Abstract
This investigation examined the effects of environmental alteration on the virulence of the oral treponemes Treponema denticola and Treponema pectinovorum. The environmental effects were assessed by using a model of localized inflammatory abscesses in mice. In vitro growth of T. denticola and T. pectinovorum as a function of modification of the cysteine concentration significantly enhanced abscess formation and size. In contrast, growth of T. denticola or T. pectinovorum under iron-limiting conditions (e.g., dipyridyl chelation) had no effect on abscess induction in comparison to that when the strains were grown under normal iron conditions. In vivo modulation of the microenvironment at the focus of infection with Cytodex beads demonstrated that increasing the local inflammation had no effect on lesion induction or size. In vivo studies involved the determination of the effects of increased systemic iron availability (e.g., iron dextran or phenylhydrazine) on the induction, kinetics, and size of lesions. T. denticola induced significantly larger lesions in mice with iron pretreatment and demonstrated systemic manifestations of the infectious challenge and an accompanying spreading lesion with phenylhydrazine pretreatment (e.g., increases in circulating free hemoglobin). In contrast, T. pectinovorum virulence was minimally affected by this in vivo treatment to increase iron availability. T. denticola virulence, as evaluated by lesion size, was increased additively by in vivo iron availability, and cysteine modified growth of the microorganism. Additionally, galactosamine sensitized mice to a lethal outcome following infection with both T. denticola and T. pectinovorum, suggesting an endotoxin-like activity in these treponemes. These findings demonstrated the ability to modify the virulence capacity of T. denticola and T. pectinovorum by environmental conditions which can be evaluated by using in vivo murine models.
Alterations in the growth environment of a bacterium, such as changes in carbon, carbohydrate, and nitrogen source, growth temperature, and iron concentration, have been shown to alter the expression of a variety of virulence factors (13, 15, 32, 48). These environmental alterations, or stress responses, also result from placing the bacterium in adverse growth environments, such as conditions of starvation, nutrient limitation, pH shift, heat shock, or change in oxygen tension. With few exceptions, all bacteria, whether living free or associated with a host, such as the periodontopathogenic bacteria, must be able to respond to these signals or cues. Adjustment to these environmental changes permits them to survive and multiply in this changing environment (11, 13, 34–36). Oral pathogens are an excellent example of bacteria that survive, grow, and multiply in a complex and rapidly changing environment. With potentially hundreds of different bacterial species occupying a periodontal niche, producing many metabolic end products and associated physicochemical changes, these bacteria are highly adapted for this niche. We have noted that hemin limitation increases the expression of new outer membrane proteins (2, 27) by Porphyromonas gingivalis. Additionally, various studies have shown that hemin limitation has a significant effect on P. gingivalis virulence in mice (22, 31, 33). Moreover, we have noted that environmental growth conditions alter the virulence potential (24) and in vitro S-layer expression of Campylobacter rectus (38).
Ultimately, to understand the disease caused by any pathogen, its virulence potential in an in vivo environment must be examined (20, 45). In vivo studies provide a means to address the role of the host and selected host-associated environmental factors on specific manifestations of disease progression. Recent work in our laboratory has shown that the murine abscess model is capable of establishing a role for the trypsin-like protease activity in P. gingivalis pathogenesis (25). Although we have some knowledge of the in vitro elaboration of putative treponeme virulence factors (i.e., hemolysin, trypsin-like enzyme, major outer sheath proteins, and lipopolysaccharide [LPS]-like molecules), we still have very little understanding of their functional role in vivo or regulation by environmental factors such as iron (28, 41, 52). The oral treponemes Treponema denticola, Treponema socranskii, Treponema pectinovorum, and Treponema vincentii have been implicated as etiological agents of severe periodontal disease in adults (19, 30, 37, 44, 46). Among the oral treponemes, T. denticola is the predominant one identified within the gingival crevice and subgingival ecology of the developing periodontal pocket and has been the most extensively studied. In vitro studies have revealed that oral treponemes elaborate a variety of proteolytic enzymes, hemolysins, esterases, collagenase, fibrinolytic enzymes, iminopeptidases, phospholipase C, hyaluronic acid, and chondroitin sulfate-degrading enzymes. The functional significance of these virulence factors has not been clearly elucidated in vivo. There are no data documenting the role of iron as a modulator of oral treponeme virulence. The murine abscess model provides the wherewithal to study the role of putative virulence factors of the oral treponemes in tissue destruction. Previously, we employed this model to explore variations in virulence among the oral treponemes, as well as the contribution of a trypsin-like protease to the virulence of T. denticola and T. pectinovorum strains (26).
The objectives of this study were to determine the role of iron, cysteine (as a sulfhydryl reducing agent), local inflammation, and endotoxin in the pathogenesis of T. denticola and T. pectinovorum in a murine abscess model. The hypotheses tested included the following. (i) Alterations in iron availability, in vitro or in vivo, modify the virulence of T. denticola and T. pectinovorum. (ii) Cysteine, which significantly enhances in vitro growth of the treponemes, increases the virulence of the species. (iii) Existing localized inflammation enhances treponeme virulence. (iv) Both T. denticola and T. pectinovorum demonstrate toxic activities in mice which had been sensitized for evaluation of endotoxin-induced lethality.
MATERIALS AND METHODS
Bacterial strains.
