Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary cystic kidney disease, with patients often having a positive family history that is characterized by a similar phenotype. However, in atypical cases, particularly those in which family history is unclear, a differential diagnosis between ADPKD and other cystic kidney diseases is important. When diagnosing ADPKD, cystic kidney diseases that can easily be excluded using clinical information include: multiple simple renal cysts, acquired cystic kidney disease (ACKD), multilocular renal cyst/multilocular cystic nephroma/polycystic nephroma, multicystic kidney/multicystic dysplastic kidney (MCDK), and unilateral renal cystic disease (URCD). However, there are other cystic kidney diseases that usually require genetic testing, or another means of supplementing clinical information to enable a differential diagnosis of ADPKD. These include autosomal recessive polycystic kidney disease (ARPKD), autosomal dominant tubulointerstitial kidney disease (ADTKD), nephronophthisis (NPH), oral-facial-digital (OFD) syndrome type 1, and neoplastic cystic kidney disease, such as tuberous sclerosis (TSC) and Von Hippel-Lindau (VHL) syndrome. To help physicians evaluate cystic kidney diseases, this article provides a review of cystic kidney diseases for which a differential diagnosis is required for ADPKD.
Keywords: cystic kidney diseases, ADPKD, differential diagnosis, hereditary
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of hereditary cystic kidney disease, affecting one in 1000–2500 individuals and is mainly caused by mutations in the PKD1 (78%) and PKD2 (15%) genes [1]. Approximately 7% of patients with ADPKD appear not to have a PKD1/2 mutation [2,3]. Among ADPKD patients, the severity of the effects of these mutations on renal survival was reported to be as follows: PKD1 truncating mutation > PKD1 non-truncating mutation > PKD2 mutation > no PKD1 or PKD2 mutation [4,5]. Proliferative cysts in both kidneys increase in number and size as kidney function impairment advances, often introducing complications such as hypertension, multiple hepatic cysts, cerebral aneurysm, heart valve disease, and colonic diverticula. Diagnosis often occurs in adulthood when symptoms are present, but a differential diagnosis of ADPKD is required to distinguish it from other cystic kidney diseases, that are also characterized by renal cysts. Patients with ADPKD often have a family history of this disease (75–90% having a parent with ADPKD [1]). However, among patients with no family history (the 10–25% who have no parent with ADPKD [1]), some 40% reportedly have no observed mutation in the PKD1 or PKD2 genes [6,7]; this suggests that careful diagnosis is required to differentiate between ADPKD and other cystic kidney diseases. The only therapeutic drug for patients with ADPKD is tolvaptan, a vasopressin V2-receptor antagonist that slows the rate of decline of the estimated glomerular filtration rate (eGFR) [8,9,10]. Accordingly, it is important to diagnose ADPKD from other cystic kidney diseases. To help physicians evaluate cystic kidney diseases, this article provides a review of the cystic kidney diseases for which a differential diagnosis is required for ADPKD (Table 1).
Table 1.
Summary of cystic kidney disease.
| Disease | Gene | Inheritance | Prevalence | Clinical Features |
|---|---|---|---|---|
| ADPKD | PKD1, PKD2 | AD | 1/1000–1/2500 | Proliferative cysts in both kidneys increase in number and size as kidney function impairment advances, often introducing complications such as hypertension, multiple hepatic cysts, cerebral aneurysm, heart valve disease, and colonic diverticula. |
| Multiple simple renal cysts | Common | Usually a simple cyst of only unilateral, but with age the number of cysts increases in both kidneys. Normally asymptomatic. | ||
| ACKD | Common | In long-term dialysis patients, the renal parenchyma in both kidneys may show atrophy and proliferation of microcysts. Asymptomatic, but screening for hemorrhage and renal cancer development is necessary. | ||
| MCDK | 1/1000–1/4300 | Renal cysts of various sizes gather. Renal prognosis is favorable if no complications such as VUR in the contralateral kidney. | ||
| URCD | Unknown | Unilateral renal cysts resembling ADPKD in imaging. No family history of PKD, no progress to ESKD, and no complications such as hepatic cysts or cerebral aneurysm. | ||
| ARPKD | PKHD1, DZIP1L | AR | 1/8000–1/40,000 | Renal cystic lesions reflecting the expansion of the collecting duct with kidney function impairment, and congenital hepatic fibrosis characterized by bile duct dysplasia and intrahepatic periportal fibrogenesis. Thrombocytopenia, splenomegaly, and portal hypertension. |
| ADTKD |
UMOD, MUC1, REN, HNF1B, SEC61A, DNAJB11 |
AD | Unknown | Tubulointerstitial fibrogenesis and progressive kidney function impairment. Poor urinary findings, slow decline in kidney function. Small renal cysts without kidney enlargement. ADTKD-UMOD, -MUC1, -REN, -SEC61A; Hyperuricemia and Gout, ADTKD-HNF1B; diabetes mellitus and liver function impairment, ADTKD-SEC61A; congenital anemia, neutropenia, and hypogammaglobulinemia. ADTKD-DNAJB11; circulatory abnormalities such as intracranial aneurysm, thoracic aorta enlargement, and carotid artery dissociation. |
| NPH |
NPHP1-20, NPHP1L, NPHP2L, TRAF31P1, AH11, CC2D2A |
AR | 1/50,000–1/100,000 | Impaired urinary concentration, chronic tubulointerstitial nephritis, renal cystic lesions and accompanying kidney function impairment and progress to ESKD by the age of 30 years. Extrarenal lesions are reportedly found in 10–20% of patients, retinitis pigmentosa, cerebellar vermis hypoplasia, gaze palsy, hepatic fibrosis, and skeletal abnormalities. |
| OFD type1 | OFD1 | X-linked | 1/50,000–1/250,000 | Progress to kidney failure in adulthood with PKD with a variety of morphological abnormalities in the oral, facial, and finger and toe. Normal to large-sized kidneys. |
| TSC | TSC1, TSC2 | AD | 1/10,000 | Hamartoma in the skin, nervous system, kidney, lung, bone, and elsewhere. Renal lesions; angiomyolipoma (AML), renal cysts, renal cell carcinoma. |
| VHL syndrome | VHL | AD | 1/50,000 | Renal cell carcinoma, pancreatic cysts, central nervous system and retinal hemangioblastoma, and pheochromocytoma. Kidney function impairment is rare. |
Abbreviations: autosomal dominant polycystic kidney disease (ADPKD), acquired cystic kidney disease (ACKD), multicystic dysplastic kidney (MCDK), unilateral renal cystic disease (URCD), autosomal recessive polycystic kidney disease (ARPKD), autosomal dominant tubulointerstitial kidney disease (ADTKD), nephronophthisis (NPH), oral-facial-digital syndrome (OFD), tuberous sclerosis (TSC), Von Hippel-Lindau (VHL), autosomal dominant (AD), autosomal recessive (AR), polycystic kidney disease (PKD), end-stage kidney disease (ESKD).
2. Cystic Kidney Diseases That Need to Be Excluded from an ADPKD Diagnosis
2.1. Multiple Simple Renal Cysts
Simple renal cysts are an acquired cystic kidney disease which primarily occur in the renal cortex; they present as thin-walled, saclike structures in the renal cortex and renal pelvic environment. Usually a simple cyst of only unilateral, but with age the number of cysts increases and the frequency with which they are present in both kidneys increases [11]. Simple renal cysts are rarely found in childhood but increase with age after the age of 30. Autopsy reports indicate the presence of at least one renal cyst in 50% of patients aged 50 years or above. Prevalence rates can vary depending on age, gender, and ethnicity [12,13,14].
Current assumptions regarding renal cyst formation indicate abnormal hypertrophic response in nephrons due to renal ischemia or obstruction, with cyst growth and compensatory hyperfiltration leading to nephron loss [14]. As renal cysts have a slow growth rate, many years may pass between onset and detection of simple renal cysts. The risk of latent kidney function impairment increases with age, and aging is associated with decreased glomerular filtration rate and increased incidence of renal cysts [15]. Simple renal cysts are normally asymptomatic (unless they are exceedingly large) and have little impact on kidney function. Typically, they increase slowly, or remain unchanged over time [11]. Symptoms include hemorrhagic cysts (2–4% of cases), infection, and rupture. Risk factors include aging, male gender, and hypertension.
Ultrasonograms show that simple renal cysts in the renal cortex and renal pelvic environment are round, anechoic, and accompanied by posterior echo enhancement [16]. Some cysts project beyond the renal parenchyma, with cysts located in the renal pelvic environment known as parapelvic renal cysts; these may easily be mistaken for hydronephrosis at first glance. Hydronephrosis and simple renal cysts can be differentiated by confirming individually isolated cysts one by one. Simple renal cysts are designated as Class I through computed tomography (CT) imaging using the Bosniak classification (Table 2) [17]. CT values are scaled from −10 to +20 Hounsfield units (HU), in the same manner as water, with smooth and sharp boundaries [16]. Contrast-enhanced CT imaging shows a uniform image with absolutely no contrast (Figure 1). Magnetic resonance imaging (MRI) displays the same signaling as water, with a T2-weighted image showing uniform hyperintensity (high-signal intensity) and a T1-weighted image exhibiting hypointensity (low-signal intensity), with no contrast whatsoever following contrast imaging. Fluid hemorrhage from the cyst interior, or the high-protein nature may appear as high absorbance on non-contrast CT imaging, and wall thickening, septum partitioning, and calcification may be observed in some cysts, giving them the name “complex cysts.” The Bosniak classification (Table 2) is helpful in the differentiation of malignant cysts; this is of utmost importance in diagnosing atypical renal cysts [17].
Table 2.
Bosniak Classification [17].
| Class | |
|---|---|
| I | Hairline-thin wall; water attenuation; no septa, calcifications, or solid components; nonenhancing |
| II | Two types: 1. Few thin septa with or without perceived (not measurable) enhancement; fine calcification or a short segment of slightly thickened calcification in the wall or septa 2. Homogeneously high-attenuating masses ≤ 3 cm that are sharply marginated and do not enhance |
| IIF | Two types: 1. Minimally thickened or more than a few thin septa with or without perceived (not measurable) enhancement that may have thick or nodular calcification 2. Intrarenal nonenhancing hyperattenuating renal masses ≥ 3 cm |
| III | Thickened or irregular walls or septa with measurable enhancement |
| IV | Soft-tissue components (i.e., nodule[s]) with measurable enhancement |
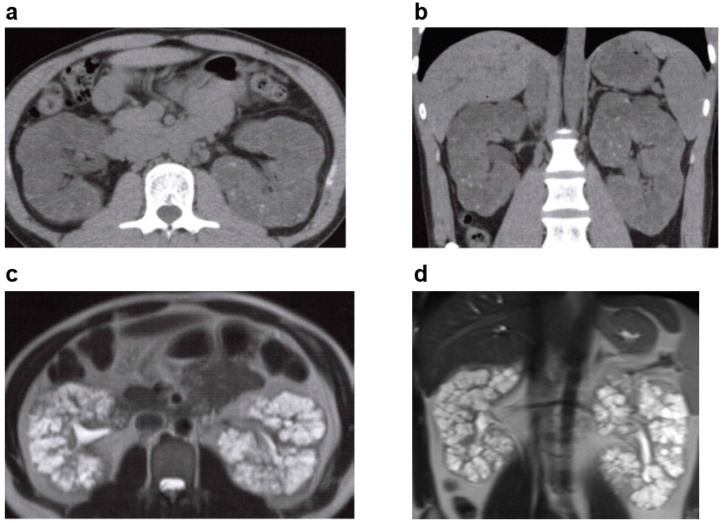
Figure 1.
Multiple simple renal cysts: 60s, male, kidney function eGFR 68 mL/min/1.73 m2, Bosniak class I (image provided by Shonan Kamakura General Hospital). Several low-density cysts (arrow) on the left kidney.
2.2. Acquired Cystic Kidney Disease (ACKD)
In long-term dialysis patients, the renal parenchyma in both kidneys may show atrophy and proliferation of microcysts. This condition is known as acquired cystic kidney disease (ACKD), and cancer may develop from these cyst walls. It has also been shown that the longer patients continue with dialysis, the greater the incidence of ACKD. Truong et al. reported that about 50% of dialysis patients developed ACKD, citing an incidence rate by dialysis interval of 13% for 2 years, 50% for 6 years, 87% for 9 years, and almost 100% for patients on dialysis for 10 years or more [18]. ACKD also appears in patients with chronic kidney disease (CKD); a report by Choyke et al. (2000) highlighted that ACKD was observed in 8–13% of patients with CKD prior to initiation of dialysis [19].
