Abstract
The essential oils (EOs) of Myrciaria floribunda (Mflo) and Myrcia sylvatica (Msyl) (Myrtaceae) were obtained by hydrodistillation. The analysis of volatile constituents was performed by GC/MS. Preliminary toxicity was assessed on Artemia salina Leach. The antioxidant capacity was measured by the ABTS•+ and DPPH• radical inhibitory activities. The results indicate that the Mflo EO had the highest yield (1.02%), and its chemical profile was characterized by high levels of hydrocarbon (65.83%) and oxygenated (25.74%) monoterpenes, especially 1,8-cineole (23.30%), terpinolene (22.23%) and α-phellandrene (22.19%). Regarding the Msyl EO, only hydrocarbon (51.60%) and oxygenated (46.52%) sesquiterpenes were identified in the sample, with (Z)-α-trans-bergamotene (24.57%), α-sinensal (13.44%), and (Z)-α-bisabolene (8.33%) at higher levels. The EO of Mflo exhibited moderate toxicity against A. salina (LC50 = 82.96 ± 5.20 µg.mL−1), while the EO of Msyl was classified as highly toxic (LC50 = 2.74 ± 0.50 µg.mL−1). In addition, relative to Trolox, the EOs of Mflo and Msyl showed significant inhibitory effects (p < 0.0001) against the DPPH• radical. This study contributes to the expansion of chemical and biological knowledge on the EOs of Myrtaceae species from the Amazon region.
Keywords: Amazonian natural products; Artemia salina; bioactive compounds; 1,8-cineole; (Z)-α-trans-bergamotene
1. Introduction
The Amazonian flora is widely studied from chemical and biological perspectives due to the existence of species used in traditional medicine for the treatment of various endemic diseases in an effort to relate the practical uses of these species with the chemical composition of their natural products [1,2]. The essential oils (EOs) of aromatic members of the Amazonian flora are extensively studied by the scientific community due to their high-value-added properties; these organisms have also aroused industrial and economic interest due to their strong prospects for wealth generation and development in the region [3,4]. Among the families of Amazonian species that produce EOs, the Myrtaceae family stands out as one of the most important of the Neotropics, with great medicinal interest [5,6].
The family Myrtaceae is represented in Brazil by 29 genera and 1193 species, of which 266 occur in the Amazon [7]. Myrtaceae species are also economically important because many of them are edible and are sources of natural products with pharmacological potential [8,9,10,11,12,13,14]. The species of the family Myrtaceae are also widely known for their antioxidant potential, especially species of the genera Eugenia, Syzygium, Myrciaria, and Eucalyptus [15,16,17,18,19,20,21,22]. The volatile compounds present in the EOs of species of this family can eliminate or inhibit the effects of free radicals of oxidizing substances, attenuating the effects of oxidative stress and reducing the possibility of occurrence of degenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, cancer, diabetes, and sclerosis [23,24,25]. The use of natural products as antioxidant substances is being increasingly studied today because they are environmentally friendly, cause no damage to the environment, and ensure greater safety of human health [26,27].
To determine possible biological activities, it is necessary to perform preliminary toxicity tests to evaluate the possible risks to human health [28,29]. Thus, the bioassay against the microcrustacean Artemia salina Leach is used to determine the level of cytotoxicity of natural products [30,31]. This test also allows the investigation of highly toxic EOs for possible uses as inputs in the manufacture of pest repellents and related products, especially in the agroindustry [32,33].
Myrciaria floribunda (H. West ex Willd.) O. Berg is one of the species of Myrtaceae found in the Amazon and is popularly known as “camboim” [34,35]. Its fruit is consumed in the form of gelatin or used as flavoring in the distilled beverage industry [36]. Myrcia sylvatica (G. Mey) DC. is a shrub popularly known as “cumatê-Folha-miúda”, “myrtle”, or “broom” that is used in folk medicine for the treatment of dysentery and intestinal diseases; like another species of Myrtaceae known as “pedra-ume-caá”, it also has potential for use against diabetes [37,38,39]. Despite the use of these species by the traditional communities of the Amazon, there are still few studies on the chemical profile and biological and antioxidant potentials of the EOs of these two species. Thus, the present study aimed to evaluate the phytochemical profile, antioxidant potential, and preliminary toxicity of the EOs of M. floribunda and M. sylvatica species.
2. Materials and Methods
2.1. Collection and Processing of Botanical Material
Shoots of M. floribunda and M. sylvatica were collected at Campina do Guarujá in the city of Bujaru, Lower Tocantins microregion, in the state of Pará, Brazil (01°57′36″ South latitude and 48°11′51″ longitude), in July 2017, following conventional botanical procedures. After collection, the material was dried in a convection oven at 35 °C for 5 days, ground, homogenized, weighed, and subjected to hydrodistillation to obtain the EO. The exsiccates, botanical identification, and registration were incorporated into the Collection of Aromatic Plants of the João Murça Herbarium collection of the Emílio Goeldi Paraense Museum, Belém, Pará, with the following registration numbers: M. floribunda MG237492 and M. sylvatica MG237516.
2.2. Production and Yield of Essential Oils
The EOs were obtained by hydrodistillation using a modified Clevenger apparatus for 3 h. After the end of distillation, the EOs were centrifuged for 5 min at 3000 rpm, dehydrated with anhydrous sodium sulfate (Na2SO4), and again centrifuged under the same conditions. Then, they were stored in amber glass ampoules and kept in a refrigerated environment at a constant temperature of 5 °C. The percentage of water in the studied samples was determined using an infrared moisture analyzer. The EO yield was calculated using the relationship between mass, oil, and moisture, as established by Santos et al. [40].
2.3. Identification of Chemical Components by GC/MS
The chemical composition of the EOs was analyzed in the Adolpho Ducke laboratory of the Goeldi Museum (LAD/MPEG, Belém, Brazil) by gas chromatography coupled to mass spectrometry (GC/MS) in a SHIMADZU QP Plus-2010 system equipped with a DB-5MS silica capillary column (30 m × 0.25 mm; 0.25 m film thickness) under the following operating conditions: carrier gas: helium, linear velocity of 36.5 cm.s−1; injection mode: splitless (2 µL of oil in 0.5 mL of hexane); injector temperature: 250 °C, temperature program: 60–250 °C with a gradient of 3 °C.min−1; temperature of the ion source and other parts: 220 °C.
The quadrupole scan rate was 39 to 500 daltons.s−1. Ionization was performed in electron impact mode at 70 eV. The identification of volatile components was based on the linear retention index (RI) and the fragmentation patterns in the mass spectra by comparison with standard samples in the NIST-11 and FFNSC-2 databases and from the literature [41]. The RIs were obtained using the homologous series of n-alkanes (C8-C40) from Sigma–Aldrich (San Luis, AZ, USA).
2.4. Preliminary Toxicity Bioassay with Artemia Salina
The preliminary toxicity of the EOs was tested against larvae of the microcrustacean A. salina. The A. salina cysts were incubated at room temperature (27–30 °C) under artificial lighting in an aquarium with artificial salt water: 46 g of NaCl, 22 g of MgCl2.6H2O, 8 g of Na2SO4, 2.6 g of CaCl2.6H2O, and 1.4 g of KCl in 2.0 L of distilled water. The pH was adjusted to 8.0–9.0 using Na2CO3.
Twenty-four hours after hatching, EO solutions were prepared in triplicate at concentrations of 100, 50, 25, 10, 5, and 1 µg.mL−1 using brine water as a vehicle and 5% dimethyl sulfoxide (DMSO) as a diluent. Ten larvae of A. salina in the meta-nauplius stage were placed in each tube. After 24 h, the mortality rate of the larvae was quantified, and the mean lethal concentration (LC50) was calculated using the Probitos statistical method, according to the methodology adapted from Góes et al. [42].
2.5. Tests of the Antioxidant Capacity of Essential Oils
2.5.1. ABTS Assay
The radical inhibition activity (AIR) of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+) was analyzed according to the initial principles proposed by Miller et al. [42] with the reaction conditions modified by Re et al. [43]. The method is based on the ability of substances to eliminate the cationic ABTS•+ radical, a blue-green chromophore with maximum absorption at 734 nm, resulting in the formation of the stable, colorless ABTS product.
Initially, ABTS (7 mM.L−1; Sigma–Aldrich; A1888; São Paulo/SP, Brazil) and potassium persulfate (140 mM.L−1; K2O8S2; Sigma–Aldrich; 216224; São Paulo/SP, Brazil) were mixed and left in the dark for 16 h to form the ABTS•+ radical (2.45 mM.L−1). Then, the radical was diluted with phosphate-buffered saline until reaching an absorbance of 0.700 ± 0.020 in an 800XI spectrophotometer (Femto; São Paulo/SP, Brazil) at 734 nm. Then, 30 μL of sample or standard was added to the solution (in triplicate), and after 5 min, the final absorbance was read. In addition, Trolox® (1 mM.L−1; Sigma–Aldrich; 23881-3; São Paulo/SP, Brazil) was used as a standard antioxidant. We calculated the inhibition activity according to the following equation:
| AIR (%) = [(Acontrol − Asample)/Acontrol × 100] | (1) |
where Acontrol represents the absorbance of the ABTS•+ radical (2.5 mM.L−1), and Asample represents the absorbance of the sample.
2.5.2. DPPH Assay
The AIR of 2,2-diphenyl-1-picrylhydrazyl (DPPH•) was determined according to the method proposed by Blois [44] with modifications. This assay assesses the total antioxidant capacity of a substance to eliminate the radical DPPH• (Sigma–Aldrich; D9132; São Paulo/SP, Brazil), a violet chromophore with absorption at 517 nm, resulting in the formation of the hydrogenated DPPH product, which is yellow or colorless.
First, DPPH• solution (0.1 mM.L−1) was prepared from the reaction between DPPH (394.32 g.mol−1; Sigma–Aldrich; A1888; São Paulo/SP, Brazil) and ethyl alcohol (PA; C2H6O; Sigma–Aldrich; 216224; São Paulo/SP, Brazil). Then, the absorbance of the DPPH• solution was read in an 800XI spectrophotometer (Femto; São Paulo/SP, Brazil) at 517 nm. Next, 50 µL of the sample or standard (Trolox; triplicate) were mixed in 950 µL of DPPH• solution and placed in a water bath at 30 °C for 30 min. Trolox was also used as a standard antioxidant. We calculated the AIR as described in the ABTS assay. Please refer to Supplemental Material S1, for better understanding of antioxidant methods.
2.6. Statistical Analysis
For analysis of the preliminary toxicity and ABTS•+ and DPPH• AIR of the EOs of M. floribunda and M. sylvatica, analysis of variance (ANOVA (Analysis of Variance),) was applied. Significant differences were compared between groups using Tukey’s post hoc analysis. In all tests, a significance level of 5% (p ≤ 0.05) was considered.
3. Results and Discussion
3.1. Analysis of the Yield and Chemical Composition of Essential Oils
The following Table 1 shows the results for the yield and chemical composition of the EOs of the two species of Myrtaceae. In total, 26 volatile constituents were identified for M. floribunda and 30 for M. sylvatica.
Table 1.
