Abstract
1,4-Napththoquinones (NQs) are clinically relevant therapeutics that affect cell function through production of reactive oxygen species (ROS) and formation of adducts with regulatory protein thiols. Reactive sulfur species (RSS) are chemically and biologically similar to ROS and here we examine RSS production by NQ oxidation of hydrogen sulfide (H2S) using RSS-specific fluorophores, liquid chromatography-mass spectrometry, UV-Vis absorption spectrometry, oxygen-sensitive optodes, thiosulfate-specific nanoparticles, HPLC-monobromobimane derivatization, and ion chromatographic assays. We show that NQs, catalytically oxidize H2S to per- and polysulfides (H2Sn, n = 2–6), thiosulfate, sulfite and sulfate in reactions that consume oxygen and are accelerated by superoxide dismutase (SOD) and inhibited by catalase. The approximate efficacy of NQs (in decreasing order) is, 1,4-NQ ≈ juglone ≈ plumbagin > 2-methoxy-1,4-NQ ≈ menadione >> phylloquinone ≈ anthraquinone ≈ menaquinone ≈ lawsone. We propose that the most probable reactions are an initial two-electron oxidation of H2S to S0 and reduction of NQ to NQH2. S0 may react with H2S or elongate H2Sn in variety of reactions. Reoxidation of NQH2 likely involves a semiquinone radical (NQ·−) intermediate via several mechanisms involving oxygen and comproportionation to produce NQ and superoxide. Dismutation of the latter forms hydrogen peroxide which then further oxidizes RSS to sulfoxides. These findings provide the chemical background for novel sulfur-based approaches to naphthoquinone-directed therapies.
Keywords: reactive sulfur species, reactive oxygen species, antioxidants, juglone, plumbagin, vitamin K
1. Introduction
Quinones form the second largest class of antitumor agents approved in the U.S. for clinical use [1]. 1,4-Naphthoquinone (1,4-NQ), a quinone derived from naphthalene, is the most basic unit of the para-substituted naphthoquinones (NQs) and the structural parent of a variety of natural and synthetic NQs historically used in herbal medicine, and recently, of considerable clinical interest [2]. A number of these compounds, juglone, plumbagin, lawsone and vitamins K1 (phylloquinone), K2 (menaquinone) and K3 (menadione) are particularly noteworthy and have been extensively investigated. Juglone is commonly derived from walnut trees and plumbagin, from the roots of the medicinal herb Plumbago zeylancia. Lawsone (henna), obtained from the Lawsonia inermis tree, has been used since ancient times to dye clothing, hair and as a temporary tattoo [3]. The vitamin K compounds are well known as essential factors in blood coagulation and to treat osteoporosis [4]. Health benefits ascribed to these compounds include anti-inflammatory, anti-diabetic, antibacterial, antifungal, anti-atherosclerotic, analgesic, anti-osteoporotic, neuroprotective and hypolipidemic, but they are perhaps best known for their anticancer and antioxidant/prooxidant activities [5,6,7,8,9].
Collectively, NQs are well known as redox-cycling and alkylating agents, although much of their biological efficacy has been attributed to redox processes through the generation of reactive oxygen species (ROS), namely superoxide (O2∙−) and hydrogen peroxide (H2O2) [10,11]. These ROS may elicit cytoprotective effects in healthy cells by activating endogenous antioxidant responses or may kill ROS-sensitive tumor cells [10]. A number of studies have shown that NQs may be reduced by NADH or NADPH in reactions catalyzed by cytochrome P450 reductase or NAD(P)H:quinone oxidoreductase-1 (NQO-1), the former being an one-electron reduction to the semiquinone and the latter a two-electron reduction to the hydroquinone (reviewed in [11]). In either case, the reduced NQ is autoxidized by O2 yielding superoxide (O2∙−) which then dismutes, either spontaneously or catalyzed by superoxide dismutase (SOD), to O2 and H2O2. The latter (H2O2) accounts for most of the endogenous antioxidant response by activating the Keap-1/Nrf2 antioxidant system. Superoxide production, or at least its removal from the catalytic site, appears to be the rate-limiting step as the rate of redox cycling by NQs is enhanced in the presence of SOD [12]. Some of the confusion surrounding the biological functions of NQs is perhaps best illustrated by the mitochondrial-targeted naphthoquinone, SKQ1 [10-(6 -plastoquinonyl)decyl] triphenylphosphonium) which has recently been used as both a prooxidant [13] and a ROS scavenger [14].
We have recently shown that polyphenolic nutraceuticals in tea, berries and spices catalyze the oxidization of H2S to polysulfides and thiosulfate [15,16]. This reaction requires oxygen and appears to be mediated by initial autoxidation of a hydroquinone in the B ring of the polyphenol. We have also shown that a variety of quinones and hydroquinones, after the latter are autoxidized, oxidize H2S to polysulfides and thiosulfate in buffer and in cells [17]. Although side-chain modifications can augment catalytic efficacy, in general, para-quinones are more effective H2S catalysts than ortho-quinones, whereas meta-quinones are completely ineffective [17]. These findings suggest that 1,4-NQs may possess similar characteristics, and if so, a number of the physiological attributes of 1,4-NQs may be explained by their effects on cellular sulfur chemistry. In this study, we examine the effects of a variety of 1,4-NQs on inorganic sulfur metabolism in aqueous buffered solutions. We show that a number of these compounds catalytically oxidize H2S to thiosulfate and to inorganic per- and polyhydrosulfides, that this process consumes oxygen, and it involves redox cycling of the NQ. Chemical structures of the naphthoquinones used in this study are shown in Figure 1.
Figure 1.
Structures of naphthoquinones used in this study; carbon positions are numbered in 1,4-naphthoquinone for reference.
2. Results
2.1. Naphthoquinones Oxidize H2S to Polysulfides
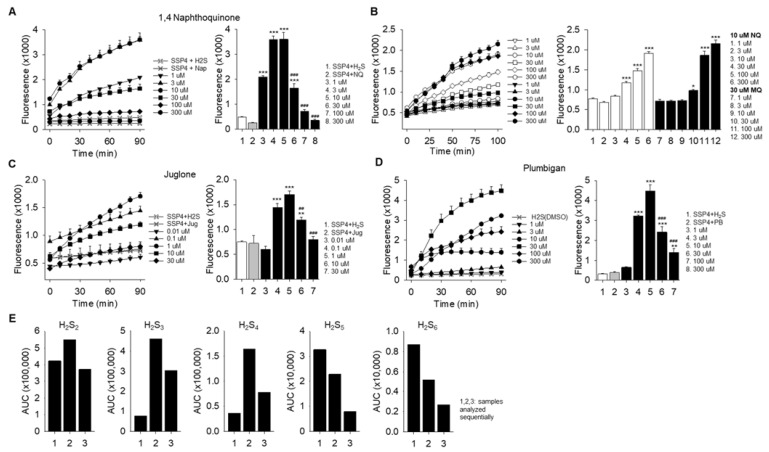
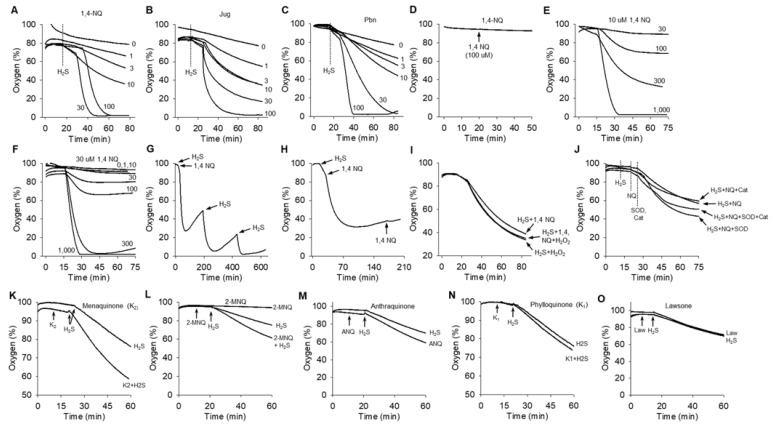
We initially examined the effect of varying concentrations of 1,4-NQ on polysulfide formation (SSP4 fluorescence) from 300 μM H2S and observed that at low concentrations 1,4-NQ concentration-dependently increased SSP4 fluorescence, whereas above 10 μM, fluorescence progressively decreased; half as much fluorescence was observed with 30 μM 1,4-NQ compared to 10 μM (Figure 2A). Conversely, increasing concentrations of H2S continuously, and concentration-dependently increased SSP4 fluorescence when incubated with either 10 or 30 μM 1,4-NQ (Figure 2B). Juglone (Jug) and plumbagin (Pbn) also increased, then decreased polysulfide production with 300 μM H2S (Figure 2C,D). Maximum polysulfide production was observed at 1 μM for Jug and 10 μM for Pbn. Similar results were observed for 2-methoxy-1,4-naphthoquinone and phylloquinone, although there was no apparent inhibition at the highest NQ concentrations, anthraquinone and lawsone were minimally efficacious and menaquinone slightly decreased fluorescence compared to SSP4 with H2S (Supplemental Figure S2). These results show that most NQs oxidize H2S to polysulfides and they suggest that the efficacy of this process is especially sensitive to the location, degree, and functional substitution (-OH, -CH3, -OCH3, etc.) substitutions on the 2, 3, and 5 positions of the NQ skeleton. The inhibitory effect of the NQs is examined in Section 2.2 below.
Figure 2.
Naphthoquinones oxidize H2S to polysulfides (SSP4 fluorescence). (A) Effect of increasing concentrations of 1,4-naphthoquinone (1,4-NQ) with constant 300 μM H2S, or (B) effect of 10 and 30 μM 1,4-NQ with increasing concentrations of H2S. (C,D) Effect of increasing concentrations of juglone (Jug) or plumbagin (Pbn) on SSP4 fluorescence from 300 μM H2S. All naphthoquinones initially increased, then decreased SSP4 fluorescence, whereas H2S concentration-dependently increased fluorescence at both concentrations of 1,4-NQ. (E) LC-ESI-HRMS identification of IAM-derivatized polysulfides produced from 1 mM H2S and 1 mM 1,4-NQ. Samples were derivatized after 10 min with 5 mM IAM and three aliquots (1, 2, 3) were analyzed serially at approximately 1 h intervals. (A–D) Mean + SE, n = 4 wells per treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to buffer; ##, p < 0.01; ###, p < 0.001 compared to maximum response.
LC-ESI-HRMS was then used to further characterize the products of the H2S/1,4-NQ reaction. In this experiment, H2S (1 mM) was added to 1,4-NQ (1 mM) and allowed to react for 10 min. IAM (10 mM) was then added, and the sample was divided into three aliquots (Samples 1, 2, 3) which were serially analyzed at approximately 1 h intervals to monitor both the identity and stability of the IAM-polysulfides. As shown in Figure 2E, multiple inorganic polysulfides were produced after 10 min incubation of H2S with 1,4-NQ. The area under the curve (AUC) for H2S2, H2S3 and H2S4 increased between samples 1 and 2 but decreased for H2S5 and H2S6. The AUC for all samples decreased from sample 2 to sample 3. This suggests that either polysulfides, or IAM-derivatized polysulfides with n > 4, are somewhat unstable and may initially decompose to lower molecular weight polysulfides over the first hour and then later decompose to other sulfur moieties that are undetectable by LC-ESI-HRMS. Using values from sample 1, the percentage of the total polysulfide detected at 10 min was 39, 42, 15, 3 and 0.8% for S2–6, respectively, and for sample 2 was 57, 32, 8 and 1%. Collectively, this suggest that a variety of polysulfides are produced by 1,4-NQ catalyzed oxidation of H2S and that H2S2 and possibly H2S3 are likely the primary products of this reaction.
2.2. Reaction of 1,4-Naphthoquinone with Polysulfides Accounts for the Apparent Inhibition of SSP4 Fluorescence
Three experiments were designed to determine if the decrease in SSP4 fluorescence at NQ concentrations above 10 μM was due to some type of interference of the NQ with the SSP4 fluorescence, or due to decreased H2S oxidation by higher NQ concentrations. In the first experiment, 5 μM SSP4 was added to 10 μM of the mixed polysulfide (K2Sn, n = 1–6) followed by 1,4-NQ to determine if 1,4-NQ interfered with the reaction between SSP4 and polysulfides. In the second experiment the order of SSP4 and 1,4-NQ addition was reversed to determine if the 1,4-NQ reacted directly with the polysulfide to produce a product undetectable by SSP4. In the third set of experiments K2Sn was incubated with SSP4 for 60 min to allow for the maximum SSP4 fluorescence to develop before adding 1,4-NQ to determine if the 1,4-NQ could reverse the reaction or if it inhibited fluorescence in other ways.
