Abstract
Simple Summary
Proton pump inhibitors are commonly prescribed medications for gastrointestinal disorders, which bring gastric acid down to normal levels. However, the effects of PPI on pancreatic risk remain unclear. Therefore, we conducted a systematic review and meta-analysis to investigate the association between PPI use and pancreatic cancer. The overall combined estimate suggested that PPI therapy was significantly associated with an increased risk of pancreatic cancer (RRadj. 1.63, 95%CI: 1.19–2.22, p = 0.002). However, this effect might be biased due to users’ definitions, exposure periods, and other confounding factors. Large epidemiological studies with controlled bias are therefore warranted to confirm or refute the association found in this study. Considering the possible carcinogenic effect of PPI, physicians should be vigilant when prescribing high-dose or long-term PPI.
Abstract
Previous epidemiological studies have shown that proton pump inhibitor (PPI) may modify the risk of pancreatic cancer. We conducted an updated systematic review and meta-analysis of observational studies assessing the effect of PPI on pancreatic cancer. PubMed, Embase, Scopus, and Web of Science were searched for studies published between 1 January 2000, and 1 May 2022. We only included studies that assessed exposure to PPI, reported pancreatic cancer outcomes, and provided effect sizes (hazard ratio or odds ratio) with 95% confidence intervals (CIs). We calculated an adjusted pooled risk ratio (RR) with 95%CIs using the random-effects model. Eleven studies (eight case–control and three cohorts) that reported 51,629 cases of pancreatic cancer were included. PPI was significantly associated with a 63% increased risk of pancreatic cancer (RRadj. 1.63, 95%CI: 1.19–2.22, p = 0.002). Subgroup analysis showed that the pooled RR for rabeprazole and lansoprazole was 4.08 (95%CI: 0.61–26.92) and 2.25 (95%CI: 0.83–6.07), respectively. Moreover, the risk of pancreatic cancer was established for both the Asian (RRadj. 1.37, 95%CI: 0.98–1.81) and Western populations (RRadj.2.76, 95%CI: 0.79–9.56). The findings of this updated meta-analysis demonstrate that the use of PPI was associated with an increased risk of pancreatic cancer. Future studies are needed to improve the quality of evidence through better verification of PPI status (e.g., patient selection, duration, and dosages), adjusting for possible confounders, and ensuring long-term follow-up.
Keywords: proton pump inhibitor, pancreatic cancer, omeprazole, meta-analysis
1. Introduction
With an annual incidence of approximately 0.5 million, pancreatic cancer is the 12th most common cancer and the seventh most frequent cause of cancer death with >0.43 million deaths annually [1]. The age-standardized incidence and mortality rate of pancreatic cancer is highest in Europe, followed by North America and Oceania [2,3]. The 5-year survival rate of pancreatic cancer varies in different counties; unfortunately, it is still less than 12% [4,5]. Early detection of pancreatic cancer and proper treatments may improve the outcomes. Numerous studies have extensively investigated the etiology of pancreatic cancer and identified several modifiable (e.g., smoking, alcohol consumption, and obesity) and non-modifiable risk factors (e.g., age, race and ethnicity, family history and genetics) [6,7,8]. The current strategy to minimize the risk of pancreatic cancer includes changing behavior, screening high-risk patients, and identifying cancer-inducing agents.
Proton-pump inhibitors (PPIs) are first-line medications in the clinical practice for the management of acid-related disorders. Since PPIs are often considered safe over-the-counter medications; therefore, less attention is paid by healthcare providers. Nowadays, adverse effects of long-term use of PPIs are gaining increasing attention, especially in the risk of gastric [9], colorectal [10], and oesophageal cancer [11]. Epidemiological studies also highlighted the association between PPIs and pancreatic cancer risk, but there was a discrepancy among the finding. Peng et al. [12] conducted a study regarding the relationship between pancreatic cancer and PPI users and indicated an increased risk of pancreatic cancer among PPIs users. However, Lassalle et al. found no increased risk of dementia among PPIs users at all [13]. Hence, the association between PPIs and pancreatic cancer remains unclear before re-evaluating the pooling effects.
Therefore, we conducted an updated systematic review and meta-analysis of existing observational studies that evaluated the association between PPI and the risk of developing pancreatic cancer.
2. Methods
We followed the guidance provided by the Cochrane Handbook [14]; thus, our study reports according to the Meta-analysis of Observational Studies in Epidemiology guidelines [15].
Data Sources and Search Strategy: PubMed, Embase, Scopus, and Web of Science were searched for published studies related to PPIs and pancreatic cancer between 1 January 2000, and 1 May 2022. Search terms used included “proton-pump inhibitor(s)” OR “omeprazole” OR “pantoprazole” OR “lansoprazole” OR “esomeprazole” OR “rabeprazole” AND “pancreatic cancer” OR “neoplasm(s)” OR “pancreatic malignancy(ies)”. The titles and abstracts of retrieved studies were screened to exclude irrelevant studies. The full texts of the remaining studies were examined to extract information that evaluated the effects of PPI on pancreatic cancer risk. The reference lists of the retrieved studies were also examined to obtain additional studies.