Chemical composition of the essential oils of the two species of Myrtaceae.
| Species | M. floribunda | M. sylvatica | ||
|---|---|---|---|---|
| Yield | 1.02% | 0.22% | ||
| IRL | IRC | Chemical Component | Area (%) | Area (%) |
| 932 | 935 | α-pinene | 3.30 | |
| 988 | 991 | Myrcene | 2.67 | |
| 1002 | 1008 | α-phellandrene | 22.19 | |
| 1014 | 1018 | α-terpinene | 1.53 | |
| 1026 | 1030 | o-cymene | 7.04 | |
| 1024 | 1033 | Limonene | 1.00 | |
| 1026 | 1036 | 1,8-cineole | 23.30 | |
| 1054 | 1059 | γ-terpinene | 5.87 | |
| 1086 | 1091 | terpinolene | 22.23 | |
| 1095 | 1100 | linalool | 0.81 | |
| 1174 | 1178 | terpinen-4-ol | 1.63 | |
| 1186 | 1191 | α-terpineol | 2.45 | |
| 1374 | 1375 | α-copaene | 0.57 | 0.50 |
| 1389 | 1387 | β-elemene | 0.52 | |
| 1411 | 1410 | α-cis-bergamotene | 0.41 | |
| 1417 | 1419 | (E)-caryophyllene | 2.21 | 4.82 |
| 1430 | 1425 | β-copaene | 0.16 | |
| 1434 | 1429 | γ-elemene | 2.89 | |
| 1432 | 1431 | α-trans-bergamotene | 7.06 | |
| 1440 | 1451 | (E)-β-farnesene | 1.79 | |
| 1457 | 1456 | β-santalene | 2.44 | |
| 1452 | 1456 | α-humulene | 0.16 | |
| 1484 | 1477 | germacrene D | 1.30 | |
| 1480 | 1481 | β-trans-bergamotene | 5.07 | |
| 1489 | 1488 | β-selinene | 0.17 | |
| 1496 | 1491 | valencene | 0.04 | |
| 1500 | 1493 | bicyclogermacrene | 3.34 | |
| 1496 | 1497 | viridiflorene | 0.51 | |
| 1506 | 1498 | (Z)-α-bisabolene | 8.33 | |
| 1500 | 1502 | α-muurolene | 0.15 | |
| 1505 | 1504 | β-bisabolene | 4.27 | |
| 1514 | 1506 | β-curcumene | 0.56 | |
| 1502 | 1509 | trans-β-guaiene | 0.11 | |
| 1514 | 1511 | (Z)-γ-bisabolene | 0.56 | |
| 1521 | 1519 | β-sesquiphellandrene | 3.94 | |
| 1522 | 1525 | δ-cadinene | 0.70 | |
| 1529 | 1527 | (E)-γ-bisabolene | 0.92 | |
| 1537 | 1537 | (E)-α-bisabolene | 0.76 | |
| 1559 | 1554 | germacrene B | 1.96 | |
| 1561 | 1564 | (E)-nerolidol | 0.61 | |
| 1564 | 1573 | davanone B | 0.04 | |
| 1577 | 1574 | spathulenol | 1.46 | |
| 1586 | 1581 | thujopsan-2-α-ol | 1.73 | |
| 1592 | 1595 | viridiflorol | 0.19 | |
| 1595 | 1597 | cubeban-11-ol | 0.06 | 0.56 |
| 1640 | 1645 | epi-α-muurolol | 1.74 | |
| 1652 | 1651 | α-cadinol | 1.25 | |
| 1670 | 1666 | epi-β-bisabolol | 0.57 | |
| 1674 | 1675 | (Z)-α-santalol | 0.39 | |
| 1690 | 1694 | (Z)-α-trans-bergamotene | 0.11 | 24.57 |
| 1755 | 1748 | α-sinensal | 13.44 | |
| 1806 | 1802 | nootkatone | 0.81 | |
| Hydrocarbon monoterpenes | 65.83 | 0.00 | ||
| Oxygenated monoterpenes | 25.74 | 0.00 | ||
| Hydrocarbon sesquiterpenes | 4.62 | 51.60 | ||
| Oxygenated sesquiterpenes | 1.01 | 46.52 | ||
| TOTAL | 99.65 | 98.12 | ||
RIL, retention index in the literature [41]; RIC, retention index calculated from a homologous series of n-alkanes (C8-C40) in a DB5-MS column. Relative area (%) calculated based on the peak areas.
The EO of M. floribunda predominantly contained hydrocarbon monoterpenes (65.82%) and oxygenated monoterpenes (25.74%). Tietbhol et al. [45] identified high levels of hydrocarbon sesquiterpenes (53.50%), oxygenated monoterpenes (16.70%), and oxygenated sesquiterpenes (16.50%) in the aromatic profile of M. floribunda. These results indicate a difference in chemical composition between the current sample and the sample reported in the literature. Regarding the EO of M. sylvatica, only hydrocarbon sesquiterpenes (51.60%) and oxygenated sesquiterpenes (46.52%) were observed in the sample. Raposo et al. [46] also identified high levels of hydrocarbon (28.00–63.90%) and oxygenated (15.30–51.40%) sesquiterpenes in the EO of the species.
3.1.1. Yield and Chemical Composition of the Essential Oil of Myrciaria floribunda
The yield of the EO of M. floribunda in the present study was 1.02%. According to Tietbohl et al. [45], the yield of the EO of fresh leaves of M. floribunda originating in Rio de Janeiro, Brazil, was 0.37%. Barbosa et al. [47] reported a yield of the EO of M. floribunda fruits of 0.60%. These comparisons indicate that the yield of the present sample is higher than that recorded in the literature. Regarding the phytochemical profile of the EO of M. floribunda, the oxygenated monoterpene 1,8-cineole, also known as eucalyptol, was the component with the highest content (23.0%), followed by the hydrocarbon monoterpenes terpinolene (22.23%), α-phellandrene (22.19%), o-cimene (7.04%), and γ-terpinene (5.87%).
Tietbohl et al. [45] evaluated the chemical composition of the EO of the fresh leaves of a specimen of M. floribunda from the Restinga de Jurubatiba National Park, Rio de Janeiro, Brazil. According to the authors, 1,8-cineole (10.40%), β-selinene (8.40%), and α-selinene (7.40%) were the volatile constituents with the highest contents. The 1,8-cineole content found in the present study was twice that recorded by those authors. In addition, α- and β-selinene were not present in the chemical composition of the sample analyzed in this study.
Tietbohl et al. [45] emphasized that the EO of M. floribunda significantly increased mortality and interrupted the metamorphosis of the parasite Trypanosoma cruzi, indicating that the secondary metabolites present in the chemical composition of this natural product are promising for use as bioinsecticides and for the environmentally appropriate control of vectors of Chagas disease. Tietbohl et al. [48] reported that the EO of another specimen found in Rio de Janeiro, Brazil, was characterized by high levels of monoterpenes (53.90%), among which 1,8-cineole was the major component (38.40%), with a concentration higher than that indicated in the present study.
Barbosa et al. [47] analyzed the chemical composition of the EO of M. floribunda fruits collected in the Brazilian state of Pernambuco. According to the authors, the hydrocarbon sesquiterpenes δ-cadinene (26.84%) and γ-cadinene (15.69%) were the major components of the sample. De Oliveira et al. [49] reported β-(Z)-o-cymene (50.80%), 1,8-cineole (3.14%), γ-terpinene (2.51%), and (E)-caryophyllene (1.16%) as the constituents with the highest contents in the EO of lyophilized isolates of fruit of M. floribunda from the Restinga de Maricá in Rio de Janeiro, Brazil.
These results indicate that the phytochemical profiles of the EOs of the fruits and leaves are different. According to Ferreira et al. [50], species from different geographic locations have different chemotypes [50]. This statement may explain the differences between the chemical composition of the present sample and those of samples reported in the literature.
The major constituent of the sample (1,8-cineole) has potential as an anti-inflammatory drug for the treatment of cancer [51]. In addition, the encapsulation of 1,8-cineole in nanofibers can prolong fungal activities against Candida species given that the isolated compound is not a strong fungal agent [52,53,54]. 1,8-Cineole also has potential as an antimicrobial, repellent, and anticancer agent and can be used in the treatment of respiratory diseases, including COVID-19 [55,56,57,58,59,60,61,62,63].
This compound also has several industrial applications, mainly in the production of pharmaceuticals and as a flavoring of foods and toothpastes [64,65,66,67]. Recent studies also point to the use of this compound as a biosolvent and anti-corrosion agent, as it is an ecological alternative to synthetic products [68,69,70].
Terpinolene has larvicidal, insecticidal, antifungal, antibacterial, antiproliferative, cytoprotective, antiviral, antimicrobial, and antibacterial activities, with activity against dangerous multidrug-resistant bacteria in the treatment of industrial wastewater [71,72,73,74].
The compound α-phellandrene also has biological activities described in the literature, including potential antifungal properties, and could potentially be used as an endosomal gel for the treatment of gout [75,76]. Regarding o-cymene, recent studies point to possible antiviral effects against COVID-19 due to its anti-inflammatory and anti-influenza activities and as an antifungal agent [77,78,79]. There are few reports of the properties of γ-terpinene in the literature. However, according to Reis et al. [80], oils containing this compound as a major component or at high levels have moderate antimicrobial activity against food pathogens.
3.1.2. Yield and Chemical Composition of Myrcia sylvatica Essential Oil
The yield obtained for the EO of M. sylvatica was 0.22%. According to Raposo et al. [46] The EO content of the leaves of a sample from Santarém, Pará, Brazil, ranged from 0.90 to 1.60%, reaching the highest content in the month of July (Amazonian dry season) and the lowest content in the month of January (rainy season). In addition, Raposo et al. [46] analyzed the yield of the EO of the fruit of the species and found a value of 1.70%. Saccol et al. [81] found an analyzed yield of 1.10% for EO from the dry leaves of a specimen from Santarém, Pará, Brazil. Rosa et al. [82] found a yield of 0.50% for the EO of M. sylvatica. These results indicate that the EO content of the present sample is lower than the results in the literature.
The EO of M. sylvatica analyzed in the present study showed the oxygenated sesquiterpene (Z)-α-trans-bergamotene as the volatile component with the highest content (24.57%), followed by the oxygenated sesquiterpene α-sinensal (13.44%) and hydrocarbon sesquiterpenes (Z)-α-bisabolene (8.33%), α-trans-bisabolene (7.06%), and β-trans-bisabolene (5.07%). Raposo et al. [46] evaluated the phytochemical profile of the EO of the leaves of a specimen of M. sylvatica collected in Santarém, Pará, Brazil. According to the authors, the hydrocarbon sesquiterpenes β-selinene (6.2–10.5%), cadalene (4.7–8.2%), α-calacorene (1.5–6.0%), δ-cadinene (0.0–6.0%), and trans-calamenene (3.5–6.5%) and the oxygenated sesquiterpene 1-epi-cubenol (5.9–9.8%) and muskatone (2.7–6.2%) characterized the chemical profile of the sample.
Raposo et al. [46] also evaluated the chemical composition of the EO of the fruit of M. sylvatica in the fertile period and found δ-cadinene (11.3%), β-selinene (6.0%), and 1-epi-cubenol (5.1%) as the volatile constituents with the highest contents. Saccol et al. [81] studied the volatile composition of the EO of the aerial parts of M. sylvatica from Santarém, Pará, Brazil. According to the authors, the hydrocarbon sesquiterpenes ar-curcumene (8.09%), β-selinene (6.48%), cadalene (6.24%), α-calamenene (5.89%), and (Z)-calamenene (5.54%) and the oxygenated sesquiterpene 1-epi-cubenol (7.41%) were the major components of the sample. The authors also found that this EO attenuated molecular and biochemical effects and physiological changes in Rhamdia quelen under different stress events.
Saccol et al. [83] analyzed the chemical composition of the EO of the aerial parts of M. sylvatica collected in Santarém, Pará, Brazil. According to the authors, α-selinene (16.08%), calamenene (11.68%), and α-calacorene (11.47%) were the volatile constituents with the highest contents. The authors also noted that this EO attenuates stress induced in the freshwater fish Sparus aurata. Saccol et al. [84] identified β-selinene (9.96%), cadalene (9.36%), α-calacorene (9.17%), and (Z)-calamene (8.17%) as major compounds of the EO of a specimen from Santarém, Pará, Brazil. Rosa et al. [82] identified (E)-caryophyllene (45.88%) and 14-hydroxy-(Z)-caryophyllene (10.15%) as the main components of the EO of a sample from Carolina, Maranhão, Brazil. According to Cascaes et al. [85], M. sylvatica has great genetic variability, which is directly responsible for the different chemotypes presented by the species.
Regarding the properties and applications of the major components, EOs containing high levels of (Z)-α-trans-bergamotene may have antibacterial and antifungal activities [86,87]. This compound is also used in industry as a flavoring agent and can be found in the aromas of cereals and other derivatives [88]. α-Sinensal is responsible for the sapodilla aroma and is also used in the food, cosmetics, and perfumery industries as a flavoring because it has an intense orange aroma accompanied by distinct floral notes [89,90]. Yi et al. [91] observed that α-sinensal showed antimicrobial activity and inhibited Gram-negative Staphylococcus aureus and Bacillus subtilis.
Lancaster et al. [92] showed that (Z)-α-bisabolene is a potential candidate insecticide against Murgantia histrionica (harlequin bug) and Phyllotreta striolata (flea beetle). There are few reports in the literature about the biological properties of the α- and β-trans-bergamotene isomers. However, Moraes et al. [93] stated that the two isomers are defensive components of insects common in Brazil. The potential of the two compounds and other derivatives as insecticides was also explored by Cribb et al. [94] for management of the southern green stink bug (Nezara viridula).
