Abstract
Kombucha is a sparkling sugared tea commonly prepared using a sugared tea infusion and fermented at ambient temperature for several days using a cellulose pellicle also called tea fungus that is comprised of acetic acid bacteria and yeast. Consumption of Kombucha has been reported as early as 220 B.C. with various reported potential health benefits and appealing sensory properties. During Kombucha fermentation, sucrose is hydrolysed by yeast cells into fructose and glucose, which are then metabolised to ethanol. The ethanol is then oxidised by acetic acid bacteria (AAB) to produce acetic acid which is responsible for the reduction of the pH and also contributes to the sour taste of Kombucha. Characterisation of the AAB and yeast in the Kombucha starter culture can provide a better understanding of the fermentation process. This knowledge can potentially aid in the production of higher quality products as these microorganisms affect the production of metabolites such as organic acids which are associated with potential health benefits, as well as sensory properties. This review presents recent advances in the isolation, enumeration, biochemical characteristics, conventional phenotypic identification system, and modern genetic identification techniques of AAB and yeast present in Kombucha to gain a better understanding of the microbial diversity of the beverage.
Keywords: Kombucha, fermentation, acetic acid bacteria, yeast, microbial identification
1. Introduction
Kombucha is a traditional fermented sparkling tea beverage with a slightly sweet and acidic flavour that has been consumed in China since around 220 B.C. The beverage is consumed for its refreshing sensory characteristics and the perceived inherent health-promoting properties [1]. It is reported that Dr Kombu introduced fermented tea to Japan around 414 A.D., where it was apparently used to alleviate digestive ailments [1,2]. The name, ‘Kombucha’ is documented to originate from “Dr Kombu” and “cha” refers to tea in Japanese. Kombucha was introduced to Russia as “Tea Kvass” and then it spread to Eastern Europe during the 20th century. The popularity of Kombucha in Russia was attributed to its purported beneficial effects on healing metabolic diseases, haemorrhoids and rheumatism [3,4]. During World War II, Kombucha was introduced to Western Europe and North Africa. In recent decades, the production of Kombucha has become global, and it is now sold as a commercial beverage with different flavours. In addition, due to the high popularity of Kombucha products, small-scale home-brewed products are often made for personal use and can frequently be found for sale at farmer’s markets and in communities.
The global market size for Kombucha has increased significantly in recent years. In 2018, Kombucha was worth USD 1.5 billion, and it is estimated to grow to around 5 billion by 2025 with an estimated compound annual growth rate (CAGR) of 23% [5]. Most commercial Kombucha products sold in New Zealand are flavoured using fruits and/or herbs in single or mixed flavours. Information on commercial Kombucha products from different brands sold in New Zealand is shown in Table 1.
Table 1.
Commercial Kombucha products sold in New Zealand.
| Brand | Origin | Packaging | Flavour(s) | Storage Conditions |
|---|---|---|---|---|
| Remedy | Australia | 330 mL (glass bottles) 250 mL (cans) 1.25 L (plastic bottles) |
Ginger lemon, Raspberry Lemonade, Cola, Wildberry, Mango Passion, Passionfruit, Apple Crisp, Peach | Chilled/ambient temperature |
| Batchwell | New Zealand | 375 mL (glass bottles) | Braeburn apple, Pineapple and Ginger, Motueka hops, Ginger and Turmeric, Early grey | Chilled |
| Daily Organics | New Zealand | 200 mL (glass bottles) 1 L (glass bottles) |
Original Kombucha, Chai spices and ginger, Lemon and ginger | Chilled |
| LO BROS | New Zealand | 330 mL (glass bottles) 250 mL (cans) 750 mL (glass bottles) |
Feijoa, Raspberry and lemon, Orange and mango, Ginger and lemon, Mango, Ginger and turmeric, Blueberry, Passionfruit, Cola, Ginger beer, Lemon lime and bitters, Pineapple and lime | Chilled/ ambient temperature |
| MAMA’S Brew Shop | New Zealand | 375 mL (glass bottles) 330 mL (cans) |
Lemongrass and ginger, Lavender and hibiscus | Chilled |
| Karma Drinks | New Zealand | 330 mL (glass bottles) | Lemon and ginger, Raspberry and lemon, Mango and passionfruit, Cherryand berry | Chilled |
| Good Buzz | New Zealand | 250 mL (cans) 328 mL (glass bottles) 375 mL (glass bottles) 888 mL (glass bottles) |
Passionfruit and guava, Blueberry and peach, Pineapple and mango, Feijoa, Raspberry and lemon, Mango, Gisborne lemon and Manuka leaf, Hawkes Bay, peach and kawakawa | Chilled or ambient temperature |
Kombucha is usually fermented from sweetened tea using a symbiotic culture of bacteria and yeast which is commonly abbreviated to SCOBY. The SCOBY is also known as tea fungus, cellulosic pellicle or consortium. The microbial community in Kombucha is diverse and varies between fermentations, but it is mainly composed of AAB and yeast, although, the presence of small amounts of lactic acid bacteria (LAB) in Kombucha has also been reported [6]. These microorganisms (LAB, AAB and yeast) have been suggested to be potential probiotics and therefore contribute to the health benefits of Kombucha [7]. However, the probiotic performance of the live cultures identified in Kombucha has rarely been studied [8]. Therefore, the probiotic potential of the microorganisms found in Kombucha should be determined using both in vitro and in vivo studies.
Many different substrates can be used for Kombucha, including green tea, oolong tea and black tea, as well as medicinal herbs including lemon balm, peppermint, thyme and sage or combinations thereof [9]. Black tea is the most common tea substrate for Kombucha fermentation [10], and sucrose is the most popular source of carbon during fermentation [2]. For Kombucha fermentation, approximately 50 to 150 g/L (5% to 15% w/v) of sucrose is dissolved in boiled water, then tea leaves or tea bags are added to the sugared hot water and allowed to infuse for around 5–10 min, followed by filtration to remove tea leaves (or removal of tea bags). Sugared tea is allowed to cool to room temperature [1]. To prevent contamination from pathogenic and spoilage microorganisms, the production process is generally carried out under highly sanitary conditions with a small portion of previously fermented Kombucha broth added to reduce the pH [2,3]. The cooled sugared tea is then transferred to a sterile wide-mouth container, and the “mushroom” (tea fungus) is placed onto the surface of the solution. The vessel is covered with a sterile cloth or paper towel to prevent insects and other undesirable cross-contamination from affecting the fermentation. The fermentation generally takes 7 to 10 days at room temperature (20 °C to 30 °C), with fermentation temperatures ranging from 18 °C to 26 °C reported as optimal [2]. During fermentation, a newly formed jelly-like daughter tea fungus membrane is formed which floats on the surface of the broth. The tea fungus is removed and retained, together with a small amount of tea broth for the next fermentation. The daughter tea fungus can grow up to 2 cm thick and covers the surface of the mother tea fungus to gain better access to oxygen [2,11,12]. After fermentation, the liquid broth is filtered through a clean cloth and stored in a sealed container at 4 °C for further processing such as packaging. With fermentation, the taste of Kombucha changes from pleasantly fruity at the beginning, to a vinegar-like flavour after longer fermentation [2].
The consumption of Kombucha has been suggested to confer numerous health benefits for humans. However, the evidence for most of these benefits is based on in vitro studies. Therefore, in vivo clinical trials are required to demonstrate any biological functions of Kombucha and to correlate them with active compounds [13]. The fermented beverage is perceived to lower blood pressure (antihypertensive) by inhibiting the angiotensin-converting enzyme (ACE) and mediating blood sugar levels (antidiabetic) and cholesterol levels [14]. Furthermore, the consumption of Kombucha may be effective in weight management by controlling appetite due to its hypolipidemic effects associated with lipase inhibition [14,15]. Kombucha has been reported to exhibit anticarcinogenic activity due to the presence of tea polyphenols and metabolites [2]. The antimicrobial activity of Kombucha against pathogenic microorganisms is mainly attributed to the action of acetic acid produced during fermentation [14,16]. The hepatoprotective activity of Kombucha is mainly due to the presence of the potential detoxifying agent, D-saccharic acid 1.4-lactone (DSL). However, there is scanty information on the potential toxicity of Kombucha associated with metabolic acidosis and hyponatremia [2,4]. Thus, the recommended daily consumption of Kombucha should be no more than 118 mL (4 fluid ounces) for healthy people [13].
The objective of this review is to document the recent advances in the microbiological characteristics, composition, and phenotypical and molecular identification techniques of Kombucha. Systematic research is recommended as the identification of the microbial characteristics of Kombucha may allow producers to effectively control the fermentation process for the production of safer, consistently high-quality products.
