Abstract
Clearance of trypanosomes from the blood of infected Cape buffalo was associated with the development of two responses: (i) complement-dependent and clone-specific lytic activity and (ii) complement-independent trypanocidal activity that was not restricted by trypanosome clone or species. This latter activity was mediated by H2O2 and required the presence of xanthine oxidase in serum but not the addition of purine substrates. Expression of the xanthine oxidase-dependent trypanocidal activity in Cape buffalo serum was coincident with, and required, a decline in its H2O2 catabolic activity. The H2O2 catabolic activity of Cape buffalo serum was due solely to catalase and declined by eightfold around the time that trypanosomes were cleared from the blood, accompanied by a fivefold drop in erythrocyte-associated catalase activity. The Cape buffalo did not develop subsequent parasitemic waves. Clearance of parasitemia in similarly infected cattle was also associated with development of trypanosome clone-specific lytic activity, but not with the acquisition of H2O2-dependent trypanocidal activity in serum, and the cattle supported recurring parasitemia. The lack of trypanocidal activity in pre- and postinfection cattle sera was due to their low content of xanthine oxidase and sustained catalase activity. These data strongly suggest that an infection-induced serum oxidative response, the efficacy of which is amplified by a decline in blood catalase, contributes to suppression of recurring parasitemia in Cape buffalo.
Cape buffalo efficiently limit the magnitude and frequency of trypanosome parasitemic waves and develop few or no signs of disease following infection with Trypanosoma brucei, T. congolense, and T. vivax (7, 8, 24, 28). Control of trypanosome parasitemia in Cape buffalo is dependent on an infection-induced mechanism that is implemented at the time of remission of the first parasitemic wave and results in the establishment of cryptic parasitemia (28). The process leading to efficient restraint of parasitemia in Cape buffalo does not prevent trypanosome antigenic variation, nor does it involve antibodies that affect growth of the parasites upon transfer into mice or inclusion in axenic cultures (28). Consequently, the control process may involve an infection-induced increase in antitrypanosome factors other than antibody in Cape buffalo blood.
Cape buffalo serum can kill all species of African trypanosomes in vitro (28). Paradoxically, the trypanocidal activity becomes evident only as serum is diluted (28). The trypanocidal serum component is xanthine oxidase (4, 21) (EC 1.1.3.22; xanthine + H2O + O2 = urate + H2O2) (21), and trypanocidal activity is due to H2O2 generated by reduction of O2 during oxidation of hypoxanthine and xanthine to uric acid (11, 21). The infection-induced change in Cape buffalo blood that leads to efficient control of trypanosome parasitemia might therefore involve an increase in the amount of H2O2 produced in blood, an increase in the life span of this oxygen radical, or both. Here we examine the impact of modifying the concentration of xanthine oxidase, purine substrate, and H2O2 catabolic activity on expression of trypanocidal activity of high concentrations of serum from naive Cape buffalo in vitro, identify the condition that allows expression of the latent oxidative capacity of serum, and show that this condition is established in trypanosome-infected Cape buffalo coincident with control of the sole parasitemic wave.
MATERIALS AND METHODS
Trypanosomes.
T. brucei subsp. brucei stock 427 clone 1 (3), T. brucei subsp. brucei GUTat 3.1 (25), T. brucei ILTtat 1.1 (hereafter referred to as A4) (18), T. congolense IL 1180 (hereafter referred to as 1180) (22), and T. congolense IL 300, IL 2642, and IL 3338 (hereafter referred to as 300, 2642, and 3338, respectively) (31) were grown in Baltz modified (1) minimal essential medium under axenic culture conditions as described previously (7, 12), harvested during exponential growth by centrifugation at 1,000 × g for 10 min, and washed three times with 137 mM NaCl in 20 mM phosphate-buffered saline (pH 7.2) containing 1% (by weight) glucose (PBS-G) before use.
Bovids and products.
Cape buffalo were bred and raised in a tsetse- and trypanosome-free environment at the Wildlife Disease Section of the Kenyan Agricultural Research Institute, Nairobi, Kenya, and used when 5 years of age. N’dama and Boran calves were bred and raised at the International Livestock Research Institute (ILRI), Nairobi, Kenya, and used at 6 months of age. All animal handling was in crushes, animals were not tranquilized, and blood collections were from a jugular vein. Blood for plasma and erythrocyte (RBC) preparation was collected into heparin (final concentration of 10 U/ml) and centrifuged (1,000 × g for 20 min at 4°C), after which plasma was used immediately or stored at −70°C. RBC were washed three times with PBS-G before use. Blood for serum preparation was collected into Vacutainers, clotted for 1 h at 21°C, and centrifuged as described above, and serum was processed in the same way as plasma. The hemoglobin contents of plasma and serum and homogenates of counted numbers of RBC were evaluated against a standard curve by using a Sigma hemoglobin kit. Serum samples from Hereford cattle were provided by Craig Beattie, USDA/ARS/Meat Animal Research Center, Clay Center, Neb.
Rabbit anticatalase Ig and immunoaffinity depletion of catalase.
