Abstract
In this study, we used a mouse model to examine the role of the adaptive immune response in alveolar bone loss induced by oral infection with the human gram-negative anaerobic bacterium Porphyromonas gingivalis. Severe combined immunodeficient mice, which lack B and T lymphocytes, exhibited considerably less bone loss than did immunocompetent mice after oral infection, suggesting that lymphocytes contribute to this process. Bone loss after oral infection was decreased in mice deficient in major histocompatibility complex (MHC) class II-responsive CD4+ T cells, but no change in bone loss was observed in mice deficient in MHC class I-responsive CD8+ T cells or NK1+ T cells. Mice lacking the cytokine gamma interferon or interleukin-6 also demonstrated decreased bone loss. These results suggest that the adaptive immune response, and in particular CD4+ T cells and the proinflammatory cytokines that they secrete, are important effectors of bone loss consequent to P. gingivalis oral infection. The studies also reinforce the utility of the mouse oral infection model in dissecting the pathobiology of periodontal disease.
Periodontal diseases are chronic inflammatory diseases which result in loss of the tooth-supporting structures including osteoclastic resorption of alveolar bone in the jaw (32). Periodontal disease in adult humans is associated with the presence of the black-pigmented gram-negative anaerobic bacterium Porphyromonas gingivalis (38, 40). There is evidence supporting a role for the adaptive immune response in human periodontal disease. Humoral and cell-mediated immune responses to P. gingivalis have been demonstrated in patients with active periodontal disease (13, 16). CD4+/CD8+ ratios appear to be increased in the peripheral blood of patients with adult periodontal disease (28) and reduced in periodontal lesions compared to peripheral blood or normal gingiva (41). Moreover, P. gingivalis-specific T lymphocytes are found in the periodontal lesion (16, 27, 31).
Adaptive immune responses are also thought to play a role in local control of bone remodeling (4, 20), and CD4+ T lymphocytes are the source of cytokines that can induce net bone resorption in vitro (4, 14). However, because of the inherent complexity of human studies, the fundamental question of whether disease results because of an inadequate immune response or because the response is actively destructive remains unresolved (16, 34, 36, 37).
Animal models are critical for elucidating the biological factors associated with pathogenic processes and repair. We have established a model in which alveolar bone loss is induced in conventional mice after oral infection with a human strain of P. gingivalis (1, 2). Using this model, in previous studies we have shown that severe combined immunodeficient (SCID) mice, which lack B and T lymphocytes and thus lack adaptive immunity, can lose bone after P. gingivalis oral infection, thus suggesting that bone loss can occur in the absence of adaptive immunity. However, since the amount of bone loss appeared to be less than that seen in similarly infected immunocompetent mice (1), in the present study we investigated the role of the adaptive immune response in P. gingivalis-induced bone loss. We used SCID mice along with mice carrying targeted knockout mutations that affect T-cell development or cytokine production to show that lymphocytes are indeed important for this pathological process through the destructive action of major histocompatibility complex (MHC) class II-dependent CD4+ T cells, the Th1 cytokine gamma interferon (IFG), and the Th2 cytokine interleukin-6 (IL-6). These results strongly suggest that because of the action of CD4+ T cells, the adaptive immune response should be considered an important effector of bone loss associated with periodontal disease.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free mice were bred and raised at The Jackson Laboratory (Bar Harbor, Maine). BALB/c background mouse strains included C.B17-SCID, IFG-deficient BALB/c-Ifngtm1Ts, and BALB/cByJ. C57BL/6 background mouse strains included beta-2-microglobulin (β2m)-deficient C57BL/6J-B2mtm1Unc (8), MHC class II H2-Aβ-deficient C57BL/6JH2-Aβo, and IL-6-deficient C57BL/6J-Il6tm1Koe (26). Mice were maintained at Bates College under the approved conditions for animal care and were quarantined from other animals. All mice were kept on a 12-h light/dark cycle and received distilled water and food ad libitum. Animals within an experiment were age-matched females, 9 to 20 weeks old at the start of experiments.
Bacteria.
P. gingivalis ATCC 53977 (A7A1-28) was maintained frozen in defibrinated sheep blood at −70°C and by weekly transfer on supplemented blood agar (Trypticase soy agar base with 0.1% yeast extract, 5.0 μg of hemin per ml, 0.5 μg of menadione per ml, and 5% defibrinated sheep blood). For experiments, bacteria were anaerobically grown under 5% CO2–10% H2–85% N2 on supplemented blood agar at 37°C for 4 to 7 days.
Oral infection.
As described previously (1), mice were given sulfamethoxazole-trimethoprim (Sulfatrim; Goldline Laboratories, Fort Lauderdale, Fla.), 10 ml/pt in deionized water, ad libitum for 10 days. This was followed by a 3-day antibiotic-free period. Mice were then infected with 109 CFU of live P. gingivalis in 100 μl of phosphate-buffered saline (PBS) with 2% carboxymethylcellulose (24) placed into the esophagus and oral cavity three times at 2-day intervals. Controls included sham-infected mice which received the antibiotic pretreatment and the carboxymethylcellulose gavage, without P. gingivalis. Forty-seven days after the first gavage, mice were euthanized by CO2.
