoenix dactylifera L.) fruit pit extract in vitro. Evid. Based Complement. Altern. Med. 2016;21:NP98–NP102. doi: 10.1177/2156587216651031. [DOI] [PubMed] [Google Scholar]
- 33.Ticona J., Bilbao-Ramos P., Flores N., Dea-Ayuela M., Bolás-Fernández F., Jiménez I., Bazzocchi I. (E)-Piplartine isolated from Piper pseudoarboreum, a lead compound against leishmaniasis. Foods. 2020;9:1250. doi: 10.3390/foods9091250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutile M., Muli M., Muita G. Safety and efficacy of Prosopis juliflora leaf extract as a potential treatment against visceral leishmaniasis. Iran. J. Parasitol. 2021;16:652–662. doi: 10.18502/ijpa.v16i4.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nargis S., Naveeda A., Attiya I., Asma A., Huma F. Evaluation of safety, antileishmanial and chemistry of ethanolic leaves extracts of seven medicinal plants: An in-vitro study. Open Chem. J. 2020;7:27. [Google Scholar]
- 36.Et-Touys A., Fellah H., Sebti F., Mniouil M., Elboury H., Talbaoui A., Bakri Y. In vitro antileishmanial activity of extracts from endemic Moroccan medicinal plant Salvia verbenaca (L.) Briq. ssp verbenaca Maire (S. clandestina Batt. non L.) Eur. J. Med. Plants. 2016;16:1–8. doi: 10.9734/EJMP/2016/27891. [DOI] [Google Scholar]
- 37.Albalawi A. Antileishmanial activity of Ziziphus spina-christi leaves extract and its possible cellular mechanisms. Microorganism. 2021;9:2113. doi: 10.3390/microorganisms9102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afrin F., Chouhan G., Islamuddin M., Want M., Ozbak H., Hemeg H. Cinnamomum cassia exhibits antileishmanial activity against Leishmania donovani infection in vitro and in vivo. PLoS Negl. Trop. Dis. 2019;13:e0007227. doi: 10.1371/journal.pntd.0007227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araújo I., de Paula R., Alves C., Faria K., Oliveira M., Mendes G., Dias E., Ribeiro R., Oliveira A., Silva S. Efficacy of lapachol on treatment of cutaneous and visceral leishmaniasis. Exp. Parasitol. 2019;199:67–73. doi: 10.1016/j.exppara.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Khalifa E., Adil A., Banaz M., Zinah A., Wasnaa S. Topical 40% Loranthus europaeus ointment as an alternative medicine in the treatment of acute cutaneous leishmaniasis versus topical 25% podophyllin solution. J. Cosmetics Dermatol. Sci. Appl. 2017;7:148–163. [Google Scholar]
- 41.Gupta G., Peine K., Abdelhamid D., Snider H., Shelton A., Rao L., Kotha S., Huntsman A., Varikuti S., Oghumu S., et al. A novel sterol isolated from a plant used by Mayan traditional healers is effective in treatment of visceral leishmaniasis caused by Leishmania donovani. ACS Infect. Dis. 2015;1:497–506. doi: 10.1021/acsinfecdis.5b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul C., Janssens J., Abel P., Osmany C., Arianna Y., Alexis D., Wagner V., Lianet M. Efficacy of four Solanum spp. extracts in an animal model of cutaneous leishmaniasis. Medicines. 2018;5:49. doi: 10.3390/medicines5020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lezama-Dávila M., McChesney D., Bastos K., Miranda A., Tiossi F., da Costa J., Bentley D., Gaitan-Puch M., Marquez I. A new antileishmanial preparation of combined solamargine and solasonine heals cutaneous leishmaniasis through different immunochemical pathways. Antimicrob. Agents Chemother. 