Abstract
Three separate studies, two involving active-immunization regimens and one involving a passive-transfer protocol, were conducted to initially screen and ultimately more fully assess several nontypeable Haemophilus influenzae outer membrane proteins or their derivatives for their relative protective efficacy in chinchilla models of otitis media. Initial screening of these antigens (P5-fimbrin, lipoprotein D, and P6), delivered singly or in combination with either Freund’s adjuvant or alum, indicated that augmented bacterial clearance from the nasopharynx, the middle ears, or both anatomical sites could be induced by parenteral immunization with P5-fimbrin combined with lipoprotein D, lipoprotein D alone, or the synthetic chimeric peptide LB1 (derived from P5-fimbrin), respectively. Data from a second study, wherein chinchillas were immunized with LB1 or lipoprotein D, each delivered with alum, again indicated that clearance of nontypeable H. influenzae could be augmented by immunization with either of these immunogens; however, when this adjuvant was used, both antibody titers in serum and efficacy were reduced. A third study was performed to investigate passive delivery of antisera directed against either LB1, lipoprotein D, nonacylated lipoprotein D, or a unique recombinant peptide designated LPD-LB1(f)2,1,3. The last three antiserum pools were generated by using the combined adjuvant of alum plus monophosphoryl lipid A. Passive transfer of sera specific for LB1 or LPD-LB1(f)2,1,3 to adenovirus-compromised chinchillas, prior to intranasal challenge with nontypeable H. influenzae, significantly reduced the severity of signs and incidence of otitis media which developed (P ≤ 0.001). Collectively, these data indicate the continued merit of further developing LB1 and LPD-LB1(f)2,1,3 as components of vaccines for otitis media.
Several outer membrane proteins of nontypeable Haemophilus influenzae (NTHI) as well as its lipooligosaccharide have been the subject of recent studies designed to assess their utility as potential immunogens against bacterial otitis media (OM) (3, 12, 13, 18, 26, 28, 30, 34, 37, 44, 53, 66). The vast majority of these studies have been performed with chinchillas as the host and with disease induced by direct inoculation of the middle ear via transbullar challenge. While the chinchilla model has its limitations, these studies have proved extremely useful in addressing questions concerning both the pathogenesis and potential prevention of OM induced by NTHI. Early studies in chinchillas by Karasic et al. (37), using purified LKP pili as the immunogen, and by Barenkamp (12), using whole killed bacterial cells, demonstrated that one could indeed immunize, either actively or passively, against NTHI-induced OM. However, the study by Karasic et al. (37) and more recent studies have demonstrated that the protection conferred is restricted largely to homologous challenge (8, 30, 53, 66). These observations, combined with the multifactorial nature of OM and the antigenic diversity of the NTHI outer membrane proteins, have led many investigators to conclude that development of a broadly effective vaccine against a group of organisms as heterogeneous as NTHI will probably require a combination of several highly conserved and immunogenic antigens to form a multicomponent vaccine (13, 18, 26).
Several current candidate immunogens, which have been shown to reduce the incidence or severity or both of OM in the chinchilla or rat host are a mixture of rPCP, P4, and P6 (26); rHtrA (44); the high-molecular-weight (HMW) adhesin proteins (13); detoxified lipooligosaccharide-protein conjugates (28); outer membrane protein P6 (18); and lipoprotein D (LPD) (3, 34). Our laboratory has similarly demonstrated that fimbrin, an NTHI adhesin (11, 36, 57, 58) homologous to outer membrane protein P5 (66), can be used as a parenteral immunogen to significantly reduce the incidence and/or severity of OM in similar direct-challenge models.
Each of the aforementioned studies has yielded valuable information about the ability of these antigens to induce antibodies effective against an overt infection in a directly challenged tympanum. One of the questions which cannot be answered in these models, however, is how effective the immunogen is against nasopharyngeal (NP) colonization and ultimately eustachian tube ascension by NTHI (15, 16, 23), both of which must occur prior to induction of a middle ear infection. To address this question and also to investigate the potential for immunizing against NP colonization, we developed a chinchilla model of adenovirus compromise to allow NTHI, inoculated into the nares, to first establish a colonized state in the nasopharynx and later, when adenovirus-induced damage compromises eustachian tube function, ascend into the middle ear space (6, 46, 47, 72).
We recently used this superinfection model to demonstrate that, as has been shown with other NTHI outer membrane proteins in the environment of the middle ear, isolated P5-homologous fimbrin protein (P5-fimbrin), delivered as a parenteral immunogen, induced significantly augmented clearance of a homologous challenge isolate from the chinchilla nasopharynx 21 days earlier than in the control cohort (8). Clearance of a heterologous isolate was, however, less rapid, occurring approximately 7 days earlier than in sham-immunized animals. In the same paper, we reported the synthesis and use of a chimeric synthetic peptide which incorporated both a 19-mer putative B-cell epitope of the mature P5-fimbrin protein of NTHI isolate 1128 and a T-cell “promiscuous” epitope from measles virus fusion protein. This 40-mer peptide, LB1, also induced significant activity, which was protective against homologous challenge when delivered as a parenteral immunogen with complete Freund’s adjuvant (CFA). Animals immunized with LB1 were able to clear NTHI from their nasopharynges 2 weeks earlier than sham-immunized chinchillas were.
In the present paper, we expand on these collective observations to first determine if any of three NTHI antigens, which are thought to induce protective activity, could be used singly or in combination as immunogens to protect against NP colonization and to induce augmented bacterial clearance from the chinchilla middle ear. The most promising immunogen(s) was then retested for efficacy. Finally, a passive-transfer protocol was combined with the aforementioned adenovirus-NTHI dual-challenge model and used to assess whether delivery of serum collected from animals immunized with the most efficacious immunogens, as determined in the two active-immunization protocols, could now be used to prevent NTHI residing in the nasopharynx from ascending the eustachian tube and inducing OM. In the latter study, we also introduced a novel recombinant peptide immunogen designated LPD-LB1(f)2,1,3, which was designed to incorporate portions of two NTHI antigens known to play a role in pathogenesis of OM. Recently, 99 clinical isolates of NTHI were examined for sequence heterogeneity in the 19-mer region of the P5-fimbrin adhesin protein that is included in the synthetic chimeric peptide, LB1. Three major groups, based on amino acid sequence diversity, were identified. The peptide LPD-LB1(f)2,1,3 was designed to contain the corresponding B-cell epitope from all three groups.
(The data in this paper were presented in part at the Twentieth and Twenty-Second Midwinter Research Meetings of the Association for Research in Otolaryngology, St. Petersburg Beach, Fla., 2 to 6 February 1997 and 14 to 18 February 1999, respectively, and the Third Extraordinary International Symposium on Recent Advances in Otitis Media, Copenhagen, Denmark, 1 to 5 June 1997.)
MATERIALS AND METHODS
Animals.
For all studies, we used healthy adult (approximately 450 to 500 g) or juvenile (approximately 300 to 350 g) chinchillas (Chinchilla lanigera) with no evidence of middle ear infection by either otoscopy or tympanometry. For the three immunization and challenge studies described, a total of 171 chinchillas were used. The mean weights of the chinchillas for each of the studies detailed below were 470 ± 60, 462 ± 9, and 302 ± 38 g, respectively. The animals were rested for 10 days upon arrival and were then bled nominally by cardiac puncture for collection of preimmune serum, which was stored at −70°C until use.
NTHI and adenovirus isolates.
All NTHI isolates used in the challenge studies are limited-passage clinical isolates cultured from either the nasopharynges (86-028NP) or middle ears (1715, 1128, and 1729) of children who underwent tympanostomy and tube insertion for chronic OM with effusion at Columbus Children’s Hospital. NTHI isolates 1128 and 86-028NP have been characterized in chinchilla models of OM (8, 11, 18, 46, 66). All the isolates were maintained frozen in skim milk plus 20% (vol/vol) glycerol until used; they were then streaked onto chocolate agar (BBL, Cockeysville, Md.) and incubated at 37°C for 18 h in a humidified atmosphere containing 5% CO2. Adenovirus serotype 1 was also recovered from a pediatric patient at Columbus Children’s Hospital and has been used in chinchilla models (6, 7, 9, 72).
Determination of the amino acid sequence variability of the fimbrin portion of the LB1 peptide.
We determined the nucleotide sequence encoding the fimbrin portion of the LB1 peptide in 53 U.S. NTHI strains from our laboratory and from 46 U.S. and European strains kindly provided by A. Forsgren (University of Lund, Malmö, Sweden).
Briefly, the DNA sequences were determined as follows. NTHI cells were lysed by boiling, and the DNA comprising the LB1(f) peptide-coding sequence was amplified with oligonucleotides derived from the nucleotide sequence of the gene encoding P5 (51). After purification, the amplified fragment was sequenced on an automated ABI sequencer with ABI-PRISM DNA sequencing-kit dye (ABI) and Amplitaq DNA polymerase FS (Perkin-Elmer, Foster City, Calif.). The resulting sequencing data were analyzed, translated, and aligned with Lasergene software (DNAstar, Inc., Madison, Wis.).
Immunogens used.
P5-fimbrin was isolated from NTHI 1128 as previously described (66), and P6 was isolated from NTHI 86-028NP, also as described (18). LPD was prepared from a recombinant Escherichia coli strain that contains plasmid pMG-LPD in which the expression of the gene encoding LPD (35) is under the control of a temperature-sensitive λpL promoter. After growth in a 20-liter fermentor, the bacteria were harvested and the recombinant product was isolated by detergent extraction (with 3% Empigen BB). LPD was further purified by successive cation-exchange, anion-exchange, and affinity (hydroxyapatite) chromatography. After concentration, the product was stored in phosphate-buffered saline (PBS; pH 6.8)–0.5% Empigen BB at −20°C until formulated for use. Protein D (PDm) is a recombinant nonacylated form of LPD lacking three N-terminal palmitate residues, whose expression and purification has been described (2, 3, 32).
LB1 is a 40-mer synthetic chimeric peptide composed of a putative B-cell epitope of the mature P5-fimbrin protein (Arg 117 to Gly 135), colinearly synthesized with a T-cell promiscuous epitope from measles virus fusion protein, and has been described previously (8). LB2 is also a 40-mer peptide and is composed of a second putative B-cell epitope of the mature P5-fimbrin protein (Tyr 163 to Thr 180) plus the same T-cell epitope. LB2 has also been described previously (8).
LPD-LB1(f)2,1,3 is a recombinant fusion peptide composed of an N-terminal LPD moiety followed by three sequential P5-fimbrin B-cell epitope peptides from LB1, called LB1(f), and a C-terminal polyhistidine purification tag. The sequence of LPD-LB1(f)2,1,3 is shown in Fig. 6. The three LB1(f) peptides incorporated into the polypeptide each represent a different NTHI group relative to this region of mature P5-fimbrin (described below) and are present in the following order: group 2a, group 1, and group 3. The gene encoding LPD-LB1(f)2,1,3 was constructed in a pMG27N-derived plasmid. This plasmid (27) is a pBR322 derivative in which the expression of a heterologous gene is under the control of a λpL promoter. The resulting plasmid has been transformed into E. coli AR58 (49). The recombinant protein was produced by growing the transformed bacteria at 30°C and then shifting the temperature to 37°C to induce the expression of the LPD-LB1(f)2,1,3 gene. After lysis of the cells, LPD-LB1(f)2,1,3 was isolated on a Qiagen nitrilotriacetate-Ni2+ column and then further purified on ion-exchange and gel filtration columns. After concentration with a Filtron Omega 10-kDa concentrator device, the protein solution was filtered through a 0.22-μm-pore-size filter. The resulting protein runs as a single band on a Coomassie brilliant blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel.
FIG. 6.
Amino acid sequence of LPD-LB1(f)2,1,3. Capital letters represent the LPD sequence; bold capital letters represent the sequences of the three LB1(f) peptides; underlined capital letters represent the histidine tag; lowercase letters represent linker regions. The box indicates the signal peptide.
First active-immunization trial.
