Abstract
Trypanosoma cruzi replicates in nucleated cells and is susceptible to being killed by gamma interferon-activated macrophages through a mechanism dependent upon NO biosynthesis. In the present study, the role of platelet-activating factor (PAF) in the induction of NO synthesis and in the activation of the trypanocidal activity of macrophages was investigated. In vitro, PAF induced NO secretion by T. cruzi-infected macrophages and the secreted NO inhibited intracellular parasite growth. The addition of a PAF antagonist, WEB 2170, inhibited both NO biosynthesis and trypanocidal activity. The inducible NO synthase/l-arginine pathway mediated trypanocidal activity, since it was inhibited by treatment with l-N-monomethyl arginine (l-NMMA), an l-arginine analog. PAF-mediated NO production in infected macrophages appears to be dependent on tumor necrosis alpha (TNF-α) production, since the addition of a neutralizing anti-TNF-α monoclonal antibody mAb inhibited NO synthesis. To test the role of PAF in mediating resistance or susceptibility to T. cruzi infection, infected mice were treated with WEB 2170, a PAF antagonist. These animals had higher parasitemia and earlier mortality than did vehicle-treated mice. Taken together, our results suggest that PAF belongs to a group of mediators that coordinate the mechanisms of resistance to infections with intracellular parasites.
Infection with the protozoan flagellate Trypanosoma cruzi causes Chagas’ disease, a major public health problem in many Latin American countries. Infection of mice with T. cruzi is a widely used model in the study of the pathophysiological mechanisms underlying the disease and host protection. There is evidence to suggest a central role for macrophage-derived nitric oxide (NO) in mediating resistance to T. cruzi infection (12, 23, 29). In macrophages, NO is generated from the guanidino nitrogen atom of l-arginine by an inducible NADPH-dependent enzyme, NO synthase (NOS) (19–21). The inducible isoform of NOS, iNOS, is induced in macrophages by cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (9, 10).
The lipid mediator platelet-activating factor (1-o-alkyl-2-acetyl-sn-glyceryl-3-phosphorocholine) (PAF) is produced by a variety of inflammatory cells, including macrophages, neutrophils, basophils, eosinophils, platelets, and endothelial cells (3, 4, 7, 18). PAF has been implicated in a number of pathological conditions, including endotoxic shock, thrombosis, allergic reactions, and a variety of other inflammatory diseases (3, 8, 14, 15). More recently, a few studies have demonstrated that PAF is also capable of activating monocytic cells to express iNOS and produce NO (28). The PAF-induced NO production in macrophages appears to have tumoricidal activity in vitro (13). Interestingly, the endogenous release of PAF appears to play an important role in the production of NO following the activation of murine macrophages with LPS (28).
In the present study, we have investigated whether PAF plays a role in mediating resistance against T. cruzi infection both in vitro and in vivo. Our results show that PAF-activated macrophages release NO that leads to trypanocidal activity and suggest a role for endogenous PAF in mediating protection against T. cruzi infection in mice.
MATERIALS AND METHODS
Experimental animals.
Female BALB/c or C3H/HeJ mice, 6 to 8 weeks old, were bred and maintained under standard conditions in the animal house of the Department of Immunology, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Parasites and experimental infections.
The Y strain of T. cruzi was used in all experiments. For experiments in vitro, trypomastigote forms were grown in and purified from the monkey kidney fibroblast cell line LLC-MK2. BALB/c mice were infected intraperitoneally with 104 blood-derived trypomastigote forms. Parasitemia levels in 5 μl of blood obtained from the tail vein were measured as previously described (17).
In vivo treatment with WEB 2170.
Infected mice received an intraperitoneal injection of the PAF antagonist WEB 2170 (10 mg/kg; Boehringer, Ingelheim, Germany) or vehicle (phosphate-buffered saline; 10 ml/kg) 20 min prior to infection and then daily for the first 15 days postinfection as previously reported (22). Parasitemia levels and mortality rates were evaluated throughout the acute phase of infection.
Macrophage cultures.