Recovery of P. gingivalis.
A sterile medium-sized paper point (Johnson & Johnson, East Windsor, N.J.) was held against the gumline of the upper molars for 5 s and then vortexed in 1 ml of prereduced brain heart infusion broth supplemented with hemin and menadione. An aliquot plated onto supplemented blood agar was incubated anaerobically for 4 weeks. P. gingivalis colonies were identified by their black pigmentation and by Gram stain reaction (1).
Flow cytometry.
Spleen cells were diluted to 2 × 107 cells per ml in flow PBS (0.2 g of KCl, 8.0 g of NaCl, 1.15 g of Na2HPO4, 0.2 g of KH2PO4, and 0.2 g of NaN3 per liter). Cells were blocked 15 min in 10 μl of normal rat immunoglobulin G (IgG) (Caltag Laboratories, South San Francisco, Calif.) per 50 μl of cells and immunostained for 30 min on ice with combinations of the following antibodies: rat IgG2b anti-mouse CD4 (L3T4) conjugated with fluoroisothiocyanate (FITC), rat IgG2a anti-mouse CD8 conjugated with either FITC or phycoerythrin (PE), and FITC- or PE-labeled rat IgG2a anti-mouse CD45R (B220) as a B-cell marker (The Jackson Laboratory), or their isotype controls (FITC- or PE-labeled rat IgG2a or rat IgG2b-FITC from Caltag Laboratories). Cells were washed free of unadsorbed antibody and resuspended at 2 × 106 cells per ml in flow PBS; 5 μl of propidium iodide was added to determine cell viability. Cells were analyzed on a FACSORT (Becton Dickinson). Granulocytes and lymphocytes were gated on the basis of forward scatter (cell size) and side scatter (cell granularity) of incident light.
P. gingivalis-specific antibody.
Blood was collected from each mouse at the time of euthanasia. Sera were stored at −70°C for later assessment of specific IgG, IgA, and IgM antibody by enzyme-linked immunosorbent assay (ELISA), as described previously (1), in polystyrene plates (Falcon; Becton-Dickinson Labware, Lincoln Park, N.J.) coated with formalin-killed whole P. gingivalis ATCC 53977. The ELISA titer was defined as the reciprocal of the highest serum dilution (expressed in log2) which produced absorbance readings more than 2 standard deviations above background levels.
Alveolar bone loss.
Horizontal bone loss around the maxillary molars was assessed by a morphometric method (24). Skulls were defleshed after 10 min of treatment in boiling water under 15-lb/in2 pressure, immersed overnight in 3% hydrogen peroxide, pulsed for 1 min in bleach, and stained with 1% methylene blue. The distance from the cementoenamel junction to the alveolar bone crest hereafter referred to as CEJ:ABC, was measured at a total of 14 buccal sites per mouse. Measurements were made under a dissecting microscope (magnification of ×40) fitted with a video image marker measurement system (model VIA 170; Boeckeler Instruments, Inc., Tucson, Ariz.) standardized to give measurements in millimeters. Bone measurements were done a total of three times in a random and blinded protocol by two evaluators. The CEJ:ABC from individual mice was subtracted from the mean CEJ:ABC from groups of sham-infected mice to give the millimeter change in bone, such that negative values indicate bone loss.
Statistics.
Differences between groups were evaluated by t test (Excel; Microsoft).
RESULTS
Adaptive immunity is important for alveolar bone loss in response to oral infection with P. gingivalis.
