Abstract
The skin is recognized as a peripheral lymphoid organ that plays an essential defensive action against external environmental stimuli. However, continuous stimulation of these factors causes chronic inflammation at the local site and occasionally causes tissue damage. Chronic inflammation is recognized as a trigger for systemic organ inflammation. Atopic dermatitis (AD) is a chronic inflammatory skin disease that is influenced by various external environmental factors, such as dry conditions, chemical exposure, and microorganisms. The pathogenesis of AD involves various Th2 and proinflammatory cytokines. Recently updated studies have shown that atopic skin-derived cytokines influence systemic organ function and oncogenesis. In this review, we focus on AD’s influence on the development of systemic inflammatory diseases and malignancies.
Keywords: atopic dermatitis, Th2, immune cell, inflammation, systemic organs
1. Introduction
The peripheral lymphoid organ plays an essential regulation action against external environmental factors such as microorganisms, chemical exposure, and medications [1,2,3,4]. Circulating immune cells and cytokines derived from the original local inflammatory peripheral organs influence other organ functions [5,6]. Therefore, excessive immunological responses in peripheral lymphoid organs may be able to influence systemic organs.
The skin is recognized as a peripheral lymphoid tissue located in the most layered organ and is influenced by external environments [7,8]. Environmental stimuli and pathogens that are exposed to the skin cause various physiological and pathological reactions [9,10]. Chronic inflammation is a representative response to these environmental stimuli, and repeated exposure to external pathogens [11,12]. The chronic inflammatory reaction exceeds local site inflammation and occasionally develops into systemic inflammatory diseases, such as cardiovascular diseases [13,14]. Because the skin is placed in the outermost and is often exposed to the external environment or microorganisms, chronic skin inflammation may become a source of circulating inflammatory cytokines and activated immune cells, which are responsible for systemic inflammatory responses. Moreover, recently updated studies have shown that chronic inflammatory skin diseases become the trigger for the development of systemic inflammatory diseases [15].
Atopic dermatitis (AD) is an inflammatory skin disease, and recent studies have elucidated the importance of local atopic skin inflammation as a trigger for inflammation of various systemic organs and oncogenesis by the Th2-mediated immunological microenvironment. This review discussed the importance of AD for systemic inflammatory diseases and the possible pathogenic role of AD.
2. Pathogenesis of AD
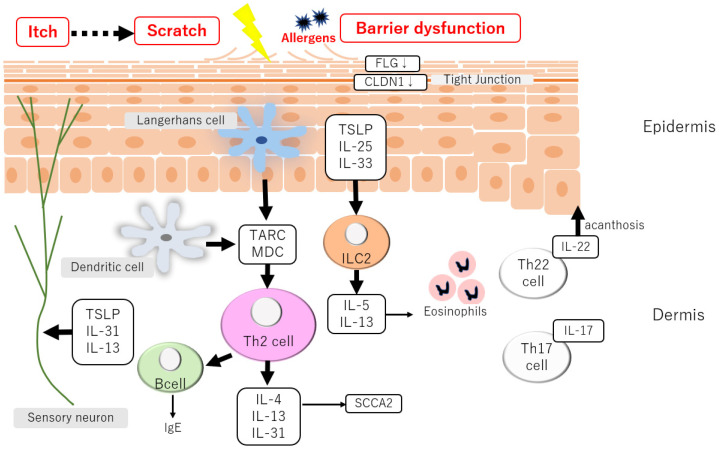
Intercellular lipids in the stratum corneum maintain the skin barrier, and they comprise the main components, such as ceramide [16] (Figure 1). Patients with AD have decreased ceramide content in the stratum corneum and decreased filaggrin or function loss due to gene mutations [17,18]. The epidermis also functions as an important barrier to external environmental factors. Tight junctions are representative components of the adhesive structure between epidermal cells, which restricts the invasion of the outside substrates into the skin [19,20]. A decrease in the constituents of tight junctions has been identified in AD [21].
Figure 1.
Pathogenesis of atopic dermatitis. Skin barrier dysfunction is a trigger for skin inflammation in AD. The following Th2 chemokines and cytokines production establishes the chronic inflammation in the skin recognized as the characteristics of clinical manifestation of AD.
In AD, skin barrier failure is a significant contributor to chronic skin inflammation [17,18]. Type 2 immune response-mediated inflammation is caused by the synthesis of thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33 by epidermal keratinocytes [22,23,24,25]. Th2 cells migrate to skin lesions in part due to the synthesis of the thymus and activation-related chemokines and macrophage-derived chemokines in atopic skin lesions [26,27]. Additionally, in AD, IL-22 helps develop epidermal thickness [28,29,30]. IL-31 plays an essential role in the development of itch in AD [31] and neutralizing antibody treatment impairs the symptoms of AD [32,33]. Each ethnicity has a specific immune cell composition for AD. In particular, Asians have a higher Th17 cell frequency, and in an animal model of AD, Th17 cells play a vital role in the worsening of dermatitis.
3. AD and Systemic Inflammatory Diseases
AD is a prolonged chronic skin inflammation, which is easily speculated for the development of systemic organ inflammation and cancers. This section introduces representative systemic inflammatory disorders related to atopic skin inflammation, such as cerebrovascular diseases, endometriosis, hypothyroidism, renal dysfunction, liver dysfunction, osteoporosis, cancers, inflammatory bowel diseases, dementia, type I diabetes, and depression.
4. Cerebrocardiovascular Diseases
Cerebrocardiovascular diseases are the most important issue for clinicians in the management of skin inflammation, and the importance of these risks has been investigated in various countries. Several epidemiological studies have identified the influence of the presence of AD and the severity of atopic skin inflammation on the risk of cardiovascular disease.
Two clinical studies have been conducted in Asian populations. A total of 301 AD patients in the Taiwanese population were investigated, and a multivariate adjustment examination revealed that AD patients aged 20 or older were at a higher incidence of ischemic stroke [13]. The severity of AD is related to a higher risk of ischemic stroke. The hazard ratios for increased ischemic stroke risk in the severity of AD with mild, moderate, and severe had 1.20 (no significant difference), 1.64, and 1.71, respectively [13].
