Abstract
This investigation focuses on the three novel polysaccharides from Cordyceps militaris and then discusses their characterization and anti-complementary activity. The three polysaccharides from C. militaris (CMP-1, CMP-2 and CMP-3) were prepared using a DEAE-52 cellulose column. The HPLC, HPGPC, FT-IR and Congo red analyses were used to characterize their monosaccharides, molecular weight and stereo conformation, which demonstrated that the three polysaccharides were homogenous polysaccharides with different molecular weights and were composed of at least ten monosaccharides with different molar ratios, and all had a triple-helix conformation. The evaluation of anti-complementary activity demonstrated that the three polysaccharides significantly inhibited complement activation through the classical pathway and alternative pathway. Preliminary mechanism studies indicated that CMP-1, CMP-2 and CMP-3 acted with C2, C5, C9, factor B, factor B, and P components in the overactivation cascade of the complement system. The analysis of the Pearson correlation and network confirmed that the ribose, glucuronic acid and galacturonic acid composition were negatively correlated with the anti-complementary activity of polysaccharides. These results suggested that the three novel polysaccharides are potential candidates for anti-complementary drugs.
Keywords: Cordyceps militaris, anti-complementary activity, polysaccharides, structure-activity relationship
1. Introduction
The complement system is considered to be the chief component of innate immunity and plays an important role in host protection, consisting of more than 40 serine proteases, receptors and inhibitors. Complement has a strict mechanism of operation to avoid damage to autologous tissues and to clear pathogens under normal conditions. However, when complement is overactivated it can cause severe complement-mediated diseases [1,2,3]. Currently, drugs with known anti-complement abilities such as glucocorticoids and rituximab have been used in the treatment of diseases associated with hypercomplementation, but their clinical application is plagued by significant adverse effects and high prices [4]. Recently, natural polysaccharides, which are complement inhibitors with high efficiency and low toxicity, have garnered the attention of researchers, serving as potential agents of complement inhibitors [5,6,7]. These reported polysaccharides with complement inhibition are mainly from plants, while a few studies have been reported on the complement activity of polysaccharides from fungi.
Cordyceps militaris (L. ex Fr.) Link. is an entomogenous fungi that belongs to the genus Codyceps and the family Hypocreaceae, which is widely distributed in China, Japan, Korea and other East Asian countries [8]. It has been used as a healthy food and folk medicine for the treatment of malaria, palpitations, fever, dizziness and chronic kidney disease [9]. Various bioactive constituents from the C. militaris have been reported, including polysaccharides, cordycepin, volatile oils and flavonoids [10,11,12]. Polysaccharides are one of the most abundant and important components in C. militaris, which can exhibit a variety of various pharmacological effects, such as antioxidant, antitumor, anti-inflammatory, immunomodulatory, antihyperlipidemic, and hepatoprotective [13,14,15,16,17]. Especially, C. militaris polysaccharides (CMPs) can upregulate the expression of TNF-α, IFN-γ, and IL-1β mRNA in mice [18]. Simultaneously, the over-activation of the complement system is involved in many autoimmune disorders and inflammatory diseases [19], and is closely related to the above immune cytokines, which imply that the polysaccharide fractions with anti-complement activity may be present in C. militaris. However, no relevant studies on the preparation and characterization of polysaccharides with anti-complement effect from C. militaris have been reported.
The present study aimed to prepare novel polysaccharides with anti-complementary activity from C. militaris. Herein, three novel polysaccharides from C. militaris (CMP-1, CMP-2 and CMP-3) were successfully prepared by a DEAE-52 cellulose column. The structural characterization of the three polysaccharides were analyzed via chromatographic and spectroscopic techniques. The complement inhibition ability and their obstructed targets were evaluated by immune haemolysis test, and the further structure–activity relationship was preliminarily explored through the Pearson correlation and network analysis.
2. Materials and Methods
2.1. Fungus Material and Reagents
Cultured Cordyceps militaris was obtained from the Yanbian Foresty Science Institute (Yanji, China), in September 2020 and identified by Prof. Gao Li (College of Pharmacy, Yanbian University). The voucher specimen (voucher number: YB-YC-2032) has been deposited at the Pharmacognosy Laboratory of the College of Pharmacy, Yanbian University. The mannose (Man), glucosamine (GlcN), ribose (Rib), rhamnose (Rha), glucuronic acid (GlcA), galacturonic acid (GalA), glucose (Glc), galactose (Gal), xylose (Xyl), arabinose (Ara) and fucose (Fuc) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The different complement components (C2, C3, C4, C5, C9, Factor B, Factor D, Factor P) were obtained from MineBio Life Sciences Ltd. (Shanghai, China). All reagents and solvents were of analytical grade. The Yanbian University Institutional Research Ethics Committee accepted all the experimental procedures and protocols (approval NO. YBU-2020-091801).
2.2. Preparation of Polysaccharides
The crude C. militaris polysaccharides (CMPs) were prepared by water extraction and the alcohol precipitation method. Briefly, the C. militaris was crushed into powder and pretreated with 80% ethanol (Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China)three times, as described in our previous work [20]. The C. militaris residues were refluxed twice with deionized water (40:1, mL/g) at 80 °C for 60 min. The extraction solution was concentrated to 1/5 of the original volume, followed by precipitation through the addition 95% ethanol and refrigerated overnight to obtain polysaccharide precipitation. Furthermore, the precipitation of polysaccharides was redissolved with deionized water, and injected into a DEAE-52 cellulose column (26 mm × 300 mm), eluted with 0, 0.1, 0.2, 0.3, 0.4 and 0.5 mol/L NaCl, and determined at 490 nm by the phenol-sulphuric acid method to draw the elution curve [21]. The main eluent fraction was collected and dialyzed, and finally lyophilized for the next analysis.
2.3. Determination of Water Solubility Index
The water soluble index (WSI) of polysaccharides was determined by Ye et al. [22]. The dried sample (0.2 g) was mixed with deionized water (2 mL) for dissolution. Then, the mixture was centrifuged at 5000 rpm for 40 min. The precipitant was collected and lyophilized to a constant weight. The WSI was calculated as follows:
| (1) |
where M0 was the weight of the sample, M1 was the weight of the lyophilized precipitant.
2.4. Determination of Homogeneity and Molecular Weights
The homogeneity and molecular weights (Mw) of the sample were measured on a Waters high-performance gel permeation chromatography (HPGPC) equipped with a RI2000 refractive index detector (RID) (Schambeck SFD GmbH, Germany), and a Shodex sugar KS-804 column (8 mm × 300 mm, Showa Denko, Japan) [23]. The mobile phase was ultrapure water at a flow rate of 1.0 mL/min at 50 °C. The N2000 GPC system (Hangzhou Sno Scientifific Instrument Co. Ltd., Hangzhou, China) was used to process data. The molecular weights of the sample were calculated by the calibration curve obtained by using a series of dextran standards.
