Abstract
Objective:
Hepatoblastoma (HB) is the most common pediatric primary malignant liver tumor, its incidence has been increasing worldwide, but recent changes in incidence and outcomes with high population coverage are not well characterized.
Methods:
We defined the incidence of HB diagnosed during 2003–2017 from United States Cancer Statistics (USCS) database, and survival during 2001–2016 from the National Program of Cancer Registries (NPCR). Data were stratified by sex, race/ethnicity, age, tumor stage, county population, and diagnosis year. Incidence trends were assessed by calculating average annual percent change (AAPC) using Joinpoint regression. Differences in overall 5-year survival were estimated using Cox regression analysis.
Results:
2178 HB cases with an annual incidence rate of 1.76 per million persons were identified and incidence increased over time (AAPC = 2.2, 95% confidence interval [CI], 0.9–3.6). The 5-year relative survival was 76.9% (95% CI: 74.9–78.8) and the risk of death was lower for cases diagnosed after 2009 (hazard ratio [HR] = 0.77, 95% CI: 0.63–0.94), higher for ages 3–7 years and 8–19 years compared to 0–2 years (HR = 1.38, 95% CI: 1.10–1.76 and 1.83, 95% CI: 1.31–2.70, respectively), for distant compared to locoregional stage (HR = 2.77, 95% CI: 2.27–3.36), and for non-Hispanic Black compared to non-Hispanic White cases (HR = 1.39, 95% CI: 1.02–1.84).
Conclusions:
HB incidence increased, and survival improved over the study period. Disparities in survival exist by age, race or ethnicity, and stage. Further studies could identify factors affecting increases in HB cases, inform future interventions, and address disparities in outcomes.
Keywords: hepatoblastoma, incidence, survival
1 |. INTRODUCTION
Hepatoblastoma (HB) is the most common liver tumor in children, accounting for over 60% of pediatric hepatic malignancies and approximately 1% of all pediatric cancers.1–3 HB typically arises within the first 5 years of life, can be associated with premature birth or low birth weight and certain inherited conditions, including familial adenomatous polyposis, Beckwith–Wiedemann syndrome, neurofibromatosis type 1, Prader–Willi syndrome, and Simpson–Golabi–Behmel syndrome.1–4 HB is rare with an incidence rate of 1.7 cases per million children between 2003 and 2014, and the incidence of HB has increased over the past four decades.1,2,5–7
In 2008, the Children’s Oncology Group (COG) established a risk-based treatment approach for HB with the AHEP0731 protocol.1,8–12 This approach involves surgical resection and chemotherapy informed by a hybrid staging method incorporating the Evans staging system and tumor histology paired with updated chemotherapeutic and surgical strategy for each risk group.1,8–12
Previous epidemiological studies of HB have predominantly relied on data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program, which has historically covered less than 30% of the US population.1,2 Given the rarity of HB and the limited sample sizes of retrospective studies in the United States, analysis of a large, recent, and comprehensive dataset ideally with 100% coverage of the population is necessary to elucidate recent epidemiological trends in HB incidence and outcomes, and is essential to gain a complete understanding of existing disease burden and the influence of these new practice changes.
In this study, we analyzed the United States Cancer Statistics (USCS) database, which covers 100% of US HB cases diagnosed between 2003 and 2017, and the National Program of Cancer Registries (NPCR) survival database, which covers 94% of US HB cases diagnosed between 2001 and 2016, to determine changes in incidence and outcomes.13,14
2 |. METHODS
2.1 |. Databases
Data related to incidence of HB were obtained from USCS database from 2003 to 2017, which included all 50 states and the District of Columbia.13 USCS comprises data from the Centers for Disease Control and Prevention’s (CDC) NPCR database and the SEER database. Data related to survival were from NPCR-funded registries that conducted active case follow-up or linkage with the CDC’s National Death Index. This database covered cases diagnosed from 2001, with follow-up through December 31, 2016, and included the District of Columbia and all US states except for Connecticut, Hawaii, Indiana, Iowa, Kansas, and New Mexico.14 Cases in both databases are deidentified. Both databases were checked for quality and meet the following criteria: (a) ≤5% of cases are based on death certificates alone; (b) ≤3% of cases are missing some information regarding sex; (c) ≤3% of cases are missing some information regarding age; (d) ≤5% of cases are missing some information regarding race; and (e) ≥97% of the registry’s records have passed single- and inter-field computerized edits that assess data validity.15
2.2 |. Inclusion criteria for incidence and survival analyses
HB cases were identified using ICD-O-3 code 8970, defined according to the International Classification of Childhood Cancer diagnostic group VIIa.16 By anatomic site code, all but two cases had code C22.0 (liver); the two remaining cases had codes C22.1 (intrahepatic bile duct) and C49.4 (connective, subcutaneous, and other soft tissue: abdomen). Cases involving patients aged <19 years with malignant, microscopically confirmed HB were included in this analysis. Only cases involving primary tumors, or the first of two or more primary tumors were included. Autopsy only or death certificate only cases were excluded. Non-Hispanic (NH) with unknown race were excluded from the relative and overall survival analyses. Cases involved in clinical trials were not included in either database.