2016;60:2732–2738. doi: 10.1128/AAC.02804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badirzadeh A., Heidari-Kharaji M., Fallah-Omrani V., Dabiri H., Araghi A., Salimi Chirani A. Antileishmanial activity of Urtica dioica extract against zoonotic cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2020;14:e0007843. doi: 10.1371/journal.pntd.0007843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Castro O., Brito L., de Moraes A., Amorim L., Sobrinho-Júnior E., de Carvalho C., Rodrigues K., Arcanjo D., Cito A., Carvalh F. In vitro effects of the neolignan 2,3-dihydrobenzofuran against Leishmania amazonensis. Basic Clin. Pharmacol. Toxicol. 2017;120:52–58. doi: 10.1111/bcpt.12639. [DOI] [PubMed] [Google Scholar]
- 46.Brito J., Passero L., Bezerra-Souza A., Laurenti M., Romoff P., Barbosa H., Ferreira E., Lago J. Antileishmanial activity and ultrastructural changes of related tetrahydrofuran dineolignans isolated from Saururus cernuus L. (Saururaceae) J. Pharm. Pharmacol. 2019;71:1871–1878. doi: 10.1111/jphp.13171. [DOI] [PubMed] [Google Scholar]
- 47.Maia M., Silva J., Nunes T., Sousa J., Rodrigues G., Monteiro A., Tavares J., Rodriqes K., Junior F., Scotti L., et al. Virtual screening and the in vitro assessment of the antileishmanial activity of lignans. Molecules. 2020;25:2281. doi: 10.3390/molecules25102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva L., Gomes K., Costa-Silva T., Romanelli M., Tempone A., Sartorelli P., Lago J. Calanolides E1 and E2, two related coumarins from Calophyllum brasiliense Cambess. (Clusiaceae), displayed in vitro activity against amastigote forms of Trypanosoma cruzi and Leishmania infantum. Nat. Prod. Res. 2020;35:5373–5377. doi: 10.1080/14786419.2020.1765347. [DOI] [PubMed] [Google Scholar]
- 49.Bortoleti B., Tomiotto-Pellissiera F., Gonçalves M., Miranda-Sapla M., Assolini J., Carloto A., Lima D.M., Silveira G.F., Almeida R.S., Costa I.N., et al. Caffeic acid has antipromastigote activity by apoptosis-like process; and anti-amastigote by TNF-α/ROS/NO production and decreased of iron availability. Phytomedicine. 2019;57:262–270. doi: 10.1016/j.phymed.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 50.Garcia A., Oliveira D., Amaral A., Jesus J., Rennó Sodero A., Souza A., Supuran C., Vermelho A., Rodrigues I., Pinheiro A. Leishmania infantum arginase: Biochemical characterization and inhibition by naturally occurring phenolic substances. J. Enzyme Inhib. Med. Chem. 2019;34:1100–1109. doi: 10.1080/14756366.2019.1616182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vieira-Araújo F., Macedo Rondon F., Pinto Vieira Í., Pereira Mendes F., Carneiro de Freitas J., Maia de Morais S. Synergism between alkaloids piperine and capsaicin with meglumine antimoniate against Leishmania infantum. Exp. Parasitol. 2018;188:79–82. doi: 10.1016/j.exppara.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Lacerda R., Freitas T., Martins M., Teixeira T., da Silva C., Candido P., Oliveira R., Junior C., Bolzani V., Danuello A., et al. Isolation, leishmanicidal evaluation and molecular docking simulations of piperidine alkaloids from Senna spectabilis. Bioorg. Med. Chem. 2018;26:5816–5823. doi: 10.1016/j.bmc.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 53.De Sarkar S., Sarkar D., Sarkar A., Dighal A., Staniek K., Gille L., Chatterjee M. Berberine chloride mediates its antileishmanial activity by inhibiting Leishmania mitochondria. Parasitol. Res. 2018;118:335–345. doi: 10.1007/s00436-018-6157-3. [DOI] [PubMed] [Google Scholar]
