Abstract
This study investigates the dry eye effect after femtosecond laser-assisted cataract surgery (FLACS) and also compares the risk of postoperative dry eye between FLACS and manual cataract surgery (MCS). We searched various databases between 1 January 2000 and 15 October 2022 and included peer-reviewed clinical studies in our review. Dry eye parameters were extracted at baseline and postoperative day one, week one, one month, and three months. Parameters included were the ocular surface discomfort index (OSDI), tear secretion (tear meniscus height, Schirmer’s test), microscopic ocular surface damage (fluorescein staining), and tear stability (first and average tear breakup time). Additionally, the differences of each parameter at each time point were compared between FLACS and MCS. In total, six studies of 611 eyes were included. On postoperative day one, increased, pooled standardised mean differences (SMDs) were noted in the OSDI, tear secretion, tear film instability, and microscopic damage. During postoperative week one, dry eye worsened. Fortunately, dry eye achieved resolution afterwards and nearly returned to the baseline level at postoperative three months. When the parameters were compared between FLACS and MCS, those of FLACS had higher severities, but most were not statistically significant. Dry eye impact was approximately the same in FLACS and MCS at postoperative three months.
Keywords: dry eye, femtosecond laser-assisted cataract surgery (FLACS), phacoemulsification, cornea
1. Introduction
Dry eye is a common postoperative complaint from patients who underwent manual cataract surgery (MCS) with conventional phacoemulsification [1,2]. Symptoms include foreign body sensation, pain, blurred vision, ocular discomfort, burning, and dryness. These symptoms negatively affect patients’ satisfaction with surgery, quality of life, and burden public health [3]. After cataract surgery, signs of dry eye include a decreased tear breakup time, decreased corneal sensitivity, and increased ocular surface staining [2,4,5]. The pathogenic factors consist of inflammation, microscopic damage, neurosensory destruction on the ocular surface, tear film instability, and hyperosmolarity [6,7,8].
Since 2010, the femtosecond laser has been used in cataract surgery. Femtosecond laser-assisted cataract surgery (FLACS) provides precise anterior capsulotomy, safe lens fragmentation, and accurate corneal incision. Thus, it uses less ultrasound energy and phacoemulsification time [9], possibly leading to less postoperative inflammation and less dry eye. However, direct contact of the ocular surface with the vacuum and sustained pressure of the suction ring during FLACS may cause hyperaemia and microscopic damage to the ocular surface. In addition, laser procedures in FLACS may potentially affect the tear film [10]. All these reasons may result in dry eye.
Previous studies comparing FLACS and MCS were primarily concerned with the refractory outcome (e.g., visual acuity and spherical equivalent) and complication rate (e.g., anterior capsule tear or posterior capsule rupture) [9,11,12,13,14,15,16]. However, very few studies have investigated post-FLACS dry eye or compared the risk of dry eye between the two surgery groups. Therefore, we conducted this meta-analysis to investigate the impact of FLACS on dry eye and then compared postoperative dry eye after FLACS and MCS.
2. Materials and Methods
2.1. Search Strategy
This study was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. We searched the PubMed, EMBASE, and Cochrane databases for studies published from 1 January 2000 to 15 October 2022, using the keywords ‘femtosecond laser-assisted cataract surgery’ and ‘dry eye’. Studies were screened first by examining the titles and abstracts and then scrutinising full texts. Bibliographies were also manually searched for the relevant literature.
2.2. Inclusion and Exclusion Criteria
Only peer-reviewed journal articles were included. They should be original, prospective, or randomised control clinical studies investigating dry eye presentation after FLACS. Reviews, meta-analyses, or conference abstracts were excluded because of repeated data. Two researchers (W.-C. Chen and Y.-Y. Chen) independently assessed the articles. A third researcher (M.-C. Hung) intervened if consensus was not reached.