As shown in Supplemental Figure S3, adding SSP4 to K2Sn before 1,4-NQ only slightly affected SSP4 fluorescence, whereas when 1,4-NQ was added first, it concentration-dependently decreased fluorescence to the extent that fluorescence was nearly completely inhibited by either 30 or 100 μM 1,4-NQ. SSP4 fluorescence was unaffected by 1,4-NQ when SSP4 was allowed to react with K2Sn for 60 min prior to addition of 1,4-NQ. Collectively, these results suggest that high concentrations of 1,4-NQ react with polysulfides to produce one or more products that are undetectable by SSP4.
2.3. Effects of Oxygen and SOD on Polysulfide Production Detected with SSP4
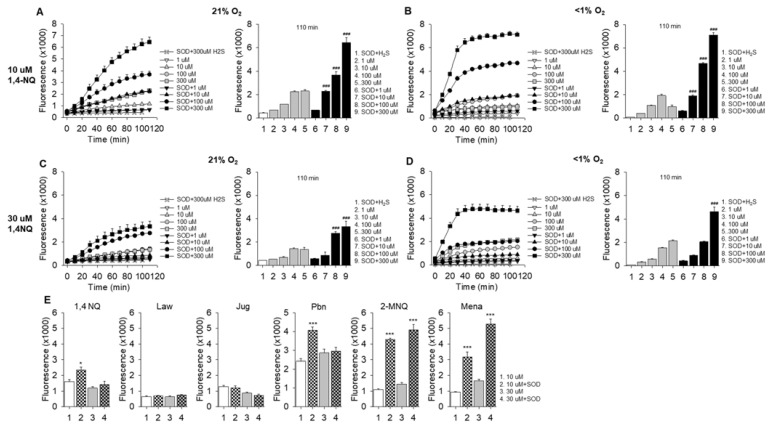
We have previously shown that low oxygen decreases SSP4-detected polysulfide production when H2S is incubated with CoQ0, whereas in normoxic solutions SOD increases polysulfides when H2S is incubated with CoQ0 [18]. This suggested that an examination of oxygen tension and SOD on 1,4-NQ reactions with H2S was also warranted. As shown in Figure 3, decreasing oxygen from 21% to <1% had minimal effects on SSP4 fluorescence at 110 min of incubation, whereas SOD increased fluorescence in both 21% and <1% O2. Overall polysulfide concentration at 110 min was greater with 10 μM than with 30 μM 1,4-NQ which is consistent with Figure 2 and the SOD-enhancement of SSP4 fluorescence is consistent with our previous observations on quinones [17,18]. However, the rate of increase in SSP4 fluorescence was considerably greater with SOD in <1% O2 than with SOD in 21% O2 with either 10 μM or 30 μM. It also appeared that there was a lag in the increase in SSP4 fluorescence over the initial 20–30 min with either 10 μM or 30 μM 1,4-NQ and SOD in 21% O2 that was not as evident in samples incubated at <1% O2. This suggests that oxidized 1,4-NQ (as provided by the supplier) is not reduced as quickly in 21% O2, as it is in <1% O2, an observation supported by absorbance spectra of these reactions (see, Section 2.6). We propose the delay in formation of reduced 1,4-NQ delays the establishment of a comproportionation reaction as discussed in Section 3.2.1.
Figure 3.
(A–D) Effects of oxygen (O2; 21% and <1%) and superoxide dismutase (SOD; 0.1 μΜ) on polysulfide production (SSP4 fluorescence) by 10 μM or 30 μM 1,4-NQ and 300 μΜ H2S. Low O2 had minimal effects on polysulfide production, whereas SOD increased production in both 21% and <1% O2. Overall polysulfide production was greater with 10 μM than with 30 μM 1,4-NQ. Bar graphs show fluorescence at 110 min. (E) Effects of SOD on polysulfide production by 10 μM or 30 μM NQs incubated with 300 μM H2S for 120 min. Mean + SE, n = 4 wells per treatment; *, p < 0.05; ***, p < 0.001 compared to similar treatment without SOD. ###, p < 0.001 compared to maximum response.
We also noticed during our experiments that there was some day-to-day variation in the absolute amount of SSP4 fluorescence produced by incubation of NQs with H2S which we attribute to a number of possible factors including H2S volatility and differences in SSP4 lot numbers and/or the degree of SSP4 autoxidation over time. While this did not affect the clear trends of NQs to oxidize H2S, we felt a study of H2S oxidation by a number of NQs examined simultaneously, in both 21% O2 and <1% O2 was warranted. As shown in Supplemental Figure S4, 1,4-NQ, plumbagin, juglone, 2-MNQ and the purported mitochondrial-targeted antioxidant, SKQ1 [14,19], concentration-dependently increased polysulfide production (SSP4 fluorescence) by oxidation of H2S. The apparent potency in 21% O2, in descending order, was plumbagin, 1,4-NQ = SKQ1, juglone, 2-MNQ. Consistent with previous experiments (described above), 30 μM NQ inhibited SSP4 fluorescence, except that this inhibition was not observed with 2-MNQ. The greatest increase in fluorescence was typically observed in the initial 20 min. Hypoxia (1% O2) typically decreased fluorescence, although it did not prevent it, and with juglone fluorescence produced by incubation of H2S with 10 and 30 μM NQs in <1% O2 was as great as maximal fluorescence at 21% O2. The effects of SOD on polysulfide production by other NQs were also examined. As shown in Figure 3E, SOD did not affect the small amount of polysulfide production by lawsone or juglone, whereas it greatly increased polysulfide production by 2-MNQ and menadione.
Collectively, these results confirm that a variety of NQs oxidize H2S to polysulfides, that slight modifications of the quinone affect overall efficacy of the reaction, and that decreasing oxygen decreases H2S oxidation but does not inhibit it, possibly because the NQs as obtained from the suppliers were mostly oxidized at the start of the reaction. The efficacy of SOD also appears to vary depending on modifications to the quinone ring.
2.4. 1,4-NQ Oxidizes H2S to Thiosulfate and Sulfite
2.4.1. Thiosulfate Detection with AgNP
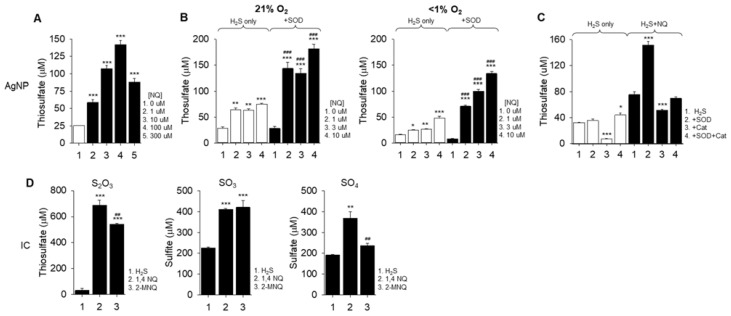
Thiosulfate is also a product of H2S oxidation by quinones [17,18]. Incubation of increasing concentrations of 1,4-NQ with 300 μM H2S exhibited a similar concentration-dependent increase in thiosulfate production from 1 to 100 μM 1,4-NQ and decrease in production at 300 μM (Figure 4A). Interestingly, the inhibitory effect on thiosulfate production with 300 μM 1,4-NQ was similar to the effect on polysulfide production, albeit the latter occurred at a lower concentration of 1,4-NQ.
Figure 4.
Effects of various parameters on thiosulfate (TS) production from 1,4-NQ (NQ) oxidation of 300 μM H2S measured with silver nanoparticles (A–C) or by ion chromatography (D). (A) NQ concentration-dependently increased TS production from 1 to 100 μM and decreased it at 300 μM. (B) Effects of increasing concentrations of NQ with or without 0.1 μM SOD in TS production in 21% or <1% O2. SOD increased TS production at both O2 tensions. Low O2 somewhat decreased TS production with or without TS but did not inhibit it. (C) Effects of 0.1 μM superoxide dismutase (SOD) and 1 μM catalase (Cat), alone or in combination, on TS production from 300 μM H2S in the absence (H2S only) or presence of 10 μM NQ. With H2S alone, TS production was decreased by Cat and slightly increased by SOD + Cat. With H2S plus NQ, TS was greatly increased by SOD, decreased by Cat but unaffected by SOD + Cat. (A–C), Mean + SE, n = 8 wells per treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to H2S from same treatment; (B), ###, p < 0.001 compared to response without SOD. (D) Ion chromatography measurements of production of TS (S2O3), sulfite (SO3) and sulfate (SO4) by incubation of 3 mM H2S with 300 μM 1,4-NQ or 2-MNQ. Mean + SE, n = 3 wells per treatment; **, p < 0.01; ***, p < 0.001 compared to H2S only; ##, p < 0.01 compared to 1,4-NQ.
Sparging the buffer with N2 and adding 1,4-NQ to H2S in a hypoxia chamber where the O2 was less than 1% somewhat decreased thiosulfate production compared to experiments simultaneously conducted in 21% O2 but did not inhibit it (Figure 4B). SOD increased thiosulfate production in both 21% and 1% O2. These results are consistent with the effects of O2 and SOD on polysulfide production measured with SSP4 (Figure 3).
The SOD-mediated increase in thiosulfate production could be due to shifting the reaction equilibrium by removing one of the products, namely superoxide, as proposed by Song and Buettner [12], or it could be due to H2O2 produced by dismutation of superoxide which would then oxidize H2S, although this reaction appears to be too slow to have a significant impact [20]. To examine these possibilities, thiosulfate production was measured in the presence of SOD and catalase (Cat, Figure 4C). With only H2S, the thiosulfate concentration was decreased by Cat and slightly increased by SOD + Cat suggesting that some of the background thiosulfate in the H2S stock was due to oxidation by oxygen. With H2S plus NQ, the thiosulfate concentration was greatly increased by SOD, decreased by Cat but unaffected by SOD + Cat. These results show that SOD augments thiosulfate formation, consistent with our previous observations on polysulfide formation. The results also show that catalase decreases thiosulfate concentration when incubated with H2S alone, or with H2S plus 1,4-NQ, and that Cat appears to completely inhibit the stimulatory effects of SOD when incubated with H2S and 1,4-NQ. This suggests that hydrogen peroxide contributes to much of the thiosulfate produced in these reactions, although it may not substantially contribute to polysulfude production from H2S [21].
2.4.2. HPLC-MBB Detection of Thiosulfate and Sulfite
Thiosulfate and sulfite were also measured using HPLC separation of MBB-derivatized samples of the reaction between 300 μM H2S (as Na2S) and 10 μM 1,4-NQ. This produced 43 μM thiosulfate and 15 μM sulfite (average of duplicate samples). These results confirm that thiosulfate is produced by 1,4-NQ oxidation of H2S, and they also provide evidence for significant production of sulfite.
2.4.3. Ion Chromatography (IC) Detection of Thiosulfate, Sulfite and Sulfate
To compensate for the relative insensitivity of the IC method, 3 mM H2S was added to 300 μM of either 1,4-NQ or 2-MNQ to evaluate production of thiosulfate, sulfite and sulfate. Both NQs significantly increased production of all three sulfoxides (Figure 4D). Upon subtracting the background sulfur compounds present or formed in the H2S blank during incubation, it was evident that thiosulfate was the predominant sulfur product followed by sulfite and then sulfate and that 2-MNQ tended to be somewhat less efficacious than 1,4-NQ in producing thiosulfate and sulfate. These results support the observations that thiosulfate was detected by both AgNP and HPLC-MBB methods and they add sulfate to the list of species produced by NQ oxidation of H2S.
2.5. H2S Reaction with Naphthoquinones Consumes O2 and Is Variously Affected by SOD and Cat
We have previously shown that para-quinones oxidize H2S to polysulfides and consume oxygen in the process [17,18]. Here, O2 consumption by NQs was measured under different conditions to determine if NQs exhibited similar characteristics, and if this was inhibited by higher concentrations of NQ. We also examined the effects of SOD and Cat on oxygen consumption by H2S and 1,4-NQ.