- 54.Azadbakht M., Davoodi A., Hosseinimehr S., Keighobadi M., Fakhar M., Valadan R., Farindnia R., Emami S., Azadbakht M., Bakhtiyari A. Tropolone alkaloids from Colchicum kurdicum (Bornm.) Stef. (Colchicaceae) as the potent novel antileishmanial compounds, purification, structure elucidation, antileishmanial activities, and molecular docking studies. Exp. Parasitol. 2020;213:107902. doi: 10.1016/j.exppara.2020.107902. [DOI] [PubMed] [Google Scholar]
- 55.Corpas-López V., Merino-Espinosa G., Díaz-Sáez V., Morillas-Márquez F., Navarro-Moll M., Martín-Sánchez J. The sesquiterpene (–)-α-bisabolol is active against the causative agents of Old World cutaneous leishmaniasis through the induction of mitochondrial-dependent apoptosis. Apoptosis. 2016;21:1071–1081. doi: 10.1007/s10495-016-1282-x. [DOI] [PubMed] [Google Scholar]
- 56.Cartuche L., Sifaoui I., López-Arencibia A., Bethencourt-Estrella C., San Nicolás-Hernández D., Lorenzo-Morales J., Pinero J., Marrero A., Fernandez J. Antikinetoplastid activity of indolocarbazoles from Streptomyces sanyensis. Biomolecules. 2020;10:657. doi: 10.3390/biom10040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimmer J., Cabral F., Sabino C., Silva C., Núñez-Montoya S., Cabrera J., Ribeiro M. Natural anthraquinones as novel photosensitizers for antiparasitic photodynamic inactivation. Phytomedicine. 2019;61:152894. doi: 10.1016/j.phymed.2019.152894. [DOI] [PubMed] [Google Scholar]
- 58.Inacio J., Fonseca M., Almeida-Amaral E. (−) -Epigallocatechin 3-O-gallate as a new approach for the treatment of visceral leishmaniasis. J. Nat. Prod. 2019;82:2664–2667. doi: 10.1021/acs.jnatprod.9b00632. [DOI] [PubMed] [Google Scholar]
- 59.Sen R., Chatterijee M. Plant-derived therapeutics for the treatment of leishmaniasis. Phytomedicine. 2011;18:1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Gervazoni L., Gonçalves-Ozório G., Almeida-Amaral E. 2′-Hydroxyflavanone activity in vitro and in vivo against wild-type and antimony-resistant Leishmania amazonensis. PLoS Negl. Trop. Dis. 2018;12:e0006930. doi: 10.1371/journal.pntd.0006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pereira I., Mendona D., Tavares G., Lage D., Ramos F., Oliveirada-Silva J., Antinarelli L., Machado A., Caravalh A., Salustiano I., et al. Parasitological and immunological evaluation of a novel chemotherapeutic agent against visceral leishmaniasis. Parasite Immunol. 2020;42:e12784. doi: 10.1111/pim.12784. [DOI] [PubMed] [Google Scholar]
- 62.Rocha V., Quintino C., Ferreira Queiroz E., Marcourt L., Vilegas W., Grimaldi G., Furrer P., Allemann E., Wolfender J., Soares M. Antileishmanial activity of dimeric flavonoids isolated from Arrabidaea brachypoda. Molecules. 2018;24:1. doi: 10.3390/molecules24010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das S., Ghosh S., De A., Bera T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017;102:996–1008. doi: 10.1016/j.ijbiomac.2017.04.098. [DOI] [PubMed] [Google Scholar]
- 64.Shilling A., Witowski C., Maschek J., Azhari A., Vesely B., Kyle D., Amsler C., McClintock J., Baker B. Spongian diterpenoids derived from the antarctic sponge Dendrilla antarctica are potent inhibitors of the leishmania parasite. J. Nat. Prod. 2020;83:1553–1562. doi: 10.1021/acs.jnatprod.0c00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonseca-Silva F., Canto-Cavalheiro M., Menna-Barreto R., Almeida-Amaral E. Effect of apigenin on Leishmania amazonensis is associated with reactive oxygen species production followed by mitochondrial dysfunction. J. Nat. Prod. 2015;78:880–884. doi: 10.1021/acs.jnatprod.5b00011. [DOI] [PubMed] [Google Scholar]