2. Microbiological Characteristics of Kombucha
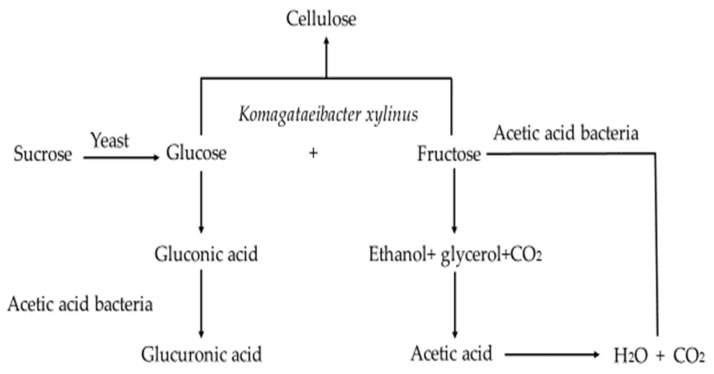
The Kombucha microbial community can be classified into two parts, that found in the floating cellulosic biofilm and that in the liquid broth [17]. An overview of the metabolic activities of the Kombucha fermentation process is summarised in Figure 1 [18]. Sucrose is hydrolysed by yeast cells into fructose and glucose, which are metabolised by yeast to produce ethanol and carbon dioxide. Glycerol can also be produced by yeast due to the high osmotic pressure and further oxidised by AAB to dihydroxyacetone (DHA) [15]. Some esters are also produced during this process, which contribute to the aroma development of Kombucha. Fructose is preferably utilised as the substrate rather than glucose [19], with the resulting ethanol produced being further metabolised by AAB to produce acetic acid, thereby reducing the ethanol content in the Kombucha. The low ethanol concentration facilitates the formation of the cellulosic pellicle [15]. Glucose is metabolised by AAB into gluconic and glucuronic acids. Autolysis of yeast provides vitamins and other nutrients to support the growth of the AAB [16]. Yeast autolysis is commonly detected during the maturation of alcoholic beverages, which may impact the aroma and flavour of Kombucha products.
Figure 1.
Metabolic activity of yeast and AAB during Kombucha fermentation. Adapted from Ref. [18].
2.1. Presence of Acetic Acid Bacteria in Fermented Foods
2.1.1. AAB Used in the Production of Fermented Products
Acetic acid bacteria play an important role in the fermented food and beverage industry. These bacteria are widespread in the environment and can be found in sugary or acidic substances such as fruits and flowers [20]. The genus Gluconobacter is easily isolated from environments abundant in sugar, while the genera Acetobacter and Gluconacetobacter are found in alcoholic environments [15]. AAB are widely used in the production of fermented food and beverage products such as vinegar, lambic beers, red wine, cocoa, and nata de coco (an edible bacterial cellulose formed from AAB in coconut water and kefir). The genera Acetobacter and Gluconacetobacter prefer to oxidise ethanol rather than glucose, while the genus Gluconobacter oxidises glycerol and glucose [15].
2.1.2. Dominant Acetic Acid Bacteria Found in Kombucha
Acetic acid bacteria and gluconic acid-producing bacteria are the dominant prokaryotes found in Kombucha starter cultures. AAB belong to the family Acetobacteraceae and are classified into acetous or acidophilic groups. Currently, AAB are divided into 17 genera, of these, Acetobacter, Gluconobacter, Gluconacetobacter and Komagataeibacter are mainly used in the food industry [20,21]. The microbial community in Kombucha varies between products, which impacts the biochemical properties of the fermented beverage [22]. The production of the cellulosic biofilm (tea fungus) floating on the surface of the tea broth in Kombucha is associated with the presence of the AAB species Komagataeibacter (K.) xylinus [1,2,16,23]. Synthesis of the cellulosic pellicle involves the production of the cellulose precursor-uridine diphospho-glucose (UDPGc), which can be synthesised from several carbon sources including ethanol, sucrose, fructose, and glycerol [16]. The presence of caffeine, theophylline and theobromine facilitates the production of the cellulose network by K. xylinus [24,25], which is also affected by sugar types, sugar concentration, and pH [15].
Other AAB isolated from Kombucha include Bacterium (B.) gluconicum [10,26], Acetobacter (A.) aceti, Acetobacter (A.) pasteurianus, Glucobacter (G.) oxygendans [27], Acetobacter (A.) musti and Gluconobacter (G.) potus [28]. Recently, species of Komagataeibacter and Gluconoacetobacter have been reported in Kombucha including Komagaeibacter (K.) kombuchae [29], Komagataeibacter (K.) saccharivorans [30], Komagataeibacter (K.) rhaeticus [31], Gluconacetobacter (G.) sacchari [32] and Gluconacetobacter sp. A4 (G. sp. A4) are the main functional AAB isolated from Kombucha which synthesise D-saccharide acid-1,4 lactone (DSL), a promising detoxifying and antioxidant agent [33]. Nitrogen-fixing Acetobacter (A.) nitrogenifigens sp. nov. and Gluconactobacter (G.) kombucahe sp.nov have also been isolated from Kombucha [34].
2.2. Lactic Acid Bacteria Isolated from Kombucha
LAB have been utilised in different food products to achieve a unique flavour and deliver health benefits [35]. The genera Lactobacillus and Bifidobacterium are commonly isolated from fermented foods [36]. LAB may be present in Kombucha; however, current information is not considered essential for its production. In certain Kombucha products, LAB have been reported to make up as much as 30% of the bacterial community. The addition of some LAB strains—Lacticaseibacillus (L.) casei and Lactiplantibacillus (L.) plantarum have been shown to enhance the antioxidant and antimicrobial activities of Kombucha [35]. Moreover, the presence of LAB during Kombucha fermentation has been reported to enhance the production of D-saccharic acid, 1,4 lactone (DSL) and glucuronic acid [33]. DSL has detoxifying properties and may be an important factor contributing to the purported hepatoprotective activity of Kombucha [37]. The LAB isolated from Kombucha are shown in Table 2.
Table 2.
LAB isolated from Kombucha.
| Species | Region | Reference |
|---|---|---|
| Lacticaseibacillus casei | NS | [35] |
| Lactiplantibacillus plantarum | China | [38] |
| Lactobacillus nagelii | United State of America (USA) | [39] |
| Lactobacillus rhamnosus | NS | [5] |
| Lactobacillus mali | USA | [39] |
| Pediococcus (P.) pentosaceus | Romania | [40] |
| P.acidiliactici | Romania | [36] |
NS = not specified.
2.3. Yeast Isolated from Kombucha
2.3.1. General Characteristics of Yeast
Yeast is a group of eukaryotic unicellular fungi which reproduce by budding or fission, with some capable of both [41]. Yeast has been used for the fermentation of foods and beverages for thousands of years due to its ability to hydrolyse different substrates to produce valuable fermented final products such as beer, wine and bread [42]. The first yeast used commercially was identified as the Saccharomyces (S.) sensu stricto complex [42]. Yeast is currently classified into Ascomycetous and Basidiomycetous yeast, according to their molecular phylogenies [41]. Yeasts are facultative anaerobes indicating that they can grow without oxygen. In the presence of oxygen, yeasts convert sugars to carbon dioxide and energy, whereas under anaerobic conditions, the sugars are converted to ethanol, glycerol and carbon dioxide [43].
S. cerevisiae, also known as baker’s yeast, is the most common food-grade yeast, widely used in bakery products, beer-brewing, and winemaking. Food-grade yeasts are also utilised as food additives, flavouring agents and as substrates for microbiological agar [43]. Some non-Saccharomyces yeast such as the genera Candida, Debaryomuces, Kluyveromyces, Yarrowia and Zygosaccharomyces are gaining attention in industry for their possible commercial applications as starter cultures for food and non-food products due to their perceived probiotic properties and impact on the development of flavour and colour in some meat products [42,44].
2.3.2. Dominant Yeast Present in Kombucha
Yeast species may vary between Kombucha products, particularly those from different regions due to environmental factors such as geographic and climatic conditions, and even contamination between starter cultures [45,46]. Common yeasts isolated from Kombucha products produced in different regions and their characteristics (morphological and metabolic) are shown in Table 3 and Table 4 [24]. Generally, the yeast population outnumbers the bacteria present in Kombucha [47].
Table 3.
Common yeast species isolated from Kombucha and their metabolic characteristics.
| Species | Morphology | Characteristics |
|---|---|---|
| Zygosaccharomyces (Z.) bailii [1] | White to cream colonies with brownish top, cylindrical or ellipsoidal shape, (3.5–6.0) × (4.5–11.5) μm in size | Tolerant to organic acids, Forms acetic acid, heat tolerance < 75 °C Growth pH > 2 and < 7 [48] |
| Zycosaccharomyces (Z.) rouxii | White to cream smooth colonies, round or oval shape |
High osmotic stress and salt/sugar tolerant, grows under low oxygen and low water activity [49] |
| Schizosaccharomyces (S.) pombe | Cream to tan, butyrous colonies, rod-shaped | Can convert malic acid to ethanol, high resistance to low water activity, low pH and wide range of temperature environments, highly sugar content tolerant [50] |
|
Saccharomycodes (S.) ludwigi [10] |
Cream, butyrous colonies, elongated shape, and swelling in the centre | Resistant to pressurised carbon dioxide, high sugar tolerant [51] |
| S. cerevisiae [27] | White to cream, butyrous colonies, spherical or ovoid shape, 2.5–10.0 µm (diameter) | Can convert glucose to ethanol, high ethanol tolerance, rapid fermentation rate [52] |
|
Brettanomyces (B.) bruxellensis [24,47] |
Distinctive elongated shape, 2.5–10.0 µm (diameter) |
Can produce high amounts of acetic acid and ethanol under aerobic conditions, high ethanol concentration (up to 15%), able to grow under low pH and oxygen environment, high efficiency to utilise nitrogen sources [53] |
Table 4.