Inclusion and Exclusion Criteria: We included all observational (cohort or case–control) studies that assessed exposure to PPI and risk of pancreatic cancer. All the observational studies needed to be published in English and provide effect sizes (OR/HR) with 95%CIs. Included studies were also required to provide clear information regarding the patients’ characteristics, inclusion, and exclusion criteria. Studies were excluded if they were reviews, editorials, case-reports, or letters to editors without data description.
Data Extraction: Two authors (TNP and MMI) developed screening guidelines and examined the appropriateness of all included studies for inclusion. The two authors extracted the following information from each study: (i) author’s first and last name, publication year, country of the participants; (ii) study design; (iii) number of participants, age, gender; (iv) inclusion and exclusion criteria, adjusted confounding factors; (v) definition of PPI exposure, long-term PPI use, individual PPI exposure; (vi) effect sizes (OR/HR), and 95%CIs.
Quality Assessment: The same two authors independently examined the quality of all included studies using the Newcastle-Ottawa Scale (NOS) recommended by the Cochrane library [16,17]. The NOS uses three parameters to assess the quality of each study: selection, comparability, and outcome (cohort studies) or exposure (case–control studies). The NOS has a maximum of nine points which are given according to the following criteria: (1) selection: a maximum of four points, (2) comparability: two points, and (3) exposure/outcome: three points. A study which receives nine points is categorized as “high” quality, seven to eight points as “medium” quality, and less than seven as “low” quality. Any discrepancy in this evaluation between the two authors was resolved by reexamination of the original study and discussion with a third author.
Data Analysis: The random-effects model (DerSimonian and Laird) was used to calculate the pooled RR and 95% CI. The heterogeneity between studies was estimated using two different methods. First, we calculated Cochran’s Q statistic for assessing heterogeneity. It tests the null hypothesis that all included studies obtain the same underlying magnitude of effect. The Q statistical test usually has insufficient power to distinguish a moderate degree of heterogeneity [18]. A p-value of <0.05 was considered to indicate significant heterogeneity. Second, we also calculated the I2 statistic to determine the proportion of total variation between studies due to heterogeneity rather than chance. In this case, values of I2 of <25%, 25 ~ <50%, 50 ~ <75%, and >75% were categorized as null, low, moderate, and high heterogeneity, respectively [19,20,21]. We investigated the presence of publication bias using Egger’s regression test (publication bias is considered if p ≤ 0.05). We also used the funnel plot of the logarithm of RRs versus their standard errors. All analyses were performed using the Comprehensive Meta-analysis Software (CMA) version V3 (Biostat Inc, Englewood, NJ, USA). All statistical tests were two-sided, and a p-value < 0.05 was considered to be statistically significant.
3. Results
Search Results: Our primary search selected a total of 482 studies. After reviewing the titles and abstracts of these 482 studies, 464 were excluded as ineligible as they were duplicates, reviews, case-reports, letters, and others which did not meet the prespecified inclusion criteria. The remaining 18 studies went through full-text evaluation. Of these, a further seven studies were excluded for reasons presented in Supplementary Figure S1. Consequently, the remaining 11 studies were included in our meta-analysis [12,13,22,23,24,25,26,27,28,29,30].
Study Characteristics: The characteristics of these 11 studies are presented in Supplementary Table S1. The 11 studies represented eight case–control and three cohort studies involving a total of 1,556,182 participants and 51,629 cases of pancreatic cancer. The 11 studies were published between 2012 and 2021. Eight studies were conducted in Western countries [13,22,23,24,26,27,29,30] (four in Europe, three in North America, and one was a collaboration of multi-centers across Europe, North America, and Australia), and three studies were conducted in Asian populations [12,25,28] (two in Taiwan, one in South Korea).
Quality of Included Studies: Two cohort studies had a NOS score of 9, and the remaining cohort study had a score of 8. For the case–control studies, four out of eight studies (50%) were of low quality (NOS score < 7), with an average NOS score of 7.27.