The developmental mechanism for ACKD remains largely unclarified. Grantham (1991) hypothesized that uremic toxic substances and electrolyte imbalance, factors specific to kidney failure, act as stimulants, with hyperplasia developing from compensatory hypertrophy of the tubular epithelium, resulting in cyst formation [20]. Other renal factors such as ischemic change, inflammation, hormonal abnormality, and precipitation of oxalic acid and other salts may also play a role [20].
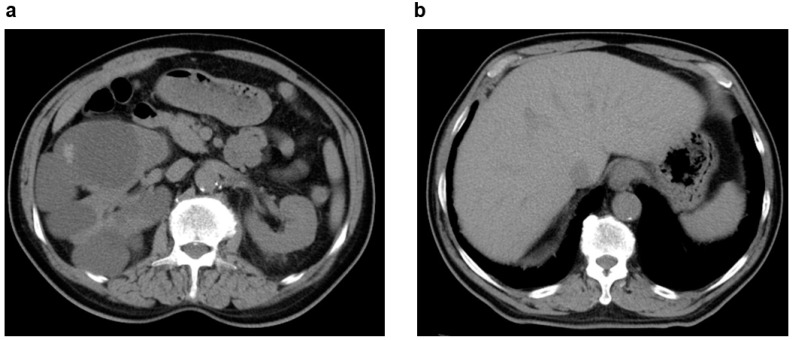
ACKD is asymptomatic, but screening is preferable as hemorrhage and renal cancer are likely complications. Ultrasound is the most common form of screening as a lower cost and non-invasive procedure. Ultrasonograms can highlight whether the kidney is atrophied or a normal size, if a thin cortex is producing high echogenicity, whether three or more cysts are present, and if it is able to detect these three symptoms in both kidneys. The absence of kidney enlargement in ACKD greatly differentiates it from ADPKD [21]. Meanwhile, CT imaging can indicate the presence of smaller cysts. Non-contrast CT images typically show proliferation of low-absorption lesions in the bilateral renal cortex, but without contrast medium it can be difficult to differentiate cysts from solid lesions. Contrast enhancement cannot characteristically be seen for cysts in contrast CT images. Imaging with MRI does, however, allow for the sharpest presentation of cysts. T1-weighted images display hypointensity and T2-weighted images, hyperintensity. In renal cancer linked to ACKD, a solid intracystic composition can often be verified by CT and MRI images. Ishikawa et al. (2011) reported the usefulness of ultrasonograms employing contrast medium in viewing intratumoral blood flow to simplify diagnosis [22]. Furthermore, Kitajima et al. (2019) reported that fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT images were useful in detecting increased FDG accumulation in some ACKD-related cases [23]. The Bosniak classification is utilized for differentiation between benign and malignant cystic kidney disease (Table 2) [17]. Contrast CT images for Bosniak Class IV cases are shown in Figure 2; cystic lesions on the left renal inferior pole show a solid densely stained area indicating a 90% or greater chance of malignant progression, so surgery is indicated in this case.
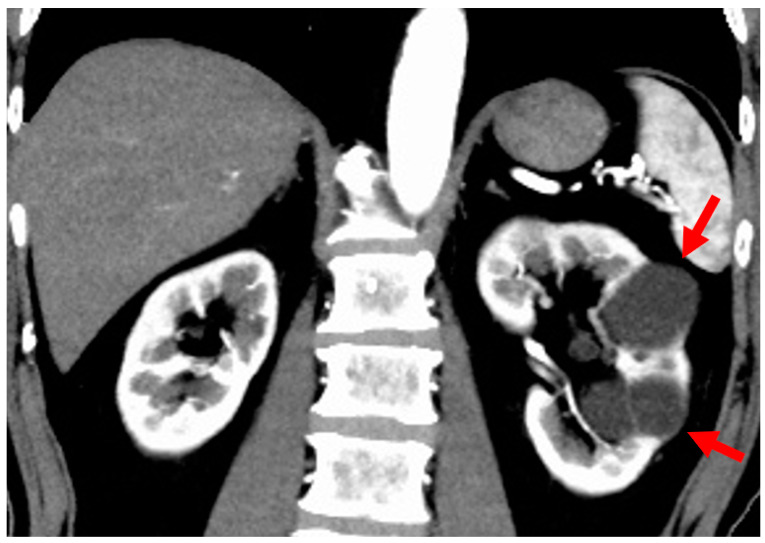
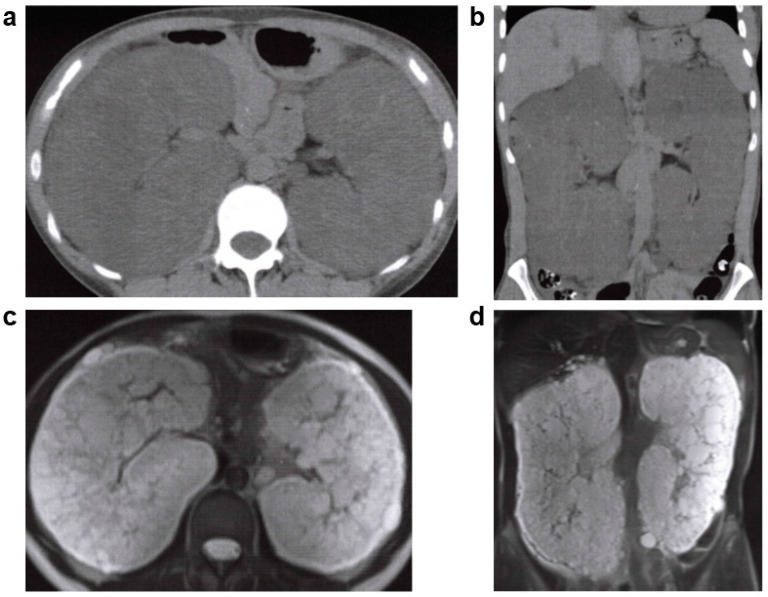
Figure 2.
Acquired cystic kidney disease (ACKD): 70s, female, history of hemodialysis 3 years (primary disease: diabetic nephropathy), Bosniak class IV (image provided by Osaka Saiseikai Nakatsu Hospital). (a) Contrast CT image (coronal section): Several low-density cysts (arrow) in both kidneys and a densely stained solid area of cystic lesions is visible on the inferior pole of the left kidney. (b) Contrast CT image (horizontal section): Several low-density cysts (arrow) in both kidneys and a densely stained solid area of cystic lesions is visible on the inferior pole of the left kidney.
Additionally, ACKD cysts are known to shrink in size following kidney transplant [24]. However, one should note that the risk of renal cancer remains [25,26,27].
2.3. Multilocular Renal Cyst (Also Referred to as Multilocular Cystic Nephroma or Polycystic Nephroma)
Multilocular renal cyst is a rare form of benign cystic kidney disease (neoplasm) which is also referred to as multilocular cystic nephroma or polycystic nephroma. Over 200 cases of this kidney cystic adenoma have been reported since the disease was first documented in 1892 [28,29,30]. Age distribution is bimodal (2 to 4 years, and patients in their forties to sixties). In patients under the age of 4 years, the ratio of males to females is 3:1, but this ratio is 1:9 in adults, with female patients predominating [29,30]. While sex hormones have been reported to play a role in this disease [31], its causality remains unclear. These lesions are noncommunicative with the renal pelvis; they are multilocular cyst lesions with no nephron structure and are largely supported by epithelial cells. Kidney tissue is normal in areas where lesions are not present.
Patients may exhibit nonspecific symptoms such as abdominal pain, hematuria, and urinary tract infections [29,30]. Ultrasonograms show alternating hypoechoic and hyperechoic cysts, with Doppler ultrasonograms helping to differentiate between benign and malignant cysts. CT imaging can verify well-defined borders of polycystic tumors (Figure 3). Calcification is rare but may be seen within the septum or on the cyst wall, complicating the differentiation from a malignant tumor. MRI imaging usually shows hypointensity on T1-weighted images and hyperintensity on T2-weighted images, with a hypointensive septum due to inclusion of tissue components (Figure 3).
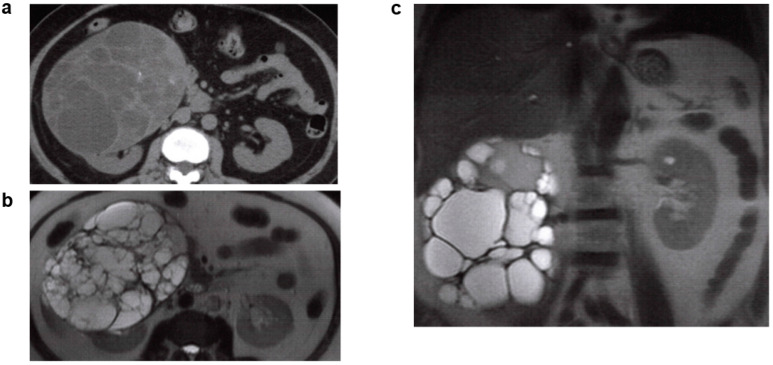
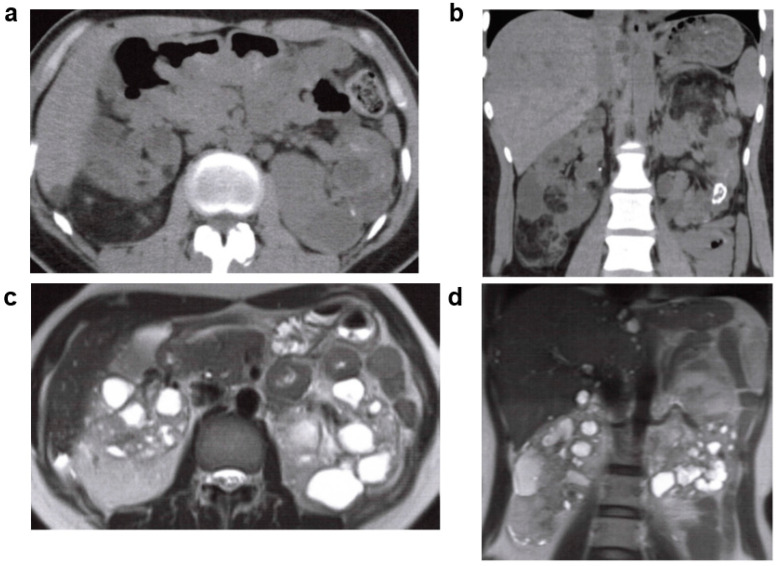
Figure 3.
Multilocular renal cyst, multilocular cystic nephroma, polycystic nephroma: 60s, female, no family history of polycystic kidney disease or end-stage kidney disease, no history of hepatic cysts, cerebral aneurysm, or heart valve disease, kidney function eGFR 82.7 mL/min/1.73 m2 (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): well-defined multilocular mass is visible on the right kidney. (b) MRI T2-weighted image (horizontal section): hyperintense polycystic mass (hypointense septum) is visible on the right kidney. (c) MRI T2-weighted image (coronal section): hyperintense polycystic mass (hypointense septum) is visible on the right kidney.
As already noted, ultrasound, CT, and MRI imaging are important in tumor identification; however, it is difficult to reach a differential diagnosis between polycystic kidney disease and renal cell carcinoma based on clinical information and imaging results alone. A definitive diagnosis requires surgical treatment in the form of a unilateral nephrectomy, or pathological evaluation following a partial nephrectomy [29,30].
2.4. Multicystic Kidney/Multicystic Dysplastic Kidney (MCDK)
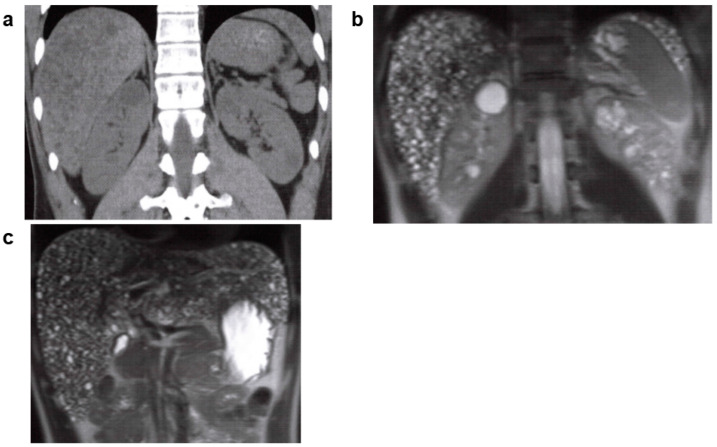
Multicystic kidney/multicystic dysplastic kidney is referred to as multicystic dysplastic kidney (MCDK) and is a cystic kidney disease expressed during the fetal stage of development as a result of the unsuccessful formation of nephrons and collecting ducts. This disease is believed to have an incidence rate of 1 in 1000–4300 individuals [32], with 70% of cases being unilateral. Most cases are diagnosed through fetal ultrasound imaging [33], and spontaneous regression occurs in 24% of cases by the age of 5 years [34], and in over 50% of cases by the age of 10 years [34,35]. Severe kidney function impairment occurs in bilateral MCDK cases, and this requires continuous observation as there is a possibility it may lead to oliguria or amenorrhea [36].
