Abstract
In contrast to most mouse strains, rats eliminate the primary schistosome burden around 4 weeks postinfection and subsequently develop protective immunity to reinfection. In rat schistosomiasis, we have shown predominant expression of a Th2-type cytokine response at the mRNA level after primary infection. In the present study, we showed a significant increase in interleukin-4 (IL-4) mRNA expression in inguinal lymph nodes early after a secondary infection. IL-5 mRNA expression showed a significant increase at days 2 and 4 postreinfection in the spleen and lymph nodes, respectively. We did not detect any gamma interferon (IFN-γ) mRNA after a challenge infection. Analysis of cytokine secretion by stimulated spleen cells after a primary infection showed predominant expression of IL-4 with maximum production on day 21, accompanied by production of IL-5 from day 11 to day 67. A significant increase in IFN-γ secretion was detected at day 21. Analysis of immunoglobulin G2b (IgG2b) and IgG2c (Th1-related isotypes) showed undetectable levels of IgG2b, but detectable levels of specific IgG2c antibodies were observed from day 42. The analysis of Th2-related isotypes showed high specific IgG1 and IgG2a antibody titers from day 29. After a secondary infection, only IL-4 and IL-5 secretion was sustained. This is supported by the increased production of Th2-related isotypes. These findings showed that S. mansoni infection can drive Th2 responses in rats in the absence of egg production which is required to induce a Th2 response in mice and are in favor of the role of Th2-type cytokines in protective immunity against reinfection.
In response to invading pathogens, a humoral, a cellular, or both types of immune response may be generated. In this context, several studies have outlined the importance of the major role T-helper (Th) lymphocytes play in the regulation of immunity to parasitic infections (4, 29). In BALB/c mice infected with Leishmania spp., protective immunity has been related to a Th1 response and pathogenesis has been related to a Th2 response (40). In murine schistosomiasis, it has been shown that a Th2 response was involved in the development of chronic infection, whereas a Th1 response participated in protection against Schistosoma mansoni infection (31, 32, 44). In humans, several immunoepidemiological studies revealed a positive correlation among high specific immunoglobulin E (IgE) levels, eosinophilia, and resistance to reinfection. Recent studies showed a correlation between the level of secreted interleukin-5 (IL-5) and resistance to reinfection, and there is now converging evidence supporting a beneficial role of the Th2 response (3, 14, 17, 36, 37). Along the same line, it has been shown, in a study performed in two areas of Kenya, that peripheral blood mononuclear cells from hepatosplenic disease patients responded to antigen stimulation with significantly higher levels of gamma interferon (IFN-γ) and tumor necrosis factor (TNF) but lower levels of IL-5 compared with non-hepatosplenic disease patients (30). Moreover, it was found that high levels of IFN-γ, TNF, soluble TNF receptors, and soluble ICAM-1 in plasma were also significantly associated with hepatosplenomegaly, suggesting the implication of a Th1-like response in the hepatosplenic disease process (30). These findings are in contrast to those obtained with the murine model, in which Th2 responses have been associated with pathology.
Studies with the rat, a semipermissive host, have implicated immune mechanisms in the rejection of worms. After a primary infection, rats established a strong resistance to reinfection (26) which is thymus dependent (7, 33). This resistance appears between 4 and 8 weeks after a primary infection and persists for at least 12 weeks (26). Several studies showed that this resistance is mainly due to ADCC mechanisms (8, 9). In this model, indirect and direct evidence supported the role of phagocytic cells and anaphylactic-type antibodies (IgG2a and IgE) in protection against S. mansoni infection both in vitro and in vivo (20, 25, 43). Nevertheless, little is known about the expression of cytokine genes involved in this mechanism. Accordingly, we have recently analyzed the pattern of cytokine mRNA expression by reverse transcription (RT)-PCR in S. mansoni-infected rats. The results showed preferential expression of Th2 cytokines before rejection of worms (10). In spleens from infected rats, a significant increase in IL-5 mRNA was observed during the early phase of infection. Analysis of pulmonary cytokine responses showed a dramatic increase in IL-4 and IL-5 mRNAs on day 7, which corresponds to the period when the parasites are present in the lungs. A significant increase in IL-2, IL-4, and IL-5 was detected in lymph nodes (LN) on day 21. IFN-γ was barely detected between day 21 and day 29 in the spleen.
In the present work, we have studied, on the one hand, the expression of IFN-γ (a Th1-type cytokine), IL-4, and IL-5 (Th2-type cytokines) at the mRNA level after a secondary infection. On the other hand, we have examined the secretion of these cytokines by cells purified from primarily and secondarily infected rat spleens after stimulation with S. mansoni antigens. Finally, in order to evaluate the functional significance of a Th2 cytokine response, we have monitored the levels of specific IgG1, IgG2a, IgG2b, and IgG2c antibodies. In rats, IgG1 and IgG2a are considered to be controlled by Th2 cytokines (1) whereas IgG2b and IgG2c are known to be controlled by Th1 cytokines (19). Taken together, our results suggest the association of Th2-type cytokines and Th2-dependent antibodies in rat protective immunity against reinfection.
MATERIALS AND METHODS
Parasites and laboratory hosts.
A Guadeloupean strain of S. mansoni was maintained in Biomphalaria glabrata snails as intermediate hosts and golden hamsters as definitive hosts. Cercariae for experimental infections were used within 1 h after collection.
Male 6- to 8-week-old inbred Fischer F344 rats (Iffa Credo) were exposed percutaneously to 2 × 103 cercariae as previously described (10) for primary infection and exposed to 2 × 103 cercariae 9 weeks later for reinfection. In preliminary infection experiments, a follow-up of the worm burden in the livers of infected rats showed reductions of 62% at day 28 and 85% at day 42 postinfection in comparison to day 21, when the maximum numbers of worms were found. Accordingly, we decided to reinfect rats at day 63 postinfection. A follow-up of the worm burden in infected-reinfected rats showed a reduction of 81% at day 21 compared to challenge control rats.