2.3 |. Variables
We stratified HB case incidence and 5-year survival data by variables, including sex, age, tumor stage, race/ethnicity, metropolitan versus non-metropolitan status by county population, and date of diagnosis. Age was stratified by the preset age categories of the USCS database: 0–4, 5–9, 10–14, and 15–19 years for incidence analysis. To align with risk-stratification groups used by the COG,1 age categories were grouped by 0–2, 3–7, and 8–19 years for survival analysis. Race/ethnicity was grouped as NH White, NH Black, NH American Indian or Alaska Native (AIAN), NH Asian or Pacific Islander (API), and Hispanic. Tumor stage was categorized as locoregional, which includes localized and regional cases, or distant (metastatic) based on merged summary stage.17,18
2.4 |. Incidence rates and trends
Rates from the USCS database are expressed per 1,000,000 persons and are age-adjusted to the 2000 US standard population. Relative risk (RR) of incidence was estimated for groups stratified separately by sex, age, and race/ethnicity using negative binomial regression. Trends in incidence are described using average annual percent change (AAPC) calculated by the Joinpoint Regression Program.19 Statistically significant AAPCs are different from zero (p < .05).
2.5 |. Relative survival analysis
Relative survival is cancer survival in the absence of other causes of death. The cohort method was used to estimate case survival within the NPCR database when all cases had a full 5 years of follow-up and the complete method was used if any of the patients had less than the full 5 years of follow-up for 5-year survival time estimates.20 We calculated relative survival using expected life tables stratified by age group, sex, race and ethnicity, metropolitan versus non-metropolitan status, and calendar year of diagnosis.21 Relative survival analyses were performed using SEER*Stat 8.3.8.22
2.6 |. Overall survival analysis
Survival curves were generated using the Kaplan–Meier method from the NPCR database. NH API and NH AIAN cases have been grouped into the category “non-Hispanic all other races,” to allow for complete analysis given the limited number of cases in these two groups. Diagnosis during 2001–2009 was compared to diagnosis during 2010–2016 to align with treatment changes instituted by the COG in 2009.1,8,9 Statistical testing for survival curves was performed using log-rank test. Multivariable Cox regression modeling was conducted to examine the effects of selected demographic and clinical variables on 5-year overall survival. Sex and metropolitan region status were not included in the multivariate model due to lack of significance on univariate modeling. Adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were generated for each of the variables in the model. A higher HR between compared groups indicates a higher risk of death, with statistical significance determined at p < .05. Multivariable analysis and generation of survival curves were performed using SAS version 9.4.
3 |. RESULTS
3.1 |. Demographic and clinical characteristics
We identified 2178 HB cases diagnosed between 2003 to 2017 within the USCS database (Table 1), and 2033 HB cases diagnosed between 2001 and 2016 within the NPCR database (Table 2). Most cases were of male patients (61.1% of USCS; 61.2% of NPCR). NH White, Hispanic, and NH Black patients comprised most HB cases (51.9%, 28.6%, and 10.4% of USCS; 52.8%, 28.2%, and 11.0% of NPCR, respectively). Most cases were of patients who lived in a metropolitan county with a population over 1 million people at the time of diagnosis (57.2% of USCS; 59.4% of NPCR). By stage, 74.9% of cases in the USCS database and 74.1% of cases in the NPCR database involved locoregional as opposed to distant tumor stage.
TABLE 1.
Incidence of hepatoblastoma
| Characteristic | Cases | % | Incidence [95% CI] | AAPC (%) [95% CI] | RR [95% CI] | RR p-value |
|---|---|---|---|---|---|---|
| Overall | 2178 | 100 | 1.76 [1.68–1.83] | 2.2* [0.9–3.6] | ||
| Sex | ||||||
| Male | 1330 | 61.1 | 2.10 [1.99–2.22] | 2.3* [0.5–4.0] | Ref. | - |
| Female | 848 | 38.9 | 1.40 [1.31–1.50] | 2.2* [0.7–3.7] | 0.68 [0.62–0.74] | <.0001 |
| Age | ||||||
| 0–4 years | 1972 | 90.5 | 6.58 [6.29–6.88] | 2.0* [0.6–3.4] | 55.03 [43.49–69.64] | <.0001 |
| 5–9 years | 133 | 6.1 | 0.44 [0.37–0.52] | 5.8* [1.1–10.7] | 3.73 [2.79–4.98] | <.0001 |
| 10–19 years | 73 | 3.4 | 0.12 [0.09–0.15] | 2.5 [−3.6–9.0] | Ref. | - |
| Race/ethnicity | ||||||
| NH White | 1130 | 51.9 | 1.76 [1.68–1.83] | 1.7* [0.3–3.1] | Ref. | - |
| NH Black | 226 | 10.4 | 1.21 [1.59–1.79] | 3.2 [−0.6–3.9] | 0.69 [0.60–0.80] | <.0001 |
| NH API | 137 | 6.3 | 2.10 [1.76–2.48] | 2.1 [−2.1–6.5] | 1.18 [0.98–1.42] | .08 |
| NHAIAN | 32 | 1.5 | 2.54 [1.73–3.58] | NR | 1.58 [1.11–2.26] | .01 |
| Hispanic | 623 | 28.6 | 2.04 [1.88–2.21] | 2.1 [−0.2–4.5] | 1.16 [1.05–1.28] | .004 |
| Stage | ||||||
| Locoregional | 1631 | 74.9 | 1.32 [1.25–1.38] | 2.7* [1.1–4.4] | Ref. | |
| Distant | 483 | 22.2 | 0.39 [0.38–0.43] | 0.7 [−1.2–2.6] | 0.30 [0.27–0.33] | <.0001 |
| Population | ||||||
| Metro >1 million | 1245 | 57.2 | 1.85 [1.75–1.96] | 2.3* [0.4–4.2] | Ref. | |
| Metro 250,000–1 million | 496 | 22.8 | 1.90 [1.73–2.07] | 0.9 [−1.4–3.3] | 1.03 [0.93–1.15] | .58 |
| <250,000 | 176 | 8.1 | 1.66 [1.42–1.92] | 5.4* [0.6–10.3] | 0.92 [0.78–1.08] | .29 |
| Non-metro | 260 | 11.9 | 1.57 [1.39–1.78] | 2.1 [−1.2–5.5] | 0.87 [0.76–1.00] | .05 |
Abbreviations: AAPC, average annual percent change; AIAN, American Indian or Alaska Native; API, Asian or Pacific Islander; CI, confidence interval; NH, non-Hispanic; Ref., reference value; RR, relative risk.