Eleven cohorts of six adult chinchillas were established. The animals received a primary immunization of 10 to 20 μg of immunogen(s) subcutaneously (s.c.) as shown in Table 1. Cohorts receiving two different isolated NTHI outer membrane proteins received them at separate injection sites. The cohort designated fimbrin/CFA was the only cohort which received a total of 150 μg of protein and was included to allow direct comparison with cohorts from our earlier studies (8, 66). The boosting dose was given 30 days later (Table 1), and all the animals were bled 10 days after the boost and immediately prior to receiving an intranasal (i.n.) inoculation of 6 × 106 50% tissue culture infective doses of adenovirus (6, 9, 72).
TABLE 1.
Immunization protocol used
| Cohort designation | Primary dose (μg) | Boosting dose (μg) | Adjuvanta |
|---|---|---|---|
| Naive | NAb | NA | NA |
| Sham/AlPO4 | NA | NA | AlPO4 |
| Sham/CFA | NA | NA | CFA/IFA |
| Fimbrinc/CFA | 100 | 50 | CFA/IFA |
| Fimbrin/AlPO4 | 10 | 10 | AlPO4 |
| Fimbrin/LPDe/AlPO4d | 10 each | 10 each | AlPO4 |
| LPD/AlPO4 | 10 | 10 | AlPO4 |
| LB1/CFA | 10 | 10 | CFA/IFA |
| LB2/CFA | 10 | 10 | CFA/IFA |
| Fimbrin/P6f/CFAd | 10 each | 10 each | CFA/IFA |
| Fimbrin/P6/AlPO4d | 10 each | 10 each | AlPO4 |
When two adjuvants are listed, the first refers to the primary dose and second refers to the boosting dose.
NA, not applicable.
Fimbrin isolated from NTHI 1128.
Antigens inoculated into separate sites.
LPD isolated as described in Materials and Methods.
P6 isolated from NTHI 86-028NP.
Seven days after the adenovirus inoculation, four animals per cohort were given an i.n. inoculation of approximately 108 CFU of NTHI (86-028NP) in 200 μl of sterile pyrogen-free saline (Abbott Laboratories, North Chicago, Ill.) as well as a bilateral transbullar (t.b.) inoculation of 2,500 CFU of NTHI/ear in 300 μl of sterile pyrogen-free saline. This t.b. inoculation is a stringent direct challenge to the middle ears and results in a consistent moderate middle ear infection (ranked 2+ on a scale of 0 to 4+ inflammation) in both ears of naive or sham-immunized animals within 48 h (19, 66). The disease course induced by this inoculation route thus provides a basis for determining protective efficacy in immune cohorts. t.b. inoculation is also necessary in active-immunization protocols because the time required to fully immunize chinchillas results in their being too old for an optimal demonstration of the synergy between i.n.-inoculated adenovirus plus NTHI in inducing bacterial OM (our unpublished observations). One animal in each cohort was predesignated for sacrifice 10 days after NTHI challenge for procurement of inferior bulla mucosa and tympanic membranes for histological examination as previously described (66).
The remaining two animals per cohort received only an i.n. inoculation of NTHI 86-028NP. These i.n.-only animals were assessed for changes in NP colonization, as were other cohort members (described below) but were used primarily to monitor any unexpected OM in adult chinchillas coinfected with adenovirus, thus allowing us to detect any artifactual induction of disease due to technique.
Second active-immunization study.
Five cohorts of 10 chinchillas each were established. The cohorts received one of the following immunogens formulated with AlPO4 (100 μg/dose): LB1 (1 μg/dose), LB1 (10 μg/dose); LPD plus LB1 (each antigen prepared separately with AlPO4 and then mixed at a ratio of 10 μg to 1 μg, respectively, per dose), LPD (10 μg/dose), or AlPO4 only (sham cohort). Immunogen formulations were prepared by SmithKline Beecham Biologicals and provided in coded vials. The chinchillas were immunized s.c. with the designated immunogen (200 μl/dose) and boosted with an identical dose 28 days later. Observers were then blinded to cohort clustering by the coding of animals. Ten days after the boost, immune serum samples were collected by cardiac puncture and all 10 animals in each cohort were then inoculated i.n. with adenovirus and 7 days later with NTHI 86-028NP at inocula identical to those described above for the first active-immunization study. Five animals from each cohort also received a bilateral t.b. inoculation of approximately 2,500 CFU of NTHI as described above for the first active-immunization study.
Production of antisera for the passive-transfer protocol.
Four cohorts of five to eight chinchillas each were used to generate antisera against selected antigens. One cohort received 10 μg of LB1 delivered in CFA followed by two boosts (10 μg in incomplete Freund’s adjuvant [IFA]) at monthly intervals. This antiserum pool served as the positive control for this study, based on data acquired in the active-immunization trials reported herein. The remaining cohorts were immunized at 10 μg/dose with LPD, PDm, or the recombinant peptide LPD-LB1(f)2,1,3. LPD and PDm were used here to determine the relative protective efficacy that one could attribute to each of these immunogens alone in the chinchilla model and also to provide information about which may be the superior choice as an H. influenzae-derived carrier protein or immunogen. LPD, PDm, and LPD-LB1(f)2,1,3 were delivered in a combined adjuvant formulation of AlPO4 plus monophosphoryl lipid A (MPL) (Ribi ImmunoChem Research, Inc., Hamilton, Mont.) (200 μg of AlPO4 and 20 μg of MPL per dose), and this was followed by two identical boosts at monthly intervals. The immunogen preparations used here (except LB1/CFA, which was prepared in-house) were also provided in coded vials by SmithKline Beecham Biologicals. All immunizations were performed via s.c. injection. The animals were bled 10 days after receiving the third immunization, and sera were pooled by cohort for passive transfer.
Passive-transfer study.
A total of 55 juvenile chinchillas were used to establish five cohorts of 11 chinchillas each. Chinchilla sera were collected and screened individually by Western blotting to determine if any animal had a significant preexisting antibody titer to NTHI outer membrane proteins prior to enrollment in the study. Seven days before the NTHI challenge, the chinchillas received adenovirus i.n., as described above, and 1 day before the challenge, they were injected intracardially (5 ml/kg) (12, 66) with a 1:5 dilution of one of the four chinchilla antiserum pools or with the diluent alone (pyrogen-free sterile saline). They were then challenged exclusively i.n. in this study by passive inhalation of approximately 108 CFU of NTHI 86-028NP per animal. Observers knew neither which antiserum was received nor which animals formed a cohort.
Clinical assessment of experimental disease.
For all three studies, the animals were blindly evaluated by otoscopy and tympanometry (EarScan, South Daytona, Fla.) daily or every 2 days from the time of adenovirus inoculation until the end of the observation period. Signs of tympanic membrane inflammation were rated on a 0 to 4+ ordinal scale (66, 72), and tympanometry plots were used to monitor changes in both middle ear pressure and tympanic membrane compliance (24, 45). Tympanometry results indicated an abnormal ear if a type B tympanogram was obtained, compliance was ≤0.5 ml, or middle ear pressure was greater than −100 daPa (24, 26, 61). Clinical signs of respiratory tract infection, including ruffling of fur, conjunctivitis, altered character of nasal or ocular secretions, wheezing, labyrinthitis, and cornering behavior, were recorded.
An NP lavage was performed on all animals, on days 1, 4, 7, 10, 14, 18, 21, 28, and 35 after NTHI challenge, by passive inhalation of 500 μl of pyrogen-free saline in 5- to 10-μl droplets through one nare with collection of this fluid, on ice, from the contralateral nare as it was exhaled, as previously described (8, 18); this procedure was also performed on days 42 and 49 in the second active-immunization study. In the passive-transfer study, an NP lavage was performed only once, 4 days after NTHI challenge, to ensure that the animals were colonized. In both active-immunization studies, epitympanic taps to collect no more than 300 μl were attempted (10, 18) (on the same schedule as the NP lavages) on any animal which had an otoscopically determined retrievable middle ear fluid (minimum 2+ inflammation score) or yielded a type B tympanogram (24) as previously described. Serial epitympanic taps with culture were not performed in the passive-transfer study to prevent potential damage to middle ear mucosa or disruption of either the disease course or bacterial clearance.
Recovered middle ear fluids and NP lavage fluids were maintained on ice until serially diluted. Middle ear fluids were plated onto chocolate agar (Becton Dickinson Microbiology Systems, Becton Dickinson and Co., Cockeysville, Md.); NP fluids were plated onto chocolate agar containing bacitracin (BBL) to discourage growth of other members of the normal NP flora. All the plates were incubated at 37°C for up to 48 h for semiquantitative determination of the number of CFU of NTHI per milliliter. Animals were also tabulated as having a “colonized” or “cleared” status based on culture results. If NTHI was cultured from either site, the animal was considered colonized for that site. If a culture-negative status was both achieved and substantiated for an additional 7 to 10 days by plate count, an animal was considered cleared as of the first day it became culture negative. Finally, blood was obtained for serum from all chinchillas in active-immunization trials, prior to sacrifice, via cardiac puncture on days 35 and/or day 49 after NTHI challenge.
Assessment of serum titer and/or specificity by ELISA and Western blotting.
Briefly, enzyme-linked immunosorbent assays (ELISA) were performed with dilutions of either pooled chinchilla serum or tapped middle ear fluids from each cohort and were assayed against NTHI whole outer membrane protein preparations (0.5 μg/well), isolated outer membrane proteins (0.2 μg/well), and LB1 or LB2 (0.2 μg/well) in 96-well microtiter plates (Dynatech, Horsham, Pa.) (8). As previously described (7, 8, 67), the titer of a serum pool or of middle ear lavage fluids was defined as the reciprocal of the dilution consistently yielding an optical density at 490 nm showing a twofold increase over that of wells containing all components but immune serum. Western blotting was performed, also as described previously (10), with pooled immune serum diluted 1:100 or 1:200 as the primary antibody and horseradish peroxidase-conjugated protein A (Zymed) diluted 1:200 as the secondary antibody. The color was developed with 4-chloro-1-naphthol (Sigma).
For some assays, in addition to chinchilla antiserum pools, a murine monoclonal antibody (MAb 2C7; a gift from Alan Lesse, State University of New York at Buffalo, Buffalo, N.Y.) produced against H. influenzae biogroup aegyptius (strain F3031), which recognizes P5 and has been described previously (14, 51, 71), was used.
Immunogold labeling of NTHI.
NTHI isolate 86-028NP was indirectly immunogold labeled by incubation with the chinchilla antiserum pools used for passive transfer or with MAb 2C7, followed by incubation with gold-conjugated protein A or gold-conjugated goat anti-mouse immunoglobulin (Ig) (5- or 10-nm diameter gold particles; EY Laboratories, Inc., San Mateo, Calif.), respectively, as previously described (5). Briefly, an 18-h culture of NTHI was suspended in either sterile double-distilled H2O or sterile 0.01 M PBS (pH 7.2) and a carbon- and Formvar-coated grid was floated on a droplet of this suspension for 5 min. The grids were then blocked by incubation in 0.5% (wt/vol) ovalbumin in PBS for 30 min, rinsed three times in PBS, and floated on the chinchilla serum pools (diluted 1:40 in PBS) or MAb 2C7 (diluted 1:75 in PBS) for 15 min at room temperature. After three rinses in PBS, the grids were floated on the appropriate gold conjugate (diluted 1:100 in PBS) for 60 min. The grids were then blotted, floated on 2.5 M PBS (pH 7.2) for 10 min to remove nonspecific gold spheres, rinsed twice with PBS, and subjected to negative staining (2% ammonium acetate, 2% ammonium molybdate) for 5 min.
Statistical methods.
All the statistical analyses were conducted by the Biometrics Laboratory of The Ohio State University School of Public Health on data compiled prior to deblinding chinchilla cohorts. Cohorts in active-immunization studies were compared for differences in relative time to bacterial clearance of the nasopharynx or middle ear, as determined by culture-negative status, via a log rank statistic, and the results were illustrated with Kaplan-Meier survival analysis curves. Orthogonal rank comparisons were additionally performed to further elaborate the differences between individual cohorts. A P value of ≤0.05 was accepted as significant.
To test for a difference in CFU of NTHI per milliliter in day 4 NP lavage fluids among cohorts in the passive-transfer study, a Kruskal-Wallis one-way analysis of variance was conducted. A P value of ≤0.05 was considered significant.