A Korean population study also demonstrated that AD patients in all aged populations had higher risks of myocardial infarction (hazard ratio (HR) = 9.43), angina (HR = 5.99), and stroke (HR = 10.61) [14].
In the European population, four studies have reported evidence of AD being related to the risk of cardiovascular diseases. A Danish population of 5,762,813 AD patients aged 15 years or older were identified, and those with severe AD receiving systemic treatment had higher risks of ischemic stroke (incidence rate ratio [IRR]: 1.51) and cardiovascular mortality (IRR: 1.46) [34], while mild AD showed a decreased risk of cardiovascular events, suggesting that lifestyle factors in severe AD seemed to be increased risk of cardiovascular disease [34].
In total, 387,439 AD patients aged 18 years or older were identified. Severe atopic eczema demonstrated a 20% higher risk of stroke (HR = 1.22, 95% confidence interval [CI] = 1.01–1.48) and a 70% increased risk of heart failure (HR = 1.69, 95% CI = 1.38–2.06) [35]. Several AD patients also had a higher cardiovascular event risk [35].
A study of 104,832 AD cases in a Swedish population aged 15 years or older has revealed that AD was related to angina pectoris risk. The odds ratio (OR) was 1.13 and the 95% CI was between 1.08 and 1.19 [36]. Severe AD was related to the risk of ischemic stroke (OR = 1.19, 95% CI = 1.07–1.33) [36].
Additionally, a study of 8686 patients with AD in a Danish population aged 18 years or older has demonstrated that AD was related to a higher fatal risk of cardiovascular events (HR = 1.45, 95% CI = 1.07–1.96) [37].
A systematic review has indicated that AD was associated with angina risk (risk ratio [RR] = 1.18, 95% CI = 1.13–1.24), myocardial infarction (RR = 1.12, 95% CI = 1.00–1.25), and stroke (RR = 1.10, 95% CI = 1.03–1.17) [38].
Essential filaggrin gene mutations, which were observed in AD aged 20 years or older, were related to the ischemic stroke risk (OR = 1.15, 95% CI = 1.02–1.30) [39]. Since the detailed molecular mechanisms of direct filaggrin-mediated ischemic stroke event have not been elucidated, this might simply indicate that filaggrin-mediated skin barrier dysfunction enhanced AD skin inflammation, leading to the development of vascular events.
Vascular inflammation is related to an increased Th2 immune response. AD patients increased the risk of cardiovascular disease [40]. Importantly, dupilumab treatment negatively regulates atherosclerosis-related genes in AD, suggesting the possible importance of systemic treatment for AD in the regulation of positive drivers of vascular inflammation [40].
Furthermore, IL-1 cytokines are released by external environmental stimuli and are recognized as worsening factors of atopic skin inflammation. IL-1 cytokines were upregulated in AD skin. IL-1β enhances TSLP production mediated by the NF-κB signaling pathway, in addition to reducing filaggrin expression in the epidermis [41]. Based on the importance of IL-1 cytokines in AD’s pathogenesis, IL-1 cytokines are also key factors in the pathogenesis of cardiovascular diseases. NLRP3 is highly expressed in atherosclerotic plaques, and cholesterol crystals were potential inducers for IL-1β release from atherosclerotic plaques [42]. The IL-1β blockade of canakinumab, which is not an application for AD, reduced vascular inflammation in patients with cardiovascular disease [43] and reduced recurrent cardiovascular events [44].
5. Endometriosis
Endometriosis is a benign disease in which the endometrium develops outside the uterus in an estrogen-dependent manner, while it also has malignant tumor-like properties exhibiting the characteristics of metastasis. Endometrial epithelial cells and stromal cells infiltrate normal muscles and connective tissues with endometrial cell proliferation and detachment in line with the female hormone cycle during menstruation, leading to tissue fibrosis, organ adhesions, and induration.
In a meta-analysis of 1821 AD patients in females between the ages of 15 and 45 years, AD was observed to be related to a higher risk of endometriosis (OR = 1.32, 95% CI = 1.09–1.55) [45].
Endometriosis develops partly because of IL-4 expression. Immunostaining of endometriotic tissues revealed IL-4-positive cells in the stroma [46]. Although the precise molecular processes of Th2 remain unknown, IL-4 in peritoneal fluid is elevated in endometriosis patients [47]. The IL-4-mediated proliferative effect was canceled by the anti-IL-4 receptor treatment, suggesting that IL-4 may have a positive regulatory role in endometriosis development. IL-4 enhances endometriotic stromal cell proliferation [46]. Endometriosis is also affected by IL-23, and IL-23 was increased in the peritoneal fluid of endometriosis patients [48].
IL-33 also contributes to endometriosis development [49]. IL-33 levels increased the inflammatory response of endometriosis and developed the proliferation of endometrial cells mediated by group 2 innate lymphoid cells [49]. Consistently, IL-33-neutralizing antibody treatment impairs the inflammatory response in endometriosis [49].
6. Hypothyroidism
The importance of hypothyroidism risk in AD patients has been elucidated; however, the number of clinical studies is limited. A United Kingdom-based population analysis of 173,709 patients with AD in an all-ages population demonstrated that AD significantly increased the risk of developing hypothyroidism (HR = 1.17, 95% CI = 1.09–1.25) [50].
IL-4 was upregulated in peripheral blood mononuclear cells in Grave’s disease [51]. Overexpressing IL-4 in thyrocytes in mice worsens hypothyroidism under a restricted iodine diet due to increased inflammatory cell infiltration in the thyroid [52], suggesting that certain conditions, such as a low dietary intake of iodine in patients with AD, might cause the development of hypothyroidism.
7. Renal Dysfunction
A clinical study has investigated chronic kidney disease risk in AD. A United Kingdom-based population analysis has reported that 56,602 chronic kidney disease patients had a history of atopic eczema aged 25 years or older (OR = 1·14, 95% CI = 1.11–1.17) [53].