Imaging findings include a large aggregation of variously sized and mutually noncommunicative botryoidal cysts (Figure 4a). Renal dynamic scintigraphy shows a decreasing glomerular filtration rate (Figure 4b), with compensatory contralateral hypertrophy [37]. Contralateral urinary tract complications occur with a 30% frequency, the most common being vesicoureteral reflux (VUR) [38]. Renal prognosis is favorable when no contralateral complications such as VUR are observed, but complications do introduce the risk of progressive kidney function impairment [39]. MCDK closely resembles occlusive nephropathy resulting from severe urinary tract obstruction, but the latter is differentiated by its tendency to progress over time. Other hereditary cystic kidney diseases are characterized by cysts found on the medulla or entire cortex, and have a functional renal parenchyma, whereas the renal parenchyma in MCDK is non-functional [36]. MCDK was thought to be a nonhereditary renal disease, but in recent years, a human HNF1B gene mutation was found to have a causal relationship with a renal developmental defect accounting for 10–30% of all cases of kidney and urinary tract congenital abnormalities (CAKUT) [40], MCDK, and autosomal dominant tubulointerstitial kidney disease (ADTKD) [41]; 1 in 10 patients with unilateral MCDK are reported to have an HNF1B gene mutation [42].
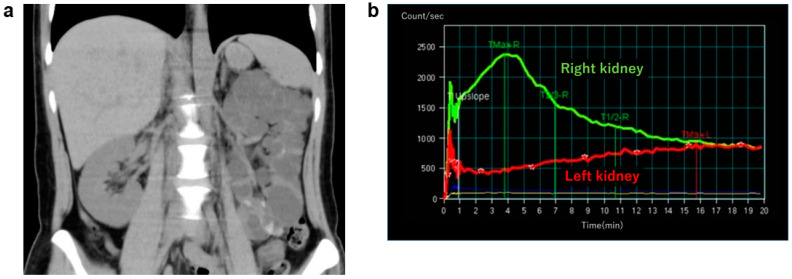
Figure 4.
Multicystic kidney/multicystic dysplastic kidney (MCDK): 30s, female, no family history of polycystic kidney disease or end-stage kidney disease, hepatic lesions, cerebral aneurysm, or heart valve disease, kidney function eGFR 70.2 mL/min/1.73 m2 (images provided by Kurume University). (a) Non-contrast CT image (coronal section), unilateral (left side) several renal cysts were observed. Cyst’s density varied from low to high absorption. High density in hemorrhagic cysts, isodense areas suggest obsolete hemorrhage. Kidney volume was 222 mL for the right kidney, and 692 mL for the left kidney. (b) Renal dynamic scintigraphy: blood flow phase/functional phase/excretion phase of the right kidney (green line) remain good, indicating normal function. The 3 phases of the left kidney (red line) are deteriorating, indicating hypofunction.
2.5. Unilateral Renal Cystic Disease (URCD)
Unilateral renal cystic disease (URCD), named by Levine et al. in 1989 [43] presents with cysts, thereby resembling ADPKD in imaging and histological findings (Figure 5) [44]. Although, it does differ from ADPKD in an absence of family history, and failure to progress to end-stage kidney disease (ESKD) [45]. Differential diagnosis is also helped by the fact that URCD has no extrarenal complications, such as hepatic cysts or cerebral aneurysm [46]. Diseases with unilateral or other cysts resembling those of URCD require a differential diagnosis; these include: multicystic dysplastic kidney, multilocular renal cysts, uteropelvic junction obstruction, non-obstructive megaureter, and simple renal cysts [46]. Differential diagnosis may also be required on the rare occasion for patients with ADPKD who have a PKD1 gene mutation and aplastic or hypoplastic kidney, or uteropelvic junction obstruction [47]. Ongoing observation is important as there are reports of unilateral cystic kidney diseases progressing over time to the bilateral stage during childhood [48].
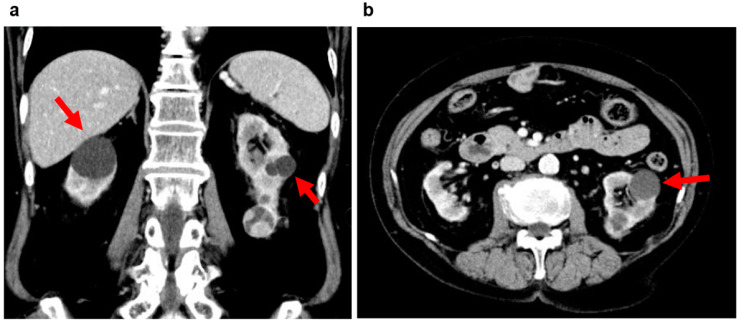
Figure 5.
Unilateral renal cystic disease: 70s, male, no family history of polycystic kidney disease or end-stage kidney disease, no hepatic cysts, cerebral aneurysm, or history of heart valve disease, kidney function eGFR 82.7 mL/min/1.73 m2 (image provide by Kurume University). (a) Non-contrast CT image (horizontal section): the right kidney shows multiple variously sized cysts, while bilateral renal volume is 1401 mL. (b) Non-contrast CT image (horizontal section): no hepatic cysts.
3. Cystic Kidney Diseases That Require a Differential Diagnosis from ADPKD
3.1. Autosomal Recessive Polycystic Kidney Disease (ARPKD)
Autosomal recessive polycystic kidney disease (ARPKD) presents as renal cystic lesions reflecting the expansion of the collecting duct with kidney function impairment, and congenital hepatic fibrosis characterized by bile duct dysplasia and intrahepatic periportal fibrogenesis [49]. The severity of these hepatic and renal lesions varies greatly from one individual to another, but it is reported to occur in one among every 8000 to 40,000 individuals [49,50,51]. Although many lesions appear in the perinatal stage or in childhood [49], they can manifest at any age, including well into adulthood. In an international ARPKD cohort study (n = 470 patients), 45 adult patients were evaluated in a cross-sectional analysis (median age, 19.9 years; average age, 21.4 years). The majority of these patients showed multiple bilateral cysts (≥10 cysts per kidney) on ultrasound, with half cases reported to be CKD stage 1–3. These patients required examination for conditions such as thrombocytopenia and splenomegaly which are derived from hepatic cysts, as well as for portal hypertension, to enable a differential diagnosis of ADPKD [52].
The PKHD1 gene (67 exons) is the causative gene and is found on 6p21.1-p12. It has a coding protein comprised of 4074 amino acids and is defined as a receptor-like protein which passes once through a cellular membrane described as fibrocystic or polyductin [53,54]. This disease has an autosomal recessive form, with 80% being homozygous or compound heterozygous, and 95% or over having one or more PKHD1 gene mutations [50,55,56,57,58]. Biallelic or truncated mutations are the most serious and usually result in embryonic or neonatal stage death, while one or more missense mutations are deemed relatively mild [55,59,60]. A recent study that analyzed PKHD1 gene missense mutations (taking amino acid location into account) in 304 patients with ARPKD (representing 277 family lineages) found less likelihood for progression to kidney failure in individuals with a missense gene mutation at amino acids 709–1837, but portal hypertension and severe hepatic complications in those with a missense gene mutation at amino acids 2625–4074 [61]. While patients within the same family lineage usually follow the same clinical course, varying levels of disease severity have been reported in about 20% of family lineages among patients within the same family lineage [50]. This suggests the possible effect, not only of the PKHD1 gene mutation, but also of environmental factors and other modified genes [59].
The second causative gene is DZIP1L, found on 3q22.1-q23 [62]. The phenotype caused by this gene mutation appears in the embryonic or early post-natal stage but is described as moderate ARPKD [62,63] as it does not lead to neonatal death.
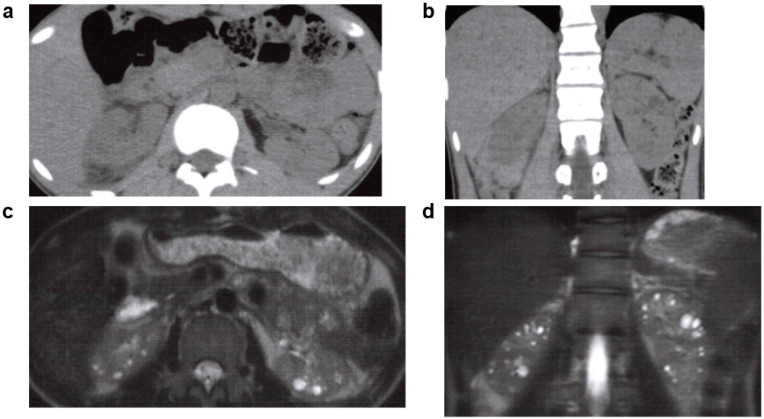
As there are no established diagnostic criteria, genetic testing should be considered for cases in which diagnosis solely through clinical conditions and imaging findings is difficult [52]. Since small cystic lesions, which are mainly dilated renal tubules rather than cysts, are diffusely present [64], the entire kidney becomes hyperechoic rather than lumpy and hypoechoic [64]. In the liver, congenital hepatic fibrosis, including hepatic swelling may be present. Typically, CT imaging reflects kidney enlargement with small embedded cysts or a striated appearance with collecting duct enlargement [64] (Figure 6). CT imaging may also be used to detect portal hypertension which is associated with congenital hepatic fibrosis. MRI imaging typically shows hypointensity on T1-weighted images and hyperintensity on T2-weighted images which is similar to ADPKD findings, but ARPKD has fewer complicated cysts associated with hemorrhaging and infection [64] (Figure 6).
Figure 6.
Autosomal recessive polycystic kidney disease (ARPKD): 30s, male, PKHD1 gene complex heterozygous mutation (compound heterozygous mutation), older brother had polycystic kidney disease (PKD) with end-stage kidney disease, no other family history (including parents) of PKD or end-stage kidney disease, no history of cerebral aneurysm or heart valve disease, kidney function eGFR 25.4 mL/min/1.73 m2 (image provided by Toranomon Hospital). (a) Non-contrast CT image (coronal section): hepatomegaly with many microcysts of low density found on the liver. Multiple low-density cysts with a diameter of 1–3 cm are seen on the corticomedullary junction of both kidneys. (b) MRI T2-weighted image (coronal section): multiple hyperintense cysts with a diameter of 1–3 cm are seen near the corticomedullary junction of both kidneys. (c) MRI T2-weighted image (coronal section): multiple hyperintense microscopic hepatic cyst-like lesions are seen on the liver.
As no established disease-specific treatment exists, patients with ARPKD are usually treated individually with symptomatic therapy. Since most patients experience hypertension, proactive antihypertensive therapy is important [52]. Kidney replacement therapy is appropriate for patients with progression of kidney function impairment, and other therapies, including hemodialysis, peritoneal dialysis, and kidney transplantation may also be considered. Hepatic complications such as cholangitis and esophageal varices are also possible.
3.2. Autosomal Dominant Tubulointerstitial Kidney Disease (ADTKD)
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a general term for a group of diseases inherited in an autosomal dominant manner and characterized by tubulointerstitial fibrogenesis and progressive kidney function impairment. First proposed in the 2015 Kidney Disease: Improving Global Outcomes (KDIGO) consensus report [41], ADTKD is currently believed to be caused by gene mutations UMOD, REN, MUC1, HNF1B, SEC61A, and DNAJB11 [65,66,67]. This disease has previously been referred to by various terms, such as familial juvenile hyperuricemic nephropathy and medullary cystic kidney disease, but with gene identification for ADTKD now available, diagnosis has become unified. For patients with kidney failure (adolescence through to old age), with a family history of kidney function impairment, who show few findings on urinary examination, and experience a slow decline in kidney function, ADTKD should be considered. As ADTKD often occurs in patients of advanced age, it is often mistaken for nephrosclerosis, or other renal diseases, and can be overlooked. Since ADTKD symptoms are nonspecific, genetic testing is required for an accurate diagnosis. Accordingly, this disease is often referred to as “ADTKD- (abnormal gene name).” HNF1B aside, the five genetic abnormalities often manifest when abnormal protein accumulates within the cell, bringing about endoplasmic reticulum stress which leads to cell dysfunction and tubulointerstitial damage [65].