For analysis of otoscopy data in the passive-transfer study, a repeated-measures analysis of variance was used to compare the pattern of responses over time (days) for chinchilla cohorts. Due to the large number of repeat observations for each animal, the analysis was divided into five parts: days 1 to 7, days 8 to 14, days 15 to 21, days 22 to 28, and days 29 to 35. The P value from each test of significance performed is presented along with any post hoc test performed. Tukey’s honestly significant difference (HSD) test was used for all post hoc multiple comparisons unless otherwise stated. Significance was assessed by using an alpha level of 0.05.
To test for significant inhibition of development of middle ear effusion in the passive-transfer study, a Z-test comparison of proportions was performed on each day the percentage of abnormal ears was >50% in sham-immunized animals. A P value of ≤0.005 was accepted as significant.
RESULTS
Characterization of immunogens.
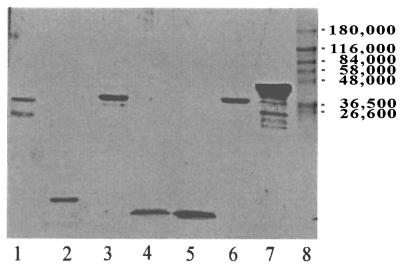
All isolated NTHI antigens, synthetic peptides, and recombinant immunogens used were characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (Fig. 1) and were assessed for purity by laser densitometry (Bio-Rad, Hercules, Calif.). The purity of each immunogen based on silver-stained preparations was as follows: P5-fimbrin, 99%; P6, 97%; LPD, 94%; LB1, 97%; LB2, 99%; PDm, 95%; LPD-LB1(f)2,1,3, 96% (predominant band). Minor bands in silver stain of LPD-LB1(f)2,1,3 (lane 7) represent degradation products. All were additionally shown to have less than 0.1% endotoxin content by weight as determined by a chromogenic Limulus assay (BioWhittaker, Walkersville, Md.).
FIG. 1.
Silver stain of the immunogens used in this study. Lanes: 1, P5-fimbrin; 2, P6; 3, LPD; 4, LB1; 5, LB2; 6, PDm; 7, LPD-LB1(f)2,1,3; 8, prestained molecular mass standards.
First active-immunization study.
Of the 66 chinchillas in this study, 5 were lost to attrition by the time of bacterial challenge and 3 animals were found dead during the study; therefore, 58 (95%) of the 61 animals completed the study as scheduled. Reciprocal pooled titers in serum, determined by cohort prior to bacterial challenge, against the immunogens delivered are shown in Table 2. All cohorts seroconverted, with each cohort demonstrating a reciprocal titer of ≥104 against the immunogen(s) delivered, with the exception of the fimbrin/P6/AlPO4 cohort, which yielded a low titer against P6 only. Cohorts immunized with any isolated NTHI outer membrane protein also demonstrated titers of ≥104 against whole outer membrane protein preparations from two NTHI isolates, as expected. Interestingly, when P5-fimbrin was used either as a single immunogen or in combination with P6, titers of ≥104 against P5-fimbrin were obtained regardless of the adjuvant used. However, simultaneous immunization of chinchillas with P5-fimbrin plus LPD had a suppressive effect on the immune response to P5-fimbrin. Immunization with LB2 induced a titer of 5 × 104 against LB2 and resulted in a reciprocal titer of 104 against isolated P6 as well as against both whole outer membrane protein preparations assayed. This response is probably due to use of the adjuvant CFA, which enhanced a preexisting immune recognition of P6, which is not uncommon in naive chinchillas.
TABLE 2.
Reciprocal titers in serum before NTHI challenge and effusion titers 4 days after challenge
| Cohort | Reciprocal titera against:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPDb
|
P5-fimbrinc
|
P6d
|
LB1
|
LB2
|
86-028NP OMPe
|
1128 OMP
|
||||||||
| Serum | Middle ear | Serum | Middle ear | Serum | Middle ear | Serum | Middle ear | Serum | Middle ear | Serum | Middle ear | Serum | Middle ear | |
| Naive | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 103 |
| Sham/AlPO4 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 |
| Sham/CFA | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 5 × 103 |
| Fimbrin/CFA | 1 × 102 | 1 × 103 | 5 × 104 | 2 × 104 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 104 | 1 × 104 | 5 × 104 | 2 × 104 |
| Fimbrin/AlPO4 | 1 × 103 | 1 × 102 | 1 × 104 | 2 × 103 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 104 | 2 × 103 | 1 × 104 | 2 × 103 |
| Fimbrin/LPD/AlPO4 | 5 × 104 | 5 × 104 | 1 × 103 | 2 × 103 | 1 × 103 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 5 × 104 | 2 × 103 | 5 × 104 | 2 × 103 |
| LPD/AlPO4 | 5 × 104 | 5 × 104 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 102 | 1 × 104 | 5 × 103 | 1 × 104 | 5 × 103 |
| LB1/CFA | 1 × 102 | 2 × 103 | 1 × 103 | 1 × 103 | 1 × 103 | 2 × 103 | 5 × 104 | 1 × 104 | 1 × 103 | 1 × 102 | 1 × 102 | 2 × 103 | 1 × 103 | 2 × 104 |
| LB2/CFA | 1 × 102 | 1 × 102 | 1 × 102 | 2 × 103 | 1 × 104 | 1 × 102 | 1 × 102 | 1 × 102 | 5 × 104 | 5 × 103 | 1 × 104 | 1 × 103 | 1 × 104 | 5 × 103 |
| Fimbrin/P6/CFA | 1 × 102 | 1 × 102 | 5 × 104 | 5 × 104 | 1 × 104 | 2 × 104 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 5 × 104 | 5 × 103 | 5 × 104 | 2 × 104 |
| Fimbrin/P6/AlPO4 | 1 × 102 | 1 × 103 | 1 × 104 | 2 × 103 | 1 × 103 | 1 × 103 | 1 × 102 | 1 × 103 | 1 × 102 | 1 × 102 | 5 × 104 | 2 × 103 | 1 × 104 | 5 × 103 |
Titers of ≥104 are shown in bold type.
LPD was isolated as described in Materials and Methods.
P5-fimbrin isolated from NTHI 1128.
P6 isolated from NTHI 86-028NP.
OMP, outer membrane protein.
Reciprocal titers against the immunogens delivered, in middle ear fluids of animals in each cohort on the first day when epitympanic taps were performed (4 days after challenge), are shown in Table 2 alongside the corresponding titers in serum. Titers in middle ear fluid largely reflect the titers in serum in these cohorts. This observation is consistent with data we reported previously showing that middle ear lavage fluids obtained from immunized chinchillas demonstrated transudation of serum antibody into the tympanum and that this phenomenon is promoted by adenovirus infection (7). The higher antibody titers in serum against P5-fimbrin and P6 obtained when CFA was used as the adjuvant than when AlPO4 was used corresponded to higher antibody titers in middle ear fluids as well.
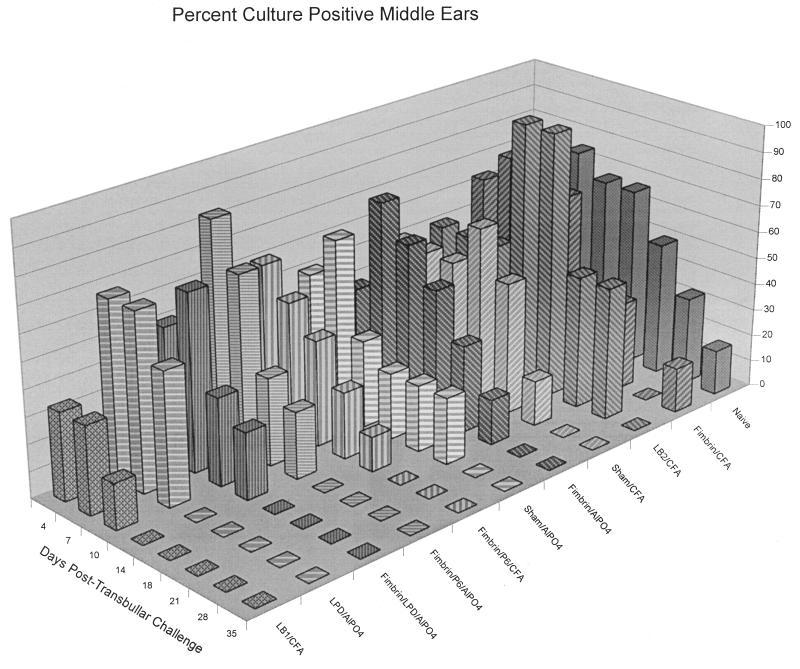
The relative clearance of NTHI from the middle ears of the animals in each of the 11 cohorts in this study is depicted in Fig. 2. The cohort immunized with LB1/CFA was cleared of culturable NTHI by 14 days after direct challenge of the middle ears. This shorter time to clearance was significant (P ≤ 0.01) relative to naive animals as well as to any of the nine remaining immunized cohorts. While the ears of all the animals in the LPD/AlPO4 cohort were similarly cleared by day 14, this was not statistically significant compared to all other cohorts (P = 0.5). The lack of significance in the LPD/AlPO4 cohort was due, in part, to the small sample size for this study (eight ears per cohort maximum); however, individual ears were also predominantly rendered culture negative on days 10 and 14 in this cohort rather than on days 1, 4, 7, or 10 as occurred in animals immunized with LB1/CFA. The next most rapidly augmented clearance of NTHI from the middle ear occurred in cohorts receiving P5-fimbrin combined with either LPD or P6, both delivered in alum. Although clearance of the middle ears 18 days after direct challenge was highly intriguing, it was not statistically significant. No evidence of significantly augmented clearance of NTHI from the middle ears of animals immunized with the following antigens was noted despite antibody titers in serum of 1 × 104 to 5 × 104 against the immunogen(s) delivered: fimbrin/CFA, fimbrin/AlPO4, LB2/CFA, or fimbrin/P6/CFA.
FIG. 2.
Three-dimensional bar graph depicting the percentage of ears per cohort which were culture positive for NTHI in middle ear fluids over time.
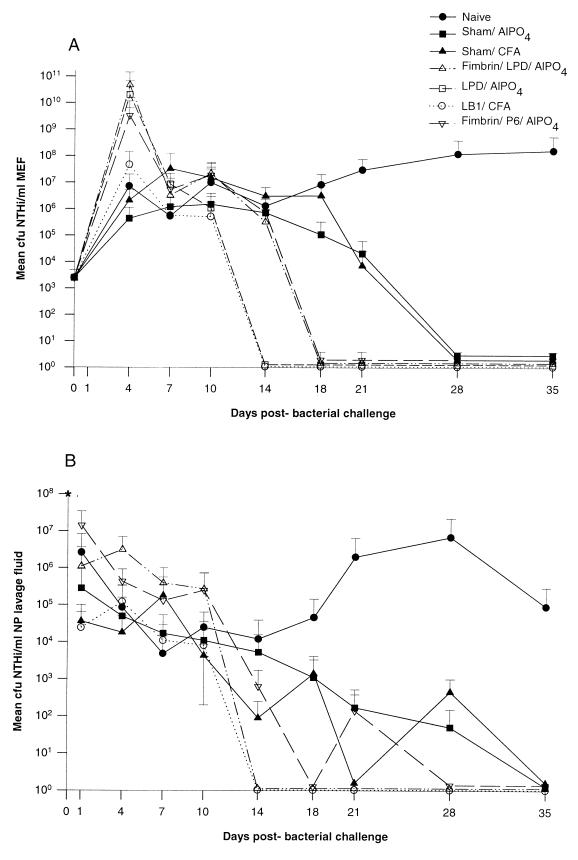
Figure 3A depicts the mean CFU of NTHI per milliliter of middle ear fluid over time for i.n.-plus-t.b. challenged animals (no OM developed in i.n.-only inoculated chinchillas) for 7 of the 11 cohorts described in Table 1. As is typical for direct challenge of the middle ear (17, 18, 66), all cohorts demonstrated a 2- to 7-log-unit increase in the CFU of NTHI per milliliter of middle ear fluid in the first 4 days after challenge, thus demonstrating initial bacterial growth in the middle ear. For the first 10 days after middle ear challenge, there were no consistent statistical differences among the 11 cohorts in terms of mean CFU of NTHI per milliliter. Thereafter, however, the relative effect of immunization, or lack thereof, was clearly discernible among the cohorts. Naive animals maintained culture-positive ears with effusions containing 106 to 108 CFU of NTHI/ml of middle ear fluid up to 5 weeks after challenge (Fig. 2 and 3A). Sterile middle ear fluids or a “dry” middle ear status was, however, ultimately achieved in all ears of all animals in the remaining 10 cohorts by the time points indicated. Both the sham/AlPO4 and the sham/CFA cohorts cleared NTHI from their middle ears on day 28, 7 days earlier than did the naive animals, which remained culture positive until day 35. This earlier time to clearance was not significant, yet these two control cohorts served to demonstrate that animals receiving adjuvant alone are given a degree of nonspecific “protection” not seen in naive animals, as has been previously reported (18).