As an example, 1,4-NQ, Jug and Pbn concentration-dependently increased O2 consumption when added to a fixed concentration of 300 μM H2S (Figure 5A–C). Contrary to the apparent inhibition of polysulfide production at higher NQ concentrations there was no evidence of any inhibitory effects on O2 consumption at the high NQ concentrations, and all the O2 was consumed in many experiments. Plumbagin appeared to be the most efficacious at the higher NQ concentrations. Baseline oxygen concentration was not affected by 100 μM 1,4-NQ in the absence of H2S (Figure 5D). In a like manner, varying the concentration of H2S resulted in a concentration-dependent increase in O2 consumption in the presence of a fixed concentration of either 10 or 30 μM 1,4-NQ without any apparent inhibitory effects (Figure 5E,F). These experiments confirm that NQ-mediated oxidation of H2S is not inhibited by high concentrations of either 1,4-NQ or H2S.
Figure 5.
Typical real-time traces of O2 consumption by naphthoquinones under various conditions. (A–C) O2 is concentration-dependently consumed during incubation of 300 μM H2S with increasing concentrations of (A) 1,4-naphthoquinone (1,4-NQ), (B) juglone (Jug) or (C) plumbagin (Pbn). (D) 100 μM 1,4-NQ does not consume O2 in the absence of H2S. (E,F) H2S concentration-dependently increases O2 consumption by 10 μM (E) and 30 μM (F) 1,4-NQ. (G,H) O2 levels begin to rise after prolonged incubation of 30 μM 1,4-NQ with 300 μM H2S and they are decreased upon subsequent addition of H2S (G) but not by 1,4-NQ (H). (I) H2O2 (100 μM) does not affect O2 consumption in the presence of 300 μM H2S or H2S plus 10 μM 1,4-NQ. (J) Superoxide dismutase (SOD, 0.1μM) increases O2 consumption by 300 μM H2S and 10 μM 1,4-NQ, whereas catalase (Cat. 1 μM) decreases it and Cat reduces the effect of SOD. O2 consumption by 300 μM H2S and 30 μM menaquinone (vitamin K2; K), 2-methoxy-1,4-NQ (2-MNQ; L), anthraquinone (M), phylloquinone (vitamin K1; N) and lawsone (O).
It also became apparent that oxygen tension began to increase after incubating H2S with high concentrations of 1,4-NQ for an extended period. We attribute this to oxygen diffusion from the atmosphere into the buffer after one, or both of the reactants had been depleted. To determine if this was due to consumption of either H2S or 1,4-NQ, or both, we added a second doses of H2S or 1,4-NQ after oxygen tension began to increase while continuously monitoring oxygen levels. As shown in (Figure 5G,H), a second dose of H2S produced an additional decrease in oxygen, whereas a second treatment with 30 μM 1,4-NQ was ineffective. These results indicate that H2S is depleted in these reactions, but 1,4-NQ is not. This supports our hypothesis that 1,4-NQ functions as a catalyst. This hypothesis is further supported by the stoichiometry of the reaction, 30 μM of 1,4-NQ will consume more than 200 μM of oxygen and over 300 μM of H2S. H2S-1,4-NQ-mediated oxygen consumption was unaffected by H2O2 (Figure 5I), suggesting that hydrogen peroxide may not contribute to the formation of various sulfur species in these reactions. This is supported by previous observations that similar concentrations of H2O2 and H2S do not produce appreciable quantities of polysulfides [21] or thiosulfate (18.9 ± 1.5 versus 21.4 ± 0.8 uM, n = 4 for 100 μM H2S versus 100 μM H2O2 plus 100 μM H2S, Olson and Gao, unpublished). Superoxide dismutase increased O2 consumption by H2S/1,4-NQ, Cat alone did not affect it, but Cat somewhat diminished the effect of SOD (Figure 5J). Menaquinone and 2-methoxy-1,4-NQ also consumed O2 (Figure 5K,L), whereas anthraquinone (Figure 5M) only slightly increased O2 consumption, O2 consumption was not affected by either phylloquinone (Figure 5N) or lawsone (Figure 5O), which is consistent with the observations that these last three compounds do not produce significant concentrations of polysulfides. Addition of SOD to the H2S NQ reaction produced a further increase in oxygen consumption by 1,4-NQ, plumbagin and juglone, less of an increase with menadione and 2-MNQ and did not appear to affect oxygen consumption by lawsone (Supplemental Figure S5).
Additional experiments were also performed to further examine whether the SOD-mediated increase in oxygen consumption in the reaction of 1,4-NQ and H2S was (a) due to removal of superoxide, as proposed by Song and Buettner [12], (b) due to reduction of oxidized NQ and establishment of a comproportionation equilibrium, or (c) due to oxygen incorporation into an unknown sulfoxide product of H2S. Addition of DTT to 1,4-NQ in the absence of H2S consumed more oxygen than addition of H2S to 1,4-NQ. SOD did not augment oxygen consumption by DTT and 1,4-NQ but, instead appeared to produce an initial transient decrease in the rate of oxygen consumption (Supplemental Figure S6A). SOD produced a similar transient decrease in oxygen consumption by DTT with 1,4-NQ and H2S, but it did not appear to affect overall consumption of oxygen (Supplemental Figure S6B). Conversely, DTT did not affect polysulfide production (SSP4 fluorescence) when incubated with H2S alone, or with H2S plus 1,4-NQ, whereas SOD decreased SSP4 fluorescence when added to H2S and 1,4-NQ (Supplemental Figure S6C). Addition of ascorbic acid to 1,4-NQ, either without or with H2S also increased oxygen consumption and these reactions were unaffected by SOD (Supplemental Figure S6D,E, respectively). As shown in Supplemental Figure S6F, oxygen consumption by H2S and 1,4-NQ was increased by tempol which is a general redox reagent against hydroxyl radicals, hydrogen peroxide and superoxide [22], but unaffected by mannitol, a weak scavenger of hydroxyl radicals and possibly other ROS [23].
To further examine the effect of a reductant on oxygen consumption by oxidized NQs, DTT was added to juglone, menadione, 2-MNQ and lawsone (Supplemental Figure S6G–J, respectively). DTT greatly increased oxygen consumption when added to juglone, whereas it produced only a modest increase when added to menadione and did not affect oxygen consumption when added to either 2-MNQ or lawsone. SOD slightly increased oxygen consumption when added to DTT with juglone or DTT with 2-MNQ, decreased oxygen consumption when added to DTT with menadione and had no effect on DTT with lawsone. Collectively, these results show that addition of reductants and SOD to NQs has effects on oxygen consumption that are specific for individual NQs and similar to those of H2S. They also suggest that autoxidation of NQs, not sulfoxide production is the primary source of oxygen consumption. These observations are generally consistent with the effect of the respective NQs on polysulfide production. The possible reactions involved are discussed in Section 3.4.2. NQ autoxidation.
2.6. Absorbance Spectra of H2S Reaction with 1,4-NQ Are Consistent with Redox Cycling
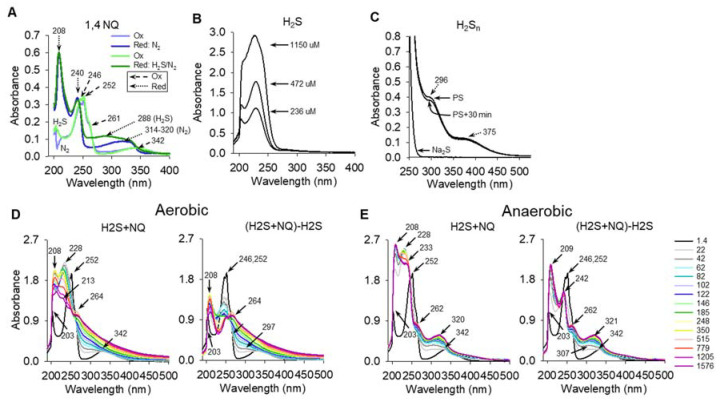
Absorbance spectra of oxidized and reduced 1,4-NQ, H2S, mixed polysulfides and 1,4-NQ plus H2S are shown in Figure 6A. Oxidized 1,4-NQ had absorbance peaks at 246 nm and 252 nm, a shoulder at 261 nm and a broad peak at ~342 nm. Reduction with H2 produced a new, strong peak at 208, a second peak at 240 and a broad peak that extended from ~314 to 320 nm. Reduction with H2S, followed by sparging with N2 to remove excess H2S, produced similar peaks at 208 nm and 240 nm, however the broad peak became even broader with a maximum absorbance around 288 nm. Na2S alone at pH 7 had peaks at 203 nm and 228 nm (Figure 6B), although the 203 peak may be a non-volatile impurity as it disappeared upon purification of sodium sulfide (not shown). Mixed polysulfides produced by incubation of elemental sulfur (S8) with Na2S produced peaks at approximately 296 nm and 375 nm (Figure 6C). The broad 288 nm peak observed after addition of H2S to 1,4-NQ (Figure 6A) may be due to a polysulfide or it could be due to formation of a sulfur-NQ adduct, which will be examined in future experiments.
Figure 6.
(A) Absorbance spectra of oxidized 1,4-NQ (60 μM, light blue/green lines) and after reduction with either N2 gas (dark blue line) or 1 mM H2S, followed by N2 gas to remove excess H2S (dark green line). (B) Spectra of different concentrations of H2S (as Na2S); (C) spectra of mixed polysulfides (H2Sn) produced by anaerobic incubation of Na2S with powdered sulfur (S8) in 200 mM phosphate buffer (pH 7.4). Numbers indicate approximate wavelength of peaks. (D,E) Absorbance spectra as a function of time of the reaction between 230 μM 1,4-NQ and 115 μM H2S in aerobic (21% O2) and anaerobic (<1% O2) buffer. Left panels (H2S + NQ) of each pair show the full spectra, in the right panels (H2S + NQ) − H2S) the H2S spectrum was subtracted from the H2S + NQ spectrum at each time point. The time, in seconds, after addition of H2S is indicated on the right of panel (E).
Time-resolved spectra of the addition of 115 μM of H2S to 230 μM 1.4 NQ (1:2 H2S:1,4-NQ) in aerobic (21% O2) or anaerobic (<1% O2) buffer are shown in the left panels in Figure 6D,E, respectively, and the right panels of each show the spectrum after the H2S absorbance was subtracted. The latter gives a clearer evaluation of the effects of H2S on the 1,4-NQ spectrum. Time after addition of H2S is shown on the far right of Figure 6E. Addition of H2S progressively decreased absorbance of the 246 nm and 252 nm (oxidized 1,4-NQ) peaks over the initial 2–3 min, whereas the 208 nm (reduced 1,4-NQ) peak became more prominent up to 248 s then decreased but it did not disappear until the end of the experiment (1576 s). The shoulder at 264 nm became a prominent peak and the broad 342 nm peak became less evident as it merges with other components. When H2S was added to 1,4-NQ in low oxygen (<1% O2), the 246 nm and 252 nm peaks completely disappeared by 42 sec and the 209 nm (208 nm) peak continued to increase throughout the duration of the experiment. The 262–263 peak became prominent as it was in 21% O2, but now it was clear that the broad 342 nm peak was lost, and it was replaced by a broad peak at 321 nm. These results suggest that H2S reduced 1,4-NQ to a hydroquinone in the absence of oxygen, whereas in the presence of oxygen 1,4-NQ was initially reduced but then became partially reoxidized, resulting in an equilibrium between oxidized and reduced species. The 264 nm peak, which could be either a semiquinone or a sulfur adduct, also became more prominent. The cause of the gently sloping decrease in absorbance from 264 nm to 500 nm observed in 21% O2 but less evident at <1% O2 is unknown. It may be due to accumulation of polysulfides (cf. Figure 6C), formation of a semiquinone [24], turbidity due to colloidal elemental sulfur, or some combination of these three.
Further characterization of the H2S-1,4-NQ reactions are shown in Supplemental Figure S7. Addition of 236 μM H2S to 115 μM 1,4-NQ (2:1 H2S:1,4-NQ) rapidly decreased the 246 nm and 252 nm peaks and produced a transient peak at 238 nm that became further blue-shifted to 228 nm by 428 s, after which the amplitude steadily decreased for the remainder of the experiment. The 208 nm peak remained prominent and only slightly decreased over the course of the experiment. A shoulder at 268 nm appeared late. The effects of H2S on the 246 nm and 252 nm peaks of oxidized 1,4-NQ became more evident after the H2S spectrum was subtracted from the H2S plus 14, NQ spectrum and they appeared to be essentially eliminated by 248 s. The peak at 268 nm also was more evident after the H2S spectrum was subtracted. By 62 s a slight shoulder appeared around 298 nm that persisted until it blended in at 350 s. Deconvolution of the H2S/1,4-NQ spectra showed a first component (a) indicative of the original oxidized 1,4-NQ, a second component (b) that is suggestive of the H2S spectrum with an additional small peak at 258 nm. The third component (c) has a peak at 207 nm, a shoulder at 252 nm and a prominent new peak at 262 nm. The 207 nm peak is suggestive of H2S, although if H2S is subtracted out then the 208 nm peak is compatible with reduced 1,4-NQ. The 252 nm peak represents the oxidized 1,4-NQ, the new 262 nm peak is somewhat similar to the late-developing peak observed in the H2S-subtracted spectrum (Supplemental Figure S7, column D).
