Abstract
β-Defensins are cationic peptides with broad-spectrum antimicrobial activity that are produced by epithelia at mucosal surfaces. Two human β-defensins, HBD-1 and HBD-2, were discovered in 1995 and 1997, respectively. However, little is known about the expression of HBD-1 or HBD-2 in tissues of the oral cavity and whether these proteins are secreted. In this study, we characterized the expression of HBD-1 and HBD-2 mRNAs within the major salivary glands, tongue, gingiva, and buccal mucosa and detected β-defensin peptides in salivary secretions. Defensin mRNA expression was quantitated by RNase protection assays. HBD-1 mRNA expression was detected in the gingiva, parotid gland, buccal mucosa, and tongue. Expression of HBD-2 mRNA was detected only in the gingival mucosa and was most abundant in tissues with associated inflammation. To test whether β-defensin expression was inducible, gingival keratinocyte cell cultures were treated with interleukin-1β (IL-1β) or bacterial lipopolysaccharide (LPS) for 24 h. HBD-2 expression increased ∼16-fold with IL-1β treatment and ∼5-fold in the presence of LPS. Western immunoblotting, liquid chromatography, and mass spectrometry were used to identify the HBD-1 and HBD-2 peptides in human saliva. Human β-defensins are expressed in oral tissues, and the proteins are secreted in saliva; HBD-1 expression was constitutive, while HBD-2 expression was induced by IL-1β and LPS. Human β-defensins may play an important role in the innate defenses against oral microorganisms.
Defenses at mucosal surfaces are multifaceted and include both adaptive immunity and the innate immune system. The latter includes a number of broad-spectrum antimicrobial proteins and peptides which may protect mucosal surfaces from the continuous exposure to microbes. To date, many of these peptides have been studied in extraoral sites. Characterization of antimicrobial factors in the oral cavity may permit new insights into oral defense mechanisms and the pathogenesis of disease (21). For example, inactivation of these factors could lead to increased microbial colonization or invasion of the oral soft tissues, increasing the risk for candidiasis and periodontal disease. Additionally, they may play a role in preventing viral infections (16). The β-defensin family of antimicrobial peptides may contribute to oral defenses but until recently has received little attention (6, 14, 20).
Mammalian defensins are cationic antimicrobial peptides, ranging from 3.5 to 4.5 kDa, which are stabilized by three intramolecular disulfide bonds (6). There are two families of human defensins, the α- and β-defensins. The β-defensins differ from the classical or α-defensins by the ordering of the three disulfide bonds between the six cysteine residues of the mature peptides (6, 27). The β-defensins contribute to a protective barrier on mucosal surfaces including the tongue, nasal, and intrapulmonary airways and the small intestine (10, 12, 26, 29). In vitro, defensins exhibit broad-spectrum antimicrobial activity against gram-positive and gram-negative bacteria, fungi, and enveloped viruses (6). Bovine lingual antimicrobial peptide (LAP) is expressed in the bovine tongue epithelia and shows a marked induction of mRNA expression in epithelia surrounding areas of inflammation (26). The expression of LAP is induced, in part, by bacterial lipopolysaccharide (LPS) and proinflammatory cytokines (4). Native bovine β-defensin peptides exhibit antimicrobial activity against both gram-negative and gram-positive bacteria and fungi (5). Although β-defensin peptides in human oral epithelial cells have received little study, it is likely that human oral tissues produce β-defensin peptides similar to those found in other animals.
To date, two human β-defensins (HBD-1 and HBD-2) have been identified. Bensch et al. isolated a 36-amino-acid β-defensin peptide (HBD-1) from dialysate hemofiltrate and cloned a partial cDNA fragment from human kidney RNA (1). HBD-1 is abundantly expressed in the urogenital tract (30) and has also been detected in respiratory and other epithelia (7, 19, 28, 31). Harder et al. isolated and purified a second human β-defensin peptide, HBD-2, from psoriatic scale extracts by using a whole Escherichia coli affinity column (9). The HBD-2 sequence had significant homology to bovine LAP and bovine tracheal antimicrobial peptide (TAP). HBD-2 was highly effective in killing gram-negative bacteria (E. coli and Pseudomonas aeruginosa) and yeasts (Candida albicans) and had a bacteriostatic effect on the gram-positive bacterium Staphylococcus aureus (9). In addition, HBD-2 mRNA expression, like that of TAP and LAP, was induced by gram-negative bacteria and proinflammatory cytokines (9). Recently, Krisanaprakornkit et al. reported the constitutive expression of HBD-1 mRNA in cultured gingival epithelial cells and noninflamed and inflamed gingival tissues (14).
