Abstract
Growing evidence supports that individual lifestyle factors contribute to the development of non-alcoholic fatty liver disease (NAFLD) without considering the coexistence and synergistic effect of lifestyle factors. Our aim is to derive a healthy lifestyle score (HLS) and estimate its association with NAFLD. In this nationwide cross-sectional study, we derived a five-item HLS including dietary pattern, body mass index, physical activity, cigarette smoking, and sleep duration. NAFLD and clinically significant fibrosis (CSF) were assessed based on vibration-controlled transient elastography (VCTE). Liver function parameters were also tested. Multivariable logistic and linear regressions were applied to investigate the association between HLS and liver diseases. Of the 3893 participants with VCTE examination, approximately 14.1% of participants possessed zero or one healthy lifestyle, 62.5% possessed two or three healthy lifestyles, and 23.4% possessed four or five healthy lifestyles. Compared with participants with a low HLS (0–1 score), the adjusted odds ratios and 95% confidence intervals for those with a high HLS (4–5 score) were 0.25 (0.19~0.33, Ptrend < 0.001) for NAFLD and 0.30 (0.18~0.50, Ptrend < 0.001) for CSF. HLS was positively associated with albumin, total protein, and total bilirubin (all Ptrend ≤ 0.001), and was inversely associated with globulin, alanine aminotransferase, and gamma-glutamyl transaminase (all Ptrend ≤ 0.003). Higher adherence to HLS is associated with lower odds of NAFLD and CSF and may improve liver function. Strategies for the promotion of a healthy lifestyle should be considered as part of NAFLD prevention.
Keywords: cross-sectional study, healthy lifestyle, non-alcoholic fatty liver disease, liver function parameters, vibration-controlled transient elastography
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), a condition characterized by the accumulation of triglycerides and other fats in liver cells in the absence of heavy drinking and secondary causes of liver disease, encompasses a continued spectrum of liver diseases ranging from simple steatosis without inflammation to advanced steatohepatitis, fibrosis, cirrhosis, and eventually hepatocellular carcinoma [1,2]. NAFLD has become the most prominent cause of chronic liver diseases worldwide, which affects up to 32% of the world’s population [3]. A study utilizing U.S. National Health and Nutrition Examination Surveys (NHANES) assessed the shift in the burden of different liver disease etiologies over the past three decades in the U.S. and reported that only the prevalence of NAFLD was growing, paralleling to the increase of obesity and diabetes [4].
Previous studies suggested that several lifestyle factors including obesity [5], tobacco smoking [6], short sleep duration [7], lack of physical activity [8], and an unhealthy diet [9], were associated with the risk of developing NAFLD. However, most studies merely focused on the association of individual lifestyle factors with NAFLD, which ignored the coexistence and synergistic effect of overall lifestyle factors. Therefore, it is essential to construct a comprehensive measure of these relevant lifestyle constituents. Prior studies have created a healthy lifestyle score (HLS) by summing the score of each lifestyle and have reported that greater adherence to a healthy lifestyle was associated with a lower risk of hypertension [10], liver cancer [11], total mortality [12,13], and cardiovascular disease-specific mortality [13]. To the best of our knowledge, only two cross-sectional studies have investigated the association between HLS and fatty liver disease (FLD) [14,15]. One study among 354 participants aged 48–77 years from Germany consisting largely of Caucasians, showed that a higher HLS was associated with lower odds of FLD determined by magnetic resonance imaging (MRI) [14]. Another study of a community-based sample of 2981 participants aged 40–75 years in China, reported that a higher HLS was associated with a lower prevalence of NAFLD determined by abdominal ultrasonography [15]. While no studies have examined the association between HLS and the risk of hepatic fibrosis. In addition, the narrow age groups in the two studies [14,15] and the sample of mainly Caucasian ethnicity in the first study [14] may limit the generalizability of the conclusions.
To add more evidence, we investigated the cross-sectional association between adherence to HLS and the odds of NAFLD and hepatic fibrosis assessed by vibration-controlled transient elastography (VCTE) in a nationally representative sample of U.S. adults aged 18 years or older. We also investigated the association between adherence to HLS and liver function parameters.
2. Methods
2.1. Study Population
In this study, participants were selected from the 2017 to 2018 cycle of the U.S. NHANES, which is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Diseases Control and Prevention (CDC) in the United States. More details on the survey protocol of NHANES have been described elsewhere [16]. The study protocol was approved by NCHS Research Ethics Review Board (Protocol #2011-17; Protocol #2018-01) and written informed consent was obtained from all participants.
A total of 9254 participants were enrolled in the survey. Individuals aged ≥ 18 years old were included in this study. Individuals were excluded if they (i) had missing dietary data (n = 873) or an unreliable energy intake (defined as <600 or >3500 kcal/day for women; <800 or >4200 kcal/day for men, n = 221); (ii) had hepatitis B virus (HBV) infection (n = 18), hepatitis C virus (HCV) infection (n = 78), or excessive alcohol consumption (>3 drinks/d for men and >2 drinks/d for women, n = 116); (iii) did not have a valid VCTE detection (partial examination: n = 314; missing steatosis value: n = 1; not receiving VCTE detection: n = 255); (iv) had missing body mass index (BMI) data (n = 21), missing sleep data (n = 32), or missing physical activity data (n = 28, Supplementary Figure S1).