FIG. 3.
(A) Dynamics of middle ear colonization in CFU of NTHI per milliliter of fluids recovered from adenovirus-infected chinchilla cohorts immunized as indicated and challenged t.b. and i.n. with NTHI 86-028NP. Only four cohorts which demonstrated moderate to significantly augmented clearance plus the naive and adjuvant-only control cohorts are included. (B) NP colonization dynamics in CFU of NTHI per milliliter of lavage fluid of adenovirus-infected chinchilla cohorts immunized as indicated and challenged with NTHI 86-028NP. Only three cohorts which demonstrated moderate to significantly augmented clearance plus naive and adjuvant-only cohorts are included. The asterisk at day 0 indicates the inoculum of NTHI instilled (108 CFU). Results are means and standard deviations (number of determinations were six per cohort for days 1 to 10 and five per cohort for days 14 to 35).
Otoscopy and tympanometry data (not shown) correlated well with bacterial culture results from middle ear fluids as an indication of the relative incidence and severity of experimental OM in each of the 11 total cohorts. Additionally, 9 days after i.n.-plus-t.b. challenge, only the LB1/CFA, LPD/AlPO4, and fimbrin/LPD/AlPO4 cohorts had one-third or fewer of the ears with a 3+ (or significant) degree of tympanic membrane inflammation.
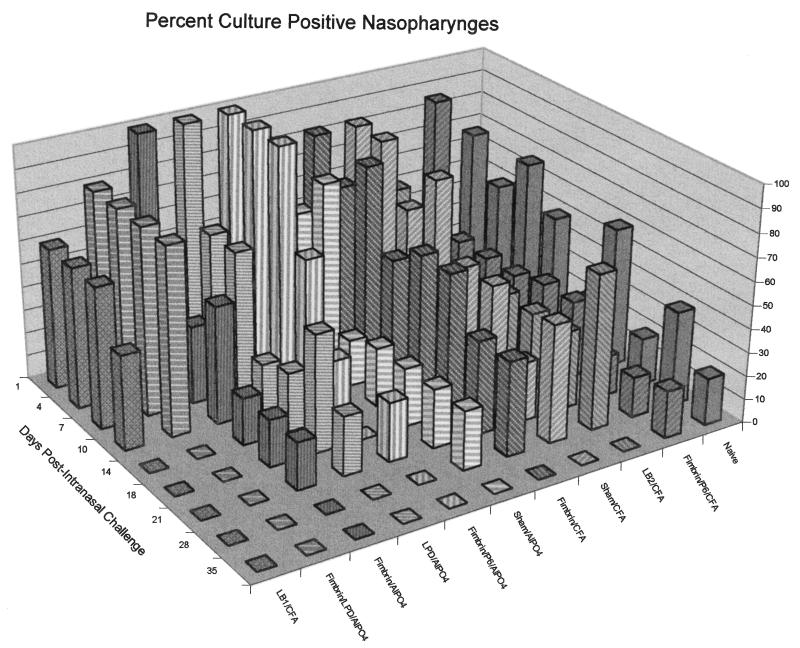
Figure 3B depicts mean CFU of NTHI per milliliter of NP lavage fluid over time for 6 of these 11 cohorts. The remaining cohorts were not afforded any demonstrable augmented clearance effect in the nasopharynx, and the results are therefore not shown. Unlike middle ear fluid culture data (Fig. 3A), which demonstrated an initial 2- to 7-log-unit increase in CFU of NTHI per milliliter, NP lavage fluids revealed a 1- to 4-log-unit decrease 1 day after challenge, indicating less initial bacterial multiplication in this site and also potentially rapidly effective clearance mechanisms. The naive cohort again provides the baseline from which relative efficacy was determined. One or two animals in the naive cohort were still colonized with ∼105 CFU of NTHI/ml of NP lavage fluid up to 5 weeks postinoculation (Fig. 3B and 4), whereas the sham/CFA and sham/AlPO4 cohorts showed a slow decrease from a colonization level of 104 to 105 CFU/ml to no culturable NTHI in any animal 35 days after challenge. These two control cohorts again serve to demonstrate the marginal, nonspecific effect of adjuvant alone on clearance of NTHI from the nasopharynx. Unlike naive animals, all other cohorts in this study achieved complete clearance of the nasopharynx in all animals by the day indicated.
FIG. 4.
Three-dimensional bar graph depicting the percentage of animals per cohort which were culture positive for NTHI in NP lavage fluids over time.
The percentage of animals in each cohort which were colonized in the nasopharynx decreased over time (Fig. 4). The two cohorts which were cleared of culturable NTHI from the nasopharynx on the earliest (day 14) were those receiving either LB1 in CFA or the combined immunogen fimbrin/LPD/AlPO4. The fimbrin/P6/AlPO4 cohort was initially considered cleared by day 18, although one animal was again culture positive on day 21. While these times to clearance were 17 to 21 days earlier than that achieved in the naive cohort, they were not statistically significant, in part due to the small sample size.
Middle ear mucosa and tympanic membrane specimens collected from one animal in each cohort 10 days after challenge showed various degrees of the hallmark signs of OM induced by t.b. inoculation of NTHI (e.g., subepithelial edema, focal bleeding, infiltration of polymorphonuclear leukocytes, and epithelial hyperplasia (data not shown). The relative severity of the histopathological signs noted correlated well with the overall assessment of the incidence and severity of induced middle ear infection, or lack thereof, reported above for each cohort on the basis of otoscopy, tympanometry, and bacterial culture data. However, tremendous histopathological changes indicative of immunopathology, as demonstrated by an infiltration of mononuclear leukocytes and extensive osteoneogenesis in inferior bulla specimens, were recorded for only two cohorts, those immunized with fimbrin/CFA or LB2/CFA.
Second active-immunization study.
The results of the first study indicated that LB1 and LPD were the most effective of the potential immunogens tested. Therefore, a second trial was established with LB1, LPD, or combinations of these immunogens. Additionally, to more closely mimic immunogen preparations which would be suitable for children, AlPO4 was used exclusively as the adjuvant in this trial. Of the 50 animals enrolled, 3 were lost to attrition by the time of bacterial challenge. Forty-one (87%) of the remainder survived until the completion of the study. Reciprocal titers in serum collected 10 days after the boosting dose against immunogens delivered were as follows: LB1 cohort (1 μg/dose), 100; LB1 cohort (10 μg/dose), 5 × 103; LPD plus LB1 cohort (10 μg and 1 μg/dose), 105 against LPD and 100 against LB1; LPD cohort (10 μg/dose), 105.
The animals in the second active-immunization trial were challenged with identical doses to those in the previous trial: all the animals were inoculated i.n. with approximately 108 CFU of NTHI 86-028NP; however, in this trial, half (5 of 10 animals in each cohort) also received a t.b. inoculation of 2.5 × 103 CFU of NTHI bilaterally. This dual-site challenge of half of the animals in each cohort and i.n.-only challenge of the other half was done to better allow a determination of the effect of parenteral immunization on NP colonization alone as well as on the clearance of NTHI from the middle ear and nasopharynx in these cohorts.
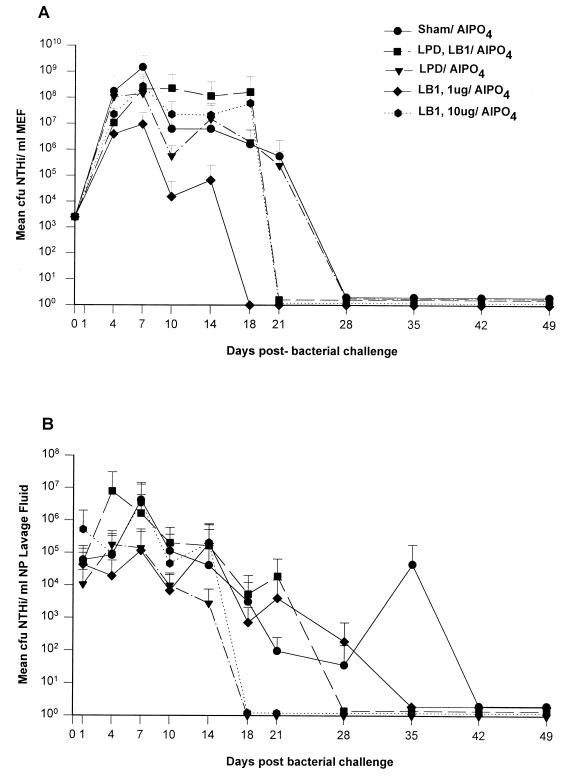
A plot of mean CFU of NTHI per milliliter of middle ear fluid for i.n.-plus-t.b.-challenged animals in each of the five cohorts over the 49-day period of observation is given in Fig. 5A. As reported in the first active-immunization study, bacterial counts in middle ear fluids increased over the first 4 days and thereafter ranged between 106 and 109 CFU of NTHI/ml for approximately 7 days after the challenge. The cohort receiving only AlPO4 maintained 105 to 107 CFU of NTHI/ml from days 10 to 21 before clearing on day 28, as did a similarly immunized cohort in the first active-immunization study (see above). Despite a reciprocal antibody titer in serum of 105 LPD-immunized animals did not clear NTHI from the middle ears earlier than controls. However, the cohort immunized with LB1 (1 μg/dose) was bacteriologically sterile on day 18. This difference in time to clearance of the middle ears was significant relative to sham-immunized controls (P ≤ 0.005). Animals immunized with either a combination of LPD and LB1 or LB1 alone (10 μg/dose) cleared bacteria from the middle ear 3 days later, on day 21 after challenge, which was not statistically significant. The somewhat paradoxical effect that the lower dosage of LB1 was more efficacious in the middle ear than the higher dosage was at least partially due to an atypically high preexisting antibody titer in serum against a whole outer membrane protein preparation derived from the NTHI challenge isolate that was demonstrated in several chinchillas randomly assigned to the low-dose LB1 cohort. Their titers were 10 times that observed in chinchillas assigned to the higher-dose LB1 cohort. This disparity was demonstrated only after the study was completed and resulted in a protocol change wherein animals were prescreened for existing titer on an individual basis, rather than by cohort, prior to enrollment in future challenge studies. Once cleared of NTHI, all cohorts remained so for an additional 21 to 31 days.
FIG. 5.
CFU of NTHI per milliliter of recovered middle ear fluids (A) or NP lavage fluids (B) in chinchillas immunized as indicated in the figure and challenged i.n. and t.b. with NTHI 86-028NP. Results are means and standard deviations.
A plot of mean CFU of NTHI per milliliter of NP lavage fluid for all animals in the same five cohorts over the 49-day observation period is given in Fig. 5B. The earliest clearance (day 18) was achieved only in cohorts inoculated with the higher dose of LB1 (10 μg/dose) or with LPD, although neither of these differences in time to clearance was statistically significant. The lack of significance was at least partly because of the small sample size due to a greater than expected number of anesthesia-related deaths in this study. Other cohorts were cleared of NTHI on days 28 to 35 after challenge, and all cohorts remained culture negative for NTHI once they were considered cleared. Overall, the cohorts immunized with LB1-containing immunogens again performed well, although both titers in serum and efficacy were notably lower than those achieved in the first study. This was largely attributed to the use of alum as the adjuvant.
PCR amplification of the LB1(f) region of P5-fimbrin.
