Abstract
Background
Aspergillus is one of the important pathogens that contribute to high mortality in patients with coronavirus disease 2019 (COVID-19) in intensive care units (ICUs). Although incidence rates of Aspergillus coinfection are high globally, a Japanese national survey reported a low incidence. This study aimed to describe the clinical characteristics of patients with COVID-19-associated pulmonary aspergillosis at our institute.
Methods
We identified patients with microbiologically confirmed COVID-19 on mechanical ventilation in the ICU. Of these patients, we identified patients in whom Aspergillus was cultured from the respiratory specimen.
Results
Of a total of 169 patients, seven had aspergillosis (4.1%), which included three patients, three patients, and one patient with possible, probable, and proven aspergillosis, respectively, according to the criteria of the European Confederation of Medical Mycology International Society. All patients received systemic steroid therapy. Two patients (one each with proven and probable aspergillosis) had tracheobronchitis diagnosed by bronchoscopy. All patients in whom Aspergillus was repeatedly isolated from samples died. The mortality rates for all cases and probable and proven cases were 57% (4/7) and 75% (3/4), respectively.
Conclusions
The incidence rate of aspergillosis in patients with COVID-19 in the ICU was higher in our institute than that reported by a Japanese national survey (4.1% vs. 0.5%). Repeated detection of Aspergillus might suggest a true Aspergillus infection, such as chronic aspergillosis, rather than colonization. In patients with severe COVID-19 patients, it is important to always keep CAPA in mind.
Keywords: COVID-19, Aspergillosis, Transbronchial aspirate, Bronchoscopy, Mechanical ventilation
1. Introduction
There is an ongoing pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 infection. It is imperative to rule out coinfections, such as fungal infections that may contribute to poor outcomes. COVID-19-associated pulmonary aspergillosis (CAPA) has been reported worldwide and is a complication contributing to high mortality [1]. The incidence rate of CAPA in critical cases of COVID-19 was 3.3%–35% in the USA and European countries [[1], [2], [3], [4], [5]]. In contrast, a national survey from 198 Japanese hospitals showed an extremely low prevalence rate (0.54%: 9 cases/1664 patients) [6]. Furthermore, only two case reports on CAPA in Japan have been published in PubMed-indexed journals [7,8]. Here, we describe the clinical characteristics of patients with CAPA at our institute.
2. Patients and methods
We conducted a retrospective review of patients with COVID-19 who received mechanical ventilation in the intensive care units (ICUs) of our emergency hospital with 487 beds in Sakai City, Osaka, Japan between February 25, 2020, and May 10, 2022. We identified 169 patients with microbiologically confirmed COVID-19 requiring mechanical ventilation in the ICU. Among these 169 patients, we identified seven patients in whom Aspergillus was cultured from the respiratory specimen. The patients were classified as having possible, probable, or proven CAPA based on the European Confederation of Medical Mycology-International Society (ECMM/ISHAM) criteria by Koehler et al. [9] All study participants provided informed consent, and the study design was approved by the appropriate ethics review board at Sakai City Medical Center, Osaka, Japan (accession number:22–279).
Transbronchial aspirates were obtained soon after intubation or at the time of admission due to an increase in sputum, fever, or an increase in serum C-reactive protein levels and white blood cell counts. Gram staining was performed and the resulting slides were evaluated by an infectious disease doctor and at least one clinical laboratory technician. If Gram staining revealed the presence of filamentous fungi, serum tests for fungal infection were conducted. If mold colonies were observed, culturing on 5% sheep agar (Kyokuto, Japan) was performed. We performed additional culture tests on Sabouraud agar (Nissui, Japan) and Potato-Dextrose-Agar (Eiken Chemical, Japan). Serum (1,3)-beta-d-glucan or Aspergillus galactomannan antigen tests were not routinely performed. Aspergillus was identified based on the morphology of colonies and direct smears by cotton blue staining.
