Abstract
Understanding the role surface proteins play in the interaction of group A streptococci with epithelial cells is an important step toward the development of new strategies to fight infections. Fibronectin-binding proteins in streptococci and staphylococci have been described as important mediators for adherence to eukaryotic cells. In the present study we describe a new Streptococcus pyogenes fibronectin-binding protein (PFBP). The gene encoding the PFBP protein (pfbp) was identified from an M12 strain genomic library. It encodes a protein of 127.4 kDa which contains the LPXTGX motif characteristic of cell wall-associated proteins in gram-positive organisms and is among the largest surface molecules described for group A streptococci. The pfbp gene is transcribed during cell growth and was present in several class I and II streptococcal strains tested. The deduced amino acid sequence of PFBP exhibits a variable N-terminal region and a conserved C-terminal region when compared to most fibronectin-binding proteins identified from other gram-positive bacteria. The N-terminal region presents a stretch of 105 amino acids with no homology with N-terminal regions of previously described fibronectin-binding molecules, while the C-terminal region contains three repeat domains that share significant similarity with the repeat regions of fibronectin-binding proteins from S. pyogenes, S. dysgalactiae, and S. equisimilis. The PFBP repeated region, when expressed on the surface of S. gordonii, a commensal organism, binds to soluble and immobilized fibronectin. This study also shows that, in addition to pfbp, a second gene homologous with that of protein F1 (which also codes for a fibronectin-binding protein) is transcribed during cell growth in the same S. pyogenes strain.
One of the early events in the infectious process is the attachment of bacteria to epithelial cells, possibly through surface proteins that serve as adhesins. The way in which such proteins interact with eukaryotic cells is still unclear, but the binding to proteins of the extracellular matrix and body fluids, such as fibronectin and fibrinogen, has been shown to be important in different bacterial genera such as the streptococci, staphylococci, mycobacteria, and porphyromonads (3, 5–8, 10, 12–19, 23, 24, 27, 32, 34, 35). As many as 10 fibronectin-binding proteins have been described in streptococci and 3 more have been described in staphylococci (3, 5, 6, 8, 10, 12–16, 18, 24, 27, 34). Protein F1, a fibronectin-binding protein from group A streptococci, has proven to be important in adherence to respiratory cells (6, 7), and many other fibronectin-binding proteins (e.g., FBP54, F2, SfbII, SfbI, FnAA, FnBB, FnB, FNZ, and GfbA) are able to competitively inhibit the binding of fibronectin to S. pyogenes, S. dysgalactiae, S. equisimilis, and group G streptococci (3, 8, 12–16, 34). The structure of most of these proteins is basically composed of a variable N-terminal region and a conserved C-terminal region. This C-terminal portion usually contains a 37- to 48-amino-acid repeat (repeated three to five times), which is the domain responsible for the fibronectin-binding activity. For protein F1 and F2, a nonrepeated domain localized just upstream of the repeated region has been reported to bind fibronectin with a higher affinity than the repeated region (8, 23, 32). For most of these proteins, the N-terminal domain has an unknown function; however, recent reports describe a fibrinogen-binding activity for this portion of protein F1 and an enzymatic function (e.g., cleavage of Apo A-1 of high-density lipoprotein) for streptococcal serum opacity factor (SOF) (11, 27). With two exceptions (3, 24), all of the streptococcal and staphylococcal fibronectin-binding proteins display the LPXTGX motif responsible for the cell wall anchorage in gram-positive bacteria (31), followed by C-terminal transmembrane and cytoplasmic domains.
Group A streptococcal strains can be classified as class I and II M types based on their reactivity with monoclonal antibodies that recognize epitopes in the conserved region of M proteins (1). In addition, class II strains produce an opacity factor which turns serum opalescent (27). The presence of fibronectin-binding proteins has been demonstrated in a wide range of different class I and II M-type strains of streptococci (13, 20), although there is no correlation between a specific streptococcal type or class and the presence or absence of a specific fibronectin-binding protein. Similarly, there are few reports of the expression of more than one fibronectin-binding protein in a given strain. In the present study we identify and characterize a new group A streptococcal fibronectin-binding protein, derived from an M12 strain, which has been named PFBP (for S. pyogenes fibronectin-binding protein). This protein exhibits significant similarity with the recently described F2 protein from S. pyogenes (8), as well as with the fibronectin-binding protein B (FnBB) from S. dysgalactiae (14) at the C-terminal end. On the other hand, the N-terminal region of the molecule exhibits a unique sequence of 105 amino acids which presents no homology with other described fibronectin-binding proteins. PFBP also contains some sequence homologies with the clumping factor and fibronectin-binding protein B of Staphylococcus aureus (10, 17).
Through recombinant technology, the repeated region of PFBP was expressed on the surface of live S. gordonii and was found to bind fibronectin. Additionally, we demonstrate the transcription of two different fibronectin-binding proteins in the same M12 strain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Nephritogenic M12 S. pyogenes strains A374 and A735 (Rockefeller University collection) were used for the genomic library construction and gene sequence completion, respectively. Strain A735 was also used for all subsequent assays performed. λgt11 (Stratagene, La Jolla, Calif.), propagated in Escherichia coli Y1090, was used as the library expression vector. Plasmid pBluescript II (Stratagene) and E. coli DH5α (Gibco-BRL, Gaithersburg, Md.) were used for DNA sequencing purposes. Plasmid pCR 2.1 (TA Cloning-Invitrogen, Carlsbad, Calif.) was used for cloning PCR-amplified DNA fragments.
Plasmid pSMB104 (Ermr) and S. gordonii GP251 (Cmr) were used for the expression of the PFBP fibronectin-binding domain (22). pSMB104 is an E. coli plasmid that does not replicate in streptococci and is able to integrate into the chromosome of appropriate S. gordonii recipient strains, such as GP251, by homologous recombination. The plasmid carries the Ermr marker and the deleted emm6.1Δ104 gene. GP251 strain is a derivative of GP230 and has been described previously (22). Wild-type S. gordonii GP204 (a spontaneous Smr mutant) (26) was used as control in some experiments. Luria-Bertani (LB) broth or agar (1.5%) was used to grow E. coli strains. Todd-Hewitt broth supplemented with 3% yeast (THY; Difco, Detroit, Mich.) or THY agar (1.5%) and brain heart infusion broth (BHI; Difco) or BHI agar (1.5%) were used to grow streptococcal strains. When appropriate, antibiotics were used at the following concentrations: ampicillin (Amp) at 50 μg/ml for E. coli and erythromycin (Erm) at 5 μg/ml, chloramphenicol (Cm) at 5 μg/ml, and streptomycin (Sm) at 500 μg/ml for S. gordonii.
Antibody to the cell wall-associated region of surface proteins.