When the 1,4-NQ concentration was increased to 230 μM with the same 236 μM H2S (1:1 H2S:1,4-NQ), the 228 nm H2S peak rapidly disappeared, and after subtraction of the H2S spectrum, the 246/252 peaks due to oxidized 1,4-NQ persisted for most of the experiment but became progressively smaller, the b component of the deconvoluted spectrum became essentially identical to the a component (oxidized 1,4-NQ) and the 258 nm peak disappeared. The peak in the c component appeared as a flat peak from 252–265 nm, perhaps reflecting some of the remaining oxidized 1,4-NQ. Further increases in H2S, with constant 230 μM 1,4-NQ (2:1 and 4:1 H2S:1,4-NQ), produced progressive decreases in both the H2S and 1,4-NQ characteristics of the a and b components, respectively, the 264 nm peak now became predominant, and an additional peak appeared at 319 nm with another small shoulder at 350 nm. Rate constants for the three deconvoluted spectra are shown in Table 1. Changes in the amplitude of the 208 nm, 228 nm, and 252 nm peaks as a function of time are shown in Supplemental Figure S7E. As expected, the main H2S peak (228 nm) decreased the fastest with a low H2S:1,4-NQ ratio, whereas it was relatively unaffected at high ratios of H2S:1,4-NQ. The 252 nm peak also was relatively unaffected at high H2S:1,4-NQ, most likely because it was rapidly reduced at the onset of the experiment.
Table 1.
Rate constants A → B (k1) and B → C (k2) for deconvoluted UV-Vis absorbance spectra of the H2S reaction with 1.4 naphthoquinone (NQ).
| μM NQ:μM H2S | k 1 | k 2 |
|---|---|---|
| 115:236 | 6.7 × 10−3 s−1 | 1.5 × 10−3 s−1 |
| 230:236 | 17.0 × 10−3 s−1 | 2.0 × 10−3 s−1 |
| 230:472 | 21.0 × 10−3 s−1 | 7.8 × 10−3 s−1 |
| 230:1150 | 22.0 × 10−3 s−1 | 3.4 × 10−3 s−1 |
2.7. 1,4-NQ Oxidation of H2S Does Not Increase DCF Fluorescence
The SOD-mediated increase in H2S oxidation by 1,4-NQ suggests that this involves a one-electron oxidation of the NQ that would concomitantly reduce oxygen to superoxide as a rate-limiting process. The superoxide that is produced would then dismute, spontaneously or catalyzed by SOD, to hydrogen peroxide and an excess of ROS is generally assumed to be one of the products of NQ reactions. In addition, with a high NQ:H2S ratio it is also possible that NQ will reduce H2O2 to hydroxyl radicals in a metal-independent Fenton reaction [1] which will also be detected by the non-specific ROS fluorophore, dichlorofluorescein (DCF) [25]. To determine if any of these possibilities result from NQ/H2S/O2 redox cycling, various reactions were monitored with DCF over 90 min. As shown in Supplemental Figure S8, DCF fluorescence was unaffected by addition of up to 30 μM of either 1,4-NQ, juglone, plumbagin, lawsone, phylloquinone, menaquinone or 2-methoxy naphthoquinone to 300 μM H2S, although all NQs slightly, but significantly, decreased fluorescence at the higher concentrations. Likewise, DCF fluorescence was essentially unaffected when the NQ:H2S ratio was increased stepwise from 1:1 to 300:1. These results suggest that either ROS are not produced in this reaction, which seems unlikely, or that if ROS are produced, they rapidly react with H2S, or more likely polysulfides, to produce sulfoxides, e.g., thiosulfate and sulfite, which was observed.
The reactions of NQ with hydrogen sulfide produce primarily superoxide anions and secondarily by dismutation of superoxide to make hydrogen peroxide. So, in the presence of SOD in living cells, hydrogen peroxide will be the predominate product, which along with molecular oxygen can react with either hydrogen sulfide [20,26,27] or polysulfides [28,29] to make sulfide, thiosulfate and finally sulfate (see Section 3). This also suggests that perhaps one of the biological effects of NQ-mediated metabolism of H2S is to rapidly quench ROS through diversion into relatively innocuous sulfoxides.
2.8. Free Iron Is Not Involved in 1,4-NQ Oxidation of H2S
Transition metals are often present as trace impurities in many reactions. These, especially iron, may catalyze autoxidation of naphthoquinones by complexing with NQ anions to overcome spin restriction and facilitate transfer of electrons to O2 [12]. To determine if iron contributed to autoxidation of NQs, 1,4-NQ was incubated with H2S in the presence of diethylenetriamine pentaacetic acid (DTPA) and polysulfide production monitored with SSP4 fluorescence. As shown in Supplemental Figure S9, DTPA did not affect NQ-catalyzed polysulfide production and it can be concluded that iron does not contribute to 1,4-NQ autoxidation.
3. Discussion
3.1. Background
Tarumi et al., 2019 [30] evaluated the rate determining steps in the Takahax process whereby H2S is oxidized to elemental sulfur by 1,4-dihydroxynaphthalene-2-sulfonate (NQSH2). Forty-six possible reactions in the initial H2S oxidation/NQ reduction reaction were identified. The energetically most favorable reaction was a one electron oxidation of H2S, and reduction of NQ (Equation (2)) followed by a one electron oxidation of a second H2S and further reduction of the semiquinone to NQH2 (Equation (4)), with molecular sulfur produced by the combination of the thiyl radicals (Equation (6)). They also predicted that reoxygenation of NQH2 was accomplished by two, sequential one electron oxidations of the NQH2, the first producing superoxide and the second using superoxide as the electron acceptor to produce peroxide (Equations (13) and (15)). Furthermore, they predict that peroxide could substitute for oxygen in Equation (13) to produce the semiquinone and hydroxyl and these products would then react with each other to produce NQ and water. The rate limiting steps in the reactions were the initial oxidation of H2S (Equation (2)) and the initial oxidation of NQH2 (Equation (13)). Consistent with this one electron process, it has also been reported that in the presence of ascorbate and oxygen, 1,4-NQ catalyzes a one electron oxidation of ascorbate and the 1,4-NQ semiquinone is oxidized by oxygen producing superoxide and 1,4-NQ [31], although the authors suggest that this only involves redox cycling between the oxidized quinone and the semiquinone. Our studies clearly show that even in the presence of oxygen reactions with H2S fully reduce 1,4-NQ to 1,4-NQH2.
3.2. Possible Mechanisms of Per- and Polysulfide, Sulfite and Thiosulfate Production by 1,4-NQ Oxidation of H2S
3.2.1. Per- and Polysulfide Production
Previous work in our lab has shown that many polyphenolic nutraceuticals readily oxidize H2S to polysulfides [15,16] and subsequent investigations demonstrated that this can be attributed to the redox active quinone [17,18]. Given the many biologically similar attributes between polyphenols and naphthoquinones, we felt it was important to examine sulfur metabolism by the latter to determine if this was a common theme in many of these nutraceuticals and related quinones and if this could readily explain their biological attributes. These studies also afforded us the opportunity to further investigate the mechanism(s) involved.
The absorption spectra shows that 1,4-NQ is oxidized when obtained from the supplier and that it is initially, and rapidly reduced by H2S, or more likely the hydrosulfide anion, HS− (Figure 6). This could occur by a single two-electron reduction that produces the hydroquinone (NQH2) and sulfane sulfur (S0; Equation (1)), or by two sequential one-electron reductions of 1,4-NQ that initially produce semiquinone (NQ·−) and hydrosulfide radicals (Equation (2)); here indicated as HS·. (Note: the dianion, S·−, may be more appropriate as the pKa appears to be near 4.0 [32]). A second reaction either between the semiquinone and hydrosulfide radicals could produce the hydroquinone and sulfane sulfur (Equation (3)), or a reaction between the semiquinone and a new hydrosulfide anion would produce a second thiyl radical (Equation (4)). The persulfide can be produced by the sulfane reacting with another hydrosulfide anion (Equation (5)), or the combination of two hydrosulfide radicals (Equation (6)). It should be noted that the pKa of persulfide is such that the hydropersulfide anion (HS2−) is more likely [33] and it has been suggested that the autoxidation of NQH2 likely proceeds in two steps starting with the reduced NQ dianion (NQ2−) [12] which is in equilibrium with the hydroquinone, the latter predominating in our experiments at neutral pH. Note also that the pKa for polysulfides becomes progressively lower as the number of sulfur atoms increases [33], but for simplicity they are represented below as the fully protonated species.
| HS− + NQ + H+ → S0 + NQH2 | (1) |
| HS− + NQ → HS∙ + NQ·− | (2) |
| HS· + NQ·− + H+ → S0 + NQH2 | (3) |
| HS− + NQ·− + 2H+ → HS· + NQH2 | (4) |
| S0 + H2S → H2S2 | (5) |
| 2HS· → H2S2 | (6) |
A hydrosulfide anion and hydrosulfide radical can also combine to form a hydropersulfide radical (Equation (7)),
| HS− + HS· → HS2·− + H+ | (7) |
which can combine with a second hydrosulfide radical to produce a polysulfide (Equation (8));
| HS2·− + HS· → H2S3 | (8) |
Alternatively, the persulfide radical could react with molecular oxygen to produce superoxide and the disulfide (Equation (9)).
| HS2·− + O2 + H+ → H2S2 + O2·− | (9) |
Polysulfides (S > 2) can also be produced by multiple reactions between sulfane and a persulfide (Equation (10)),
| nS0 + H2S2 → H2S(n+2) | (10) |
or dimerization of persulfide radicals (Equation (11)),
| 2HS2·− → H2S4 | (11) |
Persulfide radicals may also be produced by reactions of polysulfides with the quinones [34] (Equation (12)),
| H2S2 + NQ → HS2·− + NQ·− + H+ | (12) |
two of which could then form a tetrasulfide or one plus a thiyl radical would form a trisulfide.
Reoxidation of NQH2 could occur via sequential one electron steps with oxygen as the electron acceptor (Equations (13) and (14))
| NQH2 + O2 → NQ·− + O2·− + 2H+ | (13) |
| NQ·− + O2 → NQ + O2·− | (14) |
or superoxide as the electron acceptor in the second reaction (Equation (15)),
| NQ·− + O2·− + 2H+ → NQ + H2O2 | (15) |
Alternatively, this could occur through a single two electron reaction (Equation (16)),
| NQH2 + O2 → NQ + H2O2 | (16) |
although this reaction is spin forbidden and should be extremely slow. Finally, the formation of the semiquinone intermediate can also occur by comproportionation of the quinone and the hydroquinone (Equation (17)),
| NQ + NQH2 → 2NQ·− + 2H+ | (17) |
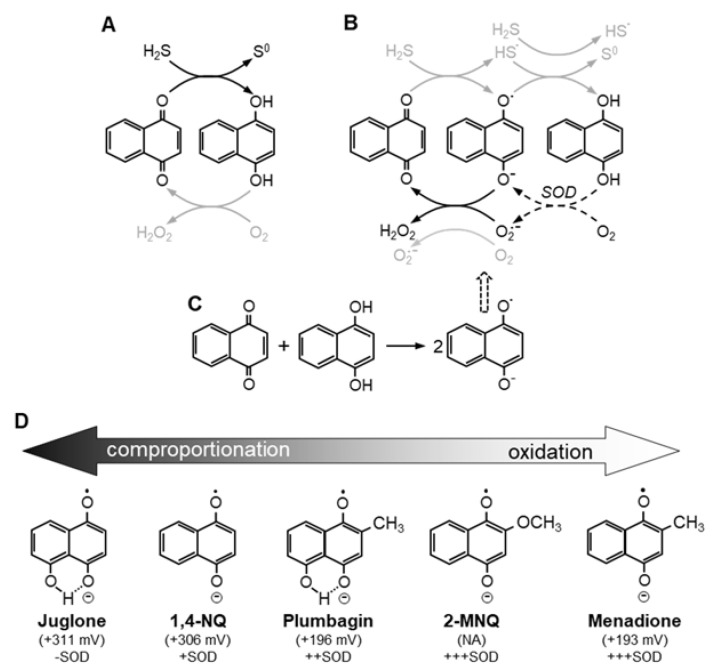
This reaction would serve to bypass the initial oxidation of NQH2 by molecular oxygen (Equation (13)) and thus overcome unfavorable energetics of the first oxidation step. Such a process is supported by our studies of the effects of SOD on SSP4 fluorescence and absorbance spectra in 21% and <1% O2 (Section 2.3 and Section 2.6, respectively). A number of these potential reactions are depicted schematically in Figure 7A–C.