The results of both active-immunization studies indicated that LB1 was capable of inducing a protective immune response. Prior to initiating another study, we wanted to determine the conservation of sequence in the P5-fimbrin protein across the region included in the LB1 peptide. To do this, 99 clinical NTHI isolates were subjected to PCR amplification and nucleotide sequencing of the 19-mer putative B-cell epitope region of mature P5-fimbrin [LB1(f)] and grouped according to analysis of the deduced amino acid sequence (Table 3). After alignment of sequences (each 13 to 22 amino acids long; approximate AA positions 110 to 140), NTHI strains from both the United States and Europe segregated into three major groups, using the criterion that assignment to a particular group required at least 75% identity to the consensus sequence of that group (Table 3). Group 2 was further divided into subgroups 2a and 2b based on limited but consistent sequence differences. Approximately 75% of these 99 isolates belonged to group 1, 16% belonged to group 2, and 8% belonged to group 3. However, due to the existence of three distinct groups and the likelihood that these three LB1(f) groups were also antigenically distinct and could potentially be combined to create a more broadly protective immunogen, the recombinant peptide LPD-LB1(f)2,1,3 was designed (Fig. 6), expressed in E. coli, and isolated as described above for use in the third chinchilla study.
TABLE 3.
Aligned NTHI group 1, 2, and 3 LB1(f) peptide sequences
| NTHI strain | Group | Consensus sequence | No. of amino acids | No. of U.S. strains (% of total)a | No. of European strains (% of total)a |
|---|---|---|---|---|---|
| 1128 | 1 | RSDYKFYEDANGTRDHKKG--- | 19 | 53 (76) | 22 (76) |
| 1715 | 2a | RSDYKLYNKNSSSNSTLKNLGE | 22 | 7 (10) | 4 (14) |
| 183 | 2b | RSDYKLYNKNSS---TLKDLGE | 19 | 3 (4) | 2 (7) |
| 1729 | 3 | RSDYKFYDN------KRID--- | 13 | 7 (10) | 1 (3) |
Of 99 U.S. and European strains tested.
Passive-immunization study.
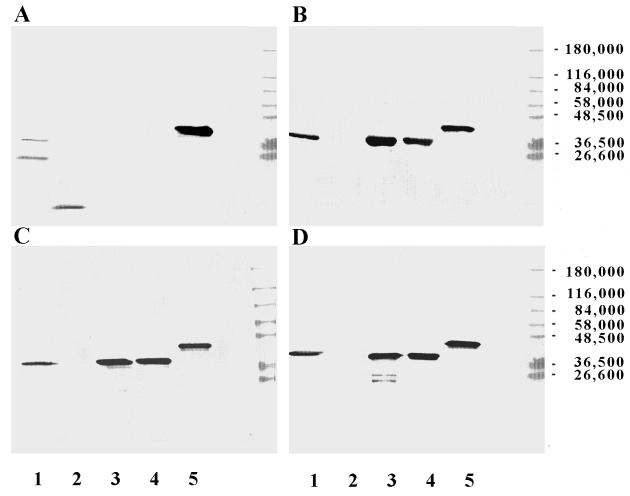
For the passive-immunization trial, antisera were generated against PDm, LPD, LB1, and LPD-LB1(f)2,1,3. These antisera were characterized by ELISA and Western blotting before being used in the passive-protection trial. Reciprocal antibody titers in serum against the immunogens delivered were determined a minimum of three separate times for each serum pool. The titers were 1 × 104 to 5 × 104 for anti-PDm, 5 × 104 for anti-LPD, 5 × 104 to 1 × 105 for anti-LB1, and 5 × 104 to 1 × 105 for anti-LPD-LB1(f)2,1,3. Recognition of immunogens by Western blotting is shown in Fig. 7 for chinchilla anti-LB1, anti-LPD, anti-PDm, and anti-LPD-LB1(f)2,1,3. Anti-LB1 recognized itself as well as the recombinant peptide and both the fully and partially denatured species of P5-fimbrin in a whole outer membrane protein preparation, as we have reported previously (8). Anti-LPD recognized an approximately 42-kDa protein (which is probably native LPD) in a whole outer membrane protein preparation, the recombinant proteins LPD and PDm, and the recombinant peptide LPD-LB1(f)2,1,3. Anti-PDm demonstrated reactivity similar to anti-LPD. Anti-LPD-LB1(f)2,1,3 recognized recombinant LPD and PDm as well as a 42-kDa protein (presumed to be LPD) within the whole outer membrane protein preparation and LPD-LB1(f)2,1,3 but did not recognize LB1 under the conditions used for this blot. However, anti-LPD-LB1(f)2,1,3 did recognize LB1 when assayed against this synthetic peptide alone (data not shown); thus, the lack of recognition of LB1 in the Western blot shown in Fig. 5 is probably due to a relatively low concentration or perhaps relatively low avidity of LB1-binding antibodies available in the anti-LPD-LB1(f)2,1,3 serum pool.
FIG. 7.
Western blot composite of the pooled chinchilla sera that were passively transferred to adenovirus-infected chinchillas prior to i.n. challenge with NTHI 86-028NP. Lanes: 1, whole NTHI 86-028NP outer membrane protein; 2, LB1; 3, LPD; 4, PDm; 5, LPD-LB1(f)2,1,3. The serum pools used were anti-LB1 (A), anti-LPD (B), anti-PDm (C), and anti-LPD-LB1(f)2,1,3 (D).
Of the 55 animals enrolled in the passive-transfer protocol, all survived until the bacterial challenge and all exhibited characteristic signs of adenovirus infection prior to bacterial challenge; these signs included conjunctivitis, tympanic membrane retraction with underpressured middle ears, cornering behavior, and ruffling of fur. Of these 55 chinchillas, 51 (93%) completed the study. All five cohorts were confirmed to be colonized with NTHI 4 days after i.n. challenge, with 75% of chinchillas demonstrating culturable NTHI in NP lavage fluids. The mean concentration of NTHI for all animals was 5.0 × 103 ± 1.0 × 104 CFU per ml of lavage fluid. There was no difference among individual cohorts in mean CFU per milliliter at this time point (P = 0.4).
Protective activity of passively delivered antiserum against the development of middle ear fluids (effusion).
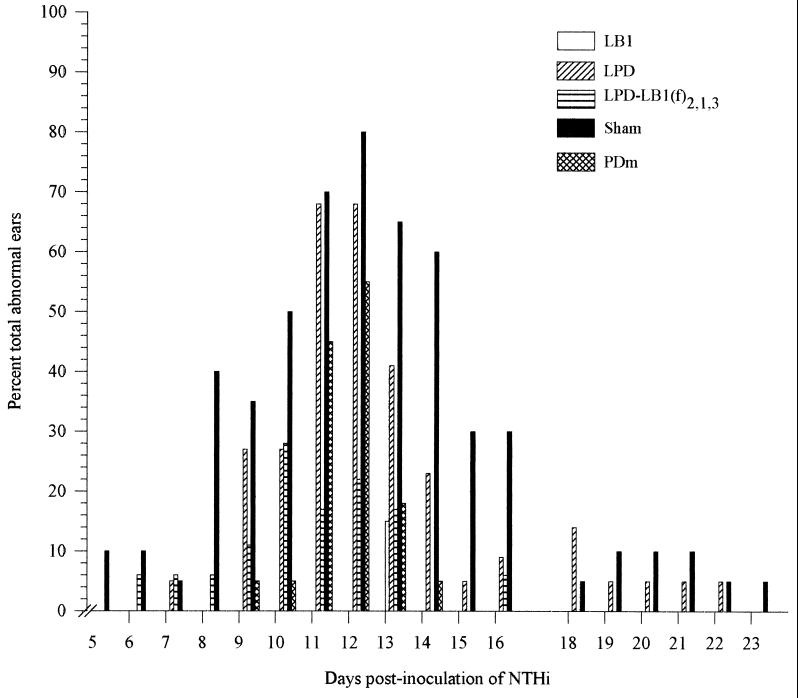
Middle ears with a directly visible effusion by otoscopy and those with either wide, flat tympanograms, tympanograms exhibiting normal pressure but low compliance (≤0.5 ml), or greatly underpressured middle ears (≥−100 daPa) were considered abnormal (24, 26, 45, 61). Based on these findings, 10% of the ears of the sham-immunized animals were abnormal on day 5, reaching 80% of ears (16 of 20) with an effusion on day 12 (Fig. 8). In the cohort receiving a 1:5 dilution of anti-LB1 serum, 3 (15%) of 20 ears were tympanometrically diagnosed as containing an effusion on day 13 only. In the cohort receiving antiserum against LPD-LB1(f)2,1,3 (diluted 1:5), 5% of ears contained an effusion on day 6, peaking at 28% (5 of 18) on day 10 after NTHI challenge. Chinchillas receiving similarly diluted anti-PDm serum demonstrated abnormal ears beginning on day 9 in 5% of ears, peaking on day 12 with 55% of ears (12 of 22) reported as containing an effusion. Within the cohort receiving anti-LPD serum (diluted 1:5), the first signs of abnormality were observed 7 days after NTHI challenge in 5% of the ears, peaking at 68% of ears (15 of 22) with effusion on days 11 and 12.
FIG. 8.
Percentage of total abnormal ears based on otoscopy and tympanometry in the five adenovirus-compromised chinchilla cohorts which received either sterile diluent or a 1:5 dilution of anti-LB1, anti-LPD, anti-PDm, or anti-LPD-LB1(f)2,1,3 by passive transfer prior to i.n. challenge with NTHI 86-028NP.
Thus, compared to sham-immunized animals, in which more than 50% of ears contained a middle ear effusion on days 11, 12, 13, and 14 after NTHI challenge, delivery of anti-immunogen serum by passive transfer reduced the incidence of OM on these four days by 100, 100, 77, and 100%, respectively, in the anti-LB1-immunized cohort; 76, 73, 74, and 100%, respectively, in the anti-LPD-LB1(f)2,1,3-immunized cohort; 36, 31, 72, and 92%, respectively, in the anti-PD-immunized cohort; and 3, 15, 37, and 62%, respectively, in the anti-LPD-immunized cohort. This difference in the percentage of ears containing an effusion was significant (P ≤ 0.001) on all four of these days for animals receiving either anti-LB1 or anti-LPD-LB1(f)2,1,3. For animals receiving anti-PDm serum, this difference was significant (P ≤ 0.001) on days 13 and 14, and for those receiving anti-LPD, this difference was significant (P = 0.02) on day 14 only.
Protective activity of passively delivered antiserum against signs of tympanic membrane inflammation.
Otoscopically determined mean tympanic membrane inflammation scores (not shown) corroborated the protective efficacy against effusion development as reported above. Inflammation began to steadily increase in all cohorts, with animals within the sham-immunized cohort showing the earliest signs of moderate (2+) OM. This occurred approximately 6 days after NTHI challenge and peaked on day 12, with inflammation greater than that attributable to adenovirus alone being maintained for approximately 12 days thereafter. This disease course is highly consistent with earlier data obtained with this model of NTHI ascension of the eustachian tube from a colonized nasopharynx (72). Up to 7 days after NTHI challenge, there were no statistically significant differences among cohorts. Throughout the remainder of the study, however, the sham-immunized cohort exhibited the greatest mean tympanic membrane inflammation scores and evidence of development of OM. Sham-immunized animals yielded a significantly greater mean inflammation score than did the cohort receiving anti-LB1 serum or the cohort receiving anti-LPD-LB1(f)2,1,3 serum on days 12 and 14, respectively (P ≤ 0.001). In addition, on all seven days from days 15 to 21 after NTHI challenge, inflammation was significantly greater in both ears of all sham-immunized animals than that in both ears of all animals in the cohorts which received antiserum directed against PDm, LB1, or LPD-LB1(f)2,1,3 (P < 0.001).
The cohort receiving anti-LB1 antiserum thus demonstrated significantly less inflammation than did the sham-immunized cohort on a total of 14 observation days during the study, including days 12 and 15 to 21 (as stated above) as well as days 25, 26, and 27 (P < 0.001) and days 29, 32, and 33 (P = 0.02). Receipt of anti-LB1 serum prevented the mean tympanic membrane inflammation from ever exceeding a 1.5+, which is the level of inflammation that can be attributed solely to i.n. inoculation of adenovirus (72). The cohort receiving anti-LB1 also exhibited significantly less inflammation than did the cohort receiving either anti-PDm or anti-LPD serum on days 25 and 28, respectively (P < 0.001). No other significant differences among cohorts were noted.
Immunogold labeling.
