Abstract
Lipopolysaccharide (LPS) is the major mediator of gram-negative septic shock. Molecules that bind LPS and neutralize its toxic effects could have important clinical applications. We showed that serum amyloid P component (SAP) neutralizes LPS. A SAP-derived peptide, consisting of amino acids 27 to 39, inhibited LPS-mediated effects in the presence of human blood. In this study, we used a pepscan of overlapping 15-mer peptides and distinguished two additional LPS-binding regions within the SAP molecule, identified in the regions spanning amino acids 61 to 75 and 186 to 200. The corresponding SAP-derived peptides, pep61-75 and pep186-200, inhibited the binding of fluorescein isothiocyanate-labeled LPS to monocytes as efficiently as a bactericidal/permeability-increasing protein (BPI)-derived 15-mer peptide comprising amino acids 85 to 99. The same SAP-derived peptides very potently inhibited LPS-induced priming of phagocytes in human blood. Also, SAP-derived pep186-200 caused a prolonged survival of actinomycin D-sensitized mice treated with LPS to induce septic shock, indicating a potential use of this peptide in the defense against serious gram-negative sepsis in humans.
Lipopolysaccharide (LPS) is the major constituent of the outer membrane of gram-negative bacteria. In the case of gram-negative infection, LPS is the well-known activator of the humoral and cellular components of the host defense system. Activation of the host defense is essential to fight gram-negative infection, but uncontrolled stimulation can also result in the serious and life-threatening symptoms of septic shock (4). Cellular activation requires CD14, which is expressed on monocytes, macrophages, and neutrophils, while CD14-negative cells, such as endothelial, epithelial, and smooth muscle cells, can be activated by LPS, via interaction of LPS with the soluble form of CD14 present in the plasma (sCD14) (2, 3, 17, 38). Another important factor in plasma is LPS-binding protein (LBP), which is able to accelerate binding of LPS to (s)CD14 100- to 1,000-fold. LBP can also accelerate movement of LPS to high-density lipoproteins, which neutralizes the capacity of LPS to stimulate cells (29, 39). Other LPS-binding proteins, such as bactericidal/permeability-increasing protein (BPI), cationic protein 18 (CAP18), and CAP37, are known to play a role in LPS-mediated effects. These are microbicidal proteins found in the azurophilic granules of neutrophils that function primarily intracellularly and at focal sites of inflammation.
Recently we described a new LPS-binding protein in human plasma (8). This protein, serum amyloid P component (SAP), belongs to the family of pentraxins. It is a decameric serum glycoprotein composed of identical 25.5-kDa subunits noncovalently associated in two pentameric rings interacting face to face. SAP is associated with all forms of amyloid deposits, including amyloid deposits in Alzheimer’s disease. Reports about the involvement of SAP in the persistence of amyloid deposits are contradictory, demonstrating its contribution to amyloidosis by stabilizing the deposits (5, 13) but also showing its inhibition of the formation of Alzheimer β-peptide fibrils (24). In mice, SAP is an acute-phase reactant, but in humans SAP is constitutively present in serum at 40 μg/ml, with a maximum twofold increase during sepsis (15, 33). Although its exact physiological function is still unclear, it is believed to play a role in the binding and clearance of host- or pathogen-derived cellular debris at sites of acute inflammation (15). SAP was demonstrated to bind all forms of LPS via the lipid A part of the molecule. Moreover, SAP was able to neutralize the biologic effects of LPS in several assays, although only at low concentrations of LBP and not in the presence of serum or in human blood. A peptide comprising amino acids 27 to 39 of the SAP sequence, called pep27-39, was identified as an LPS-binding motif within the SAP protein. In the literature, this peptide, or pep27-38, was reported to interfere with the binding of SAP to heparin and C4b-binding protein (14, 19, 27) and to support cell attachment (9). Pep27-39 was found to bind to LPS and to inhibit the LPS-induced responses in human phagocytes in the presence of serum and also in human blood.
In this study we used a panel of overlapping 15-mer peptides of SAP to look for additional LPS-binding regions within the SAP protein.
MATERIALS AND METHODS
Reagents.
LPS from Salmonella minnesota R595 (ReLPS) was obtained from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human LBP was a generous gift from H. Lichenstein (Amgen, Boulder, Colo.).
Synthetic peptides.
The sequence of SAP (amino acids 1 to 204) was divided into 39 different 15-mer peptides that progressed along the SAP sequence by initiating a new peptide every sixth amino acid. Peptides were prepared by automated simultaneous multiple peptide synthesis, set up by using a standard autosampler (Gilson 221) as described previously (37). Briefly, standard 9-fluorenylmethoxycarbonyl chemistry with in situ PyBop/N-methylmorpholine (Novabiochem, Laufelfingen, Switzerland) activation of the amino acids in a fivefold molar excess with respect to 2 μmol/peptide PAL-PEG-PS resin (Perseptive Biosystems, Framingham, Mass.) was used. Peptides were obtained as C-terminal amides after cleavage with 90 to 95% trifluoroacetic acid-containing scavenger cocktails. Most peptides were dissolved in distilled H2O to a concentration of 5 mM; others were dissolved in a mixture of dimethyl sulfoxide, 60% acetonitrile, and 8 M urea or 8 M urea acidified with acetic acid to a similar concentration. All peptides were further diluted in 0.25 M Tris-HCl (pH 7.5) to a concentration of 0.6 mM. Before use in biological assays, the peptides were diluted in Hanks’ balanced salt solution (HBSS) containing 0.2% human serum albumin (HSA; Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands). SAP-derived peptide pep186-200 was also synthesized in larger quantities by Isogen Bioscience (Maarssen, The Netherlands) for protection experiments using mice.