To prepare antibodies specific for the cell wall-associated region of surface proteins other than M protein, cell walls were prepared from 4 liters of an 18-h growth of M6(D471) strain as described previously (28), except that a Bead Beater was used to rupture the cells. The cell walls were washed once in 1% sodium dodecyl sulfate for 1 h and then four times in saline (0.8% NaCl) and finally separated into three equal aliquots. The pellet of one aliquot was suspended in phosphate-buffered saline (PBS) (pH 7.4) and digested with pronase (100 μg/ml) for 2 h at 37°C in order to digest any proteins located on the surface of the cell wall. The cell walls were then washed four times in PBS and suspended in saline. This suspension was sonicated (at maximum intensity) for three 1-min intervals in an ice bath to release the cell wall-associated fragments of surface proteins. Then, 1 ml of this material was mixed with an equal volume of Freund’s complete adjuvant, and two rabbits were immunized intradermally at several sites (1 ml of the sonicate was kept frozen at −70°C in 0.5-ml aliquots). The animals were boosted twice, within a 30-day interval, with 0.5 ml of the sonicate in Freund’s incomplete adjuvant and bled 10 days after the second boost.
To remove any antibodies directed to the cell wall-associated region of M protein, 0.5 ml of the antiserum was absorbed with recombinant M6 protein immobilized onto a column of glutardialdehyde-activated glass beads according to the manufacturer’s instructions (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). To remove antibodies reactive to the surface-exposed regions of surface proteins and cell wall carbohydrate, the M protein-absorbed serum was absorbed with whole M(−) streptococci (strain JRS75) (21) by using an equal volume of packed cells and antiserum. All absorptions were performed at 4°C with end-over-end mixing for 2 h. The final serum was unreactive with recombinant M6 protein on Western blot, but it reacted with several other proteins after digestion of the cell wall with phage lysin.
Genomic library construction and screening.
Genomic DNA from strain M12(A374) was mechanically fractionated and subsequently ligated to EcoRI linkers. The fragments were then cloned into the λgt11 vector and propagated in E. coli Y1090. A monospecific rabbit antiserum directed to the cell wall-associated region of the streptococcal M protein (described above) was used for the library screening. The protocol described previously (29) was followed.
DNA sequence determination.
The Sanger dideoxy-chain termination method (30) and automated Taq FS dye terminator fluorescence-cycle sequencing (ABI 377 DNA Sequencer XL upgrade at the Protein/DNA Technology Center at Rockefeller University, New York, N.Y.) were used to determine the sequence of the pfbp gene. For the Sanger method the Sequenase version 2.0 DNA sequencing kit (USB/Amersham) was used. The strategy followed comprised the design of nested primers based on the sequence previously confirmed at least two times. The nucleotide sequence and deduced amino acid sequence were analyzed with the GCG and DNA Star-Lasergene programs.
PCR.
In general, the PCR amplifications were performed with standard concentrations as follows: 200 μM deoxynucleoside triphosphate; 0.2 to 0.4 μM concentrations of each primer; 15 mM MgCl2; and 2.5 U of AmpliTaq polymerase (Perkin-Elmer, Norwalk, Conn.). Annealing temperatures varied depending on the primer set used.
Southern hybridization.
DNA from different M-type strains [M6(D471), M5(1RP144), M14(4RP106), M1(D710), M12(A735), M2(D444), M13(D742), M28R(T28/150A), M58(A774), and M62(A956)] from the Rockefeller University collection was extracted as follows: a phage lysin treatment of the streptococci, in order to generate protoplasts, was performed as described before (24), followed by lysis of the protoplasts and genomic DNA purification by standard protocols (29). The DNAs were subsequently digested with EcoRI, separated by agarose gel electrophoresis, and then transferred to Hybond-N or Hybond-N(+) membranes (Amersham). Two different probes were used for this assay, the PCR-amplified pfbp gene and a PCR fragment of 235 bp (P235) corresponding to the first 78 amino acids of the mature protein. The primers used for the amplification of the pfbp gene were 5′-CTGTCGCTTATGAATGTG-3′ and 5′-CGCGAATTCACTCTTTGGCTTATCTTTTTTAGG-3′ and for P235 were 5′-GGAAACAAGAAATGGAGCAAA-3′ and 5′-TCCGGCTGGTTTTATCATA-3′ (forward and reverse primers, respectively, in each case). The probe corresponding to the whole gene was labeled with [α-32P]dATP (Amersham) by random labeling (Ready-to-Go; Pharmacia, Uppsala, Sweden), and the P235 was labeled by enhanced chemiluminescence direct nucleic acid labeling (Amersham). Hybridizations were performed under stringent conditions and revealed by autoradiography with X-Omat AR films (Eastman Kodak Co., Rochester, N.Y.) in the presence of intensifying screens.
Northern hybridization.
Total RNA from M12(A735) and M6(D471) strains was extracted as previously described (2). For the M12 strain the extraction was performed at different stages during cell growth (3, 4, and 5 h [early-, mid-, and late-log phases, respectively]) at 37°C. The RNAs (10 μg) were separated by formaldehyde-agarose gel electrophoresis and transferred to a nylon (+) membrane (MSI, Westboro, Mass.) by using the Turboblotter system (Schleicher & Schuell, Inc., Keene, N.H.). For the hybridization, the PCR-amplified pfbp was used as a probe. The primers used for the pfbp gene amplification were the same as those used for the Southern hybridization.
Similarly, for the M6 strain assay, the extraction was done after 5 h (mid-log phase) of growth at 37°C, and the PCR-amplified prtF1 gene was used as a probe for the hybridization. The primers used for the prtF1-like amplification were 5′-ATGTTTGTGAGAGGAGAGAA-3′ (designed for this study) and 5′-CAATATCTCCTGTTGCAGGAAG-3′ (12) as the forward and reverse primers, respectively.
For both Northern assays the probes were labeled with [α-32P]dATP (Amersham) as described for the Southern assay. Standard hybridization protocols were followed.
Cloning and expression in S. gordonii of the PFBP repeated region.
The repeated region of PFBP (amino acids 998 to 1108) was amplified by PCR by using 5′-CCCGGATCCACTAAGGGTTCAGGTCAGGTT-3′ as the forward primer and the reverse primer described above for the Southern hybridization. The PCR DNA fragment (390 bp) was then cloned in frame, as confirmed by sequencing, with the emm6.1Δ104 gene cassette in the pSMB104 plasmid. The recombinant plasmid was then introduced into competent S. gordonii GP251 by natural transformation as described before (25, 26). Briefly, frozen competent GP251 cells (0.45 ml) were thawed (at 37°C), mixed with 0.5 μg of recombinant plasmid, and incubated at 37°C for 45 min. After the incubation, DNase I (10 μg/ml) and Mg2+ (10 mM) were added to the cells. The mixture was then added to melted BHI agar-blood (5%) and plated on a BHI agar plate, followed by incubation at 37°C for 90 min. After this period, a layer of melted BHI agar containing the antibiotic (Erm, 5 μg/ml) was added. The plates were incubated at 37°C for 48 h. Recombinant colonies were selected based on their Ermr phenotype.
