Abstract
The effect of Bordetella pertussis adenylate cyclase toxin (ACT) on platelet aggregation was investigated. This cell-invasive adenylate cyclase completely suppressed ADP (10 μM)-induced aggregation of rabbit platelets at 3 μg/ml and strongly suppressed thrombin (0.2 U/ml)-induced aggregation at 10 μg/ml. The suppression was accompanied by marked increase in platelet intracellular cyclic AMP (cAMP) content and was diminished by the anti-ACT monoclonal antibody B7E11. A catalytically inactive point mutant of ACT did not show the suppressive effect. Since an increase of cAMP content is a known cause of platelet dysfunction, these results indicate that the observed platelet inactivation was due to the catalytic activity of ACT through increase of intracellular cAMP.
The adenylate cyclase toxin (ACT) of Bordetella pertussis is a 1,706-amino acid (aa) RTX toxin (5, 18) which enters mammalian cells and converts intracellular ATP to cyclic AMP (cAMP) in an unregulated way due to its high catalytic activity in the presence of calmodulin (7, 21, 24, 55). Since calmodulin is absent from bacteria, it is likely that ACT functions exclusively in mammalian cells.
The adenylate cyclase catalytic activity of this toxin is located in the N-terminal 400-aa domain which upon interaction with mammalian cells is internalized into the target cells across the cytoplasmic membrane in the presence of calcium (17, 18, 34, 43). The invasive activity strictly depends on the integrity of the 1,300-residue-long C-terminal part of the molecule; even a small deletion of 60 aa completely impairs the invasive activity (27, 45). This C-terminal domain is also endowed with hemolytic activity (1, 12, 22, 44, 45) and is structurally closely related to RTX toxins, including α-hemolysin of Escherichia coli (8). The hemolysin domain forms a hemolytic pore on sheep and human erythrocytes (44, 45) and on artificial lipid bilayer membranes (2, 51), suggesting that ACT has a very broad target cell specificity. ACT can invade and can elevate intracellular cAMP levels in a wide variety of cell types, including isolated and established human leukocytes (7, 14, 16, 26, 41), mouse neuroblastoma N1E-115 (11) and adrenal tumor Y-1 (16) cells, Chinese hamster ovary cells (16, 20, 38, 42), and baby hamster kidney cells (54), as well as human and sheep erythrocytes (1, 13, 43, 45).
Since cAMP has been generally recognized to be an important intracellular second messenger, numerous studies have been performed to clarify the role of ACT in whooping cough disease. Mutant B. pertussis strains deficient in ACT synthesis are known to be unable to colonize the surface of respiratory tract (19, 53). In addition, ACT has been shown to inhibit phagocytic functions (7, 41) and to induce apoptosis in macrophages (23, 30). However, the primary target of ACT during B. pertussis infection has not yet been clearly identified.
The platelets are one of the major blood components, and their dysfunction results in hemorrhage, which is considered to be one of the complications of whooping cough (52). cAMP, on the other hand, is known to be a potent suppressor of platelet aggregation (25, 37, 46, 56). We demonstrate here that ACT suppresses platelet aggregation in vitro through increase of intracellular cAMP due to its catalytic activity and that ACT induces prolongation of bleeding time in vivo.
MATERIALS AND METHODS
Preparation of toxins.
Recombinant ACT (3) and its catalytically inactive derivative ACTK58Q (27) were expressed in E. coli XL1-Blue harboring separately plasmids pCACT3 (3) and pT7CT7ACT-K58Q (constructed by Peter Šebo and kind gifts from Agnes Ullmann [Institut Pasteur]). Recombinant toxins were extracted from ultrasound-disrupted cell debris, which contained 60 to 70% of total cellular adenylate cyclase activity (45, 48), with 8 M urea–50 mM Tris-HCl–0.2 mM CaCl2 (pH 7.5) and then purified to close to homogeneity by DEAE-Sepharose Fast Flow chromatography as described by Sakamoto et al. (45) (Fig. 1). Protein content was determined with a Pierce bicinchoninic acid (BCA) protein assay kit (49). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on an 8% gel was performed as described by Laemmli (35), and proteins were visualized with Coomassie brilliant blue.
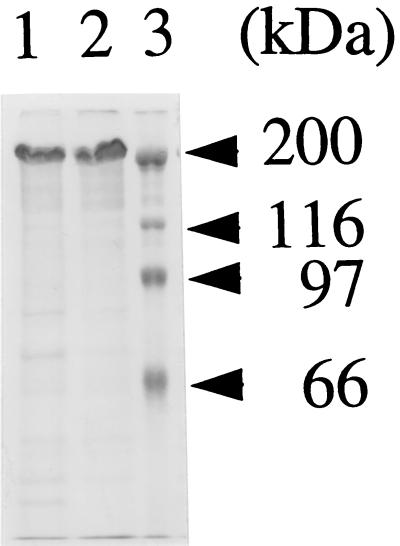
FIG. 1.
ACT and ACTK58Q preparations used in this study. ACT and ACTK58Q were prepared as described in Materials and Methods by DEAE-Sepharose Fast Flow chromatography. The preparations (20 μg of protein/lane) were loaded on an SDS–8% polyacrylamide gel and visualized by Coomassie brilliant blue staining. Lanes: 1, ACT; 2, ACTK58Q; 3, molecular weight markers.
Preparation of platelets. (i) Platelet preparation for cAMP assay.