Cell isolation.
Human neutrophils and peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood drawn from healthy volunteers as described elsewhere (35).
Binding of FITC-LPS to human monocytes.
Fluorescein isothiocyanate-labeled recombinant LPS (FITC-LPS) was prepared as described elsewhere (34) with a molar labeling efficiency of 1:1. For FITC-LPS binding studies, FITC-LPS (2.5 ng/ml) was preincubated with increasing amounts of peptides (0 to 30 μM) for 30 min at 37°C in HBSS containing 0.2% HSA. Thereafter, LBP (10 ng/ml) and PBMC (6 × 106/ml) in the same buffer were added to a final volume of 50 μl. The mixture was gently shaken for 30 min at 37°C and put on ice. Binding of FITC-LPS to monocytes was analyzed on a FACScan, using forward and sideward scatter parameters to distinguish monocytes from lymphocytes. The results were expressed as the mean fluorescence of 10,000 cells. Percent inhibition of binding was calculated with the following formula: 1 − (A − bgrA/B − bgrB) × 100, where A is the mean fluorescence of cells incubated with FITC-LPS, LBP, and peptide, B is the mean fluorescence of cells incubated with FITC-LPS plus LBP, bgrA is the background fluorescence of cells incubated with FITC-LPS plus peptide, and bgrB is the background fluorescence of cells incubated with FITC-LPS alone.
LPS-induced priming of human neutrophils.
The procedure was described in detail elsewhere (35). Briefly, freshly isolated neutrophils (105/ml) were added to a mixture of LPS (1 ng/ml) alone or with increasing amounts of peptides (0 to 30 μM) in the presence of LBP (10 ng/ml in HBSS–2% HSA). LPS and peptides were preincubated for 30 min at 37°C before cells were added. Cells were incubated with the mixtures for 30 min at 37°C under constant agitation. Next, chemiluminescence response was measured in a luminometer (Autolumat LB 953; Berthold GmbH & Co., Wildbad, Germany) after automated injection of N-formyl-methionyl-leucyl-phenylalanine (fMLP; final concentration of 1 μM) and HBSS containing 180 μM luminol (Sigma). In a whole blood assay, LPS (1 to 10 ng/ml) was preincubated in a volume of 20 μl with or without 0 to 30 μM peptides for 30 min at 37°C. Thereafter, 80 μl of human heparinized blood was added and agitated for 30 min at 37°C. Next, 900 μl of PBS–0.05% glucose was added, and 100 μl was used to measure the chemiluminescence response in a luminometer as described above. The chemiluminescence response was measured automatically over a period of 10 min. Data were analyzed with the AXIS software package (ExOxEmis, San Antonio, Tex.). Curves were obtained for all samples presenting the chemiluminescence response in counts per minute versus time. Absolute counts are obtained by calculating the area under the curve of the chemiluminescence for 10 min. In some experiments, 1 nM recombinant tumor necrosis factor alpha (TNF-α; Sigma) was used to prime blood for an enhanced fMLP response in the presence of peptides.
Heparin affinity chromatography.
About 60 μg of peptide in 100 μl of Tris-HCl (pH 7.2) was loaded onto a heparin-Sepharose column (Pharmacia), using the Pharmacia FPLC system. A linear NaCl gradient of 0 to 1 M over 20 ml (0.5 ml/min) was used to elute peptides from the column. The molarity of NaCl at which peptides eluted was used as a measure to compare heparin-binding affinities of different tested peptides.
Animal protection experiments.
Actinomycin D-sensitized BALB/c mice were used in lethality experiments to test the potential of SAP-derived peptide pep186-200 to neutralize the toxicity of ReLPS. ReLPS was preincubated with pep186-200 in 0.2% HSA for 30 min at 37°C. This mixture together with 20 μg of actinomycin D (MSD, Rahway, N.J.) in a total volume of 200 μl was intravenously injected in mice. A total of 3 pg of ReLPS with or without pep186-200 (5 mg/kg of body weight) was injected per mouse. Each group consisted of eight mice. Two additional groups of four mice each, one group injected with buffer and one injected with pep186-200 only, together with 20 μg of actinomycin D, were also tested.
RESULTS
Synthetic 15-mer SAP peptides.