Antigens.
Based on the presence of soluble cross-reactive antigen between cercariae and schistosomula (34) and in addition to the fact that in experimental rat schistosomiasis there are no adult worms nor is there egg production, we used cercarial antigens in enzyme-linked immunosorbent assay (ELISA) and stimulation experiments. Cercarial soluble antigens were prepared from cercariae homogenized in phosphate-buffered saline (PBS) containing 0.5 mM phenylmethylsulfonyl fluoride and 1 mM EDTA.
Homogenates were sonicated six times for 30 s each time by using a sonicator (Labsonic U.; B. Braun, Strasbourg, France) and centrifuged twice at 10 × 103 × g for 10 min each time at 4°C. The soluble fraction was recovered and used as the source of antigens. Measurement of protein content was performed by using the bicinchoninic acid protein assay reagent (Pierce).
Lymphocyte cultures.
Due to the limited number of cells in lymph nodes and difficulties in culturing cells from lungs, we only analyzed IFN-γ, IL-4, and IL-5 secretion by cells purified from the spleen. Spleens were removed from infected or reinfected rats, and cell suspensions were prepared as follows. Briefly, spleens were forced through fine wire mesh and splenic erythrocytes were lysed by osmotic treatment (170 mM Tris-buffered saline, 155 mM ammonium chloride solution), followed by three washes in RPMI 1640 (Gibco BRL, Courbevoie, France). Cell viability was evaluated by trypan blue dye exclusion, and 107 cells were resuspended in 1 ml of culture medium, which is RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (JRH BioSciences, Lenexa, Kans.), 50 μg of gentamicin (Schering-Plough, Levallois-Perret, France) per ml, 2 mM glutamine (Seromed, Berlin, Germany), 1% nonessential amino acids (Seromed), 1 mM sodium pyruvate (Sigma, St. Louis, Mo.), and 5 × 10−5 M β-mercaptoethanol (Merck, Darmstadt, Germany). One milliliter of a cell suspension was incubated in the presence of S. mansoni cercarial soluble antigens (50 μg/ml) or concanavalin A (ConA; 5 μg/ml; Seromed) in 24-well plates (Nunclon; Nunc, Roskilde, Denmark) at 37°C in a 5% CO2 atmosphere. Supernatants were collected after 24 h for IL-5 measurement, after 48 h for IL-4 measurement, and after 72 h for IFN-γ measurement.
Cytokine secretion. (i) IL-4 detection.
IL-4 detection was performed with the Biosource International Cytoscreen rat IL-4 kit as recommended by the manufacturer. Values for the standard curve ranged between 500 and 7.8 pg/ml for IL-4.
(ii) IL-5 detection.
For IL-5 detection, the LyH7.B13 Cell Proliferation Assay was used. The mouse B-cell line LyH7.B13, which proliferates in response to IL-5, was kindly provided by D. Fattah, GlaxoWellcome (18). Cells (5 × 104/well) were cultured in triplicate in Dulbecco modified Eagle medium (Gibco BRL) supplemented with 10% fetal calf serum (JRH BioSciences), nonessential amino acids (Seromed), and 5 × 10−5 M β-mercaptoethanol (Merck) together with appropriately diluted samples in 96-well flat-bottom plates (Falcon; Becton Dickinson). After 72 h of culture, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma) at a concentration of 50 μg was added to each well and the plate was incubated for at least 5 h at 37°C in a 5% CO2 atmosphere. A 0.1-ml volume of isopropanol-HCl (0.04 N) was then added to each well. After homogenization, color development was measured spectrophotometrically at 570 nm (Multiskan MCC/340; Titertek, Helsinki, Finland). Cytokine concentrations were calculated by reference to standard curves constructed with known amounts of recombinant purified mouse IL-5 (Genzyme, Cambridge, United Kingdom) diluted in culture medium. Values ranged between 5 × 103 and 15.62 mIU/ml for IL-5.
(iii) IFN-γ detection.
For IFN-γ detection, Nunc Immulon modules were coated at 5 μg/ml with polyclonal rabbit anti-rat IFN-γ (Biosource International) and incubated for 3 h at 37°C with 100 μl of coating buffer (0.1 M NaHCO3, 0.1 M Na2CO3, pH 9.6) per well. After three washes, plates were saturated with 200 μl of PBS–0.5% gelatin per well and incubated for 1 h at room temperature. After three washes, supernatants were added and the plates were incubated overnight at 4°C. The plates were then washed in PBS–0.1% Tween 20 and incubated for 90 min at 37°C with a biotinylated mouse anti-rat IFN-γ monoclonal antibody purified from DB-1 hybridoma cells (100 μl/well; Biosource International) diluted in PBS–0.1% Tween 20 at 1 mg/ml. Amplification with streptavidin-peroxidase conjugate (1/2,000; Amersham) was performed. Finally, after extensive washes, 100 μl of substrate solution (1 mg of o-phenylenediamine dihydrochloride [Sigma] per ml, 0.03% H2O2 in 0.1 M citrate buffer, pH 5.0) was added to the wells. The color reaction was stopped by the addition of 100 μl of HCl (2 N). A492 was measured by using a Multiskan MCC/340 spectrophotometer (Labsystems, Helsinki, Finland). Cytokine concentrations were calculated by using standard curves constructed with known amounts of the recombinant purified cytokine diluted in culture medium. Values ranged between 5 × 103 and 62.5 pg/ml for IFN-γ.
Isolation and purification of mRNA.
Each tissue mRNA sample was analyzed separately by RT-PCR. For tissue cytokine mRNA determinations, draining LN, the right lung, the spleen, and the liver were removed (two rats were infected and reinfected per group, two rats were infected but not reinfected per group, and two rats were not infected as a control) at the different time points indicated (see Fig. 1) and immediately frozen in liquid nitrogen. Frozen organs were homogenized in 1 ml of RNA PLUS solution (Bioprobe Systems). Total RNA was isolated as recommended by the manufacturer. The RNA was resuspended in RNase-free sterile water. The amount and quality of RNA were determined by spectrophotometry, and the RNA was analyzed by agarose gel electrophoresis.