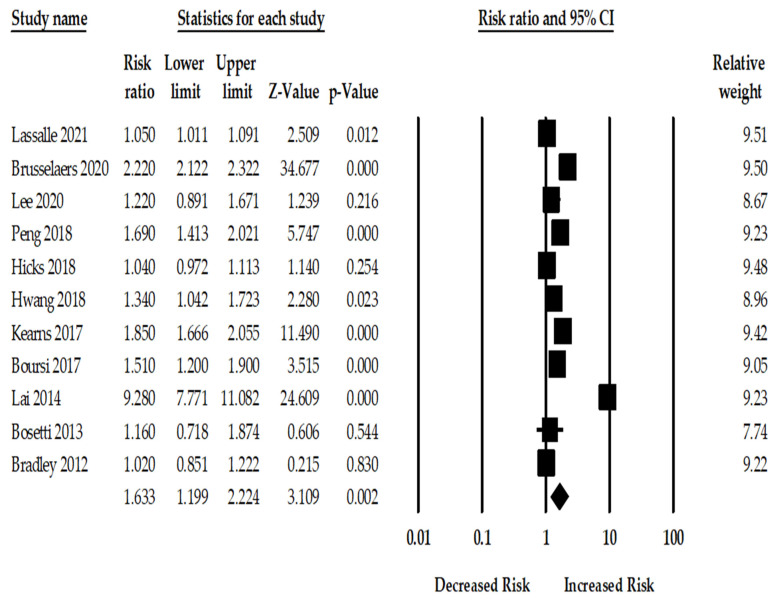
PPI Use and the Risk of Pancreatic Cancer: Among the included 11 studies, the use of PPI was associated with a statistically significant 63% increase in pancreatic cancer (RRadj. 1.63; 95%CI: 1.19–2.22, p = 0.002). There was, however, considerable heterogeneity across the studies (Q = 1172.45, I2 = 99.14, τ2 = 0.26, p < 0.001). Figure 1 shows the risk of pancreatic cancer among PPI users for the 11 included studies.
Figure 1.
Forest plot of association between PPI and pancreatic cancer risk for eleven studies [12,13,22,23,24,25,26,27,28,29,30].
Subgroup Analysis: We conducted subgroup analyses of the included 11 studies based on study design, location, adjusted factors, number of participants, study quality, and individual PPIs use (Table 1). The adjusted pooled analysis of the eight case–control studies also resulted in a significant association between PPI use and the risk of pancreatic cancer (RRadj. 1.62, 95%CI: 1.12–2.34, p = 0.01, Q = 656.94, I2 = 98.73%). The overall pooled analysis of the three cohort studies demonstrated a significant positive association with pancreatic cancer (RRadj. 1.67, 95%CI: 1.17–2.39, p = 0.004, Q = 24.57, I2 = 91.86%). However, the overall pooled analysis of individual PPI use showed a non-significant association with pancreatic cancer.
Table 1.
Subgroup analysis of all studies.
| Subgroup | No. of Study | Effect Size | 95% CI | p-Value | I 2 | Q-Value | p-Value | τ2 |
|---|---|---|---|---|---|---|---|---|
| All | 11 | 1.63 | 1.19–2.22 | 0.002 | 99.14 | 1172.45 | <0.001 | 0.26 |
| Study design | ||||||||
| Case-control | 8 | 1.62 | 1.12–2.34 | 0.01 | 98.73 | 656.94 | <0.001 | 0.27 |
| Cohort | 3 | 1.67 | 1.17–2.39 | 0.004 | 91.86 | 24.57 | <0.001 | 0.08 |
| Region | ||||||||
| Western | 8 | 1.37 | 0.98–1.81 | 0.06 | 99.04 | 734.89 | <0.001 | 0.17 |
| Asian | 3 | 2.76 | 0.79–9.56 | 0.10 | 99.14 | 233.21 | <0.001 | 1.19 |
| Methodological quality | ||||||||
| High | 3 | 1.26 | 0.97–1.62 | 0.07 | 84.13 | 12.60 | 0.002 | 0.04 |
| Moderate | 4 | 1.30 | 0.78–2.17 | 0.30 | 99.19 | 373.68 | <0.001 | 0.26 |
| Low | 4 | 2.43 | 1.03–5.73 | 0.04 | 98.88 | 269.71 | <0.001 | 0.74 |
| Sample size | ||||||||
| ≤10,000 | 5 | 1.87 | 0.73–4.80 | 0.18 | 98.85 | 350.02 | <0.001 | 1.12 |
| >10,000 | 6 | 1.44 | 1.02–2.04 | 0.03 | 99.30 | 722.77 | <0.001 | 0.18 |
| Adjusted for age | ||||||||
| Yes | 11 | 1.63 | 1.19–2.22 | 0.002 | 99.14 | 1172.45 | <0.001 | 0.26 |
| Adjusted for smoking status | ||||||||
| Yes | 3 | 1.45 | 0.83–2.53 | 0.18 | 97.48 | 79.42 | <0.001 | 0.23 |
| No | 8 | 1.70 | 1.17–2.48 | 0.005 | 98.93 | 658.24 | <0.001 | 0.27 |
| Adjusted for chronic pancreatitis | ||||||||
| Yes | 7 | 1.75 | 1.16–2.63 | 0.007 | 99.47 | 1147.15 | <0.001 | 0.29 |
| No | 4 | 1.49 | 1.18–1.87 | 0.001 | 70.63 | 10.21 | 0.01 | 0.03 |
| Adjusted for diabetes | ||||||||
| Yes | 8 | 1.70 | 1.17–2.46 | 0.005 | 99.40 | 1168.32 | <0.001 | 0.27 |
| No | 3 | 1.56 | 1.34–1.82 | <0.001 | 12.47 | 2.28 | 0.31 | 0.002 |
| Adjusted for H. pylori | ||||||||
| Yes | 2 | 1.04 | 1.01–1.08 | 0.006 | 0 | 0.05 | 0.80 | 0 |
| No | 9 | 1.81 | 1.28–2.55 | 0.001 | 97.91 | 384.49 | <0.001 | 0.26 |
| Adjusted for obesity | ||||||||
| Yes | 3 | 2.61 | 0.97–6.97 | 0.05 | 99.67 | 622.32 | <0.001 | 0.75 |
| No | 8 | 1.36 | 0.9–1.89 | 0.06 | 98.16 | 380.61 | <0.001 | 0.20 |