Platelets were prepared as described by Kitamura et al. (32, 33), with slight modification. Japanese White rabbits (3 to 4 kg) were bled (10 ml) into a 10-ml syringe containing 1/10 volume of 3.8% sodium citrate. The blood was centrifuged at 190 × g for 20 min, and the upper layer (platelet-rich plasma [PRP]) was isolated. Eventually, the lower layer, which still contained platelets, was resuspended in washing medium (135 mM NaCl, 4 mM glucose, 2 mM EDTA, 13 mM sodium acetate [pH 6.5]) and centrifuged at 190 × g for 20 min, and the upper layer was added to PRP. PRP or washing medium-containing PRP was then centrifuged again at 190 × g for 5 min to remove contaminating nonplatelet cells, and then the supernatant was centrifuged at 800 × g for 10 min to precipitate platelets. Precipitated platelets were resuspended in 10 ml of washing medium. The platelets were washed two times in washing medium by recentrifuging at 800 × g for 10 min and then once with suspension medium (135 mM NaCl, 5 mM glucose, 2 mM EDTA, 15 mM HEPES [pH 7.5]). The platelets were finally resuspended in 5 ml of suspension medium and kept at 37°C to prevent spontaneous aggregation. The final platelet preparation contained 1 nonplatelet cell per 2,000 to 10,000 platelets, which did not significantly affect cAMP measurements (data not shown).
(ii) Platelet preparation for aggregation assay.
PRP was isolated as described above except that Na,K-Tris (137 mM NaCl, 5.4 mM KCl, 11 mM glucose, 25 mM Tris-HCl [pH 7.4]) was used instead of washing medium (31). PRP or Na,K-Tris-containing PRP was recentrifuged at 800 × g for 10 min, and precipitated platelets were resuspended in 450 μl of Na,K-Tris. Then 50 μl of 112 mM citrate (pH 6.0) was added to the platelet suspension, and the suspension was kept at 37°C to prevent spontaneous aggregation. The final preparation contained 1 nonplatelet cell (which did not contribute to aggregation) per at least 1,000 platelets.
Establishment and production of monoclonal antibody B7E11.
The adenylate cyclase toxin fragment AC196–267, comprising aa 196 to 267 (corresponding to the calmodulin binding site of the catalytic domain), was expressed in E. coli and purified as described previously (39). Purified AC196–267 was kindly provided by Hélène Munier. Spleen cells of mice highly immunized with AC196–267 (a kind gift of Nicole Guiso) were fused with the murine fusion line SP2, and the specificity of antibody secreted by cloned hybridomas was identified by screening of culture supernatants in ACT and AC196–267 enzyme-linked immunosorbent assay (ELISA). For ELISA, the purified protein of interest (10 μg/ml; 100 μl/well) was directly coated overnight onto Maxisorp Microtiter plates (Nunc, Roskilde, Denmark) in alkaline carbonate buffer (pH 9.6). The wells were then blocked with phosphate-buffered saline (PBS) containing 2% bovine serum albumin for 4 h at room temperature. Hybridoma supernatants were incubated 2 h in the wells, followed by washing with PBS-bovine serum albumin containing 0.5% Tween 20. Bound antibody was detected by using goat anti-mouse antibody labeled with alkaline phosphatase and revealed with the substrate p-nitrophenyl phosphate (Sigma); plates were read at wavelength 405 nm. Antibody B7E11 was purified from hybridoma supernatant by protein A-agarose chromatography (HiTrap; Pharmacia, Uppsala, Sweden), purity was checked by SDS-PAGE, and the protein content of purified antibody was determined by the Pierce BCA protein assay.
cAMP assay.
A 125-μl portion of platelet suspension was added to 125 μl of prewarmed (37°C) suspension medium containing 4 mM CaCl2, which gave a final concentration of 1 mM in the 250-μl incubation due to the chelating effect of EDTA in the platelet suspension. To avoid protein denaturation, appropriate concentrations of ACT and ACTK58Q were added to the medium immediately before addition of platelet suspension. Urea concentration in all incubations were equilibrated to 48 mM. The mixture was then incubated at 37°C, and cAMP was extracted by the method of Kitamura et al. (32, 33). Briefly, 1 ml of ethanol containing 0.15 M HCl was added to the incubation mixture, and platelets were immediately disrupted by sonication for 10 s with a model UP 50 H sonicator (Dr Hielscher GmbH, Teltow, Germany). The sonicated extract was heated at 100°C for 10 min, and then solvent was evaporated. Residues were redissolved in 1 ml of 50 mM sodium acetate buffer (pH 6.2), and 10 to 100 μl was subjected to radioimmunoassay (1) using rabbit anti-cAMP polyclonal antiserum (ICN Biochemicals, Costa Mesa, Calif.). Sixty percent of cAMP had already accumulated during first 5 min of a 15-min incubation period and thus was already present at a level about 170 times higher than physiological cAMP levels (data not shown). Thus, to avoid spontaneous platelet aggregation during unnecessarily prolonged incubation periods, 5 min of ACT treatment was used for cAMP and aggregation assays throughout this study.
Platelet aggregometry.