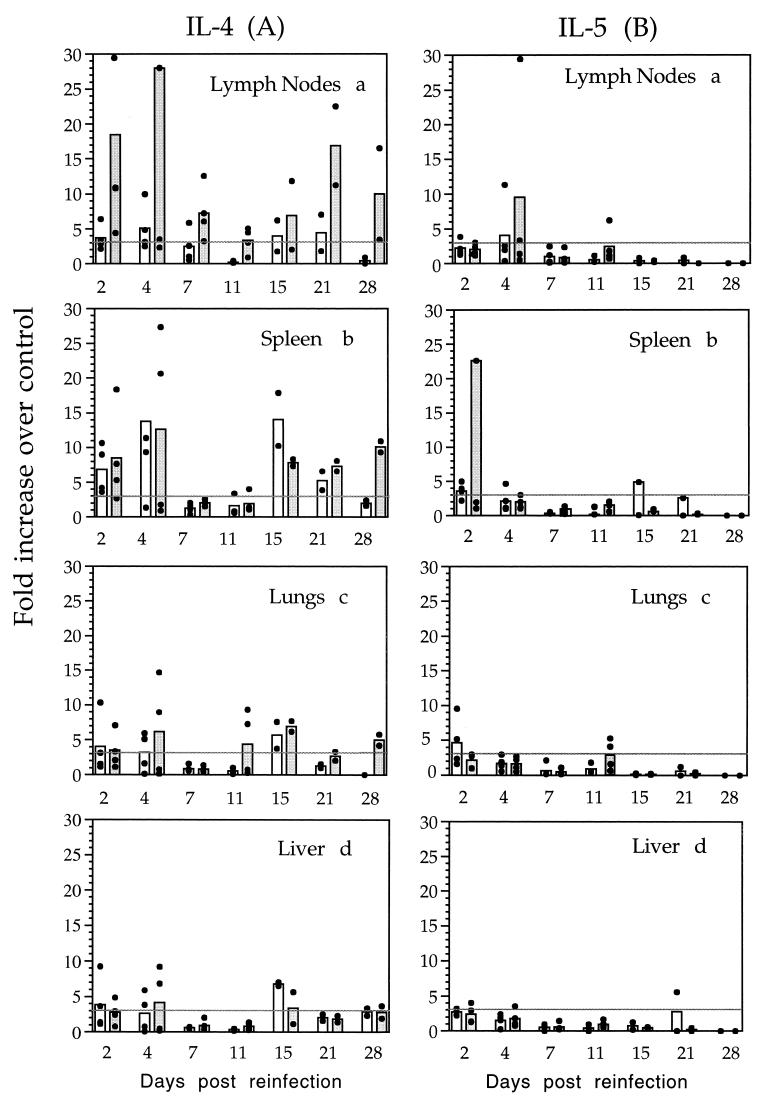
FIG. 1.
IL-4 and IL-5 mRNA expression in different tissues
in rats after primary and secondary S. mansoni infections.
RNA was purified from the spleens, inguinal LN, lungs, and livers of
infected animals (□) and infected-and-reinfected animals
( ). The
levels of different cytokines were evaluated by RT-PCR as described in
Materials and Methods. Each animal is represented by a filled circle
(●). The columns represent means of four individual animals for days
2, 4, 7, and 11 and two individual animals for days 15, 21, and 28.
Panel A represents IL-4 mRNA expression in the draining LN (a) (in
graph a, one rat presenting an 89-fold increase over the control at day
4 postreinfection is not shown), the spleen (b), the lungs (c), and the
liver (d). Panel B represents IL-5 mRNA expression in the draining LN
(a), the spleen (b) (in graph b; one rat presenting a 64-fold increase
over the control at day 2 postreinfection is not shown), the lungs (c),
and the liver (d). Results from two kinetic studies are presented as
fold increases over the control. A level threefold over the control was
considered significant.
). The
levels of different cytokines were evaluated by RT-PCR as described in
Materials and Methods. Each animal is represented by a filled circle
(●). The columns represent means of four individual animals for days
2, 4, 7, and 11 and two individual animals for days 15, 21, and 28.
Panel A represents IL-4 mRNA expression in the draining LN (a) (in
graph a, one rat presenting an 89-fold increase over the control at day
4 postreinfection is not shown), the spleen (b), the lungs (c), and the
liver (d). Panel B represents IL-5 mRNA expression in the draining LN
(a), the spleen (b) (in graph b; one rat presenting a 64-fold increase
over the control at day 2 postreinfection is not shown), the lungs (c),
and the liver (d). Results from two kinetic studies are presented as
fold increases over the control. A level threefold over the control was
considered significant.
RT-PCR detection of cytokine mRNA. (i) RT reactions.
An RT-PCR procedure was performed to determine the relative quantities of mRNA for IL-4, IL-5, IFN-γ, and β-actin. RT of RNA was performed in a 25-μl final volume containing 0.5 μg of random oligo(dT) and 2.5 μg of total RNA. The RT reaction mixture was incubated for 5 min at 65°C, and then the following were added: a mixture of all four deoxynucleoside triphosphates at 1.2 mM each, 1× reverse transcriptase buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 4 U of RNase inhibitor (Promega, Madison, Wis.), and 200 U of reverse transcriptase (Gibco BRL). The reaction mixture was incubated for 1 h at 37°C, the reaction was stopped by heating for 3 min at 92°C to denature the murine leukemia virus reverse transcriptase, and the mixture was cooled on ice for 3 min and stored at −20°C.
(ii) PCRs.