Bacterial dot blot immunoassay.
The bacterial dot blot immunoassay procedure was as described previously (9), and this assay was followed with some modifications. Overnight BHI broth cultures, with the appropriate antibiotic, of recombinant, parental, and wild-type S. gordonii were washed three times with PBS (pH 7.4) containing 0.02% sodium azide. Resuspended cells (in PBS [pH 7.4]) were diluted to an optical density of 0.2 at 650 nm (1-cm cuvette), and 25 μl of the suspension was loaded onto a nitrocellulose filter (Schleicher & Schuell) by using a Bio-Dot Apparatus (Bio-Rad Laboratories, Hercules, Calif.). In addition, 5% bovine serum albumin (BSA) and PBS were used as negative controls. The filter was air dried and then blocked overnight in PBS-Tween (0.05%). After the blocking step, the membrane was probed with a solution of human fibronectin (1 μg/ml) for 2 h at room temperature. The fibronectin-binding property of the recombinant S. gordonii was then assayed by using an anti-fibronectin monoclonal antibody diluted 1:250 in PBS-Tween (0.05%) (human fibronectin and anti-fibronectin antibody; Boehringer Mannheim). The presence of the fusion proteins on the surface of the recombinant S. gordonii was confirmed with an anti-M protein monoclonal antibody (10F5) (9) diluted 1:250 in PBS-Tween. For all purposes, an alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, Mo.) was used for the development reaction according to standard procedures.
Iodination of fibronectin with 125I.
Human fibronectin was iodinated with 125I by the chloramine-T method by using Iodobeads (Pierce Chemical Co., Rockford, Ill.). A 400-μl aliquot of a fibronectin solution (1 mg/ml) was mixed with Iodobeads previously activated with 0.1 M PBS (pH 6.5) and 1 mCi of 125I (NEN, Boston, Mass.) (24), followed by an incubation for 20 min at room temperature. The unbound 125I was removed by using a PD-10 column (Sephadex G-25; Pharmacia) equilibrated with 0.1 M PBS (pH 6.5). Fractions were collected and counted in a gamma counter to determine the specific activity.
Bacterial dot blot [125I]fibronectin-binding assay.
The dot blot was performed as described above. After the blotting, the filter was blocked overnight at room temperature in a solution containing 0.05 M Tris (pH 7.6), 0.15 M NaCl, 0.5% Tween 20, 0.04% NaN3, 1% BSA, and 0.5% gelatin. The hybridization was performed in the same solution, with the 125I-labeled fibronectin (1.1 × 106 cpm/μg), at room temperature for 3.5 h. Then, washes with a 1:1 dilution solution of the blocking buffer containing 0.3 M NaCl were performed at room temperature. The hybridization was visualized by autoradiography with X-Omat AR film (Eastman Kodak) at −70°C by using intensifying screens.
Immunofluorescence.
Recombinant and parental S. gordonii overnight cultures in BHI broth were washed twice with 1 volume of PBS (pH 7.4) containing 0.02% sodium azide. The bacteria were resuspended in PBS (pH 7.4), loaded on precleaned slides, and air dried. The samples were fixed with 4% formaldehyde and then blocked with 3% BSA in PBS. After being washed with PBS the primary monoclonal antibody (10F5 anti-M protein monoclonal antibody) diluted 1:50 in PBS was added, followed by an incubation of 1 h at room temperature in a moist chamber. A fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse immunoglobulin G diluted 1:100 in antibody diluent (Dako, Glostrup, Denmark) was then added to the previously washed slides and reincubated for 1 h at room temperature. The washed and dried slides were analyzed and photographed with a Nikon (Nikon, Inc., Melville, N.Y.) fluorescence microscope.
To test the fibronectin-binding activity of the recombinant S. gordonii, a variation of the above protocol was performed. After the blocking, 100 μl of a 50-μg/ml solution of fibronectin in PBS was added to the samples for 1 h at room temperature. Then, an anti-fibronectin monoclonal antibody diluted 1:100 in PBS was added, followed by an incubation of 1 h at room temperature. The same conjugate was used to visualize the reaction under the fluorescence microscope. Three different controls were included to assure the specificity of the reaction: (i) no fibronectin, with only anti-fibronectin monoclonal antibody and conjugate; (ii) fibronectin and conjugate, but with no anti-fibronectin monoclonal antibody; and (iii) no fibronectin or anti-fibronectin monoclonal antibody, but with only conjugate.
Immobilized fibronectin-binding assay.
Human fibronectin (100 μl) at 50 and 25 μg/ml was immobilized on a nitrocellulose filter (Schleicher & Schuell). The filter was then blocked with 3% BSA in PBS for 1 h at room temperature. Strips of the nitrocellulose membrane were incubated either with the recombinant or with the parental S. gordonii strain, processed as described before for the bacterial dot blots, for 1 h at 37°C. A solution of 3% BSA was used as a negative control. The strips were then washed with PBS-Tween (0.05%), followed by a 1-h incubation at room temperature with the 10F5 anti-M protein monoclonal antibody diluted 1:250 in PBS-Tween (0.05%). An anti-mouse conjugate coupled to alkaline phosphatase (Sigma) was used for the development reaction.
Nucleotide sequence accession number.
The nucleotide and deduced amino acid sequences of the pfbp gene have been deposited in the GenBank database under accession number AF071083.
RESULTS
Identification and characterization of pfbp.
The pfbp gene was first identified after screening a genomic library from an S. pyogenes M12(A374) strain. By using a monospecific rabbit antiserum directed to the cell wall-associated region of streptococcal surface proteins, as described in Materials and Methods, several clones were identified and selected for further study. After five rounds of purification and insert analysis, one clone was selected (λ3.1) which contained most of the pfbp gene (2.87 kb). Because the A374 strain was no longer viable since the time the original library was constructed, PCR amplifications were performed on genomic DNA from a comparable M12 S. pyogenes strain (A735) in order to complete the sequence. The sequences of the primers used for these amplifications were derived from the sequences of the fibronectin-binding proteins from S. dysgalactiae (FnBB) (14), S. equisimilis (FnB) (15), and S. pyogenes (F2) (8) (data not shown). The pfbp gene was confirmed, by sequencing, to be identical in both M12 strains.
The nucleotide and deduced amino acid sequences of the pfbp gene are shown in Fig. 1. The open reading frame starts with an ATG codon at position 196 and ends with an TAA codon, consisting of 3,486 bp. The ATG codon is preceded by two putative promoter sequences (−35 and −10) and a predicted ribosomal binding site.
