Abstract
Patients with unresolving acute respiratory distress syndrome (ARDS) have persistently elevated levels of proinflammatory cytokines in the lungs and circulation and increased rates of bacterial infections. Phagocytic cells hyperactivated with lipopolysaccharide (LPS), which induces high levels of proinflammatory cytokines in monocytic cells, are inefficient in killing ingested bacteria despite having intact phagocytic activity. On the other hand, phagocytic cells that are activated with an analogue of LPS that does not induce the expression of proinflammatory cytokines effectively ingest and kill bacteria. We hypothesized that in the presence of high concentrations of proinflammatory cytokines, bacteria may adapt and utilize cytokines to their growth advantage. To test our hypothesis, we primed a human monocytic cell line (U937) with escalating concentrations of the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-6 and with LPS. These cells were then exposed to fresh isolates of three common nosocomial pathogens: Staphylococcus aureus, Pseudomonas aeruginosa, and an Acinetobacter sp. In human monocytes primed with lower concentrations of proinflammatory cytokines (10 to 250 pg) or LPS (1 and 10 ng), intracellular bacterial growth decreased. However, when human monocytes were primed with higher concentrations of proinflammatory cytokines (1 to 10 ng) or LPS (1 to 10 μg), intracellular growth of the tested bacteria increased significantly (P <0.0001). These results were reproduced with peripheral blood monocytes obtained from normal healthy volunteers. The specificity of the cytokine activity was demonstrated by neutralizing the cytokines with specific antibodies. Our findings provide a possible mechanism to explain the frequent development of bacterial infections in patients with an intense and protracted inflammatory response.
Inflammation is an innate immune response of the host to an infectious or noninfectious assault. The most proximal expression of such a response is the elaboration of proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6. When present in optimal concentrations, these biologically active molecules recruit both specific and nonspecific immune cells to the site of assault and activate them, thereby helping to eradicate the assault and to restore homeostasis (11). However, there are occasions when the host defense response, in terms of inflammation, is exaggerated and protracted. In such cases, this primary defense process may instead cause enhanced tissue injury and maladaptive repair, leading to vital-organ dysfunction and failure (12). Acute respiratory distress syndrome (ARDS) is a frequent form of hypoxemic respiratory failure caused by the acute development of diffuse lung inflammation. We have previously reported that nonsurvivors of ARDS have a dysregulated host defense response characterized by (i) persistent elevation of pulmonary and circulatory levels of proinflammatory cytokines TNF-α, IL-1β, and IL-6 and (ii) an increased rate of nosocomial bacterial infections (6). In these patients, nosocomial bacterial infections may represent an epiphenomenon of the exaggerated and protracted inflammatory response (6). In agreement with this line of evidence, Murphy et al. (14) reported that patients with AIDS who were treated with the cytokine IL-2 had increased rates of bacterial infections. The mechanisms by which excessive inflammation might favor bacterial infections have not been investigated. If it can be proven that exaggerated inflammation favors bacterial growth, it is reasonable to assume that treatment modalities directed at controlling exaggerated inflammation might be useful in curtailing bacterial infections.
Several recent reports indicate that bacteria can utilize certain cytokines to enhance their extracellular and intracellular growth. Porat et al. (16) reported that virulent strains of Escherichia coli express receptors for IL-1β and demonstrated enhanced extracellular in vitro growth in the presence of biologically active recombinant IL-1β. Furthermore, Denis et al. (2) reported that both IL-2 and granulocyte-macrophage colony-stimulating factor enhanced the extracellular growth of virulent strains of E. coli. For these reasons, we initiated a series of experiments to study the in vitro extracellular and intracellular growth response of bacteria exposed to graded concentrations of biologically active TNF-α, IL-1β, and IL-6. The three bacterial species used for these studies were Staphylococcus aureus, Pseudomonas aeruginosa, and an Acinetobacter sp., pathogens that frequently cause nosocomial infections in patients with ARDS. We have previously reported that all three bacterial species showed concentration-dependent growth enhancement when incubated with one or more tested proinflammatory cytokines and that blockade by specific neutralizing monoclonal antibodies (MAb) significantly inhibited cytokine-induced growth (13). The effects of cytokines on extracellular bacterial growth were seen only with fresh isolates and were lost after six in vitro passages (13).