Sensitivity Analysis: Since the overall findings had high heterogeneity (I2 = 99.14%, p < 0.001), we performed a sensitivity analysis. In order to assess the overall impact of a single study on pancreatic cancer risk, a sensitivity analysis was conducted by excluding studies one by one. First, we excluded Bosetti et al.’s [29] study from the primary analysis because the main objective of the study was not directly related to PPI use and pancreatic cancer risk. The overall pooled risk of pancreatic cancer was 1.68 (95%CI:1.21–2.32, p = 0.002, I2 = 99.23%). Second, Boursi et al.’s [27] study was excluded because they assessed the risk of pancreatic cancer for those with new-onset diabetes. The adjusted pooled RR of developing pancreatic cancer among PPI users was 1.70 (95%CI:1.20–2.39, p = 0.002, I2 = 99.31%). However, the sensitivity analysis did not substantially change the pooled effect and the level of heterogeneity.
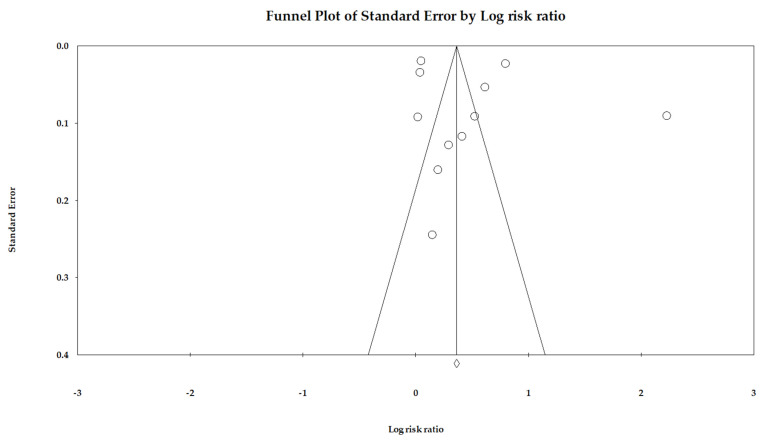
Publication Bias: We used Egger’s regression to detect overall publication bias and also generated Begg’s funnel plots (Figure 2). However, the distribution of included studies was relatively symmetric, indicating very little publication bias (p = 0.55). We utilized Duval and Tweedie’s trim-and-fill methods, and the adjusted RR was 1.43 (95% CI: 1.40–1.47); hence, the impact of this bias was probably close to null.
Figure 2.
Funnel plot.
4. Discussion
This is an updated meta-analysis of eleven observational studies involving more than 1.5 million individuals, which evaluated the effect of PPI use on the risk of pancreatic cancer. Our meta-analysis found a moderately increased risk of pancreatic cancer in people using PPI. However, this association was not statistically significant when stratified by region.
Previous meta-analyses of observational studies on PPI uses and the risk of pancreatic cancer reported that there was a positive link between them [31,32,33], which is consistent with our findings. Laoveeravat et al. [32] included seven studies with a total of 546,199 participants. Compared to patients who did not take PPI, the pooled RR of pancreatic cancer in patients receiving PPI was 1.73 (95%CI: 1.16–2.57). However, they did not conduct any subgroup and sensitivity analyses. Alkhushaym et al. [31] evaluated the effect of PPI on pancreatic cancer with a total of 700,178 participants. The findings of their study showed that PPI use was associated with a 75% increased risk of pancreatic cancer (pooled RR, 1.75 95%CI: 1.12–2.72), with high statistical significance (p < 0.001) but also high heterogeneity (I2 = 99%). This study also lacked subgroup analyses. Furthermore, Hong et al. [33] conducted a meta-analysis to determine the risk of pancreatic cancer among PPI users. They included ten observational studies with 948,782 participants. A positive association between PPI use and pancreatic cancer was observed (pooled RR 1.69, 95%CI: 1.20–2.40, I2 = 98.75%). Their study also did not provide subgroup analyses or any information regarding doses and duration. In contrast, our updated meta-analysis used a higher number of studies and conducted comprehensive subgroup analyses to assess the differential effects of PPI use on pancreatic cancer. Furthermore, our study showed the effect sizes with several confounding factors that were not addressed by previous studies.