ADTKD-UMOD appears as a mutation of the UMOD gene found on chromosome 16p12.3 [41]. The protein made by UMOD is uromodulin, also known as the Tamm–Horsfall protein, which is produced in kidney epithelial cells by the thick ascending limb (TAL) of the loop of Henle and secreted in urine. Physiologically, uromodulin is the most abundant protein excreted in urine and is the main component of urinary casts [67]. It is greatly glycosylated and enables protein polymerization. Uromodulin functions in TAL/distal ureter electrolyte transport safeguards against urinary tract infection and calcium-containing nephrolithiasis, and aids in topical and systemic immunoregulation. TAL dysfunction, which includes downregulation of Na+-K+-2Cl cotransporter, has been proven in ADTKD-UMOD [68].
Hyperuricemia is observed from childhood, and the mechanism is believed to include obstructed transitions in the luminal surface of the Na+-K+-2Cl cotransporter; this causes a slight fluid volume decrease and the compensatory appearance of a uric acid transporter in the secondary proximal renal tubule, along with an increase in uric acid [69]. Clinical observations include hyperuricemia, gouty arthritis, a decline in urine concentrating ability, and progressive kidney function impairment. Patients are reported to reach ESKD at a median (range) age of 46 (39–57) years. Histologically, kidney atrophy is due to stroma fibrogenesis and nephron atrophy, microcyst formation has also been noted. Renal size ceases to be normal due to atrophy, with 25% cyst formation (Figure 7) [70].
Figure 7.
Autosomal dominant tubulointerstitial kidney disease (ADTKD): 20s, male, UMOD gene heterozygous mutation, positive for familial hyperuricemia, no family history of polycystic kidney disease or end-stage kidney disease, no history of cerebral aneurysm or heart valve disease, began dialysis at age 20 years (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): many cysts in both kidneys, but there is no kidney enlargement. (b) Non-contrast CT image (coronal section): many cysts in both kidneys, but there is no kidney enlargement. (c) MRI T2-weighted image (horizontal section): many hyperintense cysts in both kidneys, but there is no kidney enlargement. (d) MRI T2-weighted image (coronal section): many hyperintense cysts in both kidneys, but there is no kidney enlargement.
ADTKD-MUC1 occurs due to a mutation in the MUC1 gene found on chromosome 1q22 [41]. Mutations in MUC1 are difficult to identify through exon analysis using a next-generation sequencer (based on polymerase chain reaction [PCR]) as the mutation often contains one base insertion of cytosine in the GC-rich and PCR-resistant variable number tandem repeat (VNTR) area [71]. Mucin1 is the protein encoded by MUC1 and is expressed in TAL, distal tubule, lung, small intestine, and stomach. Mucin1 safeguards the luminal surface of epithelial cells, and is a transmembrane glycoprotein involved in intracellular signaling and cell interaction. The mucin1 protein has been shown to activate cytoprotective hypoxia-inducible factor-1 (HIF-1) and the beta-catenin-dependent route in a mouse model of renal ischemic reperfusion damage [72]. It also activates the transient receptor potential cation channel, subfamily V, member 5 (TRPV5), a Ca2+ channel in the distal tubule, and acts to increase urine Ca2+ resorption [67]; this suggests that there is less mucin1 protein excretion in the urine of patients with renal calculus than in healthy individuals [72]. Mutant mucin1 protein influences endoplasmic reticulum stress and tubulointerstitial nephritis which causes cell dysfunction. Clinical observations include progressive kidney function impairment, with patients reaching ESKD at an average age of 45 years [73]. The only noted extrarenal disease is gout, which appears comparatively infrequently with ADTKD-UMOD [73]. Imaging reveals no kidney enlargement; rather, the kidney tends to show atrophy. Cyst formation has been reported in 37% of ADTKD-MUC1 patients [74]; however, not in the medulla. Histological findings show stroma fibrogenesis, nephron atrophy, and microcyst formation.
ADTKD-REN occurs due to a mutation in the REN gene found on chromosome 1q32.1 [41]. The disease is extremely rare, but in recent years, genetic and clinical spectra have been further clarified [75]. Pre-prorenin is the protein encoded by REN; it is primarily comprised of 406 amino acids expressed on granule cells of the juxtaglomerular apparatus. The N-terminal signal peptide is removed by the endoplasmic reticulum through cellular processing, allowing the formation of prorenin. Prorenin ultimately becomes renin, which is comprised of 340 amino acids, is stored in vesicles, enabling regulated exocytosis. As a result of the impaired translocation of the endoplasmic reticulum and mild endoplasmic reticulum stress caused by the signal peptide, the heterozygous REN mutation causes deteriorated synthesis of prorenin and renin [75]. This signal peptide and pro-segment mutation frequently occurs, and it is indicated at an extremely early age (about half of cases presenting before 10 years), in slight hyperkalemia, anemia, acidosis, slowly progressive CKD with a median kidney lifespan of 60 years, and early-onset gout. On the other hand, a mutation in part of the mature renin protein presents in adult-onset CKD and gout, with patients reaching ESKD in their late 60s. Renal biopsy specimens show deteriorating renin production [75], with signal peptide mutant non-glycosylated pre-prorenin reportedly accumulating in the cytoplasm [76]. When a mutation occurs in the mature renin protein, the mutant protein accumulates in the endoplasmic reticulum, activating endoplasmic reticulum stress and an unfolded protein response (UPR) [77]. As decreased renin activity is accompanied by low blood pressure and hyperkalemia, treatment is required and includes fludrocortisone. As sodium depletion also accompanies decreased renin activity, diuretics, a sodium-restricted diet, nonsteroidal anti-inflammatory drugs (NSAIDs) and RAAS-suppressing drugs should be avoided [65,76].
ADTKD-HNF1B occurs due to a mutation in the HNF1B gene found on chromosome 17q12 [41]. HNF1B encodes hepatocyte nuclear factor 1 B (HNF1B), a transcription factor which suppresses multiple genes expressed in the kidney, pancreas, and liver. As the HNF1B protein is involved in the transcription of various genes, congenital anomalies of the kidney and urinary tract, such as dysplastic kidney and renal aplasia, as well as renal cysts can be found in the kidney (Figure 8). Extrarenal diseases include juvenile-onset diabetes mellitus and liver function impairment, as well as abnormalities of the reproductive organs [67]. HNF1B is also involved in the activation of PKHD1, PKD2, and UMOD gene transcription factors which are linked to cystic disease. As HNF1B regulates transcription of the FXYD2 gene which encodes the gamma subunit of Na+-K+-ATPase expressed in distal tubules, it also impacts electrolyte regulation. Screening for extrarenal manifestations such as diabetes and liver function impairment is important.
Figure 8.
ADTKD: 40s: female, HNF1B gene heterozygous mutation, no family history of polycystic kidney disease or end-stage kidney disease, no history of cerebral aneurysm or heart valve disease, kidney function eGFR 39.2 mL/min/1.73 m2 (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): multiple cysts in both kidneys, but there is no kidney enlargement. (b) Non-contrast CT image (coronal section): multiple cysts in both kidneys, but there is no kidney enlargement. (c) MRI T2-weighted image (horizontal section): multiple hyperintense cysts of various sizes are noted near the corticomedullary junction on both kidneys, but there is no kidney enlargement. (d) MRI T2-weighted image (coronal section): multiple hyperintense cysts of various sizes are noted near the corticomedullary junction on both kidneys, but there is no kidney enlargement.
The hetero-missense mutation SEC61A1 which encodes the alpha 1 subunit of SEC61 translocon is also linked to ADTKD [67]. As this subunit constitutes a part of the translocon which transports newly synthesized protein to the endoplasmic reticulum, when the SEC61 translocon structure mutates, there is a disruption to the post-translational modification, folding, and sorting of the transmembrane protein, resulting in endoplasmic reticulum stress. Clinically, this disease is characterized as early stage, it is slowly progressive with CKD and gout occurring at an early age. Ultrasound testing reveals a normal or small size kidney, and bilateral polycystic kidneys. Renal biopsy shows tubular atrophy and interstitial fibrosis. Extrarenal symptoms include congenital anemia, neutropenia, and hypogammaglobulinemia. Growth retardation, cleft palate, spina bifida, mild cognitive impairment, and polydactyly may also be evident [66].
ADTKD-DNAJB11 is also recognized as a form of ADTKD [67]. DNAJB11 is a cofactor of BiP (GRP78), an endoplasmic reticulum chaperone which suppresses protein folding, transport, and degradation. It was recently clarified that a DNAJB11 heterozygous mutation is linked to a clinical phenotype expressed in a hybrid between ADTKD and ADPKD [78]. Patients typically transition to ESKD at a median age of 75 years, with a favorable renal prognosis compared to ADTKD-UMOD and ADTKD-MUC1 [79]. This disease is characterized by bilateral, multiple small renal cysts without kidney enlargement, and by hepatic cysts in 50% of all cases. Renal biopsies in several cases show tubulointerstitial fibrosis. Early-onset gout is not reported in these patients, but circulatory abnormalities such as intracranial aneurysm, thoracic aorta enlargement, and carotid artery dissociation have been indicated [78,79].
While six genetic abnormalities associated with ADTKD have been described here, new gene mutations are also likely to be found in patients showing histopathological findings suggesting ADTKD.
3.3. Nephronophthisis (NPH)
Nephronophthisis (NPH) is characterized by impaired urinary concentration, chronic tubulointerstitial nephritis, renal cystic lesions, and accompanying kidney function impairment and progress to ESKD by the age of 30 years [80]. NPH accounts for 15% of all diseases in which ESKD is reached by the age of 30 years, and it occurs in 1 or 2 out of every 100,000 individuals [81]. Clinically, there are three subtypes classified according to the median age at which ESKD is reached: infantile NPH (median age 1 year), juvenile NPH (median age 13 years), and adolescent/adult NPH (median age 19 years) [80,82,83], with patients reported to reach ESKD between 20 and 60 years of age [80,84,85]. This disease is autosomal recessive with 25 known causative genes: NPHP1, NPHP2/INVS, NPHP3, NPHP4, NPHP5/QCB1, NPHP6/CEP290, NPHP7/GLIS2, NPHP8/RPGRIP1L/MKS5, NPHP9/NEK8, NPHP10/SDCCAG8/SLSN7, NPHP11/TMEM67/MKS3, NPHP12/TTC21B/JBTS11, NPHP13/WDR19, NPHP14/ZNF423, NPHP15/CEP164, NPHP16/ANKS6, NPHP17/IFT172, NPHP18/CEP83, NPHP19/DCDC2, NPHP20/MAPKBP1, NPHP1L/XPNPEP3, NPHP2L/SLC41A1, TRAF3IP1, AH11/JBTS3, CC2D2A/MKS6 [81]. The NPHP1 gene is responsible for the most occurrences of NPH (20%), with the remaining genes each accounting for less than 1%. Two-thirds of all NPH cases have no clearly identifiable gene mutation [80]. Causative genes that are representative of adolescent/adult NPH include: NPHP3, NPHP4, and NPHP9/NEK8, but some cases of NPH do not clearly match up with any of these genes [80,81,86,87,88,89,90].
Clinical conditions include polyuria and polydipsia indicating an impairment to concentrate urine, hyponatremia accompanied by impaired sodium absorption, and symptoms characteristic to the progression of kidney function impairment. Typically, blood pressure is normal. There are distinct ultrasound findings, such as high luminance, normal to a slightly smaller sized kidney, and corticomedullary junction cysts, as well as the disappearance of the corticomedullary junction [64]. CT imaging shows renal parenchymal fibrogenesis, either a normal kidney size, or comparatively slightly smaller than average kidney size for the age of the patient, as well as cysts in the renal medulla and corticomedullary junction [64]. Some patients also show kidney enlargement resembling that of ARPKD [63] (Figure 9a), and MRI T2-weighted images show multiple cysts with hyperintensity [64] (Figure 9b). Pathological findings in the kidney include corticomedullary junction cysts, irregular hypertrophy, and overlapping of the tubular basement membranes, as well as tubulointerstitial nephritis [91]. Extrarenal lesions are reportedly found in 10–20% of patients, together with retinitis pigmentosa, cerebellar vermis hypoplasia, gaze palsy, hepatic fibrosis, and skeletal abnormalities. The following NPH-related diseases are based on the type of extrarenal lesions the patient presents with: Senior-Loken syndrome, Arima syndrome, Alstrom syndrome, RHYNS, Joubert syndrome (JS), Cogan syndrome, COACH, Meckel-Gruber syndrome (MKS), Boichis syndrome, Mainzer-Saldino syndrome, Bardet-Biedel syndrome, Ellis van Creveld syndrome, Jeune syndrome, and Sensenbrenner syndrome. In some patients, a different variant of the same gene produces a different phenotype and a different syndrome. For example, a nonsense mutation of the CC2D2A gene is associated with MKS, while a missense mutation is associated with JS. Similarly, the TMEM231 gene is linked to MKS, OFD, and JS.