TABLE 2.
Survival of children with hepatoblastoma
| 5-Year relative survival (%) [95% CI] |
|||||
|---|---|---|---|---|---|
| Characteristic | Cases | % | 2001–2009 | 2010–2016 | 2001–2016 |
| Overall | 2033 | 100 | 74.9 [72.1–77.5] | 79.1 [76.0–81.9] | 76.9 [74.9–78.8] |
| Sex | |||||
| Male | 1245 | 61.2 | 74.5 [70.8–77.9] | 78.0 [73.9–81.6] | 76.4 [73.8–78.8] |
| Female | 788 | 38.8 | 75.5 [70.9–79.4] | 81.0 [76.0–85.0] | 77.8 [74.5–80.7] |
| Age | |||||
| 0–2 years | 1603 | 78.8 | 78.0 [74.9–80.7] | 81.6 [78.3–84.5] | 79.7 [77.5–81.7] |
| 3–7 years | 340 | 16.7 | 64.9 [57.0–71.7] | 74.6 [65.9–81.4] | 69.6 [64.0–74.5] |
| 8–19 years | 90 | 4.4 | 56.9 [41.0–70.0] | 55.4 [36.2–71.0] | 56.4 [44.4–66.8] |
| Race/ethnicity | |||||
| NH White | 1073 | 52.8 | 76.3 [72.5–79.6] | 79.7 [75.3–83.4] | 77.7 [74.9–80.2] |
| NH Black | 224 | 11.0 | 69.5 [59.0–77.8] | 71.2 [60.8–79.3] | 70.8 [63.7–76.7] |
| NH API | 133 | 6.5 | 82.2 [70.7–89.5] | 82.7 [62.6–92.5] | 84.0 [75.9–89.6] |
| NHAIAN | 30 | 1.5 | 64.1 [29.7–85.0] | 83.7 [55.1–94.8] | 75.2 [53.8–87.7] |
| Hispanic | 573 | 28.2 | 72.5 [66.6–77.5] | 80.4 [74.8–84.9] | 76.2 [72.2–79.7] |
| Stage | |||||
| Locoregional | 1506 | 74.1 | 80.8 [77.7–83.5] | 85.0 [81.7–87.7] | 82.8 [80.6–84.7] |
| Distant | 454 | 22.3 | 55.9 [49.3–61.9] | 61.1 [53.4–67.8] | 58.1 [53.2–62.7] |
| Population | |||||
| Metro >1 million | 1208 | 59.4 | 75.8 [72.1–79.1] | 78.5 [74.3–82.2] | 77.1 [74.4–79.5] |
| Metro 250,000–1 million | 448 | 22.0 | 75.1 [68.9–80.3] | 85.6 [79.1–90.1] | 80.2 [75.9–83.7] |
| <250,000 | 153 | 7.5 | 68.9 [56.3–78.6] | 76.9 [65.2–85.1] | 72.9 [64.5–79.6] |
| Non-metro | 223 | 11.0 | 73.3 [64.2–80.5] | 70.6 [59.8–79.0] | 72.2 [65.4–77.9] |
Abbreviations: AIAN, American Indian or Alaska Native; API, Asian or Pacific Islander; CI, confidence interval; NH, non-Hispanic.