The etiology of pancreatic cancer is multifactorial; age, sex, geographical location, genetic and behavioral factors are key contributors [34,35]. Although the exact biological pathway remains unidentified, there are several plausible biological pathways which could explain the link between PPI use and pancreatic cancer. First, gastrin has a dual role which is related to meal-induced gastric acid secretion and as a trophic hormone for epithelial and enterochromaffin cells. However, gastrin and their receptor (CCK-B/gastrin-like receptor) have a shared common link to develop pancreatic cancer [36]. Second, long-term use of PPI induces hypergasterinemia which could also be a potential factor in the development of pancreatic cancer [37,38]. Third, the reduction in gastric acid due to PPI use instigates bacterial growth and secretion of nitorsamides, which can be responsible for increasing pancreatic cell overgrowth [39,40]. Finally, PPI use can impair vitamin B12 absorption [41] because B12 plays a key role in pancreatic cancer as reported in previous studies [42,43].
In the subgroup analysis discerning between study populations, the risk of pancreatic cancer was higher in the Asian than in the Western population. Regional differences are always complicated. Previous evidence demonstrated that many physiological determinants, such as genetic factors [44,45], lifestyle (e.g., eating habits, smoking, alcohol, physical activity) [46] are related to these variations. Additionally, environmental factors (pollution, socioeconomic status, and stress) [8,47] and public health services may also contribute to these differences [4,48]. However, gradual improvement of gastric disorder symptoms, early screening, and diagnosis of pancreatic cancer risk factors can improve the situation. The risk of pancreatic cancer among races cannot be fully explained by the known and suspected risk factors [49]. Our study findings for geographical differences have potential limitations of the small number of available studies. The statistically insignificant association existed both in Asia and the Western population. Thus, caution is needed when interpreting these findings.
Subgroup analyses showed an insignificant association among the studies adjusted with smoking status. Previous evidence highlighted smoking as a recognized risk factor for pancreatic cancer [50,51]. An insignificant association can be explained by a limited number of studies (only three studies were used to pool effect size). The pooled effect size of low-quality studies showed a high risk of pancreatic cancer among PPI users than moderate and high-quality studies. It is because the patient selection and potential risk of bias are often tried to control in high and moderate-quality of studies than low-quality studies. Finally, our study shows that all kinds of PPI are associated with an increased risk of pancreatic cancer, although the relationship between them was statistically insignificant. More studies with control patient selection are needed to measure possible associations.
5. Implications for Practice and Research
Due to limited evidence and lack of high-quality studies on the effect of PPI use on pancreatic cancer, we believe that the following aspects are needed to be investigated in future studies and in the real-world clinical practice.
(i) Study design: All the studies can use the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [52] to follow a standard study design and generate quality evidence. It is well known that randomized control trials cannot always answer all the questions, and they take a long time to conduct. However, observational studies have considerable importance in assessing the benefits and harms of medical interventions. Indeed, observational studies have great potential to identify the unwanted consequences of any drug treatment and are more likely to provide an appropriate direction of what is happening in real-world clinical practice [53]. The credibility of the evidence of the observational studies depends on the strengths and weaknesses in the study design, conduct, and analysis. Every study needs to properly define patient selection such as what type of PPI users they were, how long patients were taking PPI, and what were the dose limits, and what was the follow-up duration. Future studies should also classify patients into several groups, such as continuous, intermittent, and low users. Moreover, all PPI cannot have the same effects; therefore, all the studies should provide individual PPI effect on pancreatic cancer. It would be helpful for physicians and patients to consider PPI for treating or disease management. For example, if omeprazole is safer than other PPI, physician could consider omeprazole for the patients with GERD.
(ii) PPI dose and duration: All studies may provide information regarding the short and long-term users and the risk of pancreatic cancer. For example, physicians often prescribe PPIs for 15 days to treat common symptoms of GERD. However, physicians also consider the long-term use of PPIs for patients with severe erosive esophagitis or Barrett’s esophagus. However, there is no appropriate formula on how to define long-term PPI use [54], and the definitions of long-term use of PPIs always vary between studies. Significant differences in definitions of short and long-term use of PPIs make the situation complex to the pooled natural effect of extended continuous and discontinuous long-term use of PPI [55]. However, a uniform definition is essential in the clinical context. Future studies could calculate the effect of PPI use every six months (e.g., six months, 1-year, 1.5 years, and so forth). Moreover, included studies provided information regarding daily dose and the risk of pancreatic cancer but classification varied from study to study. For example, one study evaluated <30 DDD, 30–180 DDD, and >180 DDD [13]; however, another study assessed the risk of pancreatic cancer among PPI users with <30 DDD, 30–65 DDD, and 65–150 DDD [12]. Furthermore, they did not provide any information on how they calculated DDD. Therefore, it is hard to reach a clinical conclusion about dose and duration when a meta-analysis is conducted using various studies’ information. Therefore, a standard protocol is warranted to summarize the effect of various doses and durations.
