Abstract
A Brucella melitensis 16M DNA fragment of 17,119 bp, which contains a large region deleted in B. abortus strains and DNA flanking one side of the deletion, has been characterized. In addition to the previously identified omp31 gene, 14 hypothetical genes have been identified in the B. melitensis fragment, most of them showing homology to genes involved in the synthesis of a polysaccharide. Considering that 10 of the 15 genes are missing in B. abortus and that all the polysaccharides described in the Brucella genus (lipopolysaccharide, native hapten, and polysaccharide B) have been detected in all the species, it seems likely that the genes described here might be part of a cluster for the synthesis of a novel Brucella polysaccharide. Several polysaccharides have been identified as important virulence factors, and the discovery of a novel polysaccharide in the brucellae which is probably not synthesized in B. abortus might be interesting for a better understanding of the pathogenicity and host preference differences observed between the Brucella species. However, the possibility that the genes described in this paper no longer encode the synthesis of a polysaccharide cannot be excluded. Brucellae belong to the alpha-2 subdivision of the class Proteobacteria, which includes other microorganisms living in association with eucaryotic cells, some of them synthesizing extracellular polysaccharides involved in the interaction with the host cell. The genes described in this paper might be a remnant of the common ancestor of the alpha-2 subdivision of the class Proteobacteria, and the brucellae might have lost such extracellular polysaccharide during evolution if it was not necessary for survival or for establishment of the infectious process. Nevertheless, further studies are necessary to identify the entire DNA fragment missing in B. abortus strains and to elucidate the mechanism responsible for such deletion, since only 9,948 bp of the deletion was present in the sequenced B. melitensis DNA fragment.
Microorganisms belonging to the genus Brucella are gram-negative, facultative intracellular bacteria that are able to cause infections in humans and many animal species. Brucellae have been classified, according to differences in pathogenicity and host preference, into six species: Brucella melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae (24). Several biovars have been also described in some of these species and are currently differentiated by serotyping, phage typing, dye sensitivities, and culture and metabolic properties (4). A high degree of homology has been found within the Brucella genus at the DNA level, which has led to a proposal to classify the genus into just one species (77, 78). However, the preferred-host classification is still used, since the six species and some of their biovars can also be differentiated on the basis of DNA polymorphisms detected by several techniques (1, 11, 12, 20, 21, 27, 29, 30, 39, 42, 53, 54, 58, 80).
In spite of the high degree of DNA homology of the Brucella species, important divergences in DNA should exist between species to explain their differential behavior. Studies leading to the identification of DNA variability between species would be of great interest in our understanding of the differences in pathogenicity and host preference observed in the brucellae.
Small deletions in several genes have been detected in some Brucella species (19, 22, 23, 29, 67), sometimes leading to the lack of production of the encoded protein (67), and the lack of expression of an existing gene has also been reported (28, 42). Moreover, DNA deletions of several sizes and DNA inversions have been shown to exist, by restriction enzyme mapping, in some Brucella species (54), but these DNA regions have not yet been identified. Cloning and sequencing of B. melitensis 16M omp31 (79), a gene coding for a Brucella major outer membrane protein, has allowed us to verify that this gene is missing in B. abortus strains but exists in all the other Brucella species (80). Moreover, DNA bordering this gene is also missing in B. abortus, and the size of the deletion was estimated to be about 10 kb (80). Such a large deletion presumably removes genes in addition to omp31, and their identification might shed more light on the understanding of the pathogenicity and host preference differences between the brucellae. In the present work, we characterized a large DNA fragment missing in B. abortus and assigned functions to the genes involved in this deletion, giving evidence for a gene machinery involved in the synthesis of a novel Brucella polysaccharide.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. melitensis 16M and B. abortus 544 were obtained from the INRA Brucella Culture Collection, Nouzilly (BCCN), France. The cultures were grown on tryptic soy agar (BioMérieux, Marcy l’Etoile, France) supplemented with 0.1% (wt/vol) yeast extract (Difco Laboratories, Detroit, Mich.). The strains were checked for purity and for species and biovar characterization by standard procedures (4). Escherichia coli JM109 cells bearing the different recombinant plasmids used in this study were cultured overnight on Luria-Bertani medium containing 50 μg of ampicillin ml−1.
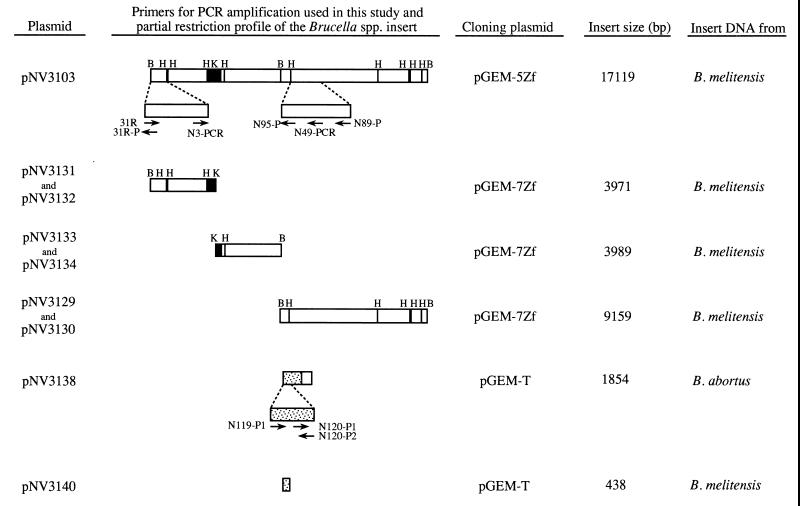
The relevant characteristics of the plasmids used in this study are shown in Fig. 1. Plasmid pNV3103 contains a B. melitensis 16M DNA fragment of 17,119 bp that includes a large fragment deleted in B. abortus and adjacent DNA bordering one side of the deletion. This recombinant plasmid was obtained by subcloning, into the NotI site of pGEM-5Zf (Promega, Madison, Wis.), the DNA insert of a phage from a B. melitensis 16M genomic library constructed in λGEM-12 XhoI half-site arms (Promega) as described previously (79). Plasmids pNV3129 and pNV3130 contain the second BamHI fragment of pNV3103 but in the opposite orientation relative to lacZ and were obtained by subcloning the insert of pNV3103 in pGEM-7Zf (Promega). Plasmids pNV3131 and pNV3132 bear the same BamHI-KpnI fragment of pNV3103 cloned in pGEM-7ZF but in the opposite orientation in relation to lacZ. Plasmids pNV3133 and pNV3134 contain the same KpnI-BamHI fragment of pNV3103 cloned in pGEM-7Zf but in the opposite orientation in relation to lacZ.
FIG. 1.
Plasmids and primers used for PCR amplification in this study. pNV3103 contains a 17,119-bp B. melitensis 16M DNA fragment and was obtained by subcloning, into pGEM-5Zf, the insert DNA of a phage from a B. melitensis 16M genomic library as described previously (79). pNV3131, pNV3132, pNV3133, p NV3134, pNV3129, and pNV3130 were obtained by subcloning the pNV3103 insert into pGEM-7Zf. The position of the previously identified omp31 gene (79) is represented in black. Plasmid pNV3138 was obtained by cloning in pGEM-T the B. abortus 544 DNA fragment amplified with primers N3-PCR and N89-P. pNV3140 was obtained by cloning in pGEM-T the B. melitensis 16M DNA fragment amplified with primers N119-P1 and N120-P2. DNA bordering the left side of the B. abortus deletion point and not cloned in the pNV3103 insert is represented as a shaded box in both pNV3138 and pNV3140. Restriction sites: B, BamHI; H, HindIII; K, KpnI. BamHI sites located at both ends of the pNV3103 insert belong to the multiple-cloning site of phage λGEM-12 and not to the B. melitensis 16M DNA insert. Primers are marked by arrows in the 5′ → 3′ direction.
pNV3138 was obtained by cloning into pGEM-T (Promega) the B. abortus 544 DNA fragment PCR amplified with primers N3-PCR and N89-P. pNV3140 bears the B. melitensis 16M DNA fragment amplified with primers N119-P1 and N120-P2 and ligated into pGEM-T.
DNA preparation.
For Brucella genomic DNA extraction, the strains were cultured for 24 h at 37°C on tryptic soy agar-yeast extract slopes and harvested, in 3 ml of sterile distilled water, by centrifugation at 2,000 × g for 10 min. The pellet was suspended in 567 μl of TE/sodium buffer (50 mM Tris, 50 mM EDTA, 100 mM NaCl [pH 8.0]). Then, 30 μl of 10% (wt/vol) sodium dodecyl sulfate (SDS) solution and 3 μl of 2% (wt/vol) proteinase K solution were added, and the mixture was kept at 37°C for 1 h. The lysed cell suspension was extracted twice with phenol-chloroform, and nucleic acids were precipitated by gently mixing the aqueous phase with 2 volumes of cold ethanol. The precipitate was dissolved in 100 μl of TE (10 mM Tris, 1 mM EDTA [pH 8.0]). The amount of DNA was measured by electrophoresis of an aliquot of each sample through 0.8% agarose gels and comparison with standard DNA solutions.
Plasmid DNA was extracted from recombinant E. coli JM109 cells by standard procedures (66).
DNA sequencing.
Insert DNA from plasmids pNV3129, pNV3130, pNV3131, pNV3132, pNV3133, and pNV3134 (obtained by subcloning the pNV3103 insert) (Fig. 1) was unidirectionally digested with exonuclease III by using the Erase-a-base system (Promega) as specified by the manufacturer. A series of plasmids differing in approximately 400 bp was obtained for each initial plasmid and used to determine the entire sequence of the 17,119-bp insert of B. melitensis 16M contained in pNV3103. pNV3138 and pNV3140 inserts were sequenced without exonuclease digestion. Plasmid DNA was obtained and purified by using the Wizard Plus SV minipreps system (Promega) as specified by the manufacturer.