The 220-kDa fraction of bovine liver catalase (EC 1.11.1.6; H2O2 + H2O2 = O2 + 2H2O; Sigma catalog no. C9322; 3,200 U/mg of protein) was isolated by fast protein liquid chromatography on a Superose 12 size exclusion gel (Pharmacia), emulsified in Freund’s complete adjuvant, and injected subcutaneously (100 μg of protein/rabbit). Rabbits were given booster doses 4 weeks later with the same amount of antigen emulsified in Freund’s incomplete adjuvant. Catalase-specific immunoglobulin (Ig) was isolated from immune serum by affinity chromatography on bovine liver catalase conjugated to cyanogen bromide-activated Sepharose 4B (5 mg of catalase/ml of gel; Pharmacia) as previously described (21); trace activity against xanthine oxidase was removed by affinity chromatography on xanthine oxidase that had been isolated from the aqueous phase of fresh cow milk by using a mouse monoclonal antibody as described previously (4) and immobilized on Sepharose 4B. Catalase-specific Ig was conjugated to cyanogen bromide-activated Sepharose 4b (3 mg of Ig/ml of gel), and catalase was removed from serum by immunoaffinity chromatography as previously described for xanthine oxidase (21). Control samples of serum were processed through anti-Cape buffalo IgG bound to Sepharose 4B (28) to demonstrate that catalase does not nonspecifically bind to immobilized rabbit Ig. Bound material was eluted with 0.1 M triethylamine (pH 11.5), neutralized by addition of 1 M potassium phosphate buffer (pH 6.4), and analyzed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (21).
Infection and parasitemia.
A combination of 5 × 104 T. brucei A4 and 5 × 104 T. congolense 1180 was inoculated into a jugular vein. Blood was collected at 1- or 2-day intervals for 18 days and periodically thereafter. Parasitemia was evaluated by the dark ground buffy coat procedure (27). Parasitological data and sera collected from cattle that had been infected with 5 × 104 T. congolense 1180 parasites alone or by exposure to T. congolense 1180-infected tsetse flies were generously provided by V. Lutje and E. Authié (ILRI).
Assay of serum trypanocidal and trypanolytic activity.
Trypanosomes (2 × 104) were incubated for 1 or 2 h at 37°C in wells of a 96-well tissue culture plate (Costar) in 100 μl of buffer composed of PBS-G supplemented with unprocessed or heat-inactivated (56°C, 30 min) Cape buffalo serum with or without 50 μM xanthine and with or without the following inhibitors, alone or in combination: 0.5 mM allopurinol, which inhibits xanthine oxidase (17); 2 mg of size-fractionated bovine liver catalase (described above)/ml; 1.5 mM (final concentration) sodium azide (NaN3) and 200 mM 3-amino-1,2,4-triazole (triazole), both of which inhibit of catalase (20); and 0.2 mM β-mercaptosuccinate, which inhibits glutathione peroxidase (23). Triplicate cultures were done for each condition tested. Trypanosomes in culture wells were examined under phase-contrast illumination using an inverted tissue culture microscope, and all organisms in each well could be categorized as either intact and motile, intact and nonmotile, or lysed and fragmented, depending on the culture condition. The intact, nonmotile trypanosomes are unable to replicate at 37°C and are noninfective in mice (21).
Catabolism of H2O2 by serum and blood cells. (i) Serum.
Assays were carried out in triplicate for each sample, and results are the means of the values obtained. Aliquots (10 μl) of diluted (25 to 0.785% [by volume]) serum (experimental group) or bovine liver catalase (0 to 4 U/ml, where 1 U of catalase converts 1 μmol of H2O2 to H2O/min at 25°C) (standard curve) were added to 10 μl of 1.25 mM H2O2 and incubated for 30 min at 25°C, after which the remaining H2O2 was detected by a modification of a standard technique (16). Briefly, 180 μl of H2O2 detection buffer (1 mM 2,4,6-tribromo-3-hydroxybenzoic acid, 0.1 mM 4-amino-antipyrine, 8 U of horseradish peroxidase/ml) was added, the mixture was incubated at 25°C for 5 min and chilled on ice to stop the reaction, and absorbance was read at 510 nm, which correlates directly with H2O2 content. The data obtained with catalase were plotted as units of catalase versus log10 optical density at 510 nm, which gave a straight line curve with y = −0.5975x − 0.0033 and r2 = 0.9902. The range of serum dilutions tested ensured a condition in which some H2O2 remained after the 30-min incubation; results were evaluated against the catalase standard curve and presented either as units of catalase activity per milliliter of serum or micromoles of H2O2 catabolized per minute. A catalase standard curve was included for each assay to allow comparison of serum samples collected on different days.
(ii) Impact of inhibitors on serum catalase activity.
The above assay was carried out with or without 1.25 mM NaN3, 0.25 mM β-mercaptosuccinate, or 0.125 M triazole, using intact serum or serum processed on anticatalase or anti-Cape buffalo Ig columns as described above.
(iii) Blood cells.
Known concentrations of RBC (with associated leukocytes) in 100 μl of PBS-G were added to 100-μl aliquots of 5 mM H2O2 in PBS-G with or without the inhibitors of catalase and glutathione peroxidase listed above, incubated for 30 min at 25°C, and centrifuged (5,000 × g for 2 min in an Eppendorf Microfuge), and remaining H2O2 was detected in 100 μl of cell-free supernatant as described above. Results were evaluated against a standard curve established with commercial catalase and were recorded as units of catalase activity per erythrocyte in the preparation. All results were presented as population mean values ± 1 standard deviation (SD), and groups were compared by the two-tailed paired t test.
Assay of H2O2 accumulation in serum.
Sera were supplemented with a saturating concentration of xanthine (400 μM), incubated for 30 min at 37°C, and centrifuged through a 3-kDa-cutoff membrane (Centricon 3; Amicon Corp.) at 4°C, and the concentration of H2O2 in the flowthrough fraction was determined as described above.