(iii) Confounding bias: In the real-world clinical setting, randomized control trials are often considered a “gold standard” method because they control for potential risk bias. However, confounding factors/effects occur in observational studies and change the outcome of interests, either directly or indirectly. These biases can weaken or strengthen or alter the actual association. All included studies adjusted confounding factors except for two studies [27,29]. However, confounding factors differed from study to study. In the future, studies can address all possible confounding factors such as age >60 years, chronic pancreatitis, diabetes, and obesity. Possible confounding factors can also be identified from previous studies. Future studies can measure similar adjustment, matching, or stratification variables in the same way. So, when a meta-analysis will be conducted, it could evaluate the effect of all possible confounding factors and assess the actual effect size of pancreatic cancer with PPI.
Limitations: Our meta-analysis has several limitations. First, all included studies in this meta-analysis were observational studies. Observational studies are often susceptible to uncontrolled biases, even if they are well designed, which may weaken the actual quality of the analysis. Second, two-thirds of the included studies in our meta-analysis came from Western countries, and only three studies were from Asia, even though the prevalence and mortality of pancreatic cancer have been rapidly increasing in Asia [34,49]. Third, we were unable to present the association between PPI and pancreatic cancer based on various duration [12,23] and dosages [12,13,23,24]. Finally, the heterogeneity among the studies was high, and several studies did not provide detailed information regarding confounding factors. Nevertheless, we used the random effect models and showed the effect sizes for different confounding factors.
6. Conclusions
We conducted an updated meta-analysis of the association between PPI use and pancreatic cancer risk in more than 1.5 million participants. The findings of this study show that PPI use was significantly associated with an increased risk of pancreatic cancer. Our robust analyses contribute to a better understanding of PPI use and pancreatic cancer risk. Since PPI is associated with an increased risk of pancreatic cancer, physicians should be cautious when prescribing PPI for GERD patients. More epidemiological and mechanistic studies are warranted to further examine the relationship between PPIs and the risk of pancreatic cancer. Future studies should also focus on adjusting possible confounders and identifying the probable mechanisms underlying this association.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14215357/s1, Figure S1: Flow diagram of the study search and selection for evaluating the risk of pancreatic cancer among PPI users; Table S1: Characteristics of the 11 studies assessing the risk of pancreatic cancer with PPI use.
Author Contributions
Conceptualization, M.M.I. and T.N.P.; Methodology, M.M.I.; Software, M.M.I.; Validation, T.N.P., M.-C.L.; Formal analysis, M.M.I.; Investigation, Y.-C.L.; Resources, M.M.I.; Data curation, M.M.I., M.-C.L.; Writing—original draft preparation, M.M.I., B.A.W.; Writing—review and editing, M.M.I. and T.N.P.; Visualization, M.M.I.; Supervision, Y.-C.L. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
All authors read and agreed to submit.
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Material).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is sponsored in part by the Ministry of Science and Technology (MOST) under grant MOST 111-2321-B-038-004 and MOST 110-2221-E-038-002-MY2, and the Higher Education Sprout Project by the Ministry of Education (MOE DP2-111-21121-01-A-02) in Taiwan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang J., Lok V., Ngai C.H., Zhang L., Yuan J., Lao X.Q., Ng K., Chong C., Zheng Z.-J., Wong M.C. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160:744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P., Sunkara T., Gaduputi V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019;10:10. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel L., Torre A., Ahmedin D. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Glob. Cancer Stat. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Hu J.-X., Zhao C.-F., Chen W.-B., Liu Q.-C., Li Q.-W., Lin Y.-Y., Gao F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021;27:4298. doi: 10.3748/wjg.v27.i27.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pourshams A., Sepanlou S.G., Ikuta K.S., Bisignano C., Safiri S., Roshandel G., Sharif M., Khatibian M., Fitzmaurice C., Nixon M.R. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019;4:934–947. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan C., Kim J., Wang Q., Lee A., Babic A., Amundadottir L., Ardanaz E., Arslan A., Beane-Freeman L., Bracci P. The age-dependent association of risk factors with pancreatic cancer. Ann. Oncol. 2022;33:693–701. doi: 10.1016/j.annonc.2022.03.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalaf N., El-Serag H.B., Abrams H.R., Thrift A.P. Burden of pancreatic cancer: From epidemiology to practice. Clin. Gastroenterol. Hepatol. 2021;19:876–884. doi: 10.1016/j.cgh.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021;18:493–502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Q.-Y., Wu X.-T., Li N., Du L., Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: A meta-analysis of 926 386 participants. Gut. 2019;68:762–764. doi: 10.1136/gutjnl-2018-316416. [DOI] [PubMed] [Google Scholar]