2.2. Definition of the HLS
A five-item HLS was derived in this study based on dietary patterns, BMI, physical activity, cigarette smoking, and sleep duration. Dietary intake was quantified via two 24-h dietary recalls. The first 24-h dietary recall was performed in person in the NHANES Mobile Examination Center (MEC), and a second 24-h dietary was conducted by telephone 3 to 10 days after the first recall. Dietary quality was assessed by the healthy eating index (HEI) scores. The HEI-2015 was calculated from two 24-h dietary recall data per participant. We defined a healthy dietary status as an HEI-2015 score in the top two-fifths of distribution.
Anthropometric measurement (height and weight) was conducted in MEC. BMI was calculated as weight (kg) divided by the square of the height (m), and the range from 18.5 to 24.9 was defined as a healthy status. Physical activity was measured using the global physical activity questionnaire through household interviews, which has been shown to have good reliability and validity in multiple populations [17,18]. A moderate-to-high level (≥500 metabolic equivalent task (MET)-minutes/week) of physical activity was defined as a healthy status. Cigarette smoking was obtained through household interviews with self-reported smoking of fewer than 100 cigarettes in life was defined as a healthy status. Sleep time was obtained using the Munich chronotype questionnaire through household interviews, a sleep duration of ≥7 h was defined as a healthy status.
Consistent with previous studies [13,19], we assigned 1 point for a healthy status and 0 points for an unhealthy status with respect to each item. The HLS was the sum of five-item scores, with a range from 0 to 5. A higher HLS denoted more adherence to a healthy lifestyle.
2.3. Assessments of Covariates
Standardized questionnaires were administrated through household interviews to collect demographic characteristics including age, sex, race/ethnicity, educational level, and income. Hypertension was defined if individuals (i) reported a history of hypertension; or (ii) had a systolic blood pressure (SBP) ≥ 140 mmHg; or (iii) had a diastolic blood pressure (DBP) ≥ 90 mmHg. Diabetes was defined if individuals (i) reported a diagnosis of diabetes; or (ii) had a hemoglobin A1c (HbA1c) level ≥ 6.5%; or (iii) had a fasting glucose level ≥ 126 mg/dL; or (iv) had a random glucose level ≥ 200 mg/dL.
2.4. Laboratory Assays and Liver Function Parameters
Liver function parameters, including serum albumin, globulin, total protein, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transaminase (GGT), were also obtained from participants. All laboratory procedures are described in detail elsewhere [20].
2.5. Ascertainments of NAFLD and Clinically Significant Fibrosis
VCTE was conducted by trained and certified technicians in MEC, using the FibroScan® model 502 V2 Touch equipped with a medium or extra-large probe. Hepatic steatosis and fibrosis were assessed by controlled attenuation parameter (CAP) and liver stiffness measurement (LSM), respectively.
According to previous studies [21,22], a CAP score ≥ 285 dB/m was used to define NAFLD (CAP ≥ 285 dB/m) vs. non-NAFLD (CAP < 285 dB/m). Furthermore, a LSM score ≥ 8.6 kPa was used to define clinically significant fibrosis (CSF: LSM ≥ 8.6 kPa) vs. non-CSF (LSM < 8.6 kPa).
2.6. Statistical Analysis
Due to the limited number of individuals among the lowest or highest lifestyle score group, participants were grouped into low HLS (0–1 score), medium HLS (2–3 score), and high HLS (4–5 score). Continuous variables were presented as a mean (standard deviation) and categorical variables were presented as percentages. Characteristics of study participants were compared across three the HLS statuses using a one-way analysis of variance for continuous variables or a chi-square test for categorical variables or the Kruskal-Wallis test for ordinal variables.
We used multivariable logistic regression to evaluate the odds ratios (ORs) and 95% confidence intervals (CIs) for HLS status in relation to NAFLD and CSF. Model 1 did not adjust for the covariates and Model 2 was performed with adjustment for age (18–39, 40–59, ≥60), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, and other races), education (less than high school, high school diploma, and more than high school), the ratio of family income to poverty (<1.30, 1.30–3.49, and ≥3.50), hypertension (yes, no), and diabetes (yes, no).
Given the limited number of CSF, we only estimated the stratified association between HLS and odds of NAFLD by age, sex, race/ethnicity, education level, the ratio of family income to poverty, hypertension, and diabetes. A Wald chi-square test was used to examine the cross-product terms between these variables and the HLS. Due to multiple tests being conducted, we used the Bonferroni correction to define the statistical significance as p < 0.007 (0.05/7) to account for the interaction effect.
Furthermore, considering departures from the normal distribution, all liver function parameters were natural logarithm transformed. Multivariable linear regression was performed to estimate the percentage change and 95% CIs for the associations between the HLS status and transformed liver function parameters, with adjustment for age, sex, race/ethnicity, education, the ratio of family income to poverty, hypertension, and diabetes. A two-sided p-value < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using R software version 4.1.0 (University of Auckland, Auckland, New Zealand).
3. Results
3.1. Characteristics of Participants across HLS
A total of 3893 participants aged 18 to 80 years (mean age 49.3 ± 18.4 years) were included in the study. The prevalence was 36.9% (1437) for NAFLD and 7.7% (301) for CSF. Approximately 14.1% of participants possessed zero or one healthy lifestyle, whereas 62.5% possessed two or three healthy lifestyles, and 23.4% possessed four or five healthy lifestyles. Participants among the high HLS were more likely to be younger, female, and of other races, had a higher education level, a higher ratio of family income to poverty, and were less likely to have a history of hypertension and diabetes. In addition, we observed that the CAP, LSM, globulin, ALT, and GGT decreased with an increased HLS, whereas albumin, total protein, total bilirubin, and AST increased with an increased HLS (Table 1). Characteristics of participants according to the NAFLD and CSF phenotypes were shown in Table S1.