Xanthine oxidase activity.
Xanthine catabolism to uric acid, with concomitant reduction of O2 to H2O2, and O2− (11), was monitored by a coupled reaction with horseradish peroxidase as described elsewhere (21) and evaluated against a standard curve that was generated by using cow milk xanthine oxidase (Sigma) for which units of activity per milligram of protein was given; 1 U of xanthine oxidase converts 1 μmol of xanthine to uric acid/min at 25°C.
RESULTS
Requirements for the generation of trypanocidal H2O2 in serum.
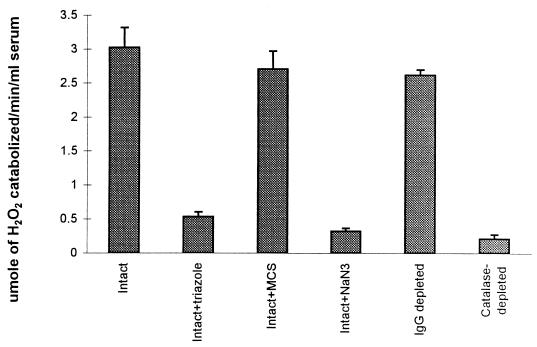
Cape buffalo sera kill all species of African trypanosomes in vitro (28). Trypanocidal activity is due to the inhibition of trypanosome glycolysis by H2O2 generated during the catabolism of hypoxanthine and xanthine by serum xanthine oxidase (21). It is detected only when the Cape buffalo sera are diluted to 25% (by volume) or lower concentrations in reaction buffer (28) and supplemented with substrates for serum xanthine oxidase (21). Three lines of evidence show that the failure of Cape buffalo serum to kill trypanosomes when used at high concentration was due to endogenous catalase activity. First, chromatography of Cape buffalo serum on an anticatalase immunoaffinity column under nonsaturating conditions removed 0.2% of serum protein, which resolved as putative catalase tetramer (240 kDa) and monomer (60 kDa) on reducing SDS-PAGE (data not shown), and abolished the capacity of serum to catabolize H2O2 (Fig. 1). Serum catalase activity was not affected by chromatography on immobilized rabbit anti-Cape buffalo Ig (Fig. 1). Second, removal of catalase revealed trypanocidal activity in high concentrations of serum containing xanthine (Table 1). This was inhibited by addition of commercial bovine catalase (Table 1). Third, inhibition of serum catalase by addition of azide or triazole abolished its capacity to catabolize H2O2 (Fig. 1) and revealed trypanocidal activity in high concentrations of Cape buffalo serum containing xanthine (data for triazole only are shown in Table 1).
FIG. 1.
Impact of immunodepletion of serum catalase, or inhibition of the enzyme, on catabolism of H2O2 in Cape buffalo serum. The H2O2 catabolic activity of intact, catalase-depleted, or IgG-depleted Cape buffalo serum was determined by incubation with 1.25 mM H2O2 for 30 min in the presence or absence of 1.25 mM NaN3, 0.25 mM β-mercaptosuccinate (MCS), or 0.125 M triazole, as indicated, and remaining H2O2 was assayed. Results presented are means of three samples tested per condition ± SD.
TABLE 1.
Impact of triazole, xanthine oxidase, xanthine, or combinations thereof on Cape buffalo and bovine serum trypanocidal activity
| Serum (45% [by vol] in PBS-G | Supplementa and/or treatment | Trypanocidal activity |
|---|---|---|
| Cape buffalo | None | No |
| Xanthine | No | |
| Triazole | No | |
| Xanthine + triazole | Yes | |
| Catalase depleted | No | |
| Catalase depleted + xanthine | Yes | |
| Catalase depleted + xanthine + catalase | No | |
| Cow | None | No |
| Xanthine | No | |
| Triazole | No | |
| Xanthine + triazole | No | |
| Xanthine oxidase | No | |
| Xanthine oxidase + triazole + xanthine | Yes | |
| Catalase depleted | No | |
| Catalase depleted + xanthine | No | |
| Catalase depleted + xanthine oxidase + xanthine | Yes |
Xanthine, 12.5 μM xanthine; triazole, 100 mM triazole; xanthine oxidase, 50 μg of cow milk xanthine oxidase/ml; catalase depleted, rendered devoid of catalase by immunoaffinity chromatography; catalase, 2 mg of catalase/ml.
Trypanocidal activity is not detected in bovine serum irrespective of the dilution tested (28). This lack of trypanocidal activity was not due solely to the presence of catalase in bovine serum because removal of catalase by immunoaffinity chromatography, or its inactivation by addition of azide (data not shown) or triazole, did not reveal trypanocidal activity in the presence of xanthine (Table 1). Bovine serum contained 0.2 ± 0.02 U of xanthine oxidase/liter (mean ± 1 SD of a mixture of samples from Zebu, Boran, and Hereford cattle). Cape buffalo serum contained 5 ± 0.2 U of xanthine oxidase/liter. Xanthine-supplemented bovine sera developed a trypanocidal concentration of H2O2 only when depleted of catalase and additionally supplemented with xanthine oxidase to mimic the content of Cape buffalo serum. A representative result obtained with serum from a Holstein heifer is shown in Table 1. These data show that the lack of expression of trypanocidal activity in bovine serum was due to its low concentration of xanthine oxidase in addition to endogenous catalase activity.