The primer sequences used were previously described (10). The primers used in this study span exons separated by at least one intron in order to avoid any amplification of contaminating genomic DNA. PCR conditions were defined for each cytokine-primer pair such that a linear relationship between the input RNA and the final PCR product was obtained. Both positive and negative controls were included in each assay to confirm that only cDNA PCR products were detected and that none of the reagents was contaminated with cDNA or previous PCR products. The PCR mixture contained a 0.8 mM deoxynucleoside triphosphate mixture, 1× PCR buffer (50 mM KCl, 10 mM Tris-HCl), 1.5 mM MgCl2, 20 pmol each of the sense and antisense primers, 2 μl of cDNA, and 1.25 U of Taq polymerase (Promega). After a first step of denaturation for 3 min at 94°C, temperature cycling of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min was initiated on a PTC 200 thermal cycler (MJ Research, Watertown, Mass.).
Analysis and quantification of PCR products.
After an appropriate number of PCR cycles (28 cycles for β-actin, IFN-γ, IL-4, and IL-5), the amplified DNA was analyzed by electrophoresis, Southern blotting, and hybridization with radioactive cytokine probes. Ten microliters of each reaction mixture was electrophoresed on a 1% agarose gel, visualized by ethidium bromide staining, and transferred by capillarity action to a Hybond-N membrane (Amersham) as previously described (10). Membranes were fixed by incubation for 2 h at 80°C and then hybridized with a cytokine-specific [γ-32P]ATP-radiolabelled oligonucleotide probe at 60°C overnight in hybridization buffer (5 × SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt’s solution, herring sperm DNA at 100 μg/ml, sodium dodecyl sulfate [SDS] at 0.5%). Previously described probes (10) were [γ-32P]ATP radiolabelled with a Ready to Go T4 polynucleotide Kinase Kit (Pharmacia). The radiolabelled membranes were washed first in 2× SSC–0.5% SDS at room temperature and then in 0.1× SSC–0.1% SDS at 60°C. Radioactive signals were quantified by using a PhosphorImager (Molecular Dynamics, Evry, France). Positive and negative controls were included in each experiment. To correct sample variations, cDNA for the β-actin gene was initially quantified and a correction factor was applied to each sample. Signal intensities were then compared with those obtained for samples from normal rats, and the results are expressed as fold increases over the control. The time study experiment was repeated twice. In our study, based on absolute intensity results of cytokine transcripts obtained from 24 controls (uninfected rats), we found that the intensity varied from one- to twofold. Therefore, for confidence, we considered differences between test and control samples to be significant when the fold increase was >3.
Analysis of antibody isotype profile by ELISA.
Rats were bled before infection and periodically afterward via the retroorbital venous plexus. Sera were aliquoted and stored at −20°C. Antibody responses to S. mansoni cercarial antigens were measured by standard ELISA techniques. Briefly, Nunc Immuno modules were coated with cercarial soluble antigens at a concentration of 10 μg/ml in PBS in a final volume of 100 μl/well by incubation at 37°C for 3 h. After washing and saturation as described above, serial dilutions of sera were added in PBS–0.1% Tween 20 and incubation was done overnight at 4°C. Plates were then washed with PBS–0.1% Tween 20 and peroxidase-conjugated anti-rat IgG1, IgG2a, IgG2b, and IgG2c antibodies (MARG-1, MARG2a, MARG-2b, and MARG-2c; Limex, Louvain, Belgium) diluted at 1/8 × 103, except MARG-2c (1/3 × 103), for 90 min at 37°C. Finally, after five washes, the reaction was developed and stopped as described above and A492 was measured by using a Multiskan MCC/340 spectrophotometer (Labsystems). The specificity of the different ELISAs was confirmed by using positive and negative sera. Results are expressed as serum titers. Titers are defined as the dilution which gives an optical density reading at least twofold higher than the mean background of normal rat serum. Titers correspond to the means of four infected animals ± the standard deviation (SD).
Statistical analysis.
Statistical analysis was performed by using Student’s t test. A value of P < 0.05 was considered significant.
RESULTS
Cytokine expression patterns in rats after secondary infection.
In our previous study, we used RT-PCR to demonstrate increased expression of mRNA encoding the Th2 cytokines in different tissues of infected rats. At the same time, mRNA for IFN-γ was undetectable except in the spleen at days 21 and 29, when it was at the limit of detection of the assay. To study the effect of a challenge infection on the Th2 response established after a primary infection, Th1 (IFN-γ) and Th2 (IL-4 and IL-5) cytokine mRNA expression was semiquantified in infected rats and infected-and-reinfected rats. Analysis of IL-4 mRNA expression (Fig. 1A) in different tissues showed that there was no significant increase in the livers, lungs, or spleens (except at day 28) of animals from the reinfected group compared to singly infected or control animals. However, in draining LN, a significant increase (P < 0.05) in IL-4 mRNA was observed at days 2 (four of four rats), 4 (three of four rats), 7 (three of four rats), and 21 (two of two rats) after reinfection. Examination of the same samples for IL-5 mRNA expression (Fig. 1B) did not show any significant increase at any time except at days 2 and 4 postreinfection in the spleens and LN of two of four rats. The follow-up of IFN-γ mRNA showed no significant expression in either infected animals or animals after challenge compared to control animals (data not shown).
Cytokine secretion patterns after infection and reinfection of rats with S. mansoni.
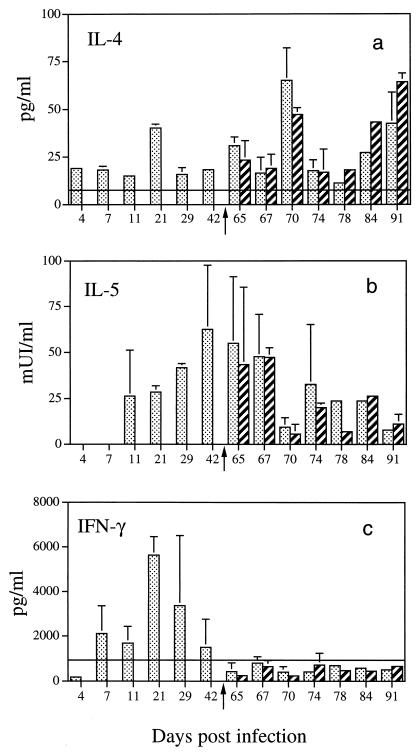
The ability of spleen cells to produce IFN-γ, IL-4, or IL-5 in vitro in response to parasitic antigens was investigated by comparison to cells incubated with medium that were used as negative controls. The kinetic study of IL-4 release by antigen-activated splenocytes during primary infection revealed significant IL-4 secretion above the constitutive secretion level with a peak at around 21 days of infection (40 pg/ml) (Fig. 2a).