IL-4 inhibition impairs renal fibrosis [54]. A mouse model of unilateral ureteral ligation-induced chronic kidney disease has demonstrated that the frequency of IL-4-producing cells was increased in the peripheral blood, and Il4-deficient mice exhibited impaired renal fibrosis mediated by the Myd88 signaling pathway [55]. Furthermore, IL-33 plays a role in chronic kidney disease development. Serum IL-33 levels were increased in chronic kidney disease patients and exhibited unfavorable survival [56]. Hence, the Th2 immune profile contributes to chronic kidney disease pathogenesis.
8. Liver Dysfunction
The risk of fatty liver disease in AD patients under 1 year of age was examined in a Japanese population-based study. Of the individuals with AD in Japan, 17.6% had non-obesity-related fatty livers [57].
Th2 cytokines are involved in fatty liver disease development. IFN-γ deficiency in mice advances the development of nonalcoholic steatohepatitis (NASH) in a transforming growth factor-β (TGF-β) and IL-13 signaling-dependent manner [58]. Consistently, TGF-β and IL-13 inhibition impairs non-alcoholic fatty liver disease-associated fibrosis [58]. By contrast, IL-4 itself may not be potent as a positive driver for fatty liver disease. Therefore, IL-13-involved skin inflammation, such as AD, may trigger the development of the fatty liver.
9. Osteoporosis
The association between the osteoporosis risk and AD has long been discussed. The incidence of osteoporosis increases in patients with AD. According to a population-based study involving 35,229 AD patients aged 20–49 years, osteoporosis was more common in AD patients (1.82 per 1000 person years) than that in non-AD patients (0.24 per 1000 person years) [59].
Additionally, a Korean population analysis of 311 patients with AD aged from 19 to 50 years has demonstrated that bone mineral density at the lumbar spine decreased in male patients with AD [60].
Although several studies have indicated that the possible osteoporosis risk in AD is associated with glucocorticoid treatment [61], recent studies have demonstrated the important incidence of osteoporosis in AD itself. Altogether, 526,808 AD patients aged 18 years or older were investigated to demonstrate an increased risk of the hip (HR = 1.10), pelvic (HR = 1.10), spinal (HR = 1.18), and wrist (HR = 1.07) fractures. The severity of AD was related to the fracture risk in spinal (HR = 2.09), pelvic (HR = 1.66), and hip (HR = 1.50) fractures, excluding the influence of oral glucocorticoid treatment [62].
Systemic treatment of AD impaired the risk of fracture and indicated that While disease-modifying antirheumatic medications (HR = 0.71; 95% CI = 0.53–0.90) and phototherapy (HR = 0.73; 95% CI = 0.56–0.95) treatment decreased the risk of fractures, severe AD was linked to a higher risk of fractures (HR = 1.31, 95% CI = 1.08–1.59) [63].
The mechanism of Th2 cytokine production in osteoporosis remains unclear. The overexpression of IL-4 reduced bone formation by osteoblasts and caused osteoporosis of both cortical and trabecular bones [64]; although, IL-4 inhibited the receptor activator of nuclear factor kappa-Β ligand (RANKL)-induced osteoclastogenesis mediated by c-Fos and NFATc1 expression in osteoclasts [65]. The non-RANKL-mediated osteoporosis mechanism might cause the pathogenesis of osteoporosis in patients with AD.
10. Cancers
Because cancer accelerates the development of tumor cells under Th2 immunological conditions [66], atopic skin inflammation may be a disadvantage in the development of malignant tumors. Moreover, several clinical studies have reported an increased risk of cancer in patients with AD.
Altogether, 15,666 patients with AD in Sweden have identified an increased future risk of cancer by 13% [67]. Representative malignancy risks were cancers of the brain (standardized incidence ratio [SIR] = 1.6, 95% CI = 1.1–2.4), lungs (SIR = 2.0, 95% CI = 1.3–2.8), esophagus (SIR = 3.5; 95% CI = 1.3–7.7), pancreas (SIR = 1.9, 95% CI = 1.0–3.4), and lymphoma (SIR = 2.0, 95% CI = 1.4–2.9) [67].
A United Kingdom-based study of 4,518,131 patients with AD has reported that the increased IRRs (excluding non-melanoma skin cancers) were 1.49 for cancer (95% CI = 1.39–1.61), 1.46 for non-melanoma skin cancers (95% CI = 1.27–1.69), 1.74 for melanoma (95% CI = 1.25–2.41), and 2.21 for lymphoma (95% CI = 1.65–2.98) [68].
A study of 31,330 patients with AD in the Danish population has demonstrated an increased risk of basal cell carcinoma (BCC) (SIR = 1.41, 95% CI = 1.07–1.83) and squamous cell carcinoma (SCC) (SIR = 2.48, 95% CI = 1.00–5.11) in patients with AD [69]. Meanwhile, a study of 557 patients with AD aged 18 years or older in the Netherlands has reported that the future risk of SCC was 13.1 (95% CI = 6.5–19.7) [70], and a meta-analysis has revealed a significantly increased risk of BCC as well (SRR = 1.34, 95% CI = 1.03–1.75) [71].
Th2 immunity is essential for oncogenesis and plays an important role in the downregulation of antitumor immune responses [72]. CCL17 overexpression indicated tumor development and lung metastasis in a melanoma model with subcutaneous or intravenous melanoma cell injection [72], leading to an increased risk of malignant tumor development in the Th2-dominant immune environment [72].
11. Inflammatory Bowel Diseases
AD increases the risk of inflammatory bowel diseases. A United Kingdom-based study with a population of 10,788 patients with AD has identified an association between AD and the risk of inflammatory bowel disease (OR = 1.107, 95% CI = 1.035–1.183) [73]. In particular, AD was linked to an increased risk of ulcerative colitis (OR = 1.149; 95% CI = 1.018–1297) [73]. Additionally, a German cohort analysis has revealed an elevated risk of inflammatory bowel illnesses among patients with AD aged 40 years or younger (n = 49,847). Ulcerative colitis had a higher RR of 1.25 (95% CI = 1.03–153) and Crohn’s disease had a RR of 1.34 (95% CI = 1.11–1.61) [74]. This link is also supported by findings from a meta-analysis of 95,291,110 patients with AD in a meta-analysis (OR = 1.35, 95% CI = 1.05–173) [75].