FIG. 1.
Complete nucleotide and deduced amino acid sequence of the pfbp gene. The putative promoters (−35 and −10) and ribosomal-binding-site sequences are shown. In the C-terminal region of the sequence, the three repeated regions (R1, R2, and R3) are underlined. The sequence ends with the LPXTGX anchoring motif (dotted underline) and is followed by the hydrophobic domain and charged tail.
The deduced amino acid sequence of the pfbp gene consists of 1,161 amino acids and has a predicted molecular mass of 127.4 kDa for the complete protein. It is mainly composed of polar amino acids (34%) and has a high content of serine (S) (113 of 1,161), threonine (T) (106 of 1,161), and glycine (G) (100 of 1,129) residues. The combined serine-threonine content represents 20% of the total protein amino acid content and, in contrast to other serine-rich fibronectin-binding proteins (e.g., SfbII) (13), PFBP does not contain localized serine-rich domains. The high glycine content distributed throughout the molecule (9%) favors the model of a compact structure.
The N-terminal region of the PFBP molecule has a stretch of about 25 hydrophobic amino acids, which may represent the leader peptide, and exhibits a potential signal peptidase cleavage site at amino acid 37 (36). The C-terminal half of the protein presents a repeated region consisting of three semiconserved repeats of 37 amino acids (R1, R2, and R3) (Fig. 1). While the first two repeats (R1 and R2) are 73% similar in sequence, the third (R3) is only 25% similar to R1 and 36% similar to R2 (CLUSTAL method, DNA Lasergene program). Dendrogram analysis of these repeats suggests that R3 is derived from a different phylogenetic origin (data not shown). The C-terminal end of PFBP contains the sequence LPATGE, a finding which is consistent with the LPXTGX motif necessary for the translocation and anchoring of most surface proteins on gram-positive bacteria (31).
Comparison of PFBP with other related molecules.
The mature protein sequence displays the common arrangement of amino acids previously described for other fibronectin-binding proteins, with a variable N-terminal domain and a conserved C-terminal fibronectin-binding domain (5, 6, 8, 10, 12–16, 27, 34). However, the N-terminal region seems to consist of two different subdomains as determined from the homology, although low (21%), with the fibronectin-binding protein B (10) and fibrinogen-binding protein (clumping factor) from S. aureus (17) (data not shown).
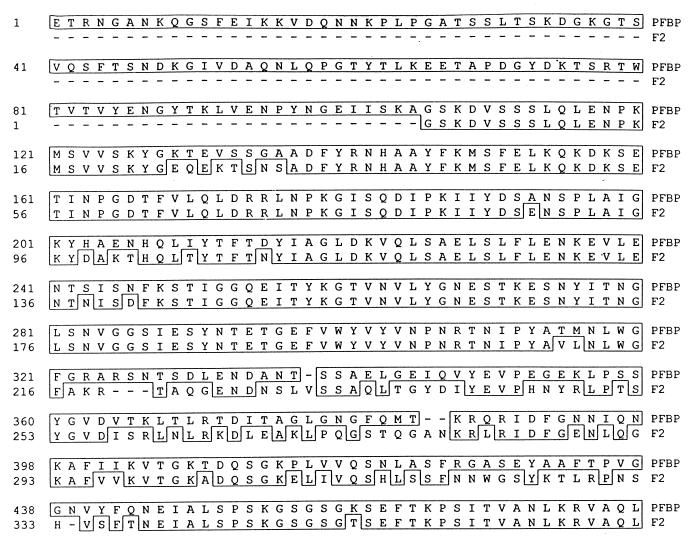
When the complete amino acid sequence of PFBP was compared against the GenBank sequences, an overall 89% sequence identity was found with the complete protein F2 of S. pyogenes recently described (8). However, the high similarity is restricted to the C-terminal region of both molecules (96%) (data not shown), which comprises the repeated region and the upstream fibronectin-binding domain (UFBD) described for F2 (8). This UFBD-like region in PFBP has an amino acid substitution at position 842, where a lysine (K) substitutes for an asparagine (N) (data not shown). In contrast, the N-terminal regions of both sequences are different, particularly in the regions from amino acids 322 to 443 in PFBP and amino acids 217 to 337 in F2 (42% identity). One of the striking differences between PFBP and protein F2 is the presence of 105 amino acids (without their leader peptides) at the N terminus of PFBP which are not found in protein F2 (Fig. 2). In fact, this 105-amino-acid segment is not present at this location in any of the reported fibronectin-binding proteins. However, this segment exhibits low sequence identity with other regions of PFBP, protein F2 (8), FnBB (14), and FnB (15).
FIG. 2.
Alignment of the N-terminal regions of PFBP and protein F2. Significant sequence divergence is exhibited between amino acids 322 to 443 in PFBP and amino acids 217 to 337 in F2. The mature PFBP protein has 105 amino acids at its N terminus that are not found in the mature F2 protein. Amino acids in boxes represent identical residues as determined by the CLUSTAL method (DNA Lasergene program).
The homology of PFBP with other fibronectin-binding proteins primarily occurs within the repeated region: 75% homology with the fibronectin-binding protein B (FnBB) and 70% homology with the fibronectin-binding protein A (FnBA) from S. dysgalactiae (14), as well as 60% homology with the fibronectin-binding protein (FnB) from S. equisimilis (15). The homology with several other fibronectin-binding proteins is only about 48% within the C-terminal region (e.g., proteins F1, Sfb, SOF, and SfbII and group G streptococcus fibronectin-binding protein GfbA) (6, 11, 12, 27, 34).
Transcription of the pfbp gene during the growth cycle.
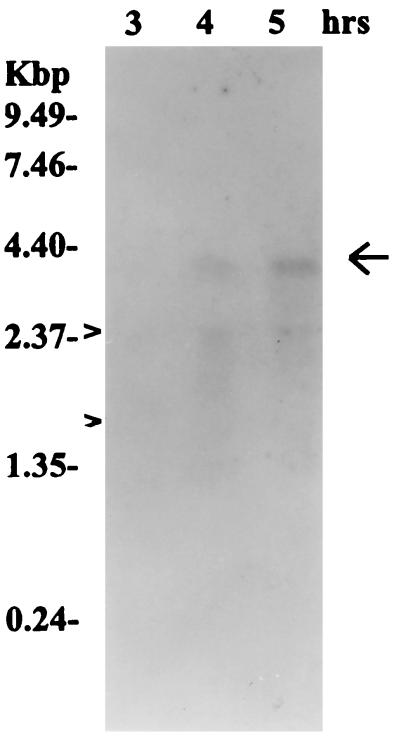
In order to determine whether, in fact, the pfbp gene is transcribed during cell growth in the M12(A735) strain, a Northern blot assay was performed. The hybridization of total RNA against the PCR-amplified pfbp gene as a probe clearly showed a band that corresponds to a fragment of about 3.4 kb (Fig. 3) and whose intensity increased during cell growth. The size of the transcript agreed with the size of the complete pfbp gene (3.29 kb), thus indicating that this gene is transcribed. However, when RNA was extracted from stationary-phase cultures, no transcription product was detected (data not shown).