C3H/HeJ and BALB/c mouse inflammatory macrophages were harvested from peritoneal cavities 3 days after the injection of 1 ml of 3% sodium thioglycolate (Difco Laboratories, Detroit, Mich.). The adherent cells were obtained after a 2- to 4-h incubation of single-cell suspensions in 24-well tissue culture plates at 37°C. The nonadherent cells were removed by exhaustive washing with Hanks’ medium. Parasites were added in a 1:1 parasite:cell ratio with or without anti-TNF-α monoclonal antibody (MAb) (XT 22.11; 50 μg/ml) and incubated for 6 h at 37°C in a humidified chamber containing 5% CO2. Culture supernatants were harvested 48 h later and stored at −20°C for later nitrite determination.
Microbicidal activity.
Peritoneal macrophages were harvested from mice 3 days after injection of 1 ml of 3% (wt/vol) sodium thioglycolate (Sigma). The cells (106/ml) were plated onto chamber slides (Nunc) and incubated overnight. Adherent cells were infected at a parasite-to-cell ratio of 1:1 for 120 min. Extracellular parasites were removed by six washes with RPMI 1640, and the cells were incubated at 37°C in 5% CO2 in the presence or absence of various concentrations of PAF, lyso-PAF (Bachem Inc.), WEB 2170 (10−5 to 10−9 M), recombinant murine IFN-γ (Life Technologies, Bethesda, Md.) (1 to 100 U/ml), or l-N-monomethyl arginine (l-NMMA; 200 mM) (Sigma). The supernatants were harvested and assayed for nitrite concentration. Growth of parasites in the macrophages was measured by counting the trypomastigotes released 5 days after the infection and by counting the intracellular amastigote forms 4 and 48 h postinfection, as previously described (25).
NO quantification.
The nitrite concentration in the culture supernatants was assayed in a microplate by mixing 0.1 ml of culture supernatant with 0.1 ml of Griess reagent (29). The absorbance at 550 nm was read 10 min later, and the NO2− concentration was determined by reference to a standard curve of 1 to 100 μM NaNO2.
Statistics.
Statistical analysis of the differences between mean values obtained for experimental groups was done by Student’s t test, and that for unpaired correlations was done by the Spearmon test.
RESULTS
PAF induces the production of NO in T. cruzi-infected macrophages.
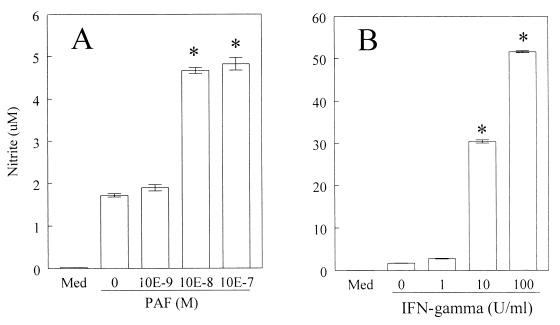
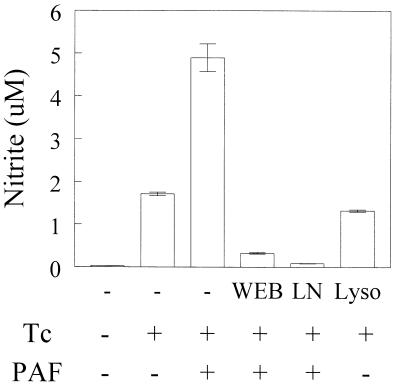
The addition of increasing concentrations of PAF (10−9 to 10−7 M) led to a significant production of NO in C3H/HeJ-derived macrophages infected with T. cruzi (Fig. 1A). As seen in Fig. 2, PAF alone induced little NO production but significantly synergized with T. cruzi infection to enhance NO levels. The levels of NO detected in response to PAF were markedly lower than those observed when infected macrophages were stimulated with IFN-γ (Fig. 1B). The effects of PAF were receptor mediated as assessed by measuring the inhibitory effects of the PAF receptor antagonist WEB 2170 on PAF-induced NO production (Fig. 3). In addition, the inactive metabolite of PAF, lyso-PAF, was ineffective in enhancing NO production by infected macrophages. PAF-induced NO production was abrogated by treatment with the NOS inhibitor l-NMMA (Fig. 3). The levels of NO production by macrophages in the presence of WEB 2170 were markedly lower than in the presence of parasite alone (Fig. 3). Similar results were obtained when BALB/c mouse macrophages were infected.