3.2. Preliminary Toxicity
In the A. salina bioassay, no mortality was observed for the control, showing that the use of 5% DMSO as a diluent is feasible [95]. The average lethal concentration (LC50) values of the EOs were calculated by fitting a logarithmic curve to the number of dead individuals and extracting the equation in Probitos. The table below shows the mortality results and the LC50 concentrations, with their respective coefficients of determination (R2) as well as the preliminary cytotoxicity classification of the EOs (Table 2).
Table 2.
Preliminary cytotoxicity of essential oils.
| Species | Concentration (µg.mL−1) |
Mortality (%) |
LC50 (µg.mL−1) |
R2 | Classification (Ramos et al. [96]) |
|---|---|---|---|---|---|
| M. floribunda | 100 50 25 10 5 1 |
56.67 50.00 33.33 0.00 0.00 0.00 |
82.96 ± 5.20 b | 0.76 | Moderate toxicity |
| M. sylvatica | 100 50 25 10 5 1 |
96.67 93.33 86.67 70.00 56.67 40.00 |
2.74 ± 0.50 a | 0.88 | High toxicity |
Values are expressed as the mean and standard deviation (n = 3) of the preliminary toxicity. Results with different lowercase letters (b or a) demonstrate that they are statistically different from each other.
The A. salina bioassay is used to efficiently evaluate the cytotoxicity of natural products aquatic environments; it is simple and fast and has low requirements [97]. The test allows the screening of a large number of toxic substances because the microcrustacean larvae are quite sensitive to various chemical constituents, and in some cases, toxicity to A. salina coincides with toxicity to mammalian cells [98,99].
Previous studies show that results of preliminary toxicity bioassay with A. salina vary according to the type of sample studied (essential oil or extract) and its chemical composition. For the brine shrimp bioassay performed with the essential oils obtained from Conobea scoparioides fresh and dried leaves, it was verified a CL50 equal to 7.8 ± 0.3 µg.mL–1 and 7.5 ± 0.3 µg mL–1, respectively. According to Ramos et al. [96], an EO is classified as toxic when LC50 is below 80 µg.mL−1, moderately toxic when LC50 is between 80 and 250 µg.mL−1, and nontoxic or slightly toxic when LC50 is higher than 250 µg.mL−1.
3.2.1. Toxicity of the Essential oil of Myrciaria floribunda
The EO of the dry leaves of M. floribunda had a mean LC50 of 82.96 ± 5.20 µg.mL−1. This result indicates that the EO of M. floribunda is moderately toxic according to the classification of Ramos et al. [96]. Barbosa et al. [47] reported that the EO of M. floribunda showed high inhibitory activity of the enzyme acetylcholinesterase, the main target of A. salina and the compound responsible for the activity of the microcrustacean.
According to Barbosa et al. [100], the EO of M. floribunda showed moderate cytotoxic potential in mammalian host cells. However, the chemical composition reported by those authors differed significantly from that of the present sample because it had high levels of sesquiterpenes. According to Tietbohl et al. [48], the EO of M. floribunda, composed mainly of 1,8-cineole (38.40%), showed acute toxicity against Oncopeltus fasciatus and Dysdercus peruvianus.
According to Caldas et al. [101] and Izham et al. [102], 1,8-cineole, the major component of the present sample, showed no indication of toxicity in tests performed with mice. Izham et al. [102] also highlighted that 1,8-cineole had a cytotoxic effect against breast cancer cells without harming the health of the mice subjected to the test. Elmhalli et al. [103] showed that the EOs of Salvadora persica and Rosmarinus officinalis, which contain 1,8-cineole as a major constituent, have moderate toxicity against nymphs of Ixodes ricinus.
Bhowal and Gopal [104] stated that 1,8-cineole has no reported negative effects in animal experiments and that the toxicity reported thus far in animal experiments appeared only after the application of high doses. According to Galan et al. [105], the amount of 1,8-cineole used commercially does not produce harmful effects to human health, and the compound has toxic effects only when administered at high doses.
Ribeiro et al. [106] reported that terpilonene showed low acute toxicity by residual contact against the mite Tetranychus urticae and was less efficient than other monoterpenes. However, Agus [107] stated that terpinolene is among the most toxic monoterpenes along with α-terpineol and linalool. According to Scherf et al. [108], terpinolene was toxic to Drosophila melanogaster, with an LC50 of 34.60 μL.L−1 at 12 h of exposure.
Scherer et al. [109] found that terpinolene and α-phellandrene showed no cytotoxic effect against L929 fibroblasts or RAW 267.7 macrophages. Martínez et al. [110] stated that α-phellandrene exhibited high toxicity against larvae of the insect Tenebrio molitor. Abdelgaleila and El-Sabrout [111] indicated that the EO of Schinus molle, consisting of 29.78% α-phellandrene, was toxic to Culex pipiens mosquito larvae. Other compounds present in lower concentrations in the EO of M. floribunda may have toxic effects, such as γ-terpinene and γ-cymene, which were likely responsible for the toxic effect of the EO of Eucalyptus camaldulensis against several cancer cells [112].
The biological activities of EOs may be related to the presence of certain constituents at a higher content and to the synergistic and/or antagonistic effects exerted by all substances present in the samples [113,114,115,116,117,118]. Considering that 1,8-cineole has low toxicity, and the other compounds present at high levels have moderate or high toxicity, the combined effects of the volatile constituents of the EO of M. floribunda may explain its moderate toxicity. In addition, monoterpenes have low toxicity when compared to sesquiterpenes and phenylpropanoids [119,120,121].
3.2.2. Toxicity of the Essential Oil of Myrcia sylvatica
Regarding the EO of M. sylvatica, the LC50 was 2.74 µg.mL−1, indicating that the natural product showed very pronounced toxicity against A. salina and that the oil of the species is highly toxic. This EO consists only of sesquiterpenes, which generally have high toxicity [122,123]. Many sesquiterpenes have shown promising potential for use as raw materials or additives in bioinsecticides, natural repellents, and biopesticides due to their high toxicities [124,125,126].
Rosa et al. [82] indicated that the EO of a specimen of M. sylvatica from Carolina, Maranhão, Brazil, showed toxicity to A. salina, with an LC50 of 79.44 µg.mL−1. The authors emphasized that the EO is composed mainly of sesquiterpenes, primarily (E)-caryophyllene and 14-hydroxy-(Z)-caryophyllene. Toxic effects of other species of Myrcia with high levels of sesquiterpenes have been described in the literature. Scalvenzi et al. [127] reported that the EO of M. splendens showed cytotoxic activity against the human tumor cell lines MCF-7 (breast) and A549 (lung) and the nontumor cell line HaCaT (human keratinocytes). Melo et al. [128] stated that the EO of M. lundiana showed toxicity against the insect Acromyrmex balzani.
According to Alves et al. [129], the major constituent of the EO of M. sylvatica is present in high content in the EO of Cordia verbenacea, which inhibited 25.9% of cowpea weevils at a concentration of 0.40 µL.cm−3. Powers et al. [87] indicated that the EOs of Santalum album and S. paniculatum, containing (Z)-α-trans-bergamotene as one of their major components, showed toxicity against Hep-G2 tumor cells (liver cancer). Regarding α-sinensal, there are no reports in the literature regarding its toxicity.
Fernandes et al. [130] found that the EOs of the leaves and stems of Eremanthus erythropappus, which contain (Z)-α-bisabolene at high levels, showed marked toxicity in mice by the MTT assay. Mahdavi et al. [131] showed that the EO of the Zingiber officinale rhizomes showed substantial toxicity against the potato moth (Phthorimaea operculella). According to the authors, this EO contains (Z)-α-bisabolene as one of its major components.
According to Fernandez et al. [132] α-trans-bergamotene was the major component of the EO of the fruits of Garcinia gardneriana and showed toxicity against Aedes aegyptus mosquito larvae. Vinturelle et al. [133] reported that the EO of Copaifera officinalis contained α-trans-bergamotene as one of the compounds with the highest content and caused 84.60% mortality in engorged females of the tick Rhipicephalus microplus. The authors attributed this toxicity to the presence of sesquiterpenes, including α-trans-bergamotene, in the chemical profile of the EO. Matsuda et al. [134] reported that the compound β-trans-bergamotene has moderate toxicity against cancer cells and has not yet shown satisfactory results for possible use as a drug.
The possible toxic effects of the five major components of the EO of M. sylvatica and the synergistic effects of the other sesquiterpenes present in the sample may have contributed to the high toxicity of the EO against the microcrustacean A. salina [135]. Thus, further studies should be conducted to evaluate the possible harmful effects of this EO and its major components on mammalian cells and human health in addition to its possible use as an additive for the production of larvicides, pesticides, and insecticides.
3.3. Antioxidant Capacity of Essential Oils
The antioxidant capacity of the EOs of M. floribunda and M. sylvatica was determined from the ABTS•+ and DPPH• AIR and by comparison with Trolox (1 mM/L), a water-soluble synthetic analog of vitamin E and a potent antioxidant.
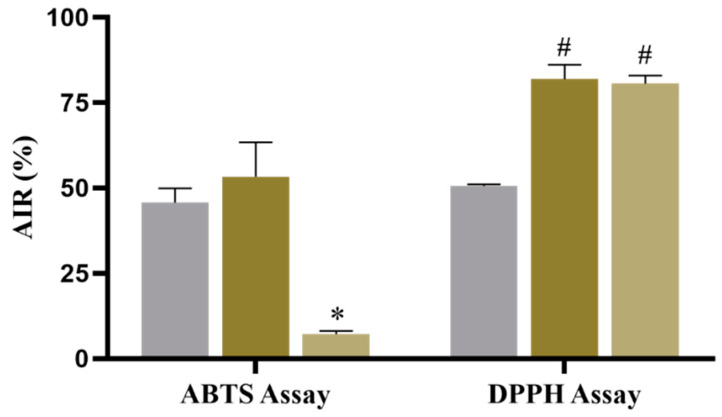
According to Ferreira et al. [136], in the ABTS assay, the reaction between ABTS and K2O8S2 generates the ABTS•+ radical, which is then reduced again to ABTS in the presence of antioxidant compounds; the degree and time scale of this reaction are dependent on the capacity antioxidant concentration, concentration of antioxidants present in the Eos, and duration of the reaction between the compounds. According to the authors, in the DPPH assay, when the antioxidant power of the EO is high, the color of the solution changes from purple to yellowish over time due to the donation of a hydrogen atom or the transfer of electrons from substances in the EO to the DPPH• radical, which then transforms into a stable diamagnetic molecule. In the present results, the RIAs of the EOs were proportional to their TEAC values (Figure 1).
Figure 1.
Percentage ABTS•+ and DPPH• radical inhibition activity (AIR) of the essential oils of Myrciaria floribunda and Myrcia sylvatica. The results are expressed as the mean and standard deviation (n = 3). * p = 0.0001 versus Trolox and Myrciaria floribunda; #
p = 0.0001 versus Trolox.  = Myrciaria floribunda;
= Myrciaria floribunda;
 = Myrcia sylvatica;
= Myrcia sylvatica;
 = Trolox.
= Trolox.
3.3.1. Antioxidant Capacity of the Essential Oil of Myrciaria floribunda
The results (Figure 1) showed that the EO of M. floribunda had an ABTS•+ AIR of 53.27 ± 8.27%, indicating that this EO had an antioxidant potential (p = 0.45) similar to that of Trolox (45.74 ± 4.16%). In the DPPH assay, the AIR of the EO was 81.91 ± 3.46%, indicating that the effect of the M. floribunda EO was superior to that of Trolox (p < 0.0001), with 50.53 ± 0.52%. These results indicate that the EO of M. floribunda has excellent antioxidant activity. This activity can be attributed to the chemical composition of the EO, mainly due to the presence of high levels of monoterpenes with antioxidant potential [137].
According to De Oliveira et al. [49], the EO of lyophilized fruits of M. floribunda showed high antioxidant potential toward the free radicals ABTS•+ and DPPH•. According to the authors, the antioxidant capacity of ABTS•+ free radicals was 550.14 μmol Trolox.g−1. In the DPPH• assay, the EC50 was 85.68 g·g−1. Moreover, the EO was characterized mainly by hydrocarbon sesquiterpenes, and its 1,8-cineole content was almost eight times lower than that recorded in the present study.
There are few reported on the antioxidant potential of the EOs of other species of Myrciaria. However, Da Costa et al. [138] stated that M. dubia fruits are a natural source of antioxidants due to their substantial contents of ascorbic acid and phenolic compounds. In addition, the antioxidant potential of the components of M. floribunda and other species of Myrciaria are well-known, and further studies are needed to verify the antioxidant effects of their volatile components [139,140].