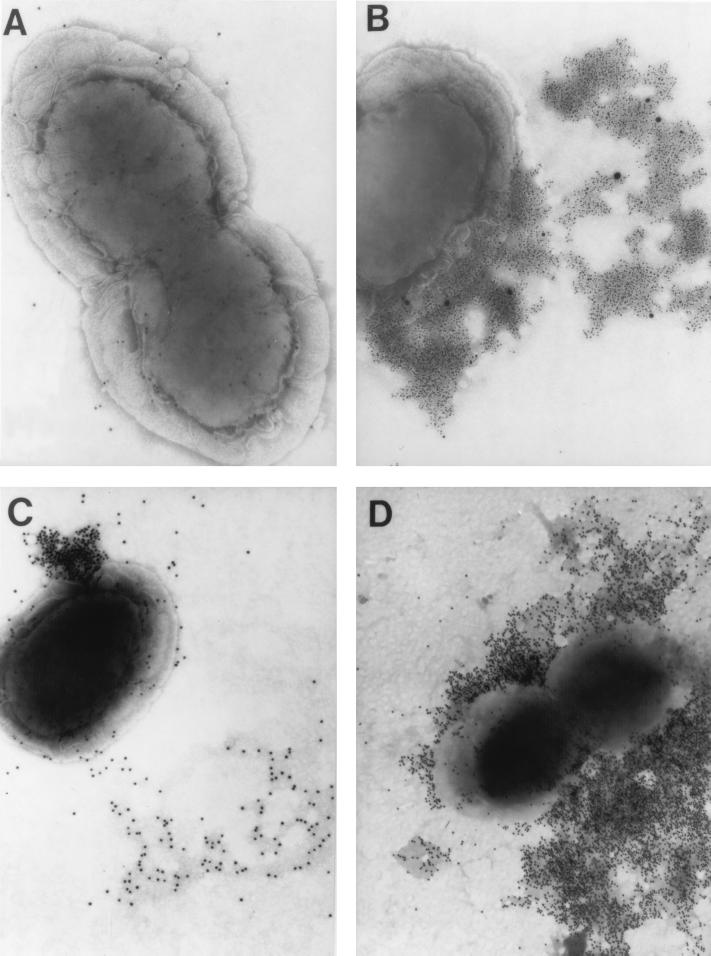
Transmission electron microscopy (TEM) observation of immunogold-labeled NTHI 86-028NP indicated that neither chinchilla anti-LPD (Fig. 9A) nor anti-PDm serum pools resulted in a significant or distinct immunolabeling pattern (data not shown). When NTHI 86-028NP was incubated with either anti-LB1 or anti-LPD-LB1(f)2,1,3 followed by gold-conjugated protein A or with MAb 2C7, a murine MAb directed against P5-fimbrin, followed by gold-conjugated goat-anti mouse IgM, IgA, plus IgG, all three sera yielded distinct immunolabeling patterns. Each of these sera and MAb 2C7 resulted in an immunolabeling pattern in which a limited region of the outer membrane was more heavily labeled rather than there being a uniform circumferential or surface labeling. Gold spheres were often tightly clustered close to the outer membrane in an almost spherical array (Fig. 9C) or fanned away from the bacterial cell and appeared to be associated with regions of the grid surface which accumulated the negative stain more heavily (Figs. 9B to D). In other TEM images (not shown), the outer membranes of bacterial cells were lightly labeled randomly; however, they were in close proximity to large clusters of gold, which were labeling bacterial cell-free masses of different sizes that had no distinct, discernible morphology. The heaviest gold-labeling patterns were obtained with anti-LPD-LB1(f)2,1,3 (Fig. 9D); however, the patterns of labeling, in terms of distribution of gold spheres, were very similar for these three antisera.
FIG. 9.
TEM composite of NTHI 86-028NP indirectly immunogold labeled with chinchilla antisera followed by either gold-conjugated protein A or gold-conjugated goat anti-mouse Igs. NTHI was incubated with chinchilla anti-LPD (A), MAb 2C7 (B), chinchilla anti-LB1 (C), or chinchilla anti-LPD-LB1(f)2,1,3 (D). Magnification, ca. ×65,000 (A and B) and ×54,000 (C and D).
Thus, immunization of chinchillas with either LB1 or LPD-LB1(f)2,1,3 resulted in polyclonal antisera containing antibodies which recognized surface-accessible epitopes on NTHI similar to those recognized by the P5-specific MAb 2C7. In addition, all three antisera recognized epitopes contained within material which was either somewhat tenuously associated with the bacterial cell (and thereby disassociated by the techniques used to prepare the specimens for microscopy) or partially released or exported as part of an active process.
DISCUSSION
Data obtained in the first active-immunization trial provided us both with an initial indication of which of the NTHI immunogens tested were the most promising (and thus of the greatest interest for further development) and with some insight into where, within the chinchilla uppermost airway, the antibodies generated were exerting a bacterial clearance effect. Both LPD and LB1 were effective in potentiating middle ear clearance when used alone, whereas combining either P6 or LPD with P5-fimbrin enhanced the ability of the chinchilla to clear NTHI from a directly challenged middle ear over that achievable with P5-fimbrin immunization alone. Combining P5-fimbrin with LPD also resulted in the ability to enhance clearance of NTHI from the nasopharynx 14 days after challenge, a phenomenon that could not be induced by either of these immunogens delivered singly. Unique to the chimeric peptide LB1, formulated in this study with CFA, was the ability to induce both notably augmented clearance of NTHI from the nasopharynx and significantly augmented clearance from the middle ears when used as a single immunogen. While use of the powerful adjuvant CFA clearly played a role here, the incorporation of a T-cell promiscuous epitope into the design of peptide LB1 probably also contributed to the marked efficacy of this immunogen. Enhancement of T-cell responses augments the clearance of NTHI from the lower airway as well (41, 42).
Any site-specific effect of the induced antibodies noted may have been due to microenvironment-sensitive altered expression of antigens by NTHI as well as to qualitative differences between the antibodies produced, since the presence of a high antibody titer in serum alone did not ensure an enhanced bacterial clearance effect in this study. The importance of a specific functional activity of antibody in addition to achieving a desired titer has been reported for other systems as well (54, 65).
In the second active-immunization study, we focused on two of the preliminarily tested immunogens, LB1 and LPD, delivering each alone and as a mixed preparation and using alum as the adjuvant. LPD was not efficacious in the middle ears of chinchillas in the second active-immunization study whereas it had been when used at an identical dose (and with the same adjuvant) in the first study despite inducing reciprocal serum antibody titers of 105 against LPD in the cohorts receiving it in both studies. It was later hypothesized that this variability in the observed results was the consequence of the use of two experimental lots of LPD with different contaminant profiles. Combining LPD with LB1 at a 10:1 ratio did not enhance the relative efficacy of either immunogen in the middle ear or in the nasopharynx in this study; in fact it significantly reduced the efficacy of LB1 (at 1 μg/dose) in the middle ears.
Finally, while LB1 at 1 or 10 μg/dose again induced either significantly augmented clearance of NTHI from the middle ears or notable but not significant clearance of NTHI from the nasopharynx, respectively, relative to control animals, delivery with alum resulted in markedly reduced titers in serum as well as reduced efficacy compared to that observed in the first active-immunization trial. This phenomenon of low antibody titers in serum being induced in chinchillas immunized with antigens prepared with alum as the adjuvant has been reported by Green et al. (26). These data, combined with those obtained in the first active-immunization trial, collectively indicated the need to induce greater antibody titers in serum and to avoid combining antigens in a manner which could result in suppression of the immune response to one or both of them.
In the final study, three of the four sera used for passive transfer were generated by using alum combined with MPL as the adjuvant. MPL is an immune response modifier (63) and was selected due to its demonstrated ability to enhance the immunogenicity of NTHI surface antigens in mice and rabbits, particularly with regard to IgG production (29). These antisera were delivered passively, and the ability to actually prevent the development of OM was now assessed instead of simply measuring augmented clearance of inoculated NTHI. Data obtained from the positive-control cohort in this study confirmed and expanded on earlier observations made with LB1 (8). Immunization with LB1 prepared with either CFA plus IFA or alum resulted in the generation of antibodies which had been shown to augment the clearance of NTHI from the nasopharynx and middle ears in the two active-immunization trials; however, in the passive-immunization study, these antibodies (again generated with CFA) were additionally shown to prevent NTHI from gaining access to the middle ear and inducing OM in adenovirus-compromised chinchillas. Delivery of anti-LB1 serum effectively prevented the development of a middle ear effusion in all ears of all animals during the entire period of observation except for 3 of 20 ears on day 13 after NTHI challenge. Moreover, animals in the LB1/CFA cohort never demonstrated a mean tympanic membrane inflammation score which exceeded that attributable to adenovirus alone. While the use of CFA probably again contributed to the demonstrated efficacy, data from the second active-immunization trial in which LB1 was delivered with alum suggest that this immunogen itself contributes largely to the protective effects noted.
Animals given a 1:5 dilution of anti-LPD-LB1(f)2,1,3 serum were also afforded significant protection from development of OM in the passive-transfer study. While mean tympanic membrane inflammation slightly exceeded that which adenovirus alone could have induced for approximately 4 days, these signs of OM were rapidly resolved. Moreover, the presence of middle ear effusion was significantly less in this cohort than that observed in sham-immunized animals on all days during the peak of the disease course.
Otoscopy showed that antiserum directed against PDm also conferred some protection to chinchillas. Animals in this cohort demonstrated significantly reduced tympanic membrane inflammation relative to sham-immunized animals from days 15 to 21 after challenge. This antiserum pool, however, was not as effective as either anti-LB1 or anti-LPD-LB1(f)2,1,3 in preventing the development of middle ear effusions or abnormal ears. The maximum percentage of abnormal ears allowed in the anti-LB1 cohort was 15%, and that for the anti-LPD-LB1(f)2,1,3 cohort was 22%, whereas 55% abnormal ears were allowed in the anti-PDm cohort. Anti-LPD serum similarly did not confer protection against induction of OM, allowing 68% of ears to develop an effusion. Nonetheless, delivery of either anti-PDm and anti-LPD did result in a significant reduction in middle ear effusions relative to sham-immunized animals on days 13 and 14 or on day 14 only, respectively. Thus, these data are very similar to those obtained in the rat model (3), wherein active immunization with either LPD or PDm did not prevent the development of acute OM; however, immunization with each augmented recovery from disease. However, the strong, early ability of anti-PDm to reduce tympanic membrane inflammation on day 12 after inoculation of NTHI was offset by its unusual inability to maintain this downward trend for the remainder of the observation period. This pattern of inflammation is highly atypical for this model and raises a suspicion of the possibility of immunoselection giving rise to “escape” mutants. The emergence of a population of NTHI in the middle ear with an altered expression of surface antigens in response to immunologic pressure has been reported for the HMW proteins (13).
The most efficacious immunogens we have tested to date, in terms of ability to prevent NTHI-induced OM, are thus LB1 and LPD-LB1(f)2,1,3. The rationale for synthesis of LB1 has been described previously (8), and its strong protective effect in the final study here indicates its continued merit. However, the immunogen LPD-LB1(f)2,1,3 is novel and serves several investigational purposes which warrant some explanation. First, the N-terminal moiety, LPD, is a well-conserved 42-kDa lipoprotein (1, 4, 21, 32, 33, 35, 68), expressed by both NTHI and type b H. influenzae (4, 33), which has glycerophosphodiester phosphodiesterase activity (34, 52, 62). LPD potentiates IgM secretion by large B cells and acts in concert with a multivalent antigen receptor cross-linking signal to mediate both increased IgM secretion and proliferation by sort-purified small B cells (67). The role of LPD in the pathogenesis of OM has been demonstrated in a rat model through the use of an LPD-deficient mutant (34) which was 100-fold less virulent than the parental isolate. A later study (3) showed that rats immunized with LPD produced bactericidal antibody with activity against both homologous and heterologous NTHI isolates and also experienced an accelerated rate of recovery from experimental OM, thus generating interest in LPD as a vaccine candidate. Similarly, a nonacylated mutated form of LPD (PDm), expressed by and isolated from the periplasmic space of E. coli (32), was found to be more easily produced and purified than LPD (3, 32) and also induced IgG and IgA in serum, which had significant bactericidal activity against both homologous and heterologous NTHI isolates (2). While neither LPD nor PDm immunization prevents the development of acute OM in rats, both result in more rapid recovery from disease, with LPD being the more efficacious (3). Thus, although PDm is more easily produced (3, 30), we selected LPD to be the fusion partner of the three LB1 peptides because of its higher immunogenicity (3) and its potential to induce more bactericidal antibodies against both NTHI and type b H. influenzae.