Catabolism of H2O2 in serum under the condition studied was mediated solely by catalase, as shown by its complete inhibition in the presence of azide and triazole and efficient catabolism in the presence of β-mercaptosuccinate, which inhibits glutathione peroxidase (23) (data for Cape buffalo only are shown in Fig. 1). Azide also completely inhibited the catabolism of H2O2 by Cape buffalo and bovine RBC, whereas addition of β-mercaptosuccinate to the incubation mixture had no effect (data not shown), indicating that under the assay conditions, catabolism of H2O2 by RBC was also mediated by catalase. To determine whether catalase in serum arose as a result of leakage from Cape buffalo and bovine RBC during blood collection, the concentrations of catalase and hemoglobin in RBC and serum were determined. Sera prepared from blood of uninfected Cape buffalo and cattle contained 2.7 ± 0.3 U of catalase/ml (mean ± 1 SD), which was equivalent to the H2O2 catabolic activity of 5 × 106 Cape buffalo and bovine RBC. The homogenate from 107 Cape buffalo and bovine RBC contained 77 ± 6 μg of hemoglobin (mean ± 1 SD), but hemoglobin was not detected in Cape buffalo or bovine plasma and serum samples. The ratio of hemoglobin to catalase was therefore much higher in RBC than in serum or plasma. Thus, although catalase is a constituent of the cell cytosol and peroxisome (6, 19) and not a secretory protein, its presence in serum did not result solely from leakage from blood cells that lysed during or after blood collection.
Infection-induced expression of serum trypanolytic and trypanocidal activities.
Cape buffalo and cattle that were infected with T. brucei A4 and T. congolense 1180 became parasitemic and subsequently cleared the parasites from their blood (Table 2). The Cape buffalo developed only a single wave of parasitemia, whereas the infected cattle developed more than one wave of parasitemia, consistent with results of previous studies (28). Clearance of trypanosomes from the blood of infected Cape buffalo and cattle was associated with the development of serum trypanolytic activity. Sera collected from the infected animals on the day of trypanosome clearance and thereafter caused lysis and fragmentation of trypanosomes during 1 h of incubation at 37°C. The trypanolytic activity was specific for the infecting organisms and was abrogated by heating the sera to 56°C for 30 min (Table 2, footnote b), consistent with the involvement of variable surface coat-specific antibodies and complement.
TABLE 2.
Parasitemia and heat-sensitive trypanolytic activity in Cape buffalo and bovine sera
| Host | Day of infection with T. brucei A4 + T. congolense 1180 | Dark ground buffy coat parasite scorea | Lowest vol (%) of serum at which trypanolytic activityb was detected on:
|
||
|---|---|---|---|---|---|
| T. brucei A4 | T. congolense 1180 | Other T. brucei and T. congolense strainsc | |||
| Cape buffalo 7810, 7813, and 7752 | 0 | 0, 0, 0 | −, −, − | −, −, − | −, −, − |
| 7 | 1, 2, 2 | −, −, − | −, −, − | ND | |
| 8 | 4, 3, 3 | −, −, − | −, −, − | ND | |
| 9 | 4, 3, 3 | 50, 50, 1.56 | 50, −, 25 | ND | |
| 11 | 0, 0, 0 | 12.5, 1.56, 1.56 | 50, 50, 25 | −, −, − | |
| 14 | 0, 0, 0 | 12.5, 1.56, 1.56 | 12.5, 3.13, 25 | −, −, − | |
| 18 | 0, 0, 1 | 6.25, 1.56, 1.56 | 6.25, 6.25, 1.56 | −, −, − | |
| 31 | 0, 0, 0 | 25, 50, 50 | −, 50, − | ND | |
| Cattle (N’dama 146 and Boran 174) | 0 | 0, 0 | −, − | −, − | −, − |
| 4 | 1, 2 | −, − | −, − | ND | |
| 7 | 0, 0 | 12.5, 25 | −, − | ND | |
| 11 | 5, 5 | 6.25, 1.56 | 12.5, 12.5 | ND | |
| 12 | 4, 3 | 3.13, 1.56 | 25, 25 | ND | |
| 14 | 1, 0 | 1.56, 1.56 | 3.13, 1.56 | −, − | |
| 16 | 0, 0 | ND | ND | ND | |
| 17 | 5, 0 | ND | ND | ND | |
| 18 | 5, 5 | ND | ND | ND | |
1, about 5 × 102 parasites/ml; 6, about 106 parasites/ml (27).
Buffalo and bovine sera were diluted in PBS-G–10% dialyzed fetal bovine serum; 2 × 104 trypanosomes were added per 100 μl of diluted serum and incubated for 2 h at 37°C, and lysed trypanosomes were evaluated. Results are recorded as the lowest percentage of serum that lysed all trypanosomes in the well. Heating of sera at 56°C for 30 min destroyed the trypanolytic activity. −, no trypanolytic activity observed; ND, not done.