FIG. 1.
PAF induces NO production by T. cruzi-infected macrophages. C3H/HeJ-derived thioglycolate-elicited peritoneal macrophages were cultured with T. cruzi trypomastigotes (Y strain) at a parasite-to-host cell ratio of 1:1 for 48 h in the presence of PAF (A) or IFN-γ (B) at 37°C in a humidified chamber containing 5% CO2. The supernatants were harvested, and the nitrite concentration was assayed by the Griess method. Bars represent the mean and standard deviation (SD) of triplicate samples from one of three independent experiments. ∗, P < 0.05 compared with the values obtained with infected cells cultured in medium (Med) alone.
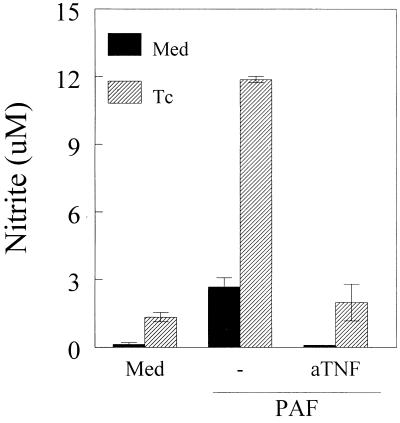
FIG. 2.
Anti-TNF-α MAb inhibits PAF-induced NO production. C3H/HeJ-derived peritoneal macrophages were cultured with T. cruzi trypomastigotes (Tc) at a parasite-to-cell ratio of 1:1 with or without PAF (10−7 M) or PAF plus anti-TNF-α MAb (aTNF) (50 μg/ml). After 48 h, the supernatants were harvested and the nitrite concentration was assayed by the Griess method. Bars represent mean ± SD of triplicate samples. The results are representative of three independent experiments. ∗, P < 0.05 compared with the values obtained with cells cultured in the presence of medium (Med) alone.
FIG. 3.
WEB 2170 and l-NMMA inhibit PAF-induced NO production. C3H/HeJ-derived peritoneal macrophages were cultured with T. cruzi trypomastigotes (Tc) at a parasite-to-cell ratio of 1:1 with or without PAF (10−7 M) plus WEB 2170 (WEB) (10−5 M), PAF plus l-NMMA (LN) (200 mM), or lyso-PAF (Lyso) (10−7 M). After 48 h, the supernatants were harvested and the nitrite concentration was assayed. Bars represent the mean and SD of triplicate samples. The results are representative of three independent experiments. ∗, P < 0.05 compared with the values obtained with cells cultured in the presence of medium alone.
Pretreatment of infected macrophages with a neutralizing MAb raised against murine TNF-α effectively inhibited the production of NO in response to PAF. Similarly, the anti-TNF-α MAb blocked the low levels of NO produced by noninfected macrophages treated with PAF (Fig. 2).
PAF-induced NO production controls the growth of T. cruzi in macrophages.
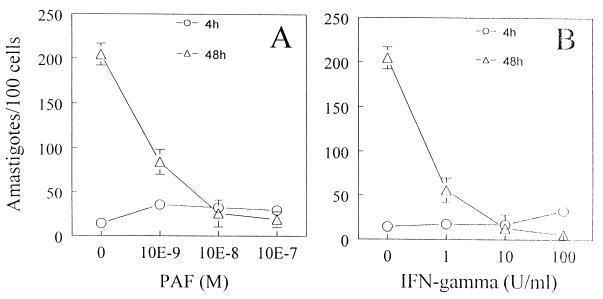
To evaluate whether PAF could affect parasite growth, infected macrophages were stimulated in vitro with PAF and parasite growth was evaluated by counting intracellular parasites soon after infection (4 h postinfection) or 48 h postinfection. The addition of PAF to infected C3H/HeJ-derived macrophages resulted in a concentration-dependent inhibition of parasite growth (Fig. 4A). For example, addition of 10−7 M PAF caused a greater than 90% reduction in parasite count compared with the outcome of incubation with medium alone. Inhibition of parasite replication by macrophages treated with IFN-γ is also shown for comparison (Fig. 4B).