3.2 |. HB incidence
The overall incidence of HB cases in the United States between 2003 and 2017 was 1.76 per million persons (95% CI: 1.68–1.83) (Table 1). The annual incidence of HB cases increased significantly during this period, with an AAPC of 2.2% (95% CI: 0.9%–3.6%). Significant AAPCs in incidence occurred amongst both male (AAPC = 2.3%, 95% CI: 0.5%–4.0%) and female cases (AAPC = 2.2%, 95% CI: 0.7%–3.7%). The incidence of HB among 0–4 and 5–9 year-old age groups were 55.03 (95% CI: 43.49–69.64) and 3.73 (95% CI: 2.79–4.98) times that of the 10–19-year-old age group, respectively. We observed significant increases in annual incidence amongst cases in the 0–4-year-old (AAPC = 2.0%, 95% CI: 0.6%–3.4%) and 5–9-year-old (AAPC = 5.8%, 95% CI: 1.1%–10.7%) age groups. A significant AAPC in incidence was also observed amongst NH White cases (AAPC = 1.7%, 95% CI: 0.3%–3.1%). AAPC amongst NH Black, NH API, and Hispanic patients were not significant. No increase was observed in the incidence of distant tumors; however, the cases involving locoregional tumor stage increased significantly during this period (AAPC = 2.7%, 95% CI: 1.1%–4.4%). Annual HB incidence increased significantly amongst cases involving patients who reside in metropolitan counties with populations over 1 million people (AAPC = 2.3%, 95% CI: 0.4%–4.2%) and metropolitan counties with populations under 250,000 people (AAPC = 5.4%, 95% CI: 0.6%–10.3%).
RR of developing HB amongst female compared to male cases was 0.68 (95% CI: 0.62–0.74). NH AIAN cases had the highest relative risk (RR = 1.58, 95% CI: 1.11–2.26) compared to NH White cases, followed by NH API cases (RR = 1.18, 95% CI: 0.98–1.42) and Hispanic cases (RR = 1.16, 95% CI: 1.05–1.28). Risk of HB amongst NH Black cases was lower than all other race/ethnicity groups (Table 1).
3.3 |. Relative survival
Five-year relative survival of HB cases diagnosed between 2001 and 2016 was 76.9% (95% CI: 74.9–78.8) (Table 2). Relative survival was not different based on date of diagnosis: 74.9% (95% CI: 72.1%–77.5) for cases diagnosed between 2001 and 2009 and 79.1% (95% CI: 76.0%–81.9%) for those diagnosed between 2010 and 2016. Relative survival of cases involving locoregional tumors was 80.8% (95% CI: 77.7%–83.5%) for those diagnosed between 2001 and 2009 and 85.0% (95% CI: 81.7%–87.7%) for those diagnosed between 2010 and 2016.
3.4 |. Overall survival
Five-year overall survival of HB cases diagnosed between 2001 and 2016 was 76.7%. Five-year survival was significantly different by age group (p < .001), with 79.4%, 69.6%, and 56.3% of the 0–2-year, 3–7-year, and 8–19-year-old age groups achieving 5-year survival, respectively (Figure 1A). Sex did not have significant effects on survival outcomes (Figure 1B). Kaplan–Meier analysis did not yield significant differences in 5-year survival by race or ethnicity: 5-year survival amongst NH other, NH White, Hispanic, and NH Black cases was 82.3%, 77.5%, 76.1%, and 70.4%, respectively (Figure 1C). County metropolitan classification did not have significant effects on survival outcomes (Figure 1D).
FIGURE 1.
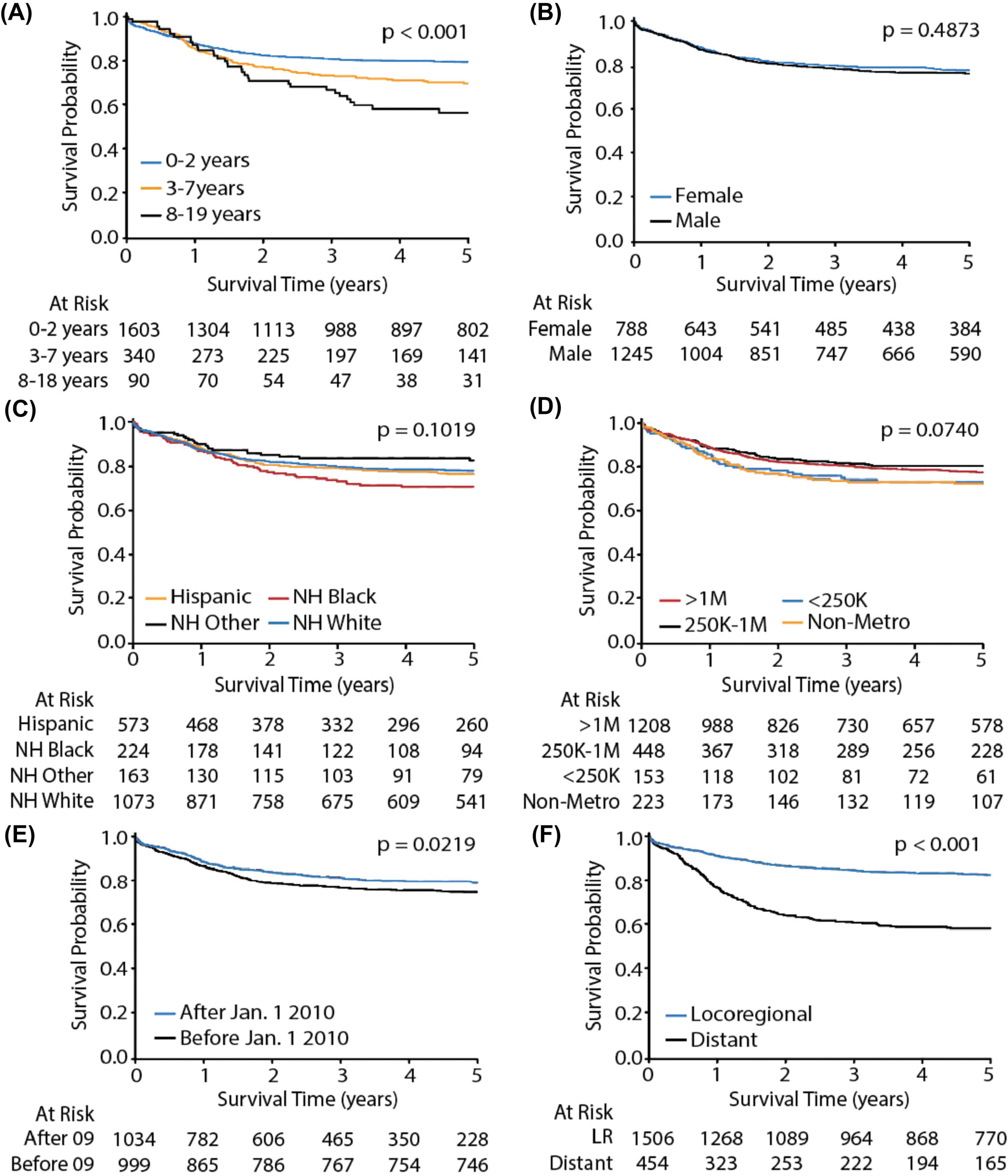
Five-year overall survival of hepatoblastoma cases of children and adolescents. Panels demonstrate subgroup analyses by (A) age, (B) sex, (C) race/ethnicity (NH = non-Hispanic), (D) county population, (E) date of diagnosis, and (D) tumor stage. LR = locoregional