The Treponema spp. used and their sources are given in Table 1. T. denticola strains were grown in GM-1 broth (1) in a Coy anaerobic chamber in an atmosphere of 85% N2, 5% CO2, and 10% H2 for 72 h at 37°C. T. pectinovorum strains were grown in GM-1 broth supplemented with 0.3% pectin (Sigma) for 24 to 72 h (49, 50). All manipulations were carried out under anaerobic conditions to ensure maximum cell viability. Culture purity was determined by dark-field and phase-contrast microscopy. Viability was estimated by the degree of motility and the presence or absence of spherical bodies of the treponemes. Growth in liquid media was estimated at an optical density of 660 nm. Log-phase cultures were harvested by centrifugation (9,000 × g for 10 min), and pellets were resuspended in the same medium under anaerobic conditions. An aliquot of the culture was removed from the chamber, and 10-fold dilutions were made for estimating total counts with a Petroff-Hausser bacterial counting chamber. After allowing sufficient time for treponemes to settle in the counting chamber, the cells were enumerated, with spherical bodies excluded.
TABLE 1.
Species and strains of oral treponemes used in this study and their sources
| Species | Strain | Source of isolate | Sourcea | Reference |
|---|---|---|---|---|
| T. denticola | ATCC 35404 | Human with periodontitis | ATCC | 5 |
| GM-1 | Human with periodontitis | AW | 43 | |
| T. pectinovorum | ATCC 33768 | Subgingival plaque | EPG | 46 |
| S1 | Human with HIVb and periodontitis | SW | 49 |
ATCC, American Type Culture Collection, Rockville, Md.; AW, Aaron Weinberg, University of Texas Health Science Center at San Antonio; EPG, E. Peter Greenberg, University of Iowa; SW, Stephen Walker, University of Texas Health Science Center at San Antonio.
HIV, human immunodeficiency virus.
Mice.
ICR (Harlan Sprague-Dawley Inc., Indianapolis, Ind.) female mice, aged 8 to 12 weeks old, were utilized. The animals were housed in isolator cages in an American Association for Accreditation of Laboratory Animal Care-accredited animal facility at the University of Texas Health Science Center at San Antonio and were provided with autoclaved TEKLAD chow (Harlan Sprague-Dawley Inc., Madison, Wis.) and water ad libitum. BALB/c mice were used for testing the lethality of the T. denticola GM-1 strain, and ICR mice were used in all other experiments.
Murine virulence model.
The murine abscess model (23, 24, 26) was used to examine the virulence capacity of the oral treponeme strains. For determination of virulence and abscess-forming capability, appropriate dilutions of the treponemes were made under anaerobic conditions and mixed with the virulence-modulating agents. Mice were injected subcutaneously (s.c.) or intraperitoneally (i.p.) within 15 to 30 min of sample preparation. After challenge, the animals were monitored daily for symptoms of infection. Progression of lesion formation for up to 3 weeks and virulence were scored on the basis of (i) onset and characteristic of lesion, (ii) size of localized abscess and/or necrotic skin lesion, (iii) size of spreading exudative hemorrhagic lesion, and (iv) death. Subcutaneous abscess and necrotic skin lesion sizes (length and width) were measured daily for 15 days with a caliper gauge, and the area was determined and expressed in square millimeters. The maximum abscess size was noted for each animal, and the mean per group was calculated. Three experimental models were used.
(i) Bacterial growth modulation.
The cysteine concentration was altered such that T. denticola and T. pectinovorum were grown in GM-1 medium (1, 49, 50) supplemented with 6, 18, or 36 mM cysteine. Logarithmic-phase broth cultures were harvested by centrifugation and resuspended in GM-1 medium (with or without cysteine) to a final concentration of 2.5 × 1011 treponemes per ml. GM-1 medium supplemented with 6, 18, or 36 mM cysteine alone was injected into groups of animals to serve as controls.
Iron-limiting GM-1 medium was produced by incubating the medium with 0.1 mM 2,2′-dipyridyl (BPD; Sigma) for 48 h (9). This BPD level was noted previously to deplete iron levels in the GM-1 medium by >90% and to decrease the growth of both of the treponemes (unpublished observations). This iron-chelated medium was then inoculated with T. denticola and T. pectinovorum. Logarithmic-phase GM-1 broth cultures (approximately 72 h of growth) with or without BPD were harvested by centrifugation and resuspended in GM-1 medium with or without 0.1 mM BPD to a final concentration of 2.5 × 1011 treponemes per ml. The treponemes were injected s.c. in mice. GM-1 medium with or without 0.1 mM BPD was injected into other animals to serve as controls.
(ii) Bacterial environment modulation in vivo.
Alterations in T. denticola virulence were assessed by the extent of abscess formation following coinoculation of mice with treponemes and Cytodex 1 dextran microcarrier beads essentially as described by Ford et al. (16). The beads induce a localized inflammatory response and can increase bacterial pathogenicity. Cytodex 1 microcarrier beads (Sigma) were suspended in phosphate-buffered saline (50 ml/g) overnight and autoclaved at 120°C for 10 min. The sterile supernatant fluid was decanted from the sedimented microcarrier beads, equal volumes of packed microcarrier beads and an appropriate concentration of T. denticola were mixed, and 0.2 ml of the mixture was injected s.c. into the posterior dorsolateral surface of each mouse for determination of abscess formation. Sterile packed Cytodex 1 beads alone were injected s.c. into a group of five animals as a negative control.