Table 1.
The characteristics of participants according to status of healthy lifestyle score †.
| Characteristics | Overall | Healthy Lifestyle Score (HLS) ‡ | p | ||
|---|---|---|---|---|---|
| Low HLS | Medium HLS | High HLS | |||
| No. of Participants | 3893 | 547 (14.05) | 2434 (62.52) | 912 (23.43) | |
| Age (%) | <0.001 | ||||
| 18–39 | 1330 (34.16) | 123 (22.49) | 817 (33.57) | 390 (42.76) | |
| 40–59 | 1172 (30.11) | 172 (31.44) | 750 (30.81) | 250 (27.41) | |
| ≥60 | 1391 (35.73) | 252 (46.07) | 867 (35.62) | 272 (29.82) | |
| Sex (Female, %) | 2030 (52.14) | 259 (47.35) | 1226 (50.37) | 545 (59.76) | <0.001 |
| Race (%) | |||||
| Non-Hispanic white | 1360 (34.93) | 230 (42.05) | 860 (35.33) | 270 (29.61) | |
| Non-Hispanic black | 876 (22.50) | 152 (27.79) | 588 (24.16) | 136 (14.91) | |
| Other races | 1657 (42.56) | 165 (30.16) | 986 (40.51) | 506 (55.48) | |
| Education (%) | <0.001 | ||||
| Less than high school | 691 (17.78) | 103 (18.83) | 479 (19.71) | 109 (11.99) | |
| High school diploma | 954 (24.55) | 160 (29.25) | 610 (25.10) | 184 (20.24) | |
| More than high school | 2241 (57.67) | 284 (51.92) | 1341 (55.19) | 616 (67.77) | |
| Family income to poverty ratio (%) | <0.001 | ||||
| <1.30 | 956 (27.74) | 164 (34.24) | 599 (27.91) | 193 (23.51) | |
| 1.30–3.49 | 1389 (40.31) | 209 (43.63) | 902 (42.03) | 278 (33.86) | |
| ≥3.50 | 1101 (31.95) | 106 (22.13) | 645 (30.06) | 350 (42.63) | |
| Hypertension (%) | 1592 (41.58) | 291 (54.39) | 1035 (43.23) | 266 (29.56) | <0.001 |
| Diabetes (%) | 732 (18.80) | 143 (26.14) | 484 (19.88) | 105 (11.51) | <0.001 |
| NAFLD (%) | 1437 (36.91) | 270 (49.36) | 989 (40.63) | 178 (19.52) | <0.001 |
| CSF (%) | 301 (7.73) | 64 (11.70) | 210 (8.63) | 27 (2.96) | <0.001 |
| Steatosis (CAP, dB/m) | 263.29 ± 62.91 | 282.21 ± 61.94 | 269.33 ± 62.63 | 235.81 ± 55.37 | <0.001 |
| Fibrosis (LSM, kPa) | 5.70 ± 4.51 | 6.38 ± 5.73 | 5.84 ± 4.75 | 4.92 ± 2.43 | <0.001 |
| Albumin (g/dL) | 4.08 ± 0.32 | 4.00 ± 0.35 | 4.06 ± 0.31 | 4.17 ± 0.31 | <0.001 |
| Globulin (g/dL) | 3.07 ± 0.42 | 3.09 ± 0.45 | 3.09 ± 0.42 | 3.03 ± 0.41 | 0.004 |
| Total protein (g/dL) | 7.15 ± 0.43 | 7.09 ± 0.43 | 7.15 ± 0.43 | 7.20 ± 0.43 | <0.001 |
| Total bilirubin (mg/dL) | 0.46 ± 0.28 | 0.43 ± 0.24 | 0.45 ± 0.26 | 0.51 ± 0.33 | <0.001 |
| ALT (IU/L) | 22.04 ± 17.16 | 21.49 ± 13.55 | 23.02 ± 17.59 | 19.77 ± 17.70 | <0.001 |
| AST (IU/L) | 21.41 ± 11.15 | 20.29 ± 8.64 | 21.79 ± 12.01 | 21.08 ± 9.99 | 0.013 |
| GGT (IU/L) | 29.87 ± 41.27 | 35.41 ± 53.22 | 31.18 ± 43.53 | 23.07 ± 21.24 | <0.001 |
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CAP, Controlled attenuation parameter; CSF, clinically significant fibrosis; GGT, Gamma-glutamyl transaminase; HLS, Healthy lifestyle score; LSM, Liver stiffness measurement; NAFLD, Non-alcoholic fatty liver diseases; NHANES, U.S. National Health and Nutrition Examination Survey. † Values were presented as mean ± SD or percentages. ‡ Low HLS: 0–1 score; Medium HLS: 2–3 score; High HLS: 4–5 score.
3.2. HLS and Odds of NAFLD and CSF
We observed a significant decline in the prevalence of NAFLD and CSF across HLS (Table 1). Compared with participants in low HLS, the adjusted ORs (95% CIs) of NAFLD for those in the high HLS group were 0.25 (0.19~0.33, Ptrend < 0.001). Similarly, a high HLS was associated with lower odds of CSF (OR = 0.30, 95% CI: 0.18~0.50, Ptrend < 0.001, Table 2).