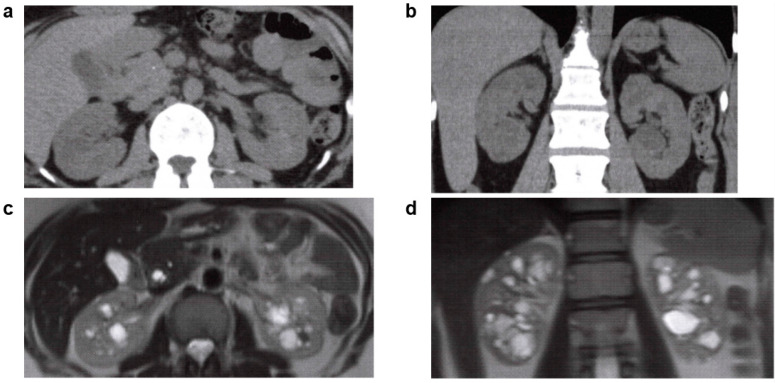
Figure 9.
Nephronophthisis (NPH): 20s, male, NPHP4 gene compound heterozygous mutation, no family history of polycystic kidney disease or end-stage kidney disease, no history of cerebral aneurysm or heart valve disease, kidney function eGFR 29.4 mL/min/1.73 m2 (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): multiple low-density regions in both kidneys. (b) Non-contrast CT image (coronal section): multiple low-absorbance regions in both kidneys. (c) MRI T2-weighted image (horizontal section): multiple hyperintense cyst-like lesions in both kidneys. (d) MRI T2-weighted image (coronal section): multiple hyperintense cyst-like lesions in both kidneys.
A diagnosis is based on clinical characteristics and confirmed by genetic testing [80,83,92]. However, as two-thirds of all cases have no clear gene mutation, a renal biopsy may be considered for a definitive diagnosis. In a report from Japan, pathological findings in the kidneys of older patients revealed a redundant thick tubular basement membrane characteristic of NPH, but genetic testing found no pathogenic mutation in the genes responsible [92].
No established disease-specific therapy exists, so symptomatic therapy is typical for individual patients [91]. Kidney replacement therapy is required if kidney function impairment progresses, and hemodialysis, peritoneal dialysis, or kidney transplant may be considered. Kidney transplantation results are reported to be comparatively favorable [93].
3.4. Multiple Abnormalities Accompanying Cystic Kidney Disease (OFD1, NPHP/SLS, JSRD, MKS, BBS, OFDS)
Cystic kidney diseases accompanied by multiple abnormalities include nephronophthisis (NPHP)/Senior-Loken syndrome (SLS), Joubert syndrome and related diseases (JSRD), Meckel syndrome (MKS), Bardet-Biedl syndrome (BBS), and orofacial digital syndrome (OFDS) [94]. Oral-facial-digital syndrome type 1 (OFD1), a type of orofacial digital syndrome, clinically manifests in adulthood and is a disease requiring differentiation from ADPKD.
3.5. Oral-Facial-Digital Syndrome (OFD) Type 1
Oral-facial-digital syndrome (OFD syndrome) is a hereditary cystic kidney disease which incorporates a variety of morphological abnormalities in the oral, facial, and finger and toe areas. Thirteen types of OFD syndrome are recognized: Type 1, Papillon-League-Psaume syndrome; type 2, Mohr syndrome; type 3, Sugarman syndrome; type 4, Baraiter-Burn syndrome; type 5, Thurston syndrome; type 6, Varadi-Papp syndrome; type 7, Whelan syndrome; type 8, Edwards syndrome; type 9, Gurrier syndrome; type 10, Figuera syndrome; type 11, Gabrielli syndrome; type 12, Moran-Barroso syndrome; and type 13, Degner syndrome [95]. The most common, OFD syndrome type 1, has an X chromosome-linked hereditary form, and as it has male lethality, the phenotype is found only in females. This disease appears in 1 in 50,000–250,000 individuals [96], with about 75% of cases being sporadic [97]. It is caused by an OFD1 gene mutation on X chromosome Xp22 [98]. OFD1 encoded by the OFD1 gene is found in centrioles and basal bodies and functions in the first stage of ciliogenesis. The OFD1 gene mutation is reportedly related to four syndromes with X chromosome-linked recessive inheritance (Joubert syndrome, retinitis pigmentosa, primary ciliary dyskinesia, and Simpson-Golabi-Behmel syndrome), which have phenotypes observed in both males and females [99].
The renal lesions are usually viewed as polycystic kidney and typically appear in adulthood (20s and 30s) [100]. Kidney function impairment can be seen from the post-natal period, but usually progresses to kidney failure in adulthood once polycystic kidney becomes apparent. Taking all age groups into account, polycystic kidney occurrence rate is 37.3%, but in patients aged 18 years and older, it is reported to be 63% [100]. Pathologically, most of the cysts are glomerulus in derivation [101]. CT and MRI imaging show kidney size to be normal to slightly large, with no change in appearance due to cysts (Figure 10). Oral lesions may result in tongue malformation (lobulation, hamartoma, split), cleft palate/high-arched palate, and tooth malformation [100]. Cephalofacial lesions typically include symptoms such as down slanted palpebral fissures, nostril underdevelopment, telecanthus, retrognathia, flat face, frontal prominence, harelip, milia (which disappears by the age of 3 years), dry and brittle hair, and hair loss [100]. Skeletal lesions which frequently manifest include brachydactyly, clinodactyly, preaxial polydactyly, broad thumb, broad hallux, and morphological defects in the finger and toe. Short stature has also been linked to this disease [100]. Associated central nervous system lesions include deformities (colpocephaly, arachnoid cysts, porencephaly, gray matter heterotopia, cerebellum malformation, abnormal gyrus, microcephaly), mental retardation, cognitive disorders, and other deformities [100]. Other symptoms include deafness and the presence of cysts on the pancreas, liver, and ovaries [100]. As this is an X chromosome-linked hereditary disease, varying degrees of X chromosome inactivity may produce differing intrafamilial/interfamilial phenotypes.
Figure 10.
Oral-facial-digital syndrome (OFD) type 1: 30s, female, OFD1 gene heterozygous mutation, no family history of polycystic kidney disease or end-stage kidney disease, no history of hepatic cysts, cerebral aneurysm, or heart valve disease, no abnormalities in urinalysis, began dialysis at age 26 years (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): multiple low-density cystic lesions are in both kidneys, accompanied by kidney enlargement. There is no change in the external appearance of the kidneys. (b) Non-contrast CT image (coronal section): multiple low-density cystic lesions are in both kidneys, accompanied by kidney enlargement. There is no change in the external appearance of the kidneys. (c) MRI T2-weighted image (horizontal section): multiple hyperintense cystic lesions are in both kidneys, accompanied by kidney enlargement. There is no change in the external appearance of the kidneys. (d) MRI T2-weighted image (coronal section): multiple hyperintense cystic lesions are in both kidneys, accompanied by kidney enlargement. There is no change in the external appearance of the kidneys.
Most oral-facial-digital deformities are diagnosed in the post-natal stage, but mild cases may not be diagnosed until the appearance of renal lesions. Renal cysts, central nervous system lesions, and skeletal deformities are also identified in other forms of ciliopathy, but tooth deformities are distinctive. Female patients suspected of having OFD1 type 1 syndrome may benefit from genetic testing for the OFD1 gene.
As established disease-specific treatment does not exist, medical care is generally provided according to individual patient symptoms. Reconstructive surgery is one option for treatable symptoms. Kidney replacement therapy may be required if kidney function impairment progresses, and hemodialysis, peritoneal dialysis, and kidney transplant may also be considered.
3.6. Neoplastic Cystic Kidney Diseases (Tuberous Sclerosis, Von Hippel-Lindau Syndrome)
3.6.1. Tuberous Sclerosis (TSC)
Tuberous sclerosis (TSC) is a hereditary systemic disease characterized by systemic hamartoma, with TSC1 and TSC2 identified as the genes responsible for this condition. Hamartin–Tuberin complex, a TSC1 and TSC2 gene product, is involved in cell proliferation through mTOR inhibition, with resulting hamartoma formation in the skin, nervous system, kidney, lung, bone, and elsewhere, together with abnormalities in the TSC1 and TSC2 genes [102,103,104]. The disease is inherited in an autosomal dominant manner and occurs in one in 10,000 individuals.
New diagnostic criteria were proposed at the second TSC Clinical Consensus Conference in 2012 and are summarized in Table 3. Criteria for genetic diagnosis were added at this time, and criteria for clinical diagnosis were modified [105].
Table 3.
Diagnostic criteria for tuberous sclerosis [105].
| Major Features | Minor Features |
|---|---|
| 1. Hypomelanotic macules (≥3, at least 5-mm diameter) 2. Angiofibromas (≥3) or fibrous cephalic plaque 3. Ungual fibromas (≥2) 4. Shagreen patch 5. Multiple retinal hamartomas 6. Cortical dysplasias 7. Subependymal nodules 8. Subependymal giant cell astrocytoma 9. Cardiac rhabdomyoma 10. Lymphangioleiomyomatosis (LAM) 11. Angiomyolipomas (AML) (≥2) |
|
| Definitive diagnosis: 2 major features or 1 major feature with 2 minor features. Alternatively, the identification of either a TSC1 or TSC2 pathogenic mutation. | |
| Possible diagnosis: Either 1 major feature or ≥2 minor features | |
Distinctive renal lesions associated with tuberous sclerosis include angiomyolipoma (AML)/renal cysts/renal cell carcinoma. A study by Wataya-Kaneda et al. (2013) found that 71% of 166 Japanese patients with tuberous sclerosis had renal lesions (61% AML, 28% renal cysts, 2.6% renal cancer) [106]. Renal lesions are reportedly the primary organ in which lesions determine the prognosis of TSC in patients aged 10 years and above [107]. Only 1% of patients with TSC require kidney replacement therapy; this is due to the high mortality rate by the age of 20 years [108]. In the proposed international diagnostic criteria (Table 3), renal AML is listed as one of the major features, while multiple renal cysts are noted as a minor feature (Figure 11) [105]. It is estimated that about 30% of TSC patients do not show typical clinical symptoms other than renal cysts, and it is thought that there are a certain number of cases that have been misdiagnosed as ADPKD [109].
Figure 11.
Tuberous sclerosis: 30s, female, wide-ranging loss of heterozygosity noted on the TSC2 gene, no family history of kidney disease, positive for hypopigmented macule/facial angiofibroma/pulmonary lymphangioleiomyomatosis, positive for epilepsy history, kidney function eGFR 55.3 mL/min/1.73 m2 (image provided by Toranomon Hospital). (a) Non-contrast CT image (horizontal section): multiple lipomas and low-density cysts in both kidneys. (b) Non-contrast CT image (coronal section): multiple lipomas and low-density cysts in both kidneys. (c) MRI T2-weighted image (horizontal section): multiple hyperintense cysts of varying sizes in both kidneys. (d) MRI T2-weighted image (coronal section): multiple hyperintense cysts of varying sizes in both kidneys.
Renal cysts complicated by TSC arise from various places of nephrons such as glomerulus, and kidney function deteriorates due to the frequent occurrence and increase in renal cysts [110]. mTOR inhibitors are recommended for renal AML [105], and while no established therapy exists for renal cysts, Siroky et al. (2017) utilized mTOR inhibitors in 15 tuberous sclerosis patients with renal cysts and reported a 71% decrease in kidney volume [111]. As TSC2, the gene responsible for tuberous sclerosis, is adjacent to PKD1, the gene responsible for ADPKD, TSC2/PKD1 contiguous gene deletion syndrome is found in 2–5% of all tuberous sclerosis patients with renal cysts [112,113]. These individuals have an increased likelihood of reaching ESKD from the ages of 20–30 years compared to ADPKD patients [114]. There is no clear evidence that treatment with tolvaptan is effective in these patients.
3.6.2. Von Hippel-Lindau (VHL) Syndrome
Von Hippel-Lindau (VHL) syndrome is a hereditary systemic disease caused by a mutation in the VHL gene. This disease is inherited in an autosomal dominant manner and occurs in one in 50,000 individuals. The VHL gene is a tumor suppressive gene found on chromosome 3p25-26, and the 25–45% of VHL syndrome patients who have this gene mutation can also present with renal cell carcinoma, pancreatic cysts, central nervous system and retinal hemangioblastoma, and pheochromocytoma [115].