To investigate what part of the SAP sequence was responsible for binding to LPS, we synthesized and tested a panel of overlapping 15-mer peptides. All peptides were analyzed by reverse-phase high-pressure liquid chromatography and contained one major peak accounting for more than 75% of the peak areas in the sample (data not shown).
Inhibition of binding of FITC-LPS to human monocytes by SAP-derived peptides.
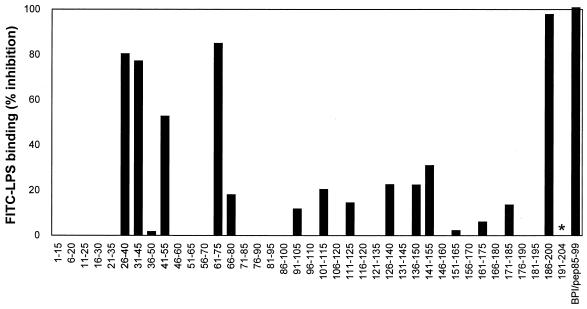
Testing the synthetic 15-mer SAP peptides showed that several peptides within the SAP sequence were able to prevent the binding of FITC-LPS to human monocytes (Fig. 1). The most active peptides were pep26-40, pep31-45, pep41-55, pep61-75, and pep186-200, with more than 50% inhibition at a concentration of 3 μM. To evaluate the relative potency of these SAP peptides, a 15-mer BPI peptide comprising amino acids 85 to 99 (BPI/pep85-99) was also tested for its capacity to inhibit binding of FITC-LPS to monocytes. BPI/pep85-99 is described as a highly potent LPS-binding and -neutralizing peptide. Comparison of 50% inhibitory concentrations (IC50s) of the peptides (Table 1) shows that pep186-200 was the most active, even more active than the BPI peptide, in preventing the FITC-LPS binding to monocytes. To exclude the possibility that the peptides prevented binding of LPS to the monocytes via binding to the monocytes itself, control experiments were performed with all active peptides. Therefore, PBMC were preincubated with each peptide for 30 min and washed three times to remove unbound peptide. Subsequent incubation of the PMBC with FITC-LPS followed by FACScan analysis showed that preincubation of PBMC with all peptides did not inhibit binding of FITC-LPS to monocytes.
FIG. 1.
Three regions within the SAP molecule inhibit the FITC-LPS binding to human monocytes. A pepscan of 10-mer overlapping 15-mer peptides of SAP was used to determine the LPS-binding and -neutralizing regions of SAP. SAP-derived peptides pep1-15 to pep186-200 and BPI/pep85-99, all 3 μM, were incubated with FITC-LPS (2.5 ng/ml) for 30 min at 37°C. This mixture was incubated with PBMC and LBP (10 ng/ml) for 30 min at 37°C, after which FITC-LPS binding to monocytes was assessed by FACScan analysis. Pep191-204 was not determined (∗). Data represent the mean of at least two separate experiments.
TABLE 1.
FITC-LPS binding to monocytes
| Peptide | IC50 ± SEM (μM) |
|---|---|
| Pep26-40 | 0.25 ± 0.03 |
| Pep31-45 | 1.11 ± 0.22 |
| Pep41-55 | 4.41 ± 0.43 |
| Pep61-75 | 0.21 ± 0.06 |
| Pep186-200 | 0.06 ± 0.01 |
| BPI/pep85-99 | 0.13 ± 0.03 |
SAP-derived peptides pep61-75 and pep186-200 inhibit the LPS-induced oxidative burst in human blood.
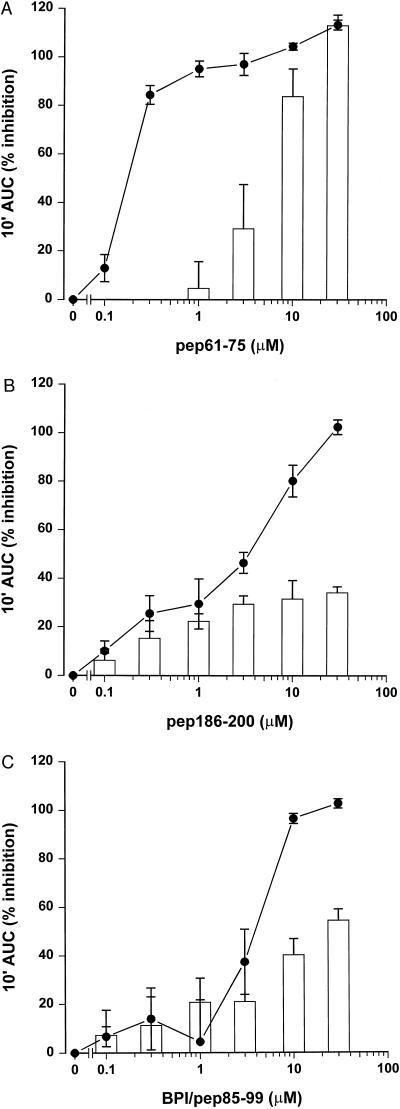
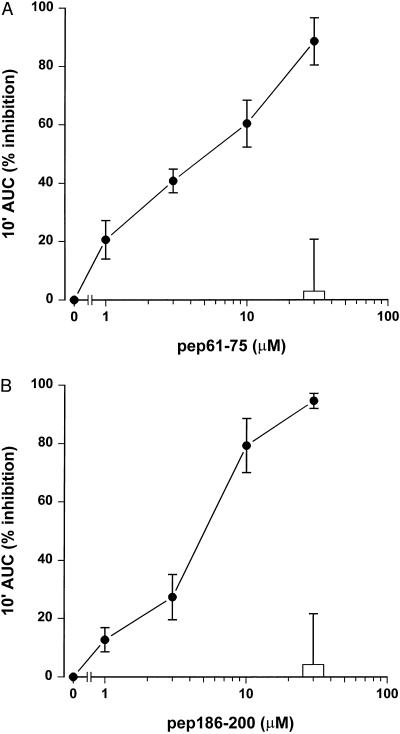
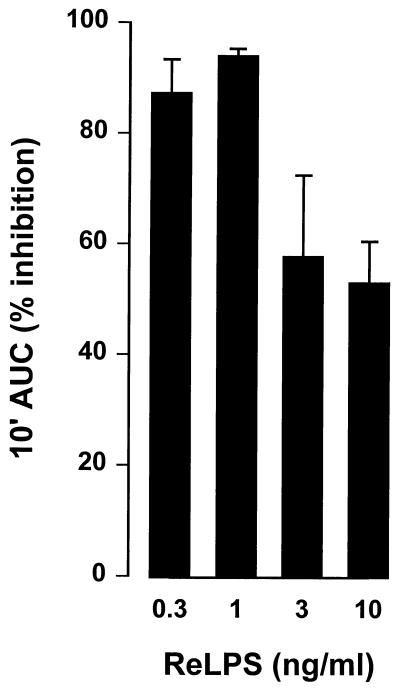