Yeast isolated from Kombucha in different regions.
| Species | Country | References |
|---|---|---|
| Brettanomyces (B.) anamalus | New Zealand | [28] |
| B. lambicus | Germany | [54] |
| B. custerisii | Germany | [54] |
| B. intermedius | NS | [24] |
| B. claussenii | NS | [24] |
|
Candida (C.) albican C. colleculosa C. kefir C. krusei |
Japan Saudi Arabia Saudi Arabia Saudi Arabia |
[24] [55] [55] [55] |
|
C. guilliermondii
C. obtuse C. stellata |
Japan/Saudi Arabia Formosa Australia |
[56] [46] [46] |
| C. famata | Indonesia | [57] |
| C. magnoliae | Indonesia | [57] |
| Dekkera (D.) bruxelensis | New Zealand | [28] |
| Hanseniaspora (H.) valbyensis | New Zealand | [28] |
| Lachancea (L.) fermentati | USA | [58] |
|
Kloeckera (K.) apiculata
Kluyceromyces (K.) africanus |
NS NS |
[24] [24] |
| Pichia (P.) fermentans | NS | [4] |
| P. membranefaciens | NS | [26] |
| P. kudriavzevii | New Zealand | [28] |
| Torulaspora (T.) delbrueckii | Australia | [46] |
| Torulopsis (T.)famata | Japan | [56] |
| Zygosaccharomyces (Z.) kombucahensis | Russia | [59] |
Note: NS = not specified.
3. Isolation of AAB and Yeast from Kombucha
3.1. Isolation, Enumeration and Preservation of AAB
AAB can be isolated from fermented foods and beverages including vinegar, cider and lambic beers [60]. However, it is difficult to isolate AAB using commercial media as AAB are nutritionally demanding [61]. Although there are many growth media designed for the isolation of AAB which are based on their metabolism and nutritional requirements, other microorganisms can also grow on these media [62]. The carbon sources utilised are mainly D-mannitol and D-glucose, with different concentrations of ethanol and acetic acid added to the medium. Nitrogen sources are mainly peptone and yeast extract, with mineral salts including KH2PO4, Na2PO4 and MgSO4 added to the medium to aid the recovery of the AAB cells [61]. The composition of culture media commonly used for the isolation of AAB are shown in Table S1 and the references [28,60,63,64,65,66,67,68,69,70,71,72,73] are cited in the Supplementary Materials.
Some AAB species are not culturable, therefore, alternative techniques such as real-time quantitative polymerase chain reaction (qPCR) are used to identify this population of AAB. Alternatively, epifluorescence staining techniques are also regarded as fast and simple methods for the enumeration of total viable/ non-viable AAB [74].
The preservation of AAB cultures is commonly achieved by sub-culturing, and storage under mineral oils, freeze-drying and cryopreservation, with frozen cultures stored at temperatures between −70 °C and −150 °C. Cell damage caused by ultra-low temperatures can be prevented by adding cryoprotectants such as glycerol (10–25%) and dimethyl sulfoxide (DMSO) (5%). However, glycerol is not suitable for the AAB that form cellulose structures such as K. xylinus. In these instances, DMSO is preferable as it can maintain stability and high viability without affecting the cellulose structure [75].
3.2. Isolation and Enumeration of Yeast
Conventional enumeration of yeast is carried out by spread plating as this technique allows microorganisms to be exposed to oxygen and avoids stress caused by hot or warm culture medium [76]. There are also numerous commercial media available for the isolation and cultivation of yeast from foods including Kombucha (Table S2) [77,78]. These media provide the basic nutrients to support the growth of yeast and lower the pH to inhibit the growth of bacteria and moulds. Antibiotics such as penicillin can also be added to prevent the growth of bacteria and moulds [77].
4. Phenotypic Characterisation and Identification of Acetic Acid Bacteria and Yeast from Kombucha
4.1. Phenotypic Characterisation of AAB
The traditional classification of AAB species includes differentiation by cellular morphology, flagellation, and physiological and biochemical properties [79]. Colony examination involves size, form, elevation, and colour. The cellular morphology of bacteria includes the cell shape, size and response to Gram reaction, motility, spore-forming and cellular arrangement [80].
AAB are commonly Gram-negative, aerobic rods or ellipsoidal non-spore forming cells, which can be single, in pairs, or clusters. Cell sizes range from 0.4 to 1.0 μm in width and 0.8 to 4.5 μm in length [81]. These bacteria are mostly catalase-positive and oxidase negative. The optimum growth temperature varies from 25 °C to 30 °C, however, some thermotolerant strains can grow at temperatures up to 42 °C [21], and some AAB can grow under acidic conditions (e.g., pH 3.5) [60].
The classification of AAB comprises five chemical properties which are: catalase positive, oxidation of ethanol to acetic acid, over-oxidation of ethanol to water and CO2, oxidation of lactate to CO2 and water, ketogenesis from glycerol and hydrolysis of D-glucose to different acids [82]. Initially, AAB were classified into Acetobacter and Gluconobacter. The genus Acetobacter has peritrichous flagella and can oxidise acetate and lactate. In contrast, the genus Gluconobacter lacks the ability to oxidise acetate and lactate, however, they can oxidise D-glucose to 2-ketogluconate and 5-ketogluconate. The main difference between the genera Acetobacter and Gluconobacter is the presence of ubiquinone 9 in the genus Acetobacter, while ubiquinone-10 is present in the genus Gluconobacter [83]. In 1997, a new genus, Gluconacetobacter, was reported with partial 16 ribosomal sequencing techniques and the species containing coenzyme Q10 [68]. Later, another new genus, Komagataeibacter, was introduced based on 16S rRNA gene sequencing, phenotypic properties and different morphology to Gluconacetobacter [60]. The 11 species from the genus Gluconacetobacter were reclassified as Komagataeibacter based on their phenotypic and genotypic characteristics. Compared with Gluconacetobacter, the species of genus Komagataeibacter are not motile, and unable to produce a water-soluble brown pigment on glucose peptone yeast extract and calcium carbonate medium. The genus Gluconacetobacter can produce 2,5-diketo-D-gluconate but not Komagataeibacter. All AAB can oxidise sugars such as glucose, fructose, galactose, mannose, ribose and xylose through the cytoplasmic hexose monophosphate pathway [65]. Furthermore, AAB can oxidise sugar alcohols such as glycerol and convert them to dihydroxyacetone (DHA). Additionally, the ability to produce a cellulose structure is mainly found in the genera Gluconacetobacter and Komagataebacter; these differential properties help to distinguish AAB at the genus or species level. The main biochemical characteristics of the genera AAB applied in the food industry are shown in Table 5.
Table 5.
Differential characteristics of the genera Acetobacter, Gluconacetobacter, Gluconobacter and Komagataeibacter commonly associated with food.
| Characteristics | Acetobacter | Gluconacetobacter | Gluconobacter | Komagataeibacter |
|---|---|---|---|---|
| Cell shape | Ellipsoidal to rods | Ellipsoidal to rods | Ellipsoidal to Rods |
Coccoid to rods |
| Cell size (μm) | 0.4–1.0 × 1.0–3.0 | 0.5–0.9 × 1.0–2.0 | 0.6–1.0 × 1.0–3.0 | 0.6–0.8 × 1.0–3.0 |
| Colony appearance | Creamy to brown | Light brown to brownish | Smooth, entire, shiny, white, pink or brown | Raised, convex to umbonate, smooth to rough, entire to irregular |
| Catalase | + | + | + | + |
| Gram staining | Gram-negative | Gram-negative | Gram-negative | Gram-negative |
| Oxidase | − | − | − | − |
| Motility | Motile or non-motile | Motile or non-motile | Non-motile | No |
| Flagellation | Peritrichous | peritrichous | polar | No |
| Oxidation of ethanol to acetic acid | + | + | + | + |
| Oxidation of acetic acid to CO2 and water | + | + | − | + |
| Oxidation of lactate/acetate | + | + | − | + |
| Production of cellulose | − | ± | − | ± |
| Growth on 0.35% acetic acid | + | + | + | + |
| Growth in the presence of 1% KNO3 | − | − | − | ND |
| Growth on methanol | ± | − | − | ND |
| Growth in 30% D-glucose | − | ± | ± | ND |
| Ketogenesis (dihydroxyacetone) from glycerol | + | + | + | + |
| Acid production from: Glycerol D-Mannitol Raffinose Sorbitol |
± − − |
+ − − − |
+ + − + |
ND − ND − |
| Production of DHA from glycerol | ± | ± | + | + |
| Production of levan-like polysaccharide from sucrose | ± | − | − | − |
| Ubiquinone type | Q9 | Q10 | Q10 | Q10 |
| G + C content (mol %) | 50.5–60.3 | 55–67 | 52–64 | 56–64 |
| Sources | Flowers, fruits, palm wine, vinegar, kefir | Rhizosphere of coffee plants, roots and stem of sugar cane | Strawberry, grape and spoiled jackfruit and sugar-rich environments | Kombucha, vinegar, wine vinegar |
4.2. Phenotypic Characterisation and Identification of Yeast from Kombucha
The phenotypic identification of yeast from Kombucha is mainly achieved by morphological, and physiological tests and the use of rapid commercial yeast identification kits such as ID 32 C [85]. Traditionally, it is time-consuming to conduct physiological (identification) tests at the species level. The morphological characteristics of yeast cells are important for their identification as they may be produced by different reproduction modes as shown in Table 6 [86]. For example, the budding formation has been observed from yeast species, Z. kombuchaensis isolated from a dried tea fungus of Russian Kombucha [59]. Cellular morphological tests are usually obtained using the wet-mount method, by suspending the culture in saline and mixing it with dyes such as India ink, lactophenol cotton blue, calcofluor white or methylene blue staining [87].