FIG. 3.
Northern hybridization with a specific probe for pfbp. The arrow indicates a transcription product of ca. 3.4 kb corresponding to the size of the whole pfbp gene. The probe was labeled with [α-32P]dATP and used at a concentration of 106 cpm/ml of hybridization solution. The sizes of the RNA molecular size markers are shown to the left. Small arrowheads on the left indicate the positions of the rRNAs on the stained agarose gel.
Presence of the pfbp gene in different M-type strains.
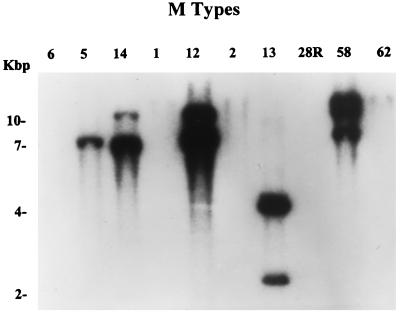
Since several fibronectin-binding proteins have been described in a variety of M serotypes (13, 20), we wanted to determine the presence of the pfbp gene in other group A streptococci. Furthermore, we were interested to determine whether a clear distribution of the pfbp gene existed between class I and II strains. For this purpose, genomic DNAs from 12 different streptococcal strains were digested with EcoRI (which does not cut the pfbp gene) and tested by Southern blot assay (Fig. 4). After hybridization with the whole pfbp gene, only three class I strains (M5, M14, and the parental strain M12) and two class II strains (M13 and M58) showed one or two copies of pfbp or a pfbp-like gene. A similar result was observed when an N-terminal-specific probe (P235) representing amino acids 1 to 78 was used (data not shown), further supporting the view that other pfbp and pfbp-like genes are present in the strains tested. Neither the M6 nor the M1 strain showed the presence of this gene under these conditions.
FIG. 4.
Southern hybridization of genomic DNAs from different M types digested with EcoRI. As a probe, the PCR-amplified pfbp gene was used to demonstrate the presence or absence of a similar gene in different class I or II M-type strains. The hybridization solution contained 106 cpm of the 32P-labeled probe per ml. The sizes of the molecular size markers are shown on the left. Class I strains: M6, M5, M14, M1, and M12. Class II strains: M2, M13, M28R, M58, and M62.
Presence of two different fibronectin-binding protein genes in the M12(A735) strain.
Certain fibronectin-binding protein genes have been shown to be present in pairs in the same bacterial strain, as is the case for fnbA and fnbB in S. dysgalactiae (14) and fnbA and fnbB in S. aureus (10). Moreover, in S. pyogenes the presence of two different fibronectin-binding protein genes has also been shown for sfbI and sfbII. Thus, we tested whether the M12(A735) strain actually possessed another fibronectin-binding protein gene. Due to the high degree of similarity between PFBP and protein F2 (8), a less-homologous protein, protein F1 (6), was used for the experiment. With specific primers derived from the reported prtF1 gene sequence [from strain M6(D471)] (6, 32), a PCR was performed with genomic DNA extracted from the M12(A735) strain. Genomic DNA from the M6(D471) strain was used as a control for the protein F1 gene amplification. A band corresponding to a fragment of approximately 2.0 kb was amplified, thus demonstrating the presence of a protein F1-like gene in the M12(A735) strain. The size of the same gene fragment in the M6 strain is around 1.8 kb (Fig. 5).
FIG. 5.
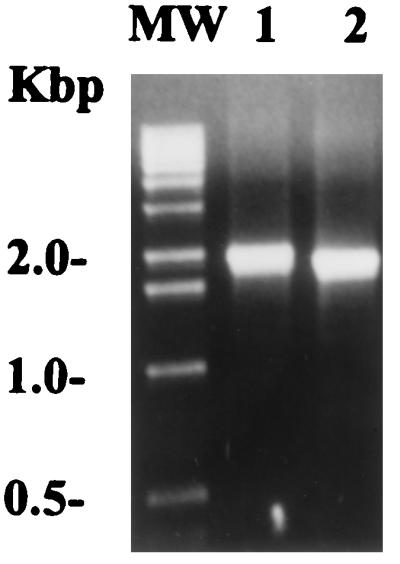
PCR amplification of a protein F1-like gene in the M12(A735) strain. The amplification was performed on genomic DNA extracted from both strains at a 60°C annealing temperature. The primers used are described in Materials and Methods. Lane 1, M12(A735) strain; lane 2, M6(D471); MW, molecular weight markers.
Cotranscription of the pfbp gene with a prtF1-like gene in the M12(A735) strain.
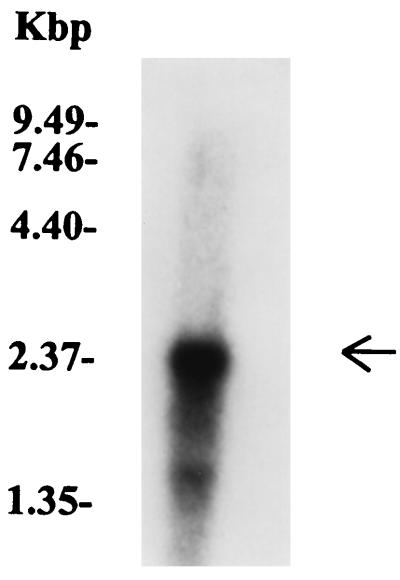
We demonstrated that the M12(A735) strain used in this study possesses two different genes, pfbp and one that was prtF1-like. Since we showed that the pfbp gene is transcribed in the M12 strain during cell growth, we wished to determine whether the prtF1-like gene was also transcribed. With the PCR-amplified prtF1 gene as a probe, a major transcription product, with an approximate size of 2.8 kb, for the prtF1 gene was detected by Northern hybridization over total RNA from the M12(A735) strain (Fig. 6). Furthermore, when the pfbp gene was probed with the prtF1 probe, no reactivity was found (data not shown), demonstrating the specificity of the probe. These results indicate that the two fibronectin-binding protein genes are indeed transcribed in the M12(A735) strain.
FIG. 6.
Northern blot hybridization. Transcription of a protein F1-like gene in the M12(A735) strain. For the assay, a specific 32P-labeled PCR probe for the prtF1 gene was used. The probe was used at a concentration of 106 cpm/ml of hybridization solution. The size of the band corresponds to a fragment of ca. 2.8 kb (arrow). The sizes of the molecular size markers are indicated on the left.