T. brucei GUTat 3, T. congolense 300, T. congolense 2642, and T. congolense 3338.
Heat inactivation of immune Cape buffalo sera abrogated trypanolytic activity and revealed low-titered, trypanocidal activity that killed trypanosomes of different clones and species (Table 3). Trypanosomes that were incubated for 2 h at 37°C in the heat-inactivated immune Cape buffalo serum completely and irreversibly lost motility but remained intact. Neither heat-inactivated immune cattle sera nor preinfection Cape buffalo sera had trypanocidal activity. The trypanocidal activity of heat-inactivated immune Cape buffalo sera was expressed without addition of purine, was not detected in preinfection sera in the absence of xanthine, was detected at both 96 and 48% (by volume) in day 11 postinfection sera, but was detected at only 96% (by volume) in day 14 postinfection sera and not at all in day 18 postinfection sera (Table 3) unless additional xanthine was supplied (data not shown). The trypanocidal activity of heat-inactivated immune Cape buffalo sera was inhibited by inclusion of allopurinol or catalase in the incubation mixture (Table 4) and was therefore due to H2O2 that was generated during catabolism of endogenous serum purine by xanthine oxidase. The H2O2-dependent trypanocidal activity of day 11 Cape buffalo sera was not affected by dialysis of the sera for 8 h at 4°C against a 1,000-fold excess volume of PBS-G, and hence the endogenous purine required for expression of xanthine oxidase-dependent trypanocidal activity was associated with macromolecules in the sera.
TABLE 3.
Trypanocidal activity in serum samples from Cape buffalo 7810, 7813, and 7752a
| Day of infection | Lowest concn (% [by vol]) of serum at which trypanocidal activityb was detected on:
|
|||
|---|---|---|---|---|
| T. brucei GUTat 3 | T. congolense 300 | T. congolense 2642 | T. congolense 3338 | |
| 0 | −, −, − | −, −, − | −, −, − | −, −, − |
| 9 | −, −, − | −, −, − | 96, −, 96 | 96, −, − |
| 11 | 48, 48, 48 | 48, 48, 48 | 48, 48, 48 | 48, 96, 48 |
| 14 | 96, 96, 96 | 96, 96, 96 | 96, 96, 96 | 96, −, 96 |
| 18 | −, −, − | −, −, − | −, −, − | −, −, − |
Data for cattle are omitted because no trypanocidal activity was detected.
Assays were set up as described for Table 2 except that test sera had been heated at 56°C for 30 min. Trypanosomes were examined after a 2-h incubation. Results are presented as the last serum dilution in which all trypanosomes were intact but nonmotile. −, no trypanocidal activity observed.
TABLE 4.
Xanthine oxidase and H2O2-dependent trypanocidal activities in immune Cape buffalo serum at day 11 of infection
| Cape buffalo | Lowest concn (% [by vol]) of serum at which trypanocidal activity was detecteda
|
|||||
|---|---|---|---|---|---|---|
|
T. brucei GUTat 3
|
T. congolense 3338
|
|||||
| No inhibitors | Allo-purinol | Catalase | No inhibitors | Allo-purinol | Catalase | |
| 7810 | 48 | 96 | − | 48 | 96 | − |
| 7813 | 48 | − | − | 48 | − | − |
| 7752 | 48 | − | − | 48 | − | − |
Incubation mixtures contained no inhibitors, 0.5 mM allopurinol, or 2 mg of bovine catalase/ml.
Infection-associated decline in Cape buffalo blood catalase activity.
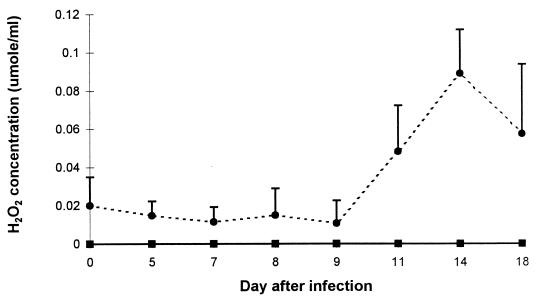
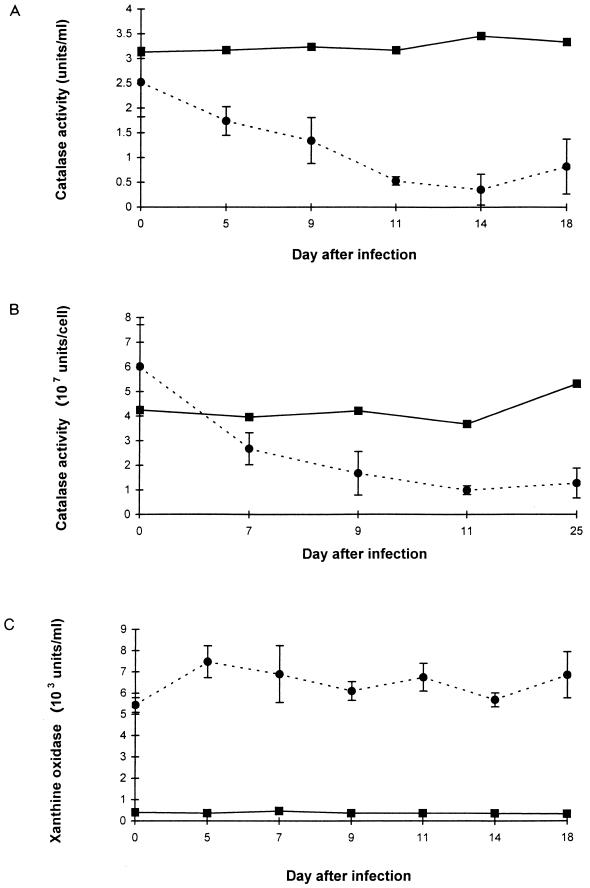
The acquisition of allopurinol- and catalase-sensitive trypanocidal activity in day 11 and day 14 heat-inactivated immune Cape buffalo sera was accompanied by a diminished capacity to destroy H2O2, evidenced by accumulation of H2O2 in the <3-kDa fraction during purine catabolism (Fig. 2). H2O2 did not accumulate in the <3-kDa fraction of xanthine-supplemented Cape buffalo sera collected before trypanosomes were cleared from the bloodstream or in bovine sera collected at any time after infection. The decline in the capacity of the immune Cape buffalo sera to catabolize H2O2 resulted from a decline in catalase activity. Indeed, both serum and RBC-associated H2O2 catabolic activities diminished significantly in the trypanosome-infected Cape buffalo (Fig. 3A and B). In contrast, neither serum nor RBC-associated catalase activities declined in the trypanosome-infected cattle (Fig. 3A and B) or in groups of N’dama and Boran cattle that were infected by needle or tsetse challenge with T. congolense 1180 alone and in which infection was allowed to progress through several waves of parasitemia (sera provided by V. Lutje and E. Authié, ILRI [data not shown]). Serum xanthine oxidase activity did not change in the infected Cape buffalo or cattle (Fig. 3C).