Table 2.
Odds ratios and 95% confidence intervals of NAFLD and CSF according to healthy lifestyle scores.
| Liver Disease | Healthy Lifestyle Score (HLS) † | Per 1 Score | Ptrend ¶ | ||
|---|---|---|---|---|---|
| Low HLS | Medium HLS | High HLS | |||
| NAFLD | |||||
| No. of cases/participants | 270/547 | 989/2434 | 178/912 | ||
| Model 1 ‡ | 1 | 0.70 (0.58~0.85) | 0.25 (0.20~0.31) | 0.66 (0.62~0.70) | <0.001 |
| Model 2 § | 1 | 0.71 (0.58~0.89) | 0.25 (0.19~0.33) | 0.66 (0.61~0.71) | <0.001 |
| CSF | |||||
| No. of cases/participants | 64/547 | 210/2434 | 27/912 | ||
| Model 1 ‡ | 1 | 0.71 (0.53~0.96) | 0.23 (0.15~0.37) | 0.69 (0.62~0.77) | <0.001 |
| Model 2 § | 1 | 0.81 (0.58~1.12) | 0.30 (0.18~0.50) | 0.76 (0.67~0.86) | <0.001 |
Abbreviations: CSF, clinically significant fibrosis; NAFLD, Non-alcoholic fatty liver diseases. † Low HLS: 0–1 score; Medium HLS: 2–3 score; High HLS: 4–5 score. ‡ Model 1 did not adjust for the covariates. § Model 2 was adjusted for age (18–39, 40–59, ≥60), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, and other races), education (less than high school, high school diploma, and more than high school), the ratio of family income to poverty (<1.30, 1.30–3.49, and ≥3.50), hypertension (yes, no), and diabetes (yes, no). ¶ The trend test was performed by using HLS as a continuous variable in the models.
When examining components in HLS, lower odds of NAFLD was observed among individuals with a healthy BMI level (OR = 0.13, 95% CI: 0.10~0.16), a healthy sleep level (OR = 0.73, 95% CI: 0.61~0.87), and a healthy HEI-2015 level (OR = 0.80, 95% CI: 0.69~0.94), whereas only a healthy BMI level (OR = 0.32, 95% CI: 0.20~0.50) and a healthy HEI-2015 level (OR = 0.66, 95% CI: 0.50~0.88) were associated with lower odds of CSF (Table 3).
Table 3.
Odds ratios and 95% confidence intervals of NAFLD and CSF by individual lifestyle factors †.
| Components of HLS | NAFLD Phenotype | CSF Phenotype | ||
|---|---|---|---|---|
| OR and 95% CI | p | OR and 95% CI | p | |
| BMI score (1 vs. 0) | 0.13 (0.10~0.16) | <0.001 | 0.32 (0.20~0.50) | <0.001 |
| Smoking score (1 vs. 0) | 0.93 (0.79~1.09) | 0.377 | 0.97 (0.74~1.28) | 0.835 |
| Sleep score (1 vs. 0) | 0.73 (0.61~0.87) | <0.001 | 0.90 (0.67~1.21) | 0.469 |
| Physical activity score (1 vs. 0) | 0.87 (0.74~1.02) | 0.087 | 0.83 (0.63~1.08) | 0.169 |
| HEI-2015 score (1 vs. 0) | 0.80 (0.69~0.94) | 0.006 | 0.66 (0.50~0.88) | 0.004 |
Abbreviations: BMI, Body mass index; CSF, clinically significant fibrosis; HEI, Healthy eating index; NAFLD, Non-alcoholic fatty liver diseases. † Model was adjusted for age (18–39, 40–59, ≥60), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, other races), education (less than high school, high school diploma, more than high school), the ratio of family income to poverty (<1.30, 1.30–3.49, ≥3.50), hypertension (yes, no), and diabetes (yes, no).
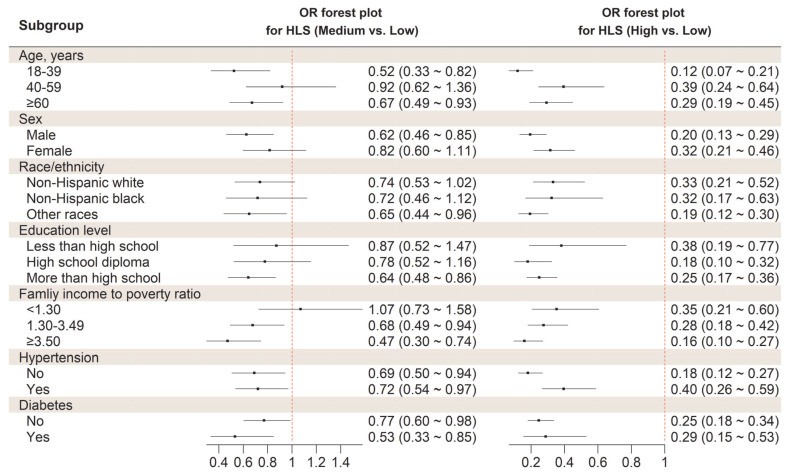
In stratified analysis, we did not find any differential association of HLS with odds of NAFLD, according to age, sex, race/ethnicity, education level, ratio of family income to poverty, and diabetes (all p for interaction > 0.007). However, the inverse association between HLS and NAFLD appeared stronger in participants who were free of hypertension, compared with hypertension patients (p for interaction = 0.005) (Figure 1).