Expression of the repeated region of PFBP on the surface of S. gordonii and demonstration of its fibronectin-binding activity.
To further characterize the biological activity of the PFBP protein as a fibronectin-binding molecule, the repeated region of this protein was expressed on the surface of S. gordonii as a fusion with the anchor region of M protein (26). Three recombinants, CR390-1, CR390-2, and CR390-4, that were erythromycin resistant and were found to bind fibronectin in a dot blot screening assay (data not shown) were selected for further study.
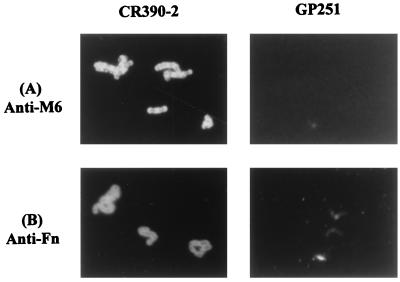
In order to confirm the expression of the fusion proteins on the surface of the three recombinant organisms, an immunofluorescence assay was performed. Recombinant CR390-2 showed a clear reactivity when an anti-M protein-specific monoclonal antibody (10F5) was used, indicating the presence of the fusion protein on the surface of S. gordonii (Fig. 7A). In contrast, the parental GP251 strain, which expresses no surface fusion protein or M protein, showed no reaction with the same antibody. A conjugate control showed no reactivity with either of the recombinant strains or the GP251 parental strain (data not shown). Similarly, by using a solution of fibronectin, followed by a reaction with an anti-fibronectin monoclonal antibody and detection with a conjugate coupled to FITC, it was possible to further demonstrate the fibronectin-binding activity of the expressed repeated region of the recombinant strain CR390-2 (Fig. 7B). As before, the GP251 parental strain was negative. The same results were obtained for recombinants CR390-1 and CR390-4 (data not shown). Furthermore, all controls included to assure the specificity of the reaction exhibited no reactivity with either the recombinants or the GP251 parental strain (data not shown). These results clearly demonstrate that the repeated region of PFBP expressed as an M-protein fusion molecule on the surface of the recombinant S. gordonii has fibronectin-binding activity.
FIG. 7.
Detection of fusion proteins on the surface of recombinant S. gordonii by immunofluorescence. (A) Recombinant CR390-2 and parental GP251 strains treated with an anti-M6 monoclonal antibody (10F5), which reacts with the M-protein portion of the fusion molecule. (B) Recombinant CR390-2 and parental GP251 strains probed with fibronectin and detected with an anti-fibronectin monoclonal antibody.
Binding of the repeated region of PFBP to soluble and immobilized fibronectin.
Fibronectin is present in a soluble form in body fluids and in a fibrillar form as part of the extracellular matrix of eukaryotic cells. Thus, it is important to assess the ability of streptococcal fibronectin-binding proteins to bind fibronectin under a variety of conditions.
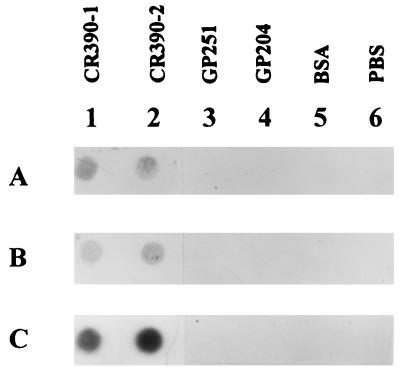
In a bacterial dot blot immunoassay, immobilized recombinant S. gordonii cells expressing the repeated region of PFBP on their surfaces (CR390-1 and CR390-2) bound soluble fibronectin (Fig. 8A, lanes 1 and 2), while the parental and wild-type strains (GP251 and GP204, respectively) (Fig. 8A, lanes 3 and 4), as well as the negative controls (BSA and PBS) (Fig. 8A, lanes 5 and 6), did not. In a second assay, 125I-labeled fibronectin was added to the same combination of organisms bound to a nitrocellulose filter. Again, the recombinant S. gordonii showed strong fibronectin-binding activity (Fig. 8C, lanes 1 and 2) in contrast to the negative signal of the control strains (Fig. 8C, lanes 3 and 4) and the negative controls (BSA and PBS) (Fig. 8C, lanes 5 and 6). A monoclonal antibody (10F5) directed to an epitope in the M-protein portion of the fusion molecule was used to further confirm the surface location and presence of the construct (Fig. 8B).
FIG. 8.
Expression of fusion proteins and binding of soluble fibronectin by recombinant S. gordonii in a bacterial dot blot immunoassay. Bacteria were immobilized on a nitrocellulose filter by using a dot blot apparatus. (A) A 1-μg/ml human fibronectin solution was added to the strip, and the reaction was developed by using an anti-fibronectin monoclonal antibody as described in Materials and Methods. (B) An anti-M protein monoclonal antibody (10F5) was added to the strip and developed as described in Materials and Methods. (C) 125I-labeled fibronectin was added to the strip and developed by autoradiography. Lanes: 1, recombinant CR390-1; 2, recombinant CR390-2; 3, GP251 S. gordonii parental strain; 4, GP204 S. gordonii wild-type strain; 5, BSA; 6, PBS.
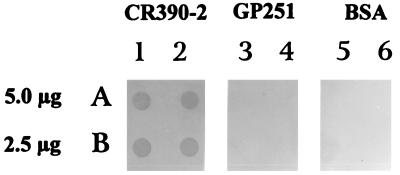
In a third assay, fibronectin was immobilized to a nitrocellulose filter in two different concentrations (5 and 2.5 μg/ml). After being blocked with a 3% BSA solution, a suspension of either recombinant (CR390-2) or parental (GP251) S. gordonii or a 3% BSA solution was added to the different filters. As shown in Fig. 9 (lanes 1 and 2), the recombinant S. gordonii surface expressing the repeated region of PFBP binds to the immobilized fibronectin, whereas the parental S. gordonii does not (lanes 3 and 4). There was no apparent difference in the adherence of the bacteria, regardless of the concentration of fibronectin immobilized on the filter.
FIG. 9.
Binding of recombinant S. gordonii to immobilized fibronectin. Human fibronectin was immobilized to nitrocellulose filters by using a dot blot apparatus: Rows: A, 5 μg/ml; B, 2.5 μg/ml. After the blocking, bacterial suspensions were added to the different strips. Lanes: 1 and 2, recombinant CR390-2; 3 and 4, GP251 S. gordonii parental strain; 5 and 6, BSA. An anti-M protein monoclonal antibody (10F5) was used to identify the presence of the microorganisms.