FIG. 2.
Accumulation of H2O2 in xanthine-supplemented sera from uninfected and trypanosome-infected Cape buffaloes and cattle. Xanthine was dissolved in Cape buffalo (●) or bovine (■) serum (1 ml) to give a final concentration of 400 μM xanthine. The preparations were incubated at 37°C for 30 min, and H2O2 in the <3-kDa fraction was assayed. Results are means and SD of sera from three Cape buffaloes and mean values only of sera from two calves before and at various days after infection with T. brucei and T. congolense 1180.
FIG. 3.
Impact of trypanosome infection on Cape buffalo and cattle. (A) Serum catalase levels; (B) RBC catalase levels; (C) serum xanthine oxidase levels. Results are means and SD of samples collected from three Cape buffaloes (●) and means of sera collected from two cattle (■) before and on various days after infection with T. brucei A4 and T. congolense 1180. Values obtained with the two cattle did not differ by more than 15% from each other on any day.
Intact, antibody-coated, and lysed trypanosomes do not inactivate Cape buffalo serum catalase.
The decline in Cape buffalo serum catalase levels that occurred after infection with trypanosomes was not due solely, if at all, to a direct interaction of catalase with trypanosomes or their products. Addition of up to 107 T. brucei A4 or T. congolense 1180 parasites per ml of preinfection Cape buffalo serum did not affect catalase activity during 1 to 24 h of incubation at 37°C. Similarly, T. brucei A4 or T. congolense 1180 that had been coated with specific Cape buffalo antibody by incubation with day 14 postinfection serum at 4°C did not affect catalase activity of preinfection Cape buffalo serum during 1 to 24 h of incubation at 37°C, even though the trypanosomes lysed during the first hour of incubation.
DISCUSSION
Control of parasitemia in trypanosome-infected Cape buffalo was associated with the development of both clone-specific trypanolytic activity and unrestricted trypanocidal activity in serum. These activities were distinguished one from another by sensitivity to inhibitors and their effects on trypanosome morphology. The trypanolytic activity required serum components that were sensitive to heating at 56°C for 30 min and was most likely due to trypanosome variable surface coat-specific antibody and complement factors. The trypanocidal activity was insensitive to heating at 56°C for 30 min but was inhibited by allopurinol and catalase, implicating involvement of xanthine oxidase and H2O2. Trypanolytic serum components caused fragmentation of the parasites during 1 h of incubation at 37°C, consistent with complement-mediated lysis of the organisms, whereas trypanocidal components caused progressive loss of trypanosome motility leading to their complete immobility without fragmentation during 2 h of incubation at 37°C, consistent with H2O2-induced decline in trypanosome glycolysis and ATP content (21).
A decline in blood (serum and RBC-associated) catalase activity was a key feature in expression of xanthine oxidase-dependent trypanocidal activity in trypanosome-infected Cape buffalo. In vitro analyses showed that the infection-associated decline in catalase activity did not result solely from an interaction of catalase with intact or lysed trypanosomes or their metabolic products and consequently must have been due to another parameter of infection. RBC-associated catalase is present predominantly in the cytosol. Therefore, the infection-associated inhibitor is likely to be of low molecular weight and to either diffuse or be transported into the cells. Catabolism of H2O2 and O2− by catalase has been shown to lead to the accumulation of inactive catalase compounds II and III (2, 10, 15), raising the possibility that the decline in blood catalase in the infected Cape buffalo may have resulted from xanthine oxidase and substrate interactions and other oxidative responses that yield H2O2 and O2−.
Several studies indicate that RBC-associated catalase is protected from oxidant-mediated inactivation by associated NADPH (2, 10, 14) but is inactivated during catabolism of H2O2 when NADPH is insufficient to sustain function, e.g., in glucose-6-phosphate dehydrogenase-deficient erythrocytes (30). It is therefore possible that the decline in blood catalase in trypanosome-infected Cape buffalo results from a combination of infection-induced oxidative responses and a constitutive, or infection-induced, inability to generate sufficient NADPH to sustain catalase activity in RBC. There is precedence for an association between defective antioxidant defenses and disease resistance. Deficiency in glucose-6-phosphate dehydrogenase confers resistance to Plasmodium falciparum malaria in people; the protective mechanism most likely involves impaired antioxidant defenses in infected RBC, leading to their surface modification and early phagocytosis (5, 29). There is also precedence for defective antioxidant defenses in sub-Saharan ungulates. It has been argued that ATP deficiency in RBC of African black rhinoceroses (and their defective glycolytic pathway and hexose monophosphate shunt) decreases the efficacy of antioxidant defenses and may be an evolutionary adaptation that confers selective advantage against common hemic parasites (26).