2.5. Determination of Monosaccharide Compositions
The monosaccharide composition of polysaccharides was analyzed by a high-performance liquid phase (Ultimate 3000 HPLC system, Thermo, Waltham, MA, USA) equipped with a diode array detector (DAD, Thermo, USA) [24]. The sample hydrolysate solution was hydrolyzed by trifluoroacetic acid (2 mol/L), added to 1-phenyl-3-methyl-5-pyrazolone solution (PMP, 0.5 mol/L) and NaOH solution (0.3 mol/L), and incubated at 70 °C for 60 min. The mixture was added to HCl solution (0.3 mol/L), and followed by extraction with chloroform for three times. The 20 µL of aqueous layer containing a PMP-labeled derivative was filtered, and injected into a Supersil ODS2 column (5 μm, 4.6 mm × 250 mm), and eluted at 0.8 mL/min with the mobile phase (acetonitrile and phosphate buffer solution, 18:82 v/v) at 30 °C. The detection wavelength was set as 245 nm. A series of diluted concentrations of standard monosaccharides were derived and analyzed for drawing standard curves to calculate the monosaccharide molar ratio of polysaccharides.
2.6. Determination of FT-IR
The FT-IR measurement (Gangdong Sci. & Tech. development Co. Ltd., Tianjin, China) was performed in the wavenumber range of 4000~400 cm−1 with KBr pellet (Dried polysaccharides: KBr powder, 2:100 mg/mg). Derivation including Savitsky-Golay algorithm with 13 smoothing factors was performed using the OMNIC 8.0 software incorporated into the instrument [25].
2.7. Congo Red Test
The triple helix structures of the samples were investigated using the Congo red, as described previously [26]. Briefly, the 2 mL of Congo red solution (80 μmol/L) and 2 mL of polysaccharides solution (2 mg/mL) were mixed with 1 mol/L the NaOH until the concentrations of NaOH in the mixtures were 0–0.5 mol/L. The absorption spectrum was scanned from 200~600 nm to obtain the maximum absorption wavelength (λmax)
2.8. Anti-Complementary Activity
2.8.1. Anti-Complementary Activity through the Classical and Alternative Pathway
The anti-complementary activity of polysaccharides on the classical pathway (CP) was evaluated as described in our previous studies [27,28]. Briefly, the 6% sheep red blood cells were added with GVB-Ca2+/Mg2+ buffer solution to a constant volume until the concentration was 2 × 109 cells/mL, then mixed with hemolysin in 1:1 ratio, incubated at 37 ℃ for 30 min, centrifuged to remove unconjugated hemolysin, and obtained sensitized sheep erythrocytes (EAs) for standby. The source of complement for the classical pathway was the normal healthy adult serum pool (NHSP). The polysaccharides solutions were prepared into different concentration gradients with GVB-Ca2+/Mg2+ buffer solution, then NHSP diluted in 1:80 was added, and EAs were added after 30 min incubation in a 37 ℃ water bath. The supernatant was collected through centrifugation, and absorbance was measured at 540 nm. The positive control was heparin. Finally, the polysaccharides concentration required for 50% hemolysis inhibition (CH50) was calculated according to the hemolysis inhibition rate of the different concentration gradients.
The anti-complementary activity of polysaccharides on the alternative pathway (AP) was determined to be similar to the above steps. The NHSP in GVB-Mg2+/EGTA as a complement source of the alternative pathway. In brief, the 2% rabbit erythrocytes (ERs) were mixed with GVB-Mg2+/EGTA buffer to a concentration of 5 × 108 cells/mL. Then, NHSP and the diluted polysaccharide solution of different concentrations were pre-incubated for 15 min, and then ERs was added to incubate at 37 °C for 30 min. The reaction vessel was placed on ice for cooling until the reaction was terminated. The supernatant was obtained by centrifugation and placed in a 96 well plate, the absorbance value was scanned at 412 nm., and the 50% hemolytic inhibition concentration (AP50) was calculated in the alternative pathway.
2.8.2. Identification of Complement Targets
The targets of polysaccharide in the complement cascade were identified by the previously reported method [29]. The critical concentration of polysaccharide was used as the test concentration of the complement target, and was selected to inhibit the concentration of polysaccharide when hemolysis was close to 100%. The different complement-depleted sera were incubated with EAs or ERs at 37 °C for 30 min. In the following operation steps, C2, C3, C4, C5 and C9 targets were identified as the same as the CP complement test, while factors B, D and P were the same as the AP test. The negative control, positive control and blank control were glucose, heparin and deletion serum without sample, respectively.
2.9. Statistical Analysis
All data were represented as mean ± standard deviation of three separate experiments. Statistical difference analysis and Pearson correlation analysis were carried out on SPSS 17.0 (SPSS Inc, Chicago, IL, USA). The network analysis of structure-activity correlation was visualized by Cytoscape 3.9.1 (National Institute of General Medical Sciences, Bethesda, MD, USA).
3. Results and Discussion
3.1. Preparation of Polysaccharides
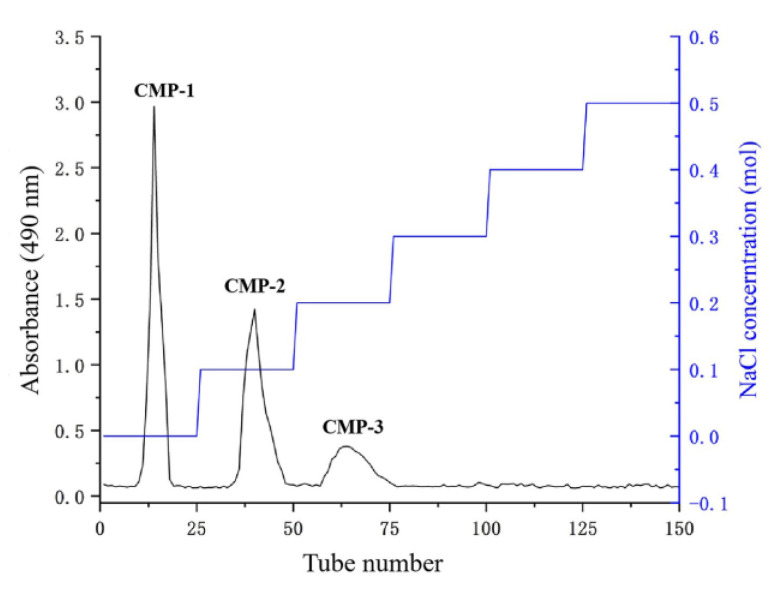
The crude CMPs were prepared by hot water extraction followed by ethanol precipitation and freeze-drying, and then isolated using a DEAE-52 cellulose column (Figure 1). The three major polysaccharides peaks (CMP-1, CMP-2 and CMP-3) were obtained, which were eluted by the deionized water, 0.1 and 0.2 mol/L NaCl solution, respectively. The polysaccharide contents of CMP-1, CMP-2 and CMP-3 were 91.78 ± 2.11%, 92.95 ± 1.17% and 94.18 ± 2.18%, respectively. The yields of CMP-1, CMP-2 and CMP-3 were 24.31 ± 1.45%, 16.52 ± 1.04% and 10.02 ± 0.52%, respectively. The water soluble index of CMP-1, CMP-2 and CMP-3 was 98.67 ± 0.62%, 98.56 ± 0.83% and 97.85 ± 0.72%, respectively.