3. Results
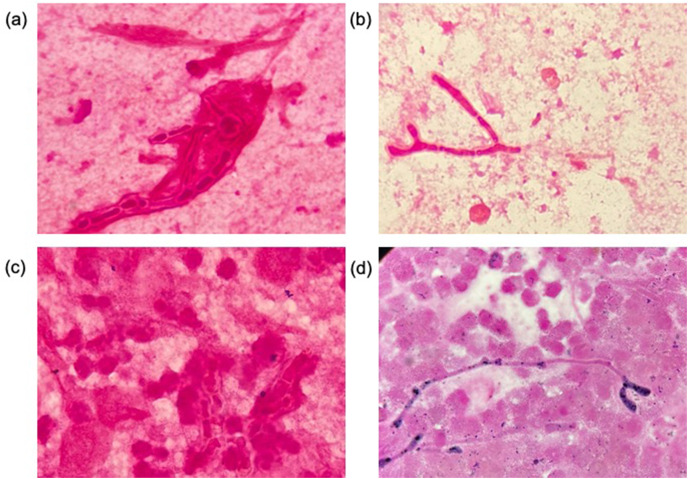
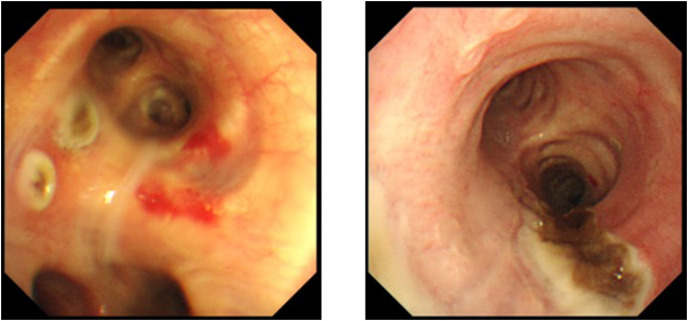
Of a total of 169 patients, seven had aspergillosis (4.1%), which included three patients, three patients, and one patient with possible, probable, and proven aspergillosis, respectively, according to the criteria of the European Confederation of Medical Mycology International Society [9]. The characteristics of the seven patients are shown in Table 1 . Two were women and five were men, and the median age of the patients was 77 (60–86) years. All patients were under mechanical ventilation in the ICU. Except for one patient, all had underlying diseases (four had diabetes, three had hypertension, one had chronic kidney disease, one had rheumatoid arthritis, and one had chronic obstructive pulmonary disease). All patients received systemic steroid therapy, tocilizumab was administered to one patient, and extracorporeal membrane oxygenation was introduced in one patient. Only one patient had received two doses of the COVID-19 vaccine. When Aspergillus isolation was confirmed, three patients had persistent or refractory fever and one patient had hemoptysis (in case 7, the patient gave a history of hemoptysis a few days after the first isolation). The recovered species included Aspergillus fumigatus in six patients and Aspergillus fumigatus and terreus in one patient. The median duration of the first isolation of Aspergillus since intubation was 5 days (1–8). Further, Gram staining revealed mold hyphae in four specimens from which Aspergillus was first isolated (Fig. 1 ). Serum Aspergillus galactomannan antigen was tested in four patients, and only one patient tested positive (case 3). Serum (1,3)-D-glucan was tested in all patients, and four patients tested positive (10.2–179.1 pg/mL). Despite receiving antifungal chemotherapies, four of six patients reported repeated positive results of Aspergillus cultures. Tracheobronchial abnormality was observed in two out of three patients who underwent bronchoscopy (Fig. 2 ). Of the seven patients, three were classified as having possible CAPA, three as having probable CAPA, and one as having proven CAPA according to the ECMM/ISHAM criteria. Antifungal treatment was administered to six of the seven patients. Finally, four of the seven patients died in the ICU. In particular, four patients with isolation of Aspergillus from repeated respiratory specimens (one possible, two probable, and one proven) died despite the administration of antifungal treatments.
Table 1.