DISCUSSION
Among streptococcal surface proteins, it is a common feature to exhibit a variable N-terminal domain in contrast to a more conserved C-terminal domain (e.g., as with M and M-like proteins). Most of the fibronectin-binding proteins in streptococci and staphylococci exhibit such characteristics (5, 6, 8, 10, 12–16, 27, 34), and while extensive analyses have been published on the functional features of the C-terminal region of these proteins (i.e., the location of the fibronectin-binding repeats), very little is known about the specific functions attributed to their variable N-terminal domains. In streptococci, only a few fibronectin-binding proteins have been reported in which the function of the N-terminal domain was identified, i.e., streptococcal SOF contains the catalytic activity to cleave Apo A-1 of high-density lipoprotein (27) and the N-terminal region of protein F1 binds fibrinogen (11).
PFBP, the fibronectin-binding protein from an M12 strain of S. pyogenes described here, exhibits the typical characteristics found in streptococcal fibronectin-binding proteins and is related to protein F2 (8). However, its variable N-terminal region contains two special features: (i) a 105-amino-acid segment located at the beginning of the molecule which is not present at this location in protein F2 or in any other fibronectin-binding protein described thus far and (ii) a region of 118 amino acids which shares only 42% homology with a comparable region in protein F2 and is flanked by regions of high homology between these two proteins. The C-terminal end of PFBP exhibits the LPXTGX motif characteristic of cell wall-associated proteins in gram-positive bacteria (31). The complete protein has a deduced molecular size of 127.4 kDa, making it one of the largest surface molecules described thus far in group A streptococci.
A special characteristic of PFBP is its high content of serine and threonine residues throughout the entire molecule. This observation is in contrast to several other C-terminal-anchored surface proteins from gram-positive bacteria, which have threonine and serine residues concentrated within the cell wall-associated region of the molecule (3a). Its high content of glycine residues scattered throughout the molecule strongly suggests that PFBP has a compact structure rather than an elongated fibrous conformation.
As in other fibronectin-binding proteins, the C-terminal domain of PFBP is not only the conserved region of the molecule but has also proven to be a fibronectin-binding domain. This domain exhibits a repeated region which consists of three semiconserved repeats that share high sequence similarity with the repeated regions of fibronectin-binding proteins F2 from S. pyogenes (97%) (8), FnBB from S. dysgalactiae (75%) (14), and FnB from S. equisimilis (60%) (15). One striking feature of the C-terminal region of PFBP is the presence of three cysteine residues, one in the R3 repeat (position 1085), one in the putative transmembrane domain (position 1141), and the third in the charged tail (position 1161). This is unusual since it is rare for gram-positive surface proteins, which are anchored through the LPXTGX motif, to contain this amino acid. The role of these residues in this molecule has not yet been determined.
We show here that the repeated region of PFBP binds both soluble and immobilized fibronectin, suggesting that this region is surface exposed and oriented to permit interaction with different conformations of the fibronectin molecule. Many studies with S. dysgalactiae and S. aureus (18, 33) have suggested that the GGX3–4I/VDF motif is responsible for the fibronectin-binding activity of these proteins. Furthermore, in S. equi subsp. zooepidemicus, the sequence VETEDT located at the C-terminal end of the repeated region has been shown to be important for the high-affinity binding activity (16). A specific motif responsible for the binding in PFBP has not been directly determined. However, the R1 and R2 repeats of PFBP have the sequences GGQGEVVDT and GGQGQVVET, respectively. In both repeats, this motif overlaps with D/ETTEDT, which shares four or five of six residues with the S. equi subsp. zooepidemicus motif. Additionally, a conserved sequence, HFDNXXP, located at the C-terminal end of the third repeat in S. pyogenes protein F2, S. dysgalactiae FnBB, and S. equisimilis FnB, is also present at the same position in the R3 repeat of PFBP. This sequence has also been suggested to be important for the fibronectin-binding activity (8). Although more experiments are required to establish whether these sequences are in fact important for the fibronectin-binding activity in PFBP, their presence and strong homology with those sequences previously reported permit us to hypothesize their role as a fibronectin-binding motif.
Fibronectin-binding proteins F1 and F2 of S. pyogenes contain nonrepeated regions located just upstream from the repeat region which have been shown to contain a second fibronectin-binding domain (8). In protein F1 (and now proposed for protein F2), the repeated and nonrepeated regions bind to different fragments of fibronectin (8, 23). Because of the high degree of similarity between protein F2 and PFBP at the C-terminal region, it is possible for us to suggest not only that indeed PFBP contains two different fibronectin-binding domains but also that these domains might bind to different fragments of the fibronectin molecule. In this study we have demonstrated, by both immunofluorescence and bacterial dot blot assays, that the repeated region of PFBP binds strongly to the human fibronectin molecule. Additional studies will permit us to determine the affinities for the different fragments of fibronectin and, alternatively, to elucidate whether PFBP is able to bind more than one molecule of fibronectin, as has been demonstrated for S. aureus (5).
Some fibronectin-binding proteins have been described as being specific for class I streptococcal strains, as in the case of protein F1 and SfbI, while others, such as SOF, belong to class II of group A streptococci (27). However, we did not find any correlation between the presence or the absence of the pfbp gene, as indicated by Southern hybridization, and the group A streptococcal class. One interesting observation obtained from this experiment was the demonstration of the presence of one or more pfbp or pfbp-like genes in 80% (four of five) of the positive strains tested, including the A735(M12) parental strain, by using either a specific N-terminal probe (corresponding to the first 78 amino acids of the mature protein) or one representing the whole molecule. An explanation for this finding relies at present on previous reports showing the existence of more than one fibronectin-binding protein gene in the same strain (10, 13, 14). Interestingly, serotypes such as M1 and M6 failed to give any signal for this gene, even after a long exposure. The absence of the pfbp gene in the M1 serotype strain was also confirmed when its sequence was not found in the M1 streptococcal genome (32a, 36).
As described above, the presence of more than one gene encoding fibronectin-binding proteins in the same strain has been demonstrated previously, by Southern blot, for group A streptococcal sfbI and sfbII (13). Also, in S. dysgalactiae (fnbA and fnbB) (14) and S. aureus (fnbA and fnbB) (10), two different genes are present. It has also been reported that most group A streptococcal strains that have the prtF1 gene do not have the prtF2 or prtF2-related genes (8). However, the expression of two different fibronectin-binding protein genes simultaneously, in the same strain, has rarely been shown. Using specific primers for the prtF1 gene, we were able to amplify a prtF1-like gene of approximately 2.0 kb in the A735(M12) strain. By Northern hybridization with specific probes for both genes, pfbp and prtF1, we were able to demonstrate transcription products for both genes in the same strain and under the same conditions of growth. The transcription product for pfbp had the expected size for the complete gene (3.4 kb). In contrast, the band obtained for the prtF1-like gene is slightly larger (2.8 kb) than the PCR amplification product (2.0 kb), which could be explained by a polycistronic transcription of the prtF1-like gene. These results clearly demonstrate that there is more than one fibronectin-binding protein transcribed in the same M12 group A streptococcal strain. However, it is unknown at this time whether both proteins are coexpressed on the surface of the bacteria and whether both contribute equally to the fibronectin-binding activity.