The effects of d-[+]-galactosamine (GalN) on the susceptibility of mice to lethal toxicity (17) following challenge with T. denticola or T. pectinovorum were also studied. T. denticola GM-1 and ATCC 35404 and T. pectinovorum S1 and ATCC 33768 were grown as described above and harvested by centrifugation, and an appropriate concentration of cells was suspended in sterile GalN (20 mg/animal; Aldrich Chemicals) and injected i.p. into mice. Control mice received GalN only, and other groups of animals received similar concentrations of treponemes alone. Symptoms of infection and death were recorded for 4 days.
(iii) Host environment modulation.
To evaluate the effect of iron pretreatment of mice on the virulence of T. denticola and T. pectinovorum, mice were administered iron dextran (ferric hydroxide dextran complex; Sigma) i.p. at 5 mg per animal 3 h prior to (41) treponeme challenge. Alternatively, administration of phenylhydrazine (Sigma) to mice elicits a rapid lysis of circulating erythrocytes with a resulting substantial increase in the levels of free hemoglobin in plasma (21). This host alteration lasts for approximately 4 days, at which time the blood levels of hemoglobin return to normal. Phenylhydrazine (2 mg/animal) was administered i.p. 3 h prior to T. denticola or T. pectinovorum challenge. Groups of animals without iron or phenylhydrazine pretreatment were also infected with the treponemes to serve as untreated controls; mice injected with iron dextran or phenylhydrazine alone served as treatment controls.
Statistical analyses.
Statistical differences in lesion size between various groups were determined by use of a Wilcoxon-Mann-Whitney U test (Minitab, State College, Pa.).
RESULTS
In vitro environmental modulation of virulence.
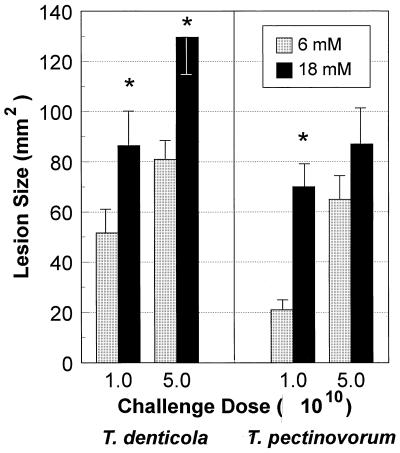
The ability of cysteine to enhance the virulence of T. denticola and T. pectinovorum, as determined by the production of soft tissue lesions in mice following s.c. challenge, was evaluated (Fig. 1). We have previously described the characteristics of the induction, progression, and gross and histologic characteristics of these lesions (26). T. denticola and T. pectinovorum grown in 18 mM cysteine induced significantly (P < 0.01) larger local abscesses than bacteria grown in 6 mM cysteine when a challenge dose of 1010 was used. Also, T. denticola grown in 36 mM cysteine induced significantly (P < 0.01) larger abscesses than bacteria grown in 6 mM cysteine, although the lesion size was similar to that of bacteria grown in 18 mM cysteine (data not shown). Similar onsets and similar lesion characteristics were elicited by both treponemes, irrespective of the availability of cysteine during in vitro cultivation. As we had previously noted, there was no mortality induced by s.c. challenge infection with these treponemes under any of the conditions.
FIG. 1.
Effect of growth of T. denticola ATCC 35404 and T. pectinovorum ATCC 33768 in cysteine-containing GM-1 medium on virulence in a murine abscess model. The bars denote the mean abscess size in square millimeters, and the error bars represent 1 standard deviation derived from groups of five mice. NT, not tested. ∗, significantly different at P < 0.01 when results for treponemes grown in 18 and 6 mM cysteine are compared. The treponeme-free negative control injected with cysteine in GM-1 medium demonstrated neither toxicity nor any lesion at the site of injection (data not shown).
We had noted previously that cultivation of various oral microorganisms under iron-depleted conditions decreased their virulence in this murine model (22). In contrast, the capacity of T. denticola and T. pectinovorum grown in 0.1 mM BPD (iron-depleted conditions) to induce abscess formation was not significantly affected in comparison to that of control treponemes cultivated under normal iron conditions (data not shown).
In vivo bacterial environment modulation.
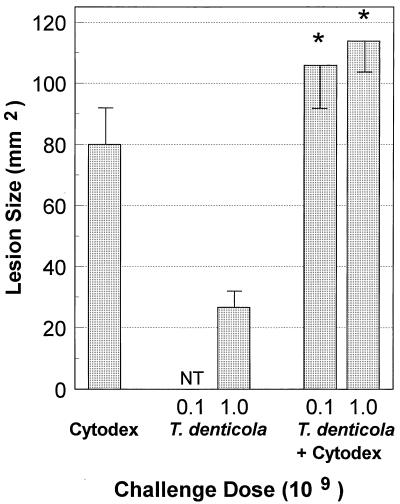
Injection of the inflammation-stimulating Cytodex 1 beads into the dorsal surface of mice induced a closed sterile abscess of approximately 80 mm2 (Fig. 2). The abscess persisted for approximately 14 days and then resolved. A similar s.c. injection of T. denticola alone at a dose of 109 bacteria/site produced a small abscess of approximately 28 mm2. In contrast to the response induced by the Cytodex 1 beads (i.e., a closed sterile abscess), the abscess induced by T. denticola was expressed through the skin and healed more slowly (i.e., 2 to 3 weeks). When 108 or 109 T. denticola bacteria were combined with the Cytodex 1 beads, the abscess formed was significantly larger (P < 0.05) than that resulting from T. denticola injection alone (Fig. 2). Interestingly, while the abscess was larger (110 to 150 mm2) in the T. denticola-Cytodex 1 bead-injected animals than in the animals injected with T. denticola or Cytodex 1 beads alone, the lesion from the combined injection appeared to be the result of an additive virulence outcome rather than a synergistic effect. Nevertheless, this local abscess persisted for more than 3 weeks and did not break through the skin, as was noted with T. denticola alone.