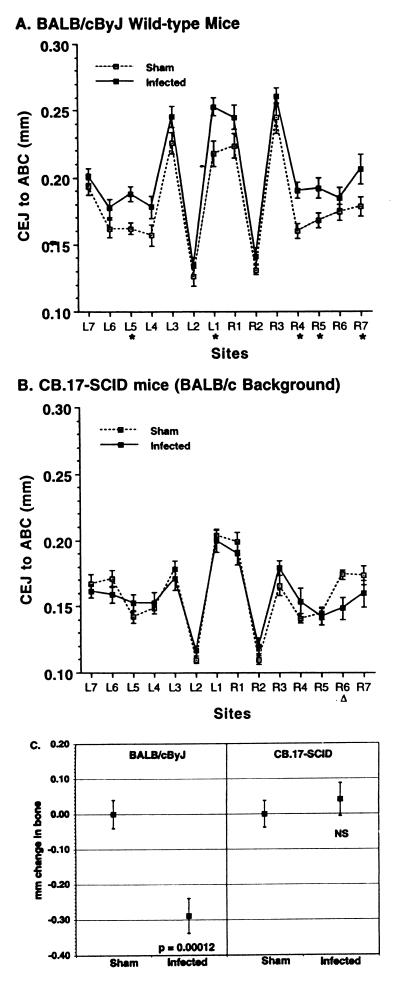
C.B17-SCID mice are homozygous for the Prkdcscid mutation and thus are deficient in both B and T lymphocytes. To determine whether there is a role for lymphocytes in alveolar bone loss, we investigated whether SCID mice showed bone loss after exposure to P. gingivalis. Figure 1 shows the mean (± standard error of the mean [SEM]) CEJ:ABC at each of the 14 measurement sites. As alveolar bone is lost, the CEJ:ABC is increased. In immunocompetent BALB/cByJ mice (Fig. 1A), the CEJ:ABC was greater in P. gingivalis-infected mice than in mice that were gavaged with PBS rather than P. gingivalis (i.e., sham-infected mice) at almost every site, indicating bone loss. The bone loss was greatest at the most mesial sites (site 1) and the sites on the second molar (sites 4 and 5). In SCID mice (Fig. 1B), there was no significant difference in CEJ:ABC in infected mice compared to sham-infected controls, despite the BALB/cJ background being essentially the same genetically as its substrain BALB/cByJ. BALB/cJ and BALB/cByJ do not differ in their bone responses after P. gingivalis oral infection (3).
FIG. 1.
Bone loss after oral infection with P. gingivalis occurred in immunocompetent mice (A) but not in immunodeficient SCID mice (B). L, left; R, right. Sites 1 to 3 are on the first molar, sites 4 and 5 are on the second molar, and sites 5 and 6 are on the third molar. Data points represent the means from 13 mice ± 1 SEM. (A) BALB/cByJ wild-type, immunocompetent mice. The CEJ:ABC was greater in infected mice than in sham-infected mice at every site, indicating bone loss. ∗, values in infected mice significantly greater than in sham-infected controls (P < 0.05). When comparisons were made on the 14-site total CEJ:ABC, values in infected mice were also significantly greater than in the sham-infected mice (P = 0.0001). (B) CB.17-SCID mice on a BALB/cJ genetic background. At every site, the CEJ:ABC in infected SCID mice was equal to or less than the distances in the sham-infected mice, indicating no bone loss. At site R6, the values in sham-infected mice were greater than in infected mice. (▵; P < 0.05). (C) Transformation of data from panels A and B to give millimeter change in bone. The 14-site total CEJ:ABC for each mouse was subtracted from the mean CEJ:ABC from groups of sham-infected mice to produce the millimeter change in bone, where negative values indicate bone loss. Squares represent the means ± 1 SEM of 13 mice per group. There was a significantly greater change in bone in infected BALB/cByJ mice than in sham-infected BALB/cByJ mice, but in SCID mice infection did not induce a change in bone (NS, not significantly different from sham-infected mice at P ≥ 0.05).
That SCID mice lacked B and T lymphocytes was confirmed by flow cytometry and by their failure to produce a specific antibody response to P. gingivalis after infection. IgG titers, but not specific IgA or IgM titers, were higher in infected BALB/cByJ mice than in sham-infected BALB/cByJ mice, thus confirming infection (data similar to those shown for BALB/cByJ mice in Table 1).
TABLE 1.
P. gingivalis-specific serum IgG titers
| Expt and strain | Group | Serum IgG titer (mean log2 titer ± 1 SEM) |
|---|---|---|
| See Fig. 4 | ||
| BALB/cByJ | Sham | 1.2 ± 0.6 |
| Infected | 13.3 ± 0.7a | |
| IFG knockout | Sham | 2.6 ± 0.3 |
| Infected | 11.6 ± 0.3a | |
| See Fig. 5 | ||
| C57BL/6J | Sham | 5.8 ± 0.6 |
| Infected | 9.2 ± 1.2a | |
| IL-6 knockout | Sham | 4.2 ± 0.5 |
| Infected | 9.4 ± 1.4a |
Different from sham-infected mice at P < 0.05.
To determine exactly how much bone resorbed in response to P. gingivalis oral infection, the 14-site total CEJ:ABC for each mouse was subtracted from the mean CEJ:ABC from groups of sham-infected mice of the same strain. The results of this data transformation are shown in Fig. 1C. P. gingivalis-infected BALB/cByJ mice lost more bone than sham-infected BALB/cByJ mice, while in SCID mice, P. gingivalis infection did not induce significant change in bone levels.
CD4+ T cells but not CD8+ T cells or NK1+ T cells are critical for alveolar bone loss bone in response to oral infection with P. gingivalis.