FIG. 2.
Cytokine secretion by spleen cells from S. mansoni-infected and infected-reinfected rats in response to in vitro antigen stimulation. Bars: ░⃞, infected rats; ▨, infected-and-reinfected rats. The secondary infection was performed at day 63 postinfection (represented by the arrow). Cells were stimulated with S. mansoni cercarial soluble antigens. Cytokine production was evaluated in 24-h culture supernatants for the measurement of IL-5, in 48-h culture supernatants for the measurement of IL-4, and in 72-h culture supernatants for the measurement of IFN-γ. The constitutive production (———) is that of uninfected animals. Panel a represents IL-4 secretion, and the constitutive production value is 7.8 ± 2.08 pg/ml. Panel b represents IL-5 secretion, and the constitutive production value is below the detection limit. Panel c represents IFN-γ production, and the constitutive production value is 1,050 ± 826 pg/ml. A significant increase in the secretion of IL-4 and IL-5 was observed after primary and secondary infections compared to uninfected rats (P < 0.05) at days 4, 7, 11, 21, 42, 65, 70, 84, and 91 for infected rats and days 70, 78, 84, and 91 for infected-reinfected rats. IFN-γ secretion was significant at day 21 postinfection (P < 0.05).
As far as IL-5 production was concerned (Fig. 2b), none was detected in the early phase of infection (days 4 and 7). However, significant production was observed on day 11, which increased to a maximum on day 42. The constitutive secretion of IL-5 by normal rats (n = 28) tested at each time point is below the detection limit of the assay.
Likewise, there was no difference between the IL-4 and IL-5 levels (Fig. 2a and b) secreted by antigen-stimulated spleen cells from infected or reinfected rats. This might be due to the fact that spleen cells purified either from infected rats or from infected-reinfected rats responded in a similar manner to in vitro antigen stimulation.
No significant IFN-γ release by spleen cells from infected rats was obtained during the early phase of primary infection (days 4 to 11) in comparison to normal rat splenocytes (1,050 ± 826 pg/ml; n = 28) (Fig. 2c). However, a significant increase in IFN-γ release was detected at day 21 and a decrease occurred thereafter (days 29 to 42). In contrast, in response to cercarial antigens, no significant secretion of IFN-γ from spleen cells of infected-reinfected rats was observed (Fig. 2c), although they produced IFN-γ in response to ConA. In fact, we did not observe impairment of spleen cell responsiveness to ConA in infected but not reinfected rats or infected-reinfected rats (6,511 ± 1,789 pg/ml [n = 37] and 7,196 ± 1,855 pg/ml [n = 41], respectively).
Kinetic analysis of antibody isotypes after infection and reinfection of rats with S. mansoni.
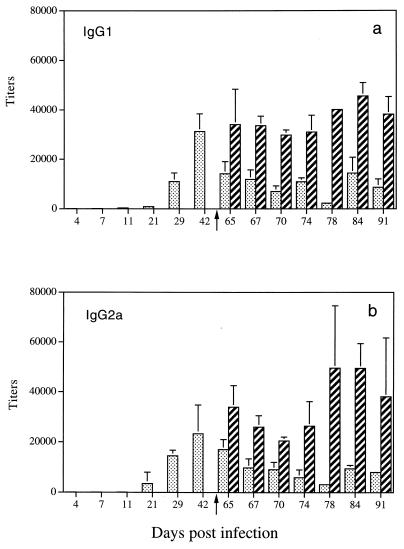
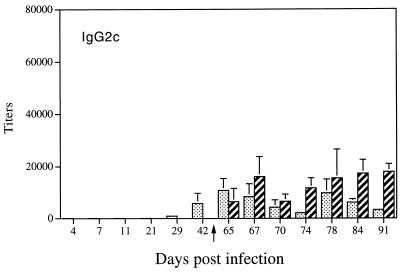
The detection of IL-4 (day 21), IL-5 (day 11 to day 70), and IFN-γ (day 21) in culture supernatants of stimulated spleen cells from infected rats raised the question of their respective roles in vivo in the development of an antibody response. We therefore performed a kinetic study to monitor the expression of specific IgG1, IgG2a antibodies, which are controlled by Th2 cytokines in the rat, and IgG2b, IgG2c antibodies, which are under the control of Th1 cytokines. As shown in Fig. 3a and b, after a primary infection, we detected anti-soluble cercarial antigen-specific IgG1 (titer, >12 × 103) and IgG2a (titer, >15 × 103) antibodies at day 29 with a maximum at day 42 (titer, >20 × 103). In contrast, undetectable levels of specific IgG2b antibodies were obtained in sera from infected rats (data not shown), whereas detectable levels of specific IgG2c antibodies were found at days 42 and 65 (titer, <10 × 103) (Fig. 4).
FIG. 3.
Distribution of Th2-related isotypes in rats after primary and secondary infections with S. mansoni. Results (mean ± SD) are expressed as serum titers. The secondary infection was performed at day 63 postinfection (represented by the arrow). Panel a represents the IgG1 antibodies. Bars: ░⃞, sera from infected rats; ▨, sera from infected-and-reinfected rats. Panel b represents the IgG2a antibodies. Bars: ░⃞, sera from infected rats; ▨, sera from infected-and-reinfected rats.
FIG. 4.