Evaluation of the quality of included articles was performed independently by two researchers (W.-C. Chen and Y.-Y. Chen) using ROBINS-I risk of bias assessment tool. A third researcher (M.-C. Hung) reassessed and made the final decision if discrepancies occurred. ROBINS-I assesses the risk of bias in 7 domains, including confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Each domain contains a set of questions (criteria). The risk of bias judgement of each domain was categorised into ‘Low risk’, ‘Moderate risk’, ‘Serious risk’, and ‘Critical risk’ of bias. Then, the overall risk of bias was judged according to the assessment of each domain.
2.3. Data Extraction
The following data were tracked from each included article: the first author, year of publication, and number/age/gender of participants. We also recorded the baseline (preoperative) and postoperative parameters regarding dry eye with: the ocular surface disease index (OSDI), tear meniscus height, Schirmer’s test, fluorescein staining, first tear breakup time, and average tear breakup time.
2.4. Definitions of Parameters
The OSDI was adopted to evaluate dry eye symptoms. The questionnaire included 12 questions about eye discomfort, visual function, and environmental triggers. A higher OSDI implies more severe dry eye [17]. Tear meniscus height was assessed via corneal topography in order to measure the height of the inferior tear meniscus [18]. A lower tear meniscus height implies a sign of dry eye. Schirmer’s test, also an index of tear secretion, was performed with sterile strips inserted at the lateral third of the lower eyelid margin [19]. The strips were removed five minutes later and the amount of wetting of the paper strips was measured. A lower Schirmer score suggests the diagnosis of dry eye. Fluorescein staining was applied to assess ocular surface damage [20]. Topical fluorescein readily enters and stains the corneal stroma where the epithelium is absent or when the epithelial cells have lost intercellular junctions. A higher score of fluorescein staining is a sign of dry eye. Tear film breakup time is a clinical evaluation of evaporative dry eye disease. Further, it is performed by instilling topical fluorescein into the eyes [21]. The number of seconds that elapsed between the last blink and the appearance of the first dry spot in the tear film was recorded as the first tear breakup time. Similarly, the average tear breakup time was recorded. A higher tear breakup time indicates tear film instability.
2.5. Statistical Analysis
Meta-analysis was performed using the Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ, USA). First, we calculated the standardised mean differences (SMDs) of each index between the post-FLACS time points and baseline. The SMD from each study was computed by dividing the mean difference between each time point and baseline by the standard deviation in order to ensure that the difference was on the same scale. Then, the SMDs were pooled to derive the overall differences between post-FLACS and baseline according to each time point. Second, we compared the differences between the FLACS and MCS groups. The SMDs from each study were pooled to derive the overall values using a similar algorithm. Thus, we could then know which surgery was favoured. The heterogeneity among the studies was determined using the I2 statistic, and an I2 statistic of ≥50% would represent high heterogeneity. Funnel plots and Egger’s test were used to assess publication bias.
3. Results
3.1. Search Results
The PRISMA flow diagram is shown in Figure 1. A total of 67 studies were identified initially. After eliminating duplicated articles (n = 8), we removed non-relevant studies by screening titles and abstracts (n = 52). Then, a full-text review was performed. Conference abstracts were excluded (n = 1). Finally, six studies were enrolled in our meta-analysis [22,23,24,25,26,27].
Figure 1.
Preferred reporting items for systemic reviews and meta-analyses (PRISM) flow diagram for searching and identifying included studies.
3.2. Evaluation of the Quality of Included Studies
Risk of bias for each study assessed by the ROBINS-I tool is presented in Table 1. The overall results showed that one study (Schargus) had low risk of bias, four studies (Yu, Shao, Zhou, and Xu) had moderate risk of bias, and one study (Ju) had severe risk of bias. None of them had critical risk of bias.
Table 1.
Risk of bias assessment for the individual studies included in the meta-analysis.