Table 6.
Morphology of yeast isolated from Kombucha.
| Different Reproduction Mode of Yeast | Morphology Characteristics of Yeast |
|---|---|
| Vegetative or asexual reproduction | Budding: new cell is produced on the surface of parent cell and then separate Fission: an asexual cell is produced by a septum grown inward from cell wall to halve the long axis of the cell. Blastoconidiation: a mother cell of stalk-like tubular sterigmata produce a terminal conidium |
| Sexual reproduction in ascomycetous yeast | Parent cell-bud conjugation Gametangial conjugation Heterothallism conjugation Conjugation between hyphae |
| Sexual reproduction in basidiomycetous yeast | Budding haplophase Dikaryotic hyphal phase or self-spore forming diplophase |
Physiological and biochemical tests (Table 7) can be conducted using representative purified yeast to characterise the species present in food samples including Kombucha [77,79]. Kurtzman, Robnett and Basehoar-Powers conducted several nitrogen assimilation and carbohydrate fermentation tests in conjunction with other growth tests to determine the phenotypic characteristics of five new ascosporogeneous strains belonging to Z. kombuchaensis, which were isolated from Kombucha [59].
Table 7.
Physiological and biochemical tests used for the characterisation of yeast.
| Physiological Test | Biochemical Test |
|---|---|
| Assimilation of carbon and nitrogen sources | Diazonium Blue B reaction |
| Fermentation of carbohydrates | Urease test |
| Growth at different temperature | |
| Growth in vitamin-free medium | |
| Growth in high osmotic pressure condition | |
| Starch hydrolysis activity |
Identification of Yeast Using Commercial Kits
Several commercial identification systems based on conventional carbon fermentation or nitrogen assimilation reactions have been designed for rapid and accurate identification of food-related yeast in combination with morphological characterisation [77]. The ability of yeast to grow on different carbon and nitrogen sources can be determined by the formation of turbidity or colour changes in the presence of a pH indicator [85]. A description of common commercial systems such as API 20 C suitable for use in identifying yeast from Kombucha is summarised in Table 8.
Table 8.
Summary of tests and accuracy of common commercial yeast identification systems.
| Commercial System | Description of Tests and Controls Included in the Kits | Incubation Condition | Accuracy (%) | Reference |
|---|---|---|---|---|
| API 20 C | 19 carbon assimilation test and 1 control test in 20 strips | 30 °C for 72 h | 98.9 | [88] |
| API Candida | 5 carbohydrate and 7 enzymes colorimetric test in 10 strips | 35 °C for 18–24 h |
97.4 | [89] |
| API 32 C | 29 assimilation tests (carbohydrate, organic acids, and amino acids); 1 negative control, 1 susceptibility test (cycloheximide) and 1 colorimetric test (esculin) in 32 wells. Includes 63 different species in database |
30 °C for 48 h | 92 | [90] |
| Auxacolor system | 13 carbohydrate tests with bromocresol purple, test for cycloheximide resistance and phenoloxidase production in 16 wells. | 37 °C for 48 h | 79.4 | [91] |
| RapID Yeast Plus system | 5 carbon assimilation tests and 13 enzymatic hydrolysis substrate tests | 30 °C for 4 h | 96 | [92] |
| The Uni-Yeast-Tek (UYT) system | 7 carbon assimilation tests, urease, Nitrate and corn meal with Tween 80 agar | 22–26 °C for 2–10 days |
99.8 | [93] |
| MicroScan yeast identification panel | 13 aminopeptidase, 3 carbohydrates, 9 glycosidase, phosphatase and urease tests. | 37 °C for 4 h | 86.9 | [94] |
| VITEK 2 YST | 4 aminopeptidase, 25 carbohydrate, esculin, 3 glycosidase, nitrate, 2 nitrogen, 9 organic acid, and urea tests | 35 °C for 18 h | 94.8 | [95] |
Recently, several commercial kits have been successfully used to characterise yeast isolated from Kombucha. For example, the API 32 C system was used to characterise the carbohydrate assimilation pattern of the yeast strain, Lanchacea fermentati isolated from Kombucha [58]. Three different yeast species, C. famata, C. krusei and C. magnoliae from Kombucha SCOBY were identified using the API 20 C kit [57]. Commercial databases (Table 8) also contain yeast species isolated from Kombucha samples; hence, these commercial yeast identification systems may also be applied in the identification of yeast isolated from Kombucha by conducting different carbohydrate and nitrogen assimilation tests and comparing the results to the database.
5. Genotypic Identification of AAB and Yeast from Kombucha
5.1. Genotypic Identification of AAB from Kombucha
It is difficult to identify AAB to species levels using only phenotypic characteristics [61]. Compared with conventional biochemical and physiological identification, molecular techniques are generally more reliable and rapid [96]. Several DNA sequence-based techniques involving DNA extraction and polymerase chain reaction (PCR) have been widely applied to identify AAB to genera, species or strain levels by comparing with reference strains [97]. Commonly used DNA-based techniques applied for the identification of AAB are shown in Table 9.
Table 9.
Commonly used molecular techniques applied in AAB identification and genotyping.
| Technique | Level | Advantage | Disadvantage |
|---|---|---|---|
| PCR-RFLP | Species | Rapid, cheap, and easy to set up; suitable for genotyping of AAB isolates | Difficult to identify small insertions and expensive, unable to discriminate closely related species |
| DGGE | Species | Rapid and cost-effective; suitable for estimation of AAB diversity | Cannot discriminate closely related species. |
| Real-time PCR | Species | Rapid, reliable and quantitative; suitable for comparing microbial abundance. | Complex and expensive |
| RAPD | Strain | Quick and simple | Low reproducibility, as the quality and concentration of template DNA influence the results |
| ALFP | Strain | Can be used for any DNA samples of any origin, and reveal multiple polymorphic bands in one lane. | Complex and sensitive |
Methods commonly used to discriminate AAB to species level include polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) of the 16S rRNA genes or 16–23S internal transcribed spacer (ITS), PCR amplification and direct sequencing of 16S rDNAs and 16S–23S ITS, denaturing gradient gel electrophoresis (DGGE) of partial 16S rRNA gene, and real-time PCR (qPCR) [97]. The PCR-RFLP method involves amplification of the targeted regions of the 16S rRNA gene followed by digestion of the PCR products with restriction enzymes. The resultant DNA fragments are then separated by polyacrylamide or agarose gel electrophoresis. AAB isolates can be identified by comparing their restriction profiles with those of the reference strains [98]. Terminal restriction fragment length polymorphism (T-RFLP) is a modified version of PCR-RFLP, and it involves the use of fluorescent-labelled primers for detecting characteristic restriction fragments. T-RFLP has been used to determine the dynamic changes of AAB during the Kombucha fermentation process, and Komagataeibacter was revealed to be the dominant genus in both the tea fungus and the broth [17]. DGGE is commonly used to determine the diversity or species composition of AAB from fermented foods such as rice vinegar [62]. The principle of this technique is to separate DNA fragments of the same length on the basis of their differences in melting point, which is determined by the nucleotide sequence of the DNA molecule [97]. Thus, DGGE has a higher resolution than RFLP or T-RFLP in the discrimination of closely related AAB species.
Real-time PCR, also known as quantitative PCR (qPCR), is a well-established method that has been successfully used in the detection and quantification of AAB present in Kombucha. During each cycle of the qPCR reaction, fluorescence released by DNA-binding fluorescent dye or oligonucleotide probes is automatically measured as a proxy of the amplified DNA [99]. The amount of target gene (i.e., 16S rRNA) in the sample is then determined by calculating the cycle threshold (Ct), which is positively correlated with colony forming unit (CFU). qPCR is thus suitable for measuring AAB abundance in commercial Kombucha SCOBY [100].
To genetically validate the taxonomic identity of an AAB isolate, the full-length 16S rRNA gene is normally amplified by PCR and subsequently sequenced using the classic method of Sanger sequencing. However, along with the technical advancement of next-generation sequencing (NGS), the Illumina MiSeq has become a popular and powerful technique for determining microbial community structure and species composition. Briefly, total DNA is extracted from a Kombucha sample and then subjected to PCR amplification using general primers that target the V1–V3 (or V3–V4) region of the 16S rRNA gene [101,102,103]. The resultant amplicons are then sequenced using the Illumina MiSeq platform. An operational taxonomic unit (OUT) is assigned at a given level of sequence similarity (e.g., 97%). Moreover, the NGS technology has recently been extended beyond the analysis of targeted rRNA genes, and shotgun metagenomic sequencing enables the analysis of whole genomic DNAs that are extracted from a particular sample [104]. For example, Arika and other researchers analysed the microbial communities of homemade Kombucha fermentations using a combination of metagenomic sequencing and 16S rRNA amplicon sequencing [105]. Both methods consistently revealed that Komagataeibacter was the dominant bacterial genus. Interestingly, a search of secondary metabolite genes in the metagenome-assembled genomes identified novel gene clusters for bacteriocin production. Bacteriocins are a group of small antimicrobial peptides against closely related bacteria. The finding may partially explain the antimicrobial properties of Kombucha.