If HBD-1 and HBD-2 play a role in oral mucosal defenses, we hypothesized that they would be expressed in a number of oral epithelia, including the salivary glands, and that they would be present in saliva. In this study, we analyzed the expression of HBD-1 and HBD-2 mRNAs in selected oral tissues by the RNase protection assay (RPA). We also tested the hypothesis that the expression of HBD-1 and/or HBD-2 was induced by a proinflammatory cytokine or bacterial LPS. Finally, we analyzed the expression of HBD-1 and HBD-2 peptides in saliva by immunoblotting, capillary electrophoresis (CE), liquid chromatography (LC), and mass spectrometry (MS).
MATERIALS AND METHODS
Tissue specimens.
Tissues were obtained from surgical discard and postmortem specimens. Samples were obtained from the tongue, gingiva, and parotid gland. The samples were immediately flash frozen in liquid nitrogen and stored at −80°C for future RNA studies. Tissue procurement procedures were approved by the Institutional Review Board at the University of Iowa.
Primary culture of gingival keratinocytes.
Healthy gingival tissue samples from crown-lengthening procedures were obtained for keratinocyte culture as described previously (11). Tissue fragments were washed in Dulbecco’s phosphate-buffered saline containing penicillin (200 IU/ml), streptomycin (200 μg/ml), amphotericin B (5 μg/ml), and gentamicin (0.1 μg/ml). To separate the epithelium from the underlying connective tissue, the specimens were incubated in Dispase II (2.4 U/ml; Boehringer Mannheim, Indianapolis, Ind.) overnight at 5°C. The epithelium was mechanically separated from the underlying connective tissue (22), and the epithelial sheets were placed in 0.25% trypsin–1 mM EDTA and vigorously pipetted to produce a cell suspension. Following trypsin neutralization by medium containing 10% fetal bovine serum, the suspension was centrifuged (208 × g for 5 min). The pellet was suspended in medium and seeded into a T-25 tissue culture flask (Corning, Corning, N.Y.) containing a feeder layer of mitomycin-treated NIH 3T3 murine fibroblasts (23). The cells were cultured in medium consisting of 3 parts Dulbecco’s modified Eagle’s medium (Sigma Chemical Co., St. Louis, Mo.) and 1 part Ham’s F12 medium (Sigma Chemical Co.) containing 10% fetal bovine serum (Intergen, Purchase, N.Y.). The medium was supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), amphotericin B (2.5 μg/ml), epidermal growth factor (10 ng/ml), cholera toxin (0.1 nM), and hydrocortisone (400 ng/ml). It was changed every 2 to 3 days, and the cultures were kept at 37°C in a humidified environment containing 5% CO2.
When the cultures reached 75 to 80% confluency, the feeder layer was removed by treatment with 0.5 mM EDTA for 5 min at 37°C and the cultures were exposed to trypsin-EDTA for 1 to 2 min (37°C). Adherent keratinocytes were incubated in trypsin-EDTA for 4 to 5 min at 37°C or until microscopic inspection demonstrated detachment of the majority of the cells. After trypsin inactivation, cell counts were determined with a hemocytometer and the cells were seeded for the experimental procedures described below. The keratinocyte nature of the cells was confirmed by immunohistochemical staining for high-molecular-mass (50-, 56.5-, 57-, 58-, 66-, and 68-kDa) cytokeratins (Dako Corp., Carpinteria, Calif.) and by histologic and ultrastructural features.
RPA.