Figure 1.
Stratified analysis on the association between HLS and odds of NAFLD. Abbreviations: HLS, Healthy lifestyle score; NAFLD, Non-alcoholic fatty liver diseases; OR, Odds ratio. The model was adjusted for age, sex, race/ethnicity, education level, the ratio of family income to poverty, hypertension, and diabetes except for variables examined in the figure.
3.3. HLS and Liver Function Parameters
After multivariable adjustment, HLS was positively associated with albumin, total protein, total bilirubin, and AST, with a percentage difference of (High HLS vs. Low HLS) of 3.25% (95% CI: 2.35~4.16%, Ptrend < 0.001), 0.94% (95% CI: 0.24~1.64%, Ptrend = 0.001), 18.60% (95% CI: 11.50~26.16%, Ptrend < 0.001), and 6.60% (2.28~11.11%, Ptrend = 0.001), respectively. In contrast, HLS was inversely associated with globulin, ALT, and GGT, with a corresponding percentage difference of −1.95% (95% CI: −3.44~−0.42%, Ptrend = 0.003), −6.36% (95% CI: −11.8~−0.58%, Ptrend = 0.010), and -19.0% (95% CI: −24.7~−12.8%, Ptrend < 0.001, Table 4), respectively.
Table 4.
Percentage change (%) and 95% confidence intervals for the associations between the healthy lifestyle score and liver function parameters †.
| Parameters | Healthy Lifestyle Score (HLS) § | Per 1 Score | Ptrend ¶ | ||
|---|---|---|---|---|---|
| Low HLS | Medium HLS | High HLS | |||
| Albumin ‡ | 0 | 0.89 (0.13~1.64) | 3.25 (2.35~4.16) | 1.04 (0.80~1.28) | <0.001 |
| Globulin ‡ | 0 | −0.48 (−1.78~0.84) | −1.95 (−3.44~−0.42) | −0.63 (−1.05~−0.22) | 0.003 |
| Total protein ‡ | 0 | 0.20 (−0.39~0.79) | 0.94 (0.24~1.64) | 0.31 (0.13~0.50) | 0.001 |
| Total bilirubin ‡ | 0 | 4.36 (−1.00~10.02) | 18.60 (11.50~26.16) | 5.89 (4.13~7.68) | <0.001 |
| ALT ‡ | 0 | 3.59 (−1.57~9.03) | −6.36 (−11.8~−0.58) | −2.11 (−3.69~−0.51) | 0.010 |
| AST ‡ | 0 | 4.82 (1.17~8.59) | 6.60 (2.28~11.11) | 1.88 (0.74~3.03) | 0.001 |
| GGT ‡ | 0 | −5.56 (−11.3~0.58) | −19.0 (−24.7~−12.8) | −6.30 (−8.16~−4.41) | <0.001 |
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; GGT, Gamma-glutamyl transaminase. † Percentage change was calculated as (eβ − 1) ∗ 100% based on multivariable linear regression with adjustment for age, sex, race/ethnicity, education, ratio of family income to poverty, hypertension, and diabetes. ‡ Liver function parameters were natural logarithm transformed. § Low HLS: 0–1 score; Medium HLS: 2–3 score; High HLS: 4–5 score. ¶ Linear trend test was performed using HLS as a continuous variable in the models.
4. Discussion
In this large cross-sectional study, we found that greater adherence to HLS was associated with lower odds of NAFLD and CSF, where normal BMI was a key contributor. Furthermore, higher adherence to HLS appeared to be linked with improved hepatic parameters.
Several lifestyle factors, such as diet, physical activity, and smoking are postulated as important modifiable risk factors for chronic diseases. Numerous studies have linked lifestyle factors with chronic diseases and mortality, showing that adherence to a healthy lifestyle is beneficial for health and longevity [10,12,19]. These lifestyle factors often operate in a synergistic manner, which cannot be captured when studying each individually [23]. Instead of examining each lifestyle factor independently, a holistic approach integrating the whole lifestyle pattern was proposed. Despite divergent definitions of HLS, two cross-sectional studies have appraised the association between HLS and FLD [14,15] and yielded similar results to our current findings. One of the studies constructed a four-item HLS by incorporating smoking, waist circumference, physical activity, and diet [14], whereas a six-item HLS including smoking, BMI, physical activity, diet, sleep, and anxiety was built in the other study [15]. The components of three constructed HLS have considerable overlap, indicating that these key individual lifestyle factors play a crucial role in modulating health outcomes.
The two previously published studies did not investigate the association of HLS with liver function parameters. Liver diseases develop silently with no signs or symptoms until the late stages. In pre-terminal stages, liver chemistries, which are commonly ordered in comprehensive metabolic profiles, may be abnormal and contribute to the early detection of diseases. Consistent with the link between HLS and VCTE-determined NAFLD, most of these liver chemistries varied significantly across HLS. On the one hand, we observed elevated serum albumin, total protein, and total bilirubin among participants with high HLS. Albumin is exclusively synthesized by the liver, and low albumin levels may be a marker of advanced diseases in chronic liver inflammation or cirrhosis [24,25]. Bilirubin is the end product of the breakdown of red blood cells. A growing body of literature shows that higher bilirubin levels are inversely associated with NAFLD as well as metabolic syndrome [26,27], although the underlying mechanisms are not well elucidated. On the other hand, a decline in serum globulin, ALT, and GGT were observed in adults with high HLS. Globulin is a group of proteins synthesized mainly in the liver by the immune system and an increase in globulin may indicate inflammatory diseases [28]. ALT and GGT are released from damaged liver cells into the blood after hepatocellular injury or death [29,30], those are considered to serve as non-invasive biomarkers of liver injury.