For identification to strain level, four PCR-based fingerprinting techniques are currently available: random amplification of polymorphic DNA (RAPD), amplified length fragments polymorphism (ALFP), enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR), and repetitive extragenic palindromic PCR (REP-PCR) [97]. The RAPD technique involves the amplification of random regions of genomic DNA with a short (~10 nucleotides) single arbitrary primer under low annealing temperatures [106]. RAPD has been successfully used to identify the dominant bacterial composition of both tea fungus and the Kombucha broth [107]. In contrast, the ALFP technique selectively amplifies a subset of digested DNA fragments to generate a unique restriction profile for each AAB genome. ALFP involves the use of a pair of specifically designed primers, which consists of three parts: a core sequence, a restriction site sequence, and a 3′ selective sequence [97]. Both REP- and ERIC-PCR target repeat sequences that are commonly found in bacterial genomes. Both techniques can type AAB isolates to strain level, but ERI-PCR is more suitable for AAB due to its higher accuracy [108]. Interestingly, REP- and ERIC-PCR can be used in combination to increase the sensitivity of these two fingerprinting techniques, as shown in a case study with cellulose-forming AAB isolates from Kombucha [109]. Molecular techniques commonly applied in the identification of the Kombucha bacterial community are summarised in Table 10. The specific organisms and primers are linked to the work in reference in Table 10.
Table 10.
Molecular techniques commonly used for AAB identification.
| Method | Microorganism | Primer Sequence (5′–3′) | Reference |
|---|---|---|---|
| 16S rRNA, Sanger sequencing | Gluconacetobacter; | 27F, AGAGTTTGATCMTGGCTCAG | [107] |
| Komagataeibacter; | 1494R, TGACTGACTGAGGYTACCTTGTTACGACTT | ||
| Gluconacetobacter; | fD1, CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG | [29] | |
| kombuchae sp. nov.; | rD1, CCCGGGATCCAAGCTTAAGGAGGTGATCCAGCC | ||
| Acetobacter aceti; | 16S d, GCTGGCGGCATGCTTAACACA | [96] | |
| 16S r, GCAGGTGATCCAGCCGCA | |||
| 16S rRNA V1-V3 region, Illumina MiSeq | Bacterial communities | Forward, TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG | [108] |
| Reverse, GTVTVGTGGGCTCGGAGATGTGTATAAGAGACAG | |||
| 16S rRNA V3-V4 region, Illumina MiSeq | Bacterial communities | Forward, CCTACGGGNGGCWGCAG | [102] [109] |
| Reverse, GACTACHVGGGTATCTAATCC | |||
| 16S rRNA, Real-time PCR | Bacterial abundance | 926f, AAACTCAAKGAATTGACGG | [100] |
| 1062r, CTCACRRCACGAGCTGAC | |||
| 16S rRNA, T-RFLP | Bacterial communities | 27F, AGAGTTTGATCMTGGCTCAG | [17] |
| 1525R, AAGGAGGTGATCCAGCC | |||
| RAPD | Komagataeibacter spp. | M13, GAGGGTGGCGGTTCT | [104] |
| AFLP | Komagataeibacter rhaeticus | A03, GACTGCGTACAGGCCCCG | [110] |
| T03, CGATGAGTCCTGACCGAG | |||
| REP-PCR | G. oxydans; A. aceti | REPIR-I, IIICGICGICATCIGGC | [105] |
| REP2-I, ICGICTTATCIGGCCTAC | |||
| ERIC-PCR | G. oxydans; A. aceti | ERIC1R, ATGTAAGCTCCTGGGGATTCAC | [105] |
| ERIC2, AAGTAAGTGACTGGGGTGAGCG | |||
| Shotgun metagenomic sequencing | Bacterial and fungal communities | [101] [102] |
5.2. Genotypic Identification of Yeast
Different rapid commercial kits such as API 20 C and API 32 C are convenient for yeast identification, however, they are associated with minor differences in biochemical profiles due to variabilities in test conditions. Results from commercial kits need to correlate to morphological observations to identify the yeast at the species level. For instance, the species from Dekkera are the anamorphs of Brettanomyces and they are deficient in sexual characteristics. Thus, the biochemical profiles of these fungi with limited morphological features are not stable and therefore difficult to differentiate [111]. Consequently, accurate identification of yeast strains often needs a combination of conventional biochemical methods and modern molecular biology techniques.
Genotypic identification of yeast is mostly focused on sequence variations in the ribosomal DNA (rDNA) region, which includes 18S, 5.8S, and 26S rRNA genes separated by two internal transcribed spacers (ITSI and ITS2). Several universal primers have been designed for genus- or species-specific identification and the D1/D2 domain of 26S rDNA is the most popular target (Table 11). Yeast strains that differ by more than 1% in the 26S rDNA D1/D2 region are generally considered distinct species [112]. As mentioned above, high-throughput sequencing techniques such as Illumina MiSeq have also been used to examine the yeast communities in Kombucha, and the analysis can target either specific rRNA genes or the whole metagenome [102]. The relative abundance of yeast in Kombucha can be estimated using the real-time PCR method with one pair of specifically designed primers, which amplify a 124 bp region in the variable D1/D2 domain of the 26S rRNA gene [113]. Finally, it should be noted that other PCR-based techniques such as AFLP, RAPD and REP-PCR have been successfully developed for genetic identification of yeast at the sub-species or strain levels [112].
Table 11.
Molecular techniques used for the identification of yeast present in Kombucha.
| Method | Microorganism | Primer Sequence (5′–3′) | Reference |
|---|---|---|---|
| 26S rDNA, D1/D2 domain | Brettanomyces/Dekkera; Pichia; B. bruxellensis; | NL1, GCATATCAATAAGCGGAGGAAAAG | [107] [104] |
| D. bruxellensis; Hanseniaspora (H.) uvarum | NL4, GGTCCGTGTTTCAAGACGG | [109] | |
| 26S rDNA, D1/D2 domain | D. bruxellensis; D. anaomala; Z. bailii; H. valbyensis | NL1, GCATATCAATAAGCGGAGGAAAAG | [108] |
| NL4, GGTCCGTGTTTCAAGACGG | |||
| 18S rDNA, D1/D2 domain | Z. kombuchaensis sp. | NS-1, GTAGTCATATGCTTGTCTC | [59] |
| NS-8A, CCTTCCGCAGGTTCACCTACGGAAACC | |||
| ITS, Illumina MiSeq | Z. bailii | ITS1F, CTTGGTCATTTAGAGGAAGTAA | [102] |
| ITS2R, GCTGCGTTCTTCATCGATGC | |||
| Real-time PCR | Brettanomyces | Yeast-F, GAGTCGAGTTGTTTGGGAATGC | [100] |
| Yeast-R, TCTCTTTCCAAAGTTCTTTTCATCTTT | |||
| PCR-ITS RFLP | D. bruxellensis; | ITS1, TCCGTAGGTGAACCTGCGG | [47] |
| ITS4, TCCTCCGCTTATTGATATGC |
6. Conclusions and Future Research on Kombucha
Kombucha is a refreshing ‘live’ fermented beverage and its popularity is partially derived from this characteristic. While previous studies have shown that phenotypic identification provides useful metabolic characteristics of the microbes in Kombucha, molecular techniques, mostly based on PCR, can provide more accurate, rapid and reliable identification of the microbiological composition of AAB and yeast at different phylogenetic classification levels. The microbial composition of the Kombucha starter culture is diverse and is largely undefined. Further, the kinetic growth pattern of dominant fermenting microbes during fermentation are poorly described. Detailed information on the dominant bacteria and yeast responsible for the fermentation of Kombucha would be useful for better control of commercial fermentation processes during the production of safe, high-quality products. This review has highlighted the need for more information on the systematic identification methods of dominant yeast and AAB in commercial Kombucha beverages. In addition, although the presence of live cultures in Kombucha has been associated with its probiotic potential, this has been scantly reported. Therefore, more studies are needed to fill this gap in the literature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11213456/s1, Table S1: Commonly used media for the isolation, cultivation and differentiation of acetic acid bacteria; Table S2: Commonly used media for the isolation, cultivation and differentiation of yeast from food products.