Second, the 19-mer of P5-fimbrin, originally selected for inclusion in LB1 due to its predicted likelihood of being a B-cell epitope, was included in the LPD-LB1 recombinant peptide because of the previously demonstrated efficacy of the 40-mer chimeric peptide LB1 in inducing rapid clearance of NTHI from the nasopharynx (8) and because of its performance in both the middle ears and nasopharynx in the two active-immunization trials reported here. However, unlike the original LB1 peptide, in which a T-cell epitope of measles virus fusion protein known for its promiscuous properties (38–40, 43) is synthesized C-terminally to the 19-mer of P5-fimbrin [LB1(f)], the recombinant peptide joins three unique LB1(f) fragments (each lacking the measles virus fusion protein moiety) C-terminally to LPD in the final expressed product. These three LB1(f) peptides represented each of the major NTHI groupings for this portion of the mature P5-fimbrin protein demonstrated here and based on derived-amino-acid sequence analysis of 99 clinical NTHI isolates. The inclusion of LB1(f) peptides from these three major groups was intended to potentially induce a greater breadth of protection against NTHI challenge, although to date we have assessed efficacy only against the group 1 isolate reported here. Antiserum against LPD-LB1(f)2,1,3, delivered passively to chinchillas at a 1:5 dilution, performed second only to anti-LB1/CFA serum in reducing the signs of OM and preventing the induction of middle ear effusions in this model; it may show equivalent or even greater efficacy when delivered without prior dilution, but this has not yet been investigated.
Thus, both LB1 and LPD-LB1(f)2,1,3 are derived from P5-fimbrin protein, which is a member of the OmpA family of proteins. The exact conformation and function of many members of this family are poorly characterized (19). However, the OmpA-like proteins play a role in pathogenicity and virulence in E. coli K1 (55, 56, 74) and NTHI (66) models of infection, and thus they are important antigens. The highly conserved C-terminal portion of the OmpA proteins suggests an association between this domain of these proteins and peptidoglycan; however, there is no obvious similarity for the N-terminal domain of OmpA family members (19). This has resulted in the somewhat controversial assignment of a universal biological function to this portion of the mature protein. Despite this incomplete understanding, the demonstrated ability of the two P5-fimbrin-derived immunogens used here to induce antibody that augments the clearance of NTHI from mucosal sites and/or prevents the development of OM in experimental models indicated that both LB1 and LPD-LB1(f)2,1,3 continue to warrant investigation as vaccine components.
LB1(f) is derived from the N-terminal half of the mature P5-fimbrin protein, specifically from a region which has little homology to other OmpA proteins (66), yet is included in one of the postulated surface-exposed loops of OmpA (48) and major outer membrane protein P5 (20). A recent report showed that nonencapsulated H. influenzae strains recovered from patients with chronic infections of the lower respiratory tract demonstrate four well-defined heterogeneous regions, including region 3, which generally corresponds to the peptide LB1(f). These concentrated areas of nonsynonymous point mutations were attributed to the long-term immunoselective pressures exerted on H. influenzae during persistent, chronic infections. PCR amplification and subsequent analysis of the deduced amino acid sequences of 99 NTHI strains in the present study similarly demonstrated heterogeneity in this region of the outer membrane protein in clinical isolates from patients with relatively acute infection of the middle ear. However, application of the restriction of 75% identity with the consensus sequences described for assignment to a particular group allowed these isolates to be arranged into three major subsets. Nonetheless, the differences among the groups, as well as those between subgroups 2a and 2b, suggest that these peptides are potentially antigenically distinct, thus leading to the design of LPD-LB1(f)2,1,3. It would also be feasible to consider a four-valent P5-fimbrin-based vaccine component, particularly if broader protective efficacy could indeed be obtained.
Data collected here, as well as in other studies from our laboratories (8, 46, 66) and those of others (36, 57, 58), will not resolve the controversy over the function of OmpA family members; however, they collectively support a model wherein this particular NTHI outer membrane protein, or portions of it, is configured in a way which is both highly accessible to antibody and capable of involvement in cell-cell or cell-substrate interactions, as are other members of the OmpA family (25, 50, 55, 56, 69, 70, 73). P5-fimbrin is an adhesin to chinchilla tracheal and middle ear epithelium (11) and eustachian tube mucus (46), human nasopharyngeal (57) and middle ear mucin (58), and both normal human oropharyngeal cells (11) and respiratory syncytial virus-infected human lung cells (36). Indirect immunogold labeling with chinchilla polyclonal antiserum against either isolated P5-fimbrin (66) or a synthetic chimeric peptide derived from P5-fimbrin (8), two murine MAbs (66), or chinchilla polyclonal antiserum against LPD-LB1(f)2,1,3 did not yield a labeling pattern which would suggest uniform circumferential distribution of the outer membrane protein. In fact, TEM suggested a protein which is expressed in discrete compartments of the bacterial outer membrane and extends, at least in part, outward from the bacterial surface or is perhaps partially released or exported.
While the mechanism(s) of the protection afforded here is not yet fully known, our goal was to attempt to immunize against NP colonization and thus also reduce the incidence of OM which developed in chinchillas. NTHI resident in the nasopharynx is known to ascend the eustachian tube of adenovirus-compromised naive chinchillas and to multiply in the normally sterile middle ear, thus inducing OM (72). Rapid elimination of NTHI colonizing the nasopharynx in LB1-immunized chinchillas, as demonstrated here in the first active-immunization trial, was probably also the mechanism which contributed to the marked ability to prevent the induction of OM in the passive-transfer protocol with anti-LB1. Similarly, passive transfer of antiserum directed against LPD-LB1(f)2,1,3, while not yet assessed specifically as an immunogen against NP colonization, also effectively prevented the development of OM; therefore, a bacterial clearance mechanism similar to that demonstrated by immunization with LB1 is possible.
Other similar approaches to immunization, in which a major protective mechanism is the inactivation of the infecting inoculum by serum IgG that has transuded onto the mucosal surface, are efficacious in preventing pneumococcal OM in children given bacterial polysaccharide immune globulin (64) or in chinchillas with experimental OM induced by homologous NTHI challenge when LKP pili were used as the immunogen (37) and form the basis of many other parenteral vaccines (59, 60, 75) and passive-transfer systems (65). In experimental models of NTHI-induced OM, protective activity afforded in the nasopharynx appears to be dependent upon both the antigen delivered and the method of immunization. s.c. immunization with isolated P6 plus CFA has no effect on NP colonization by NTHI in the chinchilla (18), whereas i.n. delivery of P6 plus cholera toxin significantly enhances NP clearance of NTHI in BALB/c mice (31). However, parenteral immunization of chinchillas with isolated P5-fimbrin protein in CFA induces significantly augmented clearance of a homologous NTHI challenge isolate from the nasopharynx (8). Adenovirus coinfection was not used in the study of P6 protective efficacy in the chinchilla, and thus the absence of virus inflammation-induced promotion of serum transudation into the mucosa of the uppermost airway (7) may have been responsible for the lack of clearance effect reported in P6-immunized chinchillas (18). Other NTHI surface antigens have not been tested for efficacy in the nasopharynx.
While the exact role of bactericidal antibody in protection is unclear, it does not appear to play a role in NP colonization (15). However, strain-specific bactericidal antibody does protect against OM (22). Thus, the bactericidal activity of immune serum may also have contributed to the clearance of NTHI seen here in the middle ears, since a correlation has been shown between the bactericidal activity of antibody directed against the HMW adhesin proteins (12), lipooligosaccharide (28) or polyribosyl ribitol phosphate-conjugated LPD and PDm (3) and protective efficacy in experimental models of human OM. However, anti-P6 antibodies with significant bactericidal activity have not been directly associated with clearance of NTHI from either the chinchilla nasopharynx (18) or the rat lung (42), although a predisposing viral infection was, again, not a component of either model.
In conclusion, the marked efficacy demonstrated by the immunogens LB1 and the recombinant peptide LPD-LB1(f)2,1,3 in the ability to induce clearance of the nasopharynges and middle ears as well as the ability of antibody directed against either to significantly prevent the induction of middle ear disease makes these immunogens highly interesting candidate components for a vaccine against NTHI-induced OM. Studies assessing the efficacy of antiserum specific for both LB1 and LPD-LB1(f)2,1,3 as immunogens against challenge by members of each of the three major NTHI groups relative to peptide LB1(f) are under way.
ACKNOWLEDGMENTS
This study was supported by grants from SmithKline Beecham Biologicals and grant R01 DC02830-03 from NIDCD, NIH.
We thank Ilija Karanfilov, Wenona Stankiewicz, Katherine Holmes, Ed Leake, John Billy, Tricia Rigel, Yumi Jeong, Tony Palanci, Susan Thompson, and Kristine Schreiner for expert technical assistance. We thank Alan Lesse for Mab2C7 and T. F. DeMaria for NTHI outer membrane protein P6. We are grateful to Jim Rauscher for chinchillas, Lynn Mitchell for biostatistical analyses, and Carrie Schreiner for preparation of the manuscript and figures.