FIG. 4.
Parasite growth is inhibited in PAF-treated macrophages. C3H/HeJ-derived peritoneal adherent cells were cultured with T. cruzi trypomastigotes at a parasite-to-host-cell ratio of 1:1 and with various concentrations of PAF (A) or IFN-γ (B) in a humidified chamber containing 5% CO2 at 37°C. The number of intracellular parasites in at least 500 cells was determined by light microscopy. Symbols represent the mean and SD of triplicate counts of 100 cells. The data are representative of three independent experiments. ∗, P < 0.05 compared to the values obtained with infected cells cultured in medium alone.
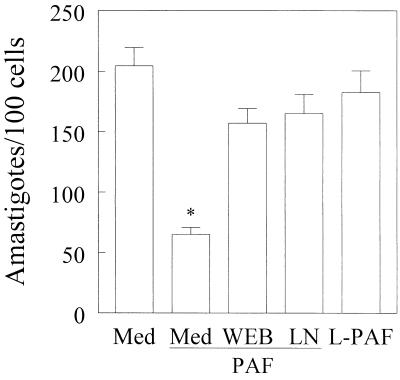
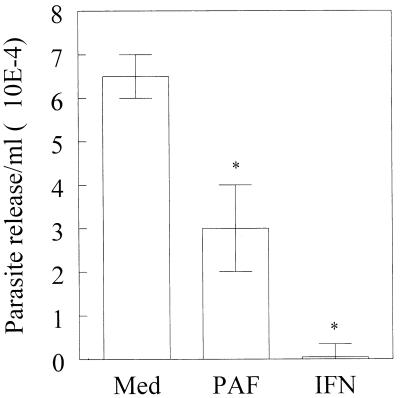
The inhibitory effects of PAF on parasite growth were counteracted by addition of a PAF antagonist, WEB 2170 (Fig. 5). Moreover, the inactive metabolite of PAF, lyso-PAF, did not induce trypanocidal activity in C3H/HeJ-derived macrophages (Fig. 5). A role for NO production in the trypanocidal activity of PAF was shown by using l-NMMA. This NOS inhibitor effectively reversed the ability of PAF to induce infected macrophages to kill T. cruzi (Fig. 5). The number of parasites released by macrophages on day 5 after infection was also significantly inhibited (by more than 53%) when exogenous PAF (10−7 M) was added (Fig. 6). Similar results were obtained when BALB/c-derived macrophages were used. For comparison, addition of 100 U of recombinant IFN-γ per ml inhibited parasite growth (99%), and IFN-γ was significantly more effective than PAF in the control of parasite replication. Together, our in vitro results demonstrate the ability of exogenous PAF to induce both NO production and trypanocidal activity in infected macrophages.
FIG. 5.
PAF-induced microbicidal activity is decreased by inhibition of the PAF receptor or iNOS activation. C3H/HeJ-derived peritoneal adherent cells were infected with T. cruzi trypomastigotes at a parasite-to-host-cell ratio of 1:1 in the absence of stimulus (Med) or with lyso-PAF (L-PAF) (10−7 M) or PAF (10−7 M) with or without WEB 2170 (10−5 M) (WEB) or l-NMMA (200 mM) (LN) and cultured for 48 h in a humidified chamber containing 5% CO2 at 37°C. Bars represent the mean and SD of triplicate counts of the number of intracellular parasites found in 100 macrophages. The data are representative of three independent experiments. ∗, P < 0.05 compared to the values obtained with infected cells cultured in medium alone.
FIG. 6.