To further understand the effects of proinflammatory cytokines on bacterial growth, we studied the intracellular growth response of S. aureus, P. aeruginosa, and Acinetobacter sp. in human monocytes (cell line U937 and cells from healthy human volunteers) primed with graded concentrations of TNF-α, IL-1β, or IL-6 or lipopolysaccharide (LPS). LPS was selected for its ability to induce the expression of TNF-α, IL-1β, and IL-6 and other inflammatory cytokines within human monocytes.
MATERIALS AND METHODS
Bacteria.
Fresh clinical bacterial isolates of S. aureus, P. aeruginosa, and Acinetobacter sp. recovered from the bronchoalveolar lavage fluid or peripheral blood of patients admitted to the University of Tennessee Bowld Hospital were used without any additional passage in vitro, to keep the biological nature of the bacterial isolates intact as far as possible. The bacteria were grown in 3 ml of RPMI-Dulbecco modified Eagle medium (RPMI-DMEM) without serum or antibiotics (Life Technologies, Bethesda, Md.) at 37°C for 8 h. Then the bacterial cultures were washed and resuspended in 1 ml of RPMI-DMEM without antibiotics to a concentration of 105 bacteria/ml.
Monocyte cell line and maintenance.
The human monocytic cell line U937 was obtained from the American Type Culture Collection, Rockville, Md. These cells were maintained in RPMI-DMEM with 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (Life Technologies). Prior to each experiment, the cells were centrifuged, resuspended in RPMI-DMEM without antibiotics or serum, and seeded to a concentration of 2 × 106 cells/ml into 12-well tissue culture plates (Costar, Cambridge, Mass.) containing RPMI-DMEM without serum or antibiotics.
Isolation of human peripheral blood monocytes.
Samples (40 ml) of blood were collected from two normal healthy volunteers by venipuncture. The blood monocytes were separated and purified by Ficoll-Hypaque gradient centrifugation and by lysing and removing any contaminating erythrocytes by standard methods.
Priming of U937 monocytic cells and normal human peripheral blood monocytes with LPS.
U937 cells or normal human peripheral blood monocytes (2 × 106 cells/ml) were exposed to graded concentrations (1, 10, 100, 1,000, 5,000, and 10,000 ng) of LPS, purified from E. coli K235 (Sigma Chemicals, St. Louis, Mo.). The cells were then incubated for 8 h at 37°C under an atmosphere of 5% CO2 prior to introduction of bacteria.
Priming of U937 monocytic cells with cytokine.
The monocytic cell line U937 (2 × 106 cells/ml) was also primed with pure biologically active recombinant cytokines (TNF-α, IL-1β, IL-6, and IL-10). These pure cytokines were obtained from R&D Systems, Minneapolis, Mn. Each cytokine was used at 1 and 10 ng based on our optimization experiments conducted on U937 cell line.
Bacterial infection of U937 monocytic cells and normal human peripheral blood monocytes.
A total of 2 × 106 monocytic cells were mixed with 6 × 106 CFU of each bacterial species and incubated at 37°C under an atmosphere of 5% CO2 for 2 h with intermittent shaking. Extracellular bacteria were then killed by treating the culture with 200 μg of gentamicin (Life Technologies) per ml. The monocytic cells containing internalized bacteria were then washed to free them of the gentamicin and the killed bacteria and were resuspended in antibiotic- and serum-free RPMI-DMEM and incubated for 12 h at 37°C under an atmosphere of 5% CO2. The experiments with U937 cells were run in triplicate, while the experiments with human monocytes were run in duplicate.
Estimation of bacterial growth.
After the specified incubation, the monocytic cells and bacteria were centrifuged. The pellets were suspended in 1.0 ml of sterile distilled water and sonicated to disrupt the monocytic cells without affecting the viability of the bacteria. The lysates were then diluted 10-fold in RPMI-DMEM without antibiotics or serum. Serially diluted lysates were then plated on Luria-Bertani (LB) agar (Difco, Detroit, Mich.) plates and incubated at 37°C for 18 h. The bacterial colonies were counted, and the results were expressed as CFU per milliliter of lysate.