Figure 7.
Proposed two-electron (A) and one-electron (B) reactions of 1,4-NQ with H2S and (C) the comproportionation reaction generating the semiquinone from oxidized and reduced 1,4-NQ. Gray arrows and chemical pathways indicate less favorable reactions, dashed gray arrows show pathway enhanced by SOD-catalyzed removal of superoxide. (D) Potential effects of ring substitutions on autoxidation or comproportionation in the initial one-electron oxidation of naphthohydroquinones. Naphthoquinones that readily comproportionate are minimally affected by SOD. Reduction potentials in in parentheses, -SOD, minimal effect of superoxide dismutase (SOD). +SOD, +++SOD moderate and large effect of SOD, respectively.
3.2.2. Sulfite and Thiosulfate Production
We have observed that, in addition to polysulfides, a variety of quinones oxidize H2S to thiosulfate, sulfite and sulfate [15,16,18]. Although we did not perform an extensive analysis of all possible sulfoxides that could be produced by the NQ/H2S reaction, we show that sulfite, sulfate and thiosulfate are produced.
There are numerous pathways for production of sulfoxides but the details of separate steps in the reactions are not entirely clear [35]. For example, Kleinjan et al. [28] studied the oxidation of polysulfides by oxygen and found production of thiosulfate which they suggest proceeds through an “nascent” elemental sulfur form at pH values less than 9. Steudel et al. [29] described the formation of thiosulfate and elemental sulfur from oxidation by molecular oxygen of polysulfides at pH > 9. At these elevated pH values, they did not see sulfide, sulfate or polythionates.
According to the studies done by Hoffmann [20], H2S is oxidized by hydrogen peroxide to make sulfite and sulfite is oxidized by peroxide to make sulfate. In our experiments superoxide is produced from oxidation of the reduced quinones and semiquinones. The well-known spontaneous dismutation of superoxide into hydrogen peroxide and oxygen will result in increasing concentrations of hydrogen peroxide which will enable the above reactions to proceed to make sulfite. Further reactions with hydrogen peroxide will lead to sulfate (Figure 4D).
Thiosulfate can arise from the reaction of elemental sulfur, derived from the two-electron reduction of quinones by hydrogen sulfide, which can then react with sulfite derived from the above reactions (Equation (18)),
| S + SO32− → S2O32− | (18) |
Sulfenic acid may be an important intermediate and can be formed from two possible reactions in our experiments (Equations (19) and (20)),
| H2S + H2O2 → HSOH + H2O | (19) |
| S + H2O → HSOH | (20) |
The sulfenic acid can react with hydrogen peroxide to give sulfoxylic acid (Equation (21)),
| HSOH + H2O2 → HOSO− + H2O + H+ | (21) |
followed by a second reaction with sulfenic acid to make thiosulfoxylic acid (Equation (22)),
| HSOH + HOSO− → HOS2O− + H2O | (22) |
Thiosulfoxylic acid can react with hydrogen peroxide to make thiosulfate (Equation (23)),
| HOS2O− + H2O2 → S2O32− + H2O + H+ | (23) |
In addition, under alkaline conditions sulfoxylic acid can react with either superoxide or hydrogen peroxide to give the sulfur dioxide anion radical which can react with itself reversibly to give dithionite [35].
3.2.3. Proposed Mechanism of 1,4-NQ Oxidization of H2S to Produce Polysulfides and Elemental Sulfur
Our experimental evidence suggests that the primary reactions are somewhat different from those offered by Tarumi et al. [30]. We propose that the initial reaction is a one-step, two-electron reduction of 1,4-NQ and concomitant oxidation of H2S to sulfane sulfur (S0) as shown in Equation (1). One-electron reduction potentials for 1,4-NQ [36] are −140 mV (NQ/NQ·−) and +306 mV (NQ·−/NQH2), whereas the one-electron reduction potential for HS−/HS +920 mV [37], making either one-electron reaction unlikely (see also Table 2). Furthermore, our spectral analysis shows that 1,4-NQ is rapidly and fully reduced to the hydroquinone (Figure 6 and Supplemental Figure S7) and this would be difficult to achieve from a semiquinone intermediate. This suggests that the NQ does not redox cycle between the oxidized NQ and the semiquinone to oxidize H2S. Conversely, the two-electron reduction potential for 1,4-NQ is +83 mV [36] and the two-electron reduction potential for sulfur/hydrosulfide (S0/HS−) is −170 millivolts [38], both of which are quite favorable. Furthermore, S0 would readily react with H2Sn (where n = 1–5) to produce the variety of polysulfides we observed with mass spectrometry (Figure 2).
Table 2.
Reduction potentials and relative toxicity (LC50) of naphthoquinones used in this study.
| NQ | pKa NQ·−, QH2 |
Q/NQ·− | NQ·−/QH2 | Q/QH2 | LC50 (μM) 1 | Superoxide Formation 1 |
|---|---|---|---|---|---|---|
| 1,4-NQ (2) | 4.1, 9.4 | −140 a | +306 | +83 | 15 | ↑ |
| Juglone (2,3,5) | 6.6 | −93, −95 | +311 | +109, +82 | 6 | ↑ |
| Plumbagin (3) | −156 | +196 | +20 | 5 | ↑↑ | |
| Lawsone (2,5) | 4.7, 8.7 | −415 b | +135 | −140, −86 | >100 | →/↑ |
| Vit K1 (4)
(phylloquinone) |
−78 | |||||
| Vit K2
(menaquinone) |
−70 | |||||
| Vit K3 (menadione) (3,5) | 9.6 | −203, −335 | +193 | −5, +20 | 40 | ↑↑↑ |
| Benzoquinone (3) | +99 | +473 | +286 | |||
| 2-methoxy 1,4-NQ (3) | 9.4 | −39 | ||||
| Anthraquinone | −445 |
1 Ref. [11], 2 Ref. [36] and references therein, 3 Ref. [12] and references therein, 4 Ref. [39], 5 Ref. [40] and references therein as half-wave potentials relative to NHE, pKa from [36,40]. Up arrows indicate degree of increase, horizontal arrow indicates no change. a, [40]; b, addition of corresponding oxidized NQ.
3.2.4. Autoxidation of 1,4-NQH2
We also suggest that reoxygenation of fully reduced 1,4-NQH2 is different from that proposed by Tarumi et al., 2019 [30]. Once 1,4-NQ is reduced by H2S (HS−) it must be reoxidized, presumably by oxygen. A two-electron oxidation of the reduced naphthoquinones by molecular oxygen is spin-forbidden [41] suggesting that reoxidation proceeds via one electron oxidation steps and goes through semiquinones as intermediates. This process could proceed by either of two steps (or both). The first involves production of the semiquinone by oxidation of the reduced quinone by molecular oxygen to yield superoxide and the semiquinone, in the second, the semiquinone is produced by comproportionation of an oxidized and reduced naphthoquinone. The one-electron reduction potentials for NQ/NQ·− and NQ·−/NQH2 are −140 mV and +306 mV, respectively, [12,41], so it would initially appear that is less likely that a substantial fraction of 1,4-NQH2 is initially oxidized by oxygen as only the second one-electron reduction mid-point potential for 1,4-NQ, is favored for autoxidation. However, although, reaction between 1,4-NQH2 with O2 as a one-electron oxidation of QH2, i.e., NQH2 +e → NQ·− + O2·− + 2H+ is also spin restricted [12,41], Song and Beutner [12] show that SOD increases autoxidation of 1,4-NQ. They [12] propose that this reaction (Equation (13)) can occur if the superoxide product is rapidly removed. Our observations that SOD increases polysulfide production (Figure 3) and oxygen consumption (Supplemental Figure S5) supports this argument. However, we propose that the oxidation of 1,4-NQ to a semiquinone is also accomplished by comproportionation of 1,4-NQ and 1,4-NQH2 the former being present in the initial stock from the supplier, and the latter having been formed by two-electron reduction of 1,4-NQ by H2S. Either way, once the semiquinone is produced it then reacts with molecular oxygen to produce 1,4-NQ and superoxide. Further discussion of the autoxidation processes of 1,4-NQ and other NQs used in this study are described in Section 3.4.
The loss of the 246/252 nm absorbance peak when 1,4-NQ was exposed to H2S in hypoxia (Figure 6E) indicates that 1,4-NQ is completely (or nearly completely) reduced to 1,4-NQH2 relatively quickly in the absence of another oxidant. That the oxidized 1,4-NQ peak only slowly decreased when H2S was added to 1,4-NQ in 21% oxygen is also indicative of redox cycling of the oxidized and reduced NQ. It is known that the semiquinone of benzoquinone has a strong absorption in the 400–500 nm range when the semiquinone is unionized and that this absorbance shifts to the 200–300 range for the ionized species at pH > 4 [24]. If the same effects hold for naphthoquinones a semiquinone adduct should also appear in the 200–300 nm range, which may be the peak we observed.
3.3. Effects of Superoxide Dismutase and Catalase
As described in the introduction, much of the biological effects of NQs are ascribed to their production of superoxide and hydrogen peroxide through reduction of molecular oxygen. We and others have observed that SOD increases redox cycling of quinones, which suggests that production of superoxide is a rate limiting step in overall reaction [12,18]. This also appears to be the case for NQ (Figure 3 and Figure 4, Supplemental Figure S5).
Regarding per- and polysulfide production, our mass spectrometry analysis (Figure 2E) suggests that persulfide production predominates in the initial reaction and that either longer chain polysulfides are produced in subsequent reactions or that they are a minor component of initial reactions. Furthermore, as SOD increased the amount of both per-/polysulfides and thiosulfate produced, (Figure 2 and Figure 3) it appears that superoxide plays little, if any, direct role in oxidation of H2S or other sulfur compounds.
Although we could not concomitantly examine the effects of Cat on polysulfide production due to Cat interference with the polysulfide-SSP4 reaction, we were able to examine the effects of both SOD and Cat on thiosulfate production (Figure 4C). Here, it was evident that Cat inhibited spontaneous thiosulfate production from H2S, it inhibited thiosulfate production in the H2S/1,4-NQ reaction, and it prevented SOD augmentation of thiosulfate production in the H2S/1,4-NQ reaction. Collectively, this suggests that hydrogen peroxide derived from superoxide dismutation contributes to some of the end products of H2S oxidation catalyzed by 1,4-NQ.
3.4. Effects of Ring Substitutions on the H2S-NQ Reaction
The two primary factors determining the reactivity of NQs are its capacity to produce free radicals and its electrophilicity [36]. Free radical production by napthoquinones depends primarily on a reduction potential that is high enough to allow efficient reduction by suitable substrates but not so high that it impedes autoxidation, electrophilicity is mainly determined by the electron density at the double bond C2-C3 carbons. Stable hydroquinones hinder electrophilic addition because they are not electrophiles. Ollinger and Brunmark [36] mainly considered free radical production by one-electron reduction of the NQ. However, as we assume reduction by H2S is a single two-electron process, free radicals would then be produced by one-electron autoxidation of the hydroquinone. Regardless, they show that the reactions of both 1,4-NQ and juglone are quite favorable, whereas the 2-hydroxyl of lawsone is deprotonated and donates electrons to the quinone ring which hinders reduction of the quinone. Our results affirm that lawsone is not an effective catalyst for H2S oxidation,
It is clear from the present experiments that substitutions on the parent 1,4-NQ variously affect H2S metabolism. These substitutions could potentially affect either the oxidation of H2S or re-oxidation of the NQ. While a detailed examination of these factors was beyond the scope of this study, evidence points to the reduction potentials of H2S and the NQs and comproportionation reactions as major contributors.
3.4.1. H2S and Reduction of Substituted NQs
The efficacy of the various H2S/NQ reactions in terms of oxidation of H2S to polysulfides and the attendant oxygen consumption appears to correlate quite well with the two-electron reduction potential of S0/HS− (−170 mV) and a two-electron reduction of the various NQs examined herein. These reactions (in decreasing order) and their reduction potentials (in parentheses) are, 1,4-NQ (+83 mV) ≈ juglone (+109 mV) ≈ plumbagin (+20 mV) > 2-methoxy-1,4-NQ (−39 mV) ≈ menadione (−5 mV, or +20 mV) >> phylloquinone (−78 mV) ≈ menaquinone (−70 mV) ≈ lawsone (−140 mV).