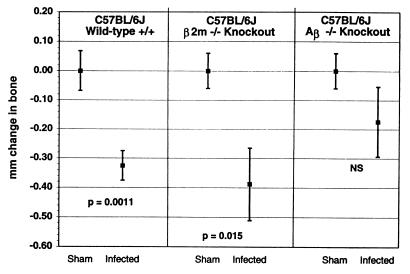
To determine whether it was the absence of T cells that accounted for the diminished bone loss seen in the SCID mice, we tested strains of mice lacking various T-cell subsets. β2m-knockout mice fail to express all class I proteins (25) and also fail to develop both conventional CD8+ T cells and a more recently described type of T cells, NK1+ T cells (6, 25). Aβ-knockout mice (19) fail to express the H2-Aα/β dimer. In the context of the H2b haplotype, such Aβ-knockout mice lack MHC class II protein expression and fail to generate MHC class II-reactive CD4+ T cells (19). As can be seen in Fig. 2, both orally infected C57BL/6J background β2m-knockout mice lacking CD8+ T cells, and wild-type, immunocompetent C57BL/6J mice underwent bone loss, whereas sham-infected animals did not. In contrast, C57BL/6J background Aβ-knockout mice did not demonstrate significant bone loss when infected with P. gingivalis (P > 0.05 compared to sham-infected Aβ-knockout mice). These results suggest that CD4+ T cells promote bone loss whereas CD8+ T cells and NK1+ T cells played no apparent role.
FIG. 2.
Bone loss after oral infection with P. gingivalis occurred in wild-type, immunocompetent mice (C57BL/6J) and in mice lacking CD8+ and NK1+ T cells (B2m-knockout mice on a C57BL/6J background) but was diminished in mice deficient in CD4+ T lymphocytes (Aβ-knockout mice on a C57BL/6J background). The CEJ:ABC from 14 sites were summed for each mouse, and the total from each mouse was subtracted from the mean totals in the sham-infected mice to give the total millimeter of change in bone. Negative values of millimeter of change in bone indicate bone loss, which was significantly greater in infected wild-type and B2m-knockout mice than in sham-infected mice of the same strain at the P values shown. In Aβ-knockout mice, the bone change in infected mice was not significantly different (NS) than in sham-infected mice at P ≥ 0.05. Data points represent the means from nine mice ± 1 SEM. The expected changes in CD4+ and CD8+ lymphocyte populations were confirmed by flow cytometry (data not shown).
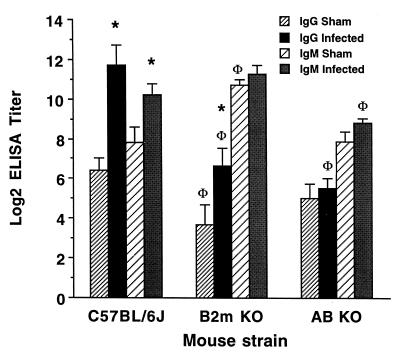
To determine whether humoral immunity contributes to bone loss, we examined P. gingivalis-specific antibody titers generated as a result of oral exposure. Orally infected Aβ-knockout mice demonstrated P. gingivalis-specific IgG and IgM antibody titers no greater than those of sham-infected controls (Fig. 3), and they did not lose bone. In comparison, orally infected C57BL/6J mice showed higher P. gingivalis-specific IgG and IgM titers than the sham-infected controls, and the infected mice lost bone. Orally infected β2m-knockout mice also showed a specific IgG response compared to sham-infected controls, but their titers were lower than those of wild-type mice, probably because they are deficient in the class I molecule FcRn (8, 17, 23) required to retard IgG catabolism (Fig. 3). That specific serum antibodies were generated indicates that a systemic adaptive immune response occurred in response to oral exposure to P. gingivalis. Moreover, these results are inconsistent with a protective role against bone loss for specific antibodies at these titers.
FIG. 3.
Influence of class I or class II proteins on the generation of P. gingivalis-specific antibodies. P. gingivalis-reactive IgG was increased in C57BL/6J and β2m-knockout (β2m KO) mice orally infected with P. gingivalis (∗, different from sham-infected controls at P < 0.05) but not in Aβ-knockout (Aβ KO) mice. P. gingivalis-reactive IgM was increased in orally infected C57BL/6J mice (∗) but not in infected β2m- or Aβ-knockout mice. Both sham-infected and infected β2m-knockout mice had less P. gingivalis-reactive IgG than sham-infected or infected C57BL/6J mice, as did infected Aβ-knockout mice (Φ, different from C57BL/6J at P < 0.05). Anti-P. gingivalis IgA was not found in any of the mouse strains tested (data not shown).
CD4+ T-cell cytokines are important for P. gingivalis-induced bone loss.