To evaluate the effects of the SAP peptides in a functional assay, we tested the capacity of SAP peptides pep61-75 and pep186-200 and BPI/pep85-99 to inhibit the LPS-induced priming of human neutrophils. Isolated neutrophils were primed with LPS (1 ng/ml) for an enhanced fMLP response in the presence of LBP (10 ng/ml) for 30 min. Figure 2 shows that addition of all three peptides resulted in a dose-dependent inhibition of the LPS-induced priming. Pep61-75 had an especially potent LPS-inhibiting effect, with an IC50 of 0.2 μM; IC50s of pep186-200 and BPI/pep85-99 were 3 and 4 μM, respectively. However, when neutrophils were primed with TNF-α instead of LPS, as a control for nonspecific effects, pep61-75 showed considerable higher inhibitory effects at the highest concentrations compared to pep186-200 and BPI/pep85-99. The effect of the SAP peptides on the LPS-induced priming of phagocytes was also studied in the presence of human blood. Figure 3 shows that both SAP peptides pep61-75 and pep186-200 were very potent in inhibiting the LPS-induced priming of phagocytes in the complex environment of human blood, with comparable IC50s of 5 μM. Both peptides did not show any effect on the oxidative burst when whole human blood was primed with TNF-α instead of LPS, indicative for the lack of toxic effect of the peptides on the phagocytes in the presence of human blood. Scrambled peptides of both pep61-75 and pep186-200, comprising the same amino acids in a random order, showed no effect on the LPS-induced priming of whole human blood (data not shown). As pep186-200 appeared to be the most promising LPS-neutralizing SAP peptide, exhibiting less toxic effects, we tested the ability of this peptide to inhibit higher concentrations of LPS. Figure 4 shows that 30 μM pep186-200 was still able to inhibit the LPS-induced priming of phagocytes up to 50% when human blood was primed with 10 ng of LPS per ml.
FIG. 2.
SAP-derived peptides inhibit the LPS-induced priming of human neutrophils. Increasing amounts of SAP-derived peptides pep61-75 (A) and pep186-200 (B) and BPI/pep85-99 (C) were incubated with 1 ng of LPS per ml (solid circles) or 1 nM TNF-α (open bars) for 30 min at 37°C. Isolated neutrophils and LBP (10 ng/ml) were added, and after 30 min at 37°C the fMLP-mediated chemiluminescence response was measured. Data represent the percent inhibition of the 10-min integral area under the curve (10′ AUC) of four separate experiments ± standard error of the mean.
FIG. 3.
Pep61-75 and pep186-200 inhibit the LPS-induced priming of human blood. Heparinized human blood was primed for 30 min at 37°C with 1 ng of ReLPS per ml and increasing amounts of pep61-75 (A) and pep186-200 (B). The fMLP-mediated chemiluminescence in 10-fold diluted blood was measured for 10 min. Data represent the percent inhibition of the 10-min integral area under the curve (10′ AUC) of five separate experiments ± standard error of the mean.
FIG. 4.