1,8-Cineole, the major component of the EOs in this study, is volatile and has been reported to have antioxidant potential [141]. EOs containing this oxygenated monoterpene as the major constituent have shown high capture power of the DPPH• and ABTS•+ radicals, as demonstrated by Boukhatem et al. [142]. According to the authors, the EO of Eucalyptus globulus is composed of more than 94% 1,8-cineole and showed good results for the inhibition of DPPH• radicals and a metal chelating activity superior to those of the synthetic antioxidants gallic acid and ascorbic acid. Limam et al. [143] also indicated that the EOs of Eucalyptus species with high levels of 1,8-cineole (>48.00%) exhibited a good ability to inhibit DPPH• radicals.
Terpilonene is a constituent with important antioxidant activities highlighted in the literature [144]. Aydin, Turkez, and Taşdemir [145] stated that terpinolene showed excellent antioxidant capacity and great potential to inhibit oxidative stress. In addition, the authors showed that the compound’s antioxidant properties make it a strong candidate for anticancer treatment, but further studies are needed to corroborate these results. According to Osanloo, Ghanbariasad, and Taghinizhad [146], the EO of Anethum graveolens is composed mainly of α-phellandrene (26.75%). According to the authors, the natural product showed a good ability to capture DPPH• radicals at concentrations above 160 µg.mL−1.
Other compounds at lower concentrations in the EO of M. floribunda, such as o-cymene, γ-terpinene, α-terpineol, myrcene, and α-pinene, also have antioxidant potential [147,148,149,150,151]. The synergistic and/or antagonistic effects of the constituents of this EO may explain its high capture of free radicals considering that the volatile components in the sample are known for their excellent antioxidant properties [152,153].
3.3.2. Antioxidant Capacity of Myrcia sylvatica Essential Oil
According to the results (Figure 1), the EO of M. sylvatica exhibited an ABTS•+ AIR of 7.20 ± 0.72%, showing a lower effect than the EO of M. floribunda (p < 0, 0001) and the standard (p < 0.0001). On the other hand, in the DPPH assay, the AIR of the EO of M. sylvatica was 80.55 ± 2.00%; this effect was similar to that of M. floribunda oil (p = 0.99) and higher than that of Trolox (p < 0.0001). These data showed that the EO of M. sylvatica had different AIR effects. We believe that this difference in effects can be attributed mainly to the oxygenated sesquiterpenes present in the EO of M. sylvatica, which are highly reactive molecules due to their oxygenation content. In addition, the ABTS•+ radical exhibits steric hindrance around its nitrogen-centered atom, which hinders reactions with other highly reactive molecules, such as oxygenated sesquiterpenes.
Saccol et al. [84] evaluated the antioxidant effects of M. sylvatica EO on the sedation process of the tambaqui (Colossoma macropomum). According to the authors, the total reactive antioxidant potential in the brain and gills of anesthetized tambaqui was higher than that of the control. Franco et al. [154] analyzed the antioxidant capacity of the EO of M. tomentosa. According to the authors, the EO showed a high percent inhibition of free radicals DPPH• (53.60 ± 0.15%) and ABTS•+ (213.00 ± 0.91). The authors also emphasized that this EO is characterized by high levels of hydrocarbon (56.74–75.82%) and oxygenated (15.09–16.83%) sesquiterpenes, mainly γ-elemene, germacrene D, (E)-caryophyllene, spathulenol, and α-zingiberene.
Gatto et al. [155] stated that the EO of M. hatschbachii had a low capacity to inhibit DPPH• free radicals (9.14 ± 0.33%). The authors also showed that the EO is composed mainly of hydrocarbon (47.81%) and oxygenated (30.05%) sesquiterpenes, mainly trans-calamenene (19.10%), (E)-caryophyllene (10.96%), and spathulenol (5.03%). Scalvenzi et al. stated that the EO of M. splendens showed an antioxidant potential six times higher than that of vitamin E for the inhibition of DPPH• free radicals. According to the authors, the EO is characterized by the presence of trans-nerolidol (67.81%) and α-bisabolol (17.51%).
Regarding the antioxidant properties of the major component of the EO of M. sylvatica, there are few reports in the literature. However, Mohankumar et al. [156] stated that (Z)-α-trans-bergamotene at high concentrations in the EO of Santalum album may be responsible for the antioxidant and oxidative-stress-modulation activities of the EO, possibly by direct elimination of free radicals and activation of the antioxidant defense system in vitro and in vivo. M. Yi et al. [91] showed that in addition to (Z)-α-trans-bergamotene, α-sinensal was one of the main factors responsible for the ABTS•+ free radical capture ability of the EO of Citrus reticulata. Saroglou et al. [157] demonstrated that the high concentration of sesquiterpenes, including (Z)-α-bisabolene, in the EO of Teucrium royleanum was responsible for the DPPH• RIA.
There are no reports on the possible antioxidant potentials of α-trans-bergamotene and β-trans-bergamotene in the literature. In general, sesquiterpenes have a lower antioxidant profile than monoterpenes [158,159]. In addition, oxygenated compounds have a greater capacity for free radical scavenging and reduced deleterious effects of lipid peroxidation, especially alcohols, phenols, and enols, due to the presence of hydroxyl groups [160]. These properties may explain the antioxidant behaviors of the EOs of M. sylvatica and M. floribunda against the ABTS•+ and DPPH• radicals.
4. Conclusions
The present study indicated that the EO of M. floribunda is characterized by high levels of hydrocarbon and oxygenated monoterpenes, predominantly 1,8-cineole, terpinolene, and α-phellandrene. Regarding the EO of M. sylvatica, only hydrocarbon and oxygenated sesquiterpenes were identified in the sample, among which (Z)-α-trans-bergamotene, α-sinensal, and (Z)-α-bisabolene were the volatile constituents with the highest contents. The preliminary toxicity results indicated that the EO of M. floribunda exhibited moderate toxicity against A. salina, while the EO of M. sylvatica showed high toxicity. In addition, the EO of M. floribunda exhibited a greater capacity to inhibit the DPPH• radical. This study contributes to the knowledge of the aromatic profile and antioxidant and biological properties of species of the family Myrtaceae from the Amazon region.
Acknowledgments
Â.A.B.d.M., L.S.d.C., and L.Q.A. thank CNPq for the scientific initiative scholarship. M.S.d.O. thanks PCI-MCTI/MPEG, Process number: 300983/2022-0. The authors would like to thank the Federal University of Pará—Propesp/PAPQ—Support Program for Qualified Publication Edital 02/2022 – PAPQ/PROPESP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11102076/s1, Supplementary Material S1, Detailed preparation method of the potential antioxidant activity.
Author Contributions
Conceptualization, Â.A.B.d.M.; methodology, Â.A.B.d.M., C.d.J.P.F., L.S.d.C., L.Q.A., L.D.d.N., O.O.F., M.M.C., E.L.P.V. and M.S.d.O.; software, Â.A.B.d.M.; formal analysis, L.D.d.N., M.S.d.O. and E.H.d.A.A.; research, Â.A.B.d.M.; writing—preparation of the original draft, S.P. and Â.A.B.d.M.; writing—revision and editing, O.O.F., E.L.P.V., C.d.J.P.F., L.D.d.N., M.M.C., M.S.d.O. and E.H.d.A.A.; visualization, L.D.d.N., M.S.d.O. and E.H.d.A.A.; supervision, L.D.d.N. and E.H.d.A.A.; project administration, E.H.d.A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cascaes M.M., Carneiro O.D.S., Nascimento L.D.D., de Moraes Â.A.B., de Oliveira M.S., Cruz J.N., Guilhon G.M.S.P., Andrade E.H.D.A. Essential oils from annonaceae species from brazil: A systematic review of their phytochemistry, and biological activities. Int. J. Mol. Sci. 2021;22:12140. doi: 10.3390/ijms222212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira O.O., Cruz J.N., de Moraes Â.A.B., de Jesus Pereira Franco C., Lima R.R., Anjos T.O.D., Siqueira G.M., Nascimento L.D.D., Cascaes M.M., de Oliveira M.S., et al. Essential oil of the plants growing in the brazilian amazon: Chemical composition, antioxidants, and biological applications. Molecules. 2022;27:4373. doi: 10.3390/molecules27144373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maia J.G.S., Andrade E.H.A. Database of the amazon aromatic plants and their essential oils. Quim. Nova. 2009;32:595–622. doi: 10.1590/S0100-40422009000300006. [DOI] [Google Scholar]
- 4.Nascimento L.D.D., Almeida L.Q., Sousa E.M.P.D., Costa C.M.L., Costa K.S.D., Andrade E.H.D.A., Faria L.J.G.D. Microwave-assisted extraction: An alternative to extract piper aduncum essential oil. Brazilian J. Dev. 2020;6:40619–40638. doi: 10.34117/bjdv6n6-558. [DOI] [Google Scholar]
- 5.García Y.M., Lemos E.E.P.D., Augusti R., Melo J.O.F. Optimization of extraction and identification of volatile compounds from myrciaria floribunda. Rev. Ciênc. Agron. 2021;52:e20207199. doi: 10.5935/1806-6690.20210031. [DOI] [Google Scholar]
- 6.Ferreira O.O., Cruz J.N.D., Nascimento L.D.D., Cascaes M.M., Pereira S.F.M., Franco C.D.J.P., Anjos T.O.D., Silva M.V.C., Oliveira M.S.D., Andrade E.H.D.A. Antimicrobial property of essential oils from myrtaceae species. In: Panda S.K., Dubey D., Govin J.N., editors. Recent Progress in Medicinal Plants: Antimicrobial Resistance and Bioactive Natural Product. Volume 51. Studium Press LLC; Houston, TX, USA: 2020. pp. 192–214. [Google Scholar]
- 7.Proença C.E.B., Amorim B.S., Antonicelli M.C., Bünger M., Burton G.P., Caldas D.K.D., Costa I.R., Faria J.E.Q., Fernandes T., Gaem P.H., et al. Myrtaceae in Flora Do Brasil 2020. [(accessed on 14 October 2022)]; Available online: https://floradobrasil2020.jbrj.gov.br/reflora/floradobrasil/FB600341.