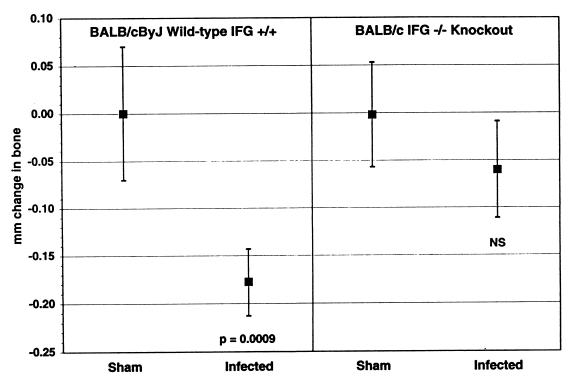
One possible way by which CD4+ T cells could cause P. gingivalis-induced bone loss is through the cytokines that the cells produce. To address this possibility, we first tested IFG-knockout BALB/c mice. IFG-knockout BALB/c mice did not lose significant levels of bone after oral infection with P. gingivalis, while infected immunocompetent BALB/cByJ mice showed considerable bone loss (Fig. 4). Uninfected IFG-knockout control mice did not differ from BALB/cByJ mice in size, weight, or CEJ:ABC (data not shown). Moreover, sham-infected IFG-knockout mice did not differ from wild-type BALB/cByJ mice in the numbers of splenic granulocytes, CD4+ T cells, or CD8+ T cells (data not shown). The normal number of CD4+T cells in the IFG-knockout mice makes it unlikely that it is the lack of CD4+ T cells per se that explains the abrogation of bone loss in CD4+ lymphocyte-deficient mice seen in Fig. 2. Nor is it likely that the bone loss results could be explained by quantitative differences in antibody responses, since the immunocompetent animals and the IFG-knockout mice responded similarly after infection (Table 1).
FIG. 4.
Alveolar bone response to oral infection in IFG-knockout BALB/cByJ mice compared to wild-type, immunocompetent BALB/cByJ mice. Infected BALB/cByJ mice lost bone (P = 0.0009 compared to sham-infected BALB/cByJ mice), but infected IFG-knockout mice did not (NS, not significantly different from IFG-knockout sham-infected mice at P ≥ 0.05). The CEJ:ABC from 14 sites were summed for each mouse, and the value from each mouse was subtracted from the mean totals from the sham-infected mice to give the total millimeters of bone lost. n = 20 ± 1 SEM.
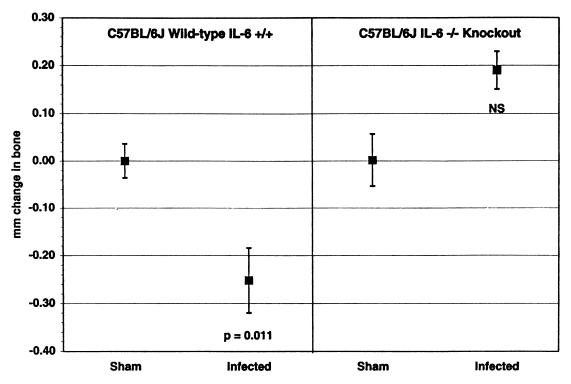
We also examined the effects of a deficiency of IL-6 on bone loss. As shown in Fig. 5, IL-6-knockout C57BL/6J mice did not lose bone after infection with P. gingivalis, while immunocompetent, wild-type C57BL/6J mice did. IL-6-deficient mice actually appeared to gain bone, although the differences between the sham-infected and P. gingivalis-infected mice did not reach significance (P = 0.08). There was no difference between sham-infected IL-6-knockout and C57BL/6J mice in CEJ:ABC or body weight, or numbers of splenic T cells, B cells, or granulocytes (data not shown), and the humoral responses to oral infection were similar in the two strains of mice (Table 1).
FIG. 5.
Alveolar bone response to oral infection in IL-6-knockout C57BL/6J mice compared to wild-type, immunocompetent C57BL/6J mice. Infected C57BL/6J mice lost bone (P = 0.011 compared to sham-infected C57BL/6J), but infected IL-6-knockout mice did not (NS, not significantly different from IL-6-knockout sham-infected mice at P ≥ 0.05). The 14-site total CEJ:ABC for each mouse was subtracted from the mean CEJ:ABC from groups of sham-infected mice to produce the millimeter mm change in bone, where negative values indicate bone loss. n = 20 ± 1 SEM.
In general, our results demonstrate that antibody titers were not correlated, either negatively or positively, with bone loss. β2m-knockout mice have less IgG than the BALB/cByJ parent strain (Fig. 3), but they have comparable bone loss (Fig. 2). Aβ-knockout mice did not develop specific antibody titers after infection (Fig. 3), yet they had less bone loss than the immunocompetent mice (Fig. 2). Both IFG- and IL-6-knockout mice had normal humoral responses after infection (Table 1) but less bone loss than the immunocompetent background mice (Fig. 4). The severity of bone loss in individual mice is also not correlated with specific antibody titers (2).
Recovery of P. gingivalis by paper points at termination of the experiments is shown in Table 2. In no case can differences in bone loss be explained by differences in the numbers of animals infected with P. gingivalis. In those animals gavaged with P. gingivalis from which P. gingivalis was not reisolated at the termination of the experiments, infection was confirmed by the presence of elevated specific antibody.