The presence of PAF decreases the release of parasites from infected macrophages. C3H/HeJ-derived peritoneal macrophages were infected with T. cruzi trypomastigotes (Y strain) at a parasite-to-host-cell ratio of 1:1 in a humidified chamber containing 5% CO2 at 37°C. Two hours later, extracellular parasites were removed and the infected cells were incubated with medium alone (Med), PAF (10−7 M), or IFN-γ (100 U/ml). On day 5 postinfection, the parasites released into the medium were counted. Bars represent the mean and SD of triplicate samples and are representative of three independent experiments. ∗, P < 0.05 compared to the values obtained with infected cells cultured in medium alone.
The blockade of PAF receptors in an animal model of acute T. cruzi infection enhances parasitemia and animal death rates.
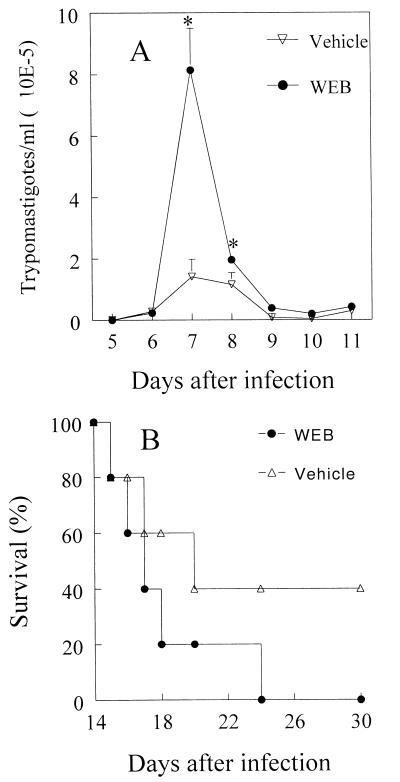
Next we examined a role for PAF in a T. cruzi infection model in mice. BALB/c mice were treated daily with WEB 2170 or vehicle for 15 days during the acute phase of infection. Parasitemia and mortality were evaluated from days 5 to 15 postinfection. Treatment during the first 15 days postinfection resulted in a significant increase in parasitemia on days 7 and 8 compared with that in infected mice receiving vehicle only. On day 8, the parasitemia in WEB 2170-treated mice was 21-fold higher than that in vehicle-treated animals (Fig. 7A). In addition, WEB 2170-treated mice had significantly lower survival rates than did vehicle-treated mice (Fig. 7B). WEB 2170-treated animals had 100% mortality around day 24 after infection, whereas vehicle-treated mice had 60% mortality at that time.
FIG. 7.
PAF mediates resistance in mice infected with T. cruzi. BALB/c mice were infected with 104 (A) or 103 (B) blood trypomastigotes and treated with WEB 2170 (●) or with vehicle (○) 4 h before and again once daily after infection with T. cruzi. Parasitemia (A) and survival rates (B) were evaluated during the acute phase. Lines represent means and SD of data obtained with 10 mice per group in one of three independent experiments. ∗, P < 0.05 compared to the values obtained for parasitemia in vehicle-treated mice.
DISCUSSION
PAF is a membrane-derived phospholipid with widely recognized proinflammatory activities. PAF is produced and exerts its biological actions in a variety of cells, including neutrophils, eosinophils, lymphocytes, and macrophages. Moreover, it has been implicated in several systemic and organ-specific disorders, such as allergic inflammation and endotoxic shock (3, 7, 16). However, much less is known about its role during infection, and, to our knowledge, there have been no studies evaluating the role of PAF during infections with the protozoan T. cruzi. It has been shown that PAF induces the production of NO in monocytic cells (28) and that NO derived from activated macrophages is cytostatic or cytotoxic for a variety of pathogens (20, 21). In the present study, we evaluated the role of PAF in modifying T. cruzi infection both in vitro and in vivo.
Pretreatment of T. cruzi-infected macrophages from C3H/HeJ or BALB/c mice with PAF induced NO production in a dose-dependent manner, although the production was lower than that induced by IFN-γ. PAF-induced NO production was shown to be inhibited by the PAF receptor antagonist WEB 2170, and lyso-PAF, an inactive metabolite, had no effect on NO production. Together, these two pieces of evidence suggest that a specific interaction between PAF and its receptor on the macrophage is responsible for the induction of NO synthesis.