- 66.Thomas S., von Salm J., Clark S., Ferlit S., Nemani P., Azhari A., Rice C., Wilson N., Kyle D., Baker B. Keikipukalides, furanocembrane diterpenes from the antarctic deep sea octocoral Plumarella delicatissima. J. Nat. Prod. 2018;81:117–123. doi: 10.1021/acs.jnatprod.7b00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colares A., Almeida-Souza F., Taniwaki N., Souza S., da Costa J., Calabrese K., Abreu-Silva A. In vitro antileishmanial activity of essential oil of Vanillosmopsis arborea (Asteraceae) Baker. Evid. Based Complement. Alternat. Med. 2013;2013:727042. doi: 10.1155/2013/727042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gervazoni L., Barcellos G., Ferreira-Paes T., Almeida-Amaral E. Use of natural products in leishmaniasis chemotherapy: An overview. Front. Chem. 2020;8:579891. doi: 10.3389/fchem.2020.579891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machado M., Dinis A., Santos-Rosa M., Alves V., Salgueiro L., Cavaleiro C., Sousa M. Activity of Thymus capitellatus volatile extract, 1, 8-cineole and borneol against Leishmania species. Vet. Parasitol. 2014;200:39–49. doi: 10.1016/j.vetpar.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto E., Campos B., Jesus J., Laurenti M., Ribeiro S., Kallas E., Fernandes M., Gomes G., Silva M., Seessa D., et al. The effect of ursolic acid on Leishmania (Leishmania) amazonensis is related to programed cell death and present therapeutic potential in experimental cutaneous leishmaniasis. PLoS ONE. 2015;10:e0144946. doi: 10.1371/journal.pone.0144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sen R., Bandyopadhyay S., Dutta A., Mandal G., Ganguly S., Saha P., Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J. Med. Microbiol. 2007;56:1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- 72.Sen R., Ganguly S., Saha P., Chatterjee M. Efficacy of artemisinin in experimental visceral leishmaniasis. Int. J. Antimicrob. Agents. 2010;36:43–49. doi: 10.1016/j.ijantimicag.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Hajaji S., Sifaoui I., Arencibia A., Batlle M., Jimenez I., Bazzocchi I., Valladares B., Akkari H., Morales J., Pinero J. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018;117:2055–2867. doi: 10.1007/s00436-018-5975-7. [DOI] [PubMed] [Google Scholar]
- 74.Brenzan M., Santos A., Nakamura C., Filho B., Ueda Nakamura T., Young M., Correa A., Junior J., Diaz J., Cortez D. Effects of (−) mammea A/BB isolated from Calophyllum brasiliense leaves and derivatives on mitochondrial membrane of Leishmania amazonensis. Phytomedicine. 2012;19:223–230. doi: 10.1016/j.phymed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Guyatt G., Sackett D., Cook D. Users’ guides to the medical literature II: How to use an article about therapy or prevention. J. Am. Med. Assoc. 1994;1:59–63. doi: 10.1001/jama.1994.03510250075039. [DOI] [PubMed] [Google Scholar]
- 76.Dey S., Mukherjee D., Chakraborty S., Mallick S., Dutta A., Ghosh J., Swapana N., Maiti S., Ghorai N., Singh C.B., et al. Protective effect of Croton caudatus Geisel leaf extract against experimental visceral leishmaniasis induces proinflammatory cytokines in vitro and in vivo. Exp. Parasitol. 2015;1512:84–95. doi: 10.1016/j.exppara.2015.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.