Author Contributions
Conceptualisation, B.W., A.N.M., writing—original draft preparation, B.W.; review and editing, B.W., K.R.-M., X.-X.Z. and A.N.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received funding from Massey University through College of Sciences REaDI Funding #SFA009.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dufresne C., Farnworth E. Tea, Kombucha, and health: A review. Food Res. Int. 2000;33:409–421. doi: 10.1016/S0963-9969(00)00067-3. [DOI] [Google Scholar]
- 2.Jayabalan R., Malbasa R.V., Loncar E.S., Vitas J.S., Sathishkumar M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food. Sci. Food Saf. 2014;13:538–550. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
- 3.Greenwalt C.J., Steinkraus K.H., Ledford R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000;63:976–981. doi: 10.4315/0362-028X-63.7.976. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V., Joshi V. Kombucha: Technology, microbiology, production, composition and therapeutic value. Int. J. Food Ferment. Technol. 2016;6:13–24. doi: 10.5958/2277-9396.2016.00022.2. [DOI] [Google Scholar]
- 5.Kim J., Adhikari K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages. 2020;6:15. doi: 10.3390/beverages6010015. [DOI] [Google Scholar]
- 6.Marsh A.J., O’Sullivan O., Hill C., Ross R.P., Cotter P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbial. 2014;38:171–178. doi: 10.1016/j.fm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Coelho R.M.D., de Almeida A.L., do Amaral R.Q.G., da Mota R.N., de Sousa P.H.M. Kombucha. Int. J. Gastron. Food Sci. 2020;22:100272. doi: 10.1016/j.ijgfs.2020.100272. [DOI] [Google Scholar]
- 8.Vargas B.K., Fabricio M.F., Ayub M.A.Z. Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Biosci. 2021;44:101332. doi: 10.1016/j.fbio.2021.101332. [DOI] [Google Scholar]
- 9.Velićanski A., Cvetković D., Markov S. Characteristics of Kombucha fermentation on medicinal herbs from Lamiaceae family. Rom. Biotechnol. Lett. 2013;18:8034–8042. [Google Scholar]
- 10.Reiss J. Influence of Different Sugars on the Metabolism of the Tea Fungus. Z. Für Lebensm.-Unters. Und-Forsch. 1994;198:258–261. doi: 10.1007/BF01192606. [DOI] [Google Scholar]
- 11.Steinkraus K.H., Shapiro K.B., Hotchkiss J.H., Mortlock R.P. Investigations into the antibiotic activity of tea Fungus/Kombucha beverage. Acta Biotechnol. 1996;16:199–205. doi: 10.1002/abio.370160219. [DOI] [Google Scholar]
- 12.Vijayaraghavan R., Singh M., Rao P.V., Bhattacharya R., Kumar P., Sugendran K., Kumar O., Pant S.C., Singh R. Subacute (90 days) oral toxicity studies of Kombucha tea. [(accessed on 20 September 2022)];Biomed. Environ. Sci. 2000 13:293–299. Available online: https://www.researchgate.net/profile/Pravin-Kumar-23/publication/11985545_Subacute_90_Days_Oral_Toxicity_Studies_of_Kombucha_Tea/links/5719be5608aed8a339e7057f. [PubMed] [Google Scholar]
- 13.Kapp J.M., Sumner W. Kombucha: A systematic review of the empirical evidence of human health benefits. Ann. Epidemiol. 2019;30:66–70. doi: 10.1016/j.annepidem.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Dutta H., Paul S.K. Production and Management of Beverages. Woodhead Publishing; Sawston, UK: 2020. Kombucha drink: Production, quality, and safety aspects; pp. 259–288. [DOI] [Google Scholar]
- 15.Laureys D., Britton S.J., De Clippeleer J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020;78:165–174. doi: 10.1080/03610470.2020.1734150. [DOI] [Google Scholar]
- 16.Villarreal-Soto S.A., Beaufort S., Bouajila J., Souchard J.P., Taillandier P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018;83:580–588. doi: 10.1111/1750-3841.14068. [DOI] [PubMed] [Google Scholar]
- 17.Chakravorty S., Bhattacharya S., Chatzinotas A., Chakraborty W., Bhattacharya D., Gachhui R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016;220:63–72. doi: 10.1016/j.ijfoodmicro.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Markov S.L., Jerinić V.M., Cvetković D.D., Lončar E.S., Malbaša R.V. Kombucha-functional beverage: Composition, characteristics and process of biotransformation. Hem. Ind. 2003;57:456–462. doi: 10.2298/HEMIND0310456S. [DOI] [Google Scholar]
- 19.Sievers M., Lanini C., Weber A., SchulerSchmid U., Teuber M. Microbiology and fermentation balance in a kombucha beverage obtained from a tea fungus fermentation. Syst. Appl. Microbiol. 1996;18:590–594. doi: 10.1016/S0723-2020(11)80420-0. [DOI] [Google Scholar]
- 20.Ray R.C. Fermented Foods. CRC Press; Boca Raton, FL, USA: 2016. Acetic Acid Bacteria: Prospective Applications in Food Biotechnology; pp. 107–121. Part I. [Google Scholar]
- 21.Lynch K.M., Zannini E., Wilkinson S., Daenen L., Arendt E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beveragesCompr. Rev. Food Sci. Food Saf. 2019;18:587–625. doi: 10.1111/1541-4337.12440. [DOI] [PubMed] [Google Scholar]
- 22.Karabiyikli S., Sengun I. Acetic Acid Bacteria. CRC Press; Boca Raton, FL, USA: 2017. Beneficial effects of acetic acid bacteria and their food products; pp. 321–342. [Google Scholar]
- 23.Jarrell J., Cal T., Bennett J. The Kombucha Consortia of Yeasts and Bacteria. Mycologist. 2000;14:166–170. doi: 10.1016/S0269-915X(00)80034-8. [DOI] [Google Scholar]
- 24.Jayabalan R., Malbaśa R.V., Sathishkumar M. Fungal Metabolites. Springer; Cham, Switzerland: 2017. Kombucha Tea: Metabolites; pp. 965–978. [DOI] [Google Scholar]
- 25.Fontana J.D., Franco V.C., De Souza S.J., Lyra I.N., De Souza A.M. Nature of Plant Stimulators in the Production of Acetobacter xylinum (“Tea Fungus”) Biofilm Used in Skin Therapy. Appl. Biochem. Biotechnol. 1991;28:341–351. doi: 10.1007/BF02922613. [DOI] [PubMed] [Google Scholar]
- 26.Jayabalan R., Chen P.-N., Hsieh Y.-S., Prabhakaran K., Pitchai P., Marimuthu S., Thangaraj P., Swaminathan K., Yun S.E. Effect of solvent fractions of kombucha tea on viability and invasiveness of cancer cells—Characterization of dimethyl 2-(2-hydroxy-2-methoxypropylidine) malonate and vitexin. Indian J. Biotechnol. 2011;10:62–75. [Google Scholar]
- 27.Liu C.-H., Hsu W.-H., Lee F.-L., Liao C.-C. The isolation and identification of microbes from a fermented tea beverage, Haipao, and their interactions during Haipao fermentation. Food Microbiol. 1996;13:407–415. doi: 10.1006/fmic.1996.0047. [DOI] [Google Scholar]
- 28.Wang B., Rutherfurd-Markwick K., Zhang X.-X., Mutukumira A.N. Isolation and characterisation of dominant acetic acid bacteria and yeast isolated from Kombucha samples at point of sale in New Zealand. Curr. Res. Food Sci. 2022;5:835–844. doi: 10.1016/j.crfs.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutta D., Gachhui R. Nitrogen-fixing and cellulose-producing Gluconacetobacter kombuchae sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 2007;57:353–357. doi: 10.1099/ijs.0.64638-0. [DOI] [PubMed] [Google Scholar]
- 30.Mukadam T.A., Punjabi K., Deshpande S.D., Vaidya S.P., Chowdhary A.S. Isolation and characterization of bacteria and yeast from Kombucha tea. Int. J. Curr. Microbiol. Appl. Sci. 2016;5:32–41. doi: 10.20546/ijcmas.2016.506.004. [DOI] [Google Scholar]
- 31.Jacek P., da Silva F.A.S., Dourado F., Bielecki S., Gama M. Optimization and characterization of bacterial nanocellulose produced by Komagataeibacter rhaeticus K3. Carbohydr. Polym. Technol. Appl. 2021;2:100022. doi: 10.1016/j.carpta.2020.100022. [DOI] [Google Scholar]
- 32.Trovatti E., Serafim L.S., Freire C.S., Silvestre A.J., Neto C.P. Gluconacetobacter sacchari: An Efficient Bacterial Cellulose Cell-factory. Carbohydr. Polym. 2011;86:1417–1420. doi: 10.1016/j.carbpol.2011.06.046. [DOI] [Google Scholar]
- 33.Yang Z., Zhou F., Ji B., Li B., Luo Y., Yang L., Li T. Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Appl. Biochem. Biotechnol. 2010;160:446–455. doi: 10.1007/s12010-008-8361-6. [DOI] [PubMed] [Google Scholar]
- 34.Dutta D., Gachhui R. Novel nitrogen-fixing Acetobacter nitrogenifigens sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 2006;56:1899–1903. doi: 10.1099/ijs.0.64101-0. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen N.K., Dong N.T., Nguyen H.T., Le P.H. Lactic acid bacteria: Promising supplements for enhancing the biological activities of kombucha. Springerplus. 2015;4:91. doi: 10.1186/s40064-015-0872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diguta C.F., Nitoi G.D., Matei F., Luta G., Cornea C.P. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods. 2020;9:1780. doi: 10.3390/foods9121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Ji B., Wu W., Wang R., Yang Z., Zhang D., Tian W. Hepatoprotective effects of kombucha tea: Identification of functional strains and quantification of functional components. J. Sci. Food Agric. 2014;94:265–272. doi: 10.1002/jsfa.6245. [DOI] [PubMed] [Google Scholar]