- 8.Batiha G.E.S., Alkazmi L.M., Wasef L.G., Beshbishy A.M., Nadwa E.H., Rashwan E.K. Syzygium Aromaticum l. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10:202. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cilingir-Kaya O.T., Bihter Gurler E. Therapeutic potential of essential oil of melaleuca quinquenervia (myrtaceae) in a rat model of ethanolinduced peptic ulcer. Trop. J. Pharm. Res. 2021;20:981–986. doi: 10.4314/tjpr.v20i5.14. [DOI] [Google Scholar]
- 10.de Paulo Farias D., Neri-Numa I.A., de Araújo F.F., Pastore G.M. A critical review of some fruit trees from the myrtaceae family as promising sources for food applications with functional claims. Food Chem. 2020;306:125630. doi: 10.1016/j.foodchem.2019.125630. [DOI] [PubMed] [Google Scholar]
- 11.Filomeno C.A., Almeida Barbosa L.C., Teixeira R.R., Pinheiro A.L., de Sá Farias E., Ferreira J.S., Picanço M.C. Chemical diversity of essential oils of myrtaceae species and their insecticidal activity against rhyzopertha dominica. Crop Prot. 2020;137:105309. doi: 10.1016/j.cropro.2020.105309. [DOI] [Google Scholar]
- 12.Ferreira O.O., da Silva S.H.M., de Oliveira M.S., Andrade E.H.D.A. Chemical composition and antifungal activity of myrcia multiflora and eugenia florida essential oils. Molecules. 2021;26:7259. doi: 10.3390/molecules26237259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira M.S.D. In: Essential Oils—Applications and Trends in Food Science and Technology. 1st ed. de Oliveira M.S., editor. Springer International Publishing; Cham, Switzerland: 2022. [Google Scholar]
- 14.Bezerra F.W.F., de Oliveira M.S., Bezerra P.N., Cunha V.M.B., Silva M.P., da Costa W.A., Pinto R.H.H., Cordeiro R.M., da Cruz J.N., Chaves Neto A.M.J., et al. Extraction of bioactive compounds. In: Inamuddin , Isloor A.M., editors. Green Sustainable Process for Chemical and Environmental Engineering and Science. Elsevier; Amsterdam, The Netherlands: 2020. pp. 149–167. [Google Scholar]
- 15.Figueiredo P.L.B., Pinto L.C., da Costa J.S., da Silva A.R.C., Mourão R.H.V., Montenegro R.C., da Silva J.K.R., Maia J.G.S. Composition, antioxidant capacity and cytotoxic activity of eugenia uniflora L. chemotype-oils from the amazon. J. Ethnopharmacol. 2019;232:30–38. doi: 10.1016/j.jep.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 16.da Costa J.S., Barroso A.S., Mourão R.H.V., da Silva J.K.R., Maia J.G.S., Figueiredo P.L.B. Seasonal and antioxidant evaluation of essential oil from eugenia uniflora L., curzerene-rich, thermally produced in situ. Biomolecules. 2020;10:328. doi: 10.3390/biom10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Costa J.S., da Cruz E.D.N.S., Setzer W.N., da Silva J.K.D.R., Maia J.G.S., Figueiredo P.L.B. Essentials oils from brazilian eugenia and syzygium species and their biological activities. Biomolecules. 2020;10:1155. doi: 10.3390/biom10081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascaes M.M., Guilhon G.M.S.P., Zoghbi M.D.G., Andrade E.H.A., Santos L.S., Kelly R., da Silva J., Trovatti Uetanabaro A.P., Araújo I.S. Flavonoids, antioxidant potential and antimicrobial activity of myrcia rufipila mcvaugh leaves (myrtaceae) Nat. Prod. Res. 2019;35:1717–1721. doi: 10.1080/14786419.2019.1629912. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro A.R.C., Cordeiro M.L.D.S., Silva L.M.P., Cadavid C.O.M., Caland R.B.D.O., Fernandes-Negreiros M.M., Queiroz M.F., Barbosa J.D.S., Aragão C.F.S., Zucolotto S.M., et al. Myrciaria tenella (DC.) O. berg (myrtaceae) leaves as a source of antioxidant compounds. Antioxidants. 2019;8:310. doi: 10.3390/antiox8080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usman L.A., Oguntoye O.S., Ismaeel R.O. Effect of seasonal variation on chemical composition, antidiabetic and antioxidant potentials of leaf essential oil of Eucalyptus globulus L. J. Essent. Oil Bear. Plants. 2020;23:1314–1323. doi: 10.1080/0972060X.2020.1862710. [DOI] [Google Scholar]
- 21.Wang W.-H., Tyan Y.-C., Chen Z.-S., Lin C.-G., Yang M.-H., Yuan S.-S., Tsai W.-C. Evaluation of the antioxidant activity and antiproliferative effect of the jaboticaba (Myrciaria cauliflora) seed extracts in oral carcinoma cells. BioMed Res. Int. 2014;2014:185946. doi: 10.1155/2014/185946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Sá L.Z.C.M., Castro P.F.S., Lino F.M.A., Bernardes M.J.C., Viegas J.C.J., Dinis T.C.P., Santana M.J., Romao W., Vaz B.G., Lião L.M., et al. Antioxidant potential and vasodilatory activity of fermented beverages of jabuticaba berry (Myrciaria jaboticaba) J. Funct. Foods. 2014;8:169–179. doi: 10.1016/j.jff.2014.03.009. [DOI] [Google Scholar]
- 23.Sihoglu Tepe A., Ozaslan M. Anti-alzheimer, anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of cinnamomum zeylanicum. Ind. Crops Prod. 2020;145:112069. doi: 10.1016/j.indcrop.2019.112069. [DOI] [Google Scholar]
- 24.Nascimento L.D.D., Silva S.G., Cascaes M.M., Costa K.S.D., Figueiredo P.L.B., Costa C.M.L., Andrade E.H.D.A., de Faria L.J.G. Drying effects on chemical composition and antioxidant activity of Lippia thymoides essential oil, a natural source of thymol. Molecules. 2021;26:2621. doi: 10.3390/molecules26092621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diniz Do Nascimento L., Antônio Barbosa De Moraes A., Santana Da Costa K., Marcos J., Galúcio P., Taube P.S., Leal Costa M., Neves Cruz J., Helena De Aguiar Andrade E., Guerreiro De Faria L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules. 2020;10:988. doi: 10.3390/biom10070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Souza Farias S.A., Da Costa K.S., Martins J.B.L. Analysis of conformational, structural, magnetic, and electronic properties related to antioxidant activity: Revisiting flavan, anthocyanidin, flavanone, flavonol, isoflavone, flavone, and flavan-3-Ol. ACS Omega. 2021;6:8908–8918. doi: 10.1021/acsomega.0c06156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueiredo P.L.B., Silva S.G., Nascimento L.D., Ramos A.R., Setzer W.N., Silva J.K.R.D., Andrade E.H.A. Seasonal study of methyleugenol chemotype of ocimum campechianum essential oil and its fungicidal and antioxidant activities. NPC Nat. Prod. Commun. 2018;13:1055–1058. doi: 10.1177/1934578X1801300833. [DOI] [Google Scholar]
- 28.Mesquita K.D.S.M., Feitosa B.D.S., Cruz J.N., Ferreira O.O., Franco C.D.J.P., Cascaes M.M., Oliveira M.S.D., Andrade E.H.D.A. Chemical composition and preliminary toxicity evaluation of the essential oil from peperomia circinnata link var. circinnata. (piperaceae) in artemia salina leach. Molecules. 2021;26:7359. doi: 10.3390/molecules26237359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadeem H.R., Akhtar S., Sestili P., Ismail T., Neugart S., Qamar M., Esatbeyoglu T. Toxicity, antioxidant activity, and phytochemicals of basil (Ocimum basilicum L.) leaves cultivated in southern punjab, pakistan. Foods. 2022;11:1239. doi: 10.3390/foods11091239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farias A.L.F., Rodrigues A.B.L., Martins R.L., Rabelo É.D.M., Farias C.W.F., de Almeida S.S.M.D.S. Chemical characterization, antioxidant, cytotoxic and microbiological activities of the essential oil of leaf of tithonia diversifolia (hemsl) A. gray (asteraceae) Pharmaceuticals. 2019;12:34. doi: 10.3390/ph12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betim F.C.M., Oliveira C.F.D., Souza A.M.D., Szabo E.M., Zanin S.M.W., Miguel O.G., Miguel M.D., Dias J.D.F.G. Ocotea nutans (nees) mez (lauraceae): Chemical composition, antioxidant capacity and biological properties of essential oil. Brazilian J. Pharm. Sci. 2019;55:1–10. doi: 10.1590/s2175-97902019000118284. [DOI] [Google Scholar]
- 32.Cossolin J.F.S., Pereira M.J.B., Martínez L.C., Turchen L.M., Fiaz M., Bozdoğan H., Serrão J.E. Cytotoxicity of piper aduncum (piperaceae) essential oil in brown stink bug euschistus heros (heteroptera: Pentatomidae) Ecotoxicology. 2019;28:763–770. doi: 10.1007/s10646-019-02072-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee M.Y. Essential oils as repellents against arthropods. Biomol. Res. Int. 2018;2018:6860271. doi: 10.1155/2018/6860271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tietbohl L.A.C., Oliveira A.P., Esteves R.S. Antiproliferative activity in tumor cell lines, antioxidant capacity and total phenolic, flavonoid and tannin contents of Myrciaria floribunda. An. Acad. Bras. Ciências. 2017;89:1111–1120. doi: 10.1590/0001-3765201720160461. [DOI] [PubMed] [Google Scholar]
- 35.de Azevedo M.M.L., Cascaes M.M., Guilhon G.M.S.P., Andrade E.H.A., Zoghbi M.D.G.B., da Silva J.K.R., Santos L.S., da Silva S.H.M. Lupane triterpenoids, antioxidant potential and antimicrobial activity of Myrciaria floribunda (H. West ex Willd.) O. Berg. Nat. Prod. Res. 2019;33:506–515. doi: 10.1080/14786419.2017.1402311. [DOI] [PubMed] [Google Scholar]
- 36.García Y.M., Ramos A.L.C.C., de Paula A.C.C.F.F., Nascimento M.H.D., Augusti R., de Araújo R.L.B., de Lemos E.E.P., Melo J.O.F. Chemical Physical characterization and profile of fruit volatile compounds from different accesses of Myrciaria floribunda (H. West Ex Wild.) O. Berg through polyacrylate fiber. Molecules. 2021;26:5281. doi: 10.3390/molecules26175281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerônimo L.B., da Costa J.S., Pinto L.C., Montenegro R.C., Setzer W.N., Mourão R.H.V., da Silva J.K.R., Maia J.G.S., Figueiredo P.L.B. Antioxidant and cytotoxic activities of myrtaceae essential oils rich in terpenoids from brazil. Nat. Prod. Commun. 2021;16:1934578X21996156. doi: 10.1177/1934578X21996156. [DOI] [Google Scholar]
- 38.Silva F.K.S., Rosário A.S., Secco R.S., Zoghbi M.G.B. Levantamento das espécies conhecidas como pedra-ume-caá (myrtaceae), com ênfase nas comercializadas na cidade de belém, pará, brasil. Biota Amaz. 2015;5:7–15. doi: 10.18561/2179-5746/biotaamazonia.v5n1p7-15. [DOI] [Google Scholar]
- 39.Oliveira E.S.C., Pontes F.L.D., Acho L.D.R., do Rosário A.S., da Silva B.J.P., Bezerra J.D.A., Campos F.R., Lima E.S., Machado M.B. QNMR quantification of phenolic compounds in dry extract of myrcia multiflora leaves and its antioxidant, anti-age, and enzymatic inhibition activities. J. Pharm. Biomed. Anal. 2021;201:114109. doi: 10.1016/j.jpba.2021.114109. [DOI] [PubMed] [Google Scholar]
- 40.Santos A.S., Alves S.D.M., Figueiredo F.J.C., Neto O.G.D.R. Descrição de Sistema e de Métodos de Extração de Óleos Essenciais e Determinação de Umidade de Biomassa em Laboratório. Ministério da Agricultura, Pecuária e Abastecimento; Belo Horizonte, Brazil: 2004. pp. 1–6. [Google Scholar]
- 41.Adams R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. pp. 804–806. [Google Scholar]
- 42.Góes D.F., Araújo J.M., Oliveira N.R.D.S., Lima R.Q.D. Atividade toxica do óleo essencial de piper duckei (piperaceae) sobre microcrustáceo artemia salina / toxic activity of essential oil of piper duckei (piperaceae) on microcrustacean artemia salina. Braz. J. Dev. 2020;6:96278–96284. doi: 10.34117/bjdv6n12-207. [DOI] [Google Scholar]
- 43.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 44.BLOIS M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 45.Tietbohl L.A.C., Mello C.B., Silva L.R., Dolabella I.B., Franco T.C., Enríquez J.J.S., Santos M.G., Fernandes C.P., Machado F.P., Mexas R., et al. Green insecticide against chagas disease: Effects of essential oil from Myrciaria floribunda (myrtaceae) on the development of Rhodnius prolixus nymphs. J. Essent. Oil Res. 2020;32:1–11. doi: 10.1080/10412905.2019.1631894. [DOI] [Google Scholar]