Until now, no approved pharmacotherapy for NAFLD exists. Practice guidance advocates that lifestyle interventions mainly consisting of weight loss, exercise, and dietary change remain the primary approach for the management of NAFLD across the disease spectrum [31,32]. NAFLD is strongly associated with obesity with a prevalence of up to 80% in obese adults, and 16% in adults with a normal BMI [33]. In this study, BMI was observed as a stronger contributor to the development of NAFLD and CSF among the five lifestyles. Obesity contributes to NAFLD by either increasing the synthesis of triglycerides in the liver or increasing the flow of fatty acids from adipose tissue to the liver [34]. Slow and controlled weight loss is the key to the improvement in the histopathological features of non-alcoholic steatohepatitis [32]. A meta-analysis of eight randomized controlled trials assessed the effect of weight loss on NAFLD [35] and suggested that losing at least 5% of body weight could improve hepatic steatosis, whereas a ≥7% body weight reduction was associated with an improvement in the NAFLD activity score. The findings from a prospective study of 293 patients with histologically proven non-alcoholic steatohepatitis indicated that greater than 10% weight loss was able to ameliorate portal inflammation and fibrosis [36].
Some dietary patterns have emerged as a novel approach to examining the association between diet and the risk of chronic liver diseases. Our recent findings [37] together with a hospital-based case-control study [38] performed in Iran consistently supported that a higher dietary insulinaemic potential was linked with an increased prevalence of hepatic steatosis and fibrosis. In addition, the dietary inflammatory index [39], and dietary approaches to stop hypertension [40] were also shown to be associated with NAFLD. In the present study, we calculated HEI-2015 to assess diet quality. The HEI-2015 was originally designed to evaluate diet for compliance with the Dietary Guidelines for Americans (DGA) 2015–2020 [41]. DGA encourages increasing the consumption of fruits, vegetables, whole grains, high-protein food from seafood, lean meats, poultry, eggs, and nuts, and limiting the intake of saturated fats, trans fats, and added sugars. An energy-restricted diet with a low calorie (1200–1600 kcal/d), low fat (less than 10% of energy from saturated fats), low carbohydrate diet (less than 50% of energy) is recommended for NAFLD patients [42].
Our findings indicated that lack of sleep was another contributor to NAFLD. Several epidemiological studies have examined the relationship between sleep duration and NAFLD and have demonstrated short sleep duration was an important risk factor for the onset of NAFLD [7,43]. This association may be driven by systemic inflammation and metabolic disorder-related pathways, including insulin resistance and adipose dysfunction [44]. Furthermore, previous studies have shown that cigarette smoking [45] and lack of physical activity [46] were associated with an increased risk of NAFLD and CSF. Although our study did not support a significant association between having healthy smoking and physical activity levels and the presence of NAFLD and CSF, most associations were inverse and thus were suggestive of possible benefits of having healthy smoking and physical activity levels. These inconsistent findings might be due to chance or no consensus on the definition of a harmful level of cigarette smoking and physical activity.
We found a significant interaction between HLS and hypertension on the odds of developing NAFLD, whereby adherence to HLS yielded a stronger health benefit against NAFLD among participants free of hypertension. Existing studies have shown a comorbidity phenomenon of hypertension and NAFLD. It is not clear whether this simply reflects shared risk factors, or whether hypertension, per se, increases the risks of NAFLD [47]. The prevalence of NAFLD increased progressively from 27.2% at optimal blood pressure (SBP < 120 mmHg and DBP < 80 mmHg) to 59.6% in participants with hypertension. Similarly, the prevalence of advanced fibrosis increased from optimal blood pressure to hypertension at 0.8% and 5.4%, respectively [48]. Therefore, this limited the health benefit of HLS among participants with hypertension.
Several limitations should be addressed. First, self-reported cigarette smoking, sleep duration, and physical activity as well as other covariates from questionnaires had measurement errors, although several methods were used to reduce measurement error in the investigation. Second, dietary information was collected based on 24-h recall. Although two 24-h recalls were conducted, it was difficult to capture the long-term dietary habits. Third, the cross-sectional design of the current study did not allow the determination of causality. Findings from our study need to be confirmed by prospective cohort studies and clinical trials in the future.
5. Conclusions
In conclusion, our findings pointed to the important association of HLS comprised of five favorable lifestyle factors with NAFLD and CSF in a representative sample of U.S. adults. With the increasing number of favorable lifestyle factors, the odds of NAFLD and CSF decreased. Strategies for the promotion of a healthy lifestyle should be considered as part of NAFLD prevention in adults.
Acknowledgments
This study uses data from the NHANES. We thank the NCHS and the US Centers for Disease Control and Prevention (CDC) for their financial support for data collection and analysis. We also thank the study participants and staff.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14214462/s1, Figure S1: Flow chart of the study participants from the 2017–2018 cycle of the NHANES; Table S1: The characteristics of participants according to NAFLD and CSF phenotypes.