Survival was significantly different based on date of diagnosis: 79.0% of cases diagnosed after January 1, 2010, achieved 5-year survival, whereas only 74.7% of cases diagnosed before this date achieved 5-year survival (p = .0219; Figure 1E). Cases involving locoregional tumors had significantly higher 5-year survival compared to those involving distant tumor stage, with 82.5% and 58.0% achieving 5-year survival, respectively (p < .001; Figure 1F).
Multivariable Cox regression analysis of 5-year overall survival was used to define independent risk markers of outcome (Figure 2). The risk of death was higher for 3–7 years (HR = 1.38, 95% CI: 1.10–1.76) and 8–19 years (HR = 1.83, 95% CI: 1.31–2.70) compared to ages 0–2 years and, distant compared to locoregional stage (HR = 2.77, 95% CI: 2.27–3.36). Notably, NH Black race or ethnicity was a marker of reduced survival (HR = 1.39, 95% CI: 1.02–1.84, p = .0345) relative to NH White race/ethnicity. This finding was statistically significant in the 0–2-year age group, but not in older age groups, likely due to the reduced frequency of HB in individuals over 2 years of age (Table S1). Other race/ethnicities were not independent risk markers of change in 5-year overall survival. Diagnosis of HB after January 1, 2010 was a significant risk marker of improved survival (HR = 0.77, 95% CI: 0.63–0.93) (Figure 2).
FIGURE 2.
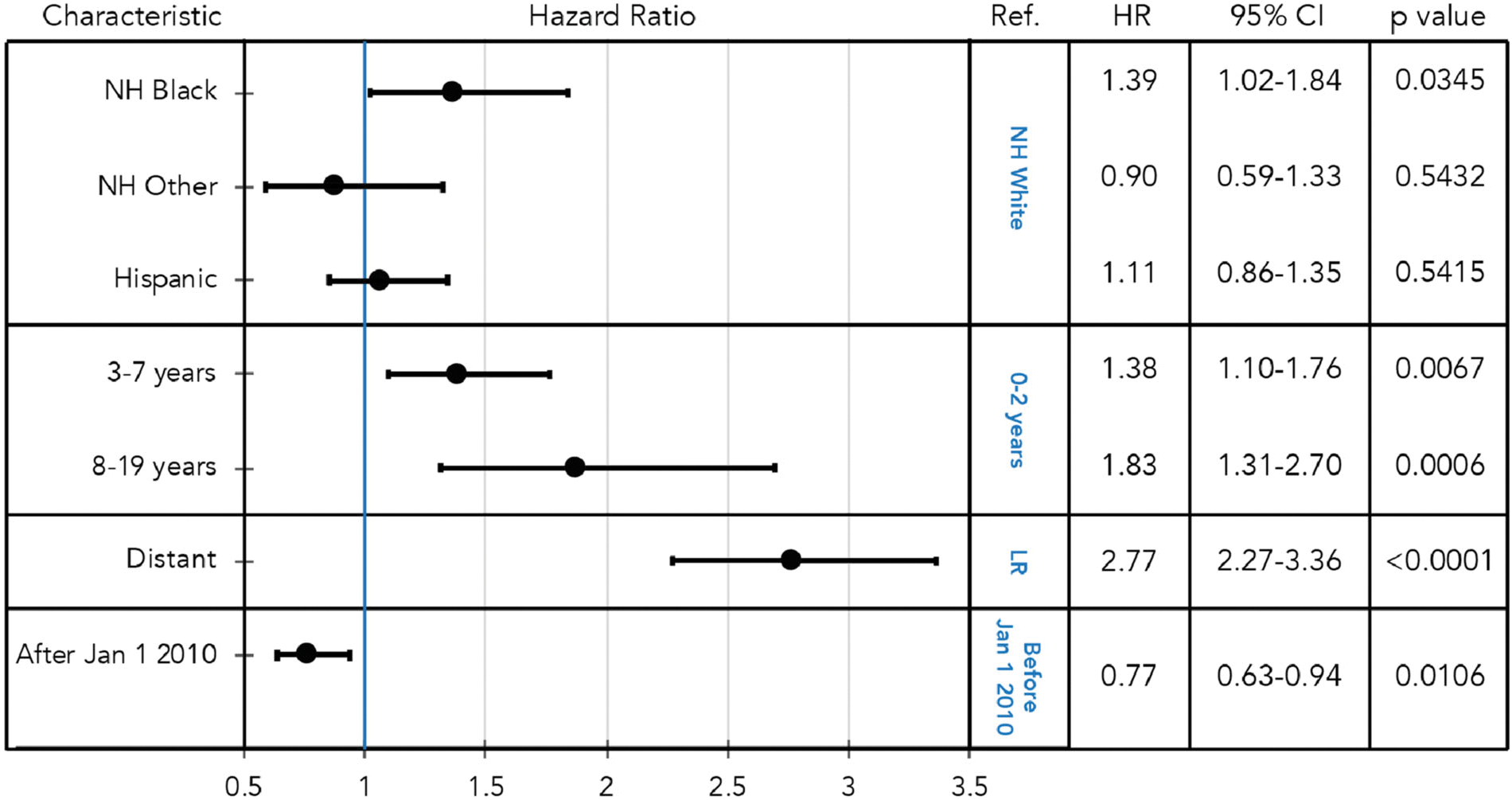
Risk factors for 5-year overall survival in children and adolescents with hepatoblastoma: hazard ratios with 95% confidence intervals (CI) are shown for indicated subgroups with corresponding p-values calculated by multivariable Cox regression analysis. Ref: reference
4 |. DISCUSSION
We show that the incidence of HB and survival has increased over the past two decades in the United States, disparities in survival exist by age, race or ethnicity, and stage, and improvements in survival after 2009 may reflect systematic changes in therapy introduced to the field.1,8–12 Our study differs from prior studies in that it utilizes databases that include between 100% and 94% of pediatric HB cases during the study period, allowing for comprehensive analysis of recent trends in incidence and survival outcomes, respectively.
The overall incidence of HB increased between 2003 and 2017. HB incidence increased significantly in individuals under 10 years of age, but not in individuals aged 10–19 years. This increase is consistent with findings from prior smaller retrospective studies with lower levels of coverage of the US population; these results collectively demonstrate the increasing burden of pediatric HB in the United States.1–3,7 Several studies have demonstrated average annual percent increases in HB incidence ranging from 2.18% to 4.3%, with one study finding a 2.6% average annual increase from 1988 to 2012 in North America amongst patients under 4 years of age.1,2,7 HB incidence may be increasing due to increasing survival amongst premature, preterm, and low birth weight births. Earlier, more accurate disease detection, improved reporting, and changes in healthcare service delivery may have also contributed to this