- 46.Raposo J.D.A., Figueiredo P.L.B., Santana R.L., da Silva Junior A.Q., Suemitsu C., da Silva R., Mourão R.H.V., Maia J.G.S. Seasonal and circadian study of the essential oil of myrcia sylvatica (g. mey) dc., a valuable aromatic species occurring in the lower amazon river region. Biochem. Syst. Ecol. 2018;79:21–29. doi: 10.1016/j.bse.2018.04.017. [DOI] [Google Scholar]
- 47.Barbosa D.C., Holandada Silva V.N., de Assis C.R.D., de Oliveira Farias de Aguiar J.C.R., DoNascimento P.H., da Silva W.V., do Amaral Ferraz Navarro D.M., Silva M.V.D., de Menezes Lima V.L., dos Santos Correia M.T. Chemical composition and acetylcholinesterase inhibitory potential, in silico, of Myrciaria floribunda (H. West Ex Willd.) O. Berg fruit peel essential oil. Ind. Crops Prod. 2020;151:112372. doi: 10.1016/j.indcrop.2020.112372. [DOI] [Google Scholar]
- 48.Tietbohl L.A.C., Barbosa T., Fernandes C.P., Santos M.G., Machado F.P., Santos K.T., Mello C.B., Araújo H.P., Gonzalez M.S., Feder D., et al. Laboratory evaluation of the effects of essential oil of myrciaria floribunda leaves on the development of dysdercus peruvianus and oncopeltus fasciatus. Rev. Bras. Farmacogn. 2014;24:316–321. doi: 10.1016/j.bjp.2014.07.009. [DOI] [Google Scholar]
- 49.de Oliveira L.M., Porte A., de Oliveira Godoy R.L., da Costa Souza M., Pacheco S., de Araujo Santiago M.C.P., Gouvêa A.C.M.S., da Silva de Mattos do Nascimento L., Borguini R.G. Chemical characterization of Myrciaria floribunda (H. West Ex willd) fruit. Food Chem. 2018;248:247–252. doi: 10.1016/j.foodchem.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira O.O., Neves da Cruz J., de Jesus Pereira Franco C., Silva S.G., da Costa W.A., de Oliveira M.S., de Aguiar Andrade E.H. First report on yield and chemical composition of essential oil extracted from myrcia eximia dc (myrtaceae) from the brazilian amazon. Molecules. 2020;25:783. doi: 10.3390/molecules25040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boukhatem M.N., Sudha T., Darwish N.H.E., Chader H., Belkadi A., Rajabi M., Houche A., Benkebailli F., Oudjida F., Mousa S.A. A New Eucalyptol-rich lavender (Lavandula stoechas L.) essential oil: Emerging potential for therapy against inflammation and cancer. Molecules. 2020;25:3671. doi: 10.3390/molecules25163671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov M., Kannan A., Stojković D.S., Glamočlija J., Calhelha R.C., Ferreira I.C.F.R., Sanglard D., Soković M. Camphor and eucalyptol—anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021;22:483. doi: 10.3390/ijms22020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saracino I.M., Foschi C., Pavoni M., Spigarelli R., Valerii M.C., Spisni E. Antifungal activity of natural compounds vs. candida spp.: A mixture of cinnamaldehyde and eugenol shows promising in vitro results. Antibiotics. 2022;11:73. doi: 10.3390/antibiotics11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra P., Gupta P., Srivastava A.K., Poluri K.M., Prasad R. Eucalyptol/β-cyclodextrin inclusion complex loaded gellan/pva nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021;609:121163. doi: 10.1016/j.ijpharm.2021.121163. [DOI] [PubMed] [Google Scholar]
- 55.Li X.W., Zhang Z.J., Hafeez M., Huang J., Zhang J.M., Wang L.K., Lu Y. Bin rosmarinus officinialis L. (lamiales: Lamiaceae), a promising repellent plant for thrips management. J. Econ. Entomol. 2021;114:131–141. doi: 10.1093/jee/toaa288. [DOI] [PubMed] [Google Scholar]
- 56.Moo C.L., Osman M.A., Yang S.K., Yap W.S., Ismail S., Lim S.H.E., Chong C.M., Lai K.S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing klebsiella pneumoniae. Sci. Rep. 2021;11:20824. doi: 10.1038/s41598-021-00249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes E.I.M., Farias E.S., Silva E.M.P., Filomeno C.A., Plata M.A.B., Picanço M.C., Barbosa L.C.A. Eucalyptus resinifera essential oils have fumigant and repellent action against hypothenemus hampei. Crop Prot. 2019;116:49–55. doi: 10.1016/j.cropro.2018.09.018. [DOI] [Google Scholar]
- 58.Rodenak-Kladniew B., Castro M.A., Crespo R., Galle M., García de Bravo M. Anti-cancer mechanisms of linalool and 1,8-cineole in non-small cell lung cancer A549 cells. Heliyon. 2020;6:e05639. doi: 10.1016/j.heliyon.2020.e05639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampath S., Veeramani V., Krishnakumar G.S., Sivalingam U., Madurai S.L., Chellan R. Evaluation of in vitro anticancer activity of 1,8-cineole–containing n-hexane extract of callistemon citrinus (curtis) skeels plant and its apoptotic potential. Biomed. Pharmacother. 2017;93:296–307. doi: 10.1016/j.biopha.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 60.Sheikh Z., Amani A., Basseri H.R., Kazemi S.H.M., Sedaghat M.M., Azam K., Azizi M., Amirmohammadi F. Repellent Efficacy of eucalyptus globulus and syzygium aromaticum essential oils against malaria vector, anopheles stephensi (diptera: Culicidae) Iran. J. Public Health. 2021;50:1668–1677. doi: 10.18502/ijph.v50i8.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panikar S., Shoba G., Arun M., Sahayarayan J.J., Usha Raja Nanthini A., Chinnathambi A., Alharbi S.A., Nasif O., Kim H.J. Essential oils as an effective alternative for the treatment of covid-19: Molecular interaction analysis of protease (mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health. 2021;14:601–610. doi: 10.1016/j.jiph.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valussi M., Antonelli M., Donelli D., Firenzuoli F. Appropriate use of essential oils and their components in the management of upper respiratory tract symptoms in patients with COVID-19. J. Herb. Med. 2021;28:100451. doi: 10.1016/j.hermed.2021.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma A.D., Kaur I. Eucalyptol (1,8 cineole) from eucalyptus essential oil a potential inhibitor of COVID-19 corona virus infection by molecular docking studies. Preprints. 2020;3:55. doi: 10.20944/preprints202003.0455.v1. [DOI] [Google Scholar]
- 64.Foudah A.I., Alam P., Alam A., Salkini M.A., Alqarni M.H., Yusufoglu H.S. Development of high-performance thin-layer chromatography (hptlc) validated method for simultaneous quantification of eucalyptol and α-pinene in lamiaceae plants. J. Pharm. Res. Int. 2019;31:1–11. doi: 10.9734/jpri/2019/v31i630354. [DOI] [Google Scholar]
- 65.Griggs J., Almohanna H., Ahmed A., Ren S., Tosti A. “Fresh Breath” on toothpaste: Peppermint as cause of cheilitis. Dermatitis. 2019;30:74–75. doi: 10.1097/DER.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 66.Hodgson A., Cochran J. Vacuum ultraviolet spectroscopy as a new tool for gc analysis of terpenes in flavors and fragrances. J. AOAC Int. 2019;102:655–658. doi: 10.5740/jaoacint.18-0284. [DOI] [PubMed] [Google Scholar]
- 67.Gogoi R., Begum T., Sarma N., Kumar Pandey S., Lal M. Chemical composition of callistemon citrinus (curtis) skeels aerial part essential oil and its pharmacological applications, neurodegenerative inhibitory, and genotoxic efficiencies. J. Food Biochem. 2021;45:e13767. doi: 10.1111/jfbc.13767. [DOI] [PubMed] [Google Scholar]
- 68.Campos J.F., Ferreira V., Berteina-Raboin S. Eucalyptol: A bio-based solvent for the synthesis of o,s,n-heterocycles. application to hiyama coupling, cyanation, and multicomponent reactions. Catalysts. 2021;11:222. doi: 10.3390/catal11020222. [DOI] [Google Scholar]
- 69.Campos J.F., Berteina-Raboin S. Eucalyptol, an all-purpose product. Catalysts. 2022;12:48. doi: 10.3390/catal12010048. [DOI] [Google Scholar]
- 70.Nabah R., Lgaz H., Zarrok H., Larouj M., Benhiba F., Ourrak K., Cherkaoui M., Zarrouk A., Touir R., Oudda H. Anti-Corrosion properties of niaouli essential oil for tinplate in 3 %NaCl medium. J. Mater. Environ. Sci. 2017;8:3730–3739. [Google Scholar]
- 71.Menezes I.O., Scherf J.R., Martins A.O.B.P.B., Ramos A.G.B., Quintans J.D.S.S., Coutinho H.D.M., Ribeiro-Filho J., de Menezes I.R.A. Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: A systematic review. Phytomedicine. 2021;93:153768. doi: 10.1016/j.phymed.2021.153768. [DOI] [PubMed] [Google Scholar]
- 72.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against culex quinquefasciatus say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 73.Pontin M., Bottini R., Burba J.L., Piccoli P. Allium sativum produces terpenes with fungistatic properties in response to infection with sclerotium cepivorum. Phytochemistry. 2015;115:152–160. doi: 10.1016/j.phytochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Mukherjee A., Ahn Y.H. Terpinolene as an enhancer for ultrasonic disinfection of multi-drug-resistant bacteria in hospital wastewater. Environ. Sci. Pollut. Res. 2022;29:34500–34514. doi: 10.1007/s11356-022-18611-6. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J.H., Sun H.L., Chen S.Y., Zeng L.I., Wang T. tao Anti-fungal activity, mechanism studies on α-phellandrene and nonanal against penicillium cyclopium. Bot. Stud. 2017;58:13. doi: 10.1186/s40529-017-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soba S.V., Babu M., Panonnummal R. Ethosomal Gel formulation of alpha phellandrene for the transdermal delivery in gout. Adv. Pharm. Bull. 2021;11:137–149. doi: 10.34172/apb.2021.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavanji S., Sayedipour S.S., Larki B., Bakhtari A. Antiviral activity of some plant oils against herpes simplex virus type 1 in vero cell culture. J. Acute Med. 2015;5:62–68. doi: 10.1016/j.jacme.2015.07.001. [DOI] [Google Scholar]
- 78.Marchese A., Arciola C., Barbieri R., Silva A., Nabavi S., Tsetegho Sokeng A., Izadi M., Jafari N., Suntar I., Daglia M., et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials. 2017;10:947. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garzoli S., Božović M., Baldisserotto A., Sabatino M., Cesa S., Pepi F., Vicentini C.B., Manfredini S., Ragno R. Essential oil extraction, chemical analysis and anti-candida activity of foeniculum vulgare miller–new approaches. Nat. Prod. Res. 2018;32:1254–1259. doi: 10.1080/14786419.2017.1340291. [DOI] [PubMed] [Google Scholar]
- 80.Reis J.B., Figueiredo L.A., Castorani G.M., Veiga S.M.O.M. Avaliação da atividade antimicrobiana dos óleos essenciais contra patógenos alimentares. Braz. J. Health Rev. 2020;3:342–363. doi: 10.34119/bjhrv3n1-025. [DOI] [Google Scholar]
- 81.Saccol E.M.H., Jerez-Cepa I., Ourique G.M., Pês T.S., Gressler L.T., Mourão R.H.V., Martínez-Rodríguez G., Mancera J.M., Baldisserotto B., Pavanato M.A., et al. Myrcia sylvatica essential oil mitigates molecular, biochemical and physiological alterations in rhamdia quelen under different stress events associated to transport. Res. Vet. Sci. 2018;117:150–160. doi: 10.1016/j.rvsc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Rosa C.S., Veras K.S., Silva P.R., Lopes Neto J.J., Cardoso H.L.M., Alves L.P.L., Brito M.C.A., Amaral F.M.M., Maia J.G.S., Monteiro O.S., et al. Composição química e toxicidade frente Aedes aegypti L. e Artemia salina Leach do óleo essencial das folhas de Myrcia sylvatica (G. Mey.) DC. Rev. Bras. Plantas Med. 2016;18:19–26. doi: 10.1590/1983-084X/15_006. [DOI] [Google Scholar]
- 83.Saccol E.M.H., Parrado-Sanabria Y.A., Gagliardi L., Jerez-Cepa I., Mourão R.H.V., Heinzmann B.M., Baldisserotto B., Pavanato M.A., Mancera J.M., Martos-Sitcha J.A. Myrcia sylvatica essential oil in the diet of gilthead sea bream (sparus aurata L.) attenuates the stress response induced by high stocking density. Aquac. Nutr. 2018;24:1381–1392. doi: 10.1111/anu.12675. [DOI] [Google Scholar]