Figure 1.
Elution profile of polysaccharides on DEAE-52 cellulose column.
3.2. Analysis of Molecular Weights
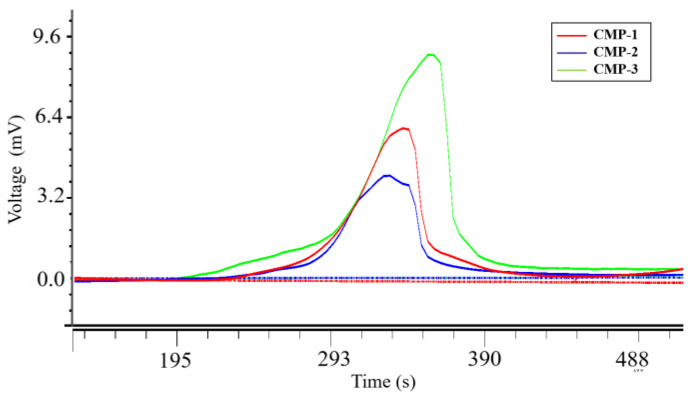
The HPGPC chromatogram of three polysaccharides are presented in Figure 2. They are all homogeneous polysaccharides demonstrated by their chromatographic curves exhibiting a single peak. The results of GPC software fitting and calculation showed that the molecular weights of CMP-1, CMP-2 and CMP-3 were 2.19 × 106 Da, 2.80 × 106 Da and 1.74 × 106 Da, respectively. Previous researchers have reported that the molecular weights of C. militaris polysaccharides were generally between ~103 Da and ~105 Da [30], and a kind of high molecular weight C. militaris polysaccharides (CMP-Ⅲ, 4.796×104 kDa) was isolated by He et al. [31]. The molecular weights of CMP-1, CMP-2 and CMP-3 were observed to be significantly higher than those of the C. militaris polysaccharides that were previously reported, which may be considered as high molecular weight polysaccharides.
Figure 2.
High performance gel permeation chromatography of CMP-1, CMP-2 and CMP-3.
3.3. Analysis of Monosaccharide Compositions
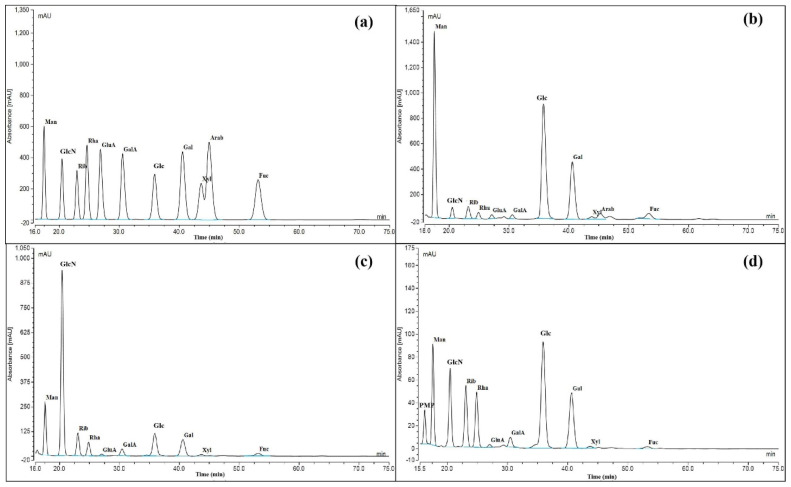
The monosaccharide composition chromatograms of the three polysaccharides and their molar ratios are shown in Figure 3 and Table 1. Compared with the monosaccharide component of CMP-1, no trace of Ara was detected in CMP-2 and CMP-3. Previous studies have shown that C. militaris polysaccharides generally contained 3 to 7 kinds of monosaccharides, which were mainly composed of Man, Glc and Gal with different molar ratios [30]. Obviously, the CMP-1, CMP-2 and CMP-3 were composed of at least 10 kinds of different monosaccharides, which were more abundant than the previously reported polysaccharides from C. militaris. Compared with polysaccharides from other sources (including other fungi, algae and plants) [32,33], they were usually composed mainly of glucose and galactose in their monosaccharide composition. However, glucosamine in these three polysaccharides was rarely found in polysaccharides from plants.
Figure 3.
High performance liquid chromatography of standard monosaccharides (a), CMP-1 (b), CMP-2 (c) and CMP-3 (d). (Mannose: Man, glucosamine: GlcN, ribose: Rib, rhamnose: Rha, glucuronic acid: GlcA, galacturonic acid: GalA, glucose: Glc, galactose: Gal, xylose: Xyl, arabinose: Ara, fucose: Fuc).
Table 1.
The molar ratios of monosaccharide composition of CMP-1, CMP-2 and CMP-3.
| Samples | Man | GlcN | Rib | Rha | GlcA | GalA | Glc | Gal | Xyl | Ara | Fuc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMP-1 | 39.35 | 4.03 | 3.98 | 2.56 | 1.62 | 1.52 | 70.52 | 26.90 | 1.00 | 3.23 | 4.23 |
| CMP-2 | 13.62 | 86.70 | 7.80 | 6.22 | 1.47 | 2.99 | 17.80 | 9.26 | 1.00 | ND | 3.02 |
| CMP-3 | 33.61 | 44.67 | 27.34 | 31.84 | 7.32 | 8.39 | 102.23 | 38.27 | 1.00 | ND | 5.79 |
Note: “ND” Not detected.
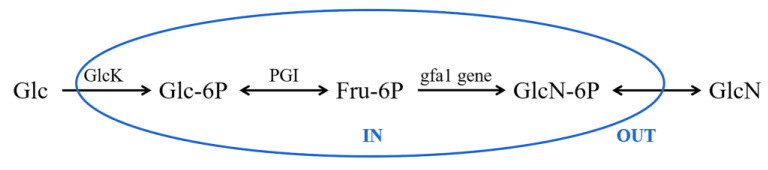
It is noteworthy that the three polysaccharides were also found to be composed of glucosamine, which were isolated from C. militaris for the first time. GlcN is an amino sugar, which was obtained by replacing the -OH group of the Glc molecule with an -NH2 group. Previous investigations demonstrated that glucosamine is widely distributed in fungi [34], and glucosamine-containing polysaccharides were also found in edible fungi, such as Helvella leucopus, Poria cocos and Lentinus edodes [35,36,37]. The biosynthetic pathway of glucosamine in C. militaris was speculated as follows (Figure 4): under the action of glucokinase (GlcK), extracellular glucose of C. militaris generates glucose-6-phosphate (Glc-6P), and further generates fructose-6-phosphate (Fru-6P) with the catalysis of phosphoisomerase (PGI) [38]. Glucosamine was synthesized by Fru-6P with glucosamine synthetase (gfa1 gene), and the glucosamine-containing polysaccharides were synthesized by dehydration condensation with other monosaccharides. Therefore, the three polysaccharides linked with glucosamine were novel C. militaris polysaccharides, and their speculated biosynthetic pathway was reasonable.