3.4.2. Autoxidation of Substituted NQs
Reoxidation (autoxidation) could also be a contributing factor to the reactivity of variously substituted NQs. In fact, this may be the limiting process, but it is clearly more complicated. As described in Section 3.2.4 for 1,4-NQ, autoxidation is unlikely to be a single, two-electron reaction. Of the two, one-electron reactions, oxidation of the hydroquinone to the semiquinone and oxidation of the semiquinone to the quinone, only the second reaction is favorable. Reduction potentials for the second, oxidation of the semiquinone to the quinone, NQ/NQ·−, are 1,4-NQ (−140 mV), juglone (−93 mV) plumbagin (−156 mV) and menadione (−203 mV). These are very favorable for reactions with superoxide, O2·−/H2O2, (+910 mV), but not for oxygen O2/O2·− (−180 mV; all oxygen potentials from [42]). This leaves the initial one-electron oxidation of the hydroquinone as the probable limiting process for all the NQs examined in the present study.
Reduced NQs can be autoxidized to the semiquinone by oxygen or semiquinones can result from comproportionation in an oxygen-independent process (Figure 7A–C). The relative importance of each process appears to correlate somewhat with the location and type of ring substitution and the effect of SOD (Figure 7D). Autoxidation is problematic for all NQs because this must start with oxygen, O2/O2·− (−180 mV), and this does not favor the initial one-electron oxidation of hydroquinones, i.e., NQ·−/NQH2; 1,4-NQ (+306 mV), juglone (+311 mV), plumbagin (+196 mV) and menadione (+193 mV). SOD provides one way around this as described in Section 3.2.4 by removing one of the reaction products, superoxide. On the other hand, formation of the semiquinone by comproportionation is favored if the NQs readily align. This would be the case for juglone and to a large extent 1,4-NQ. Conversely, we propose that ring substitutions on plumbagin, 2-MNQ and menadione present steric problems for comproportionation. This would lead to an increased dependency on the one-electron autoxidation process which is inherently slow, hence it’s augmentation by SOD.
3.5. Reconciliation with Other Studies
Munday [40] examined NQ redox reactions by measuring oxygen consumption when either SOD or oxidized NQs were added to reduced NQs. He observed that the relative rates of autoxidation were, juglone > lawsone > 2-MNQ > menadione > 1,4-NQ, but even 1,4-NQH2 was more than 50% oxidized in less than 2 min at pH 7.4. Munday also observed that addition of SOD to reduced 1,4-NQ did not significantly affect the rate of oxygen consumption, whereas SOD increased oxygen consumption by 14% when added to juglone. Conversely, when SOD was added to reduced 2-MNQ, menadione and lawsone, oxygen consumption was inhibited by 83%, 84% and 99% respectively. The inhibitory effect of SOD was attributed to inhibiting semiquinone formation by superoxide (Equation (24)),
| NQH2 + O2·− → NQ·− + H2O2 | (24) |
When the corresponding oxidized quinone was added to the reduced quinone, oxygen consumption increased by 159%, 34%, 27% and 17% with 1,4-NQH2, menadione, 2-MNQH2, and lawsone, respectively, but was unaffected with juglone (Table 3). Addition of the oxidized corresponding quinone partially overcame the inhibitory effect of SOD on the autoxidation of 2-MNQ, menadione and lawsone. Munday concluded that, (1) comproportionation was very important in autoxidation of 1,4-naphthohydroquinone, (2) neither comproportionation nor superoxide-driven reactions were significant in the autoxidation of juglone, (3) superoxide was an important chain propagator in the autoxidation of lawsone and comproportionation less important, (4) both chain propagations by superoxide and comproportionation were important in autoxidation of menadione and 2-MNQ.
Table 3.
Comparison of the relative rates of oxygen consumption (O2) during autoxidation of NQs under various conditions from Munday a and this study. Right column shows polysulfide production (SSP4 fluorescence) by SOD with NQ and H2S. a, [40]; b, addition of corresponding oxidized NQ; c, [18]. Up arrows indicate degree of increase, horizontal arrow indicates no change and down arrows indicate degree of decrease.
| Munday a | This Study | ||||||
|---|---|---|---|---|---|---|---|
| SOD (O2) | NQ(ox) (O2) b | H2S (O2) | H2S + SOD (O2) | DTT (O2) |
DTT + SOD (O2) |
H2S + SOD (SSP4) | |
| 1,4-NQ | → | ↑↑↑ | ↑↑↑ | ↑↑ | ↑↑↑ | ↓↑ | ↑ |
| 2-MNQ | ↓↓↓ | ↑↑ | ↑ | ↑ | → | ↑ | ↑↑↑ |
| Juglone | ↑ | → | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑(slight) | → |
| Menadione | ↓↓↓ | ↑↑ | ↑ | ↑ | ↑↑ | ↓ | ↑↑↑ c |
| Lawsone | ↓↓↓ | ↑ | ↑ | →(↑↓) | → | → | → |
Ollinger et al. [43] reduced NQs to hydroquionones with DT-diaphorase and observed that SOD inhibited autoxidation of 1,4-NQ and all NQs with a substitution on the quinonid ring, regardless of whether it was -OH, -CH3, or -OCH3. Conversely, SOD stimulated autoxidation when there was an -OH substitution on the benzene ring and this was unaffected by substitutions on the quinoid ring. They concluded that oxygen was important in the inital, slow oxidation and that superoxide generated in this reaction then rapidly oxidized another hydroquinone, which could be inhibited by SOD.
In the present study we used oxidized NQs and reduced them with either H2S, DTT or ascorbic acid (AA) and then examined subsequent reoxidation by measuring oxygen consumption and our results (Table 3) were quite different from those of Munday [40] or Ollinger et al. [43]. When oxidized NQs were initially reduced with H2S, SOD greatly increased O2 consumption by juglone, plumbagin and 1,4-NQ, and to a lesser extent by menadione, 2-MNQ only slightly increased oxygen consumption (although the rate of oxygen consumption appeared to accelerate near the end of the experiment) and lawsone had a negligible effect (Supplemental Figure S5). By comparison, SOD only modestly increased polysulfide production with H2S and 1,4-NQ, whereas SOD greatly increased polysulfide production H2S and either 2-MNQ or menadione, moderately increased it with plumbagin, but SOD has no effect with juglone or lawsone (Figure 3E). Because SOD did not decrease either polysulfide or thiosulfate production, we assume that the effects of SOD on oxygen consumption were on the reoxidation (autoxidation) of NQs and not on superoxide- or hydrogen peroxide-dependent production of sulfoxides.
Reducing 1,4-NQ with DTT produced a greater increase in oxygen consumption than H2S and an even greater increase that consumed all oxygen by 40 min when DTT was added along with H2S (Supplemental Figure S6A,B, respectively). With either DTT + 1,4-NQ or DTT + 1,4-NQ + H2S, subsequent addition of SOD initially, and transiently decreased O2 consumption. However, DTT did not affect polysulfide production (SSP4 fluorescence) from H2S + 1,4-NQ in the absence of SOD, whereas fluorescence was decreased when SOD was added (Supplemental Figure S6C). This suggests that DTT effectively promoted autoxidation of the 1,4-NQ by comproportionation, or that with NQ in the reaction mixture, DTT oxidation competes with sulfide oxidation in the presence of SOD and, since DTT cannot make polysulfides, the polysulfide yield drops. Adding ascorbic acid to reduce 1,4-NQ greatly increased oxygen consumption, either without or with H2S, and in both cases SOD had no effect (Supplemental Figure S6D,E). This is also suggestive of a comproportionation reaction, although we cannot rule out the possibility that superoxide may also be scavenged by DTT and ascorbic acid.
As pointed out by Munday [40] “the mechanism of hydroquinone autoxidation is complex” and by Song and Buettner [12], just because a reaction is “not thermodynamically favorable” does not mean it will not occur. Clearly, other factors such as substrate concentration, pH, solubility, and interactions with non-polar solvents used to dissolve certain NQs, can have substantial effects on these reactions that remain to be fully characterized. Nevertheless, it is interesting that although we did not find evidence to support superoxide as a chain propagator (none of our reactions were inhibited by SOD) and our oxygen consumption results were unlike those of Munday, our conclusions on the relative importance between the initial one-electron autoxidation and comproportionation for the various NQs were generally quite similar.
3.6. Significance of Reactive Sulfur Species
Most of the purported health and medicinal benefits of naphthoquinones have been attributed to either pro- or antioxidant effects and these are generally believed to be mediated through modulation of ROS. Here, we provide an alternative mechanism whereby NQs affect metabolism of reactive sulfur species (RSS). The similarities, differences, and advantages of RSS over ROS, especially in terms of biological systems, has been reviewed in detail [44] and are only briefly summarized here. Both oxygen and sulfur are chalcogens with six valence electrons. As sulfur is bigger than oxygen, sulfur’s electrons are farther from the nucleus and being more facile, sulfur will form more varied and stable reaction products. Most biological activity of ROS is attributed to peroxide (H2O2) which is similar to persulfide (H2S2), the former the result of a two-electron reduction of molecular oxygen and the latter resulting from two-electron oxidation of 2H2S. Both H2O2 and H2S2 are oxidants but H2S2 is also an effective reductant as its pKa is in the physiological range (permitting formation of nucleophilic HS2−) whereas the pKa for H2O2 is well above physiological limits. H2O is difficult to oxidize and relatively inert from a biological standpoint, whereas H2S is not only readily oxidized to other RSS, it also donates electrons to the electron transport chain as a source of energy production and it also contributes to mitochondrial stability. SOD, Cat, and the peroxiredoxin, thiorexodin and glutaredoxin systems, i.e., the endogenous antioxidant defense mechanisms, all metabolize RSS to other biologically active forms of sulfur, but unlike ROS, they do not always irreversibly inactivate RSS. Both H2O2 and H2S2 signal via cysteines on regulatory proteins and the effector responses are identical, however, excessive H2S2 is reversible but excessive H2O2 is not. Activation of the Keap1-Nrf2 axis by oxidation of Keap1 with H2O2 (sulfenylation) or persulfidation with H2S2 is arguably the best example of the duality of these systems and it contributes to the difficulty in resolving the issue of the relative contributions of ROS and RSS in health and disease. We propose that sorting out the relative roles of these two ‘reactive species’ in cellular biology and signaling can help explain the conundrum of many effector pathways activated by NQs. The work presented here illustrates the efficacy of NQ in sulfur metabolism and we suggest that this can explain some, if not much of the therapeutic effects of NQ and related nutraceuticals.
3.7. Summary
In summary we have demonstrated that naphthoquinones (NQs) can catalytically produce polysulfides and sulfoxides from hydrogen sulfide by consuming oxygen in a cyclic manner. The polysulfides produced by these reactions are mixtures of different chain lengths of sulfur with the disulfide as the predominate species. NQ-mediated oxidation of H2S is increased when SOD is present and decreased when Cat is present implicating superoxide formation in the oxidation of the semiquinone as a rate limiting step, and hydrogen peroxide, derived from superoxide dismutation, as the important compound further oxidizing polysulfides and elemental sulfur to thiosulfate and sulfite. These findings provide the chemical background for a novel sulfur-based approach to naphthoquinone-directed therapies.
4. Methods and Materials
4.1. H2S and Polysulfide Measurements in Buffer
Fluorophore experiments were performed in 96-well plates and fluorescence was measured with a SpectraMax M5e plate reader (Molecular Devices, Sunnyvale, CA. USA). Compounds were pipetted into 96-well plates and the plates were covered with tape to minimize H2S loss due to volatilization. Excitation/emission (Ex/Em) wavelengths were per manufacturer’s recommendations; 7-azido-4-methylcoumarin (AzMC, 365/450 nm), 3′,6′-Di(O-thiosalicyl)fluorescein (SSP4, 482/515 nm) and dichlorofluorescein (DCF, 500/525 nm). AzMC and SSP4 have been shown to have sufficient specificity relative to other sulfur compounds and reactive oxygen and nitrogen species (ROS and RNS, respectively) to effectively identify H2S (AzMC) and per- and polysulfides (H2S2 and H2Sn where n = 3–7 or RSnH where n > 1 or RSnR = where n > 2) [45,46,47]. As both AzMC and SSP4 are irreversible, they provide a cumulative record of H2S and polysulfide production, but they do not reflect cellular concentrations at any specific time.