Purified plasmid DNA was sequenced by primer-directed dideoxy sequencing (68) with an ABI PRISM 377 DNA sequencer (Perkin-Elmer, Foster City, Calif.) and the forward pUC19 primer. In some cases, specific Brucella DNA primers or the reverse pUC19 primer were also used.
DNA amplification and cloning of PCR-amplified DNA.
Primers for PCR amplification of B. melitensis 16M or B. abortus 544 DNA fragments were selected according to the pNV3103 or pNV3138 deduced sequence. The following primers were used (Fig. 1): 31R, 5′-TCTGCGTGATGAAATGCTGG-3′; 31R-P, 5′-CCAGCATTTCATCACGCA-3′; N3-PCR, 5′-ACTGGTTTCATTCCCGCC-3′; N95-P, 5′-GCGATAGTTCACCGTTGT-3′; N49-PCR, 5′-AGAGCAGGCGTTCCACAC-3′; N89-P, 5′-ATCAAGCCTGCGGGACAT-3′; N119-P1, 5′-TTGCTGGTCTTGCGGTGT-3′; N120-P1, 5′-CCGTGCCGATTTTTATGG-3′; and N120-P2, 5′-AAGCCTTTTCGGATGAGC-3′. Amplification reaction mixtures were prepared in volumes of 50 μl containing 1× PCR buffer (MAD-GEN, Valencia, Spain), 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1 μM each primer, 200 ng of genomic DNA, and 2.5 U of DNA polymerase Super-Therm (MAD-GEN). The temperature cycling for the amplification was performed in a PTC-100 thermocycler (MJ Research Inc., Watertown, Mass.) as follows: the first cycle was 94°C for 5 min (denaturation); the next 35 cycles were 58°C for 1 min (annealing), 72°C for 2 min (extension), and 94°C for 1 min (denaturation); the last cycle was 58°C for 1 min (annealing) and 72°C for 10 min (extension). The size of the amplified DNA was determined by electrophoresis on 0.8% agarose gels and comparison with DNA molecular weight standards (Boehringer, Mannheim, Germany). When desired, the amplified DNA was cloned into plasmid pGEM-T (Promega) as specified by the manufacturer.
DNA and protein analysis.
DNA sequences were analyzed for putative coding regions by using the DNAStrider 1.2 program (51). Searches for DNA and protein homologies were performed with the FASTA program (26a, 59). Cellular location and motifs of the predicted proteins were determined with the PSORT (57, 60a) and MOTIF programs (55a), respectively. Multiple alignments were performed with CLUSTAL W 1.74 (23a, 76).
Nucleotide sequence accession number.
The nucleotide sequences of the B. melitensis 16M inserts of pNV3103 and pNV3140 and of the B. abortus 544 insert of pNV3138 have been submitted to the DDBJ/EMBL/GenBank databases under accession no. AF076290, AF076289, and AF076288, respectively.
RESULTS
DNA sequence of the pNV3103 insert DNA.
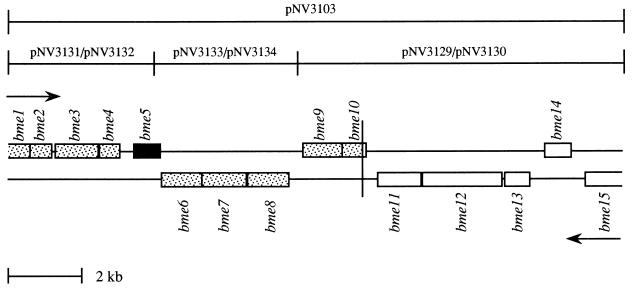
Plasmid pNV3103, which had been obtained previously, bears a B. melitensis 16M DNA insert of about 17 kb that was shown to contain a large DNA fragment, including the omp31 gene (missing in B. abortus strains) and flanking DNA (80). The entire nucleotide sequence of both strands of the pNV3103 insert was determined by sequencing, as described in Materials and Methods, the insert DNA from plasmids pNV3131, pNV3132, pNV3133, pNV3134, pNV3129, and pNV3130, obtained by subcloning the pNV3103 B. melitensis 16M insert (Fig. 1). The B. melitensis insert of pNV3103 was shown to be 17,119 bp (Fig. 2), and analysis of the nucleotide sequence with DNA Strider and FASTA has allowed us to identify 15 open reading frames (ORFs) that potentially encode proteins (Fig. 2; Tables 1 and 2).
FIG. 2.
ORF distribution of the B. melitensis 16M DNA fragment cloned in plasmid pNV3103. The ORFs deleted in B. abortus strains are shaded, except that corresponding to the previously identified omp31 (79), which is represented in black. The vertical line shows the limit of the right side of the deletion in B. abortus located between nucleotides 9948 and 9949. Arrows show the direction of ORF transcription. The extent of the B. melitensis 16M plasmids inserts used for sequencing is also marked.
TABLE 1.
Characteristics of the proteins deduced from the putative genes found in the B. melitensis 16M DNA insert of plasmid pNV3103
| Protein | Start nucleotide (putative start codon) | End nucleotide | Putative RBS sequence (position)a | Strand of transcription | No. of amino acids | Size of protein (kDa) | Cellularb localization |
|---|---|---|---|---|---|---|---|
| Bme1c | 618 | Direct | |||||
| Bme2 | 615 (ATG) | 1232 | GGAG (12) | Direct | 205 | 22.0 | P |
| Bme3 | 1310 (ATG) | 2491 | GGAG (4) | Direct | 393 | 44.7 | CM |
| Bme4 | 2503 (ATG) | 3117 | AAGG (9) | Direct | 204 | 22.0 | CM |
| Bme5d | 3514 (ATG) | 4222 | GAGG (7) | Direct | 240 | 25.3 | OM |
| Bme6 | 5431 (ATG) | 4307 | AGGAGG (9) | Reverse | 374 | 40.5 | CM |
| Bme7 | 6663 (ATG) | 5428 | GGA (9) | Reverse | 411 | 44.6 | C |
| Bme8 | 7934 (ATG) | 6684 | AGGA (10) | Reverse | 416 | 46.4 | C |
| Bme9 | 8198 (TTG) | 9268 | GGAGG (9) | Direct | 356 | 40.6 | C |
| Bme10 | 9252 (ATG) | 9992 | GGAGGT (9) | Direct | 246 | 26.5 | C |
| Bme11 | 11583 (GTG) | 10336 | GGA (5) | Reverse | 415 | 45.0 | P |
| Bme12 | 13782 (GTG) | 11593 | AAGGAG (8) | Reverse | 729 | 79.0 | CM |
| Bme13 | 14586 (ATG) | 13819 | GAGG (9) | Reverse | 255 | 28.2 | CM |
| Bme14 | 14949 (ATG) | 15707 | GGT (7) | Direct | 252 | 28.5 | CM |
| Bme15c | 16084 | Reverse |
Position of the putative RBS expressed as nucleotides upstream of the start codon.
The most probable cellular localization of each protein was determined with the PSORT program (60a). C, cytoplasm; CM, cytoplasmic membrane; OM, outer membrane; P, periplasm.
Only DNA encoding the C-terminal end of Bme1 and Bme15 was cloned in pNV3103. Therefore, the start position, RBS, number of amino acids, size, and cellular localization are not given for these proteins.
Bme5 corresponds to the previously identified outer membrane protein Omp31 (79).
TABLE 2.