Figure 4.
The putative biosynthetic pathway of glucosamine in C. militaris polysaccharides.
3.4. Analysis of FT-IR Spectra
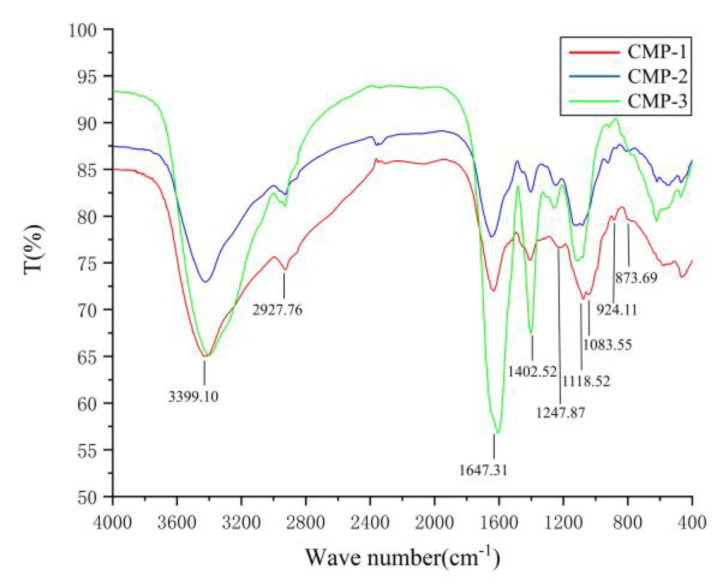
The CMP-1, CMP-2 and CMP-3 possessed similar characteristic absorption peaks of polysaccharides (Figure 5). The absorption peaks in the range of 3180–3700 cm−1 might be associated with O-H stretching and N-H stretching vibrations, where the -OH and -NH2 groups were characteristic of the polysaccharides and its attached GlcN, respectively [39]. The weak absorption at 2927 cm−1 may be caused by the methyl group on Fuc or Rha [40]. The peak at 1650 cm−1 and 1402 cm−1 were related to associated water and bending vibrations of C-H in the sugar ring, respectively [41]. The peaks at 1000–1200 cm−1 are attributed to the existence of the pyranose ring [42]. The peaks at 924.11 cm−1 and 873.69 cm−1 showed the presence of β-and α-type glycosidic linkage in the three polysaccharides [43].
Figure 5.
The FT-IR spectrum of CMP-1, CMP-2 and CMP-3.
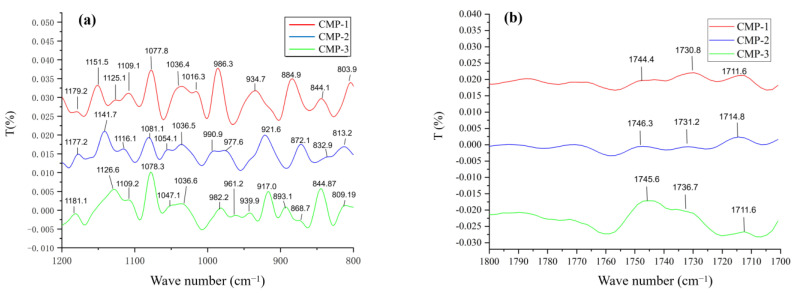
Moreover, the additional frequency band information of the three polysaccharides was obtained by the second derivative of infrared spectrum, which proved that CMP-1, CMP-2 and CMP-3 were of significant difference in the fingerprint region (800 ~ 1300 cm−1) (Figure 6a). In detail, CMP-1 has three weak absorption peaks (1109 cm−1, 1125 cm−1, 1151 cm−1) in the range of 1110 ~ 1160 cm−1, which may be attributed to side chains on α-carb\zon. Compared with CMP-1, however, there was no obvious absorption peak at 1125 cm−1 in CMP-2 and 1150 cm−1 in CMP-3, under the condition of neglecting the weak wave number shift caused by the tolerance error. The same situation was evident within the range of 990~860 cm−1; CMP-3 had six absorption peaks, while CMP-1 had only three broad absorption bands and CMP-2 had only four absorption peaks. Therefore, the second derivative proved that significant differences existed among the three polysaccharides in the fingerprint region. In particular, the weak absorption peak of 1730−1 cm in the second derivative spectrum (Figure 6b) suggested that they contained a small amount of uronic acid [44], which was consistent with the results of the monosaccharide composition.
Figure 6.
The second derivative FT-IR spectrum of three polysaccharides in the range of 800~1200 cm−1 (a) and 1700 ~ 1800 cm−1 (b).
3.5. Congo Red Test
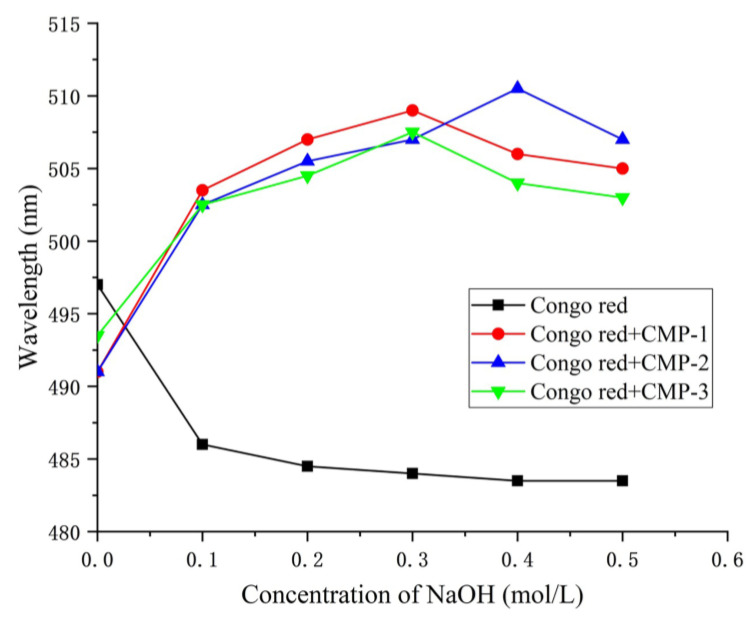
Polysaccharides containing the triple helix conformation can form complexes and increased λmax with Congo red in weakly alkaline solutions, while in strongly alkaline solutions the helix is disrupted, leading to a decrease in the λmax of the complex [45]. As shown in Figure 7, the λmax of Congo red + CMP-1, Congo red + CMP-2 and Congo red + CMP-3 increased with the increasing concentration of NaOH from 0 to 0.3 mol/L. Subsequently, the concentration of NaOH continued to increase, and the helix coil of the polysaccharide changed, indicating that the three polysaccharides all had the triple helix conformation.
Figure 7.
Change of maximum absorption wavelength of Congo red and three polysaccharide complexes.