4.2. Oxygen Dependency of Naphthoquinone Reactions with H2S in Buffer
H2S oxidation by quinones consumes O2 [17] and it is likely that NQs do the same. To verify this, O2 tension was monitored in a stirred 1 mL water-jacketed chamber at room temperature with a FireStingO2 oxygen sensing system (Pyroscience Sensor Technology, Aachen, Germany) using a non-oxygen consuming 3 mm diameter OXROB10 fiberoptic probe. The probe was calibrated with 21% O2 (room air) or nitrogen gas (0% O2). H2S, NQs and other compounds of interest were added at timed intervals and percent O2 (100% equals room air) was measured at timed intervals (usually every 0.2–0.3 s) for at least 55 min.
In other experiments the requirement for O2 in NQ-catalyzed reactions was examined by conducting parallel experiments in 96 well-plates in the presence (room air) or absence of O2, the latter achieved by bubbling phosphate-buffered saline (PBS) with N2 gas and conducting the experiments in a model 856-HYPO hypoxia chamber (Plas Labs, Inc. Lansing, MI) set to 0% O2 which effectively lowers O2 to <1%.
4.3. Thiosulfate Production; AgNP Method
Thiosulfate was measured using silver nanoparticles (AgNP) as previously described [48]. Briefly, AgNPs were prepared by reducing AgNO3 with tannic acid (TA) in the presence of HAuCl4. One mL of 20 mM AgNO3 was mixed with 200 μL of 0.5 mM in 98 mL of Milli-Q water at room temperature. One mL of 5.0 mM TA was added, and the mixture was vigorously stirred, turning yellow within 30 min. The AgNPs were stored at 4 °C until use.
For thiosulfate measurements H2S and NQs were placed in 96 well plates and the plates were covered with tape for 60 min to minimize H2S volatilization during the reaction, the tape was then removed to allow excess H2S to dissipate for an additional 60 min. Thirty μL of the buffer was then added to 200 μL of the AgNP in 96-well plates and absorbance measured after 60 min at 419 nm. Thiosulfate standards were made in either buffer or cell medium and the concentrations were plotted against (A0 − A)/A0 where A0 and are the absorbances of AgNPs without and with thiosulfate, respectively. We note that the standard curve is non-linear but reproducible.
4.4. Thiosulfate and Sulfite Production; HPLC-MBB Method
Although the AgNP method was useful for most measurements, we noted some interference with other reactions. To confirm our findings, thiosulfate was also measured using high performance liquid chromatography (HPLC, UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) with fluorescence detection (Ultimate FLD-3100 fluorescence detector, Thermo Fisher Scientific) after derivatization with monobromobimane (MBB) as described previously [49,50]. Briefly, 300 μM H2S (as Na2S) was dissolved in PBS, 10 μM 1,4-NQ was added and allowed to react for 60 min after which it opened for 1 h to remove any residual H2S by volatilization. A 50 μL aliquot was then mixed with 5 μL of 25 mM MBB in acetonitrile and incubated in the dark at room temperature for 60 min to convert thiosulfate and sulfite into thiosulfatebimane and sulfitebimane, respectively and subjected to HPLC-fluorescence analysis. A Shim-pack VP-ODS column (5 μm, 4.6 × 150 mm, Shimadzu Scientific, Kyoto, Japan) was used with mobile phases A (MilliQ water containing 0.25% formic acid) and B (methanol containing 0.25% formic acid). Samples (10 μL) were injected onto the column and run with a linear gradient (0–75% B) at a flow rate of 0.8 mL/min and a column temperature of 30 °C. The detector excitation/emission was set to 370/485 nm. Concentrations of thiosulfate and sulfite were determined based on a standard curve prepared from thiosulfate and sulfite standards derivatized with MBB and analyzed on the same day.
4.5. Thiosulfate, Sulfite and Sulfate Measurement by Ion Chromatography (IC)
As neither of the above methods could detect sulfate, we employed ion chromatography (IC) to detect sulfate. Although not as sensitive as the above methods, it also provided information on thiosulfate and sulfite.
A Dionex ICS-5000 Ion Chromatography (IC) System (ThermoFisher, Sunnyvale, CA USA) equipped with a conductivity detector, RFIC+ Eluent Degasser, and an AS-AP Autosampler was used to detect the sulfite, sulfate, and thiosulfate. Separation of ions was carried out on a Dionex™ IonPac™ AS14A IC column (4 × 250 mm) attached with a corresponding AS14A guard column (4 × 50 mm). Conductivity was detected with AERS 500 carbonate electrolytically regenerated suppressor. The suppressor voltage, flow rate, and run-time were set at 43 mA, 1.0 mL/min, and 30 min, respectively. Injection volumes on the AS-AP Autosampler were set to 25 μL for analysis. Instrument control and data reduction were carried out with Chromeleon7.2Bsoftware. Data collection rate and temperature were set at 5 Hz and 30 °C, respectively.
Eluent was prepared by diluting AS14A Eluent Concentrate with Milli-Q water to achieve a final concentration of 0.08 mM sodium carbonate/0.01 mM sodium bicarbonate. Sulfite, sulfate, and thiosulfate standard solutions were prepared with Combined Five Anion Standard diluted with Milli-Q water (1:3 v/v). Sodium thiosulfate (Na2S2O3), sodium sulfite (Na2SO3), and sodium sulfate (Na2SO4) standards were dissolved in Milli-Q water and filtered through a 0.22 μm syringe filter (Foxx Life Sciences, Salem, NH, USA) prior to analysis. NQs (300 μM) were added to 3 mM H2S and allowed to react in closed Eppendorf tubes for 1.5 h at room temperature. The caps were then opened for another 1.5 h to allow the H2S to volatize off and stop the reactions. The samples were filtered through a 0.22 μm syringe filter and collected for IC analysis.
4.6. Absorbance Spectra
Absorbance spectra were measured in either 100 or 200 mM PBS, pH 7.4, with either a Shimadzu UV-2401PC recording spectrophotometer (Shimadzu, Kyoto, Japan) with slit width of 0.5 nm, or an Agilent HP 8453 spectrometer (Agilent Technologies, Santa Clara, CA, USA).
In initial experiments, the spectra of oxidized 1,4-NQ (as supplied from the supplier and oxidized by exposure to room air), 1,4-NQ reduced with hydrogen gas (H2) and 5% palladium on asbestos or reduced after reaction with H2S (as Na2S) followed by sparging for 30 min with N2 gas to remove the dissolved H2S leaving the H2S-reduced 1,4-NQ. Quartz cuvettes with screw tops with septa and needles were used for inlet and outlet gassing with N2 or room air. In other experiments various ratios of H2S and 1,4-NQ were examined in both normoxia (21% O2) and hypoxia (~0% O2) at 6 s intervals over 1800 s. In these experiments an Na2S stock solution prepared in buffer was made anaerobic by N2 gas replacement.
4.7. Mass Spectrometry
4.7.1. LC-ESI-HRMS Protocol
Inorganic polysulfides were identified using liquid chromatography electrospray ionization high resolution mass spectrometry (LC-ESI-HRMS) using a micrOTOF-Q II Mass Spectrometer (Bruker Daltronics, Billerica, MA, USA) coupled to an UltiMate 3000 (Thermo Fisher) UHPLC system, as described previously [18]. A Waters Acquity UPLC HSS T3 column (1.8 µm, 150 mm × 2.1 mm inner diameter) was used with mobile phases A (water containing 0.1% formic acid) and B (acetonitrile containing 0.1% formic acid). Samples were diluted 10-fold in water and 20 µL was injected with a linear gradient (0–90% B, 30 min) at a flow rate of 0.4 mL/min. The mass spectrometer was used in the positive ion mode with the capillary voltage set to 2200 V and drying gas set to 8.0 L/min at 180 °C. The IAM polysulfide adducts were detected as the [M + Na]+ ion using their exact masses ±0.002 m/z: S1 (149.038, 171.020), S2 (181.010, 202.992), S3 (212.982, 234.964), S4 (244.954, 266.936), S5 (276.926, 298.908), S6 (308.898, 330.880). In these experiments, H2S (1 mM, as Na2S) was incubated in 10 mM phosphate buffer with 1 mM 1,4-NQ for 10 min, derivatized with 5 mM IAM for 30 min, and subjected to LC-ESI-HRMS within 1–2 h.
4.7.2. Justification for IAM Derivatization
In preliminary studies, H2S or K2Sx (mixture of polysulfides) were derivatized with either iodoacetamide (IAM) or tyrosine methyl ester-iodoacetamide (TME-IAM), the latter having been recently reported to be far superior to IAM and to provide exceptional stability to derivatized polysulfides [51]. IAM or TME-IAM was added to Na2S or K2Sn at 0, 10, 30 and 60 min, analyzed by LC-ESI-HRMS and the area under the curve (AUC) compared. We expected that if derivatization was rapid and the samples were stable then the AUC should be similar, if the derivatization was slower, then the AUC should increase and then plateau. In either case the TME-IAM samples would be superior to the IAM derivatized samples. However, as shown in Supplemental Figure S1A,B, the AUC for the TME-IAM samples rapidly decreased over time, whereas the IAM derivatized samples did not. We then sparged the buffer with nitrogen to determine if the decrease in AUC in the TME-IAM samples was due to oxidation of polysulfides but this did not appear to be the case (Supplemental Figure S1C). It was evident that IAM derivatized inorganic sulfur samples were considerably more stable than those derivatized with TME-IAM (Supplemental Figure S1) and all subsequent studies employed IAM derivatization. It should be noted that while the study by Kasamatsu et al. [51] demonstrated the stability of TME-IAM-derivatized organic sulfur compounds, they did not evaluate the stability of inorganic compounds. In studies currently in progress we also found that the TME-IAM was superior for organic polysulfides.
4.8. Chemicals
SSP4 was purchased from Dojindo molecular Technologies Inc. (Rockville, MD, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or ThermoFisher Scientific (Grand Island, NY, USA). ‘H2S’ is used throughout to denote the total sulfide (sum of H2S + HS−) derived from Na2S as S2− most likely does not exist under these conditions [52]. Phosphate buffered saline (PBS; in mM): 137 NaCl, 2.7 KCl, 8 Na2HPO4, 2 NaH2PO4. Phosphate buffer for absorbance measurements (PB; in mM): 200 Na2PO4. pH was adjusted with 10 mM HCl or NaOH to pH 7.4.
4.9. Statistical Analysis
Data was analyzed and graphed using QuatroPro (Corel Corporation, Ottawa, ON, Canada) and SigmaPlot 13.0 (Systat Software, Inc., San Jose, CA, USA). Statistical significance was determined with Students t-test or one-way ANOVA and the Holm-Sidak test for multiple comparisons as appropriate using SigmaStat (Systat Software, San Jose, CA, USA). Results are given as mean + SE; significance was assumed when p < 0.05.
Acknowledgments
The authors gratefully acknowledge Mijoon Lee and Nonka Sevova at the University of Notre Dame Mass Spectrometry and Proteomics Facility for excellent technical assistance. The High-performance liquid chromatography (HPLC) and Ion chromatography (IC) analyses were conducted at the Center for Environmental Science and Technology (CEST) at University of Notre Dame with the help of Jon Loftus.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113293/s1.
Author Contributions
Conceptualization, K.R.O.; Methodology, K.J.C., Z.M. and G.W.; Formal Analysis, K.R.O., K.J.C., P.J.D., Y.G., Z.M., N.M.C., A.F., D.J.G., S.M.K., K.N., C.L.V., I.N., C.J.P., E.P., B.P.V., T.A.K., G.W. and K.D.S.; Investigation, K.J.C., P.J.D., Y.G., Z.M., N.M.C., A.F., D.J.G., S.M.K., K.N., C.L.V., I.N., C.J.P., E.P., B.P.V., G.W. and K.D.S.; Resources, K.R.O.; Writing—Original Draft Preparation, K.R.O.; Writing—Review and Editing, K.R.O., K.J.C., P.J.D., Z.M., T.A.K., G.W. and K.D.S.; Supervision, K.R.O.; Funding Acquisition, K.R.O., P.J.D., T.A.K. and K.D.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
TAK is an officer and shareholder in Gerenox Inc. The authors have no other conflict of interest.