Pep186-200 neutralizes increasing amounts of LPS in the LPS-induced priming of human blood. Human blood was primed for 30 min at 37°C with 1 to 10 ng of ReLPS per ml and 30 μM pep186-200. An fMLP-induced chemiluminescence in 10-fold diluted blood was measured for 10 min. Data represent the percent inhibition of the 10-min integral area under the curve (10′ AUC) of three separate experiments ± standard error of the mean.
Binding of LPS-binding peptides to heparin.
Binding of the LPS-binding SAP-derived peptides to heparin was analyzed by heparin affinity chromatography. The NaCl concentration at which the peptides eluted from a heparin-Sepharose column were determined and used as an indicator for affinity. BPI/pep85-99, used as a control, heparin-binding peptide, eluted from the column at 0.45 M NaCl, while SAP-derived peptides pep27-39 and pep186-200 eluted at 0.15 M NaCl. Pep61-75 showed no binding affinity for heparin. Scrambled peptides of pep27-39 and pep186-200 were also tested for heparin-binding capacity, and both showed elution patterns comparable to those of the original peptides.
SAP-derived peptide pep186-200 protects mice from LPS-induced septic shock.
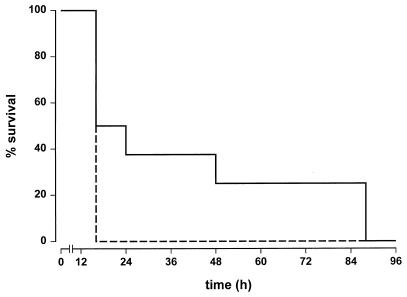
To investigate the effectivity of the SAP-derived peptides in vivo, we tested whether pep186-200 was able to inhibit LPS-induced septic shock in actinomycin D-sensitized mice. Therefore, ReLPS was preincubated for 30 min with pep186-200 at 37°C. Mice were injected with a total of 3 pg of ReLPS in the presence or absence of 5 mg of pep186-200 per kg. Although all mice eventually died, the Kaplan-Meier plot clearly shows a prolonged survival of the mice receiving ReLPS together with pep186-200 compared to ReLPS alone (Fig. 5).
FIG. 5.
Pep186-200 protects actinomycin D-sensitized mice against an LPS-induced septic shock. BALB/c mice were injected with 3 pg of ReLPS per mouse with (continuous line) or without (dashed line) pep186-200 (5 mg/kg). Each group consisted of eight mice. Two additional groups of four mice each, one group injected with buffer and one injected with pep186-200 only, were also tested; all of these mice survived (data not shown).
DISCUSSION
In this study we show that three regions within the SAP protein are able to bind LPS with LPS-neutralizing activities. We demonstrated in an earlier study that one of these three LPS-binding regions, comprised of amino acids 26 to 40, was involved in the binding of LPS. At that time a 13-mer SAP-derived peptide, pep27-39, was shown to bind to LPS with the capacity to neutralize LPS in the complex environment of human whole blood (8). In the present study, pep26-40 showed LPS-binding and -neutralizing activities comparable to those of pep27-39.
In this study, two other regions within the SAP protein were identified as LPS-binding regions. SAP-derived peptides pep61-75 and pep186-200 inhibited the binding of FITC-LPS to human monocytes as efficiently as the previously described BPI/pep85-99 (26). Moreover, both SAP-derived peptides also inhibited the LPS-induced oxidative burst of phagocytes in the presence of human blood. In the presence of only buffer containing LBP, both peptides had toxic effects on isolated neutrophils. However, these toxic effects were not evident when the peptides were tested in whole blood. Both pep61-75 and pep186-200 showed IC50s comparable with that of the previously reported SAP-derived peptide pep27-39 when used in its carboxamidomethylated form. Thus, we identified three SAP-derived peptides capable of inhibiting the LPS-induced oxidative burst of phagocytes in the complex environment of human blood. Although eventually all mice died, use of one of these peptides, pep186-200, led to a prolonged survival of LPS-injected mice, indicating a potential use of this peptide in the defense against gram-negative sepsis in humans.
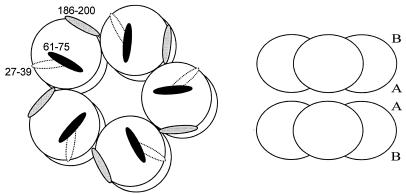
It seems strange that three regions within SAP are capable of inhibiting LPS-induced toxic effects. In Fig. 6 the approximate positions of the three LPS-binding regions within the SAP decamer are schematically drawn. The pep27-39 sequence within the SAP molecule appears not to be readily available for LPS binding, as it is partly situated on a β-strand under a short α-helix on the A-face of the SAP molecule (13). The pep61-75 and pep186-200 sequences are situated on the B-face of the SAP molecule, the presumed ligand-binding site of SAP (13). Thus, both the pep61-75 and pep186-200 sequences seem to be situated at a position within the SAP molecule that can readily be reached by LPS. Site-directed mutagenesis experiments within these three regions of SAP will be needed to elucidate the contribution of each of the regions in SAP binding to LPS. SAP is not the only LPS-binding protein with more than one recognized LPS-binding region; for BPI also, three regions are described as contributing to the total biological activity of the molecule against LPS (26). In addition, two LPS-binding regions have been found for lactoferrin (Lf) and reported to cooperate in the binding of Lf to LPS (28).