3.6. Anti-Complementary Activity of CMP-1, CMP-2 and CMP-3
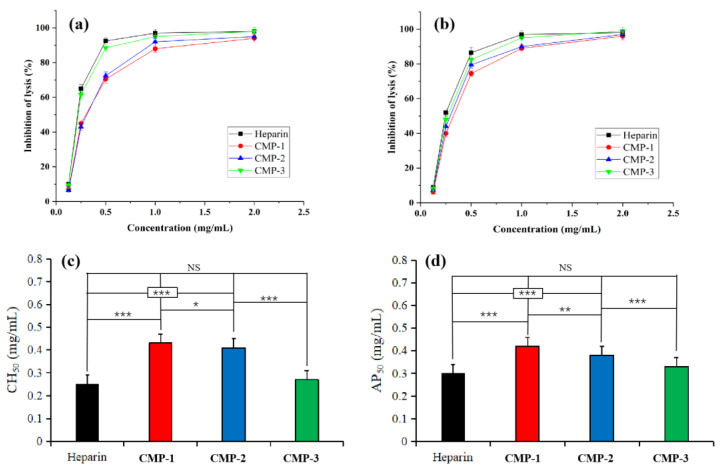
As shown in Figure 8a,b, CMP-1, CMP-2 and CMP-3 inhibited the hemolysis via classical pathway (CP) and alternative pathway (AP) in a dose-dependent manner within a concentration of 0.125 ~ 2.00 mg/mL. Furtherly, the half inhibitory concentration of the three polysaccharides on complement activation was calculated (Table 2), and the CH50 and AP50 differences between different samples were evaluated via one-way ANOVA analysis. As illustrated in Figure 8c,d, no significant difference (p > 0.05) was present between the two groups in CMP-3 (CH50 = 0.27 ± 0.04 mg/mL, AP50 = 0.33 ± 0.07 mg/mL) and heparin (CH50 = 0.25 ± 0.02 mg/mL, AP50 = 0.30 ± 0.03 mg/mL) on CP and AP, which indicated that CMP-3 had a similar anti-complementary effect with the positive drug. Although CMP-1 and CMP-2 can inhibit the activation of complement, the CH50 and AP50 values of CMP-1 (CH50 = 0.43 ± 0.07 mg/mL, AP50 = 0.42 ± 0.08 mg/mL) and CMP-2 (CH50 = 0.41 ± 0.08 mg/mL, AP50 = 0.38 ± 0.09 mg/mL) are significantly higher than those of heparin (p < 0.05), and the CH50 and AP50 values between CMP-1, CMP-2 and CMP-3 also showed significant differences (p < 0.05). The increasing order of complement inhibitory activity was as follows: CMP-1 < CMP-2 < CMP-3. These results indicated that the three polysaccharides have anti-complement activity via CP and AP, and CMP-3 had the strongest complement inhibition ability, which was worthy of further study.
Figure 8.
The anti-complementary activity of three polysaccharides with different dilution concentrations through CP (a) and AP (b). Comparison of CH50 (c) and AP50 (d). (NS: not significant, * p < 0.05, ** p < 0.01 and *** p < 0.001).
Table 2.
The anti-complementary activity of CMP-1, CMP-2 and CMP-3.
| Samples | CH50 (mg/mL) | AP50 (mg/mL) |
|---|---|---|
| CMP-1 | 0.43 ± 0.07 | 0.42 ± 0.08 |
| CMP-2 | 0.41 ± 0.08 | 0.38 ± 0.09 |
| CMP-3 | 0.27 ± 0.04 | 0.33 ± 0.07 |
| Heparin | 0.25 ± 0.02 | 0.30 ± 0.03 |
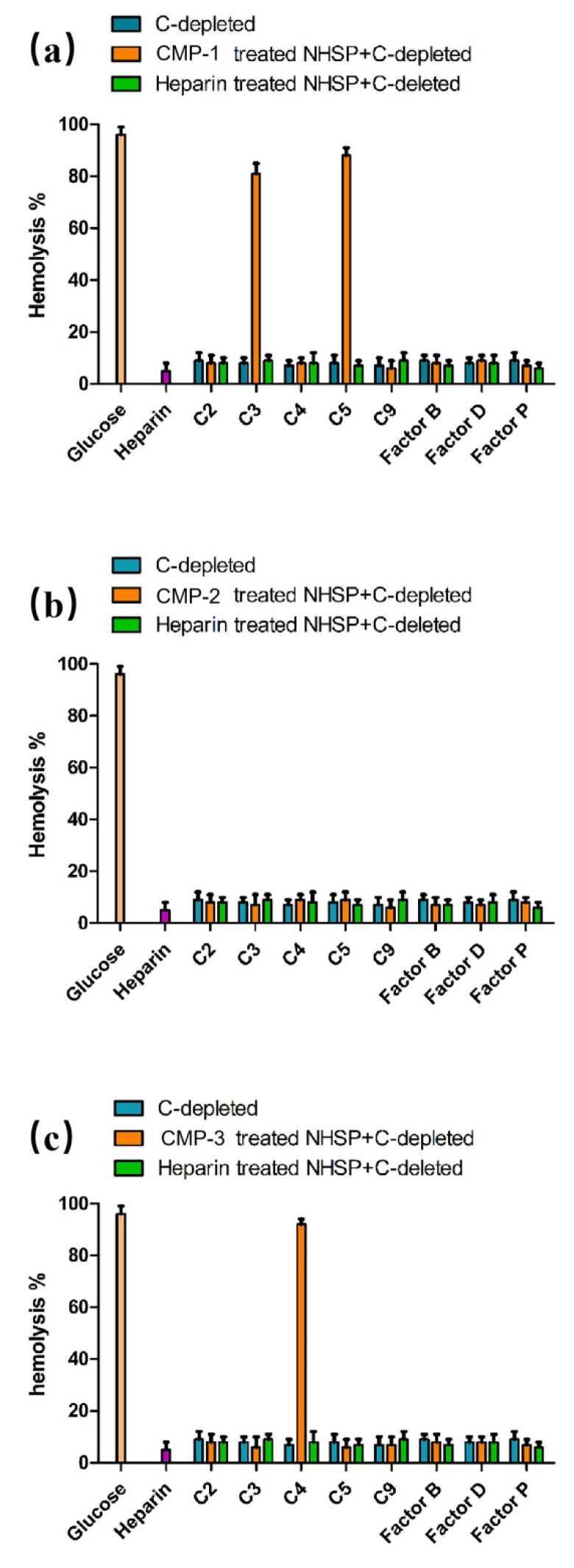
The targets of polysaccharides action were determined based on the changes in hemolysis rates, reflected by the depletion of different complement components with CMP-1, CMP-2 and CMP-3. If the polysaccharides interact with the complement component being measured, it can exhibit hemolysis that cannot be recovered [46]. As shown in Figure 9, none of the complement depleted sera independently lysed red blood cells, and their hemolytic percentages were not more than 20%. After treatment with CMP-1, the complement-depleted sera of C2, C4, C9, Factor B, Factor D and Factor P did not restore hemolytic, while the C3- and C5-depleted sera significantly restored hemolytic activity. This proved that CMP-1 was inferred to block the activation cascade of the complement system by targeting C2, C4, C9, Factor B, Factor D and Factor P, but not with C3 and C5. Similarly, Figure 9b showed that CMP-2 can inhibit complement activation by interacting with C2, C3, C4, C5, C9, Factor B, Factor D and Factor P. Additionally, Figure 9c illustrates that CMP-3 can inhibit complement activation by interacting with C2, C3, C5, C9, Factor B, Factor D and Factor P, but not with C4.