Clinical diagnostic criteria for VHL syndrome vary with family history; individuals with a clear family history are diagnosed by the presence of one VHL syndrome-related lesion, while individuals without a clear family history are diagnosed by the presence of multiple central nervous system angioblastomas or retinal hemangiomas, or by comorbidity of central nervous system angioblastoma or retinal hemangioma and VHL syndrome-related lesions [116] (Table 4).
Table 4.
Diagnostic criteria for Von Hippel Lindau syndrome [116].
| (1) With a positive family history of VHL syndrome |
| Retinal angiomas, Spinal or cerebellar hemangioblastomas, Endolymphatic sac tumors, Renal cell carcinoma, Pheochromocytoma, Pancreatic lesions (Pancreatic cysts, Pancreatic neuroendocrine tumors), Epididymal cystadenomas, etc. |
| (2) With no clear family history of VHL syndrome |
| 1. Spinal or cerebellar hemangioblastomas or Retinal angiomas (≥2) 2. Spinal or cerebellar hemangioblastomas or Retinal angiomas (≥1) along with 1 of the following: a. Renal cell carcinoma, b. pheochromocytoma, c. Pancreatic cyst or Pancreatic neuroendocrine tumors, d. Epididymal cystadenomas, e. Endolymphatic sac tumors 3. 1 of the above clinical features with identification of a heterozygous germline pathogenic mutations in VHL on molecular genetic testing |
Renal lesions associated with VHL syndrome broadly range from simple renal cysts to renal cell carcinoma and appear in 50–70% of patients with VHL syndrome [117,118,119]. These lesions are reported to appear in patients aged 20–30 years [116].
VHL syndrome is characterized by the frequent appearance of neoplastic or cystic lesions in multiple organs. In a study by Taylor et al. (2012), cysts were found in six out of 21 patients with VHL, four of whom had renal cysts [120]. Renal cysts in VHL do not produce any symptoms, and unlike ADPKD, kidney function impairment is rare. However, complex cysts may produce renal tumors. To determine the course of treatment, it is recommended to use the Bosniak classification (Table 2) in a regular image evaluation [17].
4. Summary
To help physicians evaluate cystic kidney diseases, this article provides a review of cystic kidney diseases for which a differential diagnosis is required for ADPKD. For each disease, we conduct a review and present image findings of specific cases that are representative of the disease. We believe this is a review paper that can be used in actual clinical practice.
Acknowledgments
We appreciate the cooperation of Naoki Sawa, Hiroki Mizuno and Yoshifumi Ubara, Toranomon Hospital, and Kei Fukami, Kurume University, for providing the case images. We also appreciate the assistance of Eisei Sohara, Takayasu Mori and Takuya Fujimaru, Tokyo Medical and Dental University for conducting the genetic testing used in the diagnoses of cases from Toranomon Hospital.
Author Contributions
Conceptualization, A.S., S.H. (Sumi Hidaka), T.M. (Tomofumi Moriyama), Y.S., K.S., T.M. (Toshio Mochizuki), S.M. (Satoru Muto) and S.H. (Shigeo Horie). Investigation and Writing-original draft preparation, A.S., S.H. (Sumi Hidaka), T.M. (Tomofumi Moriyama), Y.S. and K.S. Writing-review and editing, E.I., K.U., H.K. (Hiroshi Kataoka), H.K. (Haruna Kawano), M.K., M.S., T.S., S.N. (Shinya Nakatani), T.O., H.K. (Hirayasu Kai), K.K., S.M. (Shiho Makabe), S.M. (Shun Manabe), W.S., K.N., S.N. (Saori Nishio), F.H., K.H., K.M., H.H., J.H., K.T. and I.N. Supervision and Project adminiatration, S.M. (Satoru Muto). All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Written informed consent has been obtained from the patients of case images to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors have no conflict of interest.
Funding Statement
This article was supported in part by a Grant-in-Aid for Intractable Renal Diseases Research, Research on Rare and Intractable Diseases, and Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornec-Le Gall E., Alam A., Perrone R.D. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919–935. doi: 10.1016/S0140-6736(18)32782-X. [DOI] [PubMed] [Google Scholar]
- 2.Audrézet M.P., Cornec-Le Gall E., Chen J.M., Redon S., Quéré I., Creff J., Bénech C., Maestri S., Le Meur Y., Férec C. Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 2012;33:1239–1250. doi: 10.1002/humu.22103. [DOI] [PubMed] [Google Scholar]
- 3.Rossetti S., Consugar M.B., Chapman A.B., Torres V.E., Guay-Woodford L.M., Grantham J.J., Bennett W.M., Meyers C.M., Walker D.L., Bae K., et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 4.Cornec-Le Gall E., Audrézet M.P., Chen J.M., Hourmant M., Morin M.P., Perrichot R., Charasse C., Whebe B., Renaudineau E., Jousset P., et al. Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 2013;24:1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang Y.H., Conklin J., Chan W., Roslin N.M., Liu J., He N., Wang K., Sundsbak J.L., Heyer C.M., Haider M., et al. Refining Genotype-Phenotype Correlation in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016;27:1861–1868. doi: 10.1681/ASN.2015060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliuta I.A., Kalatharan V., Wang K., Cornec-Le Gall E., Conklin J., Pourafkari M., Ting R., Chen C., Borgo A.C., He N., et al. Polycystic Kidney Disease without an Apparent Family History. J. Am. Soc. Nephrol. 2017;28:2768–2776. doi: 10.1681/ASN.2016090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimaru T., Mori T., Sekine A., Mandai S., Chiga M., Kikuchi H., Ando F., Mori Y., Nomura N., Iimori S., et al. Kidney enlargement and multiple liver cyst formation implicate mutations in PKD1/2 in adult sporadic polycystic kidney disease. Clin. Genet. 2018;94:125–131. doi: 10.1111/cge.13249. [DOI] [PubMed] [Google Scholar]
- 8.Torres V.E., Chapman A.B., Devuyst O., Gansevoort R.T., Grantham J.J., Higashihara E., Perrone R.D., Krasa H.B., Ouyang J., Czerwiec F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres V.E., Chapman A.B., Devuyst O., Gansevoort R.T., Perrone R.D., Dandurand A., Ouyang J., Czerwiec F.S., Blais J.D. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant. 2018;33:477–489. doi: 10.1093/ndt/gfx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres V.E., Chapman A.B., Devuyst O., Gansevoort R.T., Perrone R.D., Koch G., Ouyang J., McQuade R.D., Blais J.D., Czerwiec F.S., et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 11.Eknoyan G. A clinical view of simple and complex renal cysts. J. Am. Soc. Nephrol. 2009;20:1874–1876. doi: 10.1681/ASN.2008040441. [DOI] [PubMed] [Google Scholar]
- 12.Terada N., Ichioka K., Matsuta Y., Okubo K., Yoshimura K., Arai Y. The natural history of simple renal cysts. J. Urol. 2002;167:21–23. doi: 10.1016/S0022-5347(05)65373-6. [DOI] [PubMed] [Google Scholar]
- 13.Rule A.D., Sasiwimonphan K., Lieske J.C., Keddis M.T., Torres V.E., Vrtiska T.J. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am. J. Kidney Dis. 2012;59:611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simms R.J., Ong A.C. How simple are ‘simple renal cysts’? Nephrol. Dial. Transpl. 2014;29((Suppl. S4)):iv106–iv112. doi: 10.1093/ndt/gfu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Said J., Brumback M.A., Moghazi S., Baumgarten D.A., O’Neill W.C. Reduced renal function in patients with simple renal cysts. Kidney Int. 2004;65:2303–2308. doi: 10.1111/j.1523-1755.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 16.Helenon O., Crosnier A., Verkarre V., Merran S., Mejean A., Correas J.M. Simple and complex renal cysts in adults: Classification system for renal cystic masses. Diagn. Interv. Imaging. 2018;99:189–218. doi: 10.1016/j.diii.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Silverman S.G., Pedrosa I., Ellis J.H., Hindman N.M., Schieda N., Smith A.D., Remer E.M., Shinagare A.B., Curci N.E., Raman S.S., et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019;292:475–488. doi: 10.1148/radiol.2019182646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truong L.D., Choi Y.J., Shen S.S., Ayala G., Amato R., Krishnan B. Renal cystic neoplasms and renal neoplasms associated with cystic renal diseases: Pathogenetic and molecular links. Adv. Anat. Pathol. 2003;10:135–159. doi: 10.1097/00125480-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Choyke P.L. Acquired cystic kidney disease. Eur. Radiol. 2000;10:1716–1721. doi: 10.1007/s003300000601. [DOI] [PubMed] [Google Scholar]
- 20.Grantham J.J. Acquired cystic kidney disease. Kidney Int. 1991;40:143–152. doi: 10.1038/ki.1991.192. [DOI] [PubMed] [Google Scholar]
- 21.Rahbari-Oskoui F., Mittal A., Mittal P., Chapman A. Renal relevant radiology: Radiologic imaging in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2014;9:406–415. doi: 10.2215/CJN.08940813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa I., Morita K., Hayama S., Nakazawa T., Araki I., Higashi K., Miyazawa K., Suzuki K., Nojima T. Imaging of acquired cystic disease-associated renal cell carcinoma by contrast-enhanced ultrasonography with perflubutane microbubbles and positron emission tomography-computed tomography. Clin. Exp. Nephrol. 2011;15:136–140. doi: 10.1007/s10157-010-0347-3. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima K., Yamamoto S., Kawanaka Y., Katsuura T., Fujita M., Nakanishi Y., Yamada Y., Hashimoto T., Suzuki T., Go S., et al. Imaging of renal cell carcinoma in patients with acquired cystic disease of the kidney: Comparison (11)C-choline and FDG PET/CT with dynamic contrast-enhanced CT. Jpn. J. Radiol. 2019;37:165–177. doi: 10.1007/s11604-018-0789-1. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa I., Yuri T., Kitada H., Shinoda A. Regression of acquired cystic disease of the kidney after successful renal transplantation. Am. J. Nephrol. 1983;3:310–314. doi: 10.1159/000166738. [DOI] [PubMed] [Google Scholar]
- 25.Leveridge M., Musquera M., Evans A., Cardella C., Pei Y., Jewett M., Robinette M., Finelli A. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J. Urol. 2011;186:219–223. doi: 10.1016/j.juro.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Stewart J.H., Vajdic C.M., van Leeuwen M.T., Amin J., Webster A.C., Chapman J.R., McDonald S.P., Grulich A.E., McCredie M.R. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transplant. 2009;24:3225–3231. doi: 10.1093/ndt/gfp331. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz A., Vatandaslar S., Merkel S., Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin. J. Am. Soc. Nephrol. 2007;2:750–756. doi: 10.2215/CJN.03661106. [DOI] [PubMed] [Google Scholar]
- 28.Jevremovic D., Lager D.J., Lewin M. Cystic nephroma (multilocular cyst) and mixed epithelial and stromal tumor of the kidney: A spectrum of the same entity? Ann. Diagn. Pathol. 2006;10:77–82. doi: 10.1016/j.anndiagpath.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson C., Palit V., Bardapure M., Thomas J., Browning A.J., Gill K., Biyani C.S. Adult multilocular cystic nephroma: Report of six cases with clinical, radio-pathologic correlation and review of literature. Urol. Ann. 2013;5:13–17. doi: 10.4103/0974-7796.106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granja M.F., O’Brien A.T., Trujillo S., Mancera J., Aguirre D.A. Multilocular Cystic Nephroma: A Systematic Literature Review of the Radiologic and Clinical Findings. Am. J. Roentgenol. 2015;205:1188–1193. doi: 10.2214/AJR.15.14548. [DOI] [PubMed] [Google Scholar]