Although the detailed mechanism remains unclear, IL-4-deficiency impaired dextran sulfate sodium-induced colitis [76]. Since IgG2- and IgG3-producing cells were increased in IL-4-deficient mice [76], these conditions may have a beneficial effect on colitis.
12. Dementia
Several clinical studies have demonstrated that patients with AD have an increased risk of dementia. AD in a total of 1059 patients was identified to be associated with a future risk of dementia (HR = 2.02, 95% CI = 1.24–3.29) [50]. In particular, AD increased the risk of Alzheimer’s disease (HR = 3.74, 95% CI = 1.17–11.97) [77]. Severity was related to the risk of dementia and moderate-to-severe AD was related to the risk of dementia (HR = 4.64, 95% CI = 2.58–8.33) [77]. However, the detailed mechanisms remain unclear. Th2 cell levels are reduced in patients with AD [78]. Further investigation of the underlying mechanisms of AD mediated by atopic skin inflammation is necessary.
13. Type I Diabetes
The risk of type I diabetes in people with AD varies greatly depending on geographic region. The risk of AD was negatively correlated with type 1 diabetes in 760 cases in a German population under five years of age (OR = 0.71, 95% CI = 0.53–096) [79]. Atopic eczema was negatively correlated with the incidence of type 1 diabetes in 545 cases of childhood-onset diabetes under the age of 15 years in a community-based study in Norway (OR = 0.55, 95% CI = 0.35–087) [80]. A lower risk of AD among 54,530 Danish twins with type 1 diabetes of 3–20 years age (OR = 0.23, 95% CI = 0.07–071) was identified [81]. These European populations have a decreased risk of type I diabetes in patients with AD.
By contrast, in a Taiwanese population-based study, 3386 patients with type I diabetes were observed to have a 1.40-fold greater incidence of AD (3.31 per 1000 person years) than patients without type I diabetes (2.35 per 1000 person years) [82]. Patients with type I diabetes had a greater overall risk of AD after controlling for relevant risk variables (HR = 1.76, 95% CI = 1.29–2.39) than in those without type I diabetes [82]. Although an analysis of another Asian population has not yet been conducted, different characteristics of systemic inflammatory diseases may exist in different countries.
14. Depression
A relationship between AD and depression has been reported. Patients with AD aged from 21 to 36 years reportedly have higher Beck Depression Inventory scores [83]. The statistical significance of AD reportedly influences psychological disorders in many countries.
In a Taiwanese population-based study, 8208 patients with AD aged 12 years or older had a higher incidence of depression (HR = 6.56, 95% CI = 3.64–1184) [84]. In a South Korean study, 1517 patients with AD had an increased risk of depression (OR = 1.79, 95% CI = 1.40–2.29) [85]. Thus, the severity of AD is related to an increased risk of depression, and moderate-to-severe AD is significantly related to depression compared with mild AD [85]. Severity was linked to depression in AD patients who had mild (HR = 1.10, 95% CI = 1.08–1.13), moderate (HR = 1.19, 95% CI = 1.15–1.23), and severe depression (HR = 1.26, 95% CI = 1.17–1.37), according to a population-based research of 526,808 individuals with AD conducted in the United Kingdom [86].
Regarding the future risk of psychological disorders in patients with AD, another Taiwanese population-based study targeting 5075 adolescents with AD aged between 10 and 17 years has also reported a higher incidence of bipolar disorder (HR = 2.51, 95% CI = 1.71–3.67) and major depression (HR = 2.45, 95% CI = 1.93–3.11) [87]. A South Korean study involving 72,435 adolescents in middle and high school has identified relationships between AD and suicidal ideation (OR = 1.26, 95% CI = 1.16–1.36) and suicide attempts (OR = 1.29, 95% CI = 1.13–1.49) [88].
Additionally, a study of 8602 Danish children with AD aged 10 years prior to the diagnosis date showed an association with a history of maternal depression (OR = 1.18, 95% CI = 1.12–1.26) [89].
Antidepressant (HR = 1.19, 95% CI = 1.04–1.36) and anxiolytic use (HR = 1.72, 95% CI = 1.57–1.90) [90] was also reportedly associated with hospital-diagnosed AD in Danish children with AD (n = 14,283).
Additionally, a United Kingdom population-based study of 11,181 children with AD has reported that severe AD was related to depression (OR = 2.38, 95% CI = 1.21–4.72) [91]. A systematic review has also revealed that AD in children and adults increases the risk of depression (OR = 2.19; 95% CI = 1.87–2.57) [92].
Although the detailed molecular mechanism of AD and psychological disorders remains unclear, higher IL-4 and IL-13 serum levels (Th2) were also observed in a group with a major depressive disorder [93]. Dupilumab treatment impairs anxiety and depressive symptoms [94,95], suggesting the possible importance of systemic therapy against the psychological disorder development in addition to a possible role of Th2 immune response in the pathogenesis of psychological disorders.
15. Summary of Inflammatory Diseases in AD
Atopic skin inflammation influences systemic organ dysfunction. Since a limited number of studies have been conducted to elucidate molecular-based mechanisms of AD-mediated inflammatory responses to inflamed systemic organ dysfunction, further investigations are desired to be clarified the detailed molecular mechanisms of AD-associated systemic organ failure.
Because the skin is a large surface covering the human body, atopic skin inflammation is easily speculated to extend into systemic organ inflammation. Several inflammatory diseases are impaired by systemic therapy against AD and reduced risks. These findings suggest that atopic-mediated systemic organ inflammation negatively regulates the development of systemic inflammatory responses and organ dysfunction. Therefore, the key factors in the pathogenesis of AD and systemic inflammatory disorders highlight the importance of systemic treatment against atopic skin inflammation.
Interestingly, different risk outcomes of AD depending on the country were observed. Type I diabetes may be a representative disease, and we might need to consider the risk of systemic inflammatory diseases based on the characteristics of AD, whose immunological pathogenesis differs worldwide.