ADP- or thrombin-induced platelet aggregation was expressed as decrease of absorbance (4) (increase of light transmittance) of platelet suspension at 700 nm. Fifty microliters of platelet suspension was added to an aggregometer cuvette (no. 3121; SSR Engineering Co., Tokyo, Japan) containing 450 μl of Na,K-Tris supplemented with 1 mM CaCl2. Rabbit fibrinogen (Sigma Chemical, St. Louis, Mo.) was added to 1 mg/ml for ADP-induced aggregation assays. ACT, ACTK58Q, dibutyryl-cAMP, or forskolin was then added, and the mixture was incubated at 37°C for 5 min. The urea concentration in the incubation mixture was kept below 45 mM, as described in the figure legends. After incubation, the cuvettes were placed in the incubation chambers of the two-chamber aggregometer (model PAT-2M; SSR Engineering), and absorbance of this initial mixture was set as 100%; absorbance of Na,K-Tris was set as 0%. Fifty microliters of 100 μM ADP or 2 U/ml of thrombin (Sigma) per incubation (final concentrations of 10 μM or 0.2 U/ml, respectively) was added gently but rapidly, using a 50-μl microsyringe, and the change in absorbance was monitored and recorded with a two-pen recorder (model U-228; Nippon Denshi Kagaku, Kyoto, Japan).
Extensive care was taken for antibody-blocking experiment in order to establish experimental conditions which allow a proper antigen-antibody reaction without impairing the aggregation potential of platelets. Because the internalization of ACT into target cells is considered to be very rapid, antibody molecules are not likely to be able to complete their binding to ACT molecules before ACT internalizes across the platelet cell membrane. On the other hand, a frequently used method, such as preincubation of antibody with ACT prior to addition of the platelet suspension, could not be successfully employed either, because of nonspecific inactivation of ACT due to self-aggregation in the absence of denaturing agents such as 8 M urea. Thus, during antibody treatment, translocation of membrane-inserted ACT molecules into platelets was prevented by not adding calcium ions (43) (but not by chelating) into the Na,K-Tris incubation medium. This mixture was incubated at 37°C for 8 min, and then a 1/20 volume of 20 mM CaCl2 was added to allow ACT internalization. After 5 min of incubation at 37°C, ADP was added and changes in absorbance at 700 nm were recorded.
Bleeding time assay.
The bleeding time assay was performed as described by Dejana et al. (10) and others (40, 50). Briefly, ACT or ACTK58Q (100 μg/mouse) was injected intravenously to female Slc: ddY mice (47) (8 weeks old) at the base of the tail. Thirty minutes after injection, the tail tip (2 mm) was cut under pentobarbital (Nembutal; 50 mg/kg of body weight, Abbott Laboratories, Chicago, Ill.) anesthesia, and the tail was immediately immersed in PBS (37°C). Bleeding time was measured from the time of tail transsection to an abrupt stopping of bleeding without rebleeding within 30 s. Bleeding times exceeding 15 min were recorded as 900 s.
RESULTS
Platelet cAMP accumulation by ACT.
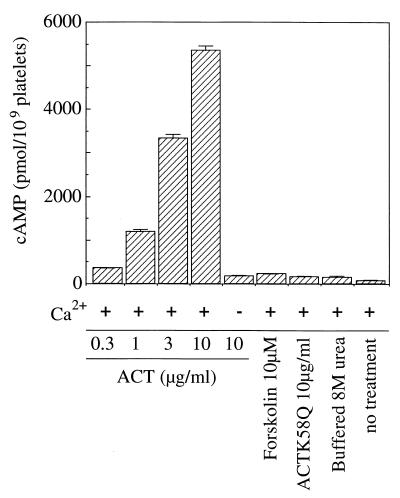
To examine whether ACT is able to act on rabbit platelets, we first measured the effect of ACT on platelet intracellular cAMP levels. Rabbit platelets (1.2 × 108/ml) were treated with 0.3 to 10 μg/ml (equivalent to a catalytic activity of 0.12 to 4.0 μmol of cAMP/min) of DEAE-purified ACT in 250 μl of suspension medium containing 1 mM CaCl2 at 37°C for 5 min, and then intracellular cAMP was extracted by ultrasonic cell disruption in ethanol-HCl and measured by radioimmunoassay (Fig. 2). ACT induced an increase in cAMP level in platelets dose dependently, up to 5 nmol/109 platelets at 10 μg/ml. In contrast, no significant increase of intracellular cAMP level was induced by 10 μg of ACT per ml in the absence of calcium, which is known to be required for internalization of the toxin into cells (43). The catalytically inactive mutant ACTK58Q did not show a significant increase of intracellular cAMP even at a concentration as high as 10 μg/ml, indicating that the cAMP increase by catalytically active ACT is a consequence of its internalization into platelets. Forskolin (10 μM) induced a slight but significant increase of intracellular cAMP compared to platelets containing urea, ACTK58Q, or ACT (without CaCl2) (Fig. 2). As cAMP levels in urea-treated platelets were significantly higher than those of untreated platelets (Fig. 2), urea treatment provided more suitable controls.
FIG. 2.
Effects of ACT on platelet intracellular cAMP accumulation. A rabbit platelet suspension (1.2 × 108/ml) was incubated with indicated concentrations of ACT, ACTK58Q, or buffered 8 M urea for 5 min at 37°C in the presence or absence of calcium chloride as described in Materials and Methods. A cAMP radioimmunoassay was performed as described in Materials and Methods. Bars indicate standard errors from triplicate assays.
Suppression of ADP-induced platelet aggregation by ACT.
We then investigated the effects of ACT on the aggregation potency of rabbit platelets through an increase of intracellular cAMP levels.