Specific oral tissues expressing HBD-1 and HBD-2 mRNAs were identified by RPA methods previously described for quantitative assessment of HBD-1 mRNA in the lung (19). Total RNA was isolated from frozen tissue samples and human cell cultures by a single-step guanidine thiocyanate-phenol-chloroform extraction (2) and stored in RNase-free water at −80°C. The [α-32P]UTP (Amersham Corp., Arlington Heights, Ill.) random-labeled HBD-1, HBD-2, and 18S ribosomal subunit (as the internal standard [Ambion Co., Austin, Tex.]) antisense riboprobes were transcribed with cDNA templates subcloned into a plasmid vector containing the T7 promoter. The radiolabeled riboprobes were hybridized to tissue-specific mRNA by using a Hybspeed RPA kit (Ambion Co.). The unprotected probes were 270 and 432 nucleotides for HBD-1 and HBD-2, respectively. The protected probes were 158 and 328 nucleotides for HBD-1 and HBD-2, respectively. In the absence of RNA, the unprotected probes were completely digested by RNases. The RNA-RNA hybrids were separated by denaturing Tris-borate-EDTA (TBE) vertical gel electrophoresis and visualized by autoradiographic methods as previously described (19).
Induction of β-defensins.
We tested for inducible β-defensin mRNA expression in primary cultures of human gingival keratinocytes (11). The cells were treated with recombinant human interleukin-1-β (IL-1β) (R & D Systems, Minneapolis, Minn.) or E. coli-derived bacterial LPS (Sigma). Cultures from four different individuals were grown on six-well plates in serum-free modified MCDB 153 medium containing 0.15 mM calcium, 50 μg of gentamicin per ml, 50 ng of amphotericin B per ml, 5 μg of insulin per ml, 0.5 μg of hydrocortisone per ml, 30 μg of bovine pituitary extract per ml, and 0.1 ng of epidermal growth factor per ml (keratinocyte growth medium; Clonetics Corp., San Diego, Calif.) by methods described previously (11). Cells (passages 2 and 3) from each subject were seeded at 4.0 × 106 cells per well into six-well tissue culture plates (Costar, Cambridge, Mass.). These cultures were maintained in the absence of a feeder layer in keratinocyte growth medium. When confluence was reached, two wells of cells from each subject were treated for 24 h with normal medium (control) or medium containing 100 ng of IL-1β per ml or 10 μg of LPS per ml. Cell viability, as assessed by commercial assays for mitochondrial dehydrogenase (MTS, CellTiter aqueous nonradioactive cell proliferation assay; Promega Corp., Madison, Wis.) was not affected by either IL-1β or LPS treatments. RNA was extracted immediately after the treatment and analyzed by RPA (19).
Purification of cationic peptides and proteins from human saliva.
Saliva was collected in 10-ml volumes from normal adult volunteers. Samples were probe-sonicated and evaluated individually by Western blotting or pooled. Cationic materials were batch-extracted from individual (10-ml) or pooled (100-ml) saliva samples with a 50% slurry of Macro-Prep CM beads (Bio-Rad, Richmond, Calif.) as previously described for other biological fluids (30). Following continuous mixing of the gel beads with the saliva overnight at 4°C, the beads were collected by centrifugation and washed with 25 mM ammonium acetate (pH 7.3). Elution of bound material was affected by addition of an equal volume of 10% acetic acid. This was repeated three times with equal volumes of 5% acetic acid. Pooled eluates were lyophilized, resuspended in 1 ml of 1% acetic acid, and further purified by reversed-phase high-pressure liquid chromatography (RP-HPLC) on a 2.1- by 250-mm Vydac C18 column (218TP52) with a mobile phase system consisting of 0.025% trifluoroacetic acid in water (solution A) and 0.021% trifluoroacetic acid–80% acetonitrile in water (solution B). Gradient conditions were 1 to 48% solution B in 60 min, 48 to 88% solution B in 30 min, and 88 to 98% solution B in 10 min at a flow rate of 0.15 ml/min. Fractions were collected at 2-min intervals, dried by vacuum centrifugation, and resuspended in 50 μl of 0.1% acetic acid.
Immunodetection of HBD-1 and HBD-2.
Cationic material from individual samples and selected fractions from RP-HPLC were electrophoresed on urea-acetic acid polyacrylamide gels together with known quantities of purified recombinant HBD standards. Recombinant HBD-1 and HBD-2 were prepared in baculovirus as reported previously (18, 30). The conditions for acid-urea polyacrylamide gel electrophoresis included 12.5% acrylamide and 4.6 M urea (pH 4). Western immunoblotting was performed with rabbit antisera to HBD-1 or HBD-2 (diluted 1:1,000) as previously described (18, 30). The secondary antibody was a horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G diluted 1:10,000. The substrate was Pierce SuperSignal.