Most representative homologies of the B. melitensis 16M hypothetical proteins encoded by pNV3103 to other proteins in the databases
| pNV3103 protein | No. of aaa | Similar proteins and source | No. of aa | Putative function | % Identity (aa overlap) | Expectation value | Accession no. |
|---|---|---|---|---|---|---|---|
| Bme1b | 205 | KdtX, Serratia marcescens | 257 | Glycosyltransferase | 28.2 (173) | 1.2 × 10−2 | U52844 |
| HI0635, Haemophilus influenzae | 254 | Unknown | 26.1 (172) | 1.4 × 10−1 | U32748 | ||
| LgtF, Neisseria meningitidis | 252 | β-1,4-Glucosyltransferase | 26.1 (176) | 3.0 | U58765 | ||
| Bme2 | 205 | ORF C, Streptomyces coelicolor | 203 | Inhibition of plasmid maintenance | 27.3 (176) | 9.9 × 10−2 | X15866 |
| HI1701, Haemophilus influenzae | 247 | Unknown | 31.3 (150) | 1.0 × 10−1 | U32843 | ||
| GdmH, Staphylococcus gallinarum | 330 | Epidermin secretion | 25.3 (162) | 1.2 × 10−1 | U61158 | ||
| EpiH, Staphylococcus epidermidis | 330 | Epidermin secretion | 26.3 (148) | 1.6 × 10−1 | U77778 | ||
| Bme3 | 393 | Hypothetical, Escherichia coli | 208 | Unknown | 24.7 (166) | 1.3 | AE000270 |
| SmpX, Synechococcus spp. | 269 | Channel protein | 24.2 (149) | 1.6 | D43774 | ||
| Bme4 | 204 | Galactoside acetyltransferase, Escherichia coli | 182 | Galactoside acetyltransferase | 39.7 (156) | 2.3 × 10−23 | D90843 |
| NodL, Rhizobium leguminosarum | 190 | Acetyltransferase | 31.3 (180) | 6.5 × 10−11 | Y00548 | ||
| NodL, Rhizobium meliloti | 183 | Acetyltransferase | 28.9 (180) | 1.6 × 10−9 | X61083 | ||
| MJ1064, Methanococcus jannaschii | 214 | Galactoside acetyltransferase | 27.1 (140) | 1.9 × 10−7 | U67549 | ||
| Bme5c | 240 | Omp31, Brucella melitensis 16M | 240 | Unknown | 100 (240) | 0 | U39453 |
| Omp25, Brucella suis | 213 | Unknown | 34.7 (245) | 3.7 × 10−6 | U39397 | ||
| Omp25, Brucella neotomae | 213 | Unknown | 34.7 (245) | 3.7 × 10−6 | U39359 | ||
| Bme6 | 374 | Hypothetical, Synechocystis spp. | 386 | Unknown | 26.6 (391) | 1.1 × 10−13 | D90901 |
| MTH370, Methanobacterium thermoautotrophicum | 395 | LPS biosynthesis, RfbU related | 30.5 (364) | 7.8 × 10−13 | AE000822 | ||
| MTH173, Methanobacterium thermoautotrophicum | 382 | LPS biosynthesis, RfbU related | 27.0 (322) | 9.5 × 10−12 | AE000805 | ||
| Bme7 | 411 | MTH450, Methanobacterium thermoautotrophicum | 411 | LPS biosynthesis, RfbU related | 33.3 (213) | 4.8 × 10−7 | AE000829 |
| YefL, Escherichia coli | 406 | Unknown | 32.5 (243) | 9.8 × 10−7 | D90842 | ||
| BplH, Bordetella pertussis | 390 | Glycosyltransferase | 29.1 (237) | 3.0 × 10−6 | X90711 | ||
| AmsK, Erwinia amylovora | 407 | EPS synthesis | 31.8 (198) | 1.3 × 10−5 | X77921 | ||
| Bme8 | 416 | Hypothetical, Synechocystis spp. | 424 | Unknown | 29.1 (409) | 0 | D90901 |
| Cap5L, Staphylococcus aureusd | 401 | Glycosyltransferase | 23.3 (390) | 5.5 × 10−21 | U81973 | ||
| YefD, Escherichia coli | 407 | Glycosyltransferase | 22.2 (388) | 1.0 × 10−10 | D90843 | ||
| WbpJ, Pseudomonas aeruginosa | 413 | Glycosyltransferase | 23.7 (367) | 1.7 × 10−10 | U50396 | ||
| Bme9 | 356 | NoeL, Rhizobium spp. | 351 | GDP–d-mannose-4,6-dehydratase | 68.1 (339) | 0 | AE000064 |
| RfbD, Vibrio cholerae MO45e | 348 | GDP–d-mannose dehydratase | 60.9 (345) | 0 | U24571 | ||
| Gmd, Yersinia enterocolitica | 372 | GDP-mannose dehydratase | 60.6 (350) | 0 | U46859 | ||
| HP0044, Helicobacter pylori | 381 | GDP–d-mannose dehydratase | 55.5 (371) | 0 | AE000526 | ||
| Bme10 | 246 | NolK, Rhizobium spp. | 314 | Nucleotide sugar epimerase/dehydrogenase | 58.1 (210) | 0 | AE000064 |
| WbcJ, Yersinia enterocolitica | 321 | Epimerase-reductase | 53.4 (204) | 0 | U46859 | ||
| WcaG, Escherichia coli | 321 | Epimerase-reductase | 51.2 (207) | 0 | U38473 | ||
| YefB, Escherichia coli | 321 | Nucleotide sugar epimerase/dehydrogenase | 51.2 (207) | 0 | D90843 | ||
| Bme11 | 415 | ExoF, Rhizobium meliloti | 421 | Biosynthesis of succinoglycan | 25.2 (409) | 1.5 × 10−18 | L05588 |
| PrsE, Rhizobium leguminosarum | 435 | Protein secretion | 25.7 (249) | 1.8 × 10−7 | Y12758 | ||
| PrsE, Rhizobium meliloti | 439 | Protein secretion | 24.7 (287) | 2.6 × 10−7 | U89163 | ||
| GumB, Synechocystis spp. | 504 | Unknown | 28.5 (172) | 9.9 × 10−5 | D90904 | ||
| Bme12 | 729 | GumC, Xanthomonas campestris | 449 | Biosynthesis of xanthan gum | 25.2 (449) | 2.6 × 10−17 | U22511 |
| ExoP, Rhizobium meliloti | 786 | Biosynthesis of succinoglycan | 23.7 (739) | 1.2 × 10−16 | L20758 | ||
| AceD, Acetobacter xylinum | 637 | EPS export | 23.1 (628) | 1.8 × 10−14 | X94981 | ||
| Bme13 | 255 | RegX, Rhodobacter sphaeroides | 212 | Response regulator | 35.3 (215) | 2.5 × 10−20 | U80081 |
| FlhR, Paracoccus denitrificans | 236 | Unknown | 30.2 (212) | 5.9 × 10−9 | AJ223460 | ||
| GlpR, Pseudomonas aeruginosa | 221 | Glycerol regulatory protein | 26.3 (209) | 7.5 × 10−9 | M60805 | ||
| EpsR, Pseudomonas solanacearum | 236 | Negative regulator of EPS synthesis | 25.9 (212) | 3.5 × 10−8 | M61197 | ||
| Bme14 | 252 | Fnr, Rhodobacter sphaeroides | 248 | Anaerobic regulatory protein | 26.0 (196) | 3.6 × 10−10 | Z49746 |
| FixK, Bradyrhizobium japonicum | 237 | Transcriptional regulator | 25.5 (212) | 8.7 × 10−10 | M86805 | ||
| AadR, Rhodopseudomonas palustris | 239 | Transcriptional regulator | 25.7 (187) | 8.8 × 10−10 | M92426 | ||
| FnrA, Pseudomonas stutzeri | 244 | Transcriptional regulator | 25.4 (209) | 2.3 × 10−9 | Z26044 | ||
| Bme15b | 344 | HI0148, Haemophilus influenzae | 379 | Unknown | 36.6 (344) | 0 | U32700 |
| YjhT, Escherichia coli | 404 | Unknown | 33.0 (342) | 7.4 × 10−19 | AE000501 |
aa, amino acid.
bme1 and bme15 were not entirely cloned in pNV3103. Therefore, the number of amino acids shown in the table corresponds to the C-terminal domain cloned in pNV3103.
Bme5 corresponds to the previously identified Omp31 (79).
Same level of homology to Cap8L from Staphylococcus aureus.
Bme9 also showed homology to other RfbD proteins from Vibrio cholerae.
(i) bme1, bme2, bme3, and bme4.
The first ORF (bme1) is located between nucleotides 1 and 618 (Fig. 2; Table 1). The deduced amino acid sequence had 28.2% identity to the C-terminal end of the kdtX product from Serratia marcescens (Table 2), which has been defined as a putative glycosyltransferase involved in core lipopolysaccharide (LPS) biosynthesis (40). A 26.1% identity was also observed to the C-terminal end of LgtF from Neisseria meningitidis (Table 2), which is a glycosyltransferase involved in the inner-core biosynthesis of lipooligosaccharide (45). It is probable that the entire bme1 gene is not present in the pNV3103 insert, since no putative ribosome binding site (RBS) has been found near an ATG codon and since homology to KdtX and LgtF covers only the C-terminal end of these proteins. The characteristic motif EX7E, found toward the C-terminal end of many glycosyltransferases (5, 70), was also found in Bme1, which suggests that the probable function of this protein is as a glycosyltransferase involved in the synthesis of a polysaccharide.
bme2 starts at position 615, slightly overlapping the end of bme1, and ends at nucleotide 1232 (Fig. 2; Table 1). The most probable location of the deduced protein was determined to be the periplasm, and a putative signal peptidase cleavage site was detected at amino acid 50. Homology was found to the proteins encoded by orfC and HI1701 of Streptomyces coelicolor and Haemophilus influenzae, respectively (Table 2). The protein encoded by orfC is involved in the inhibition of extrachromosomal maintenance of the Streptomyces plasmid SLP1 (37). GdmH and EpiH, from Staphylococcus gallinarum and Staphylococcus epidermidis, respectively, also had homology to Bme2 (Table 2). Both proteins seem to be implicated in the secretion of the lantibiotic epidermin (60).
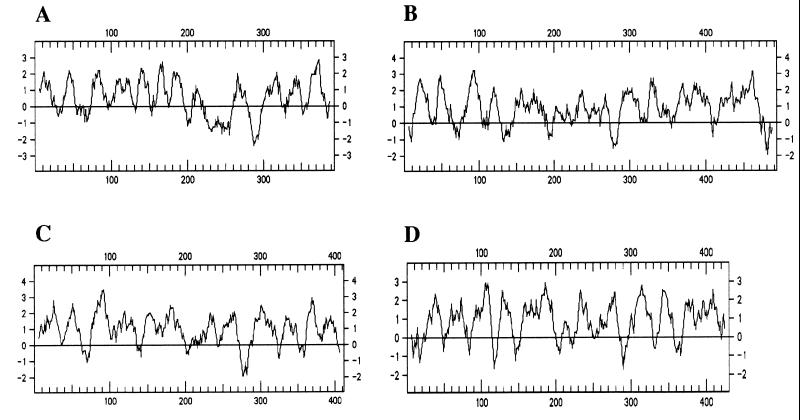
bme3, located between nucleotides 1310 and 2491, would encode a highly hydrophobic cytoplasmic membrane protein (Fig. 2; Table 1). Only the N-terminal end of the deduced protein showed homology (about 24% identity) to a hypothetical protein of E. coli of unknown function and to SmpX from Synechococcus spp. (Table 2), which has been defined as a pore-forming channel protein belonging to a family of homologous intrinsic membrane proteins involved in a variety of transport processes (46). The hydrophobicity profile of Bme3 was very similar to that of Wzx proteins (Fig. 3), reported to be involved in the transfer of polysaccharides across the cytoplasmic membrane and also described as multiple membrane-spanning proteins (73). The Wzx proteins of different polysaccharide biosynthesis clusters have little similarity at the amino acid sequence level but are predicted to have structural homology (49, 73).
FIG. 3.