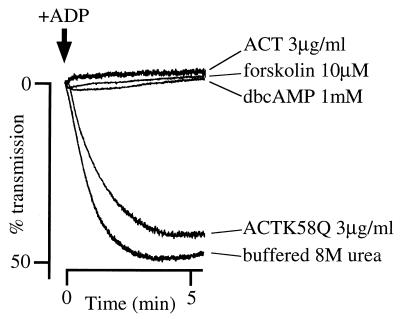
Rabbit platelets (1.5 × 108/ml) were resuspended in Na,K-Tris supplemented with 1 mM CaCl2, aggregation was initiated by the addition of 10 μM ADP, and absorbance of the platelet suspension was monitored with a platelet aggregometer. A dramatic decrease in absorbance due to aggregation was observed within 1 min after addition of ADP to control (buffered 8 M urea-treated) platelet suspension, which reflects the normal reactivity of the platelets to ADP (Fig. 3). Treatment of platelets with ACT (3 μg/ml; equivalent to a catalytic activity of 1.2 μmol of cAMP/min) for 5 min at 37°C, prior to addition of 10 μM ADP, completely suppressed the aggregation (Fig. 3). This suppression was also observed by forskolin (10 μM) and dibutyryl-cAMP (1 mM) treatments (Fig. 3). In contrast, the catalytically inactive mutant ACTK58Q at 3 μg/ml (Fig. 3) or 10 μg/ml (data not shown) did not significantly suppress ADP-induced aggregation, suggesting that the impairment of platelet function resulted from accumulation of intracellular cAMP. There was no significant difference between buffered 8 M urea-treated and untreated controls (data not shown), which was compatible with the results of Camici et al. (6).
FIG. 3.
Suppression of ADP-induced platelet aggregation by ACT. Platelets (1.5 × 108/ml) were treated with ACT (3 μg/ml) or ACTK58Q (added as 2.4 μl of a 625-μg/ml solution in buffered 8 M urea), 10 μM forskolin (added as 2.0 μl of a 2.4 mM solution in dimethyl sulfoxide), 1 mM dibutyryl-cAMP (dbcAMP; 10 μl of a 50 mM aqueous solution), or 2.4 μl of buffered 8 M urea in 500 μl of Na,K-Tris (137 mM NaCl, 5.4 mM KCl, 11 mM glucose, 25 mM Tris-HCl [pH 7.4]) containing 1 mM CaCl2 at 37°C for 5 min. Then 50 μl of 100 μM ADP was added, and changes in absorbance were recorded.
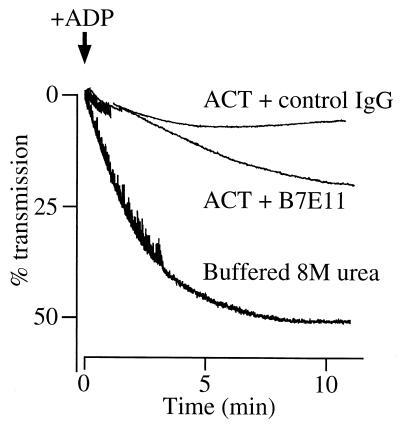
This suppressive effect of ACT was diminished by an anti-ACT monoclonal antibody, B7E11, that reacts with the calmodulin binding site of ACT, located in the middle of the catalytic domain (17). Platelets (1.5 × 108/ml) were treated sequentially with the antibody (6.6 μg/ml) and ACT (7.5 μg/ml) as described in Materials and Methods, and then ADP (10 μM) was added to initiate platelet aggregation. Monoclonal antibody B7E11 was shown to partially inhibit the suppressive effect of ACT, resulting in significant recovery of platelet aggregation (Fig. 4).
FIG. 4.
Blocking of ACT-mediated suppression of platelet aggregation by anti-ACT antibody. Anti-ACT monoclonal antibody B7E11 or control mouse immunoglobulin G (IgG; Sigma) (6.6 μg/ml; added as 20 μl of a 165-μg/ml solution in Tris-buffered saline) and ACT (7.5 μg/ml; added as a 1.25 μl of a 3-mg/ml solution in buffered 8 M urea) were sequentially added to 500 μl of platelet suspension (1.5 × 108/ml) in Na,K-Tris in the absence of calcium chloride and incubated for 8 min at 37°C; 25 μl of Na,K-Tris containing 20 mM CaCl2 was then added; after a further 5 min of incubation at 37°C, 50 μl of 100 μM ADP was added to initiate platelet aggregation and changes in absorbance were recorded.
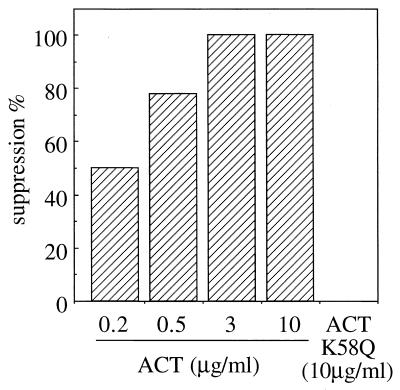
The suppressive effect of ACT on ADP-induced platelet aggregation was dose dependent. Although there was a large variation in the level of responsiveness of platelets when different platelet preparations were used, a positive correlation was always observed between the degree of suppression and ACT concentration. A typical pattern of dose dependency is shown in Fig. 5. Percent suppression (determined as described in the legend to Fig. 5) increased with ACT concentration, reaching 100% at 3 μg/ml, clearly indicating the dose dependence of the ACT effect on ADP-induced platelet aggregation.
FIG. 5.