Characteristics of patients diagnosed with COVID-19-associated pulmonary aspergillosis.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age/sex | 86/F | 77/F | 73/M | 81/M | 60/M | 70/M | 78/M |
| BMI | 25.3 | 22.9 | 29 | 22.5 | 30 | 26.6 | 24.2 |
| Aspergillus cultured year/month | 2020/December | 2020/December | 2021/May | 2021/May | 2021/August | 2022/February | 2022/March |
| Mechanical ventilation | yes | Yes | yes | yes | yes | yes | yes |
| Underlying diseases | RA, HT | DM | DM | DM | no | DM, HT, CKD | COPD, HT |
| Systemic steroid used | dexamethasone | dexamethasone | dexamethasone | dexamethasone | hydrocortisone | dexamethasone | dexamethasone |
| Tocilizumab use | no | Yes | no | no | no | no | no |
| ECMO | no | No | no | no | yes | no | no |
| Vaccination against COVID-19 | no | No | no | no | no | twice | no |
| Body temperature (°C) | 39.4 | 35.9 | 36.8 | 38.6 | 36 | 36.8 | 37.9 |
| Hemoptysis | no | No | no | no | yes | no | yes |
| CT image | ARDS | diffuse GGO | GGO, consolidation | GGO, consolidation | ARDS | GGO, pleural effusion | multiple consolidation |
| Bronchoscopy | no | No | no | yes | yes | no | yes |
| Tracheobronchial abnormality | no | No | no | no | yes | no | yes |
| Serum GM level (DOI) | ND | 0.2 | 1.5 | 0.2 | ND | ND | 0.1 |
| Serum (1,3)beta D-glucan level (pg/mL) | 179.1 | <6 | 15.8 | <6 | 13.5 | <6 | 10.2 |
| First isolation since intubation (day) | 6 | 1 | 8 | 8 | 5 | 1 | 5 |
| Cultured from | TA | TA | TA | BALF | bronchial plaque | TA | TA with bronchoscopy |
| Species of cultured Aspergillus | A. fumigatus | A. fumigatus | A. fumigatus | A. fumigatus | A. fumigatus | A. fumigatus | A. fumigatus |
| A. terreus | |||||||
| Mold hyphae on Gram staining | none | Positive | positive | positive | none | none | positive |
| Repeated cultures of Aspergillus | yes | ND | no | yes | yes | ND | yes |
| CAPA diagnosis | |||||||
| Koehler's (ref 9) | possible | Possible | probable | probable | proven | possible | probable |
| Verweij's (ref 10) | none | None | probable | probable | proven | none | probable |
| White's (ref 1) | putative | None | putative | none | proven | none | putative |
| Bassetti's (ref 11) | none | None | probable | probable | proven | none | probable |
| Anti-fungal treatment | MCFG- > L-amB | MCFG | MCFG | MCFG | MCFG- > MCFG + VRCZ | no | MCFG + VRCZ- > L-amB |
| Outcome | died | Recovered | recovered | died | died | recovered | died |
Abbreviations: COVID-19, coronavirus disease 2019; F, female; M, male; BMI, body Mass Index; RA, rheumatoid arthritis; HT, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; CT, computed tomography; ARDS, acute respiratory distress syndrome; GGO, ground glass opacity; GM, galactomannan; DOI, optical density index; ND, no data; TA, tracheal aspirates; BALF, bronchoalveolar lavage fluid; CAPA, COVID-19-associated pulmonary aspergillosis; MCFG, micafungin; L-amB, liposomal amphotericin B; VRCZ, voriconazole.
Fig. 1.
Images of Gram stained respiratory specimens ( × 1000) from (a) case 2, (b) case 3, (c) case 4, and (d) case 7.
Fig. 2.
Bronchial mucosal findings of bronchoscopy; multiple ulcerations and mucosal ulcers with black plaque in tracheobronchial mucosa can be seen (left: case 5. right: case 7).