Comparison of the hydrophobicity profiles of Bme3 and Wzx homologous proteins, determined by the method of Kyte and Doolittle (47) with a window length of 11 amino acid residues. (A) Bme3. (B) Wzx of the E. coli K-12 colanic acid cluster (accession no. U38473). (C) Wzx of the P. aeruginosa B-band LPS O-chain cluster (accession no. U50396). (D) Wzx of the Y. enterocolitica O:8 LPS O-chain cluster (accession no. U46859).
bme4 is located between nucleotides 2503 and 3117 (Fig. 2; Table 1) and would code for a putative protein which displays significant homology to several acetyltransferases (Table 2), enzymes involved in the acetylation of several substrates. The highest homology was observed to the galactoside acetyltransferase from E. coli K-12 (44) and NodL from Rhizobium leguminosarum. The latter protein acetylates various substrates, such as lipooligosaccharides, chitin fragments, and N-acetylglucosamine, and appeared to be located in the cytoplasm (10). Analysis of the amino acid sequence of the hypothetical Bme4 protein with the PSORT program identified the cytoplasmic membrane as the most probable location of the protein, rather than the cytoplasm as described for the NodL acetyltransferase. However, other acetyltransferases, including the acetyltransferase intervening in the synthesis of the Rhizobium meliloti exopolysaccharide (EPS) succinoglycan, have been described as cytoplasmic membrane proteins (36).
No regions of dyad symmetry have been found in the intergenic spaces between the four genes, which suggests that they might be transcribed as a single unit.
(ii) bme5.
bme5 corresponds to the previously identified omp31 gene (79), coding for an immunogenic major outer membrane protein in all Brucella species except in B. abortus, where omp31 was shown to be absent (80). The gene extended from nucleotides 3514 to 4236 (Fig. 2; Table 1) (79). A putative cleavage site for signal peptidase is located between amino acids 19 and 20 of the deduced protein (79).
Although no clear regions of dyad symmetry have been found following the translation termination codon of bme4, omp31 is probably not cotranscribed with bme4. Expression of omp31 has been detected with an anti-Omp31 monoclonal antibody in recombinant E. coli bearing a plasmid containing a PCR product covering omp31 and part of the intergenic region between bme4 and omp31. Expression of omp31 has been detected independently of the orientation of the PCR product in relation to the lac promoter (data not shown), which suggests that omp31 is transcribed from its own promoter.
A region of dyad symmetry that might function as rho-independent transcription terminator was detected 18 bp downstream the stop codon of the gene.
(iii) bme6, bme7, and bme8.
The three hypothetical genes bme6 to bme8 would be transcribed from the reverse strand according to the schema shown in Fig. 2 and are probably cotranscribed, since no regions of dyad symmetry have been found in the intergenic spaces. The dyad symmetry region downstream of omp31 might also function as the transcription terminator of this hypothetical operon.
bme6 is located between nucleotides 4307 (end) and 5431 (start) and would produce a 374-residue protein, whose most probable location is the cytoplasmic membrane (Fig. 2; Table 1). The highest homology level was found to a Methanobacterium thermoautotrophicum protein, which has been defined as an LPS biosynthesis RfbU-related protein (72) (Table 2). RfbU has mannosyltransferase activity in Salmonella enterica (50). The protein also showed homology to a wide number of mannosyltransferases of different microorganisms (data not shown), enzymes that act by transferring mannose for the synthesis of polysaccharide chains.
bme7 extends between nucleotides 5428 (end) and 6663 (start) and would encode a protein that is probably located in the cytoplasm (Fig. 2; Table 1). The C-terminal domain of Bme7 showed homology (Table 2) to the C-terminal end of several proteins that are supposed to be glycosyltransferases (e.g., E. coli YefL and Bordetella pertusis BplH, which is thought to be a glycosyltransferase required for LPS biosynthesis [2]).
bme8 would encode a protein of 416 amino acids located in the bacterial cytoplasm. The ORF covers the pNV3103 insert region contained between nucleotides 6684 (end) and 7934 (start) (Fig. 2; Table 1). A search for homologies revealed that Bme8 had significant homology (Table 2) to a wide number of proteins, from several microorganisms, that are supposed to function as glycosyltransferases (e.g., Cap5L and Cap8L from Staphylococcus aureus, involved in the synthesis of the type 5 and 8 capsular polysaccharides, respectively [69]; YefD from E. coli; and WbpJ from Pseudomonas aeruginosa, involved in the synthesis of LPS [16]).
As described for several glycosyltransferases (5, 70), Bme6, Bme7, and Bme8 exhibit the characteristic motif EX7E toward the C-terminal end of the protein, which accounts for the probable function of these proteins as glycosyltransferases.
(iv) bme9 and bme10.
No regions of dyad symmetry have been found between bme9 and bme10, suggesting that they might be transcribed from the same promoter as a single unit.
Bme9 has a high level of homology to GDP-mannose-4,6-dehydratases, which function as sugar oxidoreductases. The highest homology levels were found to NoeL from Rhizobium spp. (68.1% identity in a 339-amino-acid overlap) (31) and RfbD from Vibrio cholerae (60.9% identity in a 345-amino-acid overlap) (Table 2). According to the nucleotide sequence of pNV3103, the first ATG codon of bme9 is located at position 8549 and the end of the ORF is at nucleotide 9268. However, no characteristic sequence for an RBS was found upstream of the ATG codon, and homology to GDP-mannose dehydratases occurred up to the TTG codon at position 8198. A sequence (GGAGG) that might function as an RBS was found 9 bp upstream of this TTG codon. We propose that bme9 starts at this TTG codon, a codon that has been proposed as the start of translation in other genes (2, 60). If this is the case, the deduced protein would be composed of 356 amino acids and would probably be located in the cytoplasm (Table 1). The region of Bme9 extending from amino acids 66 to 118 showed 71.1% identity to a short identified fragment (53 amino acids) of an RfbD-homologous protein from B. melitensis (accession no. AF043475) and B. abortus (accession no. AF021920), involved in the synthesis of the LPS O chain (3) (Fig. 4).
FIG. 4.
Multiple alignment of amino acids 66 to 118 of Bme9 and the 53 amino acids corresponding to the published partial coding sequence of one GDP–d-mannose dehydratase from B. melitensis (accession no. AF043475) and B. abortus (accession no. AF021920 [3]). Alignment was performed with CLUSTAL W 1.74 (23a). Identical amino acids are shown by asterisks. Conserved and semiconserved substitutions are shown by colons and periods, respectively.
The bme10 start codon is located at position 9252, slightly overlapping the end of bme9, and the end of the ORF is located at nucleotide 9992 (Fig. 2; Table 1). The putative protein would probably have a cytoplasmic location (Table 1). Homology (58.1% identity) was found between Bme10 and NolK from Rhizobium spp. (Table 2), which is proposed to be a NAD-dependent nucleotide sugar epimerase-dehydrogenase required for the biosynthesis of lipochito-oligosaccharidic Nod factors, involved in the nodulation process of the Rhizobium-legume symbiosis (31). Bme10 also showed 53.4 and 51.2% identity to WbcJ from Yersinia enterocolitica serotype O:8 and WcaG from E. coli, respectively (Table 2). Both proteins have been described as sugar epimerase-reductases involved in the conversion of GDP-4-keto-6-deoxy-mannose to GDP-fucose, one of the sugars of the Y. enterocolitica O:8 LPS O antigen (85) and the E. coli EPS colanic acid (73). Although the bme10 ORF ended at nucleotide 9992, homology of Bme10 was found to similar proteins after the stop codon with a change in the reading frame. Close to the stop codon of this other reading frame, located at position 10231, was found a region of dyad symmetry that might act as the transcription terminator of the probably cotranscribed bme9 and bme10. It is possible that the ORF originally finished at this position but that a single nucleotide has been removed during evolution, thereby changing the length of the gene. Additional studies are necessary to elucidate whether this probable reduction of the gene size has altered the function of the encoded protein.
(v) bme11, bme12, and bme13.
The three genes bme11 to bme13 would be transcribed from the reverse strand of the pNV3103 insert, and they might be transcribed as a single unit, since no regions of dyad symmetry have been found in the intergenic spaces. The dyad-symmetry domain found downstream of bme10 might also function as transcription terminator of this three-gene operon transcribed from the reverse strand.
Bme11 shows 25% identity to ExoF from Rhizobium meliloti (Table 2), which is involved in the biosynthesis of the EPS succinoglycan and is reported to be located in the periplasm but probably anchored to the cytoplasmic membrane through its amino terminus (63). ExoF was initially thought to be required for the addition of the first sugar to the lipid carrier of succinoglycan (63). However, although no homology to other proteins in the database was reported for ExoF when the sequence was published (56), later comparisons revealed homology of ExoF to GumB from Xanthomonas campestris, postulated to be involved in the polymerization of the repeating unit of xanthan gum, and KpsD from E. coli, needed for translocation of the capsular polysaccharide (35). The possible role of ExoF in polymerization or translocation of succinoglycan in R. meliloti was then taken into account (35). Local homology was also detected between Bme11 and GumB from X. campestris and between Bme11 and PrsE from R. leguminosarum and R. meliloti, a protein that has been implicated in the secretion of proteins required for the production of low-molecular-weight succinoglycan (83). The first ATG codon of bme11 was located at position 11238 of the pNV3103 insert, 355 bp downstream of the stop codon of bme12. No region of dyad symmetry has been found between the two genes, and homology to exoF extended up to close to a GTG codon located at nucleotide 11583, 10 bp downstream of the bme12 stop codon. A putative RBS (GGA) was detected 5 bp upstream of this GTG. We propose that this GTG codon is the initiation codon, although the real start codon is difficult to establish, since other GTG codons are present between the first GTG codon and the first ATG of the ORF. Accordingly, the ORF would extend from nucleotides 10336 (end) to 11583 (start) and would encode a protein of 415 amino acids with a putative signal peptidase cleavage site at amino acid 26 and the most probable localization in the periplasm (Fig. 2; Table 1).