ACT dose dependency in suppression of platelet aggregation. Platelets (108/ml) were treated with indicated concentrations of ACT (added as 2.4 μl of solutions of appropriate concentrations in buffered 8 M urea) or buffered 8 M urea solution (2.4 μl) in 500 μl of Na,K-Tris containing 1 mM CaCl2 at 37°C for 5 min. Then 50 μl of 100 μM ADP was added, and changes in absorbance were recorded. Percent suppression was calculated from the results of ACT-urea pairs in a two-chamber platelet aggregometer analyzed in parallel as follows: % suppression = 1 − (maximum aggregation of ACT-treated platelets/maximum aggregation of urea-treated platelets) × 100.
Suppression of thrombin-induced platelet aggregation by ACT.
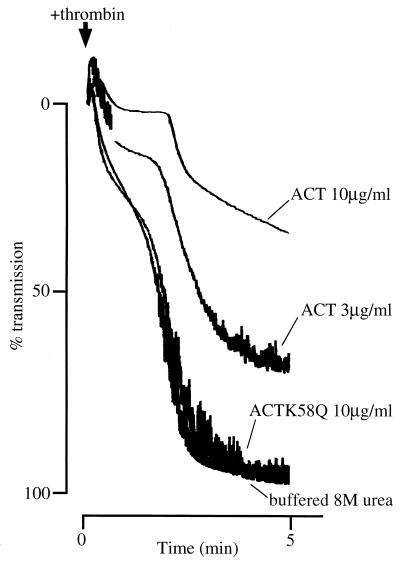
Figure 6 shows the effect of ACT on thrombin-induced platelet aggregation. Thrombin (0.2 U/ml) more strongly induced aggregation on control platelets, and this aggregation was suppressed by 3 μg of ACT per ml. Stronger suppression was achieved by treatment with higher concentrations of ACT (up to 10 μg/ml), again showing a clear dose dependence, which may reflect higher intracellular cAMP accumulation evoked by 10 μg/ml than by 3 μg/ml, as shown in Fig. 2. The same concentration (10 μg/ml) of a catalytically inactive mutant ACTK58Q did not suppress thrombin-induced aggregation. There was no significant difference between buffered 8 M urea-treated and untreated controls (data not shown). These results clearly indicate that the suppression of ADP- and thrombin-induced platelet aggregation was due to the catalytic activity of ACT through increase of intracellular cAMP.
FIG. 6.
Suppression of thrombin-induced platelet aggregation by ACT. Platelets (1.7 × 108/ml) were treated with ACT (3 or 10 μg/ml; added as 2.4 μl of a 625-μg/ml solution or 2.9 μl of a 1.7-mg/ml solution in buffered 8 M urea, respectively) or ACTK58Q (10 μg/ml; added as 2.9 μl of a 1.7-mg/ml solution in buffered 8 M urea) or 2.9 μl of buffered 8 M urea in 500 μl of Na,K-Tris (137 mM NaCl, 5.4 mM KCl, 11 mM glucose, 25 mM Tris-HCl [pH 7.4]) containing 1 mM CaCl2 at 37°C for 5 min. Then 50 μl of thrombin (2 U/ml) was added, and changes in absorbance were recorded.
In vivo effect of ACT on mouse bleeding time.
We then examined whether this suppressive effect of ACT on platelet aggregation would occur in vivo in mice. ACT and ACTK58Q were injected intravenously into the tail vein of mice at the base of the tail, to avoid destruction of tail tissue near the tail tip. Thirty minutes after injection, the tail tip (2 mm) was cut with a sharp razor blade under pentobarbital anesthesia, the tail was immediately immersed in PBS (37°C), and bleeding times were measured for eight to nine mice per group. Significance of difference between groups was confirmed with Student’s t test. Table 1 shows the bleeding times of ACT-treated, ACTK58Q-treated, and control (buffered 8 M urea-treated) mice. The result clearly show a significant prolongation of bleeding time by ACT treatment, compared to ACTK58Q-treated and control groups, indicating the in vivo suppressive activity of ACT on blood coagulation.
TABLE 1.
Bleeding times of ACT-treated micea
| Treatment | No. of mice | Mean bleeding time (s) ± SE | Pb |
|---|---|---|---|
| ACT (100 μg/mouse) | 9 | 749 ± 100 | |
| ACTK58Q (100 μg/mouse) | 9 | 242 ± 93 | <0.01 |
| Buffered 8 M urea | 8 | 275 ± 129 | <0.01 |
One hundred fifty microliters of toxin solution in 8 M urea containing 20 mM Tris HCl (pH 7.5) and 0.2 mM CaCl2 was intravenously injected to female Slc: ddY mice (8w). Tail tip (2 mm) was then severed under pentobarbital anesthesia, and the tail was immediately immersed in PBS (37°C). Bleeding time was measured from the time of tail transsection to an abrupt stopping of bleeding without rebleeding within 30 s. Bleeding times exceeding 15 min were recorded as 900 s.
Student’s t test against ACT-treated group.