TABLE 2.
Recovery of P. gingivalis from oral cavities of infected mice at termination of experiments
DISCUSSION
In this study, we addressed the role of the adaptive immune response in P. gingivalis-induced alveolar bone loss. To do so, we exploited strains of mice carrying disrupted genes that are important to the adaptive immune response. The mouse strain genetic backgrounds that were studied were BALB/c and C57BL/6. We find that the BALB/c background is more susceptible to P. gingivalis-induced bone loss than the C57BL/6J background when the two strains are run in the same experiment (3), but the use of the C57BL/6J background was necessitated in the indicated experiments because of mouse availability. Importantly, the C57BL/6J background showed sufficient susceptibility in independent experiments to yield interpretable results. Our results are consistent with there being a strong T-cell cytokine-mediated immunological component to the alveolar bone loss which accompanies periodontal disease. The deletion of T cells, and in particular CD4+ T cells, was associated with decreased alveolar bone loss after oral infection with P. gingivalis. This implies a destructive role for CD4+ T cells in alveolar bone loss. In contrast, the lack of CD8+ and NK+ T cells had no significant effect on bone loss.
We also examined the consequences of deficiencies in two T-cell cytokines, IFG and IL-6, on alveolar bone loss. Although these cytokines are sometimes considered to be Th1 and Th2 markers, IL-6 can be secreted by both subsets (11), and both cytokines are secreted by other cells in addition to CD4+ T cells (39, 44, 45). CD4+ T cells isolated from inflamed gingiva of periodontitis patients expressed mRNA for both IFG and IL-6 (15). IFG plays a role in regulating the proliferation and function of activated T cells. IL-6 is a proinflammatory cytokine with the ability to induce bone resorption in vitro (22, 29), and IL-6-knockout mice show impaired inflammatory responses to infection (26).
Some investigators have proposed that Th1 CD4+ T cells are destructive (12). Our finding that IFG-knockout mice had less bone loss in response to infection (Fig. 4) lends support to the hypothesis of a destructive role for Th1 cells. Moreover, the deletion of IL-6 also decreased bone loss and possibly resulted in bone increase (Fig. 5). Others have shown a correlation between the progression of periodontal disease in humans and the presence in inflamed gingiva of cells producing Th2 cytokines, including IL-6 (42, 46).
One possible mechanism by which T cells could influence bone is by secreting bone-resorptive cytokines in response to oral infection. IL-6 is one such cytokine (26). P. gingivalis fimbriae induce human peripheral blood mononuclear cells to secrete IL-6 in vitro (30), and mononuclear cells from inflamed gingiva of periodontitis patients secrete IL-6 (14). IL-6 secretion by bone calvaria cells (29) and by human periodontal ligament fibroblasts (45) is stimulated in vitro by P. gingivalis lipopolysaccharide. Others have found that IL-6-deficient mice are protected against osteoporotic bone loss induced by estrogen depletion (33). T-cell-deficient nude mice do not lose bone after injection of endotoxin into their gingiva, while normal mice or nude mice reconstituted with T cells lose bone (43).
In contrast to IL-6, IFG is not known to have direct bone-resorptive activity, and so its participation in promoting bone loss is likely indirect. One possible mechanism is through the ability of IFG to increase the expression of MHC class II molecules on antigen-presenting cells (10), in turn leading to the activation of CD4+ T cells that cause bone loss.
It is somewhat surprising that immunodeficient mice lost less bone after infection. People with AIDS have reduced numbers of CD4+ T cells, and some have rapidly progressing periodontal disease. However, recent studies indicate that the incidence of periodontal disease is not higher in people who are seropositive than in those who are seronegative for human immunodeficiency virus (18, 21). Since nonlymphocytic cell types can produce bone-resorptive cytokines, bone loss may proceed by a different mechanism in AIDS patients. In addition, organ transplant recipients taking certain immunosupressant drugs, such as cyclosporin, develop osteoporosis, but this bone loss is an effect of the drug, not of an abrogation of immunity (9). Despite the fact that these immunosupressant drugs appear to work on T cells, the osteopenia that they induce is also T-cell dependent. T-cell-deficient rats do not lose bone when treated with cyclosporin A (7). Findings presented in older reports that bone loss is increased in rats immunosuppressed by cyclophosphamide (35) may have been due to the drug, not to the immune suppression. Organ recipients immunosuppressed with other drugs (prednisone and imuran) which do not themselves induce bone resorption had less periodontal inflammation and no worsening of periodontal parameters, despite high plaque loads (5).
In conclusion, we have shown that CD4+ but not CD8+ T cells were associated with bone resorption in response to oral infection with P. gingivalis. At least two CD4+ T-cell cytokines, IFG and IL-6, were also associated with bone loss. As the immune system responds to bacterial infection, the cytokines that it secretes may affect the balance of resorption and deposition that constitutes bone remodeling, resulting in enhanced resorption.