Author Contributions
Y.Z. (Yu Zhu) and H.Y. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: W.Y. and Y.Z. (Yu Zhu); Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Y.Z. (Yu Zhu) and H.Y.; Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Y.Z. (Yu Zhu); Obtained funding: W.Y.; Administrative, technical, or material support: W.W. and W.Y.; Study supervision: W.W. and W.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The NCHS Research Ethics Review Board approved the NHANES study protocols (Protocol #2011-17 and Protocol #2018-01). And the study design was confirmed in accordance with the Helsinki Declaration.
Informed Consent Statement
All participants provided informed consent before enrollment.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction (https://www.cdc.gov/nchs/nhanes/index.htm, accessed on 11 April 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82073651), Anhui Provincial Natural Science Foundation (2008085MH262 and 2108085QH357), Anhui Provincial Education Department (gxyqZD2021099), and grants from Anhui Medical University (2021xkjT007, XJ201935). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hardy T., Oakley F., Anstee Q.M., Day C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R., Priyadarshi R.N., Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J. Clin. Transl. Hepatol. 2020;8:76–86. doi: 10.14218/JCTH.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Stepanova M., Younossi Y., Golabi P., Mishra A., Rafiq N., Henry L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 5.Pang Q., Zhang J.Y., Song S.D., Qu K., Xu X.S., Liu S.S., Liu C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J. Gastroenterol. 2015;21:1650–1662. doi: 10.3748/wjg.v21.i5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zein C.O., Unalp A., Colvin R., Liu Y.C., McCullough A.J., Nonalcoholic Steatohepatitis Clinical Research N. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 2011;54:753–759. doi: 10.1016/j.jhep.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura T., Hashimoto Y., Hamaguchi M., Obora A., Kojima T., Fukui M. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: A population-based longitudinal study. J. Gastrointestin. Liver Dis. 2019;28:73–81. doi: 10.15403/jgld.2014.1121.281.alc. [DOI] [PubMed] [Google Scholar]
- 8.Gerber L., Otgonsuren M., Mishra A., Escheik C., Birerdinc A., Stepanova M., Younossi Z.M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment. Pharmacol. Ther. 2012;36:772–781. doi: 10.1111/apt.12038. [DOI] [PubMed] [Google Scholar]
- 9.Abenavoli L., Boccuto L., Federico A., Dallio M., Loguercio C., Di Renzo L., De Lorenzo A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health. 2019;16:3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai G., Zhang J., Zhao C., Wang Y., Qi Y., Zhang B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens. Res. 2017;40:196–202. doi: 10.1038/hr.2016.119. [DOI] [PubMed] [Google Scholar]
- 11.Song C., Lv J., Yu C., Zhu M., Yu C., Guo Y., Yang L., Chen Y., Chen Z., Jiang T., et al. Adherence to Healthy Lifestyle and Liver cancer in Chinese: A prospective cohort study of 0.5 million people. Br. J. Cancer. 2022;126:815–821. doi: 10.1038/s41416-021-01645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun C., Li K., Xu H., Wang X., Qin P., Wang S., Liang B., Xu L. Association of healthy lifestyle score with all-cause mortality and life expectancy: A city-wide prospective cohort study of cancer survivors. BMC Med. 2021;19:158. doi: 10.1186/s12916-021-02024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y.B., Chen C., Pan X.F., Guo J., Li Y., Franco O.H., Liu G., Pan A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ. 2021;373:n604. doi: 10.1136/bmj.n604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch M., Borggrefe J., Schlesinger S., Barbaresko J., Groth G., Jacobs G., Lieb W., Laudes M., Muller M.J., Bosy-Westphal A., et al. Association of a lifestyle index with MRI-determined liver fat content in a general population study. J. Epidemiol. Community Health. 2015;69:732–737. doi: 10.1136/jech-2014-204989. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y.Y., Zhong Q.W., Zhong H.L., Xiong F., Ke Y.B., Chen Y.M. Higher Healthy Lifestyle Score is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and older Chinese adults: A community-based cross-sectional study. Public Health Nutr. 2021;24:5081–5089. doi: 10.1017/S1368980021000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention NHANES Procedure Manuals. [(accessed on 11 April 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2017.
- 17.Bull F.C., Maslin T.S., Armstrong T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health. 2009;6:790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 18.Keating X.D., Zhou K., Liu X., Hodges M., Liu J., Guan J., Phelps A., Castro-Pinero J. Reliability and Concurrent Validity of Global Physical Activity Questionnaire (GPAQ): A Systematic Review. Int. J. Environ. Res. Public Health. 2019;16:4128. doi: 10.3390/ijerph16214128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Schoufour J., Wang D.D., Dhana K., Pan A., Liu X., Song M., Liu G., Shin H.J., Sun Q., et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ. 2020;368:l6669. doi: 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention NHANES Laboratory Methods. [(accessed on 11 April 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2017.