The addition of a neutralizing anti-TNF-α MAb blocked the PAF-mediated NO production. One interesting possibility raised by these results is that PAF induces TNF-α synthesis, which, in turn, leads to iNOS induction and NO production in infected macrophages. In addition, the ability of WEB 2170 to inhibit the production of NO by T. cruzi-infected macrophages (Fig. 2 and data not shown) raises the possibility that PAF plays an essential role in signal transduction pathways necessary for NO production, possibly via induction of TNF-α (11). We are presently addressing these possibilities.
Since PAF increased NO production in infected macrophages, we investigated whether it could induce a cytotoxic or cytostatic effect on intracellular growth of T. cruzi. We found a direct correlation between the ability of PAF to induce NO production and its capacity to decrease intracellular parasite replication, since PAF-induced NO production and microbicidal activity were both inhibited by either WEB 2170 or l-NMMA. Moreover, the addition of lyso-PAF had no effect on intracellular parasite growth or NO production. Interestingly, we also found that PAF potentiates macrophage trypanocidal activity when added simultaneously with low doses of IFN-γ (data not shown). These data suggest that PAF binding and signaling through a specific receptor lead to parasite killing via the NO pathway, in the early stages of parasite infection in vivo. These results are in agreement with the data showing an ability of PAF to induce NO production in different situation (27); however, they are the first to show the importance of PAF in resistance against T. cruzi infection in vitro.
To evaluate the role of PAF in the control of parasite replication in vivo, we treated T. cruzi-infected mice with WEB 2170, a PAF receptor antagonist. We found significantly higher parasitemia and earlier mortality in WEB 2170-treated mice, demonstrating a decreased resistance of these animals to infection with T. cruzi. Similar results were obtained with another PAF receptor antagonist, UK-74505 (data not shown). Since interleukin-12 (IL-12) is induced during T. cruzi infections (1) and this cytokine was found to be involved in the induction of PAF synthesis by polymorphonuclear cells and NK cells (2), it seems reasonable to suggest that PAF is produced endogenously in T. cruzi-infected mice and, according to the results presented above, mediates resistance to the infection by controlling parasite replication. However, the role of T. cruzi-induced IL-12 on PAF induction during in vivo infections remains to be elucidated. Recently, it has been shown that Candida albicans-infected mice produced PAF, which is involved in resistance to infection via production of TNF-α (15). Since NO is thought to be involved in the control of C. albicans replication, it is possible that the PAF-mediated resistance to infection with this fungus is mediated by NO, which is the end product of an activation cascade initiated by PAF and TNF-α. The possible interaction between endogenously produced PAF and TNF-α in T. cruzi-infected mice is currently being investigated in our laboratories.
The results of the present study suggest that PAF is produced during T. cruzi infection and, together with parasite-induced proinflammatory cytokines, including TNF-α (25), IFN-γ (5, 24, 26), and IL-12 (1), mediates resistance to infection. Thus, neutralization of these proinflammatory cytokines or blockade of the PAF receptor leads to exacerbation of parasitemia and early death, suggesting that these molecules might be involved in the effector responses to the parasite. Another relevant issue concerns a possible role for PAF in acute myocarditis in T. cruzi-infected mice. Since this molecule is a chemoattractant for several leukocyte populations, it is possible that trypomastigote forms present in the heart induce PAF secretion, which in turn leads to inflammatory-cell infiltration and production of proinflammatory cytokines in this tissue (6). Finally, it will be important to define a role for PAF during infection with other protozoa, such as Leishmania major and Toxoplasma gondii, for which protective responses are also dependent on NO production.
ACKNOWLEDGMENTS
This work was supported by grants from FAPESP (96/4118-9 and 97/11640-6), CNPq, and WHO/TDR (970728).
We thank Luisa K. P. Arruda, Dragana Jancovic, and George Yap for critical reading and comments.