- 38.Pei J., Jin W., Abd El-Aty A., Baranenko D.A., Gou X., Zhang H., Geng J., Jiang L., Chen D., Yue T. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control. 2020;110:106923. doi: 10.1016/j.foodcont.2019.106923. [DOI] [Google Scholar]
- 39.Yang J., Lagishetty V., Kurnia P., Henning S.M., Ahdoot A.I., Jacobs J.P. Microbial and chemical profiles of commercial Kombucha products. Nutrients. 2022;14:670. doi: 10.3390/nu14030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogdan M., Justine S., Filofteia D.C., Petruta C.C., Gabriela L., Roxana U.E., Florentina M., Camelia Filofteia D., Călina Petruța C., Gabriela L. Lactic acid bacteria strains isolated from Kombucha with potential probiotic effect. Roman. Biotechnol. Lett. 2018;23:13592–13598. [Google Scholar]
- 41.Deak T., Beuchat L.R. Comparison of the SIM, API 20C, and ID 32C Systems for Identification of Yeasts Isolated from Fruit Juice Concentrates and Beverages. J. Food Prot. 1993;56:585–592. doi: 10.4315/0362-028X-56.7.585. [DOI] [PubMed] [Google Scholar]
- 42.Buzzini P., Lachance M.-A., Yurkov A. Yeasts in Natural Ecosystems: Diversity. Springer; Cham, Switzerland: 2017. [DOI] [Google Scholar]
- 43.Bekatorou A., Psarianos C., Koutinas A.A. Production of food grade yeasts. Food Technol. Biotechnol. 2006;44:407–415. [Google Scholar]
- 44.Fleet G. Yeasts in Food and Beverages. Springer; Berlin/Heidelberg, Germany: 2006. The commercial and community significance of yeasts in food and beverage production; pp. 1–12. [DOI] [Google Scholar]
- 45.Lachance M.-A., Starmer W.T., Rosa C.A., Bowles J.M., Barker J.S.F., Janzen D.H. Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 2001;1:1–8. doi: 10.1016/S1567-1356(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 46.Teoh A.L., Heard G., Cox J. Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 2004;95:119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Matei B., Diguță C.F., Popa O., Cornea C.P., Matei F. Molecular identification of yeast isolated from different kombucha sources. Ann. Univ. Dunarea Jos Galati. Fascicle VI-Food Technol. 2018;42:17–25. [Google Scholar]
- 48.Thomas D., Davenport R. Zygosaccharomyces bailii—A profile of characteristics and spoilage activities. Food Microbiol. 1985;2:157–169. doi: 10.1016/S0740-0020(85)80008-3. [DOI] [Google Scholar]
- 49.Escott C., Del Fresno J.M., Loira I., Morata A., Suárez-Lepe J.A. Zygosaccharomyces rouxii: Control strategies and applications in food and winemaking. Fermentation. 2018;4:69. doi: 10.3390/fermentation4030069. [DOI] [Google Scholar]
- 50.Loira I., Morata A., Palomero F., González C., Suárez-Lepe J.A. Schizosaccharomyces pombe: A promising biotechnology for modulating wine composition. Fermentation. 2018;4:70. doi: 10.3390/fermentation4030070. [DOI] [Google Scholar]
- 51.Vejarano R. Saccharomycodes ludwigii, control and potential uses in winemaking processes. Fermentation. 2018;4:71. doi: 10.3390/fermentation4030071. [DOI] [Google Scholar]
- 52.Choonut A., Saejong M., Sangkharak K. The production of ethanol and hydrogen from pineapple peel by Saccharomyces cerevisiae and Enterobacter aerogenes. Energy Procedia. 2014;52:242–249. doi: 10.1016/j.egypro.2014.07.075. [DOI] [Google Scholar]
- 53.Agnolucci M., Tirelli A., Cocolin L., Toffanin A. Brettanomyces bruxellensis yeasts: Impact on wine and winemaking. World J. Microbiol. Biotechnol. 2017;33:180. doi: 10.1007/s11274-017-2345-z. [DOI] [PubMed] [Google Scholar]
- 54.Mayser P., Fromme S., Leitzmann C., Grunder K. The yeast spectrum of the ‘tea fungus Kombucha’. Mycoses. 1995;38:289–295. doi: 10.1111/j.1439-0507.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 55.Ramadani A., Abulreesh H. Isolation and Identification of Yeast Flora in Local Kombucha Sample: Al Nabtah. Umm Al Qura Univ. J. App. Sci. 2010;2:42–51. [Google Scholar]
- 56.Kozaki M., Kakuo K., Koizumi A. Microorganisms of zoogloeal mats formed in tea decoction. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1972;13:89–96. doi: 10.3358/shokueishi.13.89. [DOI] [Google Scholar]
- 57.Urbahillah A., Jayus J., Nurhayati N. Improving SCOBY starter using co-culture of tapai and bakery yeast. Biodivers. J. Biol. Divers. 2021;22:4617–4624. doi: 10.13057/biodiv/d221055. [DOI] [Google Scholar]
- 58.Bellut K., Krogerus K., Arendt E.K. Lachancea fermentati strains isolated from kombucha: Fundamental insights, and practical application in low alcohol beer brewing. Front. Microbiol. 2020;11:764. doi: 10.3389/fmicb.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurtzman C.P., Robnett C.J., Basehoar-Powers E. Zygosaccharomyces kombuchaensis, a new ascosporogenous yeast from ‘Kombucha tea’. FEMS Yeast Res. 2001;1:133–138. doi: 10.1111/j.1567-1364.2001.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 60.Yamada Y., Yukphan P. Genera and Species in Acetic Acid Bacteria. Int. J. Food Microbiol. 2008;125:15–24. doi: 10.1016/j.ijfoodmicro.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 61.Gomes R.J., Borges M.F., Rosa M.F., Castro-Gomez R.J.H., Spinosa W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018;56:139–151. doi: 10.17113/ftb.56.02.18.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sengun I.Y., Karabiyikli S. Importance of acetic acid bacteria in food industry. Food Control. 2011;22:647–656. doi: 10.1016/j.foodcont.2010.11.008. [DOI] [Google Scholar]
- 63.Sievers M. Family II. Acetobacteraceae. Bergey’s Man. Systmatic Bacteriol. 2005;2:41–50. [Google Scholar]
- 64.Gullo M., Giudici P. Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. Int. J. Food Microbiol. 2008;125:46–53. doi: 10.1016/j.ijfoodmicro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 65.Cleenwerck I., Camu N., Engelbeen K., De Winter T., Vandemeulebroecke K., De Vos P., De Vuyst L. Acetobacter ghanensis sp. nov., a novel acetic acid bacterium isolated from traditional heap fermentations of Ghanaian cocoa beans. Int. J. Syst. Evolut. Microbiol. 2007;57:1647–1652. doi: 10.1099/ijs.0.64840-0. [DOI] [PubMed] [Google Scholar]
- 66.Mamlouk D., Gullo M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013;53:377–384. doi: 10.1007/s12088-013-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carr J.G., Passmore S.M. Methods for identifying acetic acid bacteria. Identif. Methods Microbiol. 1979;14:33–47. [Google Scholar]
- 68.Cleenwerck I., De Wachter M., Gonzalez A., De Vuyst L., De Vos P. Differentiation of species of the family Acetobacteraceae by AFLP DNA fingerprinting: Gluconacetobacter kombuchae is a later heterotypic synonym of Gluconacetobacter hansenii. Int. J. Syst. Evol. Microbiol. 2009;59:1771–1786. doi: 10.1099/ijs.0.005157-0. [DOI] [PubMed] [Google Scholar]
- 69.Wu J.J., Ma Y.K., Zhang F.F., Chen F.S. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012;30:289–297. doi: 10.1016/j.fm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Entani E., Ohmori S., Masai H., Suzuki K.-I. Acetobacter polyoxogenes sp. nov., a new species of an acetic acid bacterium useful for producing vinegar with high acidity. J. Gen. Appl. Microbiol. 1985;31:475–490. doi: 10.2323/jgam.31.475. [DOI] [Google Scholar]
- 71.Hestrin S., Schramm M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954;58:345. doi: 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohmori S., Masai H., Arima K., Beppu T. Isolation and identification of acetic acid bacteria for submerged acetic acid fermentation at high temperature. Agric. Biol. Chem. 1980;44:2901–2906. doi: 10.1080/00021369.1980.10864432. [DOI] [Google Scholar]
- 73.Fugelsang K.C. Wine Microbiology. 2nd ed. Ripol Classic; Moscow, Russia: 2007. [Google Scholar]
- 74.Bartowsky E.J., Henschke P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008;125:60–70. doi: 10.1016/j.ijfoodmicro.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Wiegand C., Klemm D. Influence of protective agents for preservation of Gluconacetobacter xylinus on its cellulose production. Cellulose. 2006;13:485–492. doi: 10.1007/s10570-005-9022-3. [DOI] [Google Scholar]
- 76.Da Silva N., Taniwaki M.H., Junqueira V.C.A., de Arruda Silveira N.F., Okazaki M.M., Gomes R.A.R. Microbiological Examination Methods of Food and Water: A Laboratory Manual. CRC Press; Boca Raton, FL, USA: 2018. p. 25. [Google Scholar]