FIG. 2.
Effect of sterile Cytodex 1 microcarrier bead-induced abscess on the virulence of T. denticola ATCC 35404. Mice were challenged with 108 or 109 bacteria as described in Materials and Methods. NT, not tested. The bars represent the mean abscess size in square millimeters, and the error bars represent 1 standard deviation derived from groups of five mice.
GalN sensitization increases the susceptibility and sensitivity of mice to the lethal effects of gram-negative bacteria and endotoxin (LPS) (17). In this study, GalN was mixed with different concentrations of T. denticola and T. pectinovorum and administered i.p. to groups of mice (Table 2). The results demonstrated that T. denticola GM-1 and ATCC 35404 were uniformly lethal at doses of 1010 or 109 bacteria. Similarly, T. pectinovorum S1 and ATCC 33768 caused 80 to 100% lethality at similar doses. Below a dose of 109 bacteria, minimal toxicity was noted with the treponemes. GalN or treponemes injected separately in control animals had no lethal effect at any dose tested.
TABLE 2.
GalN effects on lethality of treponeme infection in mice
| Treponeme straina | GalNb | Treponeme dose | Lethalityc |
|---|---|---|---|
| T. denticola GM-1 | + | 107 | 1/5 |
| + | 108 | 1/5 | |
| + | 109 | 4/4 | |
| + | 1010 | 5/5 | |
| − | 109 | 0/5 | |
| − | 1010 | 0/5 | |
| T. denticola 35404 | + | 109 | 4/5 |
| + | 1010 | 5/5 | |
| − | 109 | 0/5 | |
| − | 1010 | 0/5 | |
| T. pectinovorum S1 | + | 107 | 0/5 |
| + | 108 | 0/5 | |
| + | 109 | 8/10 | |
| + | 1010 | 10/10 | |
| − | 107 | 0/5 | |
| − | 108 | 0/5 | |
| − | 109 | 0/5 | |
| − | 1010 | 0/5 | |
| T. pectinovorum 33768 | + | 109 | 5/5 |
| + | 1010 | 5/5 | |
| − | 109 | 0/5 | |
| − | 1010 | 0/5 | |
| None | + | − | 0/5 |
T. denticola GM-1 was tested in BALB/c mice; all other strains were tested in ICR mice and provided comparable results irrespective of the mouse strain used.
GalN was mixed with an appropriate concentration of treponemes and administered i.p. to groups of mice. Control animals received either GalN or an appropriate concentration of treponemes i.p.
Number of animals that died/total number of animals infected.
Host environment modulation.
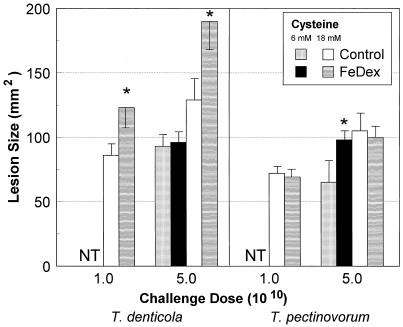
The in vivo administration of iron-containing compounds (e.g., iron dextran, hemin, hemoglobin, or transferrin) has been shown to enhance the virulence of many bacterial pathogens (28, 42, 51, 52), although these studies have generally emphasized microorganisms which cause systemic infections. Mice pretreated with iron dextran and infected with T. denticola (grown in 18 mM cysteine) induced at least 50% larger lesions (P < 0.025) than untreated T. denticola-challenged mice (Fig. 3). No abscess or symptoms of toxicity were observed in control mice that received iron dextran alone. In contrast, iron dextran had no effect on the virulence of infection with T. pectinovorum (grown in 18 mM cysteine) in comparison to that in untreated control animals (Fig. 3).
FIG. 3.
Effect of administration of iron dextran (FeDex) to mice on the virulence of T. denticola ATCC 35404 and T. pectinovorum ATCC 33768 cultivated in medium with cysteine at various concentrations (6 or 18 mM). Animals were challenged with 1 × 1010 or 5 × 1010 bacteria as described in Materials and Methods. The bars represent the maximum mean lesion size in square millimeters, and the error bars represent 1 standard deviation derived from groups of five mice. NT, not tested. ∗, significantly different (P < 0.01) when results of growth in 18 mM cysteine with host iron pretreatment and growth in 18 mM cysteine alone are compared. Mice injected with iron dextran alone demonstrated neither toxicity nor any lesion at the site of injection (data not shown).
The experimental design also enabled us to evaluate the capacity of T. denticola and T. pectinovorum to utilize multiple virulence-enhancing environmental signals in the murine lesion model. As such, the treponemes were cultured in 6 or 18 mM cysteine (Fig. 3) and subsequently used to challenge mice which had been pretreated with iron dextran to increase in vivo iron availability. The results demonstrated that the environmental conditions, that is, elevated cysteine and iron pretreatment, contributed additively to the resulting lesion size of T. denticola. Interestingly, under growth conditions with lower cysteine concentrations, iron pretreatment had no effect on the virulence of T. denticola but enhanced T. pectinovorum virulence significantly (Fig. 3).