4. Discussion
The diagnosis and treatment of CAPA remain challenging. Reports from early 2020 showed that the incidence rate of CAPA in critical cases of COVID-19 was 3.3%–35% in the USA and European countries. In contrast, a national survey from 198 Japanese hospitals by Takazono et al. [6] showed an extremely low prevalence rate (0.5%). Takazono et al. pointed out that studies from the USA or Europe were not based on defined diagnostic criteria. However, recently, a few criteria, including the ECMM/ISHAM criteria have been proposed for CAPA diagnosis [1,[9], [10], [11]]. Kariyawasam et al. performed a systematic review and meta-analysis using these criteria and reported a prevalence of CAPA of 10% (95% confidence interval: 8%–13%) [12]. We applied these criteria to our patients and found that the number of patients with CAPA was seven, four, four, and four according to the criteria of Koehler, Verweij, White, and Bassetti, respectively (Table 1). The incidence of CAPA based on all these criteria was higher in the current study than in the survey by Takazono et al. although serum (1,3) beta-D-glucan or serum Aspergillus galactomannan examinations were not routinely performed. In some countries outside Japan, galactomannan tests using serum or bronchoalveolar fluid (BALF), and PCR tests for Aspergillus species using plasma, serum, whole blood or respiratory specimens are performed to prove Aspergillus involvement. Bartoletti et al. described the usefulness of Aspergillus galactomannan examinations on BALF for the diagnosis of CAPA [13]. Therefore, we might have missed other CAPA cases that could have been diagnosed if more sensitive tests had been performed. For the environmental aspects, during the COVID-19 epidemic, we used four wards for severe COVID-19 patients. Hospital construction or renovation is one of the reasons for nosocomial infection of Aspergillus [14]. These wards were in the same floor, however all wards were separated each other. During the study period, the patients of Aspergillosis were found from all four wards without the bias. In two wards, the renovation of the room for isolation of COVID-19 patients were done. However, CAPA patients were not in these wards at least one month after the renovation work except one (admitted three weeks after renovation; case 4). Thus, the reason for difference of incident rate could be related to the status of implementation of bronchoscopy and microbiological examination of respiratory samples. The American Association for Bronchology and Interventional Pulmonology states that bronchoscopy is recommended in patients with COVID-19 only when the intervention is considered lifesaving, including interventions for secondary infections [15], and when there is a concern that oxygenation would worsen by performing bronchoscopy. In our study, all patients with repeated Aspergillus isolation from samples died despite for treatment with anti-fungal drugs. For CAPA treatment, voriconazole or isavuconazole is recommended [9], however, all our patients were treated with micafungin as anti-fungal drug empirically to avoid the side effects or drug-drug interactions, and all the cases were diagnosed as possible or probable CAPA initially. Echinocandin is mainly used for the salvage therapy for invasive Aspergillosis with high safety [[16], [17], [18]]. This might be the one reason why some of the cultures did not turn to be negative. However, in these cases, we changed or added anti-fungal drugs to liposomal amphotericin-B or voriconazole with therapeutic drug monitoring (Table 1), and cultures were still positive after changing drugs. All the isolates were identified morphologically. Recently, the identification of Aspergillus is based on genetic testing or mass spectrometry results. However, genetic testing can only be performed at some medical institutions. In our institute, we did not possess mass spectrometer during this study period. Another reason for repeated cultures were drug resistance. We could not perform drug susceptibility testing on the isolates because of complexity and difficulty of the implementation as well. Thus, drug susceptibility testing at advanced medical institutions might be necessary.
We found fungal hyphae on Gram staining in four patients, and all samples were positive for cultures. In Japan, the importance and effectiveness of Gram staining is being emphasized for patients with ventilator-associated pneumonia [19]. Thus, Gram staining could provide a clue for the diagnosis of CAPA. In addition, Tashiro et al. described the importance of repeated isolation of the identical Aspergillus species and detection of anti-Aspergillus antibodies and/or Aspergillus antigens in sera for the diagnoses of Aspergillosis [20]. Repeated detection of Aspergillus might suggest a true Aspergillus infection, such as chronic aspergillosis, rather than colonization.
Based on our case series, it is important to keep CAPA in mind based on the clinical course and imaging findings, and to obtain specimens with good sensitivity to detect Aspergillus by brochofiberscopy while performing (1,3) beta-D-glucan and Aspergillus galactomannan examinations as appropriate. By obtaining the strains, identification and drug susceptibility testing should be performed leading to more appropriate antifungal treatment.
In conclusion, although galactomannan examinations are not performed routinely, the incidence of CAPA at our institute was not as low as that in previous reports from Japan (4.1%). Repeated detection of Aspergillus might suggest a true Aspergillus infection, such as chronic aspergillosis, rather than colonization. In patients with severe COVID-19 patients, it is important to always keep CAPA in mind.
Authorship statement
Conceptualization: Y Ogawa, Writing: Y Ogawa.
Review and editing: Y Ogawa, K Kasahara.
Investigation and Supervison: K Murata, K Hasegawa, K Nishida, I Gohma.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data from this study are not publicly available.
Declaration of competing interest
None.
Acknowledgements
None to be reported.