- 84.Saccol E.M.H., Toni C., Pês T.S., Ourique G.M., Gressler L.T., Silva L.V.F., Mourão R.H.V., Oliveira R.B., Baldisserotto B., Pavanato M.A. Anaesthetic and Antioxidant effects of Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. essential oils on tambaqui (Colossoma macropomum) Aquac. Res. 2017;48:2012–2031. doi: 10.1111/are.13034. [DOI] [Google Scholar]
- 85.Cascaes M.M., Guilhon G.M.S.P., de Aguiar Andrade E.H., das Graças Bichara Zoghbi M., da Silva Santos L. Constituents and pharmacological activities of Myrcia (Myrtaceae): A review of an aromatic and medicinal group of plants. Int. J. Mol. Sci. 2015;16:23881–23904. doi: 10.3390/ijms161023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashrafi B., Beyranvand F., Ashouri F., Rashidipour M., Marzban A., Kheirandish F., Veiskarami S., Ramak P., Shahrokhi S. Characterization of phytochemical composition and bioactivity assessment of pseudotrachydium kotschyi essential oils. Med. Chem. Res. 2020;29:1676–1688. doi: 10.1007/s00044-020-02594-5. [DOI] [Google Scholar]
- 87.Powers C.N., Osier J.L., McFeeters R.L., Brazell C.B., Olsen E.L., Moriarity D.M., Satyal P., Setzer W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules. 2018;23:1549. doi: 10.3390/molecules23071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turgumbayeva A., Ustenova G., Datkhayev U., Rahimov K., Abramavicius S., Tunaityte A., Zhakipbekov K., Kozhanova K., Tulemissov S., Ustenova O., et al. Safflower (Carthamus Tinctorius L.) a potential source of drugs against Cryptococcal infections, malaria and Leishmaniasis. Phyton. 2020;89:137–146. doi: 10.32604/phyton.2020.07665. [DOI] [Google Scholar]
- 89.Lasekan O., Yap S.P. Characterization of the aroma compounds in fresh and dried sapodilla (Manikara Zapota, L.) by the application of aroma extract dilution analysis. CYTA—J. Food. 2018;16:801–806. doi: 10.1080/19476337.2018.1485748. [DOI] [Google Scholar]
- 90.Silvestre W.P., Agostini F., Muniz L.A.R., Pauletti G.F. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016;178:90–94. doi: 10.1016/j.jfoodeng.2016.01.011. [DOI] [Google Scholar]
- 91.Yi F., Jin R., Sun J., Ma B., Bao X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT. 2018;95:346–353. doi: 10.1016/j.lwt.2018.05.011. [DOI] [Google Scholar]
- 92.Lancaster J., Lehner B., Khrimian A., Muchlinski A., Luck K., Köllner T.G., Weber D.C., Gundersen-Rindal D.E., Tholl D. An IDS-type sesquiterpene synthase produces the pheromone precursor (Z)-α-Bisabolene in Nezara viridula. J. Chem. Ecol. 2019;45:187–197. doi: 10.1007/s10886-018-1019-0. [DOI] [PubMed] [Google Scholar]
- 93.Moraes M.C.B., Pareja M., Laumann R.A., Borges M. The chemical volatiles (semiochemicals) produced by neotropical stink bugs (Hemiptera: Pentatomidae) Neotrop. Entomol. 2008;37:489–505. doi: 10.1590/S1519-566X2008000500001. [DOI] [PubMed] [Google Scholar]
- 94.Cribb B.W., Siriwardana K.N., Walter G.H. Unicellular pheromone glands of the pentatomid bugnezara viridula (heteroptera: Insecta): Ultrastructure, classification, and proposed function. J. Morphol. 2006;267:831–840. doi: 10.1002/jmor.10442. [DOI] [PubMed] [Google Scholar]
- 95.Chanda S., Baravalia Y. Brine shrimp cytotoxicity of caesalpinia pulcherrima aerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat. Prod. Res. 2011;25:1955–1964. doi: 10.1080/14786419.2010.530600. [DOI] [PubMed] [Google Scholar]
- 96.Ramos S.C.S., de Oliveira J.C.S., da Câmara C.A.G., Castelar I., Carvalho A.F.F.U., Lima-Filho J.V. Antibacterial and cytotoxic properties of some plant crude extracts used in northeastern folk medicine. Rev. Bras. Farmacogn. 2009;19:376–381. doi: 10.1590/S0102-695X2009000300007. [DOI] [Google Scholar]
- 97.Ribeiro I.A.T.A., Sá J.L.F., Lima M.V., Veras S.T.S., Aguiar J.C.R.O.F., Aires A.L., Albuquerque M.C.P.A., da Silva M.V., Melo A.M.M.A., Navarro D.M.A.F., et al. Toxic effect of croton rudolphianus leaf essential oil against biomphalaria glabrata, schistosoma mansoni cercariae and artemia salina. Acta Trop. 2021;223:106102. doi: 10.1016/j.actatropica.2021.106102. [DOI] [PubMed] [Google Scholar]
- 98.Santana de Oliveira M., Pereira da Silva V.M., Cantão Freitas L., Gomes Silva S., Nevez Cruz J., Aguiar Andrade E.H. Extraction yield, chemical composition, preliminary toxicity of bignonia nocturna (bignoniaceae) essential oil and in silico evaluation of the interaction. Chem. Biodivers. 2021;18:cbdv.202000982. doi: 10.1002/cbdv.202000982. [DOI] [PubMed] [Google Scholar]
- 99.do Nascimento J.C., David J.M., Barbosa L.C., de Paula V.F., Demuner A.J., David J.P., Conserva L.M., Ferreira J.C., Guimarães E.F. Larvicidal activities and chemical composition of essential oils from Piper klotzschianum (Kunth) C. DC.(Piperaceae) Pest Manag. Sci. 2013;69:1267–1271. doi: 10.1002/ps.3495. [DOI] [PubMed] [Google Scholar]
- 100.Barbosa D.C.D.S., Holanda V.N., Ghosh A., Maia R.T., da Silva W.V., Lima V.L.D.M., da Silva M.V., dos Santos Correia M.T., de Figueiredo R.C.B.Q. Leishmanicidal and cytotoxic activity of essential oil from the fruit peel of Myrciaria floribunda (H. West ex Willd.) O. Berg: Molecular docking and molecular dynamics simulations of its major constituent onto Leishmania enzyme targets. J. Biomol. Struct. Dyn. 2021;39:1–16. doi: 10.1080/07391102.2021.1978320. [DOI] [PubMed] [Google Scholar]
- 101.Caldas G.F.R., Limeira M.M.F., Araújo A.V., Albuquerque G.S., Silva-Neto J.D.C., Silva T.G.D., Costa-Silva J.H., Menezes I.R.A.D., Costa J.G.M.D., Wanderley A.G. Repeated-doses and reproductive toxicity studies of the monoterpene 1, 8-cineole (eucalyptol) in Wistar rats. Food Chem. Toxicol. 2016;97:297–306. doi: 10.1016/j.fct.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 102.Izham M.N.M., Hussin Y., Rahim N.F.C., Aziz M.N.M., Yeap S.K., Rahman H.S., Masarudin M.J., Mohamad N.E., Abdullah R., Alitheen N.B. Physicochemical characterization, cytotoxic effect and toxicity evaluation of nanostructured lipid carrier loaded with eucalyptol. BMC Complement. Med. Ther. 2021;21:254. doi: 10.1186/s12906-021-03422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elmhalli F., Garboui S.S., Borg-Karlson A.K., Mozūraitis R., Baldauf S.L., Grandi G. The repellency and toxicity effects of essential oils from the libyan plants salvadora persica and rosmarinus officinalis against nymphs of ixodes ricinus. Exp. Appl. Acarol. 2019;77:585–599. doi: 10.1007/s10493-019-00373-5. [DOI] [PubMed] [Google Scholar]
- 104.Bhowal M., Gopal M. Eucalyptol: Safety and pharmacological profile. RGUHS J. Pharm Sci. 2016;5:125–131. doi: 10.5530/rjps.2015.4.2. [DOI] [Google Scholar]
- 105.Galan D.M., Ezeudu N.E., Garcia J., Geronimo C.A., Berry N.M., Malcolm B.J. Eucalyptol (1, 8-cineole): An underutilized ally in respiratory disorders? J. Essent. Oil Res. 2020;32:103–110. doi: 10.1080/10412905.2020.1716867. [DOI] [Google Scholar]
- 106.Ribeiro N.C., da Camara C.A.G., Melo J.P.R., de Moraes M.M. Acaricidal properties of essential oils from agro-industrial waste products from citric fruit against tetranychus urticae. J. Appl. Entomol. 2019;143:731–743. doi: 10.1111/jen.12642. [DOI] [Google Scholar]
- 107.Agus H.H. Terpene toxicity and oxidative stress. In: Patel V.B., Preedy V.R., editors. Toxicology: Oxidative Stress and Dietary Antioxidants. Academic Press; Cambridge, UK: 2021. pp. 33–42. [Google Scholar]
- 108.Scherf J.R., Barbosa dos Santos C.R., Sampaio de Freitas T., Rocha J.E., Macêdo N.S., Mascarenhas Lima J.N., Melo Coutinho H.D., Bezerra da Cunha F.A. Effect of terpinolene against the resistant Staphylococcus aureus strain, carrier of the efflux pump QacC and β-lactamase gene, and its toxicity in the Drosophila melanogaster model. Microb. Pathog. 2020;149:104528. doi: 10.1016/j.micpath.2020.104528. [DOI] [PubMed] [Google Scholar]
- 109.de Christo Scherer M.M., Marques F.M., Figueira M.M., Peisino M.C.O., Schmitt E.F.P., Kondratyuk T.P., Endringer D.C., Scherer R., Fronza M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability. 2019;28:94–99. doi: 10.1016/j.jtv.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 110.Martínez L.C., Plata-Rueda A., Colares H.C., Campos J.M., Dos Santos M.H., Fernandes F.L., Serrão J.E., Zanuncio J.C. Toxic effects of two essential oils and their constituents on the mealworm beetle, tenebrio molitor. Bull. Entomol. Res. 2018;108:716–725. doi: 10.1017/S0007485317001262. [DOI] [PubMed] [Google Scholar]
- 111.Abdelgaleil S.A.M., El-Sabrout A.M. Composition, toxicity and developmental potential of three essential oils on the west nile virus mosquito, Culex pipiens L. Int. J. Pest Manag. 2020;66:1–9. doi: 10.1080/09670874.2020.1864508. [DOI] [Google Scholar]
- 112.Mubarak E.E., Ali L.Z., Ahmed I.F.A., Ahmed A.B.A., Taha R.M. Essential oil compositions and cytotoxicity from various organs of eucalyptus camaldulensis. Int. J. Agric. Biol. 2015;17:320–326. [Google Scholar]
- 113.Andrade-Ochoa S., Sánchez-Aldana D., Chacón-Vargas K.F., Rivera-Chavira B.E., Sánchez-Torres L.E., Camacho A.D., Nogueda-Torres B., Nevárez-Moorillón G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–antagonism effects. Insects. 2018;9:25. doi: 10.3390/insects9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kwiatkowski P., Łopusiewicz Ł., Pruss A., Kostek M., Sienkiewicz M., Bonikowski R., Wojciechowska-Koszko I., Dołęgowska B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against mrsa strains. Int. J. Mol. Sci. 2020;21:7106. doi: 10.3390/ijms21197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng Y.X., Wang Y., Geng Z.F., Zhang D., Almaz B., Du S.S. Contact toxicity and repellent efficacy of valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 2020;190:110106. doi: 10.1016/j.ecoenv.2019.110106. [DOI] [PubMed] [Google Scholar]
- 116.Cascaes M.M., Silva S.G., Cruz J.N., Oliveira S.D., Oliveira J., Antonio A., Moraes B.D., Augusto F., Santana K., Diniz L., et al. First report on the annona exsucca dc. essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021;36:4009–4012. doi: 10.1080/14786419.2021.1893724. [DOI] [PubMed] [Google Scholar]
- 117.Souza da Silva Júnior O., de Jesus Pereira Franco C., Barbosa de Moraes A.A., Cruz J.N., Santana da Costa K., Diniz do Nascimento L., Helena de Aguiar Andrade E. In silico analyses of toxicity of the major constituents of essential oils from two Ipomoea L. species. Toxicon. 2021;195:111–118. doi: 10.1016/j.toxicon.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 118.Da Silva Júnior O.S., Franco C.D.J.P., Moraes Â.A.B.D., Pastore M., Cascaes M.M., do Nascimento L.D., Oliveira M.S.D., Andrade E.H.D.A. Chemical variability of volatile concentrate from two Ipomoea L. species within a seasonal gradient. Nat. Prod. Res. 2022;36:1–8. doi: 10.1080/14786419.2022.2070618. [DOI] [PubMed] [Google Scholar]
- 119.Brari J., Thakur D.R. Fumigant toxicity and cytotoxicity evaluation of monoterpenes against four stored products pests. Int. J. Dev. Res. 2015;5:5661–5667. [Google Scholar]
- 120.Santos P.L., Matos J.P.S.C.F., Picot L., Almeida J.R.G.S., Quintans J.S.S., Quintans-Júnior L.J. Citronellol, A Monoterpene Alcohol with Promising Pharmacological Activities—A Systematic Review. Volume 123. Elsevier Ltd.; Amsterdam, The Netherlands: 2019. [DOI] [PubMed] [Google Scholar]