FIG. 6.
Schematic representation of decameric SAP showing the positions of the three LPS-binding regions. The three LPS-binding regions are depicted only on one pentamer of SAP. Pep27-39 (dashed area) is situated on the A-face of the SAP molecule between the two adjacent pentamers. Pep61-75 (black area) and pep186-200 (gray area) are situated on the B-face (ligand-binding site) of the SAP molecule (left). A side view of the SAP decamer showing the A-faces (A) and B-faces (B) is shown on the right.
We showed in an earlier study that SAP is an LPS-binding protein with neutralizing capacities. SAP is also reported to bind heparin. This places SAP among other LPS-binding and -neutralizing proteins with heparin-binding capacities. For BPI, CAP18, CAP37, and Lf, the LPS-binding regions have also been demonstrated to bind heparin (21, 26, 31, 36). In addition, apolipoproteins B and E (Apo B and E), also LPS-binding proteins with neutralizing capacities (12, 32), are reported to bind heparin (7), although it is not known whether these LPS- and heparin-binding regions overlap. In the case of SAP, two regions were found to bind LPS and heparin. SAP-derived peptide pep27-39 was previously found to bind heparin (19). The second LPS-binding peptide, pep186-200, partly overlaps a region within SAP, comprising amino acids 192 to 204, described to bind heparin (18, 19). Using heparin-Sepharose affinity chromatography, we showed that pep186-200 indeed bound heparin to the same extent as pep27-39. There seems to be a high resemblance between SAP and other LPS-binding proteins in that their LPS-binding regions also bind heparin. However, on the basis of sequence homology between these LPS/heparin-binding regions, SAP shows no resemblance with any of these regions of other LPS-binding proteins. Table 2 shows the heparin- and (proposed) LPS-binding regions of several LPS-binding proteins. Recently consensus binding sites for heparin-binding proteins have been described (7, 20). For example, BPI and CAP18, in contrast to pep27-39 and 186-200, show a high degree of structural similarity between the cationic/hydrophobic motif of LPS-binding molecules and the known consensus sequences of heparin binding proteins: XBBXBX or XBBBXXBX, where B is a basic amino acid and X is any amino acid, mostly hydrophobic (22, 23, 25, 26). The BPI peptide used in this study as a positive control for LPS binding and neutralization is one of the described LPS- and heparin-binding peptides showing high similarity to these consensus heparin-binding sequences (26). Also the LPS-binding proteins Apo B and E show alternating basic and hydrophobic amino acids resembling both heparin-binding consensus sequences (7, 20). Although the LPS-binding regions within Apo E and B have not been defined yet, we hypothesize them to be situated in the described heparin-binding sites of the molecules. For Lf, a small heparin-binding domain containing alternating basic and hydrophobic amino acids has been described. The same domain was also involved in the binding of Lf to LPS (10, 11). Also, a second region within Lf is thought to be involved in heparin as well as LPS binding (11, 28, 36). This region contains an N-terminal stretch of only four arginines, indicating in this case a major role of basic charged amino acids in the binding to heparin and LPS. Indeed, binding sites containing four contiguous basic amino acids were also predicted to recognize heparin (20). A same N-terminal Arg33-Arg36 cluster was found to play a major role in the binding of hepatocyte growth factor to heparin (1). However, not all described LPS-binding proteins or peptides possess these consensus heparin-binding sequences. A synthetic peptide, based on amino acids 20 to 44 of CAP37, is reported to bind and neutralize LPS in vitro and in vivo. However, although this peptide binds to heparin, it does not contain the consensus heparin-binding sequences (6, 30, 31). While the whole CAP37 protein is strongly basic, the net charge of the CAP20-44 peptide is only +2. The authors propose that the LPS-binding property of the CAP20-44 peptide is due to the combination of its hydrophobicity (56% of its amino acids), basic charge, and relatively small size (30). Screening our pepscan of SAP-derived peptides for charge and hydrophobicity, we found six peptides with a net charge ≥2 and a hydrophobicity of >50% hydrophobic amino acids. Of the three SAP-derived peptides with LPS-binding capacities, only pep186-200 (two basic and 53% hydrophobic amino acids) belongs to this group of peptides. The remaining five SAP-derived peptides with a net charge of ≥2 and a hydrophobicity of >50% showed no LPS-binding activities. This finding indicates that charge and hydrophobicity are, at least, not the only factors determining LPS-binding capacity. Moreover, the scrambled pep186-200, containing the same amino acids in random order, showed no LPS-binding capacities, which implies that the sequence of amino acids also plays an important role in the LPS-binding capacities of the SAP-derived peptides. Also the small size of the peptides, improving access to the lipid A target, could play a role.
TABLE 2.