Author Contributions
M.I. and Y.S. organized this paper’s conceptualization and wrote this manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kedmi R., Najar T.A., Mesa K.R., Grayson A., Kroehling L., Hao Y., Hao S., Pokrovskii M., Xu M., Talbot J., et al. A RORγt(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. 2022;610:737–743. doi: 10.1038/s41586-022-05089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabashima K., Honda T., Ginhoux F., Egawa G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019;19:19–30. doi: 10.1038/s41577-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 3.Yoshioka M., Sawada Y., Saito-Sasaki N., Yoshioka H., Hama K., Omoto D., Ohmori S., Okada E., Nakamura M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021;11:5493. doi: 10.1038/s41598-021-85009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawada Y., Nakamura M., Tokura Y. Generalized fixed drug eruption caused by pazufloxacin. Acta Derm.-Venereol. 2011;91:600–601. doi: 10.2340/00015555-1132. [DOI] [PubMed] [Google Scholar]
- 5.Tomura M., Honda T., Tanizaki H., Otsuka A., Egawa G., Tokura Y., Waldmann H., Hori S., Cyster J.G., Watanabe T., et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Investig. 2010;120:883–893. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray E.E., Ramírez-Valle F., Xu Y., Wu S., Wu Z., Karjalainen K.E., Cyster J.G. Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurabielle C., Link V.M., Bouladoux N., Han S.J., Merrill E.D., Lightfoot Y.L., Seto N., Bleck C.K.E., Smelkinson M., Harrison O.J., et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc. Natl. Acad. Sci. USA. 2020;117:16465–16474. doi: 10.1073/pnas.2003022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada Y., Nakatsuji T., Dokoshi T., Kulkarni N.N., Liggins M.C., Sen G., Gallo R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021;6:eabe1935. doi: 10.1126/sciimmunol.abe1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid B., Künstner A., Fähnrich A., Bersuch E., Schmid-Grendelmeier P., Busch H., Glatz M., Bosshard P.P. Dysbiosis of skin microbiota with increased fungal diversity is associated with severity of disease in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2022;36:1811–1819. doi: 10.1111/jdv.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu Z., Zhu Z., Liu X., Chen B., Yin H., Gu C., Fang X., Zhu R., Yu T., Mi W., et al. A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis. J. Exp. Med. 2022;219 doi: 10.1084/jem.20212397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amar Y., Schneider E., Köberle M., Seeholzer T., Musiol S., Hölge I.M., Gschwendtner S., Krappmann D., Steiger K., Biedermann T., et al. Microbial dysbiosis in a mouse model of atopic dermatitis mimics shifts in human microbiome and correlates with the key pro-inflammatory cytokines IL-4, IL-33 and TSLP. J. Eur. Acad. Dermatol. Venereol. 2022;36:705–716. doi: 10.1111/jdv.17911. [DOI] [PubMed] [Google Scholar]
- 12.Hrestak D., Matijašić M., Čipčić Paljetak H., Ledić Drvar D., Ljubojević Hadžavdić S., Perić M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022;23:3503. doi: 10.3390/ijms23073503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su V.Y., Chen T.J., Yeh C.M., Chou K.T., Hung M.H., Chu S.Y., Su K.C., Chang Y.S., Lin Y.H., Liu C.J. Atopic dermatitis and risk of ischemic stroke: A nationwide population-based study. Ann. Med. 2014;46:84–89. doi: 10.3109/07853890.2013.870018. [DOI] [PubMed] [Google Scholar]
- 14.Jung H.J., Lee D.H., Park M.Y., Ahn J. Cardiovascular comorbidities of atopic dermatitis: Using National Health Insurance data in Korea. Allergy Asthma Clin. Immunol. 2021;17:94. doi: 10.1186/s13223-021-00590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashiro T., Sawada Y. Psoriasis and Systemic Inflammatory Disorders. Int. J. Mol. Sci. 2022;23:4457. doi: 10.3390/ijms23084457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imokawa G., Abe A., Jin K., Higaki Y., Kawashima M., Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 17.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J., et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 18.Nomura T., Sandilands A., Akiyama M., Liao H., Evans A.T., Sakai K., Ota M., Sugiura H., Yamamoto K., Sato H., et al. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J. Allergy Clin. Immunol. 2007;119:434–440. doi: 10.1016/j.jaci.2006.12.646. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K., Kubo A., Fujita H., Yokouchi M., Ishii K., Kawasaki H., Nomura T., Shimizu H., Kouyama K., Ebihara T., et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:856–864. doi: 10.1016/j.jaci.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Yokouchi M., Kubo A., Kawasaki H., Yoshida K., Ishii K., Furuse M., Amagai M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J. Dermatol. Sci. 2015;77:28–36. doi: 10.1016/j.jdermsci.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 21.De Benedetto A., Rafaels N.M., McGirt L.Y., Ivanov A.I., Georas S.N., Cheadle C., Berger A.E., Zhang K., Vidyasagar S., Yoshida T., et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011;127:773–786.e7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y.H., Angkasekwinai P., Lu N., Voo K.S., Arima K., Hanabuchi S., Hippe A., Corrigan C.J., Dong C., Homey B., et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hvid M., Vestergaard C., Kemp K., Christensen G.B., Deleuran B., Deleuran M. IL-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction? J. Investig. Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 25.Imai Y., Yasuda K., Sakaguchi Y., Haneda T., Mizutani H., Yoshimoto T., Nakanishi K., Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vestergaard C., Yoneyama H., Murai M., Nakamura K., Tamaki K., Terashima Y., Imai T., Yoshie O., Irimura T., Mizutani H., et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Investig. 