Funding Statement
This research was supported in part by National Science Foundation Grant NO. IOS2012106 (KRO), National Institute of Health Grant NO R01NS094535 (TAK, PJD), Welch Foundation Grant BE-0048 (TAK) and Biomedical Research Foundation at Central Arkansas Veteran’s Healthcare System (KDS).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanchez-Cruz P., Santos A., Diaz S., Alegria A.E. Metal-independent reduction of hydrogen peroxide by semiquinones. Chem. Res. Toxicol. 2014;27:1380–1386. doi: 10.1021/tx500089x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu H.Y., Wang P.F., Lin H.Y., Tang C.Y., Zhu H.L., Yang Y.H. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem. Biol. Drug Des. 2018;91:681–690. doi: 10.1111/cbdd.13141. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury A.R., Maddy A.J., Egger A.N. Henna as a Hair Dye: A Current Fashion Trend with Ancient Roots. Dermatology. 2019;235:442–444. doi: 10.1159/000500828. [DOI] [PubMed] [Google Scholar]
- 4.Mladěnka P., Macáková K., Kujovská Krčmová L., Javorská L., Mrštná K., Carazo A., Protti M., Remião F., Nováková L. Vitamin K-sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022;80:677–698. doi: 10.1093/nutrit/nuab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad T., Suzuki Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants. 2019;8:91. doi: 10.3390/antiox8040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajalakshmi S., Vyawahare N., Pawar A., Mahaparale P., Chellampillai B. Current development in novel drug delivery systems of bioactive molecule plumbagin. Artif. Cells Nanomed. Biotechnol. 2018;46((Suppl. S1)):209–218. doi: 10.1080/21691401.2017.1417865. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y.T., Li Y., Chu P., Ma X.D., Tang Z.Y., Sun Z.L. Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development. Biomed. Amp. Pharmacother. 2022;148:112785. doi: 10.1016/j.biopha.2022.112785. [DOI] [PubMed] [Google Scholar]
- 8.Yadav A.M., Bagade M.M., Ghumnani S., Raman S., Saha B., Kubatzky K.F., Ashma R. The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts. Biol. Chem. 2022;403:211–229. doi: 10.1515/hsz-2021-0290. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z., Zhang J., Chen L., Guo Q., Yang B., Zhang W., Kang W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. BioMed Res. Int. 2020;2020:1–10. doi: 10.1155/2020/6940953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor N., Kandwal P., Sharma G., Gambhir L. Redox ticklers and beyond: Naphthoquinone repository in the spotlight against inflammation and associated maladies. Pharm. Res. 2021;174:105968. doi: 10.1016/j.phrs.2021.105968. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L.O., Hou X., Jacob C. 1,4-naphthoquinones: From oxidative damage to cellular and inter-cellular signaling. Molecules. 2014;19:14902–14918. doi: 10.3390/molecules190914902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y., Buettner G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goleva T.N., Lyamzaev K.G., Rogov A.G., Khailova L.S., Epremyan K.K., Shumakovich G.P., Domnina L.V., Ivanova O.Y., Marmiy N.V., Zinevich T.V., et al. Mitochondria-targeted 1,4-naphthoquinone (SkQN) is a powerful prooxidant and cytotoxic agent. Biochim. Biophys. Acta Bioenerg. 2020;1861:148210. doi: 10.1016/j.bbabio.2020.148210. [DOI] [PubMed] [Google Scholar]
- 14.Swiderska A., Coney A.M., Alzahrani A.A., Aldossary H.S., Batis N., Ray C.J., Kumar P., Holmes A.P. Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants. 2021;10:840. doi: 10.3390/antiox10060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson K.R., Briggs A., Devireddy M., Iovino N.A., Skora N.C., Whelan J., Villa B.P., Yuan X., Mannam V., Howard S., et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox. Biol. 2020;37:101731. doi: 10.1016/j.redox.2020.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson K.R., Gao Y., Briggs A., Devireddy M., Iovino N.A., Licursi M., Skora N.C., Whelan J., Villa B.P., Straub K.D. “Antioxidant” berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021;165:67–78. doi: 10.1016/j.freeradbiomed.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Olson K.R., Gao Y., Straub K.D. Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. Int. J. Mol. Sci. 2021;22:961. doi: 10.3390/ijms22020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson K.R., Clear K., Derry P., Gao Y., Ma Z., Wu G., Kent T., Straub K.D. Coenzyme Q10 and related quinones oxidize H2S to polysulfides and thiosulfate. Free Radic. Biol. Med. 2022;182:119–131. doi: 10.1016/j.freeradbiomed.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes C., Videira A.J.C., Veloso C.D., Benfeito S., Soares P., Martins J.D., Goncalves B., Duarte J.F.S., Santos A.M.S., Oliveira P.J., et al. Cytotoxicity and Mitochondrial Effects of Phenolic and Quinone-Based Mitochondria-Targeted and Untargeted Antioxidants on Human Neuronal and Hepatic Cell Lines: A Comparative Analysis. Biomolecules. 2021;11:1605. doi: 10.3390/biom11111605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M.R. Kinetics and mechanism of oxidation of hydrogen sulfide by hydrogen peroxide in acidic solution. Environ. Sci. Technol. 1977;13:1406–1414. doi: 10.1021/es60159a014. [DOI] [Google Scholar]
- 21.Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2017;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillbe C.E., Sage F.J., Gutteridge J.M. Commentary: Mannitol: Molecule magnifique or a case of radical misinterpretation? Free Radic. Res. 1996;24:1–7. doi: 10.3109/10715769609087994. [DOI] [PubMed] [Google Scholar]
- 24.Wilson I., Wardman P., Lin T.S., Sartorelli A.C. One-electron reduction of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. J. Med. Chem. 1986;29:1381–1384. doi: 10.1021/jm00158a010. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson M., Kurz T., Brunk U.T., Nilsson S.E., Frennesson C.I. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 26.Chen K.Y., Morris J.C. Kinetics of oxidation of aqueous sulfide by O2. Environ. Sci. Technol. 1972;6:529–537. doi: 10.1021/es60065a008. [DOI] [Google Scholar]
- 27.O’Brien D.J., Birkner F.B. Kinetics of oxygenation of reduced sulfur species in aqueous solution. Environ. Sci. Technol. 1977;11:1114–1120. doi: 10.1021/es60135a009. [DOI] [Google Scholar]
- 28.Kleinjan W.E., de Keizer A., Janssen A.J. Kinetics of the chemical oxidation of polysulfide anions in aqueous solution. Water Res. 2005;39:4093–4100. doi: 10.1016/j.watres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Steudel R., Holdt G., Nagorka R. On the Autoxidation of Aqueous Sodium Polysulfide. Z. Für Nat. B. 1986;41b:1519–1522. doi: 10.1515/znb-1986-1208. [DOI] [Google Scholar]
- 30.Tarumi M., Matsuzaki Y., Suzuki K. Theoretical study on the redox reaction mechanism of quinone compounds in industrial processes. Chem. Eng. Sci. 2019;199:381–387. doi: 10.1016/j.ces.2019.01.006. [DOI] [Google Scholar]
- 31.Roginsky V.A., Barsukova T.K., Bruchelt G., Stegmann H.B. Kinetics of redox interaction between substituted 1,4-benzoquinones and ascorbate under aerobic conditions: Critical phenomena. Free Radic. Res. 1998;29:115–125. doi: 10.1080/10715769800300131. [DOI] [PubMed] [Google Scholar]
- 32.Lykakis I.N., Ferreri C., Chatgilialoglu C. The sulfhydryl radical (HS(.)/S(.-)): A contender for the isomerization of double bonds in membrane lipids. Angew. Chem. Int. Ed. Engl. 2007;46:1914–1916. doi: 10.1002/anie.200604525. [DOI] [PubMed] [Google Scholar]
- 33.Kamyshny A., Jr., Goifman A., Gun J., Rizkov D., Lev O. Equilibrium distribution of polysulfide ions in aqueous solutions at 25 degrees C: A new approach for the study of polysulfides’ equilibria. Environ. Sci. Technol. 2004;38:6633–6644. doi: 10.1021/es049514e. [DOI] [PubMed] [Google Scholar]
- 34.Abiko Y., Nakai Y., Luong N.C., Bianco C.L., Fukuto J.M., Kumagai Y. Interaction of Quinone-Related Electron Acceptors with Hydropersulfide Na2S2: Evidence for One-Electron Reduction Reaction. Chem. Res. Toxicol. 2019;32:551–556. doi: 10.1021/acs.chemrestox.8b00158. [DOI] [PubMed] [Google Scholar]
- 35.Makarov S.V., Horvath A.K., Makarova A.S. Reactivity of Small Oxoacids of Sulfur. Molecules. 2019;24:2768. doi: 10.3390/molecules24152768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ollinger K., Brunmark A. Effect of hydroxy substituent position on 1,4-naphthoquinone toxicity to rat hepatocytes. J. Biol. Chem. 1991;266:21496–21503. doi: 10.1016/S0021-9258(18)54666-4. [DOI] [PubMed] [Google Scholar]
- 37.Das T.N., Huie R.E., Neta P., Padmaja S. Reduction potential of the sulfhydryl radical: Pulse radiolysis andlLaser flash photolysis studies of the formation and reactions of ·SH and HSSH—in aqueous solutions. J. Phys. Chem. A. 1999;103:5221–5226. doi: 10.1021/jp9907544. [DOI] [Google Scholar]
- 38.Collman J.P., Ghosh S., Dey A., Decreau R.A. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl. Acad. Sci. USA. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner G.C., Kassner R.J., Kamen M.D. Redox potentials of certain vitamins K: Implications for a role in sulfite reduction by obligately anaerobic bacteria. Proc. Natl. Acad. Sci. USA. 1974;71:253–256. doi: 10.1073/pnas.71.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munday R. Autoxidation of naphthohydroquinones: Effects of pH, naphthoquinones and superoxide dismutase. Free Radic. Res. 2000;32:245–253. doi: 10.1080/10715760000300251. [DOI] [PubMed] [Google Scholar]
- 41.Roginsky V.A., Barsukova T.K., Stegmann H.B. Kinetics of redox interaction between substituted quinones and ascorbate under aerobic conditions. Chem. Biol. Interact. 1999;121:177–197. doi: 10.1016/S0009-2797(99)00099-X. [DOI] [PubMed] [Google Scholar]
- 42.Koppenol W.H., Stanbury D.M., Bounds P.L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Ollinger K., Buffinton G.D., Ernster L., Cadenas E. Effect of superoxide dismutase on the autoxidation of substituted hydro- and semi-naphthoquinones. Chem. Biol. Interact. 1990;73:53–76. doi: 10.1016/0009-2797(90)90108-Y. [DOI] [PubMed] [Google Scholar]
- 44.Olson K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020;33:1125–1142. doi: 10.1089/ars.2020.8132. [DOI] [PubMed] [Google Scholar]
- 45.Bibli S.I., Luck B., Zukunft S., Wittig J., Chen W., Xian M., Papapetropoulos A., Hu J., Fleming I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018;18:295–304. doi: 10.1016/j.redox.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson K.R., Gao Y. Effects of inhibiting antioxidant pathways on cellular hydrogen sulfide and polysulfide metabolism. Free Radic. Biol. Med. 2019;135:1–14. doi: 10.1016/j.freeradbiomed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Olson K.R., Gao Y., Arif F., Patel S., Yuan X., Mannam V., Howard S., Batinic-Haberle I., Fukuto J., Minnion M., et al. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants. 2019;8:639. doi: 10.3390/antiox8120639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong C., Wang Z., Zhang Y., Ma X., Iqbal M.Z., Miao L., Zhou Z., Shen Z., Wu A. High-Performance Colorimetric Detection of Thiosulfate by Using Silver Nanoparticles for Smartphone-Based Analysis. ACS Sens. 2017;2:1152–1159. doi: 10.1021/acssensors.7b00257. [DOI] [PubMed] [Google Scholar]
- 49.Ran M., Wang T., Shao M., Chen Z., Liu H., Xia Y., Xun L. Sensitive Method for Reliable Quantification of Sulfane Sulfur in Biological Samples. Anal. Chem. 2019;91:11981–11986. doi: 10.1021/acs.analchem.9b02875. [DOI] [PubMed] [Google Scholar]
- 50.Togawa T., Ogawa M., Nawata M., Ogasawara Y., Kawanabe K., Tanabe S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem. Pharm. Bull. 1992;40:3000–3004. doi: 10.1248/cpb.40.3000. [DOI] [PubMed] [Google Scholar]
- 51.Kasamatsu S., Ida T., Koga T., Asada K., Motohashi H., Ihara H., Akaike T. High-Precision Sulfur Metabolomics Innovated by a New Specific Probe for Trapping Reactive Sulfur Species. Antioxid. Redox Signal. 2021;34:1407–1419. doi: 10.1089/ars.2020.8073. [DOI] [PubMed] [Google Scholar]
- 52.May P.M., Batka D., Hefter G., Konigsberger E., Rowland D. Goodbye to S(2-) in aqueous solution. Chem. Commun. 2018;54:1980–1983. doi: 10.1039/C8CC00187A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Material.