References
- 1.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Permpalung N., Chiang T.P.U., Massie A.B., Zhang S.X., Avery R.K., Nematollahi S., et al. Coronavirus disease 2019-associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis. 2022;74:83–91. doi: 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupont D., Menotti J., Turc J., Miossec C., Wallet F., Richard J.C., et al. Pulmonary aspergillosis in critically ill patients with coronavirus Disease 2019 (COVID-19) Med Mycol. 2021;59:110–114. doi: 10.1093/mmy/myaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado M., Valerio M., Álvarez-Uría A.A., Olmedo M., Veintimilla C., Padilla B., et al. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses. 2021;64:132–143. doi: 10.1111/myc.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutsaert L., Steinfort N., Van Hunsel T., Bomans P., Naesens R., Mertes H., et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takazono T., Mukae H., Izumikawa K., Hasegawa N., Yokoyama A. COVID-19 associated pulmonary aspergillosis: a nationwide survey by the Japanese Respiratory Society. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00402-2021. 00402-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imoto W., Himura H., Matsuo K., Kawata S., Kiritoshi A., Deguchi R., et al. COVID-19-associated pulmonary aspergillosis in a Japanese man: a case report. J Infect Chemother. 2021;27:911–914. doi: 10.1016/j.jiac.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwanaga Y., Kawanami T., Yamasaki K., Sakakibara H., Ikushima I., Ikegami H., et al. A fatal case of COVID-19-associated invasive pulmonary aspergillosis. J Infect Chemother. 2021;27:1102–1107. doi: 10.1016/j.jiac.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassetti M., Azoulay E., Kullberg B.J., Ruhnke M., Shoham S., Vazquez J., et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the Intensive Care Unit working group. Clin Infect Dis. 2021;72:S121–S127. doi: 10.1093/cid/ciaa1751. [DOI] [PubMed] [Google Scholar]
- 12.Kariyawasam R.M., Dingle T.C., Kula B.E., Vandermeer B., Sligl W.I., Schwartz I.S. Defining COVID-19-associated pulmonary aspergillosis: systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:920–927. doi: 10.1016/j.cmi.2022.01.027. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73(11):e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanamori H., Rutala W.A., Sickbert-Bennett E.E., Weber D.J. Review of fungal outbreaks and infection prevention in healthcare settins during construction and renovation. Clin Infect Dis. 2015;61(3):433–444. doi: 10.1093/cid/civ297. [DOI] [PubMed] [Google Scholar]
- 15.Wahidi M.M., Lamb C., Murgu S., Musani A., Shojaee S., Sachdeva A., et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchology Interv Pulmonol. 2020;27 doi: 10.1097/LBR.0000000000000681. e52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiemenz J.W., II Raad, Maertens J.A., Hachem R.Y., Saah A.J., Sable C.A., et al. II Raad Efficacy of caspofungin as salvage therapy for invasive aspergillosis compared to standard therapy in a historical cohort. Eur J Clin Microbiol Infect Dis. 2010;29(11):1387–1394. doi: 10.1007/s10096-010-1013-0. [DOI] [PubMed] [Google Scholar]
- 17.Tokimatsu I., Kushima H., Iwata A., Hashinaga K., Umeki K., Ohama M., et al. Intern Med. 2007;46(11):775–779. doi: 10.2169/internalmedicine.46.6193. [DOI] [PubMed] [Google Scholar]
- 18.Kotsopoulou M., Papadaki C., Anargyrou K., Spyridonidis A., Baltadakis I., Papadaki H.A., et al. Effectiveness and safety of micafungin in managing invasive fungal infections among patients in Greece with hematologic disorders: the ASPIRE study. Infect Dis Ther. 2019;8(2):255–268. doi: 10.1007/s40121-019-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimura J., Yamakawa K., Ohta Y., Nakamura K., Hashimoto H., Kawada M., et al. Effect of Gram stain-guided initial antibiotic therapy on clinical response in patients with ventilator-associated pneumonia: the GRACE-VAP randomized clinical trial. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tashiro T., Izumikawa K., Tashiro M., Takazono T., Morinaga Y., Yamamoto K., et al. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med Mycol. 2011;49(6):581–587. doi: 10.3109/13693786.2010.548084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are not publicly available.