1999;104:1097–1105. doi: 10.1172/JCI7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestergaard C., Bang K., Gesser B., Yoneyama H., Matsushima K., Larsen C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Investig. Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 28.Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 30.Esaki H., Brunner P.M., Renert-Yuval Y., Czarnowicki T., Huynh T., Tran G., Lyon S., Rodriguez G., Immaneni S., Johnson D.B., et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J. Allergy Clin. Immunol. 2016;138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Sonkoly E., Muller A., Lauerma A.I., Pivarcsi A., Soto H., Kemeny L., Alenius H., Dieu-Nosjean M.C., Meller S., Rieker J., et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 32.Ruzicka T., Hanifin J.M., Furue M., Pulka G., Mlynarczyk I., Wollenberg A., Galus R., Etoh T., Mihara R., Yoshida H., et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017;376:826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 33.Kabashima K., Furue M., Hanifin J.M., Pulka G., Wollenberg A., Galus R., Etoh T., Mihara R., Nakano M., Ruzicka T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018;142:1121–1130.e7. doi: 10.1016/j.jaci.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Andersen Y.M.F., Egeberg A., Gislason G.H., Hansen P.R., Skov L., Thyssen J.P. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016;138:310–312.e3. doi: 10.1016/j.jaci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Silverwood R.J., Forbes H.J., Abuabara K., Ascott A., Schmidt M., Schmidt S.A.J., Smeeth L., Langan S.M. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: Population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivert L.U., Johansson E.K., Dal H., Lindelöf B., Wahlgren C.F., Bradley M. Association Between Atopic Dermatitis and Cardiovascular Disease: A Nationwide Register-based Case-control Study from Sweden. Acta Derm.-Venereol. 2019;99:865–870. doi: 10.2340/00015555-3235. [DOI] [PubMed] [Google Scholar]
- 37.Thyssen J.P., Skov L., Egeberg A. Cause-specific mortality in adults with atopic dermatitis. J. Am. Acad. Dermatol. 2018;78:506–510. doi: 10.1016/j.jaad.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Ascott A., Mulick A., Yu A.M., Prieto-Merino D., Schmidt M., Abuabara K., Smeeth L., Roberts A., Langan S.M. Atopic eczema and major cardiovascular outcomes: A systematic review and meta-analysis of population-based studies. J. Allergy Clin. Immunol. 2019;143:1821–1829. doi: 10.1016/j.jaci.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varbo A., Nordestgaard B.G., Benn M. Filaggrin loss-of-function mutations as risk factors for ischemic stroke in the general population. J. Thromb. Haemost. JTH. 2017;15:624–635. doi: 10.1111/jth.13644. [DOI] [PubMed] [Google Scholar]
- 40.Villani A.P., Pavel A.B., Wu J., Fernandes M., Maari C., Saint-Cyr Proulx E., Jack C., Glickman J., Choi S., He H., et al. Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy. 2021;76:3107–3121. doi: 10.1111/all.14859. [DOI] [PubMed] [Google Scholar]
- 41.Bernard M., Carrasco C., Laoubi L., Guiraud B., Rozières A., Goujon C., Duplan H., Bessou-Touya S., Nicolas J.F., Vocanson M., et al. IL-1β induces thymic stromal lymphopoietin and an atopic dermatitis-like phenotype in reconstructed healthy human epidermis. J. Pathol. 2017;242:234–245. doi: 10.1002/path.4887. [DOI] [PubMed] [Google Scholar]
- 42.Paramel Varghese G., Folkersen L., Strawbridge R.J., Halvorsen B., Yndestad A., Ranheim T., Krohg-Sørensen K., Skjelland M., Espevik T., Aukrust P., et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016;5:e003031. doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker P.M., Howard C.P., Walter V., Everett B., Libby P., Hensen J., Thuren T. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 44.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 45.Barbieri J.S., Shin D.B., Margolis D.J. Atopic Dermatitis Is Associated with Preeclampsia and Endometriosis. JID Innov. 2022;2:100123. doi: 10.1016/j.xjidi.2022.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.OuYang Z., Hirota Y., Osuga Y., Hamasaki K., Hasegawa A., Tajima T., Hirata T., Koga K., Yoshino O., Harada M., et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am. J. Pathol. 2008;173:463–469. doi: 10.2353/ajpath.2008.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podgaec S., Abrao M.S., Dias J.A., Jr., Rizzo L.V., de Oliveira R.M., Baracat E.C. Endometriosis: An inflammatory disease with a Th2 immune response component. Hum. Reprod. 2007;22:1373–1379. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 48.Andreoli C.G., Genro V.K., Souza C.A., Michelon T., Bilibio J.P., Scheffel C., Cunha-Filho J.S. T helper (Th)1, Th2, and Th17 interleukin pathways in infertile patients with minimal/mild endometriosis. Fertil. Steril. 2011;95:2477–2480. doi: 10.1016/j.fertnstert.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Miller J.E., Lingegowda H., Symons L.K., Bougie O., Young S.L., Lessey B.A., Koti M., Tayade C. IL-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis. JCI Insight. 2021;6:e149699. doi: 10.1172/jci.insight.149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Lusignan S., Alexander H., Broderick C., Dennis J., McGovern A., Feeney C., Flohr C. Atopic dermatitis and risk of autoimmune conditions: Population-based cohort study. J. Allergy Clin. Immunol. 2022;150:709–713. doi: 10.1016/j.jaci.2022.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Song R.H., Qin Q., Wang X., Yan N., Meng S., Shi X.H., He S.T., Zhang J.A. Differential cytokine expression detected by protein microarray screening in peripheral blood of patients with refractory Graves’ disease. Clin. Endocrinol. 2016;84:402–407. doi: 10.1111/cen.12778. [DOI] [PubMed] [Google Scholar]
- 52.Merakchi K., Djerbib S., Soleimani M., Dumont J.E., Miot F., De Deken X. Murine Thyroid IL-4 Expression Worsens Hypothyroidism on Iodine Restriction and Mitigates Graves Disease Development. Endocrinology. 2022;163:bqac107. doi: 10.1210/endocr/bqac107. [DOI] [PubMed] [Google Scholar]