ACKNOWLEDGMENTS
We thank Teresa Hopkins and Cornelia Sfintescu for their contributions to this project, and we thank Len Shultz and Dave Serreze for critical reading of the manuscript.
This work was supported by Public Health Service grants R29 DE10728 (to P.J.B.) and R01 AI24544 (to D.C.R.) from the National Institutes of Health and by a grant to Bates College from the Howard Hughes Medical Institute.
REFERENCES
- 1.Baker P J, Evans R T, Roopenian D C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 2.Baker, P. J., S. Carter, M. Dixon, R. T. Evans, and D. C. Roopenian. Serum antibody response to oral infection precedes but does not prevent Porphyromonas gingivalis-induced alveolar bone loss in mice. Oral Microbiol. Immunol., in press. [DOI] [PubMed]
- 3.Baker, P. J., M. Dixon, and D. C. Roopenian. Genetic control of susceptibility to alveolar bone loss in mice. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 4.Baron R, Vignery A, Horowitz M. Lymphocytes, macrophages, and the regulation of bone remodeling. In: Peck W A, editor. Bone and mineral research annual 2: a yearly survey of developments in the field of bone and mineral metabolism. New York, N.Y: Elsevier Science Publishers; 1983. pp. 175–243. [Google Scholar]
- 5.Been V, Engel D. The effects of immunosuppressive drugs on periodontal inflammation in human renal allograft patients. J Periodontol. 1982;53:245–248. doi: 10.1902/jop.1982.53.4.245. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A. Mouse NK1+ T cells. Curr Biol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 7.Buchinsky F J, Ma Y, Mann G N, Rucinski B, Bryer H P, Romero D F, Jee W S S, Epstein S. T lymphocytes play a critical role in the development of cyclosporin A-induced osteopenia. Endocrinology. 1996;137:2278–2285. doi: 10.1210/endo.137.6.8641176. [DOI] [PubMed] [Google Scholar]
- 8.Christianson G J, Vekasi S, Niles J, Roopenian S L, Roths J B, Roopenian D C. β2 microglobulin-deficient mice show abnormal Ig homeostasis and are protected from lupus-like autoimmunity. J Immunol. 1997;159:4780–4792. [PubMed] [Google Scholar]
- 9.Cvetkovic M, Mann G N, Romero D F, Liang X G, Ma Y, Jee W E E, Epstein S. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation. 1994;57:1231–1237. doi: 10.1097/00007890-199404270-00016. [DOI] [PubMed] [Google Scholar]
- 10.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 11.Daugelat S, Kaufmann S H E. Role of Th1 and Th2 cells in bacterial infections. In: Romagnani S, editor. Chemical immunology: Th1 and Th2 cells in health and disease. S. Basel, Switzerland: Karger AG; 1996. pp. 66–97. [PubMed] [Google Scholar]
- 12.Eastcott J W, Yamashita K, Taubman M A, Harada Y, Smith D J. Adoptive transfer of cloned T helper cells ameliorates periodontal disease in nude rats. Oral Microbiol Immunol. 1994;9:284–289. doi: 10.1111/j.1399-302x.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 13.Ebersole J L, Taubman M A, Smith D J, Frey D E, Haffajee A D, Socransky S S. Human serum antibody responses to oral microorganisms. IV. Correlation with homologous infection. Oral Microbiol Immunol. 1987;12:53–59. doi: 10.1111/j.1399-302x.1987.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujihashi K, Kono Y, Beagley K W, Yamamoto M, McGhee J R, Mestecky J, Kiyono H. Cytokines and periodontal disease: immunopathological role of interleukins for B cell responses in chronic inflamed gingival tissues. J Periodontol. 1993;64:400–406. [PubMed] [Google Scholar]
- 15.Fujihashi K, Yamamoto M, Hiroi T, Bamberg T V, McGhee J R, Kiyono H. Selected Th1 and Th2 cytokine mRNA expression by CD4+ T cells isolated from inflamed human gingival tissues. Clin Exp Immunol. 1996;103:422–428. doi: 10.1111/j.1365-2249.1996.tb08297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genco R J. Host responses in periodontal diseases: current concepts. J Periodontol. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 17.Ghetie V, Hubbard J G, Kim J-K, Tsen M-F, Lee Y, Ward S E. Abnormally short half-lifes of IgG in β2-microglobulin deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie G, Marino R. Oral manifestations of HIV infection: a Panamerican perspective. J Oral Pathol Med. 1993;22:2–7. doi: 10.1111/j.1600-0714.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 19.Gosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 20.Gruber H E. Bone and the immune system. Proc Soc Exp Biol Med. 1991;197:219–225. doi: 10.3181/00379727-197-43249. [DOI] [PubMed] [Google Scholar]