Comparison of proposed LPS/heparin-binding regions of several LPS-binding proteins
| LPS-binding proteins (amino acids) | Amino acid sequencea | Reference |
|---|---|---|
| BPI (85–100) | N I K I S G K W K A Q K R F L K M | 23 |
| LBP (84–99) | S I R V Q G R W K V R K S F F K L | 23 |
| LALFb (32–50) | H Y R I K P T F R R L K W K Y K G K F | 22 |
| CAP18 (106–137) | G L R K R L R K F R N K I K E K L K K I G Q K I Q G L L P K L A | 25 |
| Lf (28–34) | R K V R G P P | 10 |
| Apo E (141–165)c | L R K L R K R L L R D A D D L Q K R L A V Y Q A G | 7 |
| Apo B (3361–3385)c | T R L T R K R G L K L A T A L S L S N K F V E G S | 7 |
| CAP37 (20–44) | N Q G R H F C G G A L I H A R F V M T A A S C F Q | 30 |
| SAP (27–39) | E K P L Q N F T L C F R A | 8 |
| SAP (61–75)d | L L V Y K E R V G E Y S L Y I | |
| SAP (186–200) | Q A L N Y E I R G Y V I I K P |
Boldface residues represent basic amino acids; italic residues represent hydrophobic amino acids.
Limulus anti-LPS factor.
Heparin-binding sequence not yet described as LPS-binding sequence.
SAP-derived sequence which binds LPS but not heparin.
We show a protective effect of a 15-mer SAP-derived peptide, pep186-200, against LPS-induced septic shock in mice. Although eventually all mice died, mice receiving LPS preincubated with pep186-200 clearly demonstrated attenuation of lethality, which indicates a beneficial interventional influence on parameters that determine long-term survival and which may provide a window of time for other therapeutic support. For other LPS-neutralizing peptides derived from other LPS-binding proteins, it has been shown that increasing the length of a peptide markedly enhances its LPS-neutralizing effect (16). Currently, we are investigating this possibility for pep186-200. The possibility of a peptide being able to protect against the severe clinical symptoms of LPS-induced septic shock is a promising development. Peptides are easily synthesized in large quantities at a low cost. The next step would be the development of peptoids (peptide mimics) that are resistant for degradation by proteinases in vivo, thus creating an LPS-binding compound with a prolonged half-life in vivo. Production of LPS-binding peptoids with LPS-neutralizing capacities would be a major step forward in the development of therapeutic agents against the severe clinical symptoms of septic shock.
REFERENCES
- 1.Aoyama H, Naka D, Yoshiyama Y, Ishii T, Kondo J, Mitsuka M, Hayase T. Isolation and conformational analysis of fragment peptide corresponding to the heparin-binding site of hepatocyte growth factor. Biochemistry. 1997;36:10286–10291. doi: 10.1021/bi962700f. [DOI] [PubMed] [Google Scholar]
- 2.Bazil V, Baudys M, Hilgert I, Stefanova I, Low M G, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 3.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 4.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 5.Botto M, Hawkins P N, Bickerstaff M C M, Herbert J, Bygrave A E, McBride A, Hutchinson W L, Tennent G A, Walport M J, Pepys M B. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 6.Brackett D J, Lerner M R, Lacquement M A, He R, Pereira H A. A synthetic lipopolysaccharide-binding peptide based on the neutrophil-derived protein CAP37 prevents endotoxin-induced responses in conscious rats. Infect Immun. 1997;65:2803–2811. doi: 10.1128/iai.65.7.2803-2811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Atherosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 8.de Haas C J C, Van der Tol M E, Van Kessel K P M, Verhoef J, Van Strijp J A G. A synthetic lipopolysaccharide (LPS)-binding peptide based on amino acids 27–39 of serum amyloid P component inhibits LPS-induced responses in human blood. J Immunol. 1998;161:3607–3615. [PubMed] [Google Scholar]
- 9.Dhawan S, Fields R L, Robey F A. A novel peptide from amyloid P component supports cell attachment. Biochem Biophys Res Commun. 1990;171:1284–1290. doi: 10.1016/0006-291x(90)90825-8. [DOI] [PubMed] [Google Scholar]
- 10.Elass Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias P S, Mazurier J, Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emancipator K, Csako G, Elin R J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emsley J, White H E, O’Hara B P, Oliva G, Srinivasan N, Tickle I J, Blundell T L, Pepys M B, Wood S P. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 14.Garcia de Frutos P, Hardig Y, Dahlback B. Serum amyloid P component binding to C4b-binding protein. J Biol Chem. 1995;270:26950–26955. doi: 10.1074/jbc.270.45.26950. [DOI] [PubMed] [Google Scholar]
- 15.Gewurz H, Zhang X H, Lint T F. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 16.Gray B H, Haseman J R, Mayo K H. B/PI-derived synthetic peptides: synergistic effects in tethered bactericidal and endotoxin neutralizing peptides. Biochim Biophys Acta. 1995;1244:185–190. doi: 10.1016/0304-4165(95)00004-u. [DOI] [PubMed] [Google Scholar]