increase; however, these variables alone likely incompletely explain the increasing pediatric HB burden, which may have been precipitated by complex genetic and environmental interactions.1,3,5,7 We also observed a previously undescribed, significant increase in the incidence of locoregional HB (adjusted for age) without a concurrent, significant increase in distant case incidence. Improved access to and increased use of imaging modalities, including magnetic resonance imaging and ultrasound, for pediatric tumors, and the standardization of HB diagnosis and staging may have contributed to this change by allowing for more expedient identification of HB at an earlier stage.23–25
Five-year overall survival increased during 2001–2016, and survival for cases diagnosed at earlier ages had better overall 5-year survival outcomes when compared to cases involving individuals older than 7 years of age. This survival difference may be related to the poor responsiveness to chemotherapy of specific subsets of HBs, including the recently recognized entity “hepatocellular neoplasm, not otherwise specified.” This subtype includes biphasic features of both HB and hepatocellular carcinoma and is more prevalent in older patients.26–28 As established in prior studies, age and stage at diagnosis remain amongst the most significant factors impacting case survival.1,2 Overall survival outcomes significantly improved between 2001 and 2016 when comparing 5-year outcomes before and after January 1, 2010. This improvement in case survival may reflect systematic changes in therapy introduced to the field in 2009.1,8,9 The COG AHEP0731 study established an updated risk-based treatment approach involving surgical intervention based on PRETEXT (Pretreatment Extent of Tumor) stage and postoperative chemotherapy informed by Evans staging and tumor histology. The very low-risk stratum enrolled children with completely resectable HB consisting of pure fetal histology (PFH) who did not receive chemotherapy. The low-risk stratum enrolled children with Stage I non-PFH, non-small cell undifferentiated (SCU), or Stage II non-SCU HB. These patients were treated with two cycles of cisplatin (CDDP), 5-fluorouracil (5FU), and vincristine (VCR).11 The intermediate-risk stratum included patients with Stages I and II HB containing SCU components or Stage III HB without SCU involvement. These patients received six cycles of CDDP, 5FU, VCR, and doxorubicin (DOX).10 Lastly, the high-risk stratum enrolled patients with any Stage IV disease or any stage HB with the initial alpha-fetoprotein level <100 ng/ml. The treatment of high-risk patients included an investigational upfront window containing two cycles of VCR and irinotecan (VI) and later with the addition of temsirolimus (VIT) followed by CDDP, VCR, 5FU, and DOX for six cycles with an additional two cycles of VI or VIT for patients with response to the upfront window.12 PRETEXT 3 extensive multifocal, PRETEXT 3 +V, PRETEXT 3 +P, or PRETEXT 4 extensive multifocal tumors were candidates for orthotopic liver transplantation.10 These updated treatment regimens may have been employed outside of clinical trials and, in combination with improved supportive care, could have affected survival of patients regardless of enrollment in a clinical study.