Distribution of Th1-related isotype IgG2c in rats after primary and secondary infections with S. mansoni. Results (mean ± SD) are expressed as serum titers. The secondary infection was performed at day 63 postinfection (represented by the arrow). Bars: ░⃞, sera from infected rats; ▨, sera from infected-and-reinfected rats.
After a secondary infection, the follow-up of IgG1 and IgG2a antibodies showed a significant increase in their titers (>25 × 103) as early as 4 days postreinfection (Fig. 3a and b, respectively) compared with those of rats after a primary infection. Analysis of specific IgG2b antibodies in the same sera revealed very low titers (<250) (data not shown). However, we still detected specific IgG2c antibodies in sera from reinfected rats with a stable titer compared to infected rats (Fig. 4), except at day 28 postreinfection.
DISCUSSION
The implication of Th1 versus Th2 responses in protective immunity was mainly described in mouse models since both molecular and cellular immunology studies were facilitated by the availability of sensitive assays for cytokines and the production of transgenic and knockout mice. However, in certain infections, the susceptibility or resistance and the nature of the immune response described in mice are different from those observed in humans. For instance, in murine schistosomiasis, the establishment of a chronic infection was associated with a Th2 response; however, such a response has been shown to correlate with resistance to reinfection in humans (22, 37). Studies with a semipermissive host, the rat, showed that, as in humans, the humoral response is crucial for protective immunity against S. mansoni reinfection. In order to better understand the link between cytokines and the control of S. mansoni infection in the rat, we analyzed Th1 and Th2 cytokine expression and the related isotypes expressed after primary and secondary infections.
The findings reported in this paper show predominant expression of IL-4 with maximum production on day 21 after a primary infection. This is accompanied by IL-5 production from day 11 to day 65. These observations confirm previous studies that defined a role for IL-4 and IL-5 in the expression of IgE antibodies and eosinophilia, respectively. IL-4 is a well-known cytokine which acts to promote IgE antibody production (16). Thus, IL-4 expressed during experimental rat schistosomiasis corroborates the detection of IgE in sera from infected rats (15, 38, 39). However, recent studies have shown that IL-13 has IL-4-like effects on human B cells by switching to the IgE isotype (12, 35). In mice, in vitro studies showed that IL-13 does not act on B cells to produce IgE (45). Moreover, mice with a disrupted IL-4 gene failed to produce IgE in response to parasitic infection (28, 42). In S. mansoni-infected rats, preliminary results from our laboratory showed an increase in IL-13 mRNA expression after a primary infection (data not shown). Whether this increase is involved in IgE synthesis or not has to be further investigated.
IL-5 is the major eosinopoietic cytokine (13, 41), and eosinophils are produced in large numbers in S. mansoni-infected rats (8, 27). Several in vitro and in vivo studies indicate that these cells may play a critical role in protection against S. mansoni by destroying larvae (9) in the presence of anaphylactic antibodies (5, 6). In addition, histological examination of the livers of infected rats revealed eosinophil-rich cell populations associated with live and dead worms (27). In humans, Roberts et al. have shown that resistance to reinfection by S. mansoni is correlated with the expression of IL-5 by peripheral blood mononuclear cells after stimulation in vitro (37). Thus, IL-5 expression in infected rats might be crucial in protective immunity. However, further experiments with neutralizing anti-IL-5 antibodies or with anti-receptor IL-5 antibodies will be required for a clear-cut demonstration.
Since both IL-4 and IL-5 were induced early during infection and because of their known effects on the expression of the humoral response that has been well reported in experimental rat schistosomiasis (20, 21, 39), the detection of IFN-γ in the supernatants of stimulated spleen cells at day 21 was unexpected. It is worthy of note that rats demonstrate strong resistance to initial infection and eliminate the large majority of the parasite population approximately 1 month after exposure (26). The presence of IFN-γ prior to the elimination period, in addition to the fact that the antibody response was not fully developed (see below), strongly suggests that cell-mediated immunity (CMI) could be involved, at least in part, in S. mansoni expulsion previously described as a “self-cure” phenomenon (11). Additional experiments are necessary in order to study the role of IFN-γ produced in vivo on the course of infection.
In order to evaluate the role of induced cytokines on the humoral response and in the light of recent studies which showed that IgG2b and IgG2c are controlled by Th1 cytokines through the production of IFN-γ (19) and that IgG1 and IgG2a may reflect the establishment of a Th2 response (1), we monitored the expression of these isotypes after a primary infection. No detectable specific IgG2b antibody levels were present in the sera of infected rats. However, detectable levels of specific IgG2c antibodies were present from day 42 after infection and remained stable at day 65. The absence of any increase in IgG2c antibodies may be the consequence of the transient expression of IFN-γ at day 21. It is important to note that rat IgG2b was described as the most potent complement-mediated hemolytic antibody because of its high capacity to bind the C1q protein required for activation of the complement pathway (2). In contrast, it has been shown that rat IgG2c antibodies produced in S. mansoni-infected rats, as well as an IgG2c monoclonal antibody, specifically inhibited the eosinophil-dependent cytotoxicity mediated by IgG2a antibodies (20). IgG2c antibodies were also able to inhibit the in vivo protection conferred by IgG2a antibodies (21). Thus, the presence of IgG2c antibodies supported the production of IFN-γ in vivo and suggests that IFN-γ may have a dual role, i.e., (i) in the expression of protective immunity through CMI mechanisms and (ii) in the production of blocking antibodies which inhibit the participation of effector antibodies at the surface target antigen (21) and/or at the effector cell level (24).
Measurement of specific IgG1 and IgG2a antibodies allowed us to detect a rapid increase in titers starting at day 21 after infection with a peak at day 42. These results, in addition to those showing the expression of IgE antibodies (39), are consistent with the idea that a primary infection is characterized by predominant production of Th2 cytokines which are involved in the elicitation of effector antibodies. Although the overall response after a primary infection is a Th2-type response supporting the involvement of an ADCC mechanism in protective immunity, the participation of IFN-γ in CMI cannot be ruled out.