Neutralization of biological activities of cytokines.
The specificity of the cytokine activity was tested by neutralizing cytokines with specific MAb. As specified by the manufacturer (R&D Systems), 300 ng of anti-IL-1β MAb was mixed with 5 ng of recombinant human IL-1β to neutralize its biological activity, 600 ng of anti-IL-6 MAb was mixed with 5 ng of recombinant human IL-6, and 4 μg of anti-TNF-α MAb was mixed with 5 ng of recombinant TNF-α. The mixtures of pure recombinant human cytokines and specific MAb were incubated at 4°C for 1 h and then added to U937 cells. Subsequently, 6 × 106 CFU of bacteria was added to each culture and incubated at 37°C for 12 h. The recovery of intracellular bacteria was assessed by estimating the CFU per milliliter of the cell lysate as described above. The specificity of action of cytokines was further tested by incubating pure recombinant cytokines with equivalent amounts of normal mouse immunoglobulin G and then assessing the effects of this mixture on intracellular bacterial growth.
RNA extraction.
Total cellular RNA was isolated by a modification of a previously described procedure (8). Following 8 h of priming with LPS, one batch of U937 cells (2 × 106 cells) from each group mentioned above was harvested, washed, and lysed with Trizole reagent (Life Technologies). Total cellular RNA was extracted from the cell lysate by chloroform extraction followed by ethanol precipitation and was stored as dry pellets or as aliquot of aqueous solutions at −70°C.
RT reactions.
The reverse transcription (RT) reactions were performed in accordance with a procedure described by Kanangat et al. (8). Samples (5 μg) of total cellular RNA were reverse transcribed with avian myeloblastosis virus (AMV) reverse transcriptase and oligo(dT)18 primer (Promega Corp., Madison, Wis.). The reaction mixture, in addition to AMV reverse transcriptase and oligo(dT) primers, consisted of 5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100, 2 mM deoxynucleoside triphosphate, and 40 U of RNase inhibitor (Promega). The mixture was incubated for 15 min at ambient temperature and for a further 90 min at 42°C, heated at 99°C for 5 min, and cooled on ice.
PCR.
PCR was done by a previously described method (8). A 5-μl sample of the RT mixture (cDNA) was used in a 25.0-μl PCR for qualitative detection of β-actin (as control for RNA isolation and RT efficiency), TNF-α, IL-1β, and IL-6. All these reactions were done in separate tubes to avoid possible competition among different target and primer pairs. The reaction mixture consisted of 1.5 to 2.5 mM MgCl2, 01% Triton X-100, 125 μM each dATP, dCTP, and dTTP, 50 mM Tris-HCl (pH 8.3), and 1.0 U of Taq DNA polymerase (Life Technologies). The conditions for PCR were denaturation at 94°C for 90 s, annealing at 55°C for 60 s, and extension at 72°C for 120 s. These cycles were repeated 35 times for each mRNA mentioned above. The primers were used at 15 pmol per reaction. The PCR products were analyzed on a 2.5% gel (Life Technologies), stained with ethidium bromide (Sigma Chemical Co.), and photographed. The intensity of the bands was measured with the Alpha Imager 2000 documentation and analysis system (Alpha Inotech Corp., San Leandro, Calif.).
Statistical analysis.
Bacterial growth, measured in 106 CFU per milliliter, was transformed by taking the natural logarithm since the variance of growth increased with increasing concentrations of the priming substances. For each combination of bacterial species and priming substance, one-way analysis of variance was used to compare bacterial growth after incubation for 8 h in medium containing various concentrations of the priming substance (TNF-α, IL-1β, IL-6, and LPS). Preplanned contrasts were used to determine whether intracellular growth changed after addition of a given concentration of priming substance compared to intracellular growth of unprimed cells (controls).
RESULTS
The intracellular growth of S. aureus, P. aeruginosa, and the Acinetobacter sp. was affected by the concentration of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) or LPS used to prime the human monocytes.
Intracellular bacterial growth in cytokine-primed U937 monocytic cells.