DISCUSSION
We observed that ACT suppresses ADP- and thrombin-induced aggregation of rabbit platelets in vitro. We also observed a large delay of bleeding time in vivo in ACT-treated mice, probably resulting from the suppression of platelet aggregation. The in vitro suppression was dependent on ACT concentration in the incubation medium and reached complete suppression of ADP-induced aggregation at a high concentration such as 3 μg/ml (Fig. 3). The suppression was accompanied by a marked increase in intracellular cAMP levels (Fig. 2), and the catalytically inactive mutant ACTK58Q showed no such suppressive effect (Fig. 3). As cAMP is a widely recognized suppressor of platelet aggregation (25, 37, 46, 56), the results clearly indicate that ACT suppresses platelet aggregation by increasing intracellular cAMP through its catalytic activity. This observation is also supported by the fact that the suppressive effect was diminished by adding the anti-ACT monoclonal antibody B7E11, directed against the calmodulin binding site in the catalytic domain of ACT (Fig. 4). This antibody is also able to inhibit the internalization of ACT into sheep erythrocytes (data not shown), suggesting that the partial inhibition of ACT action by this antibody was due to blocking of ACT entry into platelets but not to inhibition of the catalytic activity of ACT. Preliminary experiments indicate that this antibody does not exert a significant inhibitory effect on the adenylate cyclase catalytic activity of ACT. This is often the case; among a large number of monoclonal or polyclonal antibodies (raised in rabbits, guinea pigs, or mice) against ACT, inhibition of adenylate cyclase activity was observed only once (51a).
cAMP is known to suppress both ADP- and thrombin-induced platelet aggregation (25, 37, 46, 56). ADP and thrombin trigger platelet activation by release of calcium from intracellular pools, through activation of phospholipase C and subsequent release of inositol 1,4,5-triphosphate (15). Alternatively, ADP is considered to stimulate calcium influx by opening of calcium channels which are coupled with membrane ADP receptors (15). Various mechanisms have been proposed for cAMP-mediated platelet inactivation, including (i) promotion of calcium efflux through activation of plasma membrane Ca2+-ATPase by cAMP-dependent protein kinase (9, 29), (ii) reduction of phospholipase C/inositol 1,4,5-triphosphate-mediated calcium mobilization from intracellular stores by interacting with phospholipase C (15), and (iii) reduction of the binding and response of platelets to thrombin (36). Although the mechanism of cAMP-mediated suppression of platelet aggregation has not yet been clearly elucidated, our results are fully consistent with all of these proposed mechanisms, as ACT simply produces supraphysiological amount of intracellular cAMP in platelets. In contrast, the increase of intracellular cAMP by 10 μM forskolin was significant but lower than that induced by 0.3 μg of ACT per ml (Fig. 2), although the same concentration of forskolin completely suppressed ADP-induced platelet aggregation (Fig. 3) whereas 0.2 or 0.5 μg ACT per ml did not (Fig. 5). The reason for this discrepancy remains unclear. One possible explanation could be the difference between the mechanisms of action of forskolin and ACT. Forskolin increases cAMP concentration by activating an exquisitely regulated cellular adenylate cyclase (29), while the calmodulin-activated invasive ACT catalyzes high-level synthesis of cAMP, which might alter cellular physiology in a different way.
Both ADP- and thrombin-induced platelet aggregation were suppressed by ACT in a dose-dependent manner. However, the ADP-induced aggregation appeared to be more sensitive to the suppressive effects of ACT than that induced by thrombin under our experimental conditions. An ACT concentration of 3 μg/ml was sufficient to completely suppress the ADP-induced platelet aggregation (Fig. 3), while concentrations as high as 10 μg/ml did not bring about a complete suppression of thrombin-induced aggregation (Fig. 6). This could be due to the difference between the mechanisms of platelet aggregation induced by these two agents. In fact, ADP-induced aggregation was partially reversible (data not shown), and the maximum reduction of absorbance did not exceed 50% (Fig. 3). In contrast, thrombin-induced aggregation was biphasic and irreversible, forming a cluster tightly attached to the stirring bar in the aggregometer cuvette, and the maximum reduction of absorbance reached almost 100% (Fig. 6). In addition, these agents may not be equally effective as inducers of platelet aggregation at the concentrations used in this study.
Numerous experimental data (7, 23, 30, 41) have shown that ACT serves to disable the host defense function of immune effector cells during the early stage of B. pertussis infection. In addition to these actions, we reported here the effects of ACT on platelet aggregation and bleeding time not only in vitro but also in vivo; however, the significance of these biological activities in pertussis disease is not yet clear. Pertussis disease is often accompanied by complications such as subconjunctival hemorrhage, skin petechiae, epistasis, hemoptysis, and occasionally intracranial hemorrhage (52). These symptoms are generally regarded as a result of venous congestion (52); however, these hemorrhagic symptoms resemble platelet dysfunction and could also be attributed, at least in part, to ACT. On the other hand, ACT is not considered to circulate in the bloodstream in clinical pertussis cases, as evidenced by the facts that (i) viable B. pertussis bacteria have been very rarely detected in circulating blood of patients (28, 52) and (ii) the presence of ACT in circulating blood has, to our knowledge, never been reported. To clarify whether ACT contributes to the symptoms of pertussis, further experimental evidence is needed. In vivo and in vitro analysis of platelet functions of patients and experimentally infected animals would help to elucidate the role of ACT in pertussis disease.
ACKNOWLEDGMENTS
We thank Agnes Ullmann, Hiroko Sato, and Yoshichika Arakawa for critical reading of the manuscript and continuous encouragement throughout this work.
N.H. was supported by the European Community (Human Capital and Mobility Programme).