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | |
|---|---|---|---|---|---|---|---|---|
| Yu [22] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Shao [23] | Low | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Schargus [24] | Low | Low | Low | Low | Low | Low | Low | Low |
| Ju [25] | Moderate | Low | Low | Low | Severe | Moderate | Moderate | Severe |
| Zhou [26] | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Xu [27] | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
D1 = Bias due to confounding; D2 = bias in selection of participants into the study; D3 = bias in classification of interventions; D4 = bias due to deviations from intended interventions; D5 = bias due to missing data; D6 = bias in measurement of outcomes; D7 = bias in selection of the reported result; Low = low risk of bias; Moderate = moderate risk of bias; and Severe = severe risk of bias.
3.3. Characteristics of Included Studies
The characteristics of the studies included in the meta-analysis are presented in Table 2. A total of 678 eyes from 611 patients were enrolled in six studies, with 359 eyes receiving FLACS and 319 eyes receiving MCS. Of the included studies, two were randomised controlled trials and four were prospective cohort studies. Five studies were conducted in China, whereas one study was performed in Germany. The mean age of the participants was 60 to 70 years in most studies.
Table 2.
Characteristics of the studies included in meta-analysis.
| Author | Year | Type | Country | Study Population | Num of Patients | Num of Eyes | Age, Year (Mean ± SD) | Male (n, %) | Cataract Grading | Phaco Time (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yu [22] | 2015 | PCS | China | FLACS | 73 | 73 | 69.0 ± 10.6 | 34 (46.6) | NS 1+ (24.7%), NS 2+ (53.4%), NS 3+ (17.8%), NS 4+ (4.1%) | 35.5 ± 18.4 |
| MCS | 64 | 64 | 71.8 ± 10.1 | 27 (42.2) | NS 1+ (23.4%), NS 2+ (54.7%), NS 3+ (18.8%), NS 4+ (3.1%) | 46.7 ± 26.7 | ||||
| Shao [23] | 2018 | RCT | China | FLACS | 123 | 150 | 65.7 ± 11.8 | 67 (44.7) | NR | NR |
| MCS | 110 | 150 | 69.1 ± 12.6 | 62 (41.3) | NR | NR | ||||
| Schargus [24] | 2020 | RCT | Germany | FLACS | 17 | 17 | 67.4 ± 9.7 | 7 (41.2) | NR | NR |
| MCS | 17 | 17 | 66.0 ± 7.5 | 9 (52.9) | NR | NR | ||||
| Ju [25] | 2019 | PCS | China | FLACS | 38 | 38 | 72.6 ± 8.7 | 16 (42.1) | NR | NR |
| Zhou [26] | 2018 | PCS | China | FLACS | 26 | 26 | 63.2 ± 8.6 | 11 (42.3) | NS 1+ (0%), NS 2+ (38.5%), NS 3+ (61.5%), NS 4+ (0%) | NR |
| MCS | 27 | 27 | 60.6 ± 6.4 | 10 (37.0) | NS 1+ (0%), NS 2+ (40.7%), NS 3+ (59.3%), NS 4+ (0%) | NR | ||||
| Xu [27] | 2019 | PCS | China | FLACS | 55 | 55 | 64.5 ± 7.6 | 25 (45.5) | NS 1+ (20.0%), NS 2+ (36.4%), NS 3+ (30.9%), NS 4+ (12.7%) | 37.7 ± 10.5 |
| MCS | 61 | 61 | 63.2 ± 8.6 | 27 (44.3) | NS 1+ (23.0%), NS 2+ (34.4%), NS 3+ (31.1%), NS 4+ (11.5%) | 48.0 ± 13.6 |
Num= number; PCS= prospective cohort study; RCT= randomised controlled trial randomised control trial; FLACS= femto-second laser cataract surgery; MCS = manual cataract surgery; NS = nuclear sclerotic cataract; NR = not reported; and Phaco = phacoemulsification.