- 17.Haziot A, Tsuberi B Z, Goyert S M. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J Immunol. 1993;150:5556–5565. [PubMed] [Google Scholar]
- 18.Heegaard N H, Mortensen H D, Roepstorff P. Demonstration of a heparin-binding site in serum amyloid P component using affinity capillary electrophoresis as an adjunct technique. J Chromatogr A. 1995;717:83–90. doi: 10.1016/0021-9673(95)00644-3. [DOI] [PubMed] [Google Scholar]
- 19.Heegaard N H H, Heegaard P M H, Roepstorff P, Robey F A. Ligand-binding sites in human serum amyloid P component. Eur J Biochem. 1996;239:850–856. doi: 10.1111/j.1432-1033.1996.0850u.x. [DOI] [PubMed] [Google Scholar]
- 20.Hileman R E, Fromm J R, Weiler J M, Linhardt R J. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Hirata M, Shimomura Y, Yoshida M, Wright S C, Larrick J W. Endotoxin-binding synthetic peptides with endotoxin-neutralizing, antibacterial and anticoagulant activities. Prog Clin Biol Res. 1994;388:147–159. [PubMed] [Google Scholar]
- 22.Hoess A, Watson S, Siber G R, Liddington R. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 Å resolution. EMBO J. 1993;12:3351–3356. doi: 10.1002/j.1460-2075.1993.tb06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubacek J A, Büchler C, Aslanidis C, Schmitz G. The genomic organization of the genes for human lipopolysaccharide binding protein (LBP) and bactericidal permeability increasing protein (BPI) is highly conserved. Biochem Biophys Res Commun. 1997;236:427–430. doi: 10.1006/bbrc.1997.6970. [DOI] [PubMed] [Google Scholar]
- 24.Janciauskiene S, Garcia de Frutos P, Carlemalm E, Dahlback B, Eriksson S. Inhibition of Alzheimer beta-peptide fibril formation by serum amyloid P component. J Biol Chem. 1995;270:26041–26044. doi: 10.1074/jbc.270.44.26041. [DOI] [PubMed] [Google Scholar]
- 25.Larrick J W, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J M, Warren H S, Wright S C. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–240. [PubMed] [Google Scholar]
- 26.Little R G, Kelner D N, Lim E, Burke D J, Conlon P J. Functional domains of recombinant bactericidal/permeability increasing protein (rBPI23) J Biol Chem. 1994;269:1865–1872. [PubMed] [Google Scholar]
- 27.Loveless R W, Floyd O S, Raynes J G, Yuen C T, Feizi T. Human serum amyloid P is a multispecific adhesive protein whose ligands include 6-phosphorylated mannose and the 3-sulphated saccharides galactose, N-acetylgalactosamine and glucuronic acid. EMBO J. 1992;11:813–819. doi: 10.1002/j.1460-2075.1992.tb05118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann D M, Romm E, Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 29.Parker T S, Levine D M, Chang J C, Laxer J, Coffin C C, Rubin A L. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun. 1995;63:253–258. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira H A, Erdem I, Pohl J, Spitznagel J K. Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci USA. 1993;90:4733–4737. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen P B, Bjorn S, Hastrup S, Nielsen P F, Norris K, Thim L, Wiberg F C, Flodgaard H. Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett. 1996;390:109–112. doi: 10.1016/0014-5793(96)00639-4. [DOI] [PubMed] [Google Scholar]
- 32.Rensen P C, Oosten M, Bilt E, Eck M, Kuiper J, Berkel T J. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J Clin Investig. 1997;99:2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steel D M, Whitehead A S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 34.Troelstra A, Antal-Szalmas P, de Graaf-Miltenburg L A M, Weersink A J L, Verhoef J, Van Kessel K P M, Van Strijp J A G. Saturable CD14-dependent binding of fluorescein-labeled lipopolysaccharide to human monocytes. Infect Immun. 1997;65:2272–2277. doi: 10.1128/iai.65.6.2272-2277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troelstra A, Giepmans B N G, Van Kessel K P M, Lichenstein H S, Verhoef J, Van Strijp J A G. Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol. 1997;61:173–178. doi: 10.1002/jlb.61.2.173. [DOI] [PubMed] [Google Scholar]
- 36.Van Berkel P H C, Geerts M E J, van Veen H A, Mericskay M, de Boer H A, Nuijens J H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Zee R, Anderton S M, Buskens C A F, Alonso de Velasco E, Van Eden W. Heat shock protein T cell epitopes as immunogenic carriers in subunit vaccines. In: Maya H L S, editor. Peptides 1994, proceedings of the Twenty-Third European Peptide Symposium. Leiden, The Netherlands: Escom Science Publishers B. V.; 1995. pp. 841–842. [Google Scholar]
- 38.Wright S D, Ramos R A, Hermanowski Vosatka A, Rockwell P, Detmers P A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wurfel M M, Kunitake S T, Lichenstein H, Kane J P, Wright S D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]