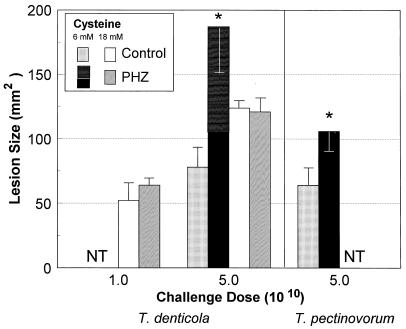
In an experiment with a similar design, we noted that T. denticola and T. pectinovorum cultivated in 6 mM cysteine were significantly (P < 0.01) more virulent in mice treated with phenylhydrazine than in untreated mice (Fig. 4). As interesting, T. denticola caused an exudative necrotic spreading lesion in the abdomen, in addition to inducing a local abscess. This spreading lesion appeared to be initiated by a spirochetemia based upon the clinical presentation of the mice, which demonstrated gross manifestations (i.e., ruffled fur, body posture, weight loss, or change in eating behavior) of infection for up to 6 days postinfection. In contrast, phenylhydrazine treatment had no significant effect on s.c. abscess formation by T. denticola cultured in 18 mM cysteine (Fig. 4). None of the phenylhydrazine-treated control animals died nor demonstrated any acute toxic symptoms to the treatment except for a brief (<24-h) period of malaise with ruffled hair.
FIG. 4.
Effect of administration of phenylhydrazine (PHZ) to mice on the virulence of T. denticola ATCC 35404 and T. pectinovorum ATCC 33768 cultivated in medium with cysteine at various concentrations (6 or 18 mM). Animals were challenged with 1 × 1010 or 5 × 1010 bacteria as described in Materials and Methods. The bars represent the maximum mean lesion size in square millimeters, and the error bars represent 1 standard deviation derived from groups of five mice. The stacked bars for PHZ treatment and T. denticola challenge represent the localized abscess (solid bar) and total (spreading necrotic lesion plus local abscess) (horizontally lined bar) lesion sizes in the mice. NT, not tested. ∗, significantly different (P < 0.01) from results for PHZ treatment of control infected mice. Mice injected with PHZ alone demonstrated neither toxicity nor any lesion at the site of injection except for a brief (<24-h) period of malaise with ruffled hair (data not shown).
DISCUSSION
In vitro studies of environmental regulation of bacterial virulence have provided a number of important observations regarding strategies used by pathogens to respond and survive under changing environmental conditions in vivo. However, recent evidence, including sophisticated in vivo expression technologies (20, 45), have demonstrated the importance of the in vivo environment in delineating significant virulence determinants which contribute to the host-parasite interactions resulting in manifestations of disease. An in vivo expression technology vector (pPGIVET) has been constructed and used to determine the expression of hemagglutinin genes hagB and hagC of P. gingivalis during an infectious process in mice (30). Thus, these genetic approaches to evaluate the virulence mechanisms of oral microorganisms have been initiated, but the capabilities of these approaches are still quite limited. The experiments described in this report examine the oral treponemes T. denticola and T. pectinovorum as members of the putative periodontopathic microbiota in the subgingival sulcus (30, 44, 46). In particular, we used more classic approaches to specifically evaluate the ability of in vitro and in vivo environmental conditions to modify the virulence capacity of oral treponemes by using a murine model (14, 23, 24).
Our initial investigation expanded previous in vitro observations from studies using cysteine (9) or iron levels (3, 4, 7) to modulate the growth characteristics of oral microorganisms. These have been suggested to result in the up- and down-regulation of selected surface and cytoplasmic molecules that aid in the ability of the bacteria to utilize alternative mechanisms for acquisition of critical nutrients and cofactors. We have noted that cysteine can stimulate or enhance enzyme activities of P. gingivalis, although high levels of this sulfhydryl-containing amino acid can actually abrogate the virulence of P. gingivalis (unpublished observation). Cysteine has also been identified as a protease activator, although it had been shown previously to exhibit a minimal effect on a number of T. denticola enzyme functions in vitro (26). However, previous studies in our laboratory have clearly shown that cell-associated hemolytic and hemoxidative activities of T. denticola are cysteine dependent (6). Further studies of these biologic activities described the isolation and characterization of a 46-kDa protein from T. denticola, referred to as cystalysin, with hemolytic activity different from that of complement and classical hemolysin (8). Its preferred substrate compound was cysteine with a sulfur atom adjacent to both a β-methylene and an α-amino group (9). The cystalysin exhibited its enzymatic activity by degrading cysteine, yielding H2S and pyruvate, which increased the growth of the bacteria and caused the hemolytic activity (9). In contrast, T. pectinovorum does not express a cystalysin activity. We hypothesized that cysteine would significantly increase the in vivo virulence of T. denticola, via these cystalysin activities. The results showed that, in fact, the virulence of T. denticola was significantly increased by growth in media with elevated levels of cysteine. However, this amino acid also appeared to modify the characteristics of T. pectinovorum and increased its lesion-inducing capacity. Thus, while the T. denticola cystalysin may be a virulence factor affected by the cysteine, it appears that the oral treponemes may have multiple mechanisms for interacting with this molecule in the environment.