- 10.Ma T., Wu M., Jia S., Yang L. Proton pump inhibitors and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Int. J. Color. Dis. 2020;35:2157–2169. doi: 10.1007/s00384-020-03717-5. [DOI] [PubMed] [Google Scholar]
- 11.Brusselaers N., Engstrand L., Lagergren J. Maintenance proton pump inhibition therapy and risk of oesophageal cancer. Cancer Epidemiol. 2018;53:172–177. doi: 10.1016/j.canep.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y.-C., Lin C.-L., Hsu W.-Y., Lu I.-T., Yeh H.-Z., Chang C.-S., Kao C.-H. Proton pump inhibitor use is associated with risk of pancreatic cancer: A nested case–control study. Dose-Response. 2018;16:1559325818803283. doi: 10.1177/1559325818803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassalle M., Le Tri T., Afchain P., Camus M., Kirchgesner J., Zureik M., Dray-Spira R. Use of Proton Pump Inhibitors and Risk of Pancreatic Cancer: A Nationwide Case–Control Study Based on the French National Health Data System (SNDS) Use of Proton Pump Inhibitors and Risk of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2021:OF1–OF8. doi: 10.1158/1055-9965.EPI-21-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; London, UK: 2011. [(accessed on 2 June 2022)]. Version 5.1.0. [Updated March 2011] Available online: www.cochrane-handbook.org. [Google Scholar]
- 15.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Proceedings of the 3rd Symposium on Systematic Reviews: Beyond the Basics; Oxford, UK. 3–5 July 2000. [Google Scholar]
- 17.Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Volume 2. Ottawa Hospital Research Institute; Ottawa, ON, Canada: 2011. pp. 1–12. [Google Scholar]
- 18.Thompson S.G., Pocock S.J. Can meta-analyses be trusted? Lancet. 1991;338:1127–1130. doi: 10.1016/0140-6736(91)91975-Z. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y., Zhang D., Kang S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2013;137:869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 20.Islam M.M., Iqbal U., Walther B., Atique S., Dubey N.K., Nguyen P.-A., Poly T.N., Masud J.H.B., Li Y.-C.J., Shabbir S.-A. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–191. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 21.Poly T.N., Lin M.-C., Syed-Abdul S., Huang C.-W., Yang H.-C., Li Y.-C.J. Proton Pump Inhibitor Use and Risk of Gastric Cancer: Current Evidence from Epidemiological Studies and Critical Appraisal. Cancers. 2022;14:3052. doi: 10.3390/cancers14133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brusselaers N., Sadr-Azodi O., Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J. Gastroenterol. 2020;55:453–461. doi: 10.1007/s00535-019-01652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.K., Merchant S.A., Schneider J.L., Jensen C.D., Fireman B.H., Quesenberry C.P., Corley D.A. Proton pump inhibitor use and risk of gastric, colorectal, liver, and pancreatic cancers in a community-based population. Off. J. Am. Coll. Gastroenterol. ACG. 2020;115:706–715. doi: 10.14309/ajg.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 24.Hicks B., Friis S., Pottegård A. Use of proton pump inhibitors and risk of pancreatic cancer. Pharmacoepidemiol. Drug Saf. 2018;27:926–930. doi: 10.1002/pds.4576. [DOI] [PubMed] [Google Scholar]
- 25.Hwang I.C., Chang J., Park S.M. Association between proton pump inhibitor use and the risk of pancreatic cancer: A Korean nationwide cohort study. PLoS ONE. 2018;13:e0203918. doi: 10.1371/journal.pone.0203918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearns M.D., Boursi B., Yang Y.-X. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol. 2017;46:80–84. doi: 10.1016/j.canep.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boursi B., Finkelman B., Giantonio B.J., Haynes K., Rustgi A.K., Rhim A.D., Mamtani R., Yang Y.-X. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology. 2017;152:840–850.e3. doi: 10.1053/j.gastro.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai S.-W., Sung F.-C., Lin C.-L., Liao K.-F. Use of proton pump inhibitors correlates with increased risk of pancreatic cancer: A case-control study in Taiwan. Kuwait Med. J. 2014;46:44–48. [Google Scholar]
- 29.Bosetti C., Lucenteforte E., Bracci P., Negri E., Neale R., Risch H., Olson S., Gallinger S., Miller A., Bueno-de-Mesquita H.B. Ulcer, gastric surgery and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case–Control Consortium (PanC4) Ann. Oncol. 2013;24:2903–2910. doi: 10.1093/annonc/mdt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley M., Murray L., Cantwell M., Hughes C. Proton pump inhibitors and histamine-2-receptor antagonists and pancreatic cancer risk: A nested case–control study. Br. J. Cancer. 2012;106:233–239. doi: 10.1038/bjc.2011.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkhushaym N., Almutairi A.R., Althagafi A., Fallatah S.B., Oh M., Martin J.R., Babiker H.M., McBride A., Abraham I. Exposure to proton pump inhibitors and risk of pancreatic cancer: A meta-analysis. Expert Opin. Drug Saf. 2020;19:327–334. doi: 10.1080/14740338.2020.1715939. [DOI] [PubMed] [Google Scholar]