- 77.Deak T. Handbook of Food Spoilage Yeasts. 2nd ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 204–205. [DOI] [Google Scholar]
- 78.Atlas R.M. Handbook of Microbiological Media. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
- 79.Kurtzman C.P., Fell J.W., Boekhout T., Robert V. The Yeasts. Elsevier; Amsterdam, The Netherlands: 2011. Methods for isolation, phenotypic characterization and maintenance of yeasts; pp. 87–110. [DOI] [Google Scholar]
- 80.Schifferdecker A.J., Dashko S., Ishchuk O.P., Piskur J. The Wine and Beer Yeast Dekkera bruxellensis. Yeast. 2014;31:323–332. doi: 10.1002/yea.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malimas T., Vu H.T.L., Muramatsu Y., Yukphan P., Tanasupawat S. Acetic Acid Bacteria. 1st ed. CRC Press; Boca Raton, FL, USA: 2017. Systematics of acetic acid bacteria; pp. 3–43. [Google Scholar]
- 82.Frateur J. Extrait de La CeUuZe 63. Joseph Van In & Cie; Lierre, Belgium: 1950. Essai Sur La Systematique Des Acetobacters. fascicule 3. [Google Scholar]
- 83.Yamada Y., Okada Y., Kondo K. Isolation and characterization of “polarly flagellated intermediate strains” in acetic acid bacteria. J. Gen. Appl. Microbiol. 1976;22:237–245. doi: 10.2323/jgam.22.237. [DOI] [Google Scholar]
- 84.Saichana N., Matsushita K., Adachi O., Frebort I., Frebortova J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015;33:1260–1271. doi: 10.1016/j.biotechadv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Lin C., Fung D. Conventional and rapid methods for yeast identification. Crit. Rev. Microbiol. 1987;14:273–289. doi: 10.3109/10408418709104441. [DOI] [PubMed] [Google Scholar]
- 86.Boekhout T., Robert V. Yeasts in Food. 1st ed. Elsevier; Amsterdam, The Netherlands: 2003. [Google Scholar]
- 87.Sangeetha J., Thangadurai D. Laboratory Protocols in Fungal Biology. Springer; Berlin/Heidelberg, Germany: 2013. Staining techniques and biochemical methods for the identification of fungi; pp. 237–257. [DOI] [Google Scholar]
- 88.St Germain G., Beauchesne D. Evaluation of the MicroScan rapid yeast identification panel. J. Clin. Microbiol. 1991;29:2296–2299. doi: 10.1128/jcm.29.10.2296-2299.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fricker-Hidalgo H., Lebeau B., Kervroedan P., Faure O., Ambroise-Thomas P., Grillot R. Auxacolor, a new commercial system for yeast identification: Evaluation of 182 strains comparatively with ID 32C. Proc. Ann. Biol. Clin. 1995;53:221–225. [PubMed] [Google Scholar]
- 90.Ramani R., Gromadzki S., Pincus D.H., Salkin I.F., Chaturvedi V. Efficacy of API 20C and ID 32C Systems for Identification of Common and Rare Clinical Yeast Isolates. J. Clin. Microbiol. 1998;36:3396–3398. doi: 10.1128/JCM.36.11.3396-3398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milan E.P., Malheiros E.S., Fischman O., Colombo A.L. Evaluation of the AUXACOLOR system for the identification of clinical yeast isolates. Mycopathologia. 1997;137:153–157. doi: 10.1023/A:1006801616134. [DOI] [PubMed] [Google Scholar]
- 92.Espinel-Ingroff A., Stockman L., Roberts G., Pincus D., Pollack J., Marler J. Comparison of RapID yeast plus system with API 20C system for identification of common, new, and emerging yeast pathogens. J. Clin. Microbiol. 1998;36:883–886. doi: 10.1128/JCM.36.4.883-886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowman P., Ahearn D. Evaluation of the Uni-Yeast-Tek kit for the identification of medically important yeasts. J. Clin. Microbiol. 1975;2:354–358. doi: 10.1128/jcm.2.4.354-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Land G., Vinton E., Adcock G., Hopkins J. Improved auxanographic method for yeast assimilations: A comparison with other approaches. J Clin. Microbiol. 1975;2:206–217. doi: 10.1128/jcm.2.3.206-217.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aubertine C., Rivera M., Rohan S., Larone D. Comparative study of the new colorimetric VITEK 2 yeast identification card versus the older fluorometric card and of CHROMagar Candida as a source medium with the new card. J. Clin. Microbiol. 2006;44:227–228. doi: 10.1128/JCM.44.1.227-228.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abd El-Salam S.S. 16S rRNA gene sequence detection of acetic acid bacteria isolated from tea Kombucha. N. Y. Sci. J. 2012;5:55–61. [Google Scholar]
- 97.Andrés-Barrao C., Barja F., Pérez R.O., Chappuis M.-L., Braito S. Acetic Acid Bacteria. CRC Press; Boca Raton, FL, USA: 2017. Identification Techniques of Acetic Acid Bacteria: Comparison between MALDI-TOF MS and Molecular Biology Techniques; pp. 162–192. [Google Scholar]
- 98.Rasmussen H.B. Gel Electrophoresis-Principles and Basics. InTechopen; Rijeka, Croatia: 2012. Restriction fragment length polymorphism analysis of PCR-amplified fragments (PCR-RFLP) and gel electrophoresis-valuable tool for genotyping and genetic fingerprinting. [DOI] [Google Scholar]
- 99.Kubista M., Andrade J.M., Bengtsson M., Forootan A., Jonák J., Lind K., Sindelka R., Sjöback R., Sjögreen B., Strömbom L. The real-time polymerase chain reaction. Mol. Asp. Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Harrison K., Curtin C. Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms. 2021;9:1060. doi: 10.3390/microorganisms9051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reva O.N., Zaets I.E., Ovcharenko L.P., Kukharenko O.E., Shpylova S.P., Podolich O.V., de Vera J.P., Kozyrovska N.O. Metabarcoding of the kombucha microbial community grown in different microenvironments. AMB Express. 2015;5:124. doi: 10.1186/s13568-015-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coton M., Pawtowski A., Taminiau B., Burgaud G., Deniel F., Coulloumme-Labarthe L., Fall A., Daube G., Coton E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017;93:fix048. doi: 10.1093/femsec/fix048. [DOI] [PubMed] [Google Scholar]
- 103.Savary O., Mounier J., Thierry A., Poirier E., Jourdren J., Maillard M.-B., Penland M., Decamps C., Coton E., Coton M. Tailor-made microbial consortium for Kombucha fermentation: Microbiota-induced biochemical changes and biofilm formation. Food Res. Int. 2021;147:110549. doi: 10.1016/j.foodres.2021.110549. [DOI] [PubMed] [Google Scholar]
- 104.Pradhan S., Prabhakar M.R., Karthika Parvathy K., Dey B., Jayaraman S., Behera B., Paramasivan B. Metagenomic and physicochemical analysis of Kombucha beverage produced from tea waste. J. Food Sci. Technol. 2022;59:1–9. doi: 10.1007/s13197-022-05476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arikan M., Mitchell A.L., Finn R.D., Gurel F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020;85:455–464. doi: 10.1111/1750-3841.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar N.S., Gurusubramanian G. Random amplified polymorphic DNA (RAPD) markers and its applications. Sci. Vis. 2011;11:116–124. [Google Scholar]
- 107.Gaggìa F., Baffoni L., Galiano M., Nielsen D.S., Jakobsen R.R., Castro-Mejía J.L., Bosi S., Truzzi F., Musumeci F., Dinelli G. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients. 2018;11:1. doi: 10.3390/nu11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.González A., Hierro N., Poblet M., Rozes N., Mas A., Guillamón J. Application of molecular methods for the differentiation of acetic acid bacteria in a red wine fermentation. J. Appl. Microbiol. 2004;96:853–860. doi: 10.1111/j.1365-2672.2004.02220.x. [DOI] [PubMed] [Google Scholar]
- 109.La China S., Vero L.D., Anguluri K., Brugnoli M., Mamlouk D., Gullo M. Kombucha tea as a reservoir of cellulose producing bacteria: Assessing diversity among Komagataeibacter isolates. Appl. Sci. 2021;11:1595. doi: 10.3390/app11041595. [DOI] [Google Scholar]
- 110.Semjonovs P., Ruklisha M., Paegle L., Saka M., Treimane R., Skute M., Rozenberga L., Vikele L., Sabovics M., Cleenwerck I. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biotechnol. 2017;101:1003–1012. doi: 10.1007/s00253-016-7761-8. [DOI] [PubMed] [Google Scholar]
- 111.Kurtzman C.P., Robnett C.J. Identification and Phylogeny of Ascomycetous Yeasts from Analysis of Nuclear Large Subunit (26S) Ribosomal DNA Partial Sequences. Antonie Van Leeuwenhoek. 1998;73:331–371. doi: 10.1023/A:1001761008817. [DOI] [PubMed] [Google Scholar]
- 112.Pincus D., Orenga S., Chatellier S. Yeast identification—Past, present, and future methods. Med. Mycol. 2007;45:97–121. doi: 10.1080/13693780601059936. [DOI] [PubMed] [Google Scholar]
- 113.Mackay I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004;10:190–212. doi: 10.1111/j.1198-743X.2004.00722.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.