Infected rats develop, as in certain human populations (3, 23), a strong immunogically mediated resistance to reinfection (6, 33). These observations, together with the data presented above, led us to check whether reexposure to S. mansoni may deviate the Th2 polarized response established after a primary infection or transiently induce the expression of IFN-γ which could be involved in immunity to reinfection. Our results clearly evidenced, after a secondary infection of rats by S. mansoni, the absence of IFN-γ expression at the mRNA and protein levels, excluding the possibility that IFN-γ contributes to immunity to reinfection. The absence of IFN-γ expression in vivo was further confirmed by the isotype profile induced after reexposure. Indeed, there was neither production of IgG2b nor an increase in specific IgG2c antibody titers, except at day 28 after reinfection. Whatever the explanation, sustained expression of IFN-γ would elicit the production of blocking antibodies which, in turn, could contribute to rat susceptibility to reinfection. Thus, the absence of IFN-γ expression and, consequently, of IgG2c antibodies may favor resistance to reinfection. Our data showed that IL-4 and IL-5 already expressed during infection are still present after reexposure in the majority of infected rats. The persistence of a Th2-type response is further confirmed by the increase of IgG1 and IgG2a antibodies in sera from reinfected rats, suggesting the involvement of an IFN-γ-independent mechanism in immunity to reinfection.
In conclusion, our results showed the sequence of cytokine expression involved in the initiation and development of a Th2-type response in rats after primary and secondary infections with S. mansoni. Particularly striking is the fact that S. mansoni infection can drive Th2 responses in rats in the absence of egg production, which is required to induce a Th2 response in mice. From our study, it can be suggested that IFN-γ expression may play a role in immunity to infection through CMI mechanisms. However, sustained expression of IFN-γ could elicit the production of blocking IgG2c antibodies. After reexposure, the absence of IFN-γ production, in addition to the development of effector antibodies, suggests that Th2 cells are critical for the expression of immunity to reinfection. Future studies will be aimed at elucidating the specific role of each cytokine in experimental rat schistosomiasis.
ACKNOWLEDGMENTS
This work was supported by the Unité INSERM U. 167, by MESR ACCSV no. 7, and by the UNDP/World Bank/WHO special program Programme for TDR (B20/181/163). We acknowledge the support of Volvic S.A. for maintenance of the schistosome life cycle. C.C. is a doctoral fellow of the MESR (96-5-10989).
We are grateful to R. J. Pierce for helpful discussions and to J. Fontaine and C. Godin for technical assistance.
REFERENCES
- 1.Blinder J, Graser E, Hancook W W, Wasowska B, Sayegh M H, Volk H D, Kupiek Weglinski J W. Downregulation of intragraft IFN-gamma expression correlates with increased IgG1 alloantibody response following intrathymic immunomodulation of sensitized rat recipients. Transplantation. 1995;60:1516–1524. doi: 10.1097/00007890-199560120-00025. [DOI] [PubMed] [Google Scholar]
- 2.Brüggemann M, Teale C, Clark M, Bondon C, Waldmann H. A matched set of rat/mouse chimeric antibodies: identification and biological properties of rat H chain constant regions μ, γ1, γ2a, γ2b, γ2c, ɛ and α. J Immunol. 1989;142:3145–3150. [PubMed] [Google Scholar]
- 3.Butterworth A E, Hagan P. Immunity in human schistosomiasis. Parasitol Today. 1987;3:11–16. doi: 10.1016/0169-4758(87)90091-3. [DOI] [PubMed] [Google Scholar]
- 4.Capron A. Immunity to schistosomes. Curr Opin Immunol. 1992;4:419–424. doi: 10.1016/s0952-7915(06)80033-6. [DOI] [PubMed] [Google Scholar]
- 5.Capron M, Capron A, Torpier G, Bazin H, Bout D, Joseph M. Eosinophil-dependent cytotoxicity in rat schistosomiasis. Involvement of IgG2a antibody and role of mast cells. Eur J Immunol. 1978;8:127–133. doi: 10.1002/eji.1830080211. [DOI] [PubMed] [Google Scholar]
- 6.Capron M, Bazin H, Joseph M, Capron A. Evidence for IgE dependent cytotoxicity by rat eosinophils. J Immunol. 1981;126:1764–1768. [PubMed] [Google Scholar]
- 7.Capron M, Capron A, Abdel-Hafez S K, Bazin H, Joseph M, Philips S M. Immunologic response of athymic rats to Schistosoma mansoniinfection. II. Antibody dependent mechanisms of resistance. J Immunol. 1983;131:1475–1480. [PubMed] [Google Scholar]
- 8.Capron M, Nogueira-Queiroz J A, Papin J P, Capron A. Interactions between eosinophils and antibodies in vivoprotective role against rat schistosomiasis. Cell Immunol. 1984;83:60–72. doi: 10.1016/0008-8749(84)90225-9. [DOI] [PubMed] [Google Scholar]
- 9.Capron M, Capron A. Rats, mice and men-models for immune effector mechanisms against schistosomiasis. Parasitol Today. 1986;2:69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- 10.Cêtre C, Cocude C, Pierrot C, Godin C, Capron A, Capron M, Khalife J. In vivo expression of cytokine mRNA in rats infected with Schistosoma mansoni. Parasite Immunol. 1998;20:135–142. doi: 10.1046/j.1365-3024.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 11.Cioli D, Dennert G. The course of Schistosoma mansoniinfection in thymectomized rats. J Immunol. 1976;117:59–65. [PubMed] [Google Scholar]
- 12.Cocks B G, De Waal Malefyt R, Galizzi J P, De Vries J E, Aversa G G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;6:657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 13.Coffman R L, Seymour B W P, Hudak S, Jackson J, Rennick D. Antibody to IL5 inhibits helminth induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 14.Couissinier P, Dessein A J. Schistosoma specific helper T cell clones from subjects resistant to infection by Schistosoma mansoniare Th0/2. Eur J Immunol. 1995;25:2295–2302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- 15.Cutts L, Wilson R A. Elimination of a primary schistosome infection from rats coincides with elevated IgE titres and mast cell degranulation. Parasite Immunol. 1997;19:91–102. doi: 10.1046/j.1365-3024.1997.d01-184.x. [DOI] [PubMed] [Google Scholar]