- 53.Schonmann Y., Mansfield K.E., Mulick A., Roberts A., Smeeth L., Langan S.M., Nitsch D. Inflammatory skin diseases and the risk of chronic kidney disease: Population-based case-control and cohort analyses. Br. J. Dermatol. 2021;185:772–780. doi: 10.1111/bjd.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B., Jiang J., Liang H., Xiao P., Lai X., Nie J., Yu W., Gao Y., Wen S. Natural killer T cell/IL-4 signaling promotes bone marrow-derived fibroblast activation and M2 macrophage-to-myofibroblast transition in renal fibrosis. Int. Immunopharmacol. 2021;98:107907. doi: 10.1016/j.intimp.2021.107907. [DOI] [PubMed] [Google Scholar]
- 55.Braga T.T., Correa-Costa M., Guise Y.F., Castoldi A., de Oliveira C.D., Hyane M.I., Cenedeze M.A., Teixeira S.A., Muscara M.N., Perez K.R., et al. MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol. Med. 2012;18:1231–1239. doi: 10.2119/molmed.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gungor O., Unal H.U., Guclu A., Gezer M., Eyileten T., Guzel F.B., Altunoren O., Erken E., Oguz Y., Kocyigit I., et al. IL-33 and ST2 levels in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS ONE. 2017;12:e0178939. doi: 10.1371/journal.pone.0178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimata H. Increased incidence of fatty liver in non-obese Japanese children under 1 year of age with or without atopic dermatitis. Public Health. 2006;120:176–178. doi: 10.1016/j.puhe.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Hart K.M., Fabre T., Sciurba J.C., Gieseck R.L., 3rd, Borthwick L.A., Vannella K.M., Acciani T.H., de Queiroz Prado R., Thompson R.W., White S., et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci. Transl. Med. 2017;9:eaal3694. doi: 10.1126/scitranslmed.aal3694. [DOI] [PubMed] [Google Scholar]
- 59.Wu C.Y., Lu Y.Y., Lu C.C., Su Y.F., Tsai T.H., Wu C.H. Osteoporosis in adult patients with atopic dermatitis: A nationwide population-based study. PLoS ONE. 2017;12:e0171667. doi: 10.1371/journal.pone.0171667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S., Choi J., Cho M.K., Kim N.H., Kim S.G., Kim K.J. Bone mineral density and osteoporosis risk in young adults with atopic dermatitis. Sci. Rep. 2021;11:24228. doi: 10.1038/s41598-021-03630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aalto-Korte K., Turpeinen M. Bone mineral density in patients with atopic dermatitis. Br. J. Dermatol. 1997;136:172–175. doi: 10.1111/j.1365-2133.1997.tb14890.x. [DOI] [PubMed] [Google Scholar]
- 62.Lowe K.E., Mansfield K.E., Delmestri A., Smeeth L., Roberts A., Abuabara K., Prieto-Alhambra D., Langan S.M. Atopic eczema and fracture risk in adults: A population-based cohort study. J. Allergy Clin. Immunol. 2020;145:563–571.e8. doi: 10.1016/j.jaci.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin T.L., Wu C.Y., Yen J.J., Juan C.K., Chang Y.L., Ho H.J., Chen Y.J. Fracture risks in patients with atopic dermatitis: A nationwide matched cohort study. Ann. Allergy Asthma Immunol. 2021;127:667–673.e2. doi: 10.1016/j.anai.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Lewis D.B., Liggitt H.D., Effmann E.L., Motley S.T., Teitelbaum S.L., Jepsen K.J., Goldstein S.A., Bonadio J., Carpenter J., Perlmutter R.M. Osteoporosis induced in mice by overproduction of interleukin 4. Proc. Natl. Acad. Sci. USA. 1993;90:11618–11622. doi: 10.1073/pnas.90.24.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamel Mohamed S.G., Sugiyama E., Shinoda K., Hounoki H., Taki H., Maruyama M., Miyahara T., Kobayashi M. Interleukin-4 inhibits RANKL-induced expression of NFATc1 and c-Fos: A possible mechanism for downregulation of osteoclastogenesis. Biochem. Biophys. Res. Commun. 2005;329:839–845. doi: 10.1016/j.bbrc.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 66.Larsen K.M., Minaya M.K., Vaish V., Peña M.M.O. The Role of IL-33/ST2 Pathway in Tumorigenesis. Int. J. Mol. Sci. 2018;19:2676. doi: 10.3390/ijms19092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagströmer L., Ye W., Nyrén O., Emtestam L. Incidence of cancer among patients with atopic dermatitis. Arch. Dermatol. 2005;141:1123–1127. doi: 10.1001/archderm.141.9.1123. [DOI] [PubMed] [Google Scholar]
- 68.Arana A., Wentworth C.E., Fernández-Vidaurre C., Schlienger R.G., Conde E., Arellano F.M. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U.K. Br. J. Dermatol. 2010;163:1036–1043. doi: 10.1111/j.1365-2133.2010.09887.x. [DOI] [PubMed] [Google Scholar]
- 69.Jensen A.O., Svaerke C., Körmendiné Farkas D., Olesen A.B., Kragballe K., Sørensen H.T. Atopic dermatitis and risk of skin cancer: A Danish nationwide cohort study (1977–2006) Am. J. Clin. Dermatol. 2012;13:29–36. doi: 10.2165/11593280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Garritsen F.M., van der Schaft J., van den Reek J.M., Politiek K., van Os-Medendorp H., van Dijk M., Hijnen D.J., de Graaf M., Bruijnzeel-Koomen C.A., de Jong E.M., et al. Risk of Non-melanoma Skin Cancer in Patients with Atopic Dermatitis Treated with Oral Immunosuppressive Drugs. Acta Derm.-Venereol. 2017;97:724–730. doi: 10.2340/00015555-2637. [DOI] [PubMed] [Google Scholar]
- 71.Gandini S., Stanganelli I., Palli D., De Giorgi V., Masala G., Caini S. Atopic dermatitis, naevi count and skin cancer risk: A meta-analysis. J. Dermatol. Sci. 2016;84:137–143. doi: 10.1016/j.jdermsci.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Morimura S., Sugaya M., Oka T., Suga H., Miyagaki T., Tsunemi Y., Asano Y., Sato S. Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2021;22:2025. doi: 10.3390/ijms22042025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meisinger C., Freuer D. Causal Association Between Atopic Dermatitis and Inflammatory Bowel Disease: A 2-Sample Bidirectional Mendelian Randomization Study. Inflamm. Bowel Dis. 2021;28:1543–1548. doi: 10.1093/ibd/izab329. [DOI] [PubMed] [Google Scholar]