- 31.Adsay N.V., Eble J.N., Srigley J.R., Jones E.C., Grignon D.J. Mixed epithelial and stromal tumor of the kidney. Am. J. Surg. Pathol. 2000;24:958–970. doi: 10.1097/00000478-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Scala C., McDonnell S., Murphy F., Leone Roberti Maggiore U., Khalil A., Bhide A., Thilaganathan B., Papageorghiou A.T. Diagnostic accuracy of midtrimester antenatal ultrasound for multicystic dysplastic kidneys. Ultrasound Obstet. Gynecol. 2017;50:464–469. doi: 10.1002/uog.17305. [DOI] [PubMed] [Google Scholar]
- 33.Cardona-Grau D., Kogan B.A. Update on Multicystic Dysplastic Kidney. Curr. Urol. Rep. 2015;16:67. doi: 10.1007/s11934-015-0541-7. [DOI] [PubMed] [Google Scholar]
- 34.Wacksman J., Phipps L. Report of the Multicystic Kidney Registry: Preliminary findings. J. Urol. 1993;150:1870–1872. doi: 10.1016/S0022-5347(17)35918-9. [DOI] [PubMed] [Google Scholar]
- 35.Rabêlo E.A., Oliveira E.A., Silva J.M., Bouzada M.C., Sousa B.C., Almeida M.N., Tatsuo E.S. Conservative management of multicystic dysplastic kidney: Clinical course and ultrasound outcome. J. Pediatr. 2005;81:400–404. doi: 10.2223/JPED.1391. [DOI] [PubMed] [Google Scholar]
- 36.Chetty S. Multicystic dysplastic kidney. Am. J. Obstet. Gynecol. 2021;225:B21–B22. doi: 10.1016/j.ajog.2021.06.046. [DOI] [PubMed] [Google Scholar]
- 37.Gaither T.W., Patel A., Patel C., Chuang K.W., Cohen R.A., Baskin L.S. Natural History of Contralateral Hypertrophy in Patients with Multicystic Dysplastic Kidneys. J. Urol. 2018;199:280–286. doi: 10.1016/j.juro.2017.06.075. [DOI] [PubMed] [Google Scholar]
- 38.Erlich T., Lipsky A.M., Braga L.H. A meta-analysis of the incidence and fate of contralateral vesicoureteral reflux in unilateral multicystic dysplastic kidney. J. Pediatr. Urol. 2019;15:77.e71–77.e77. doi: 10.1016/j.jpurol.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Mansoor O., Chandar J., Rodriguez M.M., Abitbol C.L., Seeherunvong W., Freundlich M., Zilleruelo G. Long-term risk of chronic kidney disease in unilateral multicystic dysplastic kidney. Pediatr. Nephrol. 2011;26:597–603. doi: 10.1007/s00467-010-1746-0. [DOI] [PubMed] [Google Scholar]
- 40.Raaijmakers A., Corveleyn A., Devriendt K., van Tienoven T.P., Allegaert K., Van Dyck M., van den Heuvel L., Kuypers D., Claes K., Mekahli D., et al. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol. Dial. Transplant. 2015;30:835–842. doi: 10.1093/ndt/gfu370. [DOI] [PubMed] [Google Scholar]
- 41.Eckardt K.U., Alper S.L., Antignac C., Bleyer A.J., Chauveau D., Dahan K., Deltas C., Hosking A., Kmoch S., Rampoldi L., et al. Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management—A KDIGO consensus report. Kidney Int. 2015;88:676–683. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama M., Nozu K., Goto Y., Kamei K., Ito S., Sato H., Emi M., Nakanishi K., Tsuchiya S., Iijima K. HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 2010;25:1073–1079. doi: 10.1007/s00467-010-1454-9. [DOI] [PubMed] [Google Scholar]
- 43.Levine E., Huntrakoon M. Unilateral renal cystic disease: CT findings. J. Comput. Assist. Tomogr. 1989;13:273–276. doi: 10.1097/00004728-198903000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Middlebrook P.F., Nizalik E., Schillinger J.F. Unilateral renal cystic disease: A case presentation. J. Urol. 1992;148:1221–1223. doi: 10.1016/S0022-5347(17)36866-0. [DOI] [PubMed] [Google Scholar]
- 45.Punia R.P., Mohan H., Bal A., Bansal V.K. Unilateral and segmental cystic disease of the kidney. Int. J. Urol. 2005;12:308–310. doi: 10.1111/j.1442-2042.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 46.Hwang D.Y., Ahn C., Lee J.G., Kim S.H., Oh H.Y., Kim Y.Y., Lee E.S., Han J.S., Kim S., Lee J.S. Unilateral renal cystic disease in adults. Nephrol. Dial. Transplant. 1999;14:1999–2003. doi: 10.1093/ndt/14.8.1999. [DOI] [PubMed] [Google Scholar]
- 47.Poster D., Kistler A.D., Krauer F., Blumenfeld J.D., Rennert H., Weishaupt D., Wüthrich R.P., Serra A.L. Kidney function and volume progression in unilateral autosomal dominant polycystic kidney disease with contralateral renal agenesis or hypoplasia: A case series. Am. J. Kidney Dis. 2009;54:450–458. doi: 10.1053/j.ajkd.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Porch P., Noe H.N., Stapleton F.B. Unilateral presentation of adult-type polycystic kidney disease in children. J. Urol. 1986;135:744–746. doi: 10.1016/S0022-5347(17)45837-X. [DOI] [PubMed] [Google Scholar]
- 49.Bergmann C., Guay-Woodford L.M., Harris P.C., Horie S., Peters D.J.M., Torres V.E. Polycystic kidney disease. Nat. Rev. Dis. Prim. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergmann C., Senderek J., Windelen E., Küpper F., Middeldorf I., Schneider F., Dornia C., Rudnik-Schöneborn S., Konrad M., Schmitt C.P., et al. Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD) Kidney Int. 2005;67:829–848. doi: 10.1111/j.1523-1755.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 51.Guay-Woodford L.M., Bissler J.J., Braun M.C., Bockenhauer D., Cadnapaphornchai M.A., Dell K.M., Kerecuk L., Liebau M.C., Alonso-Peclet M.H., Shneider B., et al. Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: Report of an international conference. J. Pediatr. 2014;165:611–617. doi: 10.1016/j.jpeds.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgmaier K., Kilian S., Bammens B., Benzing T., Billing H., Büscher A., Galiano M., Grundmann F., Klaus G., Mekahli D., et al. Clinical courses and complications of young adults with Autosomal Recessive Polycystic Kidney Disease (ARPKD) Sci. Rep. 2019;9:7919. doi: 10.1038/s41598-019-43488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward C.J., Hogan M.C., Rossetti S., Walker D., Sneddon T., Wang X., Kubly V., Cunningham J.M., Bacallao R., Ishibashi M., et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 54.Onuchic L.F., Furu L., Nagasawa Y., Hou X., Eggermann T., Ren Z., Bergmann C., Senderek J., Esquivel E., Zeltner R., et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am. J. Hum. Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furu L., Onuchic L.F., Gharavi A., Hou X., Esquivel E.L., Nagasawa Y., Bergmann C., Senderek J., Avner E., Zerres K., et al. Milder presentation of recessive polycystic kidney disease requires presence of amino acid substitution mutations. J. Am. Soc. Nephrol. 2003;14:2004–2014. doi: 10.1097/01.ASN.0000078805.87038.05. [DOI] [PubMed] [Google Scholar]
- 56.Losekoot M., Haarloo C., Ruivenkamp C., White S.J., Breuning M.H., Peters D.J. Analysis of missense variants in the PKHD1-gene in patients with autosomal recessive polycystic kidney disease (ARPKD) Hum. Genet. 2005;118:185–206. doi: 10.1007/s00439-005-0027-7. [DOI] [PubMed] [Google Scholar]
- 57.Gunay-Aygun M., Tuchman M., Font-Montgomery E., Lukose L., Edwards H., Garcia A., Ausavarat S., Ziegler S.G., Piwnica-Worms K., Bryant J., et al. PKHD1 sequence variations in 78 children and adults with autosomal recessive polycystic kidney disease and congenital hepatic fibrosis. Mol. Genet Metab. 2010;99:160–173. doi: 10.1016/j.ymgme.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunay-Aygun M., Font-Montgomery E., Lukose L., Tuchman M., Graf J., Bryant J.C., Kleta R., Garcia A., Edwards H., Piwnica-Worms K., et al. Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2010;5:972–984. doi: 10.2215/CJN.07141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossetti S., Harris P.C. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 2007;18:1374–1380. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 60.Erger F., Brüchle N.O., Gembruch U., Zerres K. Prenatal ultrasound, genotype, and outcome in a large cohort of prenatally affected patients with autosomal-recessive polycystic kidney disease and other hereditary cystic kidney diseases. Arch. Gynecol. Obstet. 2017;295:897–906. doi: 10.1007/s00404-017-4336-6. [DOI] [PubMed] [Google Scholar]
- 61.Burgmaier K., Brinker L., Erger F., Beck B.B., Benz M.R., Bergmann C., Boyer O., Collard L., Dafinger C., Fila M., et al. Refining genotype-phenotype correlations in 304 patients with autosomal recessive polycystic kidney disease and PKHD1 gene variants. Kidney Int. 2021;100:650–659. doi: 10.1016/j.kint.2021.04.019. [DOI] [PubMed] [Google Scholar]
- 62.Lu H., Galeano M.C.R., Ott E., Kaeslin G., Kausalya P.J., Kramer C., Ortiz-Brüchle N., Hilger N., Metzis V., Hiersche M., et al. Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat. Genet. 2017;49:1025–1034. doi: 10.1038/ng.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergmann C. Early and Severe Polycystic Kidney Disease and Related Ciliopathies: An Emerging Field of Interest. Nephron. 2019;141:50–60. doi: 10.1159/000493532. [DOI] [PubMed] [Google Scholar]
- 64.Dillman J.R., Trout A.T., Smith E.A., Towbin A.J. Hereditary Renal Cystic Disorders: Imaging of the Kidneys and Beyond. Radiographics. 2017;37:924–946. doi: 10.1148/rg.2017160148. [DOI] [PubMed] [Google Scholar]
- 65.Devuyst O., Olinger E., Weber S., Eckardt K.U., Kmoch S., Rampoldi L., Bleyer A.J. Autosomal dominant tubulointerstitial kidney disease. Nat. Rev. Dis. Prim. 2019;5:60. doi: 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 66.Bolar N.A., Golzio C., Zivna M., Hayot G., Van Hemelrijk C., Schepers D., Vandeweyer G., Hoischen A., Huyghe J.R., Raes A., et al. Heterozygous Loss-of-Function SEC61A1 Mutations Cause Autosomal-Dominant Tubulo-Interstitial and Glomerulocystic Kidney Disease with Anemia. Am. J. Hum. Genet. 2016;99:174–187. doi: 10.1016/j.ajhg.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mabillard H., Sayer J.A., Olinger E. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease. Nephrol. Dial. Transpl. 2021:gfab268. doi: 10.1093/ndt/gfab268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labriola L., Olinger E., Belge H., Pirson Y., Dahan K., Devuyst O. Paradoxical response to furosemide in uromodulin-associated kidney disease. Nephrol. Dial. Transpl. 2015;30:330–335. doi: 10.1093/ndt/gfu389. [DOI] [PubMed] [Google Scholar]
- 69.Schaeffer C., Cattaneo A., Trudu M., Santambrogio S., Bernascone I., Giachino D., Caridi G., Campo A., Murtas C., Benoni S., et al. Urinary secretion and extracellular aggregation of mutant uromodulin isoforms. Kidney Int. 2012;81:769–778. doi: 10.1038/ki.2011.456. [DOI] [PubMed] [Google Scholar]