ESI-LC-MS, CE, and Edman degradation.
Immunoblot-positive fractions from RP-HPLC were analyzed by LC-MS with a Hewlett-Packard 1100 MSD equipped with an electrospray ionization (ESI) source, a Hewlett-Packard 1100 series HPLC apparatus, and a 0.3- by 250-mm LC Packings capillary column packed with Vydac 218TPC18 RP material. CE was performed with a Hewlett-Packard 3D CE apparatus equipped with a Hewlett-Packard extended-light-path fused-silicate capillary column (75 μm [inner diameter] by 80.5 cm [total length]). The experiments were performed at 20,000 V in 0.1 M sodium phosphate (pH 2.9). Edman degradation was conducted on a polyvinylidene difluoride membrane in the gas phase with an Applied Biosystems Procise sequencer.
RESULTS
β-Defensin mRNA expression in oral tissues.
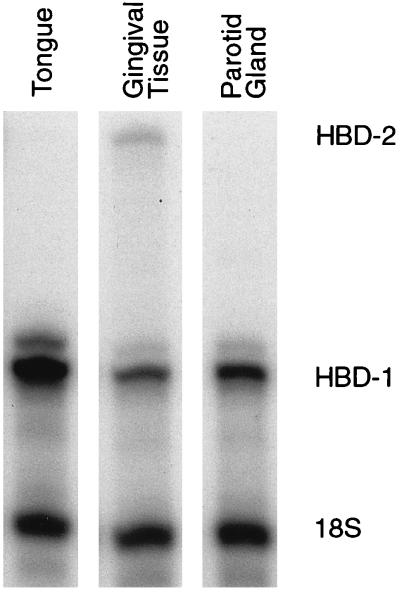
HBD-1 mRNA expression was detected in gingival, parotid gland, and lateral tongue tissue (Fig. 1). In contrast, expression of HBD-2 was detectable only in gingival tissue (Fig. 1) and at low levels in gingival keratinocytes (see Fig. 2A). The expression of HBD-2 mRNA was most abundant in gingival tissues with associated inflammation (data not shown). To determine whether β-defensin expression in oral epithelia was inducible, further experiments were performed with a gingival keratinocyte cell culture model.
FIG. 1.
β-Defensin expression in oral tissues by RPA. HBD-1 mRNA, represented by a single protected fragment of 158 bp, was detected in the lateral tongue, gingival tissue, and the parotid gland. In contrast, expression of HBD-2, represented by a single protected fragment of 328 bp, was detectable by RPA only in gingival tissue and was most abundant in gingival tissues associated with inflammation (data not shown).
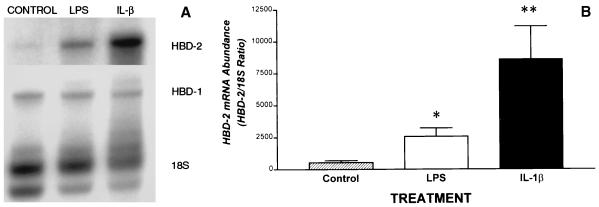
FIG. 2.
Regulation of β-defensin expression in gingival keratinocytes. (A) A representative autoradiograph demonstrates that by using quantitative RPA, moderate HBD-1 and low HBD-2 mRNA expression were detected in 20 μg of total RNA from gingival keratinocyte cultures under control conditions (CONTROL). Following a 24-h treatment of separate wells of the same culture with 100 ng of IL-1β per ml (IL-β), HBD-2 mRNA expression dramatically increased. Treatment with 10 μg of LPS per ml (LPS) also induced HBD-2 expression. Expression of HBD-1 mRNA was unchanged in the presence of either LPS or IL-1β. The results are representative of three similar experiments. (B) Quantitation of HBD-2 mRNA in gingival keratinocytes following induction with IL-1β or LPS. The radioactive counts from 32P-labeled riboprobes were normalized to the abundance of the 18S ribosomal subunit. Results are mean and standard error. ∗, LPS versus control, P = 0.05; ∗∗, IL-1β versus control, P < 0.05 (analysis of variance). There was no significant change in HBD-1 mRNA abundance in response to LPS or IL-1β (data not shown).