- 16.De Vries J E, Gauchat J F, Aversa G G, Punnonen J, Gascan H, Yssel H. Regulation of IgE synthesis by cytokines. Curr Opin Immunol. 1991;3:851–858. doi: 10.1016/s0952-7915(05)80003-2. [DOI] [PubMed] [Google Scholar]
- 17.Dunne D, Butterworth A E, Fulford A J C, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R J, Sturrock R F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 18.Fattah D, Quint D J, Proudfoot A, O’Malley R, Zanders E D, Champion B R. In vitro and in vivostudies with purified recombinant human interleukin-5. Cytokine. 1990;2:112–121. doi: 10.1016/1043-4666(90)90005-e. [DOI] [PubMed] [Google Scholar]
- 19.Gracie J A, Bradley J A. IL-12 induces IFNγ dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 20.Grzych J M, Capron M, Bazin H, Capron A. In vitro and in vivo effector function of rat IgG2a monoclonal anti-Schistosoma mansoniantibodies. J Immunol. 1982;129:2739–2743. [PubMed] [Google Scholar]
- 21.Grzych J M, Capron M, Dissous C, Capron A. Blocking activity of rat monoclonal antibodies in experimental schistosomiasis. J Immunol. 1984;133:998–1004. [PubMed] [Google Scholar]
- 22.Grzych J M, Pearce E, Cheever A, Caulada Z A, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 23.Hagan P, Blumenthal U J, Dunne D, Simpson A J G, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 24.Khalife J, Capron M, Grzych J M, Bazin H, Capron A. Fc receptors on eosinophils: isotype dependent cell activation. J Immunol. 1985;135:2780–2784. [PubMed] [Google Scholar]
- 25.Kigoni E P, Elsas P P, Lenzi H L, Dessein A J. IgE antibody and resistance to infection II. Effect of IgE suppression on the early and late skin reaction and resistance of rats to Schistosoma mansoniinfection. Eur J Immunol. 1986;16:589–595. doi: 10.1002/eji.1830160602. [DOI] [PubMed] [Google Scholar]
- 26.Knopf P M, Nutman T B, Reasoner J A. Schistosoma mansoni: resistance to reinfection. Exp Parasitol. 1977;41:74–82. doi: 10.1016/0014-4894(77)90131-x. [DOI] [PubMed] [Google Scholar]
- 27.Knopf P M. Schistosoma mansoni: peripheral and tissue eosinophilia in infected rats. Exp Parasitol. 1979;47:232–245. doi: 10.1016/0014-4894(79)90076-6. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Rajewsky K, Muller W. Generation and analysis of IL-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T R, Sad S. The expanding universe of T cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 30.Mwatha J K, Kimani G, Kamau T, Mbugua G G, Ouma J H, Mumo J, Fulford A J C, Jones F M, Butterworth A E, Roberts M B, Dunne D. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-γ, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 31.Pearce E J, Sher A. Functional dichotomy in the CD4+ T cell response to Schistosoma mansoni. Exp Parasitol. 1991;73:110–116. doi: 10.1016/0014-4894(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 32.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philips S M, Bentley A G, Linette G, Doughty B L, Capron M. The immunologic response of congenitally athymic rats to Schistosoma mansoni infection I. In vivostudies of resistance. J Immunol. 1983;131:1466–1474. [PubMed] [Google Scholar]
- 34.Pierrot C, Godin C, Liu J L, Capron A, Khalife J. Schistosoma mansonielastase: an immune target regulated during the parasite life-cycle. Parasitology. 1996;113:519–526. doi: 10.1017/s0031182000067561. [DOI] [PubMed] [Google Scholar]
- 35.Punnonen J, Aversa G, Cocks B G, McKenzie A N J, Menon S, Zurawski G, Malefyt R D W, De Vries J E. Interleukin-13 induces interleukin-4 independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rihet P, Demeure C E, Bourgeois A, Prata A, Dessein A J. Evidence for association between human resistance to schistosomiasis and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 37.Roberts M, Butterworth A E, Kimani G, Kamau T, Fulford A J C, Dunne D, Ouma J H, Sturrock R F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousseaux R, Capron M, Bazin H, Capron A. Quantitative determination of specific IgE antibodies against S. mansoni: a follow-up study of two strains of infected rats. Correlation with protective immunity. Immunology. 1978;35:33–39. [PMC free article] [PubMed] [Google Scholar]
- 39.Rousseaux-Prevost R, Bazin H, Capron A. IgE in experimental schistosomiasis. I. Serum IgE levels after infection by Schistosoma mansoniin various strains of rats. Immunology. 1977;33:501–505. [PMC free article] [PubMed] [Google Scholar]
- 40.Scott P, Pearce E, Cheever A W, Coffman R L, Sher A. Role of cytokines and CD4+ T cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 41.Sher A, Coffman R L, Hieny S, Scott P, Cheever A W. IL-5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with S. mansoni. Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urban J F, Noben-Trauth N, Donaldson D D, Madden K B, Morris S C, Collins M, Finkelman F D. IL-13, IL-4Ra, and Stat-6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 43.Verwaerde C, Joseph M, Capron M, Pierce R J, Dammoneville M, Velge F, Auriault C, Capron A. Functional properties of a rat monoclonal IgE antibody specific for Schistosoma mansoni. J Immunol. 1987;138:4441–4446. [PubMed] [Google Scholar]
- 44.Yamashita T, Boros D L. IL4 influences IL2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992;149:3659–3664. [PubMed] [Google Scholar]
- 45.Zurawski G, De Vries J E. Interleukin-13, an IL-4 like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]