- 121.Wojtunik-Kulesza K.A. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules. 2022;27:1716. doi: 10.3390/molecules27051716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amina M., Alam P., Parvez M.K., Al-Musayeib N.M., Al-Hwaity S.A., Al-Rashidi N.S., Al-Dosari M.S. Isolation and validated hptlc analysis of four cytotoxic compounds, including a new sesquiterpene from aerial parts of plectranthus cylindraceus. Nat. Prod. Res. 2018;32:804–809. doi: 10.1080/14786419.2017.1363750. [DOI] [PubMed] [Google Scholar]
- 123.Pang X., Almaz B., Qi X.J., Wang Y., Feng Y.X., Geng Z.F., Xi C., Du S.S. Bioactivity of essential oil from atalantia buxifolia leaves and its major sesquiterpenes against three stored-product insects. J. Essent. Oil-Bear. Plants. 2020;23:38–50. doi: 10.1080/0972060X.2020.1729245. [DOI] [Google Scholar]
- 124.Barreto I.C., de Almeida A.S., Sena Filho J.G. Taxonomic insights and its type cyclization correlation of volatile sesquiterpenes in vitex species and potential source insecticidal compounds: A review. Molecules. 2021;26:6405. doi: 10.3390/molecules26216405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hussain A., Rizwan-Ul-Haq M., AlJabr A.M., Al-Ayedh H. Lethality of sesquiterpenes reprogramming red palm weevil detoxification mechanism for natural novel biopesticide development. Molecules. 2019;24:1648. doi: 10.3390/molecules24091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang F., Park Y.L., Gutensohn M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry. 2020;180:112532. doi: 10.1016/j.phytochem.2020.112532. [DOI] [PubMed] [Google Scholar]
- 127.Scalvenzi L., Grandini A., Spagnoletti A., Tacchini M., Neill D., Ballesteros J., Sacchetti G., Guerrini A. Myrcia splendens (Sw.) DC.(syn. M. fallax (Rich.) DC.)(Myrtaceae) essential oil from Amazonian Ecuador: A chemical characterization and bioactivity profile. Molecules. 2017;22:1163. doi: 10.3390/molecules22071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melo C.R., Blank A.F., Oliveira B.M.S., Santos A.C.C., Cristaldo P.F., Araújo A.P.A., Bacci L. Formicidal activity of essential oils of myrcia lundiana chemotypes on acromyrmex balzani. Crop Prot. 2021;139:105343. doi: 10.1016/j.cropro.2020.105343. [DOI] [Google Scholar]
- 129.Alves M.S., Santos D.P., Silva L.C.P., Pontes E.G., Souza M.A.A. Essential oils composition and toxicity tested by fumigation against Callosobruchus maculatus (Coleoptera: Bruchidae) pest of stored cowpea. Rev. Virtual Quim. 2015;7:2387–2399. doi: 10.5935/1984-6835.20150142. [DOI] [Google Scholar]
- 130.Fernandes C.C., Andrade P.M.D., Santos T.C.L.D., Santiago M.B., Crotti A.E.M., Martins C.H.G., Magalhães L.G., Miranda M.L.D. In vitro evaluation of anticaries, antimycobacterial, antileishmanial and cytotoxic activities of essential oils from eremanthus erythropappus and of α-bisabolol, their major sesquiterpene. Aust. J. Crop Sci. 2020;14:236–243. doi: 10.21475/ajcs.20.14.02.p1876. [DOI] [Google Scholar]
- 131.Mahdavi V., Rafiee-Dastjerdi H., Asadi A., Razmjou J., Achachlouei B.F. Synthesis of Zingiber officinale essential oil-loaded nanofiber and its evaluation on the potato tuber moth, Phthorimaea operculella (Lepidoptera: Gelechiidae) J. Crop Prot. 2018;7:39–49. [Google Scholar]
- 132.Fernandez C.M.M., Lorenzetti F.B., Kleinubing S.A., de Andrade J.P.P., Bortolucci W.D.C., Gonçalves J.E., Júnior R.P., Cortez D.A.G., Gazim Z.C., Filho B.P.D. Chemical composition and insecticidal activity of Garcinia gardneriana (Planchon & Triana) Zappi (Clusiaceae) essential oil. Bol. Latinoam. y del Caribe de Plantas Med. y Aromát. 2021;20:503–514. doi: 10.37360/blacpma.21.20.5.37. [DOI] [Google Scholar]
- 133.Vinturelle R., Mattos C., Meloni J., Lamberti H.D., Nogueira J., da Silva Vaz Júnior I., Rocha L., Lione V., Folly E. Evaluation of essential oils as an ecological alternative in the search for control Rhipicephalus microplus (Acari: Ixodidae) Vet. Parasitol. Reg. Stud. Rep. 2021;23:100523. doi: 10.1016/j.vprsr.2020.100523. [DOI] [PubMed] [Google Scholar]
- 134.Matsuda S., Tsunematsu Y., Matsushita T., Ogata Y., Hachiya S., Kishimoto S., Miyoshi N., Watanabe K. Toward engineered biosynthesis of drugs in human cells. ChemBioChem. 2022;23:2022. doi: 10.1002/cbic.202100645. [DOI] [PubMed] [Google Scholar]
- 135.Ambrož M., Boušová I., Skarka A., Hanušová V., Králová V., Matoušková P., Szotáková B., Skálová L. The influence of sesquiterpenes from myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules. 2015;20:15343–15358. doi: 10.3390/molecules200815343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferreira O.O., Franco C.D.J.P., Varela E.L.P., Silva S.G., Cascaes M.M., Percário S., de Oliveira M.S., Andrade E.H.D.A. Chemical Composition and Antioxidant Activity of Essential Oils from Leaves of Two Specimens of Eugenia florida DC. Molecules. 2021;26:5848. doi: 10.3390/molecules26195848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ola O.S., Sofolahan T.A. A monoterpene antioxidant, linalool, mitigates benzene-induced oxidative toxicities on hematology and liver of male rats. Egypt. J. Basic Appl. Sci. 2021;8:39–53. doi: 10.1080/2314808X.2021.1898141. [DOI] [Google Scholar]
- 138.da Costa J.S., Andrade W.M.S., de Figueiredo R.O., Santos P.V.L., Freitas J.J.D.S., Setzer W.N., da Silva J.K.R., Maia J.G.S., Figueiredo P.L.B. Chemical composition and variability of the volatile components of myrciaria species growing in the amazon region. Molecules. 2022;27:2234. doi: 10.3390/molecules27072234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Plaza M., Batista Â.G., Cazarin C.B.B., Sandahl M., Turner C., Östman E., Maróstica Júnior M.R. Characterization of antioxidant polyphenols from myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- 140.Santos I.B.D.S., Santos dos Santos B., Oliveira J.R.S.D., Costa W.K., Zagmignan A., da Silva L.C.N., Ferreira M.R.A., Lermen V.L., Lermen M.S.B.D.S., da Silva A.G., et al. Antioxidant action and in vivo anti-inflammatory and antinociceptive activities of myrciaria floribunda fruit peels: Possible involvement of opioidergic system. Adv. Pharmacol. Pharm. Sci. 2020;2020:1258707. doi: 10.1155/2020/1258707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu G., Guo J., Sun C. Eucalyptol ameliorates early brain injury after subarachnoid haemorrhage via antioxidant and anti-inflammatory effects in a rat model. Pharm. Biol. 2021;59:114–120. doi: 10.1080/13880209.2021.1876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boukhatem M.N., Boumaiza A., Nada H.G., Rajabi M., Mousa S.A. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice) Appl. Sci. 2020;10:5581. doi: 10.3390/app10165581. [DOI] [Google Scholar]
- 143.Limam H., Ben Jemaa M., Tammar S., Ksibi N., Khammassi S., Jallouli S., Del Re G., Msaada K. Variation in chemical profile of leaves essential oils from thirteen tunisian eucalyptus species and evaluation of their antioxidant and antibacterial properties. Ind. Crops Prod. 2020;158:112964. doi: 10.1016/j.indcrop.2020.112964. [DOI] [Google Scholar]
- 144.Chambre D.R., Moisa C., Lupitu A., Copolovici L., Pop G., Copolovici D.M. Chemical composition, antioxidant capacity, and thermal behavior of satureja hortensis essential oil. Sci. Rep. 2020;10:21322. doi: 10.1038/s41598-020-78263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aydin E., Türkez H., Taşdemir Ş. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arch. Ind. Hyg. Toxicol. 2013;64:415–424. doi: 10.2478/10004-1254-64-2013-2365. [DOI] [PubMed] [Google Scholar]
- 146.Osanloo M., Ghanbariasad A., Taghinezhad A. Antioxidant and anticancer activities of Anethum graveolens L., Citrus limon (L.) Osbeck and Zingiber officinale Roscoe essential oils. Tradit. Integr. Med. 2021;6:333–347. doi: 10.18502/tim.v6i4.8266. [DOI] [Google Scholar]
- 147.De Castro J.A.M., Monteiro O.S., Coutinho D.F., Rodrigues A.A.C., Da Silva J.K.R., Maia J.G.S. Seasonal and circadian study of a thymol/γ-terpinene/p-cymene type oil of Ocimum gratissimum L. and its antioxidant and antifungal effects. J. Braz. Chem. Soc. 2018;30:930–938. doi: 10.21577/0103-5053.20180237. [DOI] [Google Scholar]
- 148.Julaeha E., Dewi K.S., Nurzaman M., Wahyudi T., Herlina T. Chemical compositions and antioxidant activities of indonesian citrus essential oils and their elucidation using principal component analysis. Preprints. 2020;11:86. doi: 10.20944/preprints202011.0586.v1. [DOI] [Google Scholar]
- 149.Xanthis V., Fitsiou E., Voulgaridou G.P., Bogadakis A., Chlichlia K., Galanis A., Pappa A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants. 2021;10:127. doi: 10.3390/antiox10010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aydin E., Türkez H., Geyikoğlu F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia. 2013;68:1004–1009. doi: 10.2478/s11756-013-0230-2. [DOI] [Google Scholar]
- 151.Türkez H., Aydın E. In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicol. Ind. Health. 2016;32:168–176. doi: 10.1177/0748233713498456. [DOI] [PubMed] [Google Scholar]
- 152.Ciesla L.M., Wojtunik-Kulesza K.A., Oniszczuk A., Waksmundzka-Hajnos M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method. Flavour Fragr. J. 2016;31:412–419. doi: 10.1002/ffj.3330. [DOI] [Google Scholar]
- 153.Olszowy-Tomczyk M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020;19:63–103. doi: 10.1007/s11101-019-09658-4. [DOI] [Google Scholar]
- 154.Franco C.D.J.P., Ferreira O.O., Antônio Barbosa de Moraes Â., Varela E.L.P., Nascimento L.D.D., Percário S., de Oliveira M.S., Andrade E.H.D.A. Chemical composition and antioxidant activity of essential oils from Eugenia patrisii vahl, E. punicifolia (Kunth) dc., and Myrcia tomentosa (Aubl.) dc., leaf of family Myrtaceae. Molecules. 2021;26:3292. doi: 10.3390/molecules26113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gatto L.J., Fabri N.T., Souza A.M.D., Fonseca N.S.T.D., Furusho A.D.S., Miguel O.G., Dias J.D.F.G., Zanin S.M.W., Miguel M.D. Chemical composition, phytotoxic potential, biological activities and antioxidant properties of myrcia hatschbachii D. legrand essential oil. Braz. J. Pharm. Sci. 2020;56:1–9. doi: 10.1590/s2175-97902019000318402. [DOI] [Google Scholar]
- 156.Mohankumar A., Kalaiselvi D., Levenson C., Shanmugam G., Thiruppathi G., Nivitha S., Sundararaj P. Antioxidant and stress modulatory efficacy of essential oil extracted from plantation-grown Santalum album L. Ind. Crops Prod. 2019;140:111623. doi: 10.1016/j.indcrop.2019.111623. [DOI] [Google Scholar]
- 157.Saroglou V., Arfan M., Shabir A., Hadjipavlou-Litina D., Skaltsa H. Composition and antioxidant activity of the essential oil of Teucrium royleanum Wall. ex Benth growing in Pakistan. Flavour Fragr. J. 2007;22:154–157. doi: 10.1002/ffj.1774. [DOI] [Google Scholar]
- 158.Iannone M., Ovidi E., Vitalini S., Laghezza Masci V., Iriti M., Tiezzi A., Garzoli S., Marianelli A. From hops to craft beers: Production process, vocs profile characterization, total polyphenol and flavonoid content determination and antioxidant activity evaluation. Processes. 2022;10:517. doi: 10.3390/pr10030517. [DOI] [Google Scholar]
- 159.Zárybnický T., Boušová I., Ambrož M., Skálová L. Hepatotoxicity of monoterpenes and sesquiterpenes. Arch. Toxicol. 2018;92:1–17. doi: 10.1007/s00204-017-2062-2. [DOI] [PubMed] [Google Scholar]
- 160.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.