The first ATG codon of bme12 was located at position 13701, but not clear region for an RBS was found near this ATG codon. However, an RBS sequence (AAGGAG) was detected 8 bp upstream of a GTG codon located at nucleotide 13782, which was considered to be the start codon of bme12. Accordingly, bme12 would extend from nucleotides 11593 (end) to 13782 (start) and would code for a protein of 729 amino acids that is probably located in the cytoplasmic membrane (Fig. 2; Table 1). A search for homology to other proteins in the database revealed that Bme12 is 25.2% identical (449 amino acid overlap) to GumC from X. campestris, a protein of 449 amino acids required for the production of the EPS xanthan gum. A 23.7% identity, covering the entire protein, was detected to ExoP from R. meliloti, reported to be implicated in succinoglycan chain length determination and export (8). AceD from Acetobacter xylinum, involved in EPS export, also showed homology to Bme12 (Table 2).
bme13 extended from nucleotides 13819 (end) to 14586 (start) and would encode a protein with the most probable location in the cytoplasmic membrane (Fig. 2; Table 1). A search for protein motifs revealed a 28-amino-acid region, located toward the C terminus of Bme13 (amino acids 201 to 228), characteristic for bacterial regulator proteins of the LuxR family (43, 74) (data not shown). Bme13 showed homology to proteins which are members of the environmentally responsive two-component regulatory systems (Table 2). Interestingly, two of the homologous proteins, GlpR from Pseudomonas aeruginosa and EpsR from Pseudomonas solanacearum, have been defined as regulator proteins involved in the biosynthesis of EPSs (18, 71).
(vi) bme14.
bme14 would be transcribed from the direct strand of pNV3103 and extended from nucleotides 14949 to 15707, encoding a putative protein probably located in the cytoplasmic membrane (Fig. 2; Table 1). Several regions of dyad symmetry have been found between bme14 and bme15, the gene located downstream of bme14 that would be transcribed from the reverse strand, and that might function as transcription terminators of both genes. Bme14 showed homology to several proteins included in a family of transcriptional regulators responding under low-oxygen concentrations (e.g., FnrL from Rhodobacter sphaeroides [84] and FixK from Bradyrhizobium japonicum [6]) (Table 2).
(vii) bme15.
bme15, which would be transcribed from the reverse strand of pNV3103, seems not to be entirely cloned in pNV3103, since no RBS sequence was found near a start codon and homology was observed only to the C-terminal end of other proteins in the database (Table 2). The highest level of homology was found to two proteins of unknown function (HI0148 from H. influenzae and YjhT from E. coli) (Table 2). The partial ORF extended from nucleotide 16084 (end) to the end of the pNV3103 insert (Fig. 2; Table 1).
Identification of the B. abortus deletion endpoints in the B. melitensis insert of pNV3103.
According to previous results (80), it was thought that the DNA fragment deleted in B. abortus strains would extend from a point close to the left end of the pNV3103 B. melitensis insert to a point located between the fifth and the sixth HindIII sites of the insert (Fig. 1). Therefore, in an attempt to identify the endpoints of the B. abortus deletion, PCR amplification of B. abortus DNA was performed with a primer covering a region near the left end of the pNV3103 insert (primer 31R or N3-PCR) and another one covering a region of the HindIII fragment mentioned above (N95-P, N49-PCR, or N89-P) (Fig. 1). Successful amplification was obtained only with primers N3-PCR and N89-P, which amplified a B. abortus DNA fragment of 1,854 bp that was cloned in plasmid pGEM-T to give plasmid pNV3138 (Fig. 1). Surprisingly, analysis of the nucleotide sequence of this fragment revealed that the last 799 bp is also present in the pNV3103 insert, corresponding to the 799 nucleotides located upstream of primer N89-P, while the first 1,055 bp was not cloned in pNV3103 (Fig. 5). Therefore, PCR amplification occurred through specific hybridization of primer N89-P and nonspecific hybridization of primer N3-PCR. According to previous work, it was thought that the entire DNA fragment missing in B. abortus was cloned in pNV3103 (80). However, sequencing of the pNV3138 insert made this hypothesis unlikely. Two hypotheses were possible, at this point, to explain this unexpected result. The first is that the B. melitensis DNA fragment cloned in pNV3103 does not contain the left side of the DNA deleted in B. abortus, and the second is that the DNA missing in B. abortus has been replaced by another DNA fragment absent in B. melitensis. To clarify this point, B. melitensis DNA was PCR amplified by using primers N119-P1 and N120-P2 (Fig. 1), which were located in the region of the B. abortus pNV3138 insert that was not cloned in the B. melitensis pNV3103 insert. A fragment of 438 bp was obtained, cloned into pGEM-T to give pNV3140 (Fig. 1), and sequenced, revealing that this fragment corresponds to the fragment located between both primers in B. abortus (Fig. 5). Therefore, DNA bordering the left side of the fragment missing in B. abortus was also present in B. melitensis, in agreement with the hypothesis of a large deletion in B. abortus that was not entirely cloned in the B. melitensis DNA insert of pNV3103. In an attempt to identify the DNA fragment absent in B. abortus that was not cloned in pNV3103, PCR amplification of B. melitensis DNA was performed with primers N120-P1 and 31R-P (Fig. 1), with unsuccessful results. In our PCR reaction conditions, fragments of about 2 kb have been successfully amplified but amplification of fragments of 3 kb with specific primers was not obtained (data not shown). Therefore, it seems likely that the DNA deleted in B. abortus strains and not cloned in pNV3103 is large size and would be greater than 2 kb, since no amplification of B. melitensis DNA was obtained with primers N120-P1 and 31R-P. Although the left side of the B. abortus deletion has not been identified, the limit of its right side has been determined to be located between nucleotides 9948 and 9949, near the end of bme10, of the pNV3103 B. melitensis insert (Fig. 2 and 5). Therefore, the DNA deleted in B. abortus accounts for more than 9,948 nucleotides, and bme1 to bme9 and most of bme10 are missing in this species.
FIG. 5.
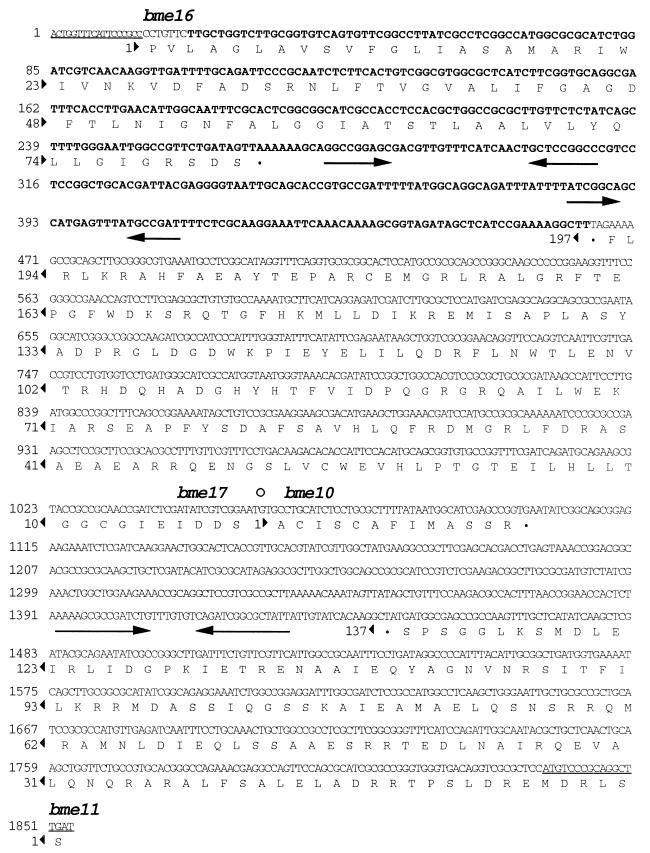
Nucleotide sequence of the B. abortus 544 DNA fragment amplified with primers N3-PCR and N89-P and cloned in pNV3138. The primers are underlined. The primer N3-PCR sequence is shown in small capitals because it is likely that it hybridized nonspecifically, and it is not included in the sequence submitted to the database. The position delimiting both sides of the B. abortus deletion is shown by an open circle. The B. melitensis DNA fragment amplified with primers N119-P1 and N120-P2 is shown in bold type. Potential coding regions are marked and translated into amino acids, although bme10 and bme17 are probably not translated in B. abortus since both genes seem to be interrupted by the deletion. bme10 and bme11 correspond to partial sequences of the same genes found in the B. melitensis insert of pNV3103. Regions of dyad symmetry that might function as rho-independent transcription terminators are shown by inverted arrows.
In addition to the ends of bme10 and bme11, two other potential coding regions, designated bme16 and bme17, were found in the B. abortus insert of pNV3138 (Fig. 5). The hypothetical bme16 would be transcribed from the direct strand and would extend from nucleotide 19 (the region corresponding to primer N3-PCR was not taken into account since this primer probably hybridized nonspecifically with the B. abortus DNA) to nucleotide 267 (Fig. 5). The deduced amino acid sequence showed homology (the highest homology was 52.7% identity in 74 amino acid overlap) to several uracil permeases (e.g., accession no. AE000202, X76083, U32802, and M59757) (data not shown), enzymes intervening in the de novo pyrimidine nucleotide biosynthesis (34, 61). Since no RBS was found near an ATG codon and homology covered only the C-terminal end of the uracil permeases, it is likely that bme16 has not been entirely cloned in pNV3138. Two regions of dyad symmetry that might act as rho-independent transcription terminators were found downstream of bme16 (Fig. 5). In addition to bme10, the deletion in B. abortus strains appears to interrupt another hypothetical gene, bme17, whose putative product showed homology to an E. coli protein of unknown function (accession no. U73857) just covering the C terminus (25% identity in a 160-amino-acid overlap) (Fig. 5). Deletion in B. abortus has probably led to the loss of expression of both bme10 and bme17. Sequencing of the B. melitensis DNA corresponding to the left side of the B. abortus deletion will be necessary to determine the entire sequence of the hypothetical bme17.