- 21.Vilar-Gomez E., Nephew L.D., Vuppalanchi R., Gawrieh S., Mladenovic A., Pike F., Samala N., Chalasani N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2022;75:1491–1506. doi: 10.1002/hep.32207. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui M.S., Vuppalanchi R., Van Natta M.L., Hallinan E., Kowdley K.V., Abdelmalek M., Neuschwander-Tetri B.A., Loomba R., Dasarathy S., Brandman D., et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019;17:156–163.e2. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Thani M., Al Thani A.A., Al-Chetachi W., Al Malki B., Khalifa S.A., Haj Bakri A., Hwalla N., Nasreddine L., Naja F. A ‘High Risk’ Lifestyle Pattern Is Associated with Metabolic Syndrome among Qatari Women of Reproductive Age: A Cross-Sectional National Study. Int. J. Mol. Sci. 2016;17:698. doi: 10.3390/ijms17060698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston D.E. Special considerations in interpreting liver function tests. Am. Fam. Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- 25.Agrawal S., Dhiman R.K., Limdi J.K. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2016;92:223–234. doi: 10.1136/postgradmedj-2015-133715. [DOI] [PubMed] [Google Scholar]
- 26.Kwak M.S., Kim D., Chung G.E., Kang S.J., Park M.J., Kim Y.J., Yoon J.H., Lee H.S. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012;18:383–390. doi: 10.3350/cmh.2012.18.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitek L., Tiribelli C. Bilirubin: The yellow hormone? J. Hepatol. 2021;75:1485–1490. doi: 10.1016/j.jhep.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Ye Y., Chen W., Gu M., Xian G., Pan B., Zheng L., Zhang Z., Sheng P. Serum globulin and albumin to globulin ratio as potential diagnostic biomarkers for periprosthetic joint infection: A retrospective review. J. Orthop. Surg. Res. 2020;15:459. doi: 10.1186/s13018-020-01959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W.R., Flamm S.L., Di Bisceglie A.M., Bodenheimer H.C., Public Policy Committee of the American Association for the Study of Liver Disease Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 30.Kunutsor S.K. Gamma-glutamyltransferase-friend or foe within? Liver Int. 2016;36:1723–1734. doi: 10.1111/liv.13221. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A., Dalbeni A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021;22:2350. doi: 10.3390/ijms22052350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 33.Milic S., Lulic D., Stimac D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014;20:9330–9337. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabner G.F., Xie H., Schweiger M., Zechner R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021;3:1445–1465. doi: 10.1038/s42255-021-00493-6. [DOI] [PubMed] [Google Scholar]
- 35.Musso G., Cassader M., Rosina F., Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 36.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005. quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y., Peng Z., Lu Y., Li H., Zeng X., Zhang Z., Li X., Hu C., Hu A., Zhao Q., et al. Higher dietary insulinaemic potential is associated with increased risk of liver steatosis and fibrosis. Liver Int. 2022;42:69–79. doi: 10.1111/liv.15057. [DOI] [PubMed] [Google Scholar]
- 38.Sohouli M.H., Sayyari A.A., Lari A., Nameni G., Lotfi M., Fatahi S., Saneie S., Gaman M.A., Moodi F., Raee P., et al. Association of dietary insulinaemic potential and odds of non-alcoholic fatty liver disease among adults: A case-control study. J. Hum. Nutr. Diet. 2021;34:901–909. doi: 10.1111/jhn.12865. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez-Velez R., Garcia-Hermoso A., Izquierdo M., Correa-Rodriguez M. The Dietary Inflammatory Index and hepatic health in the US adult population. J. Hum. Nutr. Diet. 2022;35:968–979. doi: 10.1111/jhn.12962. [DOI] [PubMed] [Google Scholar]
- 40.Xiao M.L., Lin J.S., Li Y.H., Liu M., Deng Y.Y., Wang C.Y., Chen Y.M. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr. 2020;23:674–682. doi: 10.1017/S1368980019002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs-Smith S.M., Pannucci T.E., Subar A.F., Kirkpatrick S.I., Lerman J.L., Tooze J.A., Wilson M.M., Reedy J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wijarnpreecha K., Thongprayoon C., Panjawatanan P., Ungprasert P. Short sleep duration and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016;31:1802–1807. doi: 10.1111/jgh.13391. [DOI] [PubMed] [Google Scholar]
- 44.Marjot T., Ray D.W., Williams F.R., Tomlinson J.W., Armstrong M.J. Sleep and liver disease: A bidirectional relationship. Lancet Gastroenterol. Hepatol. 2021;6:850–863. doi: 10.1016/S2468-1253(21)00169-2. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y., Dai M., Bi Y., Xu M., Xu Y., Li M., Wang T., Huang F., Xu B., Zhang J., et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): A population-based study in China. J. Epidemiol. 2013;23:115–121. doi: 10.2188/jea.JE20120067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D., Konyn P., Cholankeril G., Ahmed A. Physical Activity Is Associated With Nonalcoholic Fatty Liver Disease and Significant Fibrosis Measured by FibroScan. Clin. Gastroenterol. Hepatol. 2022;20:e1438–e1455. doi: 10.1016/j.cgh.2021.06.029. [DOI] [PubMed] [Google Scholar]
- 47.Ma C., Yan K., Wang Z., Zhang Q., Gao L., Xu T., Sai J., Cheng F., Du Y. The association between hypertension and nonalcoholic fatty liver disease (NAFLD): Literature evidence and systems biology analysis. Bioengineered. 2021;12:2187–2202. doi: 10.1080/21655979.2021.1933302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciardullo S., Monti T., Sala I., Grassi G., Mancia G., Perseghin G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertension. 2020;76:562–568. doi: 10.1161/HYPERTENSIONAHA.120.15220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction (https://www.cdc.gov/nchs/nhanes/index.htm, accessed on 11 April 2021).