Regulation of β-defensin expression in cultured gingival keratinocytes.
As shown in Fig. 2A, using a quantitative RPA, we found moderate HBD-1 and low HBD-2 mRNA expression in gingival keratinocyte cultures under basal conditions. Following a 24-h treatment of separate wells of the same culture with 100 ng of IL-1β per ml, there was a ∼16-fold increase (n = 4) in HBD-2 mRNA expression above the control (Fig. 2). Treatment of cells with 10 μg of LPS per ml for 24 h also induced a ∼fivefold increase in HBD-2 expression (Fig. 2). In contrast, HBD-1 mRNA levels were unchanged in the presence of either IL-1β or LPS (Fig. 2A).
RP-HPLC.
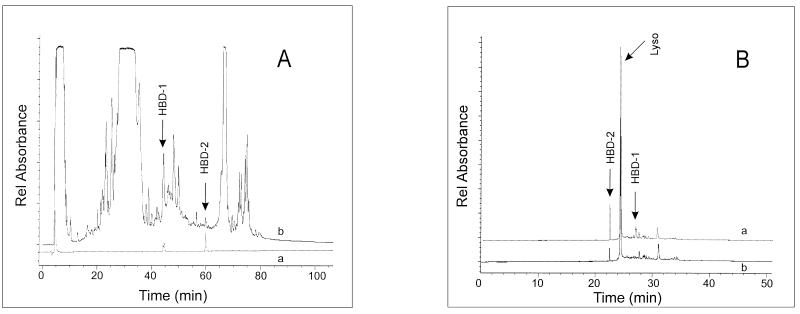
Cationic species batch-extracted from pooled saliva with Macro-Prep CM beads were fractionated by HPLC. Peptides eluting from this column with increasing mobile phase were monitored by measuring the absorbance at 206 nm, and 0.3-ml fractions were collected at 2-min intervals. The elution positions of purified recombinant HBD-1 and HBD-2 were independently assessed. These data are presented as composite chromatograms in Fig. 3A. Candidate peaks for HBD-1 and HBD-2 were selected by comparing the absorbance profiles and mobilities of the pooled salivary proteins with recombinant β-defensin standards.
FIG. 3.
Composite chromatograms of salivary cationic peptides and proteins analyzed by RP-HPLC and CE. (A) RP-HPLC chromatogram, monitored at 206 nm, of peptides eluting with increasing mobile phase (tracing b). The elution positions of purified recombinant HBD-1 and HBD-2 were independently monitored at 206 nm (tracing a). (B) Analysis of HBD-2 immunoreactive fraction 32 (64 min, 53.3% solution B [Fig. 4]) by CE. Absorbance was monitored at 200 nm. The migration times of purified recombinant HBD-1 and HBD-2 were assessed both independent of and in combination with ions present in fraction 32. The lower absorbance trace (tracing b) depicts the ion profile of fraction 32 alone, and the upper trace (tracing a) depicts that of fraction 32 supplemented with HBD-1 and HBD-2 standards. Fraction 32 contains a peptide ion at 22.5 min that has a mobility identical to that of the HBD-2 standard. Lyso, lysozyme.
Immunodetection of HBD-1 and HBD-2 in fractionated saliva.