Previous studies have clearly shown that environmental iron has a significant regulatory effect on the growth (3) and virulence expression (22) of P. gingivalis. We have noted that cultivation of oral treponemes under iron-limited conditions alters outer membrane protein profiles (7), enzymatic activities (9), and hemolytic activities (8). Recently, we have observed that T. denticola and T. pectinovorum have a minimal capacity to survive under continued iron-depleted conditions in vitro (unpublished observations), in contrast to the ability of P. gingivalis to survive and grow for three to seven passages in media devoid of iron (unpublished data). Thus, we hypothesized that depleting the environment of iron would have a profound effect on virulence expression by the oral treponemes. Although iron is essential for the in vitro growth of oral treponemes and appears to alter putative virulence characteristics, including toxins, cytotoxins, and hemolysins (6), depletion of this molecule during a single in vitro passage had a negligible impact on in vivo virulence. These results suggest that the treponemes may be less affected by the dramatic fluctuations in environmental iron in the gingival sulcus and, as such, are less dependent upon the local availability of iron for maintenance of their virulence capability.
Iron is a necessary factor for the survival and growth of prokaryotes, which is clearly evident in the numerous evolutionary strategies that have been developed by bacteria to acquire iron from the natural environment (51). Various studies have begun to contribute to a clearer understanding of mechanisms by which oral anaerobes acquire iron from the environment (3, 4, 18), particularly with respect to P. gingivalis. We have recently reported preliminary observations concerning the accession of iron in vivo by P. gingivalis (22). Additionally, we have determined that P. gingivalis virulence is enhanced by pretreatment of mice with iron dextran, hemin, and transferrin (unpublished data). This has been suggested to result from increased iron availability and altered regulation of virulence determinants. However, very little is known concerning the characteristics of iron acquisition and utilization by oral treponemes. To this end, we assessed iron utilization by T. denticola and T. pectinovorum in vivo in mice. Specifically, numerous studies have demonstrated that iron loading of mice significantly increases the virulence of systemic pathogens, such as Vibrio vulnificus (21), Escherichia coli (48), and Yersinia enterocolitica (41), although this model has never been used to examine localized, tissue-destructive pathogens. In our study, iron dextran administration significantly enhanced the virulence (abscess size) of T. denticola. Similarly, phenylhydrazine pretreatment of mice, which has been reported to induce hemoglobinemia in mice, significantly enhanced lesion formation by T. denticola. The phenylhydrazine pretreatment also substantially altered the virulence characteristics of T. denticola either by a direct effect of the increased iron on bacterial virulence mechanisms or by altering host resistance and allowing the T. denticola to spread from the infection site. Both iron dextran and phenylhydrazine pretreatments appeared to have less of an effect on the virulence of T. pectinovorum in vivo. One interpretation of these findings is that the alterations were at the level of the microorganism rather than the host, although further studies will be required to validate this concept. Additionally, the minimal effect of host iron changes on T. pectinovorum suggested that this microorganism either requires less iron for in vivo survival and virulence expression or does not express the capacity to utilize either of these host iron sources but acquires its iron by other mechanisms. Again, these options need to be evaluated in future studies.
Extensive inflammation, which enhances delivery of soluble and cellular serum components to the sulcular environment, results in dramatic changes in the milieu of the sulcus, from health to periodontitis. Extensive data have shown that the oral treponemes emerge as dominant members of the oral ecology at gingival sites with increased inflammation (19, 37). This change in the inflammatory environment may be expected to result in alterations in a variety of local cues which require bacterial responsiveness to the milieu. Thus, we tested the synergistic effect of cysteine and iron, based upon the findings that cysteine and in vivo iron individually enhance T. denticola virulence. We noted that cysteine and iron contributed additively to the virulence of T. denticola. In contrast, these two environmental cues appeared to act independently with respect to the virulence of T. pectinovorum. These findings suggest that the two signals may be acting upon different aspects of each microorganism’s survival and virulence strategies as expressed in this murine model.
It has also not been determined whether the increase in treponemes associated with gingivitis and periodontitis (19, 30, 37, 44) represents the treponemes contributing to the induction of an inflammatory environment or if their increase in the plaque results from the creation of a more favorable ecological niche at the inflammation sites. Thus, we hypothesized that locally induced inflammation would create a more favorable environment and increase the ability of T. denticola to induce tissue-destructive lesions, which could be evaluated in a murine model. We had previously reported that Cytodex 1 microcarrier beads, which create a localized inflammatory milieu in vivo, enhance the infectivity and pathogenicity of C. rectus as assessed by the murine abscess model (24). We evaluated the ability to affect treponeme virulence by altering the local in vivo environment of the bacteria. The abscess-promoting agent Cytodex microcarrier beads was evaluated for increasing local inflammation and for potentially providing a more favorable environment for the treponemes to establish an infectious focus. The results indicate that within the limitations of this model, local inflammation appears to have a minimal effect on virulence expression by treponemes. Of potential importance is that this model may be used to explore other modifications of the local in vivo environment and to determine their effect on treponeme virulence.
The LPS from gram-negative bacteria is capable of eliciting a wide range of biological responses and is implicated in the pathogenesis of a variety of diseases. Members of the genus Treponema have an outer envelope resembling that of gram-negative bacteria. Several reports have described an LPS-like component in T. denticola (10, 29, 53, 54), Serpulina (Treponema) hyodysenteriae (39, 40), and the nonpathogenic Reiter treponeme (12). Injection of GalN makes mice increasingly susceptible to the lethal effects of endotoxin (17). This model has been suggested to be a quantitative measure of the endotoxicity of LPS, particularly as related to the toxic activity of the lipid A portion of the molecule (47). We have recently identified an LPS-like macromolecule isolated from the outer sheath of T. pectinovorum (unpublished data). Thus, we hypothesized that LPS-like macromolecules could contribute to the pathogenicity of oral treponemes and evaluated this biological activity in a galactosamine sensitization model in mice. The results demonstrated a general lack of toxicity of both treponemes when injected i.p. into normal mice. However, both treponemes were lethal for mice that were concomitantly treated with GalN and elicited systemic changes normally associated with endotoxic activities. The majority of GalN-sensitized animals died within 8 h of bacterial challenge, consistent with an LPS-mediated effect (17). The systemic manifestations of endotoxemia have been identified as resulting from the ability of LPS to activate host cells to produce circulating levels of cytokines, such as interleukin 1β, interleukin 6, and tumor necrosis factor alpha, which are the direct effectors of endotoxic shock and death. Therefore, while this study did not identify cytokines in the murine serum, nor specifically identify the capacity of the LPS to affect host responses, the results suggest that examination of the factor(s) that elicited the lethal outcome would be important in understanding treponeme-mediated pathogenicity.