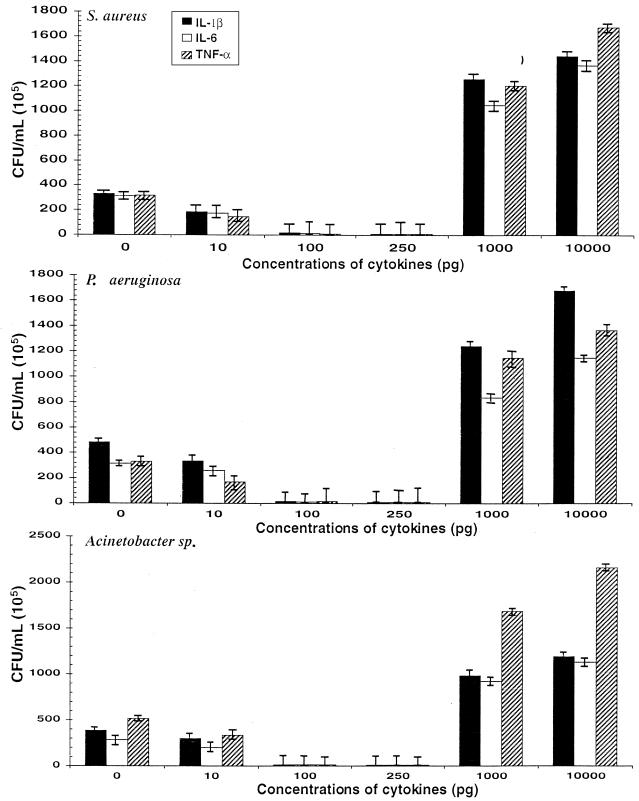
Figure 1 shows the intracellular growth of the tested bacteria in U937 monocytic cells primed with graded concentrations of IL-1β, IL-6, or TNF-α. A concentration-dependent biphasic growth response was observed for all three bacteria, and this response was similar among the tested cytokines. The intracellular growth of three bacteria progressively decreased as the concentration of priming cytokines increased from 10 to 250 pg. This trend, however, reversed when the priming concentration reached and exceeded 1,000 pg, and intracellular bacterial growth increased manyfold. Compared to the control (no cytokines present), the intracellular growth of S. aureus (Fig. 1, top) in U937 monocytic cells primed with IL-1β, IL-6, or TNF-α progressively decreased at cytokine concentrations of 10 pg (P = 0.002, 0.007, and 0.002, respectively), 100 pg (P < 0.0001 for all three cytokines), and 250 pg (P < 0.0001 for all three cytokines); similar results were obtained for P. aeruginosa (Fig. 1, middle) at priming cytokine concentrations of 10 pg (P = 0.05, 0.37, and 0.007, respectively), 100 pg (P < 0.0001 for all three cytokines), and 250 pg (P < 0.0001 for all three cytokines) and for the Acinetobacter sp. (Fig. 1, bottom) at cytokine concentrations of 10 pg (P = 0.05, 0.36, and 0.007, respectively), 100 pg (P < 0.0001 for all three cytokines), and 250 pg (P < 0.0001 for all three cytokines). Compared to control, the intracellular growth of all three bacterial isolates increased significantly at a priming concentration of 1,000 pg (P < 0.001 for all cytokines), and 10,000 pg (P < 0.0001 for all cytokines).
FIG. 1.
Intracellular bacterial growth of S. aureus, P. aeruginosa, and Acinetobacter sp. in U937 cells primed with graded concentrations of IL-1β, IL-6, and TNF-α. U937 cells (2 × 106) were primed with 0, 10, 100, and 250 pg and 1 and 10 ng of IL-1β, IL-6, and TNF-α. The primed cells were mixed with 6 × 106 CFU of each tested bacterium and incubated for 2 h. Extracellular bacteria were killed with gentamicin (see Materials and Methods). The cells with internalized bacteria were incubated at 37°C for 12 h under an atmosphere of 5% CO2. They were then lysed, serially diluted, and cultured onto LB agar plates. The CFU per milliliter was estimated after 16 to 18 h of incubation. A concentration-dependent biphasic growth response was observed for all three bacteria, and this response was similar among the tested cytokines. The intracellular growth of three bacteria progressively decreased as the concentration of priming cytokines increased from 10 to 250 pg. However, this trend reversed when the priming concentration reached and exceeded 1,000 pg, and intracellular bacterial growth increased manyfold (P < 0.0001 for all cytokines).