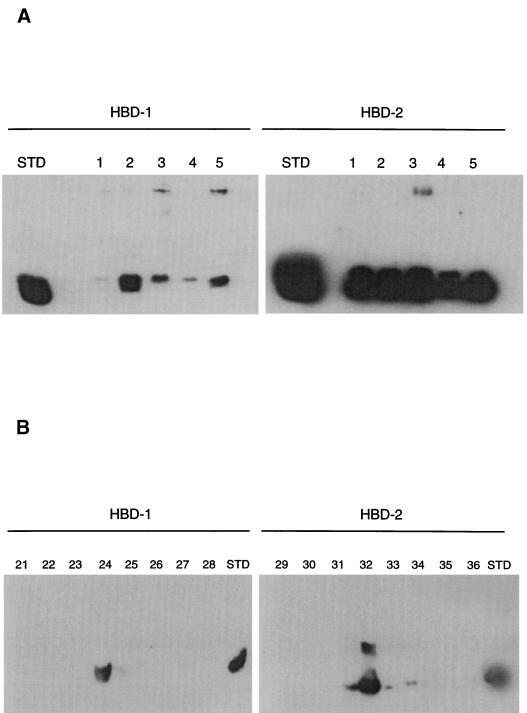
In a pilot experiment, cationic peptides and proteins were extracted from the saliva of five subjects and screened for the presence of HBD-1 or HBD-2 by Western blotting. All the samples showed immunoreactive bands consistent with HBD-1 and HBD-2 (Fig. 4A). There was considerable variability of HBD-1 abundance between samples while HBD-2 abundance was less variable. More than one band was immunoreactive with HBD-1 antisera in some of the samples (Fig. 4A, left). Such heterogeneity in HBD-1 peptides has been previously found in the urogenital tract (30). Samples probed for HBD-2 immunoreactivity also showed more than a single species (Fig. 4A, right). Further studies were performed with RP-HPLC-fractionated materials. Based on the observed retention times for HBD-1 and HBD-2 standards of 45 and 60 min (Fig. 3A), respectively, select fractions were taken for urea-acetic acid gel electrophoresis and Western analysis with specific rabbit polyvalent antisera to HBD-1 and HBD-2. Specifically, fractions 21 to 28 were selected to screen for HBD-1 immunoreactivity and fractions 29 to 36 were selected to screen for HBD-2 immunoreactivity. The Western blot results are shown in Fig. 4B. Peak reactivity with HBD-1 and HBD-2 antisera was observed in fractions 24 and 32, respectively.
FIG. 4.
Western blot analysis of HBD-1 and HBD-2 in saliva and fractions obtained by RP-HPLC. (A) Western blots of cationic peptides and proteins extracted from saliva from five different subjects (lanes 1 to 5). Samples were subjected to urea-acetic acid gel electrophoresis, and Western analysis was performed with specific rabbit polyvalent antisera to HBD-1 (left) and HBD-2 (right). All the samples contained immunoreactive material. (STD, recombinant peptide standard). (B) Immunodetection of HBD-1 and HBD-2 in fractionated saliva. Based on the observed retention times for HBD-1 and HBD-2 standards of 45 and 60 min (Fig. 3A), respectively, select fractions were taken for urea-acetic acid gel electrophoresis and Western analysis with specific rabbit polyvalent antisera to HBD-1 and HBD-2. Fractions 21 to 28 were screened for HBD-1 immunoreactivity. Peak reactivity was seen in fraction 24 (48 min, 38.4% solution B). Fractions 29 to 36 were screened for HBD-2 immunoreactivity. Peak reactivity with HBD-2 antiserum was observed in fraction 32 (64 min, 53.3% solution B).
CE, Edman degradation, and LC-MS analysis of fractionated saliva.
We used CE to profile peptide and protein components of the immunoreactive HBD-1 and HBD-2 fractions 24 and 32, respectively, and to corroborate our preliminary identifications based on gel mobilities and reactivities with specific antisera. These fractions were also subsequently analyzed by Edman degradation and ESI-LC-MS. Fraction 24 proved far too complex to obtain useful data by CE or ESI-LC-MS. Interestingly, Edman analysis revealed a major protein component with an N-terminal sequence Arg-Ile-Gly-Arg-Phe-Gly-Tyr-Gly-Tyr-Gly-Pro. A database search (with BLAST) identified this component as being derived from statherin precursor, a tyrosine-rich acidic peptide from parotid saliva.
In contrast, HBD-2 fraction 32, by virtue of its position in a less trafficked position of the chromatogram, was amenable to analysis by CE. These data are shown in Fig. 3B. Peptide ions present in fraction 32 were separated according to their different migration velocities under conditions of uniform field strength at pH 2.9 and monitored by measurement of absorbance at 200 nm. The migration times of purified recombinant HBD-1 and HBD-2 were assessed both independent of and in combination with the ions present in fraction 32. The lower absorbance trace depicts the ion profile of fraction 32 alone, and the upper trace depicts that of fraction 32 supplemented with HBD-1 and HBD-2 standards in a ratio of 8:2 (vol/vol). The results of this study clearly indicate the presence in fraction 32 of a peptide ion at 22.5 min which has a mobility identical to that of the HBD-2 standard. Subsequent LC-MS and Edman analysis of fraction 32 confirmed the presence of the 41-amino-acid form of HBD-2 (observed molecular weight, 4,327.56; calculated molecular weight, 4328.06) with the N-terminal sequence Gly-Ile-Gly-Asp-Pro-Val-Thr. Edman analysis further revealed the major peptide ion observed by CE at 24.3 min to be the mature form of lysozyme C (data not shown). From these data, we estimate the concentration of HBD-2 in saliva to be about 150 ng/ml. This is undoubtedly a conservative estimate, since we have no means of quantitatively assessing the efficiency of recovery at each step of the purification.