The adherence of group A streptococci to eukaryotic cells is an important step in their ability to invade, colonize, and cause infection. How these microorganisms actually adhere to cells has not yet been elucidated, but binding fibronectin has proven to be in some way related to the process (7). The fact that more than one fibronectin-binding protein could be expressed on a single M-type strain, as described here, further reinforces the hypothesis that binding fibronectin and other components of the eukaryotic extracellular matrix might be an important step in the development of an infection. Furthermore, since environmental conditions have been shown to play a role in the expression of some fibronectin-binding proteins (e.g., proteins F1 and F2) (4, 8), the modulation of the expression of one, two, or more fibronectin-binding proteins on the surface of streptococci might determine the fate of the bacteria and enhance or repress its capacity to adhere, invade, and disseminate. A better understanding of the role fibronectin-binding proteins play in the infectious process will certainly improve our chances of controlling group A streptococcal infections.
ACKNOWLEDGMENTS
We want to thank Kevin Jones, Vijay Pancholi, and Ambrose Cheung for their advice and helpful suggestions, as well as Anne Derbise, Patricia Fontan, and Patricia Ryan for their support and enlightened discussions. We also thank C. D. Easterbrook for encouragement.
This study was supported in part by grants from the National Institutes of Health (AI-11822) and SIGA Pharmaceuticals (to V.A.F.).
REFERENCES
- 1.Bessen D, Jones K F, Fischetti V A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989;169:269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung A L, Eberhardt K, Fischetti V A. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 3.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Fischetti, V. A. Unpublished data.
- 4.Fogg G C, Caparon M. Constitutive expression of fibronectin binding in Streptococcus pyogenes as a result of anaerobic activation of rofA. J Bacteriol. 1997;179:6172–6180. doi: 10.1128/jb.179.19.6172-6180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fröman G, Switalski L M, Speziale P, Höök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987;262:6564–6571. [PubMed] [Google Scholar]
- 6.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffe J, Natanson-Yaron S, Caparon M G, Hanski E. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two domains. Mol Microbiol. 1996;21:373–384. doi: 10.1046/j.1365-2958.1996.6331356.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones K F, Khan S A, Erickson B W, Hollingshead S K, Scott J R, Fischetti V A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986;164:1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jönsson K, Signäs C, Müller H P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 11.Katerov V, Andreev A, Schalén C, Totolian A A. Protein F, a fibronectin-binding protein of Streptococcus pyogenes, also binds human fibrinogen: isolation of the protein and mapping of the binding region. Microbiology. 1998;144:119–126. doi: 10.1099/00221287-144-1-119. [DOI] [PubMed] [Google Scholar]
- 12.Kline J B, Xu S, Bisno A L, Collins C M. Identification of a fibronectin-binding protein (GfbA) in pathogenic group G streptococci. Infect Immun. 1996;64:2122–2129. doi: 10.1128/iai.64.6.2122-2129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreikemeyer B, Talay S R, Chhatwal G S. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindgren P-E, McGavin M J, Signäs C, Guss B, Gurusiddappa S, Höök M, Lindberg M. Two different genes coding for fibronectin-binding proteins from Streptococcus dysgalactiae. The complete nucleotide sequence and characterization of the binding domains. Eur J Biochem. 1993;214:819–827. doi: 10.1111/j.1432-1033.1993.tb17985.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindgren P-E, Signäs C, Rantamäki L, Lindberg M. A fibronectin-binding protein from Streptococcus equisimilis: characterization of the gene and identification of the binding domain. Vet Microbiol. 1994;41:235–247. doi: 10.1016/0378-1135(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 16.Lindmark H, Jacobsson K, Frykberg L, Guss B. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect Immun. 1996;64:3993–3999. doi: 10.1128/iai.64.10.3993-3999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 18.McGavin M J, Gurusiddappa S, Lindgren P-E, Lindberg M, Raucci G, Höök M. Fibronectin receptors from Streptococcus dysgalactiae and Staphylococcus aureus. Involvement of conserved residues in ligand binding. J Biol Chem. 1993;268:23946–23953. [PubMed] [Google Scholar]
- 19.Murakami Y, Iwahashi H, Yasuda H, Umemoto T, Namikawa I, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbrillin is one of the fibronectin-binding proteins. Infect Immun. 1996;64:2571–2576. doi: 10.1128/iai.64.7.2571-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 21.Norgren M, Caparon M G, Scott J R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989;57:3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oggioni M R, Pozzi G. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene. 1996;169:85–90. doi: 10.1016/0378-1119(95)00775-x. [DOI] [PubMed] [Google Scholar]
- 23.Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon M G, Yamada K M, Akiyama S K, Vlodavsky I, Hanski E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- 24.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozzi G, Musmanno R A, Lievens P M-J, Oggioni M R, Plevani P, Manganelli R. Method and parameters for genetic transformation of Streptococcus sanguis challis. Res Microbiol. 1990;141:659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 26.Pozzi G, Oggioni M R, Manganelli R, Fischetti V A. Expression of M6 protein gene of Streptococcus pyogenes in Streptococcus gordonii after chromosomal integration and transcriptional fusion. Res Microbiol. 1992;143:449–457. doi: 10.1016/0923-2508(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 27.Rakonjac J V, Robbins J C, Fischetti V A. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect Immun. 1995;63:622–631. doi: 10.1128/iai.63.2.622-631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salton M R, Horne R W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951;7:177. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 32.Sela S, Aviv A, Tovi A, Burstein I, Caparon M G, Hanski E. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993;10:1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 32a.Streptococcus pyogenes Genome Sequencing Project. University of Oklahoma. http://www.genome.ou.edu/strep.html. [16 February 1999, last date accessed.]
- 33.Sun Q, Smith G M, Zahradka C, McGavin M J. Identification of D motif epitopes in Staphylococcus aureus fibronectin-binding protein for the production of antibody inhibitors of fibronectin binding. Infect Immun. 1997;65:537–543. doi: 10.1128/iai.65.2.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talay S R, Valentin-Weigand P, Jerlström P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thole J E R, Schöningh R, Janson A A M, Garbe T, Cornelisse Y E, Clark-Curtiss J E, Kolk A H J, Ottenhoff T H M, De Vries R R P, Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992;6:153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 36.von Heijne G. A new method for predicting signal sequences cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]