Figure 9.
The targets of CMP-1 (a), CMP-2 (b) and CMP-3 (c) in the complement activation cascade.
3.7. Correlation analysis of structure and anti-complementary activity
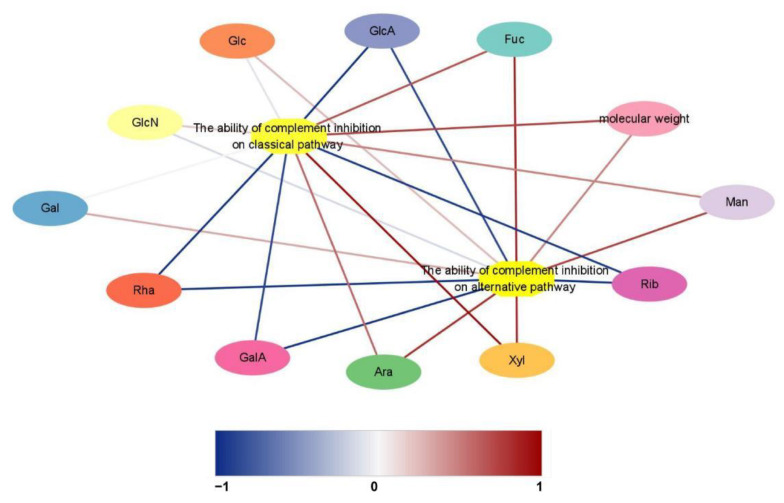
The Pearson correlation is a more commonly used method for calculating correlation coefficients. It is one of the most fundamental approaches for revealing the direction and degree of linear correlation between two variables, and this correlation analysis technique has been used to explore the structure and bioactivity relationships of polysaccharides in recent years [47,48,49]. The correlation between the monosaccharide composition, molecular weight and anti-complementary activity of CMPs was analyzed, and the results and visualizations are shown in Table 3 and Figure 10. The structure activity correlation increased with the absolute value of Pearson’s correlation coefficient (r). In other words, the correlation coefficient values close to −1/1 suggested a stronger correlation between structural characterization and anti-complementary activity. Conversely, the correlation coefficient close to 0 was weak. The Rib and GalA composition of CMPs were significantly negatively correlated with the ability of the complement inhibition on the alternative pathway (p < 0.05). The correlation coefficients were −0.999 and −0.990, respectively. Meanwhile, a notable linear correlation was found between the GlcA composition and the ability of the complement inhibition on the classical pathway, and the correlation coefficient was −0.990 (p < 0.05). Although other monosaccharides showed different degrees of correlation with the anti-complementary activity of CMPs, the correlation was not significant (p > 0.05).
Table 3.
Pearson correlation analysis of monosaccharide composition, molecular weight and anti-complementary activity of CMPs.
| Value | Pearson | The Ability of Complement Inhibition on Classical Pathway-CH50 |
The Ability of Complement Inhibition on Alternative Pathway-AP50 |
|---|---|---|---|
| Man | r | 0.492 | 0.757 |
| p | 0.336 | 0.226 | |
| GlcN | r | 0.193 | −0.149 |
| p | 0.438 | 0.452 | |
| Rib | r | −0.954 | −0.999 * |
| p | 0.097 | 0.013 | |
| Rha | r | −0.987 | −0.983 |
| p | 0.052 | 0.058 | |
| GlcA | r | −0.990 * | −0.886 |
| p | 0.044 | 0.154 | |
| GalA | r | −0.885 | −0.990 * |
| p | 0.154 | 0.044 | |
| Glc | r | −0.092 | 0.250 |
| p | 0.471 | 0.420 | |
| Gal | r | −0.012 | 0.327 |
| p | 0.492 | 0.394 | |
| Xyl | r | 0.976 | 0.845 |
| p | 0.070 | 0.175 | |
| Ara | r | 0.596 | 0.832 |
| p | 0.297 | 0.187 | |
| Fuc | r | 0.680 | 0.888 |
| p | 0.262 | 0.152 | |
| molecular weight | r | 0.748 | 0.480 |
| p | 0.231 | 0.341 |
Note: * p < 0.05; The absolute value of r = 0.8–1.0, extremely strong correlation; the absolute value of r = 0.6–0.8, strong correlation; the absolute value of r = 0.4–0.6, moderate correlation; the absolute value of r = 0.2–0.4, weak correlation; the absolute value of r = 0–0.2, extremely weak correlation or no correlation.
Figure 10.
Network analysis of monosaccharide composition and molecular weight associated with the anti-complementary activity of CMPs. Red lines represent the positive correlation between structure and activity (r > 0.99). Blue lines represent the negative correlation between structure and activity (r > 0.99). The darker the line color, the higher is the significance of the correlation between structure and bioactivity.
4. Conclusions
In the present study, the three polysaccharides (CMP-1, CMP-2 and CMP-3) were isolated from C. militaris for the first time. The results of monosaccharide composition, molecular weight, characteristic groups and stereo conformation showed that CMP-1, CMP-2 and CMP-3 were homogenous polysaccharides, composed of at least 10 monosaccharides with different molar ratios, and exhibited a triple helix structure with pyranoid polysaccharides. In addition, these three polysaccharides can interact with different complement components, and thus exhibited strong complement inhibitory activity. This laid the theoretical basis for the clinical application of C. militaris polysaccharides as novel anti-complementary drugs in the therapeutic process. The correlation analysis between the anti-complementary activity and polysaccharides showed that ribose, glucuronic acid and galacturonic acid played a major role in the complement inhibitory ability of C. militaris polysaccharides, which provided a new way to investigate the structure–activity relationship of C. militaris polysaccharides. However, the type and sequence of glycosidic linkage of CMP-1, CMP-2 and CMP-3 are unknown. Therefore, more analytical techniques, such as gas chromatography-mass spectrometry and nuclear magnetic resonance, can be used to investigate their structures in future research. The mechanism of biological activity in vivo needs to be further studied.