DISCUSSION
The above studies demonstrate the distribution of human β-defensin mRNAs in oral epithelial tissues and the parotid gland. Expression of HBD-1 mRNA was observed in tongue and parotid gland tissues, and both HBD-1 and HBD-2 mRNAs were detected in the gingiva and cultured gingival keratinocytes. Of note, HBD-2 expression was induced by IL-1β and LPS whereas HBD-1 expression remained unchanged in the presence of inflammatory stimuli. Both HBD-1 and HBD-2 peptides were detected in saliva. The widespread expression of β-defensins in oral tissues suggests that they contribute to host defenses in the oral cavity.
In our studies of cultured gingival keratinocytes, an interesting contrast was noted between HBD-1 and HBD-2. Consistent with previous reports (14, 31), HBD-1 mRNA expression showed no significant change in response to IL-1β or LPS. In contrast to HBD-1, HBD-2 expression was induced markedly by IL-1β and to a lesser extent by LPS. This suggests different roles for these peptides in oral defenses. The induction of HBD-2 by IL-1β and LPS is similar to that of bovine LAP and TAP (5, 24, 26). Harder et al. showed that HBD-2 expression was induced in skin keratinocytes in the presence of tumor necrosis factor alpha, gram-negative and gram-positive bacteria, and C. albicans (9). Similarly, HBD-2 expression is inducible in airway epithelia (9, 28). We speculate that HBD-1 plays a constitutive role in oral defenses while HBD-2 expression is induced in response to local infection or inflammation.
Both human β-defensin peptides were readily detected in saliva. A conservative estimate for the concentrations of HBD-1 and HBD-2 in saliva is ∼150 ng/ml. We speculate that these concentrations may be sufficient to be microbicidal for some organisms, especially considering that they may act synergistically with other microbicidal factors present in saliva. Such factors include human salivary histatin 5, lactoferrin, mucin glycoprotein, and lysozyme (15, 25). Further studies of the activity of HBD-1 and HBD-2 against a spectrum of oral microorganisms including pathogenic species are needed. The concentrations of HBD-1 and HBD-2 peptides in saliva are somewhat at odds with data obtained with the cultured gingival keratinocytes. Perhaps the constant exposure of the oral mucosa to microorganisms serves to induce the production of HBD-2, even in the absence of overt oral disease. Because the parotid glands expressed HBD-1 mRNA but not HBD-2 mRNA, we speculate that the HBD-2 peptide detected in the saliva arose from induced expression by keratinocytes in the mouth. Alternatively, HBD-2 may also be secreted from other salivary glands. Zhao et al. previously reported the expression of HBD-1 mRNA in salivary epithelia (31), and Harder et al. observed HBD-2 mRNA expression in the salivary gland, although the gland of origin was not stated (9). We did not detect HBD-2 mRNA expression in the parotid gland. The differences in these results may reflect the sensitivity of the detection system (RPA versus reverse transcription-PCR), the degree of inflammation present in the tissue sampled, or the specific salivary gland studied (major or minor).
These results suggest that epithelial β-defenses may play an important role in the mucosal defenses of the mouth. HBD-1 may provide a basal antimicrobial activity at mucosal surfaces to guard against infections at and away from the site of invasion. This might explain the greater expression of HBD-1 than HBD-2 in the kidney and salivary glands, where epithelial surface fluids are excreted away from site of origin, preventing ascending infections (30). However, further studies are needed to understand the antimicrobial activity of HBD-1 and HBD-2 peptides against relevant oral microorganisms and how the activity or expression may be altered in states such as periodontal disease or immunosuppression. In addition to their antimicrobial properties, β-defensins attract monocytes (17), suggesting a possible interaction between antimicrobial expression and inflammation. This relationship is an example of the ability of the peptide to generate a robust local response to microbial and viral infections. Inducible antibiotics like defensins may work to repair injured mucosal sites, given that defensins exhibit growth factor activity, in addition to microbicidal activity, in vitro and in vivo (17).