REFERENCES
- 1.Akkoyunlu M, Forsgren A. Local and systemic antibody levels against protein D of Haemophilus influenzae following immunization and infection in rats. APMIS. 1996;104:709–717. [PubMed] [Google Scholar]
- 2.Akkoyunlu M, Janson H, Ruan M, Forsgren A. Biological activity of serum antibodies to a nonacylated form of lipoprotein D of Haemophilus influenzae. Infect Immun. 1996;64:4586–4592. doi: 10.1128/iai.64.11.4586-4592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akkoyunlu M, Melhus A, Capiau C, Van Opstal O, Forsgren A. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect Immun. 1997;65:5010–5016. doi: 10.1128/iai.65.12.5010-5016.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akkoyunlu M, Ruan M, Forsgren A. Distribution of protein D and immunoglobulin D-binding protein, in Haemophilus strains. Infect Immun. 1991;59:1231–1238. doi: 10.1128/iai.59.4.1231-1238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakaletz L O, Barenkamp S J. Localization of high molecular weight adhesion proteins of nontypable Haemophilus influenzae by immunoelectron microscopy. Infect Immun. 1994;62:4460–4468. doi: 10.1128/iai.62.10.4460-4468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakaletz L O, Daniels R L, Lim D J. Modeling adenovirus type 1-induced otitis media in the chinchilla: effect on ciliary activity and fluid transport function of eustachian tube mucosal epithelium. J Infect Dis. 1993;168:865–872. doi: 10.1093/infdis/168.4.865. [DOI] [PubMed] [Google Scholar]
- 7.Bakaletz L O, Holmes K A. Evidence for transudation of specific antibody into the middle ears of parenterally immunized chinchillas after an upper respiratory tract infection with adenovirus. Clin Diagn Lab Immunol. 1997;4:223–225. doi: 10.1128/cdli.4.2.223-225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakaletz L O, Leake E R, Billy J M, Kaumaya P T P. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine. 1997;15:955–961. doi: 10.1016/s0264-410x(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 9.Bakaletz L O, Murwin D M, Billy J M. Adenovirus serotype 1 does not act synergistically with Moraxella (Branhamella) catarrhalis to induce otitis media in the chinchilla. Infect Immun. 1995;63:4188–4190. doi: 10.1128/iai.63.10.4188-4190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakaletz L O, Tallan B M, Andrzejewski W J, DeMaria T F, Lim D J. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins of nontypable Haemophilus influenzae. Infect Immun. 1989;57:3226–3229. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakaletz L O, Tallan B M, Hoepf T, DeMaria T F, Birck H G, Lim D J. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun. 1988;56:331–335. doi: 10.1128/iai.56.2.331-335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barenkamp S J. Protection by serum antibodies in experimental nontypeable Haemophilus influenzae otitis media. Infect Immun. 1986;52:572–580. doi: 10.1128/iai.52.2.572-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein J M, Bronson P M, Wilson M E. Immunoglobulin G subclass response to major outer membrane proteins of nontypeable Haemophilus influenzae in children with acute otitis media. Otolaryngol Head Neck Surg. 1997;116:363–371. doi: 10.1016/S0194-59989770275-4. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein J M, Faden H S, Ogra P L. Nasopharyngeal colonization by nontypeable Haemophilus influenzae in children: the effect of serum bactericidal antibody. Otolaryngol Head Neck Surg. 1991;105:406–410. doi: 10.1177/019459989110500309. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein J M, Howard F F, Dryja D M, Wactawski-Wende J. Micro-ecology of the nasopharyngeal bacterial flora in otitis-prone and non-otitis-prone children. Acta Otolaryngol. 1993;113:88–92. doi: 10.3109/00016489309135772. [DOI] [PubMed] [Google Scholar]
- 17.DeMaria T F, Apicella M A, Nichols W A, Leake E R. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect Immun. 1997;65:4431–4435. doi: 10.1128/iai.65.11.4431-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Mot R, Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both Gram-positive and Gram-negative bacteria, possibly the interaction of these domains with peptidoglycan. Mol Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 20.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duim B, Ruiter P, Bowler L D, Dankert J, van Alphen L. Sequence variation in the hpd gene of nonencapsulated Haemophilus influenzae isolated from patients with chronic bronchitis. Gene. 1997;191:57–60. doi: 10.1016/s0378-1119(97)00030-9. [DOI] [PubMed] [Google Scholar]
- 22.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children. The systemic immune response to nontypeable Haemophilus influenzae. J Infect Dis. 1989;160:999–1004. doi: 10.1093/infdis/160.6.999. [DOI] [PubMed] [Google Scholar]
- 23.Freijd A, Bygdeman S, Rynnel-Dagöö B. The nasopharyngeal microflora of otitis-prone children, with emphasis on H. influenzae. Acta Otolaryngol. 1984;97:117–126. doi: 10.3109/00016488409130971. [DOI] [PubMed] [Google Scholar]
- 24.Giebink G S, Heller K A, Harford E R. Tympanometric configurations and middle ear findings in experimental otitis media. Ann Otol Rhinol Laryngol. 1982;91:20–24. doi: 10.1177/000348948209100106. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Duarte O G, Dehio M, Guzman C A, Chhatwal G S, Dehio C, Meyer T F. Binding of vitronectin to opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect Immun. 1997;65:3857–3866. doi: 10.1128/iai.65.9.3857-3866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross M, Sweet R W. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985;5:1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu X X, Sun J, Jin S, Barenkamp S J, Lim D J, Robbins J B, Battey J. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect Immun. 1997;65:4488–4493. doi: 10.1128/iai.65.11.4488-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu X X, Tsai C M, Ueyama T, Barenkamp S J, Robbins J B, Lim D J. Synthesis, characterization, and immunologic properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun. 1996;64:4047–4053. doi: 10.1128/iai.64.10.4047-4053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase E M, Campagnari A A, Sarwar J, Shero M, Wirth M, Cumming C U, Murphy T. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1991;59:1278–1284. doi: 10.1128/iai.59.4.1278-1284.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotomi M, Saito T, Yamanaka N. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine. 1998;16:1950–1956. doi: 10.1016/s0264-410x(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 32.Janson H, Hedén L O, Forsgren A. Protein D, the immunoglobulin D-binding protein of Haemophilus influenzae, is a lipoprotein. Infect Immun. 1992;60:1336–1342. doi: 10.1128/iai.60.4.1336-1342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janson H, Hedén L O, Grubb A, Ruan M, Forsgren A. Protein D and immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequence, and expression in Escherichia coli. Infect Immun. 1991;59:119–125. doi: 10.1128/iai.59.1.119-125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janson H, Melhus A, Hermansson A, Forsgren A. Protein D, the glycerophosphodiester phosphodiesterase from Haemophilus influenzae with affinity for human immunoglobulin D, influences virulence in a rat otitis model. Infect Immun. 1994;62:4848–4854. doi: 10.1128/iai.62.11.4848-4854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janson H, Ruan M, Forsgren A. Limited diversity of the protein D gene (hpd) among encapsulated and nonencapsulated Haemophilus influenzae strains. Infect Immun. 1993;61:4546–4552. doi: 10.1128/iai.61.11.4546-4552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Z, Nagata N, Molina E, Bakaletz L O, Hawkins H, Patel J A. Fimbriae-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect Immun. 1999;67:187–192. doi: 10.1128/iai.67.1.187-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karasic R B, Beste D J, To S C M, Doyle W J, Wood S W, Carter M J, To A C C, Brinton C C. Evaluation of pilus vaccines for prevention of experimental otitis media caused by nontypeable Haemophilus influenzae. Pediatr Infect Dis J. 1989;8:S062–S065. doi: 10.1097/00006454-198901001-00021. [DOI] [PubMed] [Google Scholar]
- 38.Kaumaya P T P, Feng N, Seo Y H, Kobs-Conrad S, VanBuskirk A M, Sheridan J F. Immunogenicity and antigenicity of a promiscuous T-cell epitope and a topographic B-cell determinant of the protein antigen LDH-C4. In: Smith J A, Rivier J E, editors. Peptides: chemistry and biology. Leiden, The Netherlands: ESCOM; 1992. pp. 883–885. [Google Scholar]
- 39.Kaumaya P T P, Kobs-Conrad S, Seo Y H, Lee H, VanBuskirk A M, Feng N, Sheridan J F, Stevens V. Peptide vaccines incorporating a “promiscuous” T-cell epitope bypass certain haplotype restricted immune responses and provide broad spectrum immunogenicity. J Mol Recognit. 1993;6:81–94. doi: 10.1002/jmr.300060206. [DOI] [PubMed] [Google Scholar]
- 40.Kobs-Conrad S, Gerdau A, Kaumaya P T P. Multivalent B-cell and T-cell epitope vaccine design. In: Smith J A, Rivier J E, editors. Peptides: chemistry and biology. Leiden, The Netherlands: ESCOM; 1992. pp. 886–888. [Google Scholar]
- 41.Kyd J M, Cripps A W. Modulation of antigen-specific T and B cell responses influence bacterial clearance of nontypeable Haemophilus influenzae from the lung in a rat model. Vaccine. 1996;14:1471–1478. doi: 10.1016/s0264-410x(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 42.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lairmore M D, Di George A M, Conrad S F, Trevino A V, Lal R B, Kaumaya P T P. Human T-lymphotropic virus type 1 peptides in chimeric and multivalent constructs with promiscuous T-cell epitopes enhance immunogenicity and overcome genetic restriction. J Virol. 1995;69:6077–6089. doi: 10.1128/jvi.69.10.6077-6089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loosmore S M, Yang Y P, Oomen R, Shortreed J M, Coleman D C, Klein M H. The Haemophilus influenzae HtrA protein is a protective antigen. Infect Immun. 1998;66:899–906. doi: 10.1128/iai.66.3.899-906.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margolis R H, Hunter L L, Rykken J R, Giebink G S. Effects of otitis media on extended high-frequency hearing in children. Ann Otol Rhinol Laryngol. 1993;102:1–5. doi: 10.1177/000348949310200101. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto N, Bakaletz L O. Selective adherence of nontypeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto N, Bakaletz L O. Kinetics of the ascension of NTHi from the nasopharynx to the middle ear coincident with adenovirus-induced compromise in the chinchilla. Microb Pathog. 1997;23:119–126. doi: 10.1006/mpat.1997.0140. [DOI] [PubMed] [Google Scholar]
- 48.Morona R, Klose M, Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984;159:570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mott J E, Grant R A. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci USA. 1985;82:88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Movva N R, Nakamura K, Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980;143:317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- 51.Munson R S, Jr, Grass S, West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993;61:4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munson R S, Jr, Sasaki K. Protein D, a putative immunoglobulin D-binding protein produced by Haemophilus influenzae, is glycerophosphodiester phosphodiesterase. J Bacteriol. 1993;175:4569–4571. doi: 10.1128/jb.175.14.4569-4571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelton S I, Bolduc G, Guiati S, Liu Y, Rice P A. Program and abstracts of the 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1990. Protection from experimental otitis media in chinchillas due to nontypeable Haemophilus influenzae following immunization with outer membrane protein 1 (P1), abstr. 610; p. 188. [Google Scholar]
- 54.Perkins A, Jonsdottir H, Briem H, Griffiths E, Plikaytis D, Hoiby E A. Immunogenicity of two efficacious outer membrane protein-based serogroup meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 55.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAc-β-1-4GlcNac epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy M S, Murphy T F, Faden H S, Bernstein J M. Middle ear mucin glycoprotein: purification and interaction with nontypeable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–180. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 59.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis—serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 60.Robbins J B, Schneerson R, Szu S C. Hypothesis: how licensed vaccines confer protective immunity. Adv Exp Med Biol. 1996;397:169–182. doi: 10.1007/978-1-4899-1382-1_22. [DOI] [PubMed] [Google Scholar]
- 61.Rosenfeld R M, Doyle W J, Griffiss J M, Gibson B W. Third-generation cephalosporins in the treatment of acute pneumococcal otitis media: an animal study. Arch Otolaryngol Head Neck Surg. 1992;118:49–52. doi: 10.1001/archotol.1992.01880010053015. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki K, Munson R S., Jr Protein D of Haemophilus influenzae is not a universal immunoglobulin D-binding protein. Infect Immun. 1993;61:3026–3031. doi: 10.1128/iai.61.7.3026-3031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneerson R, Fattom A, Szu S C, Bryla D, Ulrich J T, Rudbach J A, Schiffman G, Robbins J B. Evaluation of monophosphoryl lipid A (MPL) as an adjuvant: Enhancement of the serum antibody response in mice to polysaccharide-protein conjugates by concurrent injection with MPL. J Immunol. 1991;147:2136–2140. [PubMed] [Google Scholar]
- 64.Shurin P A, Rehmus J M, Johnson C E, Marchant C D, Carlin S A, Super D M, Van Hare G F, Jones P K, Ambrosino D M, Siber G R. Bacterial polysaccharide immune globulin for prophylaxis of acute otitis media in high-risk children. J Pediatr. 1993;123:801–810. doi: 10.1016/s0022-3476(05)80865-0. [DOI] [PubMed] [Google Scholar]
- 65.Siber G R, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson C M, Walsh E E, Piedra P A, Hemming V G, Prince G A. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 66.Sirakova T, Kolattukudy P E, Murwin D M, Billy J M, Leake E R, Lim D J, DeMaria T F, Bakaletz L O. The role of fimbriae expressed by nontypable Haemophilus influenzae (NTHi) in the pathogenesis of and protection against otitis media and the relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snapper C M, Rosas F R, Jin L, Wortham C, Kehry M R, Mond J J. Bacterial lipoproteins may substitute for cytokines in the humoral immune response to T cell-independent type II antigens. J Immunol. 1995;155:5582–5589. [PubMed] [Google Scholar]
- 68.Song X M, Forsgren A, Janson H. The gene encoding protein D (hpd) is highly conserved among Haemophilus influenzae type b and nontypeable strains. Infect Immun. 1995;63:696–699. doi: 10.1128/iai.63.2.696-699.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sperandio V, Bailey C, Giron J A, DiRita V J, Silveira W D, Vettore A L, Kaper J B. Cloning and characterization of the gene encoding the OmpU outer membrane protein of Vibrio cholerae. Infect Immun. 1996;64:5406–5409. doi: 10.1128/iai.64.12.5406-5409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sperandio V, Giron J A, Silveira W D, Kaper J B. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect Immun. 1995;63:4433–4438. doi: 10.1128/iai.63.11.4433-4438.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spinola S M, Griffiths G E, Shanks K L, Blake M S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993;61:1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki K, Bakaletz L O. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62:1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virji M, Makepeace K, Ferguson D J P, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 74.Weiser J N, Gotschlich E C. Outer membrane protein A (Omp A) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willems J L, Kamerbeek J, Geuijen A W, Top J, Gielen H, Gaastra W, Mooi F R. The efficacy of a whole cell pertussis vaccine and fimbriae against Bordetella pertussis and Bordetella parapertussis infections in a respiratory mouse model. Vaccine. 1998;16:410–416. doi: 10.1016/s0264-410x(97)80919-x. [DOI] [PubMed] [Google Scholar]