- 21.Holmstrup P, Westergaard J. Periodontal diseases in HIV-infected patients. J Clin Periodontol. 1994;21:270–280. doi: 10.1111/j.1600-051x.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 22.Jilka R L, Hangoc G, Girasole G, Passeri G, Williams D C, Abrams J S, Boyce B, Broxmeyer H, Manolagas S C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 23.Junghans R P, Anderson C L. The Bambrell protection receptor (FcRp) for Ig is the β2-microglobulin-containing neo-intestinal transport receptor (FcRn) Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klausen B, Evans R T, Sfintescu C. Two complementary methods of assessing periodontal bone level in rats. Scand J Dent Res. 1989;97:494–499. doi: 10.1111/j.1600-0722.1989.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 25.Koller B H, Marrack P, Kappler J W, Smithies O. Normal development of mice deficient in β2M, MHC Class I proteins and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 26.Kopf M, Baumann H, Freer G, Feudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 27.Mahanonda D, Seymour G J, Halliday J W, Powell L W, Good M F. Limit dilution analysis of peripheral blood T-lymphocytes specific to periodontopathic bacteria. Clin Exp Immunol. 1989;75:245–251. [PMC free article] [PubMed] [Google Scholar]
- 28.Mather A, Michalowicz B S. Cell-mediated immune system regulation in periodontal diseases. Crit Rev Oral Biol Med. 1997;8:76–89. doi: 10.1177/10454411970080010401. [DOI] [PubMed] [Google Scholar]
- 29.Miyata Y, Takeda H, Kitano S, Hanazawa S. Porphyromonas gingivalis lipopolysaccharide-stimulated bone resorption via CD14 is inhibited by broad-spectrum antibiotics. Infect Immun. 1997;65:3513–3519. doi: 10.1128/iai.65.9.3513-3519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa T, Uchida H. A peptide, ALTTE, within the fimbrial subunit from Porphyromonas gingivalis, induces production of interleukin 6, gene expression and protein phosphorylation in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 1995;11:197–206. doi: 10.1111/j.1574-695X.1995.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Kono Y, McGhee M L, McGhee J R, Roberts J E, Hamada S, Kiyono H. Porphyromonas gingivalis-specific serum IgG and IgA antibodies originate from immunoglobulin-secreting cells in inflamed gingiva. Clin Exp Immunol. 1991;83:237–244. doi: 10.1111/j.1365-2249.1991.tb05621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page R C, Schroeder H E. Periodontitis in man and other animals: a comparative review. S. Basel, Switzerland: Karger; 1982. [Google Scholar]
- 33.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan G A, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranney R R. Immunologic mechanisms of pathogenesis in periodontal diseases: an assessment. J Periodontal Res. 1991;26:243–254. doi: 10.1111/j.1600-0765.1991.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 35.Sallay K, Sanavi F, Ring I, Pham P, Behling U H, Nowotny A. Alveolar bone destruction in the immunosuppressed rat. J Periodontal Res. 1982;17:263–274. doi: 10.1111/j.1600-0765.1982.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 36.Seymour G J, Gemmell E, Reinhardt R A, Eastcott J, Taubman M A. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontal Res. 1993;28:478–486. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 37.Shenker B J. Immunologic dysfunction in the pathogenesis of periodontal diseases. J Clin Periodontol. 1987;14:489–498. doi: 10.1111/j.1600-051x.1987.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 38.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Takashiba S, Nagai A, Takigawa M, Myoukai F, Kurihara H, Murayama Y. Assessment of interleukin-6 in the pathogenesis of periodontal disease. J Periodontol. 1994;65:147–153. doi: 10.1902/jop.1994.65.2.147. [DOI] [PubMed] [Google Scholar]
- 40.Tanner A C R, Haffer C, Bratthall G T, Visconti R A, Socransky S S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979;6:278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 41.Taubman M A, Stoufi E D, Ebersole J L, Smith D J. Phenotypic studies of cells from periodontal disease tissues. J Periodontal Res. 1984;19:587–590. doi: 10.1111/j.1600-0765.1984.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 42.Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol. 1997;107:166–174. doi: 10.1046/j.1365-2249.1997.d01-880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ukai T, Hara Y, Kato I. Effects of T cell adoptive transfer into nude mice on alveolar bone resorption induced by endotoxin. J Periodontal Res. 1996;31:414–422. doi: 10.1111/j.1600-0765.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 44.van Snick J. IL-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 45.Yamaji Y, Kubota T, Sasaguri K, Sato S, Suzuki Y, Kumada H, Umemoto T. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharide. Infect Immun. 1995;63:3576–3581. doi: 10.1128/iai.63.9.3576-3581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamakazi K, Nakajima T, Gemmell E, Polak B, Seymour G J, Hara K. IL-4 and IL-6-producing cells in human periodontal disease tissue. J Oral Pathol Med. 1994;23:347–353. doi: 10.1111/j.1600-0714.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]