We identify that in the survival of patients with HB, racial and ethnic disparities exist, which have not been previously described, but have been observed with other cancers, including hepatocellular carcinomas, brain and central nervous system tumors, leukemias, and lymphomas.29–32 Specifically, we found that NH Black race or ethnicity was a significant risk marker of reduced overall survival in multivariable Cox regression analysis. Race or ethnicity overall was not found to have a significant effect on overall unadjusted survival by Kaplan–Meier analysis, and the Cox regression results indicate a significant difference in survival when comparing NH Black case specifically to NH White cases and also adjusting for the effects of the other covariates in the model. This disparity in survival may be the result of racial and ethnic disparities in access to healthcare and quality of healthcare delivered in the United States or differences in tumor biology.30–32
This study has limitations. Age groups provided by the USCS database are preset and do not specifically match age groups used in the analysis of the NPCR survival database, which more closely reflects the age groups currently used in the clinical risk stratification and treatment of HB and serve as important prognostic factors.33 Second, there were insufficient numbers to examine data for AIAN and API groups in the survival analysis. Third, the comparison of 5-year survival before and after January 1, 2010 may not be sufficient to associate recent improvements in survival with updated risk stratification-based therapies for pediatric HB. These databases do not allow for analysis of 5-year survival stratified by clinically determined risk or treatment regimen. No cases involved in clinical trials are included in either dataset. Lastly, all cases are deidentified and do not allow for verification of presence in both datasets; however, presence in both datasets is likely given their high case coverage.
The incidence of pediatric HB in the United States increased in recent decades, which was accompanied by an increase in 5-year survival outcomes before and after January 1, 2010. Despite these improvements in survival, disparities exist by age, tumor stage, and race or ethnicity, particularly of NH Black patients. These data may inform risk stratification of HB cases and encourage the investigation of existing disparities in survival outcomes. Innovative interventions are needed to address HB in patients older than age 7 years and those with distant disease. Systemic healthcare changes, including improved access to high-quality care amongst patients from racial and ethnic minority groups in the United States, might help address observed disparities in HB survival by race or ethnicity.
Supplementary Material
ACKNOWLEDGMENTS
We thank the U.S. Cancer Statistics database, the National Program of Cancer Registries, and the Centers for Disease Control and Prevention for data availability. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- 5FU
5-fluorouracil
- AAPC
average annual percent change
- AIAN
American Indian or Alaska Native
- API
Asian or Pacific Islander
- CDC
Centers for Disease Control and Prevention
- CDDP
cisplatin
- CI
confidence interval
- COG
Children’s Oncology Group
- DOX
doxorubicin
- HB
hepatoblastoma
- HR
hazard ratio
- NH
non-Hispanic
- NPCR
National Program of Cancer Registries
- PFH
pure fetal histology
- RR
relative risk
- SCU
small cell undifferentiated
- SEER
Surveillance, Epidemiology, and End Results
- USCS
United States Cancer Statistics
- VCR
vincristine
- VI
vincristine/irinotecan
- VIT
vincristine/irinotecan/temsirolimus
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest relevant to this article.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request by contacting uscsdata@cdc.gov. The data are not publicly available due to privacy and legal restrictions.