The findings in this study clearly demonstrate the ability to modify the virulence of oral treponemes by altering the in vitro and in vivo environments to which these bacteria are subjected. This model can be manipulated to evaluate the contribution of putative virulence components of oral treponemes within the host.
ACKNOWLEDGMENTS
This study was supported by USPHS grant DE-11368 from the National Institute for Dental Research.
We gratefully acknowledge the assistance of Hiroshi Nitta. We also thank Stephen Walker for supplying the T. pectinovorum strains used in these studies.
REFERENCES
- 1.Blakemore R P, Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976;128:616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramanti T E, Holt S C. Iron-regulated outer membrane proteins in the periodontopathic bacterium, Bacteroides gingivalis. J Biochem Biophys Res Commun. 1990;166:1146–1154. doi: 10.1016/0006-291x(90)90986-w. [DOI] [PubMed] [Google Scholar]
- 3.Bramanti T E, Holt S C. Roles of porphyrins and host iron transport proteins in regulation of growth of Porphyromonas gingivalis W50. J Bacteriol. 1991;173:7330–7339. doi: 10.1128/jb.173.22.7330-7339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramanti T E, Holt S C. Localization of a Porphyromonas gingivalis 26-kilodalton heat-modifiable, hemin-regulated surface protein which translocates across the outer membrane. J Bacteriol. 1992;174:5827–5839. doi: 10.1128/jb.174.18.5827-5839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S L, Siboo R, Chin Quee T, Johnson J L, Mayberry W R, Chan E C S. Comparative study of six random oral spirochetes. Serological heterogeneity of Treponema denticola. J Periodontal Res. 1985;20:602–612. doi: 10.1111/j.1600-0765.1985.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 6.Chu L, Kennell W, Holt S C. Characterization of hemolysis and hemoxidation activities by Treponema denticola. Microb Pathog. 1994;16:183–195. doi: 10.1006/mpat.1994.1019. [DOI] [PubMed] [Google Scholar]
- 7.Chu L, Song M, Holt S C. Effect of iron regulation on expression and hemin binding function of outer-sheath proteins from Treponema denticola. Microb Pathog. 1994;16:321–335. doi: 10.1006/mpat.1994.1033. [DOI] [PubMed] [Google Scholar]
- 8.Chu L, Ebersole J L, Kurzban G, Holt S C. Cystalysin, a 46-kilodalton cysteine desulfhydrase from Treponema denticola, with hemolytic and hemoxidative activities. Infect Immun. 1997;65:3231–3238. doi: 10.1128/iai.65.8.3231-3238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu L, Ebersole J L, Kurzban G P, Holt S C. Cystalysin, a 46 kDa l-cysteine desulfhydrase from Treponema denticola: biochemical and biophysical characterization. Clin Infect Dis. 1999;28:442–450. doi: 10.1086/515164. [DOI] [PubMed] [Google Scholar]
- 10.Cockayne A, Sanger R, Ivic A, Strugnell R A, Macdougall J H, Russell R R B, Penn C W. Antigenic and structural analysis of Treponema denticola. J Gen Microbiol. 1989;135:3209–3218. doi: 10.1099/00221287-135-12-3209. [DOI] [PubMed] [Google Scholar]
- 11.Cutler C W, Eke P I, Genco C A, Van Dyke T E, Arnold R R. Hemin-induced modifications of the antigenicity and hemin-binding capacity of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 1996;64:2282–2287. doi: 10.1128/iai.64.6.2282-2287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro G, del Carpio C. A lipopolysaccharide antigen of the treponema. Nature (London) 1958;181:991–992. doi: 10.1038/181991b0. [DOI] [PubMed] [Google Scholar]
- 13.DiRita V J, Mekalanos J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- 14.Ebersole J L, Kesavalu L, Schneider S L, Machen R L, Holt S C. Comparative virulence of periodontopathogens in a mouse abscess model. Oral Dis. 1995;1:115–128. doi: 10.1111/j.1601-0825.1995.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford C W, Hamel J C, Stapert D, Yancey R J. Establishment of an experimental model of a Staphlococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J Med Microbiol. 1989;28:259–266. doi: 10.1099/00222615-28-4-259. [DOI] [PubMed] [Google Scholar]
- 17.Galanos C, Freudenberg M A, Feutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genco C A, Odusanya B M, Brown G. Binding and accumulation of hemin in Porphyromonas gingivalis are induced by hemin. Infect Immun. 1994;62:2885–2892. doi: 10.1128/iai.62.7.2885-2892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 20.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helms S D, Oliver J D, Travis J C. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984;45:345–349. doi: 10.1128/iai.45.2.345-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesavalu L, Ebersole J L, Holt S C. Hemin as a modulator of Porphyromonas gingivalis virulence in a murine model. J Dent Res. 1996;75:205. . (Abstr. 1483.) [Google Scholar]
- 23.Kesavalu L, Ebersole J L, Machen R L, Holt S C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992;60:1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesavalu L, Holt S C, Crawley R R, Borinski R, Ebersole J L. Virulence of Wolinella recta in a murine abscess model. Infect Immun. 1991;59:2806–2817. doi: 10.1128/iai.59.8.2806-2817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesavalu L, Holt S C, Ebersole J L. Trypsin-like protease activity of Porphyromonas gingivalis as a potential virulence factor in a murine lesion model. Microb Pathog. 1996;20:1–10. doi: 10.1006/mpat.1996.0001. [DOI] [PubMed] [Google Scholar]