Author Contributions
Conceptualization, Z.H. and J.S.; methodology, Z.H. and J.W.; software, Z.H. and L.J.; investigation, Z.H., J.W., T.Z. and Y.D.; formal analysis, Y.D.; data curation, J.S.; visualization, Z.H.; writing—original draft preparation, Z.H.; writing—review and editing, J.S.; supervision, W.Z.; project administration, G.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 81760627, and the Jilin Provincial Education Department, grant number JJKH20200534KJ, and the administration of traditional Chinese Medicine of Jilin Province, grant number 2020140, and Jilin Provincial Science and Technology Department, grant number 20220508073RC.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lutsep H.L., Clark W.M. Current status of neuroprotective agents in the treatment of acute ischemic stroke. Curr. Neurol. Neurosci. Rep. 2001;1:13–18. doi: 10.1007/s11910-001-0072-0. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin V.D., Hajishengallis G., Yang K., Lambris J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harboe M., Thorgersen E.B., Mollnes T.E. Advances in assay of complement function and activation. Adv. Drug Deliv. Rev. 2011;12:976–987. doi: 10.1016/j.addr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Quintana L.F., Kronbichler A., Blasco M., Zhao M.H., Jayne D. ANCA associated vasculitis: The journey to complement-targeted therapies. Mol. Immunol. 2019;112:394–398. doi: 10.1016/j.molimm.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Huo J.Y., Lu Y., Xia L., Chen D.F. Structural characterization and anticomplement activities of three acidic homogeneous polysaccharides from Artemisia annua. J. Ethnopharmacol. 2020;247:112281. doi: 10.1016/j.jep.2019.112281. [DOI] [PubMed] [Google Scholar]
- 6.Xia L., Li B.B., Lu Y., Chen D.F. Structural characterization and anticomplement activity of an acidic polysaccharide containing 3-O-methyl galactose from Juniperus tibetica. Int. J. Biol. Macromol. 2019;132:1244–1251. doi: 10.1016/j.ijbiomac.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Du D.S., Lu Y., Cheng Z.H., Chen D.F. Structure characterization of two novel polysaccharides isolated from the spikes of Prunella vulgaris and their anticomplement activities. J. Ethnopharmacol. 2016;193:345–353. doi: 10.1016/j.jep.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Huang S.J., Tsai S.Y., Lee Y.L., Mau J.L. Nonvolatile taste components of fruit bodies and mycelia of Cordyceps militaris. LWT--Food Sci. Technol. 2006;39:577–583. doi: 10.1016/j.lwt.2005.05.002. [DOI] [Google Scholar]
- 9.Hsu C.H., Sun H.L., Sheu J.N., Ku M.S., Hu C.M., Chan Y., Lue K.H. Effects of the immunomodulatory agent Cordyceps militaris on airway inflammation in a mouse asthma model. Pediatr. Neonatol. 2008;49:171–178. doi: 10.1016/S1875-9572(09)60004-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen R.Z., Jin C.G., Li H.P., Liu Z.Q., Lu J., Li S.Z., Yang S.M. Ultrahigh pressure extraction of polysaccharides from Cordyceps militaris and evaluation of antioxidant activity. Sep. Purif. Technol. 2014;134:90–99. doi: 10.1016/j.seppur.2014.07.017. [DOI] [Google Scholar]
- 11.Bai K.C., Sheu F. A novel protein from edible fungi Cordyceps militaris that induces apoptosis. J. Food. Drug. Anal. 2018;26:21–30. doi: 10.1016/j.jfda.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling J.Y., Sun Y.J., Zhang H., Lv P., Zhang C.K. Measurement of cordycepin and adenosine in stroma of Cordyceps sp. by capillary zone electrophoresis. J. Biosci. Bioeng. 2002;94:371–374. doi: 10.1016/S1389-1723(02)80181-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Wu G., Huang Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013;58:18–22. doi: 10.1016/j.ijbiomac.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Jing Y.S., Cui X.L., Chen Z.Y., Huang L.J., Song L.Y., Liu T., Lv W.J., Yu R.M. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014;102:288–296. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Rao Y.K., Fang S.H., Wu W.S., Tzeng Y.M. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator’s production and human cancer cell proliferation. J. Ethnopharmacol. 2010;131:363–367. doi: 10.1016/j.jep.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Cheung J.K., Li J., Cheung A.W., Zhu Y., Zheng K.Y., Bi C.W., Duan R., Choi R.C., Lau D.T., Dong T.T., et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J. Ethnopharmacol. 2009;124:61–68. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Xu N., Zhang J., Zhao H., Lin L., Jia S., Jia L. Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 2015;131:355–362. doi: 10.1016/j.carbpol.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Liu J.Y., Feng C.P., Li X., Chang M.C., Meng J.L., Xu L.J. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016;86:594–598. doi: 10.1016/j.ijbiomac.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kirschfink M. Controlling the complement system in inflammation. Immunopharmacology. 1997;38:51–62. doi: 10.1016/S0162-3109(97)00057-X. [DOI] [PubMed] [Google Scholar]
- 20.Sun J.F., Jin M., Zhou W., Diao S.B., Li G. A new ribonucleotide from Cordyceps militaris. Nat. Prod. Res. 2017;31:1–7. doi: 10.1080/14786419.2017.1323210. [DOI] [PubMed] [Google Scholar]
- 21.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.T., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 22.Ye G.B., Chen Y.H., Wang C.L., Yang R.R., Bin X.Y. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018;120:1315–1321. doi: 10.1016/j.ijbiomac.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Luo D.H. Identification of structure and antioxidant activity of a fraction of polysaccharide purified from Dioscorea nipponica Makino. Carbohydr. Polym. 2008;71:544–549. doi: 10.1016/j.carbpol.2007.06.023. [DOI] [Google Scholar]
- 24.Zhang Y., Wang J., Yang J., Li Y., Zhang W., Liu S., Yang G., Yan Z., Liu Y. Microwave-assisted enzymatic extraction, partial characterization, and antioxidant potential of polysaccharides from Sagittaria trifolia Tuber. Chem. Biodiversity. 2022;19:e202200219. doi: 10.1002/cbdv.202200219. [DOI] [PubMed] [Google Scholar]
- 25.Manohar N., Jayramudu J., Suchismita S., Rajkumar K., Babulreddy A., Sadiku E.R., Priti R., Maurya D.J. A unique application of second order derivative ftir-atr spectra for compositional analyses of natural rubber and polychloroprene rubber and their blends. Polym. Test. 2017;62:447–453. doi: 10.1016/j.polymertesting.2017.07.030. [DOI] [Google Scholar]