Specificity of the biological activities of individual cytokines.
The specificity of the individual cytokine action was examined by the addition of specific antibodies to individual cytokines in the growth medium. Figure 2 shows that when the cytokines are neutralized by specific antibodies prior to incubation with monocytic cells, the cells exposed to such neutralized cytokines were unable to enhance the replication of the tested bacteria.
FIG. 2.
Specificity of cytokine action on intracellular bacterial growth. The biological activities of respective cytokines were neutralized by mixing pure recombinant cytokines with specific neutralizing antibodies as specified by the manufacturer (R&D Systems) and incubating the mixture at 4°C for 1 h (see Materials and Methods). The neutralized cytokines were then added to the cells before they were exposed to the bacteria. When the cytokines were neutralized by specific antibodies before being incubated with monocytic cells, the cells exposed to such neutralized cytokines were unable to enhance the replication of the tested bacteria.
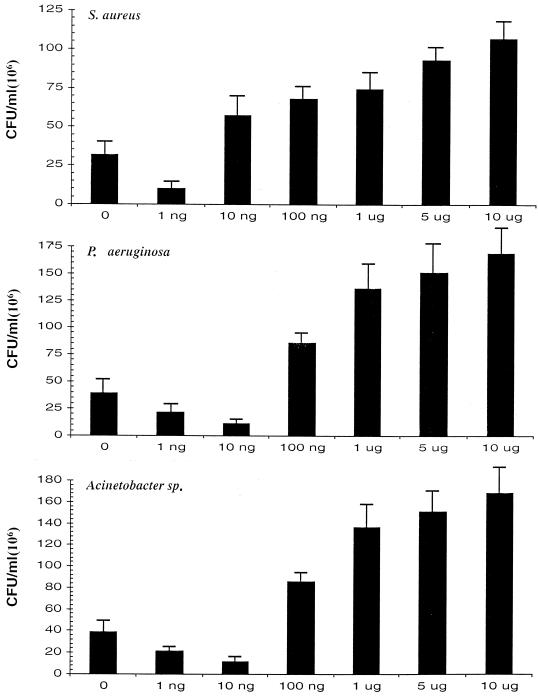
Intracellular bacterial growth in LPS-primed U937 monocytic cells.
Since LPS induces the production of a wide variety of cytokines by monocytes, we tested the ability of our three bacterial species to grow intracellularly in LPS-primed U937 cells. The growth of S. aureus, P. aeruginosa, and Acinetobacter sp. in presence of graded concentrations of LPS is shown in Fig. 3. At a priming concentration of 1.0 ng, a reduction in intracellular growth, in comparison to the control (without LPS), was observed for all three bacteria (P = 0.02, 0.055, and 0.03, respectively). However, at concentrations of 100 ng or higher, we observed a significant increase in intracellular bacterial growth for all three isolates (P < 0.0001 for all three bacteria).
FIG. 3.
Intracellular bacterial growth in LPS-primed U937 monocytic cells. U937 cells were pulsed with graded concentrations of LPS for 8 h, and 2 × 106 cells were mixed with 6 × 106 CFU of the tested bacteria. The cell-bacterium mixture was incubated for 2 h. The extracellular bacteria were killed with gentamicin (see Materials and Methods). The cells with intracellular bacteria were incubated for at 37°C for 12 h and then lysed and serially diluted. The cell lysates were then cultured on LB agar plates, and the CFU per milliliter was estimated after 16 h of incubation at 37°C. At a priming concentration of 1.0 ng/ml, a reduction in intracellular growth in comparison to control (with no LPS) was observed for all three bacteria (P = 0.02, 0.055, and 0.03, respectively). However, at concentrations of 100 ng or higher, a significant increase in intracellular bacterial growth was observed for all three isolates (P < 0.0001 for all three bacteria).
Proinflammatory cytokine mRNA expression in LPS-primed U937 monocytic cells.
To determine whether the induced intracellular growth enhancement of the tested bacteria was due to the induction of proinflammatory cytokines in LPS-primed U937 monocytic cells, the amounts of these cytokines (TNF-α, IL-1β, and IL-6) at the transcriptional (mRNA) level were estimated in U937 cells primed with LPS by using a semiquantitative RT-PCR approach. Figure 4 shows that in U937 monocytic cells, LPS enhances the expression of TNF-α, IL-1β, and IL-6 in a concentration-dependent manner.