REFERENCES
- 1.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz R, Maier E, Ladant D, Ullmann A, Šebo P. Adenylate cyclase toxin of Bordetella pertussis: evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J Biol Chem. 1994;269:27231–27239. [PubMed] [Google Scholar]
- 3.Betsou F, Šebo P, Guiso N. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun. 1993;61:3583–3589. doi: 10.1128/iai.61.9.3583-3589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Born G V R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 5.Brownlie R M, Coote J G, Parton R, Schultz J E, Rogel A, Hanski E. Cloning of the adenylate cyclase genetic determinant of Bordetella pertussis and its expression in Escherichia coli and B. pertussis. Microb Pathog. 1988;4:335–344. doi: 10.1016/0882-4010(88)90061-7. [DOI] [PubMed] [Google Scholar]
- 6.Camici M, Evangelisti L, Raspolli-Galletti M. The effect of oxalic acid on the aggregability of human platelet rich plasma. Prostaglandins Leukot Med. 1986;21:107–110. doi: 10.1016/0262-1746(86)90168-x. [DOI] [PubMed] [Google Scholar]
- 7.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 8.Coote J G. Structural and functional relationships among the RTX toxin determinants of Gram-negative bacteria. FEMS Microbiol Rev. 1992;88:137–162. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 9.Dean W L, Chen D, Brandt P C, Vanaman T C. Regulation of platelet plasma membrane Ca2+-ATPase by cAMP-dependent and tyrosine phosphorylation. J Biol Chem. 1997;272:15113–15119. doi: 10.1074/jbc.272.24.15113. [DOI] [PubMed] [Google Scholar]
- 10.Dejana E, Calloni A, Quintana A, de Gaetano G. Bleeding time in laboratory animals. II. A comparison of different assay conditions in rats. Thromb Res. 1979;15:191–197. doi: 10.1016/0049-3848(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 11.Donovan M G, Storm D R. Evidence that the adenylate cyclase secreted from Bordetella pertussis does not enter animal cells by receptor-mediated endocytosis. J Cell Physiol. 1990;145:444–449. doi: 10.1002/jcp.1041450308. [DOI] [PubMed] [Google Scholar]
- 12.Ehrmann I E, Gray M C, Gordon V M, Gray L S, Hewlett E L. Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett. 1991;278:79–83. doi: 10.1016/0014-5793(91)80088-k. [DOI] [PubMed] [Google Scholar]
- 13.Ehrmann I E, Weiss A A, Goodwin M S, Gray M C, Barry E, Hewlett E L. Enzymatic activity of adenylate cyclase toxin from Bordetella pertussis is not required for hemolysis. FEBS Lett. 1992;304:51–56. doi: 10.1016/0014-5793(92)80587-7. [DOI] [PubMed] [Google Scholar]
- 14.Friedman E, Farfel Z, Hanski E. The invasive adenylate cyclase of Bordetella pertussis: properties and penetration kinetics. Biochem J. 1987;243:145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger J, Walter U. Properties and regulation of human platelet cation channels. EXS. 1993;66:281–288. doi: 10.1007/978-3-0348-7327-7_22. [DOI] [PubMed] [Google Scholar]
- 16.Gentile F, Raptis A, Knipling L G, Wolff J. Bordetella pertussis adenylate cyclase: penetration into host cells. Eur J Biochem. 1988;175:447–453. doi: 10.1111/j.1432-1033.1988.tb14215.x. [DOI] [PubMed] [Google Scholar]
- 17.Glaser P, Elmaoglou-Lazaridou A, Krin E, Ladant D, Bârzu O, Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989;8:967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988;2:19–30. [PubMed] [Google Scholar]
- 19.Goodwin M S, Weiss A A. Adenylate cyclase toxin is critical for colonization and Pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon V M, Leppla S H, Hewlett E L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988;56:1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon V M, Young W W, Jr, Lechler S M, Gray M C, Leppla S H, Hewlett E L. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 22.Gross M K, Au D C, Smith A L, Storm D R. Targeted mutations that ablate either the adenylate cyclase or hemolysin function of the bifunctional cyaA toxin of Bordetella pertussis abolish virulence. Proc Natl Acad Sci USA. 1992;89:4898–4902. doi: 10.1073/pnas.89.11.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanski E, Farfel Z. Bordetella pertussis invasive adenylate cyclase: partial resolution and properties of its cellular penetration. J Biol Chem. 1985;290:5526–5532. [PubMed] [Google Scholar]
- 25.Haslam R J, Davidson M M L, Davies T, Lynham J A, McClenaghan M D. Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res. 1978;9:533–552. [PubMed] [Google Scholar]
- 26.Hewlett E L, Gray L, Allietta M, Ehrmann I E, Gordon V M, Gray M C. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J Biol Chem. 1991;266:17503–17508. [PubMed] [Google Scholar]
- 27.Iwaki M, Ullmann A, Šebo P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol Microbiol. 1995;17:1015–1024. doi: 10.1111/j.1365-2958.1995.mmi_17061015.x. [DOI] [PubMed] [Google Scholar]
- 28.Janda W M, Santos E, Stevens J, Celig D, Terrile L, Schreckenberger P C. Unexpected isolation of Bordetella pertussis from a blood culture. J Clin Microbiol. 1994;32:2851–2853. doi: 10.1128/jcm.32.11.2851-2853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson J S, Nied L E, Haynes D H. Cyclic AMP stimulates Ca2+-ATPase-mediated Ca2+ extrusion from human platelets. Biochim Biophys Acta. 1992;1105:19–28. doi: 10.1016/0005-2736(92)90158-i. [DOI] [PubMed] [Google Scholar]