- 74.Schmitt J., Schwarz K., Baurecht H., Hotze M., Fölster-Holst R., Rodríguez E., Lee Y.A.E., Franke A., Degenhardt F., Lieb W., et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J. Allergy Clin. Immunol. 2016;137:130–136. doi: 10.1016/j.jaci.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 75.Lee H., Lee J.H., Koh S.J., Park H. Bidirectional relationship between atopic dermatitis and inflammatory bowel disease: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020;83:1385–1394. doi: 10.1016/j.jaad.2020.05.130. [DOI] [PubMed] [Google Scholar]
- 76.Stevceva L., Pavli P., Husband A., Ramsay A., Doe W.F. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun. 2001;2:309–316. doi: 10.1038/sj.gene.6363782. [DOI] [PubMed] [Google Scholar]
- 77.Pan T.L., Bai Y.M., Cheng C.M., Tsai S.J., Tsai C.F., Su T.P., Li C.T., Lin W.C., Chen T.J., Liang C.S., et al. Atopic dermatitis and dementia risk: A nationwide longitudinal study. Ann. Allergy Asthma Immunol. 2021;127:200–205. doi: 10.1016/j.anai.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Niu C. Relation of CDC42, Th1, Th2, and Th17 cells with cognitive function decline in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2022;9:1428–1436. doi: 10.1002/acn3.51643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenbauer J., Herzig P., Giani G. Atopic eczema in early childhood could be protective against Type 1 diabetes. Diabetologia. 2003;46:784–788. doi: 10.1007/s00125-003-1108-6. [DOI] [PubMed] [Google Scholar]
- 80.Stene L.C., Joner G. Atopic disorders and risk of childhood-onset type 1 diabetes in individuals. Clin. Exp. Allergy. 2004;34:201–206. doi: 10.1111/j.1365-2222.2004.01864.x. [DOI] [PubMed] [Google Scholar]
- 81.Thomsen S.F., Duffy D.L., Kyvik K.O., Skytthe A., Backer V. Relationship between type 1 diabetes and atopic diseases in a twin population. Allergy. 2011;66:645–647. doi: 10.1111/j.1398-9995.2010.02517.x. [DOI] [PubMed] [Google Scholar]
- 82.Lin C.H., Wei C.C., Lin C.L., Lin W.C., Kao C.H. Childhood type 1 diabetes may increase the risk of atopic dermatitis. Br. J. Dermatol. 2016;174:88–94. doi: 10.1111/bjd.14166. [DOI] [PubMed] [Google Scholar]
- 83.Arima M., Shimizu Y., Sowa J., Narita T., Nishi I., Iwata N., Ozaki N., Hashimoto S., Matsunaga K. Psychosomatic analysis of atopic dermatitis using a psychological test. J. Dermatol. 2005;32:160–168. doi: 10.1111/j.1346-8138.2005.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 84.Cheng C.M., Hsu J.W., Huang K.L., Bai Y.M., Su T.P., Li C.T., Yang A.C., Chang W.H., Chen T.J., Tsai S.J., et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: A nationwide longitudinal study. J. Affect. Disord. 2015;178:60–65. doi: 10.1016/j.jad.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 85.Kim S.H., Hur J., Jang J.Y., Park H.S., Hong C.H., Son S.J., Chang K.J. Psychological Distress in Young Adult Males with Atopic Dermatitis: A Cross-Sectional Study. Medicine. 2015;94:e949. doi: 10.1097/MD.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schonmann Y., Mansfield K.E., Hayes J.F., Abuabara K., Roberts A., Smeeth L., Langan S.M. Atopic Eczema in Adulthood and Risk of Depression and Anxiety: A Population-Based Cohort Study. J. Allergy Clin. Immunol. Pract. 2020;8:248–257.e16. doi: 10.1016/j.jaip.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei H.T., Lan W.H., Hsu J.W., Huang K.L., Su T.P., Li C.T., Lin W.C., Chen T.J., Bai Y.M., Chen M.H. Risk of developing major depression and bipolar disorder among adolescents with atopic diseases: A nationwide longitudinal study in Taiwan. J. Affect. Disord. 2016;203:221–226. doi: 10.1016/j.jad.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Lee S., Shin A. Association of atopic dermatitis with depressive symptoms and suicidal behaviors among adolescents in Korea: The 2013 Korean Youth Risk Behavior Survey. BMC Psychiatry. 2017;17:3. doi: 10.1186/s12888-016-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamann C.R., Egeberg A., Silverberg J.I., Gislason G., Skov L., Thyssen J.P. Exploring the association between parental psychiatric disease and childhood atopic dermatitis: A matched case-control study. J. Eur. Acad. Dermatol. Venereol. 2019;33:725–734. doi: 10.1111/jdv.15321. [DOI] [PubMed] [Google Scholar]
- 90.Vittrup I., Andersen Y.M.F., Droitcourt C., Skov L., Egeberg A., Fenton M.C., Mina-Osorio P., Boklage S., Thyssen J.P. Association between hospital-diagnosed atopic dermatitis and psychiatric disorders and medication use in childhood. Br. J. Dermatol. 2021;185:91–100. doi: 10.1111/bjd.19817. [DOI] [PubMed] [Google Scholar]
- 91.Kern C., Wan J., LeWinn K.Z., Ramirez F.D., Lee Y., McCulloch C.E., Langan S.M., Abuabara K. Association of Atopic Dermatitis and Mental Health Outcomes Across Childhood: A Longitudinal Cohort Study. JAMA Dermatol. 2021;157:1200–1208. doi: 10.1001/jamadermatol.2021.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rønnstad A.T.M., Halling-Overgaard A.S., Hamann C.R., Skov L., Egeberg A., Thyssen J.P. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018;79:448–456.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 93.Pavón L., Sandoval-López G., Eugenia Hernández M., Loría F., Estrada I., Pérez M., Moreno J., Avila U., Leff P., Antón B., et al. Th2 cytokine response in Major Depressive Disorder patients before treatment. J. Neuroimmunol. 2006;172:156–165. doi: 10.1016/j.jneuroim.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Miniotti M., Lazzarin G., Ortoncelli M., Mastorino L., Ribero S., Leombruni P. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of Dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol. Ther. 2022;35:e15407. doi: 10.1111/dth.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lönndahl L., Lundqvist M., Bradley M., Johansson E.K. Dupilumab Significantly Reduces Symptoms of Prurigo Nodularis and Depression: A Case Series. Acta Derm.-Venereol. 2022;102:adv00754. doi: 10.2340/actadv.v102.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.