DISCUSSION
In a previous study, a large DNA fragment, containing the gene encoding the Brucella Omp31 and DNA bordering both sides of this gene, was found to be missing in B. abortus strains but not in the other Brucella species (80). According to Southern blot hybridization and PCR amplification results, the size of this fragment was suggested to be about 10 kb, and it was thought that the entire DNA sequence absent in B. abortus was contained in the B. melitensis insert of plasmid pNV3103 (80). In the present work, we analyzed the nucleotide sequence of the pNV3103 insert, searching for the limit of the DNA missing in B. abortus and the genes involved in this deletion.
A search for the ORFs of the B. melitensis pNV3103 DNA insert and homology of the deduced proteins to other proteins in the database has allowed us to identify 14 putative new genes, in addition to omp31 (Fig. 2; Tables 1 and 2). Most of the proteins encoded by these genes exhibited a significant degree of homology to proteins involved in the biosynthesis process of several polysaccharides from different bacteria (Table 2), which point to the possibility of the implication of this group of genes in the synthesis of a Brucella polysaccharide.
Four proteins (Bme1, Bme6, Bme7, and Bme8) showed a significant degree of homology to several sugar transferases (Table 2), enzymes that act by transferring the sugar from one nucleotide-sugar complex to the growing chain of a polysaccharide. In the synthesis of a polysaccharide, a sugar transferase is needed for each different linkage between sugars (62), which would mean that the hypothetical Brucella polysaccharide would be composed of at least four sugar units. Bme4 shows homology to acetyltransferases and might act by acetylating one or more sugars of the polysaccharide. Bme9 and Bme10 are homologous to GDP–d-mannose dehydratases and epimerase-reductases, respectively, which might be involved in the modification of some of the sugars before their incorporation into the growing polysaccharide chain. A partial amino acid sequence for B. melitensis and B. abortus proteins homologous to GDP–d-mannose dehydratases has been found in the database (accession no. AF043475 and AF021920, respectively). The two partial sequences were identical, but homology to the equivalent fragment of Bme9 reached only 71.1% identity (Fig. 4). The GDP–d-mannose dehydratase from B. abortus is involved in the synthesis of the smooth-LPS (S-LPS) O chain (3). Bme9 might be involved in the synthesis of a different polysaccharide, since genes for the synthesis of each individual polysaccharide are usually arranged in independent clusters (62).
Biosynthesis of polysaccharides occurs through two different mechanisms. The Wzy-dependent pathway involves Wzx, which would transfer the repeat polysaccharide units across the cytoplasmic membrane to the periplasmic face, a polymerase (Wzy) of the polysaccharide units, and Wzz, which would regulate the polysaccharide length (81). The second pathway requires the ATP binding and transmembrane components of an ATP binding cassette transporter, the polymerase is not needed, and regulation of the polysaccharide length is independent of Wzz (81). Homologous proteins with bacterial ATP binding cassette transporters have not been found among the hypothetical proteins supposed to be encoded by the B. melitensis DNA fragment cloned in pNV3103. Although no homologous proteins with Wzy or Wzx have been identified among the pNV3103 hypothetical proteins, it must be noted that Wzy and Wzx of different polysaccharide biosynthesis clusters have little similarity at the amino acid sequence level (49, 73). Wzx, involved in the transfer of polysaccharides across the cytoplasmic membrane, has been described as a multiple membrane-spanning protein (73). Bme3 is predicted to be a highly hydrophobic protein located in the cytoplasmic membrane, with multiple membrane-spanning segments, that showed a hydrophobicity profile very similar to other Wzx-like proteins (Fig. 3), and it might have a Wzx function, allowing the translocation of the polysaccharide to the periplasm. Moreover, the N-terminal end of Bme3 showed homology to SmpX from Synechococcus spp. (accession no. D43774), which has been defined as a pore-forming channel protein belonging to a family of homologous intrinsic membrane proteins involved in a variety of transport processes (46). Polymerization of the polysaccharide subunits might be mediated by Bme11 and/or Bme12, which might also participate in the transport of the polysaccharide. Both proteins showed homology to other proteins that have been implicated in the polymerization or export of several EPSs (see Results), which points to the possibility that the genes identified in the B. melitensis insert of pNV3103 are part of a pathway for the synthesis of an EPS. This possibility is reinforced by the presence of an outer membrane protein, Bme5, among the proteins found to be encoded in this DNA fragment. Bme5 corresponds to the previously identified Omp31 (79), a protein that is located in the outer membrane of Brucella spp. and for which a possible role as porin has been suggested (79). Bme5 might be involved in the secretion of a hypothetical EPS, as has been suggested for other outer membrane proteins (32, 73). Bme2, probably located in the periplasm, shows only local homology to proteins involved in the secretion of the lantibiotic epidermin (60) and might also contribute to the secretion of an EPS.
Two genes, bme13 and bme14, encoding proteins homologous to regulatory proteins have been identified in the pNV3103 insert. It is difficult to assign them a regulatory function in the synthesis of the hypothetical polysaccharide, but several clusters for the synthesis of other bacterial polysaccharides are controlled by the action of regulatory proteins (18, 38, 74). Although bme15 was not entirely cloned in pNV3103, Bme15 exhibited a significant level of homology to two proteins of unknown function (Table 2), and it is difficult to determine whether it might be involved in the synthesis of a polysaccharide or have an unrelated function.
The number of genes in polysaccharide clusters is normally between 6 and 19, depending on the complexity of the polysaccharide (62). Most of the 15 hypothetical genes found in the pNV3103 B. melitensis DNA insert are supposed to be involved in the synthesis of a polysaccharide, but sequencing of adjacent DNA to both sides of the pNV3103 insert will be necessary to determine whether there are more genes that might be implicated in the synthesis of the hypothetical polysaccharide.
Although it was thought that the pNV3103 insert of B. melitensis contained the entire fragment missing in B. abortus strains (80), results presented in this paper demonstrate that the pNV3103 insert does not contain this entire DNA fragment. In spite of this, the exact point delimiting one side of the DNA absent in B. abortus has been determined to be located between nucleotides 9948 and 9949 of the pNV3103 insert, close to the end of bme10. Therefore, the fragment missing in B. abortus is at least 9,948 bp and covers 10 of the 15 genes identified in the pNV3103 insert (from bme1 to bme10). According to the PCR results presented in this paper, it seems likely that more than 2 kb of the B. abortus deletion remains unknown, and further studies are necessary to completely identify the DNA missing in B. abortus and to clarify the mechanism responsible for this deletion.
If genes contained in the pNV3103 insert are members of a cluster that efficiently synthesize a polysaccharide, it seems likely that the polysaccharide would not be present in B. abortus strains, since 10 of the 15 genes of the pNV3103 insert are deleted in B. abortus. Several polysaccharides have been identified in the genus Brucella. S-LPS has been widely studied since it is an important diagnostic and protective antigen and it is considered as a virulence factor in infections caused by the brucellae. Two S-LPS types, A and M, have been found to exist in smooth Brucella spp., with differences residing in the O chain of the S-LPS (13, 52, 82). The O chain of type A S-LPS is composed of a homopolymer of α-1,2-linked residues of 4,6-dideoxy-4-formamido-d-mannopyranose, and the O chain of type M S-LPS is composed of pentasaccharide repeats of the same sugar but with four α-1,2-linked residues and one α-1,3-linked residue (13, 14, 17). The brucellae have been classified into A>M, M>A, or A+M+ according to the distribution of both S-LPS types, and strains A>M and M>A have been shown to exist in all the species which can exist in smooth phase. Therefore, it seems unlikely that the genes contained in the pNV3103 insert are involved in the synthesis of the Brucella O-polysaccharide chain, since no differences have been found between the O chains of the different species. Moreover, the DNA fragment cloned in pNV3103 also seems to exist in rough Brucella strains, since omp31 (bme5) has been amplified from both smooth and rough strains (80). It is also unlikely that these genes are involved in the synthesis of the native hapten, a polysaccharide with the same structure of the S-LPS O chain and that also exists in B. abortus (86). Another polysaccharide, polysaccharide B, a polymer of cyclic β-1,2-glucan, has been found in the genus Brucella, but it has been shown to exist in both B. melitensis and B. abortus (15).
Therefore, if the group of genes identified in the B. melitensis insert of pNV3103 are part of the gene machinery leading to the synthesis of a polysaccharide, it is probable that this polysaccharide has not yet been described in the brucellae. The Brucella S-LPS has been described as an important virulence factor (3, 64, 65), and capsular polysaccharides and EPS have also been described as virulence factors in other microorganisms. Identification of a novel Brucella polysaccharide might be of great interest for a better understanding of the differences in pathogenicity and host preference observed between the brucellae. In an attempt to identify a polysaccharide present in B. melitensis but absent in B. abortus, SDS-polyacrylamide gel electrophoresis (PAGE) followed by periodate-silver staining for polysaccharides has been performed with B. melitensis 16M and B. abortus 544 cells grown in solid medium and with the supernatant recovered after centrifugation of the resuspended cells. However, these studies proved unsuccessful, since no differences in the polysaccharide profile were found between these organisms (data not shown). LPS and capsular polysaccharides, including LPS of Brucella spp. (26, 33), are currently detected by this technique (5, 9, 85). However, while periodate-silver staining after SDS-PAGE is a good method for detection of LPSs, other polysaccharides not attached to a lipid moiety are unable to migrate in SDS-PAGE, as has been shown for the Brucella native hapten (7). The possibility cannot be excluded that B. melitensis produces a polysaccharide not detectable by the technique we used or that the polysaccharide is synthesized only when contact with the host is established and is not detected under the conditions usually employed in the laboratory. Another possibility that should be taken into account is that the genes found in B. melitensis no longer lead to the synthesis of a polysaccharide. Brucellae are members of the alpha-2 subdivision of the class Proteobacteria and are genetically close to bacteria of the same group, which are associated with eucaryotic cells (25, 55). Bacteria from the genus Rhizobium and Agrobacterium, living in association with plant cells, produce EPS which is important for the interaction with the host eucaryotic cell (48, 75). The common ancestor of the alpha-2 subdivision of the class Proteobacteria has been suggested to have EPS, and the genes discovered in B. melitensis might be a remnant from this common ancestor. Modifications, during the course of evolution, in one or more of the genes involved in the synthesis of this polysaccharide might have led to the loss of this polysaccharide, which would not be necessary for survival or the establishment of the infection process. The alpha-2 subdivision of the class Proteobacteria contains several motile members, and recently, although the brucellae are defined as nonmotile, genes encoding homologs of several flagellum-related proteins have been found in B. abortus (41). One of the hypothesis made by Halling to explain this fact was that the brucellae may have lost motility during evolution because it is not essential and may even be detrimental for their cycle as animal pathogens (41).