- 70.Bollee G., Dahan K., Flamant M., Moriniere V., Pawtowski A., Heidet L., Lacombe D., Devuyst O., Pirson Y., Antignac C., et al. Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin. J. Am. Soc. Nephrol. 2011;6:2429–2438. doi: 10.2215/CJN.01220211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirby A., Gnirke A., Jaffe D.B., Baresova V., Pochet N., Blumenstiel B., Ye C., Aird D., Stevens C., Robinson J.T., et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat. Genet. 2013;45:299–303. doi: 10.1038/ng.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu S.M., Bleyer A.J., Anis K., Herlitz L., Zivna M., Hulkova H., Markowitz G.S., Jim B. Autosomal Dominant Tubulointerstitial Kidney Disease due to MUC1 Mutation. Am. J. Kidney Dis. 2018;71:495–500. doi: 10.1053/j.ajkd.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Olinger E., Hofmann P., Kidd K., Dufour I., Belge H., Schaeffer C., Kipp A., Bonny O., Deltas C., Demoulin N., et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int. 2020;98:717–731. doi: 10.1016/j.kint.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 74.Ayasreh N., Bullich G., Miquel R., Furlano M., Ruiz P., Lorente L., Valero O., Garcia-Gonzalez M.A., Arhda N., Garin I., et al. Autosomal Dominant Tubulointerstitial Kidney Disease: Clinical Presentation of Patients with ADTKD-UMOD and ADTKD-MUC1. Am. J. Kidney Dis. 2018;72:411–418. doi: 10.1053/j.ajkd.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Zivna M., Kidd K., Zaidan M., Vyletal P., Baresova V., Hodanova K., Sovova J., Hartmannova H., Votruba M., Treslova H., et al. An international cohort study of autosomal dominant tubulointerstitial kidney disease due to REN mutations identifies distinct clinical subtypes. Kidney Int. 2020;98:1589–1604. doi: 10.1016/j.kint.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bleyer A.J., Zivná M., Hulková H., Hodanová K., Vyletal P., Sikora J., Zivný J., Sovová J., Hart T.C., Adams J.N., et al. Clinical and molecular characterization of a family with a dominant renin gene mutation and response to treatment with fludrocortisone. Clin. Nephrol. 2010;74:411–422. doi: 10.5414/CNP74411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaeffer C., Izzi C., Vettori A., Pasqualetto E., Cittaro D., Lazarevic D., Caridi G., Gnutti B., Mazza C., Jovine L., et al. Autosomal Dominant Tubulointerstitial Kidney Disease with Adult Onset due to a Novel Renin Mutation Mapping in the Mature Protein. Sci. Rep. 2019;9:11601. doi: 10.1038/s41598-019-48014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cornec-Le Gall E., Olson R.J., Besse W., Heyer C.M., Gainullin V.G., Smith J.M., Audrézet M.P., Hopp K., Porath B., Shi B., et al. Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am. J. Hum. Genet. 2018;102:832–844. doi: 10.1016/j.ajhg.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huynh V.T., Audrezet M.P., Sayer J.A., Ong A.C., Lefevre S., Le Brun V., Despres A., Senum S.R., Chebib F.T., Barroso-Gil M., et al. Clinical spectrum, prognosis and estimated prevalence of DNAJB11-kidney disease. Kidney Int. 2020;98:476–487. doi: 10.1016/j.kint.2020.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo F., Tao Y.H. Nephronophthisis: A review of genotype-phenotype correlation. Nephrology. 2018;23:904–911. doi: 10.1111/nep.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Snoek R., van Setten J., Keating B.J., Israni A.K., Jacobson P.A., Oetting W.S., Matas A.J., Mannon R.B., Zhang Z., Zhang W., et al. NPHP1 (Nephrocystin-1) Gene Deletions Cause Adult-Onset ESRD. J. Am. Soc. Nephrol. 2018;29:1772–1779. doi: 10.1681/ASN.2017111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hildebrandt F., Attanasio M., Otto E. Nephronophthisis: Disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf M.T. Nephronophthisis and related syndromes. Curr. Opin. Pediatr. 2015;27:201–211. doi: 10.1097/MOP.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Georges B., Cosyns J.P., Dahan K., Snyers B., Carlier B., Loute G., Pirson Y. Late-onset renal failure in Senior-Loken syndrome. Am. J. Kidney Dis. 2000;36:1271–1275. doi: 10.1053/ajkd.2000.19845. [DOI] [PubMed] [Google Scholar]
- 85.Hoefele J., Nayir A., Chaki M., Imm A., Allen S.J., Otto E.A., Hildebrandt F. Pseudodominant inheritance of nephronophthisis caused by a homozygous NPHP1 deletion. Pediatr. Nephrol. 2011;26:967–971. doi: 10.1007/s00467-011-1761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.König J., Kranz B., König S., Schlingmann K.P., Titieni A., Tönshoff B., Habbig S., Pape L., Häffner K., Hansen M., et al. Phenotypic Spectrum of Children with Nephronophthisis and Related Ciliopathies. Clin. J. Am. Soc. Nephrol. 2017;12:1974–1983. doi: 10.2215/CJN.01280217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hudson R., Patel C., Hawley C.M., O’Shea S., Snelling P., Ho G., Holman K., Bennetts B., Crawford J., Francis L., et al. Adult-Diagnosed Nonsyndromic Nephronophthisis in Australian Families Caused by Biallelic NPHP4 Variants. Am. J. Kidney Dis. 2020;76:282–287. doi: 10.1053/j.ajkd.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 88.Akira M., Suzuki H., Ikeda A., Iwasaki M., Honda D., Takahara H., Rinno H., Tomita S., Suzuki Y. Atypical histological abnormalities in an adult patient with nephronophthisis harboring NPHP1 deletion: A case report. BMC Nephrol. 2021;22:261. doi: 10.1186/s12882-021-02466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macia M.S., Halbritter J., Delous M., Bredrup C., Gutter A., Filhol E., Mellgren A.E.C., Leh S., Bizet A., Braun D.A., et al. Mutations in MAPKBP1 Cause Juvenile or Late-Onset Cilia-Independent Nephronophthisis. Am. J. Hum. Genet. 2017;100:323–333. doi: 10.1016/j.ajhg.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stokman M.F., van der Zwaag B., van de Kar N., van Haelst M.M., van Eerde A.M., van der Heijden J.W., Kroes H.Y., Ippel E., Schulp A.J.A., van Gassen K.L., et al. Clinical and genetic analyses of a Dutch cohort of 40 patients with a nephronophthisis-related ciliopathy. Pediatr. Nephrol. 2018;33:1701–1712. doi: 10.1007/s00467-018-3958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugimoto K., Miyazawa T., Enya T., Nishi H., Miyazaki K., Okada M., Takemura T. Clinical and genetic characteristics of Japanese nephronophthisis patients. Clin. Exp. Nephrol. 2016;20:637–649. doi: 10.1007/s10157-015-1180-5. [DOI] [PubMed] [Google Scholar]
- 92.Fujimaru T., Kawanishi K., Mori T., Mishima E., Sekine A., Chiga M., Mizui M., Sato N., Yanagita M., Ooki Y., et al. Genetic Background and Clinicopathologic Features of Adult-onset Nephronophthisis. Kidney Int. Rep. 2021;6:1346–1354. doi: 10.1016/j.ekir.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamiwka L.A., Midgley J.P., Wade A.W., Martz K.L., Grisaru S. Outcomes of kidney transplantation in children with nephronophthisis: An analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. Pediatr. Transpl. 2008;12:878–882. doi: 10.1111/j.1399-3046.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 94.Harris P.C., Torres V.E. Polycystic kidney disease. Annu. Rev. Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gurrieri F., Franco B., Toriello H., Neri G. Oral-facial-digital syndromes: Review and diagnostic guidelines. Am. J. Med. Genet. A. 2007;143a:3314–3323. doi: 10.1002/ajmg.a.32032. [DOI] [PubMed] [Google Scholar]
- 96.Romio L., Wright V., Price K., Winyard P.J., Donnai D., Porteous M.E., Franco B., Giorgio G., Malcolm S., Woolf A.S., et al. OFD1, the gene mutated in oral-facial-digital syndrome type 1, is expressed in the metanephros and in human embryonic renal mesenchymal cells. J. Am. Soc. Nephrol. 2003;14:680–689. doi: 10.1097/01.ASN.0000054497.48394.D2. [DOI] [PubMed] [Google Scholar]
- 97.Feather S.A., Woolf A.S., Donnai D., Malcolm S., Winter R.M. The oral-facial-digital syndrome type 1 (OFD1), a cause of polycystic kidney disease and associated malformations, maps to Xp22.2-Xp22.3. Hum. Mol. Genet. 1997;6:1163–1167. doi: 10.1093/hmg/6.7.1163. [DOI] [PubMed] [Google Scholar]
- 98.Ferrante M.I., Giorgio G., Feather S.A., Bulfone A., Wright V., Ghiani M., Selicorni A., Gammaro L., Scolari F., Woolf A.S., et al. Identification of the gene for oral-facial-digital type I syndrome. Am. J. Hum. Genet. 2001;68:569–576. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morleo M., Franco B. OFD Type I syndrome: Lessons learned from a rare ciliopathy. Biochem. Soc. Trans. 2020;48:1929–1939. doi: 10.1042/BST20191029. [DOI] [PubMed] [Google Scholar]
- 100.Macca M., Franco B. The molecular basis of oral-facial-digital syndrome, type 1. Am. J. Med. Genet. C Semin. Med. Genet. 2009;151c:318–325. doi: 10.1002/ajmg.c.30224. [DOI] [PubMed] [Google Scholar]
- 101.Feather S.A., Winyard P.J., Dodd S., Woolf A.S. Oral-facial-digital syndrome type 1 is another dominant polycystic kidney disease: Clinical, radiological and histopathological features of a new kindred. Nephrol. Dial. Transplant. 1997;12:1354–1361. doi: 10.1093/ndt/12.7.1354. [DOI] [PubMed] [Google Scholar]
- 102.Curatolo P., Bombardieri R., Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 103.Dabora S.L., Jozwiak S., Franz D.N., Roberts P.S., Nieto A., Chung J., Choy Y.S., Reeve M.P., Thiele E., Egelhoff J.C., et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rakowski S.K., Winterkorn E.B., Paul E., Steele D.J., Halpern E.F., Thiele E.A. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int. 2006;70:1777–1782. doi: 10.1038/sj.ki.5001853. [DOI] [PubMed] [Google Scholar]
- 105.Northrup H., Krueger D.A. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013;49:243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wataya-Kaneda M., Tanaka M., Hamasaki T., Katayama I. Trends in the prevalence of tuberous sclerosis complex manifestations: An epidemiological study of 166 Japanese patients. PLoS ONE. 2013;8:e63910. doi: 10.1371/annotation/c511ee3c-f91a-4cfe-bad7-6ef58579717e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Umeoka S., Koyama T., Miki Y., Akai M., Tsutsui K., Togashi K. Pictorial review of tuberous sclerosis in various organs. Radiographics. 2008;28:e32. doi: 10.1148/rg.e32. [DOI] [PubMed] [Google Scholar]
- 108.Schillinger F., Montagnac R. Chronic renal failure and its treatment in tuberous sclerosis. Nephrol. Dial. Transpl. 1996;11:481–485. doi: 10.1093/oxfordjournals.ndt.a027315. [DOI] [PubMed] [Google Scholar]
- 109.Barua M., Pei Y. Diagnosis of autosomal-dominant polycystic kidney disease: An integrated approach. Semin. Nephrol. 2010;30:356–365. doi: 10.1016/j.semnephrol.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Bissler J.J., Siroky B.J., Yin H. Glomerulocystic kidney disease. Pediatr. Nephrol. 2010;25:2049–2056. doi: 10.1007/s00467-009-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siroky B.J., Towbin A.J., Trout A.T., Schäfer H., Thamann A.R., Agricola K.D., Tudor C., Capal J., Dixon B.P., Krueger D.A., et al. Improvement in Renal Cystic Disease of Tuberous Sclerosis Complex after Treatment with Mammalian Target of Rapamycin Inhibitor. J. Pediatr. 2017;187:318–322.e312. doi: 10.1016/j.jpeds.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 112.Curatolo P., Maria B.L. Tuberous sclerosis. Handb. Clin. Neurol. 2013;111:323–331. doi: 10.1016/b978-0-444-52891-9.00038-5. [DOI] [PubMed] [Google Scholar]
- 113.Martignoni G., Bonetti F., Pea M., Tardanico R., Brunelli M., Eble J.N. Renal disease in adults with TSC2/PKD1 contiguous gene syndrome. Am. J. Surg. Pathol. 2002;26:198–205. doi: 10.1097/00000478-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 114.Sampson J.R., Maheshwar M.M., Aspinwall R., Thompson P., Cheadle J.P., Ravine D., Roy S., Haan E., Bernstein J., Harris P.C. Renal cystic disease in tuberous sclerosis: Role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 1997;61:843–851. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pei Y. Diagnostic approach in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2006;1:1108–1114. doi: 10.2215/CJN.02190606. [DOI] [PubMed] [Google Scholar]
- 116.Lonser R.R., Glenn G.M., Walther M., Chew E.Y., Libutti S.K., Linehan W.M., Oldfield E.H. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 117.Maher E.R., Neumann H.P., Richard S. von Hippel-Lindau disease: A clinical and scientific review. Eur. J. Hum. Genet. 2011;19:617–623. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chittiboina P., Lonser R.R. Von Hippel-Lindau disease. Handb. Clin. Neurol. 2015;132:139–156. doi: 10.1016/b978-0-444-62702-5.00010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ashouri K., Mohseni S., Tourtelot J., Sharma P., Spiess P.E. Implications of Von Hippel-Lindau Syndrome and Renal Cell Carcinoma. J. Kidney Cancer VHL. 2015;2:163–173. doi: 10.15586/jkcvhl.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taylor S.R., Singh J., Sagoo M.S., Lightman S.L. Clinical and molecular features associated with cystic visceral lesions in von hippel-lindau disease. Open Ophthalmol. J. 2012;6:83–85. doi: 10.2174/1874364101206010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.