RBC-associated and serum catalase levels declined to their lowest levels around 14 days after infection of Cape buffalo and remained depressed until at least 30 days after infection. In contrast, the concentration of xanthine oxidase in serum was not affected by the infection. Trypanocidal activity in the absence of added xanthine was restricted to Cape buffalo sera that were collected on days 11 and 14 after infection. Taken together, these data suggest that a short-lived elevation in serum purine content arose around day 11 after infection, i.e., coincident with clearance of trypanosomes from the blood, and declined after 14 days of infection. The serum purine that supported xanthine oxidase-mediated trypanocidal activity in postinfection Cape buffalo serum was not removed by dialysis (25-kDa cutoff) and hence was associated with serum macromolecules. Mining of this sequestered resource by trypanosomes and accompanying exposure to serum xanthine oxidase may have resulted in the generation of reactive oxygen intermediates in the vicinity of trypanosomes rather than a generalized oxidative response in blood.
Infected Cape buffalo do not completely eliminate trypanosomes upon remission of the first parasitemic wave. Rather, parasitemia is maintained at 1 to 10 trypanosomes/ml of blood (28). Although H2O2 that is generated during substrate catabolism by xanthine oxidase and by other host oxidative responses can kill trypanosomes (21), it can also have more subtle effects, including prolongation of the time that trypanosomes spend in G1 of their cell cycle and an increase in the time taken to clear variable surface glycoprotein-specific antibody from their surface (2a). Prolongation of the life span of H2O2 in plasma due to decreased blood catalase may allow expression of these more subtle antitrypanosome effects of H2O2, at times when the serum purine content is inadequate to support the generation of a trypanocidal concentration of H2O2, and in this way enhance the capacity of infected Cape buffalo to control trypanosomes by acquired immune responses.
Infected cattle developed heat-sensitive trypanosome clone-specific trypanolytic activity but not catalase-sensitive trypanocidal activity in serum. Their inability to mount this latter response was due to a combination of low serum xanthine oxidase activity and sustained blood catalase activity throughout infection. The absence of catalase-sensitive trypanocidal activity in bovine serum correlated with, and may contribute to, recurring parasitemia. If so, elucidation of the mechanisms that regulate serum xanthine oxidase concentration and blood catalase activity in Cape buffalo may suggest strategies to develop trypanosomiasis-resistant cattle.
ACKNOWLEDGMENTS
We thank the Kenya Agricultural Research Institute and ILRI, Nairobi, Kenya, for provision of Cape buffalo and experimental facilities. We also thank John Wanda and Yanli Li for excellent technical assistance and George Orinda, Simon Barasa Okuku, and Josiah Orinda for expert handling of Cape buffalo.
This research was supported by NIH 1 RO1 AI 35646 and by a grant from USAID to expedite collaboration between ILRI and the University of Massachusetts.
REFERENCES
- 1.Baltz T, Baltz D, Giroud C, Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985;4:1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biscout D J, Field M J, Gouet P, Jouve H M. Simulations of electron transfer in the NADPH-bound catalase from Proteus mirabilis PR. Biochim Biophys Acta. 1995;1251:172–176. doi: 10.1016/0167-4838(95)00123-c. [DOI] [PubMed] [Google Scholar]
- 2a.Black, S. J. Unpublished data.
- 3.Black S J, Vandeweerd V. Serum lipoproteins are required for multiplication of Trypanosoma brucei brucei under axenic conditions. Mol Biochem Parasitol. 1989;37:65–72. doi: 10.1016/0166-6851(89)90103-5. [DOI] [PubMed] [Google Scholar]
- 4.Black S J, Wang Q, Makadzange T, Li Y-L, Van Praagh A V, Loomis M, Seed J R. Anti-Trypanosoma brucei activity of non-primate zoo sera. J Parasitol. 1999;85:48–53. [PubMed] [Google Scholar]
- 5.Cappadoro M, Giribaldi G, O’Brien E, Turrini F, Mannu F, Ulliers D, Simula G, Luzzatto L, Arese P. Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood. 1998;92:2527–2534. [PubMed] [Google Scholar]
- 6.de Duve C, Beaufay H, Jacques P, Rahman L-Y, Sellinger O Z, Wattiaux B, de Coninck S. Intracellular localization of catalase and some oxidases in rat liver. Biochim Biophys Acta. 1989;1000:321–322. [PubMed] [Google Scholar]
- 7.Dwinger R H, Grootenhuis J G, Murray M, Moloo S K, Gettinby G. Susceptibility of buffaloes, cattle and goats to infection with different stocks of Trypanosoma vivax transmitted by Glossina morsitans centralis. Res Vet Sci. 1986;41:307–315. [PubMed] [Google Scholar]
- 8.Grootenhuis J G, Dwinger R H, Dolan R B, Moloo S K, Murray M. Susceptibility of African buffalo and Boran cattle to Trypanosoma congolense transmitted by Glossina morsitans centralis. Vet Parasitol. 1990;35:219–231. doi: 10.1016/0304-4017(90)90057-i. [DOI] [PubMed] [Google Scholar]