Although small deletions in several genes have been identified in some Brucella species (19, 22, 23, 29, 67) and unidentified DNA deletions of several sizes exist in some brucellae (54, 80), this is the first report describing the characterization of a large DNA fragment deleted in one of the Brucella species. In spite of the high level of DNA homology reported for this genus (77), important divergences between the brucellae should exist at the DNA level that are able to explain their differences in pathogenicity and host preference. Here we have found several genes that seem to be implicated in the synthesis of a polysaccharide not yet described in the genus Brucella. Although this polysaccharide has not been detected, the possibility cannot be excluded that it was not detected under the conditions we used or that it is synthesized only in contact with the host. Discovery of a novel Brucella polysaccharide, probably not synthesized in B. abortus, would be of great interest since it might play an important role in virulence and might explain some of the differences in pathogenicity and host preference among the brucellae.
ACKNOWLEDGMENTS
We thank Manuel Sánchez Hernández for his valuable help with DNA sequence determination, and we thank Jean Michel Verger and Maggy Grayon for providing the Brucella DNA.
Nieves Vizcaíno was supported by an EEC fellowship.
REFERENCES
- 1.Allardet-Servent A, Bourg G, Ramuz M, Pages M, Bellis M, Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988;170:4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen C A, Adams L G, Ficht T A. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect Immun. 1998;66:1008–1016. doi: 10.1128/iai.66.3.1008-1016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alton G G, Jones L M, Angus R D, Verger J M. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 5.Amor P A, Whitfield C. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 1997;26:145–161. doi: 10.1046/j.1365-2958.1997.5631930.x. [DOI] [PubMed] [Google Scholar]
- 6.Anthamatten D, Scherb B, Hennecke H. Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J Bacteriol. 1992;174:2111–2120. doi: 10.1128/jb.174.7.2111-2120.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aragón V, Díaz R, Moreno E, Moriyón I. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. 1996;178:1070–1079. doi: 10.1128/jb.178.4.1070-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker A, Niehaus K, Pühler A. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol Microbiol. 1995;16:191–203. doi: 10.1111/j.1365-2958.1995.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 9.Bik E M, Bunschoten A E, Willems R J L, Chang A C Y, Mooi F R. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol Microbiol. 1996;20:799–811. doi: 10.1111/j.1365-2958.1996.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 10.Bloemberg G V, Thomas-Oates J E, Lugtenberg B J J, Spaink H P. Nodulation protein NodL of Rhizobium leguminosarum O-acetylates lipooligosaccharides, chitin fragments and N-acetylglucosamine in vitro. Mol Microbiol. 1994;11:793–804. doi: 10.1111/j.1365-2958.1994.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Bricker B J, Halling S M. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–2666. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bricker B J, Halling S M. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J Clin Microbiol. 1995;33:1640–1642. doi: 10.1128/jcm.33.6.1640-1642.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bundle D R, Cherwonogrodzky J W, Gidney M A J, Meikle P J, Perry M B, Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989;57:2829–2836. doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bundle D R, Cherwonogrodzky J W, Perry M B. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry. 1987;26:8717–8726. doi: 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- 15.Bundle D R, Cherwonogrodzky J W, Perry M B. Characterization of Brucella polysaccharide B. Infect Immun. 1988;56:1101–1106. doi: 10.1128/iai.56.5.1101-1106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrows L L, Charter D F, Lam J S. Molecular characterization of the Pseudomonas aeruginosa serotype O5 (PAO1) B-band lipopolysaccharide gene cluster. Mol Microbiol. 1996;22:481–495. doi: 10.1046/j.1365-2958.1996.1351503.x. [DOI] [PubMed] [Google Scholar]
- 17.Caroff M, Bundle D R, Perry M B, Cherwonogrodzky J W, Duncan J R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984;46:384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman M R, Kao C C. EpsR modulated production of extracellular polysaccharides in the bacterial wilt pathogen Ralstonia (Pseudomonas) solanacearum. J Bacteriol. 1998;180:27–34. doi: 10.1128/jb.180.1.27-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cloeckaert A, Debbarh H S A, Vizcaíno N, Saman E, Dubray G, Zygmunt M S. Cloning, nucleotide sequence, and expression of the Brucella melitensis bp26 gene coding for a protein immunogenic in infected sheep. FEMS Microbiol Lett. 1996;140:139–144. doi: 10.1016/0378-1097(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 20.Cloeckaert A, Verger J M, Grayon M, Grépinet O. Restriction site polymorphism of the genes encoding the major 25 kDa and 36 kDa outer-membrane proteins of Brucella. Microbiology. 1995;141:2111–2121. doi: 10.1099/13500872-141-9-2111. [DOI] [PubMed] [Google Scholar]
- 21.Cloeckaert A, Verger J M, Grayon M, Grépinet O. Polymorphism at the dnaK locus of Brucella species and identification of a Brucella melitensis species-specific marker. J Med Microbiol. 1996;45:200–205. doi: 10.1099/00222615-45-3-200. [DOI] [PubMed] [Google Scholar]
- 22.Cloeckaert A, Verger J M, Grayon M, Vizcaíno N. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol Lett. 1996;145:1–8. doi: 10.1111/j.1574-6968.1996.tb08547.x. [DOI] [PubMed] [Google Scholar]
- 23.Cloeckaert A, Verger J M, Grayon M, Zygmunt M S, Grépinet O. Nucleotide sequence and expression of the gene encoding the major 25-kilodalton outer membrane protein of Brucella ovis: evidence for antigenic shift, compared with the other Brucella species, due to a deletion in the gene. Infect Immun. 1996;64:2047–2055. doi: 10.1128/iai.64.6.2047-2055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.CLUSTAL W Website. 19 January 1999, revision date. [Online.] http://www2.ebi.ac.uk/clustalw/. [27 January 1999, last date accessed.]
- 24.Corbel M J, Brinley-Morgan W J. Genus Brucella Meyer and Shaw 1920, 173AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 377–388. [Google Scholar]
- 25.De Ley J, Mannheim W, Segers P, Lievens A, Denijn M, Vanhoucke M, Gillis M. Ribosomal ribonucleic acid cistron similarities and taxonomic neighborhood of Brucella and CDC group Vd. Int J Syst Bacteriol. 1987;37:35–42. [Google Scholar]
- 26.Dubray G, Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1986;138:27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- 26a.FASTA Website. 1 November 1996, revision date. [Online.] http://genome.eerie.fr/bin/fasta-guess.cgi. [30 September 1998, last data accessed.]