- 26.Kesavalu L, Walker S, Holt S C, Crawley R R, Ebersole J L. Virulence characteristics of oral treponemes in a murine model. Infect Immun. 1997;65:5096–5102. doi: 10.1128/iai.65.12.5096-5102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S J, Chu L, Holt S C. Isolation and characterization of a hemin-binding cell envelope protein from Porphyromonas gingivalis. Microb Pathog. 1996;21:65–70. doi: 10.1006/mpat.1996.0043. [DOI] [PubMed] [Google Scholar]
- 28.Kochan I, Kvach J T, Wiles T I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977;135:623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- 29.Kurimoto T, Suzuki M, Watanabe T. Chemical composition and biological activities of lipopolysaccharides extracted from Treponema denticola and Treponema vincentii. Odontology (Tokyo) 1990;78:208–232. [PubMed] [Google Scholar]
- 30.Lee S-W, Hillman J D, Progulske-Fox A. The hemagglutinin genes hagB and hagC of Porphyromonas gingivalis are transcribed in vivo as shown by use of a new expression vector. Infect Immun. 1996;64:4802–4810. doi: 10.1128/iai.64.11.4802-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh P D, McDermid A S, McKee A S, Baskerville A. The effect of growth rate and hemin on the virulence and proteolytic activity of Porphyromonas gingivalis W50. Microbiology. 1994;140:861–865. doi: 10.1099/00221287-140-4-861. [DOI] [PubMed] [Google Scholar]
- 32.Maurelli A T. Temperature regulation of virulence genes in pathogenic bacteria: a general strategy for human pathogens. Microb Pathog. 1989;7:1–10. doi: 10.1016/0882-4010(89)90106-x. [DOI] [PubMed] [Google Scholar]
- 33.McKee A S, McDermid A S, Baskerville A, Dowsett A B, Ellwood D C, Marsh P D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986;52:349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melton A R, Weiss A A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989;171:6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 37.Moore W E C, Moore L V H. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 38.Nitta, H., C. Shipley, S. C. Holt, and J. L. Ebersole. Environmental stress effects and S-layer expression by Campylobacter rectus. Submitted for publication.
- 39.Nuessen M E, Birmingham J R, Joens L A. Biological activity of a lipopolysaccharide extracted from Treponema hyodysenteriae. Infect Immun. 1982;37:138–142. doi: 10.1128/iai.37.1.138-142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuessen M E, Joens L A, Glock R D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983;131:997–999. [PubMed] [Google Scholar]
- 41.Robins-Browne R M, Prpic J K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985;47:774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robins-Browne R M, Cianciosi S, Bordun A-M, Wauters G. Pathogenicity of Yersinia kristensenii for mice. Infect Immun. 1991;59:162–167. doi: 10.1128/iai.59.1.162-167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sela M N, Weinberg A, Borinsky R, Holt S C, Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral factors. Infect Immun. 1988;56:589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonson L G, Goodman C H, Bial J J, Morton H E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988;56:726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slauch J M, Mahan M J, Mekalanos J J. In vivo expression technology for selection of bacterial genes specifically induced in host tissues. Methods Enzymol. 1994;235:481–492. doi: 10.1016/0076-6879(94)35164-3. [DOI] [PubMed] [Google Scholar]
- 46.Smibert R M, Burmeister J A. Treponema pectinovorum sp. nov. isolated from humans with periodontitis. Int J Syst Bacteriol. 1983;33:852–856. [Google Scholar]
- 47.Takayama K, Qureshi N, Ribi E, Cantrell J L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984;6:439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- 48.Waalwijk A, MacLaren D M, De Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983;42:245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker S G, Ebersole J L, Holt S C. Identification, isolation, and characterization of the 42-kilodalton major outer membrane protein (MompA) from Treponema pectinovorum ATCC 33768. J Bacteriol. 1997;179:6441–6447. doi: 10.1128/jb.179.20.6441-6447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber F H, Canale-Parola E. Pectinolytic enzymes of oral spirochetes from humans. Appl Environ Microbiol. 1984;48:61–67. doi: 10.1128/aem.48.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 52.Wright A C, Simpson L M, Oliver J D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yotis W W, Sharma V K, Gopalsami C, Chegini S, McNulty J, Hoerman K, Keene J, Jr, Simonson L G. Biochemical properties of the outer membrane of Treponema denticola. J Clin Microbiol. 1991;29:1397–1406. doi: 10.1128/jcm.29.7.1397-1406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yotis W W, Macaluso F, Gopalsami C. Immunochemical features of a macromolecule of Treponema denticola. J Basic Microbiol. 1995;35:255–268. doi: 10.1002/jobm.3620350411. [DOI] [PubMed] [Google Scholar]