FIG. 4.
Proinflammatory cytokine mRNA expression in LPS-primed U937 monocytic cells. U937 cells were pulsed with graded concentrations of LPS (see Materials and Methods). The cells were harvested after 8 h of incubation and lysed. Total cellular RNA was isolated with Trizole reagent (see Materials and Methods) and subjected to RT with AMV reverse transcriptase. The cDNA thus obtained was amplified by PCR with specific primers for IL-1β, IL-6, and TNF-α. β-Actin was used as an internal control. The levels of expression of various cytokines were expressed as ratios of cytokines to β-actin. In U937 monocytic cells, LPS enhances the expression of TNF-α, IL-1β, and IL-6 in a concentration-dependent manner.
Intracellular growth of S. aureus in LPS primed human peripheral blood monocytes.
The above experiment was repeated with peripheral blood monocytes obtained from normal healthy human volunteers and primed with graded concentrations of LPS. As shown in Fig. 5, the observed response was similar to the one observed with U937 monocytic cells. Compared to the control (without LPS), intracellular growth of S. aureus was significantly decreased (P < 0.0001) when monocytes were primed with a low concentration of LPS (10 ng), while intracellular growth was significantly enhanced (P < 0.0001) at higher concentrations of LPS (1,000 to 10,000 ng).
FIG. 5.
Intracellular growth of S. aureus in LPS-primed human peripheral blood monocytes. The peripheral blood monocytes isolated from two normal healthy volunteers were exposed to graded concentrations of LPS and then infected with S. aureus (see Materials and Methods). The extracellular bacteria were killed by exposing the culture to a mixture of antibiotics. The cells with internalized S. aureus were incubated for 12 h, after which they were lysed and the CFU per milliliter was assessed. The observed response was similar to that observed with U937 monocytic cells.
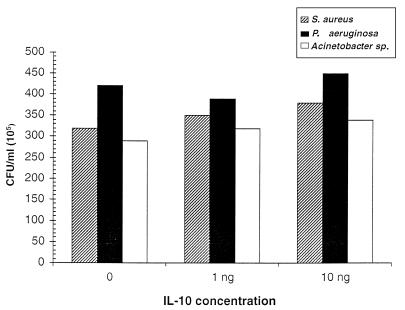
Intracellular bacterial growth in U937 monocytic cells primed with IL-10.
To determine the effect on intracellular growth of priming with an anti-inflammatory cytokine, we primed U937 cells with graded concentrations of IL-10 before exposing them to the three bacterial isolates. As shown in Fig. 6, the intracellular growth of the tested bacteria was unaffected by IL-10 priming.
FIG. 6.
Intracellular bacterial growth in U937 monocytic cells primed with IL-10. U937 cells were treated with 0, 1, and 10 ng of IL-10 and subsequently infected with the tested bacteria. The extracellular bacteria were removed by treating the cells with a mixture of antibiotics and by washing. Intracellular bacterial growth was assessed by determining the CFU per milliliter of lysed cells. The intracellular growth of the tested bacteria was unaffected by IL-10 priming.
DISCUSSION
In the present study, we found that the intracellular growth of S. aureus, P. aeruginosa and the Acinetobacter sp. in human monocytes was affected by the degree of cell activation obtained by exposure to graded concentrations of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 or of LPS. In monocytes primed with lower concentrations of cytokines (10 to 250 pg) or LPS (1 and 10 ng), intracellular bacterial growth decreased. However, when monocytes were primed with higher concentrations of cytokines (1 to 10 ng) or LPS (1 to 10 μg), intracellular growth of the tested bacteria increased significantly (P < 0.0001). The specificity of the cytokine activity was demonstrated by neutralizing the cytokines with specific antibodies.