- 27.Fekete A, Bantle J A, Halling S M, Stich R W. Amplification fragment length polymorphism in Brucella strains by use of polymerase chain reaction with arbitrary primers. J Bacteriol. 1992;174:7778–7783. doi: 10.1128/jb.174.23.7778-7783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ficht T A, Bearden S W, Sowa B A, Adams L G. DNA sequence and expression of the 36-kilodalton outer membrane protein gene of Brucella abortus. Infect Immun. 1989;57:3281–3291. doi: 10.1128/iai.57.11.3281-3291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ficht T A, Bearden S W, Sowa B A, Marquis H. Genetic variation at the omp2 porin locus of the brucellae: species-specific markers. Mol Microbiol. 1990;4:1135–1142. doi: 10.1111/j.1365-2958.1990.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 30.Ficht T A, Husseinen H S, Derr J, Bearden S W. Species-specific sequences at the omp2 locus of Brucella type strains. Int J Syst Bacteriol. 1996;46:329–331. doi: 10.1099/00207713-46-1-329. [DOI] [PubMed] [Google Scholar]
- 31.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 32.Frosch M, Müller D, Bousset K, Müller A. Conserved outer membrane protein of Neisseria meningitidis involved in capsule expression. Infect Immun. 1992;60:798–803. doi: 10.1128/iai.60.3.798-803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garin-Bastuji B, Bowden R A, Dubray G, Limet J N. Dodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of smooth-lipopolysaccharide heterogeneity among Brucella biovars related to A and M specificities. J Clin Microbiol. 1990;28:2169–2174. doi: 10.1128/jcm.28.10.2169-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghim S-Y, Neuhard J. The pyrimidine biosynthesis operon of the thermophile Bacillus caldolyticus includes genes for uracil phosphoribosyltransferase and uracil permease. J Bacteriol. 1994;176:3698–3707. doi: 10.1128/jb.176.12.3698-3707.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glucksmann M A, Reuber T L, Walker G C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J Bacteriol. 1993;175:7033–7044. doi: 10.1128/jb.175.21.7033-7044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant S R, Lee S C, Kendall K, Cohen S N. Identification and characterization of a locus inhibiting extrachromosomal maintenance of the Streptomyces plasmid SLP1. Mol Gen Genet. 1989;217:324–331. doi: 10.1007/BF02464900. [DOI] [PubMed] [Google Scholar]
- 38.Gray J X, Djordjevic M A, Rolfe B G. Two genes that regulate exopolysaccharide production in Rhizobium sp. strain NGR234: DNA sequences and resultant phenotypes. J Bacteriol. 1990;172:193–203. doi: 10.1128/jb.172.1.193-203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimont F, Verger J M, Cornelis P, Limet J, Lefèvre M, Grayon M, Régnault B, Van Broeck J, Grimont P A D. Molecular typing of Brucella with cloned DNA probes. Res Microbiol. 1992;143:55–65. doi: 10.1016/0923-2508(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 40.Guasch J F, Piqué N, Climent N, Ferrer S, Merino S, Rubires X, Tomas J M, Regué M. Cloning and characterization of two Serratia marcescens genes involved in core lipopolysaccharide biosynthesis. J Bacteriol. 1996;178:5741–5747. doi: 10.1128/jb.178.19.5741-5747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halling S M. On the presence and organization of open reading frames of the nonmotile pathogen Brucella abortus similar to class II, III, and IV flagelar genes and to LcrD virulence superfamily. Microb Comp Genomics. 1998;3:21–29. doi: 10.1089/omi.1.1998.3.21. [DOI] [PubMed] [Google Scholar]
- 42.Halling S M, Zehr E S. Polymorphism in Brucella spp. due to highly repeated DNA. J Bacteriol. 1990;172:6637–6640. doi: 10.1128/jb.172.12.6637-6640.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henikoff J, Wallace C, Bown J P. Finding protein similarities with nucleotide sequence databases. Methods Enzymol. 1990;183:111–132. doi: 10.1016/0076-6879(90)83009-x. [DOI] [PubMed] [Google Scholar]
- 44.Itoh T, Aiba H, Baba T, Fujita K, Hayashi K, Inada T, Isono K, Kasai H, Kimura S, Kitakawa M, Kitagawa M, Makino K, Miki T, Mizobuchi K, Mori H, Mori T, Motomura K, Nakade S, Nakamura Y, Nashimoto H, Nishio Y, Oshima T, Saito N, Sampei G, Seki Y, Sivasundaram S, Tagami H, Takeda J, Takemoto K, Wada C, Yamamoto Y, Horiuchi T. A 460-kb DNA sequence of the Escherichia coli K-12 genome corresponding to the 40.1–50.0 min region on the linkage map. DNA Res. 1996;3:379–382. doi: 10.1093/dnares/3.6.379. [DOI] [PubMed] [Google Scholar]
- 45.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashiwagi S, Kanamaru K, Mizuno T. A Synechococcus gene encoding a putative pore-forming intrinsic membrane protein. Biochim Biophys Acta. 1995;1237:189–192. doi: 10.1016/0005-2736(95)00124-l. [DOI] [PubMed] [Google Scholar]
- 47.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 48.Leigh J A, Reed J W, Hanks J F, Hirsch A M, Walker G C. Rhizobium meliloti mutants that fail to succinilate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Cole R A, Reeves P R. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J Bacteriol. 1996;178:2102–2107. doi: 10.1128/jb.178.7.2102-2107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu D, Haase A M, Lindqvist L, Lindberg A A, Reeves P R. Glycosyl transferases of O-antigen biosynthesis in Salmonella enterica: identification and characterization of transferase genes of groups B, C2, and E1. J Bacteriol. 1993;175:3408–3413. doi: 10.1128/jb.175.11.3408-3413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marck C. “DNA Strider”: a “C” program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meikle P J, Perry M B, Cherwonogrodzky J W, Bundle D R. Fine structure of A and M antigens from Brucella biovars. Infect Immun. 1989;57:2820–2828. doi: 10.1128/iai.57.9.2820-2828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercier E, Jumas-Bilak E, Allardet-Servent A, O’Callaghan D, Ramuz M. Polymorphism in Brucella strains detected by studying distribution of two short repetitive DNA elements. J Clin Microbiol. 1996;34:1299–1302. doi: 10.1128/jcm.34.5.1299-1302.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michaux-Charachon S, Bourg G, Jumas-Bilak E, Guigue-Talet P, Allardent-Servent A, O’Callaghan D, Ramuz M. Genome structure and phylogeny in the genus Brucella. J Bacteriol. 1997;179:3244–3249. doi: 10.1128/jb.179.10.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno E, Stackebrandt E, Dorsch M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.MOTIF Website. 21 April 1998, revision date.. [Online.] http://www.motif.genome.ad.jp./. [30 September 1998, last date accessed.]
- 56.Müller P, Weng M W, Quandt J, Arnold W, Pühler A. Genetic analysis of Rhizobium meliloti exoYFQ operon: ExoY is homologous to sugar transferases and ExoQ represents a transmembrane protein. Mol Plant-Microbe Interact. 1993;6:55–65. doi: 10.1094/mpmi-6-055. [DOI] [PubMed] [Google Scholar]
- 57.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 58.Ouahrani S, Michaux S, Widada J S, Bourg G, Tournebize R, Ramuz M, Liautard J P. Identification and sequence analysis of IS6501, an insertion sequence in Brucella spp.: relationship between genomic structure and the number of IS6501 copies. J Gen Microbiol. 1993;139:3265–3273. doi: 10.1099/00221287-139-12-3265. [DOI] [PubMed] [Google Scholar]
- 59.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peschel A, Schnell N, Hille M, Entian K D, Götz F. Secretion of the lantibiotics epidermin and gallidermin: sequence analysis of the genes gdmT and gdmH, their influence on epidermin production and their regulation by EpiQ. Mol Gen Genet. 1997;254:312–318. doi: 10.1007/s004380050421. [DOI] [PubMed] [Google Scholar]
- 60a.PSORT Website. 1 December 1996, revision date. [Online] http://psort.nibb.ac.jp:8800/. [30 September 1998, last date accessed.]
- 61.Quinn C L, Stephenson B T, Switzer R L. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J Biol Chem. 1991;266:9113–9127. [PubMed] [Google Scholar]
- 62.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 63.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 64.Riley L K, Robertson D C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984;46:224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riley L K, Robertson D C. Brucellacidal activity of human and bovine polymorphonuclear leukocyte granule extracts against smooth and rough strains of Brucella abortus. Infect Immun. 1984;46:231–236. doi: 10.1128/iai.46.1.231-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 67.Sangari F J, García-Lobo J M, Agüero J. The Brucella abortus vaccine strain B19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol Lett. 1994;121:337–342. doi: 10.1111/j.1574-6968.1994.tb07123.x. [DOI] [PubMed] [Google Scholar]
- 68.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 70.Saxena I M, Brown R M, Fevre M, Geremia R A, Henrissat B. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schweizer H P. The agmR gene, an environmentally responsive gene, complements defective glpR, which encodes the putative activator for glycerol metabolism in Pseudomonas aeruginosa. J Bacteriol. 1991;173:6798–6806. doi: 10.1128/jb.173.21.6798-6806.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Mao J-I, Rice P, Nölling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevenson G, Andrianopoulos K, Hobbs M, Reeves P R. Organization of the Escherichia coli K-12 gene cluster responsible for the production of the extracellular polysaccharide colanic acid. J Bacteriol. 1996;178:4885–4893. doi: 10.1128/jb.178.16.4885-4893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stout V, Torres-Cabassa A, Maurizi M R, Gutnick D, Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991;173:1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomashow M F, Karlinsey J E, Marks J R, Hurlbert R E. Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol. 1987;169:3209–3216. doi: 10.1128/jb.169.7.3209-3216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verger J M, Grimont F, Grimont P A D, Grayon M. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1985;35:292–295. [Google Scholar]
- 78.Verger J M, Grimont F, Grimont P A D, Grayon M. Taxonomy of the genus Brucella. Ann Inst Pasteur Microbiol. 1987;138:235–238. doi: 10.1016/0769-2609(87)90199-2. [DOI] [PubMed] [Google Scholar]
- 79.Vizcaíno N, Cloeckaert A, Zygmunt M S, Dubray G. Cloning, nucleotide sequence, and expression of the Brucella melitensis omp31 gene coding for an immunogenic major outer membrane protein. Infect Immun. 1996;64:3744–3751. doi: 10.1128/iai.64.9.3744-3751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vizcaíno N, Verger J M, Grayon M, Zygmunt M S, Cloeckaert A. DNA polymorphism at the omp-31 locus of Brucella spp.: evidence for a large deletion in Brucella abortus, and other species-specific markers. Microbiology. 1997;143:2913–2921. doi: 10.1099/00221287-143-9-2913. [DOI] [PubMed] [Google Scholar]
- 81.Whitfield C, Amor P A, Köplin R. Modulation of the surface architecture of Gram-negative bacteria by the action of surface polymer:lipid A-core ligase and by determinants of polymer chain length. Mol Microbiol. 1997;23:629–638. doi: 10.1046/j.1365-2958.1997.2571614.x. [DOI] [PubMed] [Google Scholar]
- 82.Wilson G S, Miles A A. The serological differentiation of smooth strains of the Brucella group. Br J Exp Pathol. 1932;13:1–13. [Google Scholar]
- 83.York G M, Walker G C. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol Microbiol. 1997;25:117–134. doi: 10.1046/j.1365-2958.1997.4481804.x. [DOI] [PubMed] [Google Scholar]
- 84.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Radziejewska-Lebrecht J, Krajewska-Pietrasik D, Toivanen P, Skurnik M. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol Microbiol. 1997;23:63–76. doi: 10.1046/j.1365-2958.1997.1871558.x. [DOI] [PubMed] [Google Scholar]
- 86.Zygmunt M S, Dubray G, Bundle D R, Perry M B. Purified native haptens of Brucella abortus B19 and B. melitensis 16M reveal the lipopolysaccharide origin of the antigens. Ann Inst Pasteur Microbiol. 1988;139:421–433. doi: 10.1016/0769-2609(88)90105-6. [DOI] [PubMed] [Google Scholar]