REFERENCES
- 1.Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford). 2013;15(10):741–746. 10.1111/hpb.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun. 2019;39(1):62. 10.1186/s40880-019-0411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2019;23(2):79–95. 10.1177/1093526619875228 [DOI] [PubMed] [Google Scholar]
- 4.Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59(5):776–779. 10.1002/pbc.24215 [DOI] [PubMed] [Google Scholar]
- 5.Aronson DC, Meyers RL. Malignant tumors of the liver in children. Semin Pediatr Surg. 2016;25(5):265–275. 10.1053/j.sempedsurg.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Siegel DA, Li J, Henley SJ, et al. Geographic variation in pediatric cancer incidence - United States, 2003–2014. MMWR Morb Mortal Wkly Rep. 2018;67:707–713. 10.15585/mmwr.mm6725a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard AK, Spector LG, Fortuna G, Marcotte EL, Poynter JN. Trends in international incidence of pediatric cancers in children under 5 years of age: 1988–2012. JNCI Cancer Spectr. 2019;3(1):pkz007. 10.1093/jncics/pkz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer. 2012;59(5):818–821. 10.1002/pbc.24217 [DOI] [PubMed] [Google Scholar]
- 9.Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic Tumors International Collaboration. Lancet Oncol. 2017;18(1):122–131. 10.1016/S1470-2045(16)30598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzenstein HM, Malogolowkin MH, Krailo MD, et al. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: a Children’s Oncology Group study. Cancer. 2022;128(5):1057–1065. 10.1002/cncr.34014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzenstein HM, Langham MR, Malogolowkin MH, et al. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children’s Oncology Group, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):719–727. 10.1016/S1470-2045(18)30895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzenstein HM, Furman WL, Malogolowkin MH, et al. Vincristine/irinotecan upfront window treatment of high-risk hepatoblastoma: a report from the Children’s Oncology Group (COG) AHEP0731 study committee. Cancer. 2017;123(12):2360–2367. 10.1002/cncr.30591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. United States Cancer Statistics. Published March 8, 2021. Accessed April 4, 2021. https://www.cdc.gov/cancer/uscs/index.htm
- 14.CDC. National Program of Cancer Registries (NPCR). Published March 31, 2021. Accessed April 4, 2021. https://www.cdc.gov/cancer/npcr/index.htm
- 15.CDC. Publication criteria: U.S. Cancer statistics data visualizations tool technical notes. Published June 23, 2021. Accessed February 20, 2022. https://www.cdc.gov/cancer/uscs/technical_notes/criteria/index.htm
- 16.Steliarova-Foucher E, Stiller C, Kaatsch P. International classification of childhood cancer, third ed. Cancer. 2005;103(7):1457–1467. 10.1002/cncr.20910 [DOI] [PubMed] [Google Scholar]
- 17.SEER Training. Review of staging systems. Accessed April 4, 2021. https://training.seer.cancer.gov/collaborative/intro/systems_review.html
- 18.U.S. Cancer Statistics Public Use Database, CDC. Merged summary stage Variable. Published March 8, 2021. Accessed April 4, 2021. https://www.cdc.gov/cancer/uscs/public-use/dictionary/merged-summary-stage.htm
- 19.Joinpoint. Joinpoint regression program. Accessed April 4, 2021. https://surveillance.cancer.gov/joinpoint/
- 20.Siegel DA, Richardson LC, Henley SJ, et al. Pediatric cancer mortality and survival in the United States, 2001–2016. Cancer. 2020;126(19):4379–4389. 10.1002/cncr.33080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SEER. Surveillance, Epidemiology, and End Results Program. Accessed April 4, 2021. https://seer.cancer.gov/index.html
- 22.SEER. SEER*Stat Software. Accessed April 4, 2021. https://seer.cancer.gov/seerstat/index.html
- 23.Smith-Bindman R, Kwan ML, Marlow EC, et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA. 2019;322(9):843. 10.1001/jama.2019.11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handa A, Nozaki T, Makidono A, et al. Pediatric oncologic emergencies: clinical and imaging review for pediatricians. Pediatr Int. 2019;61(2):122–139. 10.1111/ped.13755 [DOI] [PubMed] [Google Scholar]
- 25.Towbin AJ, Meyers RL, Woodley H, et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48(4):536–554. 10.1007/s00247-018-4078-z [DOI] [PubMed] [Google Scholar]
- 26.López-Terrada D, Alaggio R, De Dávila MT, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014; 27(3):472–491. 10.1038/modpathol.2013.80 [DOI] [PubMed] [Google Scholar]
- 27.Geramizadeh B, Foroughi R, Shojazadeh A. Hepatocellular malignant neoplasm, not otherwise specified: a new name in liver tumors: a brief narrative review of published cases. Gastrointest Tumors. 2021;8(2): 96–100. 10.1159/000513962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou S, Malvar J, Chi Y-Y, et al. Independent assessment of the children’s hepatic tumors international collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. 10.1001/jamanetworkopen.2021.48013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haizel-Cobbina J, Spector LG, Moertel C, Parsons HM. Racial and ethnic disparities in survival of children with brain and central nervous tumors in the United States. Pediatr Blood Cancer. 2021;68(1):e28738. 10.1002/pbc.28738 [DOI] [PubMed] [Google Scholar]
- 30.Siegel DA, Li J, Ding H, Singh SD, King JB, Pollack LA. Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States. Pediatr Blood Cancer. 2019;66(2):e27501. 10.1002/pbc.27501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delavar A, Barnes JM, Wang X, Johnson KJ. Associations between race/ethnicity and US childhood and adolescent cancer survival by treatment amenability. JAMA Pediatr. 2020;174(5):428–436. 10.1001/jamapediatrics.2019.6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathur AK. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145(12):1158–1163. 10.1001/archsurg.2010.272 [DOI] [PubMed] [Google Scholar]
- 33.Haeberle B, Rangaswami A, Krailo M, et al. The importance of age as prognostic factor for the outcome of patients with hepatoblastoma: analysis from the Children’s Hepatic tumors International Collaboration (CHIC) database. Pediatr Blood Cancer. 2020;67(8):e28350. 10.1002/pbc.28350 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request by contacting uscsdata@cdc.gov. The data are not publicly available due to privacy and legal restrictions.