- 30.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4070. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitagawa S, Kotani K, Kametani F. Inhibitory mechanism of cis-polyunsaturated fatty acids on platelet aggregation: the relation with their effects on Ca2+ mobilization, cyclic AMP levels and membrane fluidity. Biochim Biophys Acta. 1990;1054:114–118. doi: 10.1016/0167-4889(90)90212-v. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Matsuo H, Eto T. Isolation and characterization of peptides which act on rat platelets, from a pheochromocytoma. Biochem Biophys Res Commun. 1992;185:134–141. doi: 10.1016/s0006-291x(05)80966-0. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 34.Ladant D, Michelson S, Sarfati R S, Gilles A-M, Predeleanu R, Bârzu O. Characterization of the calmodulin-binding and of the catalytic domains of Bordetella pertussis adenylate cyclase. J Biol Chem. 1989;264:4015–4020. [PubMed] [Google Scholar]
- 35.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Lerea K M, Glomset J A, Krebs E G. Agents that elevate cAMP levels in platelets decrease thrombin binding. J Biol Chem. 1987;262:282–288. [PubMed] [Google Scholar]
- 37.Marquis N R, Vigdahl R L, Tavormina P A. Platelet aggregation. I. Regulation by cyclic AMP and prostaglandin E1. Biochem Biophys Res Commun. 1969;36:965–972. doi: 10.1016/0006-291x(69)90298-8. [DOI] [PubMed] [Google Scholar]
- 38.Mouallem M, Farfel Z, Hanski E. Bordetella pertussis adenylate cyclase toxin: intoxication of host cells by bacterial invasion. Infect Immun. 1990;58:3759–3764. doi: 10.1128/iai.58.11.3759-3764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munier H, Bouhss A, Gilles A-M, Palibroda N, Bârzu O, Mispelter J, Craescu C T. Structural characterization by nuclear magnetic resonance spectroscopy of a genetically engineered high-affinity calmodulin-binding peptide derived from Bordetella pertussis adenylate cyclase. Arch Biochem Biophys. 1995;320:224–235. doi: 10.1016/0003-9861(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 40.Novak E K, Sweet H O, Prochazka M, Parentis M, Soble R, Reddington M, Cairo A, Swank R T. Cocoa: a new mouse model for platelet storage pool deficiency. Br J Haematol. 1988;69:371–378. doi: 10.1111/j.1365-2141.1988.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 41.Pearson R D, Symes P, Conboy M, Weiss A A, Hewlett E L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987;139:2749–2754. [PubMed] [Google Scholar]
- 42.Raptis A, Knipling L, Wolff J. Dissociation of catalytic and invasive activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1989;57:1725–1730. doi: 10.1128/iai.57.6.1725-1730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogel A, Hanski E. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. Translocation of the toxin across the membrane. J Biol Chem. 1992;267:22599–22605. [PubMed] [Google Scholar]
- 44.Rogel A, Meller R, Hanski E. Adenylate cyclase toxin from Bordetella pertussis. The relationship between induction of cAMP and hemolysis. J Biol Chem. 1991;266:3154–3161. [PubMed] [Google Scholar]
- 45.Sakamoto H, Bellalou J, Šebo P, Ladant D. Bordetella pertussis adenylate cyclase toxin: structural and functional independence of the catalytic and hemolytic activities. J Biol Chem. 1992;267:13598–13602. [PubMed] [Google Scholar]
- 46.Salzman E W, Rubino E B, Sims R V. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. III. The role of cyclic AMP in platelet aggregation. Ser Haemat. 1970;3:100–113. [PubMed] [Google Scholar]
- 47.Sato H, Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in a intracerebral or aerosol challenge system. Infect Immun. 1984;46:415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sebo P, Glaser P, Sakamoto H, Ullmann A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed Escherichia coli system. Gene. 1991;104:19–24. doi: 10.1016/0378-1119(91)90459-o. [DOI] [PubMed] [Google Scholar]
- 49.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 50.Swank R T, Reddington M, Novak E K. Inherited prolonged bleeding time and platelet storage pool deficiency in the Subtle Gray (sut) mouse. Lab Anim Sci. 1996;46:56–60. [PubMed] [Google Scholar]
- 51.Szabo G, Gray M C, Hewlett E L. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium- and polarity-dependent manner. J Biol Chem. 1994;269:22496–22499. [PubMed] [Google Scholar]
- 51a.Ullmann, A. Personal communication.
- 52.Walker E. Clinical aspects of pertussis. In: Wardraw A C, Parton R, editors. Pathogenesis and immunity of pertussis. Chichester, England: John Wiley & Sons; 1988. pp. 273–282. [Google Scholar]
- 53.Weiss A A, Hewlett E L, Myers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 54.Westrop G D, Campbell G, Kazi Y, Billcliffe B, Coote J G, Parton R, Freer J H, Edwards J G. A new assay for the invasive adenylate cyclase toxin of Bordetella pertussis based on its morphological effects on the fibronectin-stimulated spreading of BHK21 cells. Microbiology. 1994;140:245–253. doi: 10.1099/13500872-140-2-245. [DOI] [PubMed] [Google Scholar]
- 55.Wolff J, Cook G H, Goldhammer A R, Berkowitz S A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci USA. 1980;77:3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zieve P D, Greenough W B., III Adenyl cyclase in human platelets: activity and responsiveness. Biochem Biophys Res Commun. 1969;35:462–466. doi: 10.1016/0006-291x(69)90368-4. [DOI] [PubMed] [Google Scholar]