- 9.Henderson G B, Fairlamb A H, Cerami A. Trypanothione-dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol Biochem Parasitol. 1987;24:39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- 10.Hillar A, Nicholls P, Switala J, Loewen P C. NADPH binding and control of catalase compound II formation: comparison of bovine, yeast, and Escherichia coli enzymes. Biochem J. 1994;300:531–539. doi: 10.1042/bj3000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- 12.Hirumi H, Hirumi K. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology. 1991;102:225–236. doi: 10.1017/s0031182000062533. [DOI] [PubMed] [Google Scholar]
- 13.Houston M, Chumley P, Radi R, Rubbo H, Freeman B A. Xanthine oxidase reaction with nitric oxide and peroxynitrite. Arch Biochem Biophys. 1998;355:1–8. doi: 10.1006/abbi.1998.0675. [DOI] [PubMed] [Google Scholar]
- 14.Kirkman H N, Gaetani G F. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Natl Acad Sci USA. 1984;81:4343–4347. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lardinois O M, Mestdagh M M, Rouxhet P G. Reversible inhibition and irreversible inactivation of catalase in the presence of hydrogen peroxide. Biochim Biophys Acta. 1996;1295:222–238. doi: 10.1016/0167-4838(96)00043-x. [DOI] [PubMed] [Google Scholar]
- 16.Le Tissier P R, Peters J, Skidmore C J. Development of an assay method for purine catabolic enzymes in the mouse and its adaptation for use on an auto analyzer. Anal Biochem. 1994;222:168–175. doi: 10.1006/abio.1994.1469. [DOI] [PubMed] [Google Scholar]
- 17.Massey V, Komai H, Palmer G. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo [3.4-d] pyrimidines. J Biol Chem. 1970;245:2837–2844. [PubMed] [Google Scholar]
- 18.Miller E N, Turner M J. Analysis of antigenic types appearing in first relapse populations of Trypanosoma brucei. Parasitology. 1981;82:63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- 19.Moreno S, Mugnaini E, Ceru M P. Immunocytochemical localization of catalase in the central nervous system of the rat. J Histochem Cytochem. 1997;43:1253–1267. doi: 10.1177/43.12.8537642. [DOI] [PubMed] [Google Scholar]
- 20.Mueller S, Arnhold J. Fast and sensitive chemiluminescence determination of H2O2 concentration in stimulated human neutrophils. Immunopharmacol Immunotoxicol. 1995;17:705–717. doi: 10.1002/bio.1170100406. [DOI] [PubMed] [Google Scholar]
- 21.Muranjan M, Wang Q, Li Y-L, Hamilton E, Otieno-Omondi F P, Wang J, Van Praagh A, Grootenhuis J G, Black S J. The trypanocidal Cape buffalo serum protein is xanthine oxidase. Infect Immun. 1997;65:3806–3814. doi: 10.1128/iai.65.9.3806-3814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nantulya V M, Musoke A J, Rurangirwa F R, Moloo S K. Resistance of cattle to tsetse-transmitted challenge with Trypanosoma brucei or Trypanosoma congolense after recovery from syringe-passaged infections. Infect Immun. 1984;43:735–738. doi: 10.1128/iai.43.2.735-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naseem K M, Chirico S, Mohammadi B, Bruckdorfer K R. The synergism of hydrogen peroxide with plasma-S-nitrosothiols in the inhibition of platelet activation. Biochem J. 1996;318:759–766. doi: 10.1042/bj3180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olubayo R O, Grootenhuis J G, Rurangirwa F R. Susceptibility of African buffalo and Boran cattle to intravenous infection with Trypanosoma congolense (IL1180) bloodstream forms. Trop Med Parasitol. 1990;41:181–184. [PubMed] [Google Scholar]
- 25.Onyango R J, Van Hoeve K, de Raadt P. The epidemiology of Trypanosoma rhodesiense sleeping sickness in Alego location, central Nyanza, Kenya. 1. Evidence that cattle may act as reservoir hosts of trypanosomes infective to man. Trans R Soc Trop Med Hyg. 1966;60:175–182. doi: 10.1016/0035-9203(66)90024-1. [DOI] [PubMed] [Google Scholar]
- 26.Paglia D E. Acute episodic hemolysis in the African black rhinoceros as an analogue of human glucose-6-phosphate dehydrogenase deficiency. Am J Hematol. 1993;42:36–45. doi: 10.1002/ajh.2830420109. [DOI] [PubMed] [Google Scholar]
- 27.Paris J, Murray M, McOdimba F. A comparative evaluation of the parasitological techniques currently available for the diagnosis of African trypanosomiasis in cattle. Acta Trop. 1982;39:307–316. [PubMed] [Google Scholar]
- 28.Reduth D, Grootenhuis J G, Olubayo R O, Muranjan M, Otieno-Omondi F P, Morgan G A, Brun R, Williams D J L, Black S J. African buffalo serum contains novel trypanocidal protein. J Eukaryot Microbiol. 1994;41:95–103. doi: 10.1111/j.1550-7408.1994.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 29.Ruwende C, Khoo S C, Snow R W, Yates S N, Kwiatkowski D, Gupta S, Warn P, Allsopp C E, Gilbert S C, Peschu N, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature (London) 1995;376:246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 30.Scott M D, Wagner T C, Chiu D T. Decreased catalase activity is the underlying mechanism of oxidant susceptibility in glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim Biophys Acta. 1993;1118:163–168. doi: 10.1016/0925-4439(93)90106-b. [DOI] [PubMed] [Google Scholar]
- 31.Wilkes J M, Mulugeta W, Wells C, Peregrine A S. Modulation of mitochondrial electrical potential: a candidate mechanism for drug resistance in African trypanosomes. Biochem J. 1997;326:755–761. doi: 10.1042/bj3260755. [DOI] [PMC free article] [PubMed] [Google Scholar]