- 32.Laoveeravat P., Thavaraputta S., Vutthikraivit W., Suchartlikitwong S., Mingbunjerdsuk T., Motes A., Nugent K., Rakvit A., Islam E., Islam S. Proton pump inhibitors and histamine-2 receptor antagonists on the risk of pancreatic cancer: A systematic review and meta-analysis. QJM Int. J. Med. 2020;113:100–107. doi: 10.1093/qjmed/hcz234. [DOI] [PubMed] [Google Scholar]
- 33.Hong H.-E., Kim A.-S., Kim M.-R., Ko H.-J., Jung M.K. Does the use of proton pump inhibitors increase the risk of pancreatic cancer? A systematic review and meta-analysis of epidemiologic studies. Cancers. 2020;12:2220. doi: 10.3390/cancers12082220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosetti C., Bertuccio P., Negri E., La Vecchia C., Zeegers M.P., Boffetta P. Pancreatic cancer: Overview of descriptive epidemiology. Mol. Carcinog. 2012;51:3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Z., Zheng R., He Y., Sun X., Wang N., Chen T., Chen W. Risk factors for pancreatic cancer in China: A multicenter case-control study. J. Epidemiol. 2016;26:64–70. doi: 10.2188/jea.JE20140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith J.P., Fantaskey A., Liu G., Zagon I. Identification of gastrin as a growth peptide in human pancreatic cancer. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 37.Triadafilopoulos G., Taddei A., Bechi P., Freschi G., Ringressi M.N., Degli’Innocenti D.R., Castiglione F., Masini E., Majewski M., Wallner G. Barrett’s esophagus: Proton pump inhibitors and chemoprevention I. Ann. New York Acad. Sci. 2011;1232:93–113. doi: 10.1111/j.1749-6632.2011.06047.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith J.P., Fonkoua L.K., Moody T.W. The role of gastrin and CCK receptors in pancreatic cancer and other malignancies. Int. J. Biol. Sci. 2016;12:283. doi: 10.7150/ijbs.14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried M., Siegrist H., Frei R., Froehlich F., Duroux P., Thorens J., Blum A., Bille J., Gonvers J.J., Gyr K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23–26. doi: 10.1136/gut.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorens J., Froehlich F., Schwizer W., Saraga E., Bille J., Gyr K., Duroux P., Nicolet M., Pignatelli B., Blum A. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut. 1996;39:54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam J.R., Schneider J.L., Zhao W., Corley D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 42.Wei D.-H., Mao Q.-Q. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: A meta-analysis. Nutr. J. 2020;19:1–12. doi: 10.1186/s12937-020-00628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Wang X., Sun X., Lu S., Liu S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine. 2018;97:e0114. doi: 10.1097/MD.0000000000010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brotherton L., Welton M., Robb S.W. Racial disparities of pancreatic cancer in Georgia: A county-wide comparison of incidence and mortality across the state, 2000–2011. Cancer Med. 2016;5:100–110. doi: 10.1002/cam4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J., Siegel R., Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. J. Natl. Cancer Inst. 2013;105:1694–1700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 46.Yadav D., Lowenfels A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanini S., Renzi S., Limongi A.R., Bellavite P., Giovinazzo F., Bermano G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur. J. Cancer. 2021;145:53–70. doi: 10.1016/j.ejca.2020.11.040. [DOI] [PubMed] [Google Scholar]
- 48.Yu J., Yang X., He W., Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int. J. Cancer. 2021;149:993–1001. doi: 10.1002/ijc.33617. [DOI] [PubMed] [Google Scholar]
- 49.Pourhoseingholi M.A., Ashtari S., Hajizadeh N., Fazeli Z., Zali M.R. Systematic review of pancreatic cancer epidemiology in Asia-Pacific Region: Major patterns in GLOBACON 2012. Gastroenterol. Hepatol. Bed Bench. 2017;10:245. [PMC free article] [PubMed] [Google Scholar]
- 50.Iodice S., Gandini S., Maisonneuve P., Lowenfels A.B. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbeck’s Arch. Surg. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 51.Lynch S.M., Vrieling A., Lubin J.H., Kraft P., Mendelsohn J.B., Hartge P., Canzian F., Steplowski E., Arslan A.A., Gross M. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandenbroucke J.P., Von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Gac. Sanit. 2009;23:158. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 53.Papanikolaou P.N., Christidi G.D., Ioannidis J.P. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006;174:635–641. doi: 10.1503/cmaj.050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghunath A., O’morain C., McLoughlin R. the long-term use of proton-pump inhibitors. Aliment. Pharmacol. Ther. 2005;22:55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]
- 55.Haastrup P.F., Jarbøl D.E., Thompson W., Hansen J.M., Søndergaard J., Rasmussen S. When does proton pump inhibitor treatment becom, e long term? A scoping review. BMJ Open Gastroenterol. 2021;8:e000563. doi: 10.1136/bmjgast-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Material).