- 26.Jia R.B., Li Z.R., Ou Z.R., Wu J., Sun B., Lin L., Zhao M. Physicochemical Characterization of Hizikia fusiforme polysaccharide and its hypoglycemic activity via mediating insulin-stimulated blood glucose utilization of skeletal muscle in type 2 diabetic rats. Chem. Biodiversity. 2020;17:e2000367. doi: 10.1002/cbdv.202000367. [DOI] [PubMed] [Google Scholar]
- 27.Wang J.M., Sun J.F., Jin L., Wang M.J., Huang Y.Y., Jin M., Zhou W., Li G. A new monoterpenoid glycoside and a new phenolic glycoside isolated from Dracocephalum moldavica and their anti-complementary activity. Nat. Prod. Res. 2021;6:1–11. doi: 10.1080/14786419.2021.1957885. [DOI] [PubMed] [Google Scholar]
- 28.Jin L., Zhou W., Hu Z.Y., Huang Y.Y., Diao S.B., Sun J.S., Li G. A new megastigmane glycoside, a new organic acid glycoside and other constituents with anticomplementary activity from Artemisia halodendron. Nat. Prod. Res. 2022;25:1–6. doi: 10.1080/14786419.2022.2104273. [DOI] [PubMed] [Google Scholar]
- 29.Fan H., Liu F., Bligh S.W., Shi S., Wang S. Structure of a homofructosan from Saussurea costus and anti-complementary activity of its sulfated derivatives. Carbohydr. Polym. 2014;105:152–160. doi: 10.1016/j.carbpol.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X., Wen C.T., Duan Y.Q., Zhang H.H., Ma H.L. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019;132:906–914. doi: 10.1016/j.ijbiomac.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 31.He B.L., Zheng Q.W., Guo L.Q., Huang J.Y., Yun F., Huang S.S., Lin J.F. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020;145:11–20. doi: 10.1016/j.ijbiomac.2019.12.115. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T., Guo Q.W., Xin Y., Liu Y. Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus. Arabian J. Chem. 2022;15:104163. doi: 10.1016/j.arabjc.2022.104163. [DOI] [Google Scholar]
- 33.Borovkova V.S., Malyar Y.N., Sudakova I.G., Chudina A.I., Skripnikov A.M., Fetisova O.Y., Kazachenko A.S., Miroshnikova A.V., Zimonin D.V., Ionin V.A., et al. Molecular characteristics and antioxidant activity of Spruce (Picea abies) Hemicelluloses isolated by catalytic oxidative delignification. Molecules. 2022;27:266. doi: 10.3390/molecules27010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Q., Gao X. Categories and biomanufacturing methods of glucosamine. Appl. Microbiol. Biotechnol. 2019;103:1–7. doi: 10.1007/s00253-019-10084-x. [DOI] [PubMed] [Google Scholar]
- 35.Di Z., Zhu S. Purification, characterization, antioxidant and anticancer activities of novel polysaccharides extracted from Bachu mushroom. Int. J. Biol. Macromol. 2018;107:1086–1092. doi: 10.1016/j.ijbiomac.2017.09.088. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y., Wu M.Q., Zhang W.Q., Xu Z.Z., Xia W.J. Correlation analysis between HPLC fingerprint of polysaccharides from poria cocos and immunological activity. J. East China Univ. Sci. Technol. 2020;46:672–679. doi: 10.14135/j.cnki.1006-3080.20190629003. [DOI] [Google Scholar]
- 37.Zhao X.T., Zhang Y.J., Fu M., Li J.W., Zhu S., Fan L.P. Fingerprint chromatography analysis of lentinan by PMP-HPLC and its relationship with immunoactivity. Sci. Technol. Cereals Oils Foods. 2021;29:61–69. doi: 10.16210/j.cnki.1007-7561.2021.03.008. [DOI] [Google Scholar]
- 38.Li X., Li K., Wang J.Q., Sui S.S., Wang J.B., Guo C.Z., Li P.W. Advances in microbial synthesis of glucosamine. J. Qilu Univ. Technol. 2021;35:19–22. doi: 10.16442/j.cnki.qlgydxxb.2021.01.004. [DOI] [Google Scholar]
- 39.Liu Z.Q., Zhang Y.J., Ai C.Q., Tian W.G., Wen C.G., Song S., Zhu B.B. An acidic polysaccharide from Patinopecten yessoensis skirt prevents obesity and improves gut microbiota and metabolism of mice induced by high-fat diet. Food Res. Int. 2022;154:110980. doi: 10.1016/j.foodres.2022.110980. [DOI] [PubMed] [Google Scholar]
- 40.He Y.L., Chen H., Ye Z.Y., Zhang X.M., Ye H.L., Ye M. Structural characterization and bioactivities of a novel polysaccharide obtained from Lachnum YM38 together with its zinc and selenium derivatives. Process Biochem. 2022;122:282–298. doi: 10.1016/j.procbio.2022.08.035. [DOI] [Google Scholar]
- 41.Liu X.X., Gu L.B., Zhang G.J., Liu H.M., Zhang Y.T., Zhang K.P. Structural characterization and antioxidant activity of polysaccharides extracted from Chinese yam by a cellulase-assisted method. Process Biochem. 2022;121:178–187. doi: 10.1016/j.procbio.2022.06.023. [DOI] [Google Scholar]
- 42.Liao N., Chen S., Ye X., Zhong J., Wu N., Dong S., Yang B., Liu D. Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, Corbicula fluminea. Food Funct. 2013;4:539–548. doi: 10.1039/c2fo30178d. [DOI] [PubMed] [Google Scholar]
- 43.Nie C.Z.P., Zhu P.L., Ma S.P., Wang M.C., Hu Y.D. Purification, characterization and immunomodulatory activity of polysaccharides from Stem lettuce. Carbohydr. Polym. 2018;188:236–242. doi: 10.1016/j.carbpol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Dong H., Zhang Q., Li Y., Li L., Lan W., He J., Li H., Xiong Y., Qin W. Extraction, characterization and antioxidant activities of polysaccharides of Chuanminshen violaceum. Int. J. Biol. Macromol. 2016;86:224–232. doi: 10.1016/j.ijbiomac.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Wang M.C., Zhao S.W., Zhu P.L., Nie C.Z.P., Ma S.P., Wang N.F., Du X.F., Zhou Y.B. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018;107:882–890. doi: 10.1016/j.ijbiomac.2017.09.062. [DOI] [PubMed] [Google Scholar]
- 46.Chen M.M., Wu J.J., Shi S.S., Chen Y.L., Wang H.J., Fan H.W., Wang S.C. Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr. Polym. 2016;152:241–252. doi: 10.1016/j.carbpol.2016.06.110. [DOI] [PubMed] [Google Scholar]
- 47.Xiong G.Y., Ma L.S., Zhang H., Li Y.P., Zou W.S., Wang X.F., Xu Q.S., Xiong J.T., Hu Y.P., Wang X.Y. Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022;194:484–498. doi: 10.1016/j.ijbiomac.2021.11.092. [DOI] [PubMed] [Google Scholar]
- 48.Wang J.J., Shi S., Li F.F., Du X., Kong B.H., Wang H., Xia X.F. Physicochemical properties and antioxidant activity of polysaccharides obtained from sea cucumber gonads via ultrasound-assisted enzymatic techniques. LWT--Food Sci. Technol. 2022;160:113307. doi: 10.1016/j.lwt.2022.113307. [DOI] [Google Scholar]
- 49.Liang X.Y., Ye Y., Zhu Y.H., Xiao J.R., Qiao Y.B. Multivariate comparative analysis of chemical constituent changes and antioxidant properties of polysaccharides in ribes stenocarpum maxim. at different maturity stages on the Qinghai-Tibet Plateau. Sci. Hortic. 2022;308:111556. doi: 10.1016/j.scienta.2022.111556. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.