In vivo, bacteria are normally ingested and killed by phagocytes. A recent study has shown that in mice depleted of alveolar macrophages, intratracheal administration of Klebsiella pneumoniae was associated with impaired bacterial clearance and increased mortality compared to controls, despite a significant increase in TNF-α expression and a threefold increase in polymorphonuclear cell recruitment (1). When mice are pretreated with an analogue of LPS (SDZ MRL 953) that substantially downregulates proinflammatory cytokine response in phagocytic cells, the animals are more resistant to bacterial infections (reviewed in reference 9). Corroborating studies by Harris et al. (5) have shown that endotoxin can induce the suppression of pulmonary antibacterial defenses against S. aureus despite preservation of the activation and phagocytic status of the cells (5). LPS is known to induce the expression and secretion of a wide variety of proinflammatory cytokines in monocytes, and our findings are in accordance with this concept. It is probable that the effect of LPS on intracellular replication of bacteria is mediated through certain cytokines or the resultant expressed transcription factors or growth-enhancing proteins. Additional research is required to precisely understand the mechanisms of the observed phenomenon.
Our findings indicate that there is a threshold of cellular activation at which phagocytic cells effectively kill ingested bacteria. Above this threshold of cellular activation, however, the intracellular micromilieu becomes favorable to the survival and replication of the ingested bacteria. The mechanisms by which bacteria survive in these activated cells and the reasons why such activated cells are unable to efficiently kill ingested bacteria are not understood. The ability of bacteria to resist intracellular killing might render them more virulent. It is known that rapidly replicating microbes such as bacteria are able to express novel genes that are required for their survival in a particular niche (4). It is possible that bacteria are able to utilize one or more of the proteins that are upregulated within the cells by the cytokines during an inflammatory response. Identification of novel genes expressed by bacteria, along with the dissection of various proteins that are expressed within the cells, may provide useful information on bacterial virulence.
In the present study, TNF-α, IL-1β, and IL-6 were selected because of their established role in inflammation. We have previously shown that ARDS nonsurvivors have persistent elevation of pulmonary and circulatory TNF-α, IL-1β, and IL-6 levels over time (12) and that bacterial infections in these patients are likely to be an epiphenomenon of exaggerated and protracted inflammation (6). Two studies reported that the intracellular growth of Mycobacterium avium-intracellulare complex was enhanced in human peripheral blood monocytes activated with the cytokines IL-3, IL-6, and granulocyte-macrophage colony-stimulating factor (3, 18). IL-6 is a unique cytokine with multifaceted activities. Depending on the sites of action, IL-6 could act as pro- or anti-inflammatory cytokine. In addition to our work (6), two studies have described an association between high circulating IL-6 levels and increased rate of infections (17, 19). Previous reports have shown that bacteria have receptors for proinflammatory cytokines. Porat et al. (16) reported that virulent strains of E. coli have receptors for IL-1β and that IL-1β enhances the growth of these bacteria. Luo et al. (10) reported that TNF-α could bind efficiently to many strains of gram-negative bacteria and that TNF-α–bacterium complexes can interact with TNF-α receptors present on eukaryotic cells. They also showed that TNF-α binding enhances bacterial invasion of HeLa cells and phagocytosis by human and murine macrophages (10).
Anti-inflammatory cytokines also promote bacterial growth. Two studies have shown that IL-10 and IL-4 can enhance the intracellular replication of bacteria. Park and Skerrett (15) reported that priming of human monocytes with IL-10 significantly enhanced the intracellular growth of Legionella pneumophila. In the present study, intracellular bacterial growth of the tested isolates was not affected by IL-10 priming (Fig. 6). This discrepancy could be due to the cell lines chosen or to the bacterial species tested, since L. pneumophila is an intracellular bacterium. Hultgren et al. (7) reported reduced growth of S. aureus in the joints of an IL-4-deficient mouse and showed that exposure of macrophages to IL-4 reduced the intracellular killing of S. aureus without impairing phagocytosis. From these studies, it appears that bacteria can adapt to the host innate and specific immune response by diverting such responses toward their own growth advantage and survival within the host.
We have previously shown that the proinflammatory cytokines TNF-α, IL-1β, and IL-6 enhance the in vitro extracellular growth of S. aureus, Acinetobacter sp., and P. aeruginosa in a concentration-dependent manner (13). The findings of the present study expand on this report to provide additional evidence in support of the hypothesis that inflammation has a bidirectional effect on bacterial growth. A mild to moderate degree of local inflammation provides an environment favorable to the host, where extracellular and intracellular bacterial growth is not promoted and phagocytic cells are efficient in killing the ingested bacteria. In contrast, an exaggerated and protracted local inflammation may compromise the resolution of an infection or favor the development of a new infection by promoting both intracellular and extracellular growth of bacteria above the clearance ability of the host.