Finally, β-defensins and other antimicrobial peptides may have therapeutic applications for the treatment of diseases in oral tissues (8, 13). For example, defensins inactivate many enveloped viruses that can penetrate mucosal surfaces (3, 16). Thus, topical application of antimicrobial peptides may have utility in the treatment of oral diseases including periodontitis or candidiasis (21). Clarification of the mechanisms of β-defensin induction may also prove useful for therapeutic applications designed to enhance innate immunity.
ACKNOWLEDGMENTS
We thank Tom Ganz for many helpful discussions and for providing the HBD-1 and HBD-2 antisera. We are grateful to Elena Rus, Protein Structure Facility, University of Iowa, for assistance with protein sequence analysis. We thank Connie C. Organ for technical assistance and Larry McCray for assistance in obtaining clinical tissue samples.
REFERENCES
- 1.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 2.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 3.Daher K A, Selsted M E, Lehrer R I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T, Lehrer R I. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 7.Goldman M J, Anderson M G, Stolzenberg E D, Kari P U, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:1–9. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 8.Hancock R E W, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 9.Harder J, Bartels J, Christophers E, Schroder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861–862. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 10.Huttner K M, Brezinski D J C, Mahoney M M, Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J Nutr. 1998;128:297S–299S. doi: 10.1093/jn/128.2.297S. [DOI] [PubMed] [Google Scholar]
- 11.Johnson G K, Poore T K, Payne J B, Organ C C. Effect of smokeless tobacco extract on human gingival keratinocyte levels of prostaglandin E2 and interleukin-1. J Periodontol. 1996;67:116–124. doi: 10.1902/jop.1996.67.2.116. [DOI] [PubMed] [Google Scholar]
- 12.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 13.Kelley K J. Using host defenses to fight infectious diseases. Nat Biotechnol. 1996;14:587. doi: 10.1038/nbt0596-587. [DOI] [PubMed] [Google Scholar]
- 14.Krisanaprakornkit S, Weinberg A, Perez C N, Dale B A. Expression of the peptide antibiotic human β-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamkin M S, Oppenheim F G. Structural features of salivary function. Crit Rev Oral Biol Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- 16.Lehrer R I, Daher K, Ganz T, Selsted M E. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Wang L, Jia H P, Zhao C, Heng H H Q, Schutte B C, McCray P B, Jr, Ganz T. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 19.McCray P B, Jr, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaki K T, Bodeau A L, Ganz T, Selsted M E, Lehrer R I. In vitro sensitivity of oral, gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun. 1990;58:3934–3940. doi: 10.1128/iai.58.12.3934-3940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyasaki K T, Lehrer R I. β-Sheet antibiotic peptides as potential dental therapeutics. Int J Antimicrob Agents. 1998;9:269–280. doi: 10.1016/s0924-8579(98)00006-5. [DOI] [PubMed] [Google Scholar]
- 22.Oda D, Watson E. Human oral epithelial cell culture. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol. 1990;26:589–595. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- 23.Rheinwald J G, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 24.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenkels L C, Veerman E C, Nieuw Amerongen A V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med. 1995;6:161–175. doi: 10.1177/10454411950060020501. [DOI] [PubMed] [Google Scholar]
- 26.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 27.Selsted M E, Tang Y Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 28.Singh P K, Jia H P, Wiles K, Hesselberth J, Liu L, Conway B D, Greenberg E P, Valore E V, Welsh M J, Ganz T, Tack B F, McCray P B., Jr Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarver A P, Clark D P, Diamond G, Russell J P, Bromage H E, Tempst P, Cohen K S, Jones D E, Sweeney R W, Wines M, Hwang S, Bevins C L. Enteric β-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with cryptosporidium parvum infection. Infect Immun. 1998;66:1045–1056. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Jr, Ganz T. Human β-defensin-1, an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]