REFERENCES
- 1.Aliberti J C, Cardoso M A, Martins G A, Gazzinelli R T, Vieira L Q, Silva J S. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussolati B, Mariano F, Cignetti A, Guarini A, Cambi V, Foa R, Piccoli G, Camussi G. Platelet-activating factor synthesized by IL-12-stimulated polymorphonuclear neutrophils and NK cells mediates chemotaxis. J Immunol. 1998;161:1493–1500. [PubMed] [Google Scholar]
- 3.Braquet P, Touqui L, Shen T Y, Vargaftig B B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- 4.Camussi G, Aglietta M, Coda R, Bussolino F, Piacibello W, Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981;42:191–199. [PMC free article] [PubMed] [Google Scholar]
- 5.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekar B, Melby P C, Troyer D A, Colston J T, Freeman G L. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am J Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- 7.Chao W, Olson M S. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chignardi M, Le Couedic J P, Tence M, Vargaftig B B, Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979;279:799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- 9.Ding A, Nathan C F, Stuher D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 10.Drapier J C, Wietzerbin J, Hibbs J B., Jr Interferon-gamma and tumor necrosis factor induce the l-arginine dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 11.Dubois C, Bissonnette E, Rola-Pleszczynski M. Platelet-activating factor (PAF) enhances tumor necrosis factor production by alveolar macrophages. Prevention by PAF receptor antagonists and lipoxygenase inhibitors. J Immunol. 1989;143:964–970. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Oswald I P, Hienry S, James S L, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 13.Howard A D, Erickson K L. The induction and augmentation of macrophage tumoricidal responses by platelet-activating factor. Cell Immunol. 1995;164:105–112. doi: 10.1006/cimm.1995.1148. [DOI] [PubMed] [Google Scholar]
- 14.Im S-Y, Ko H-M, Ko Y-S, Kim J-W, Lee H-K, Ha T-Y, Lee H B, Oh S-J, Bai S, Chung K-C, Lee Y-B, Kang H-S, Chun S-B. Augmentation of tumor metastasis by platelet-activating factor. Cancer Res. 1996;56:2662–2665. [PubMed] [Google Scholar]
- 15.Im S-Y, Choi J-H, Ko H-M, Han S-J, Chun S-B, Lee H-K, Ha T-Y. A protective role of platelet-activating factor in murine candidiasis. Infect Immun. 1997;65:1321–1326. doi: 10.1128/iai.65.4.1321-1326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao W-H, Kume K, Fukuchi Y, Ikuta K, Miyazaki J-I, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo R C, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64:475–482. [PubMed] [Google Scholar]
- 18.Mencia-Huerta J M, Benveniste J. Platelet-activating factor and macrophage. I. Evidence for the release from rat and mouse peritoneal macrophages and not from mastocytes. Eur J Immunol. 1979;9:409–415. doi: 10.1002/eji.1830090512. [DOI] [PubMed] [Google Scholar]
- 19.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;30:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 20.Nathan C, Xie Q-W. Nitric oxide synthases: roles, tools and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 21.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1993;6:3051–3064. [PubMed] [Google Scholar]
- 22.Negro-Alvarez J M, Lopez J C M, Martinez J L O, Aleman A A, Rubio del Barrio R. Platelet-activating factor antagonists. Allergol Immunopathol. 1997;25:249–258. [PubMed] [Google Scholar]
- 23.Petray P, Rottenberg M E, Grinstein S, Orn A. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 1994;16:193–199. doi: 10.1111/j.1365-3024.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 24.Plata F, Wietzerbin J, Ons F G, Falcoff E, Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984;14:930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- 25.Silva J S, Vespa G N R, Cardoso M A G, Aliberti J C S, Cunha F Q. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steil A A, Garcia Rodriguez M C, Alonso A, Crespo M S, Bosca L. Platelet-activating factor: the effector of protein-rich plasma extravasation and nitric oxide synthase induction in rat immune complex peritonitis. Br J Pharmacol. 1995;114:895–901. doi: 10.1111/j.1476-5381.1995.tb13288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo C, Wu C C, Mitchell J A, Gross S S, Thiemermann C, Vane J R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipolysaccharide. Circ Res. 1993;73:991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- 29.Vespa G N R, Cunha F Q, Silva J S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]