ACKNOWLEDGMENTS
This investigation received financial support from the Assisi Foundation of Memphis and the University of Tennessee Medical Group.
We gratefully acknowledge Vivian Gomez for preparation of the manuscript and figures.
REFERENCES
- 1.Broug-Holub E, Toews G B, Van Iwaarden F, Strieter R M, Kunkel S L, Paine R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denis M, Campbell D, Gregg E O. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia coli. Infect Immun. 1991;59:1853–1856. doi: 10.1128/iai.59.5.1853-1856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis M, Gregg E O. Recombinant tumor necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990;71:139–141. [PMC free article] [PubMed] [Google Scholar]
- 4.Falkow S. Invasion and intracellular sorting of bacteria: searching for bacterial genes expressed during host/pathogen interactions. J Clin Investig. 1997;100:239–243. doi: 10.1172/JCI119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris S E, Nelson S, Astry C L, Bainton B G, Summer W R. Endotoxin-induced suppression of pulmonary antibacterial defenses against Staphylococcus aureus. Am Rev Respir Dis. 1988;138:1439–1443. doi: 10.1164/ajrccm/138.6.1439. [DOI] [PubMed] [Google Scholar]
- 6.Headley A S, Tolley E, Meduri G U. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 7.Hultgren O, Kopf M, Tarkowski A. Staphylococcus aureus-induced septic arthritis and septic death is decreased in IL-4 deficient mice: role of IL-4 as promoter of bacterial growth. J Immunol. 1998;160:5082–5087. [PubMed] [Google Scholar]
- 8.Kanangat S, Thomas J, Gangappa S, Babu J S, Rouse B T. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression: implications in immunopathogenesis and protection. J Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 9.Kiani A, Tschiersch A, Gaboriau E, Otto F, Seiz A, Knopf H, Stütz P, Färber L, Haus U, Galanos C, Mertelsmanh R, Engelhardt R. Downregulation of the proinflammatory cytokine response to endotoxin by nontoxic lipid A analogue SDZ MRL 953 in cancer patients. Blood. 1997;90:1673–1683. [PubMed] [Google Scholar]
- 10.Luo G, Niesel D W, Shaban R A, Grimm E A, Klimpel G R. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993;61:830–835. doi: 10.1128/iai.61.3.830-835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meduri G U, Estes R J. Pathogenesis of ventilator-associated pneumonia: The lower respiratory tract. Intensive Care Med. 1995;21:452–461. doi: 10.1007/BF01707417. [DOI] [PubMed] [Google Scholar]
- 12.Meduri G U. The role of the host defense response in the progression and outcome of ARDS: pathophysiological correlations and response to glucocorticoid treatment. Eur Respir J. 1996;9:2650–2670. doi: 10.1183/09031936.96.09122650. [DOI] [PubMed] [Google Scholar]
- 13.Meduri, G. U., S. Kanangat, J. Stefan, E. Tolley, and D. Schaberg. Cytokines IL-1β, IL-6, and TNF-α enhance in vitro growth of bacteria. Am. J. Respir. Crit. Care. Med., in press. [DOI] [PubMed]
- 14.Murphy P M, Lane H C, Gallin J I, Fauci A S. Marked disparity in incidence of bacterial infections in patients with the acquired immunodeficiency syndrome receiving interleukin-2 or interferon-γ. Ann Intern Med. 1988;108:36–41. doi: 10.7326/0003-4819-108-1-36. [DOI] [PubMed] [Google Scholar]
- 15.Park D R, Skerrett S J. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ: differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- 16.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 17.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiratsuchi H, Johnson J L, Ellner J L. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991;146:3165–3170. [PubMed] [Google Scholar]
- 19.Wagner J A. Is IL-6 a cytokine and a neurotropic factor? J Exp Med. 1996;183:2417–2419. doi: 10.1084/jem.183.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]