3.4. Outcome Assessment of FLACS Group
Table 3 presents the three parameters (OSDI, tear meniscus height, and Schirmer’s test) at baseline and postoperative time points. Table 4 shows the values of the other three parameters (fluorescein staining, first tear breakup time, and average tear breakup time). The postoperative time points include day one, week one, one month, and three months.
Table 3.
Post-operative changes in OSDI, tear meniscus height, and Schirmer’s test.
| Study | Group | Num of Eyes | OSDI | Tear Meniscus Height | Schirmer’s Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Day | 1 Week | 1 Month | 3 Months | Baseline | 1 Day | 1 Week | 1 Month | 3 Month | Baseline | 1 Day | 1 Week | 1 Month | 3 Months | |||
| Yu [22] | FLACS | 73 | 22.9 ± 4.2 | NR | 11.0 ± 5.5 | 9.1 ± 6.0 | NR | 0.25 ± 0.12 | 0.32 ± 0.19 | 0.27 ± 0.13 | 0.28 ± 0.16 | NR | 9.2 ± 7.0 | 10.3 ± 8.5 | 7.2 ± 6.4 | 7.6 ± 7.2 | NR |
| MCS | 64 | 23.7 ± 5.8 | NR | 8.8 ± 4.9 | 8.0 ± 4.9 | NR | 0.24 ± 0.15 | 0.30 ± 0.17 | 0.25 ± 0.15 | 0.26 ± 0.14 | NR | 9.4 ± 7.4 | 11.0 ± 8.6 | 7.3 ± 6.3 | 8.6 ± 6.9 | NR | |
| Shao [23] | FLACS | 150 | 0.5 ± 0.2 | 5.3 ± 0.5 | 5.0 ± 0.5 | 2.2 ± 0.7 | 0.6 ± 0.3 | 0.37 ± 0.09 | 0.41 ± 0.13 | 0.22 ± 0.07 | 0.32 ± 0.05 | 0.36 ± 0.07 | 10.9 ± 4.1 | 11.3 ± 4.9 | 7.6 ± 3.7 | 8.8 ± 2.6 | 11.2 ± 5.0 |
| MCS | 150 | 0.5 ± 0.4 | 4.0 ± 0.3 | 3.5 ± 0.6 | 1.8 ± 0.7 | 0.5 ± 0.4 | 0.35 ± 0.08 | 0.44 ± 0.11 | 0.20 ± 0.06 | 0.30 ± 0.06 | 0.37 ± 0.06 | 9.4 ± 4.0 | 10.7 ± 3.7 | 7.2 ± 3.3 | 8.0 ± 2.7 | 10.1 ± 5.4 | |
| Schargus [24] | FLACS | 17 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 13.5 ± 7.9 | NR | NR | 12.3 ± 7.9 | 12.0 ± 8.3 |
| MCS | 17 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 12.7 ± 8.2 | NR | NR | 14.9 ± 8.2 | 17.2 ± 8.7 | |
| Ju [25] | FLACS | 38 | 8.4 ± 2.1 | 17.5 ± 5.5 | 16.0 ± 6.7 | 13.5 ± 3.6 | 11.7 ± 3.0 | 0.32 ± 0.11 | 0.41 ± 0.13 | 0.31 ± 0.07 | 0.30 ± 0.09 | 0.29 ± 0.07 | 12.9 ± 3.2 | 13.4 ± 2.6 | 10.6 ±2.3 | 11.4 ± 3.0 | 11.6 ± 2.6 |
| Zhou [26] | FLACS | 26 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 12.8 ± 1.9 | NR | 12.1 ±1.5 | 12.2 ± 2.2 | 12.2 ± 1.7 |
| MCS | 27 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 13.5 ± 2.5 | NR | 11.9 ±1.5 | 11.3 ± 1.4 | 13.0 ± 2.1 | |
| Xu [27] | FLACS | 55 | 24.5 ± 6.5 | NR | 10.4 ± 4.2 | 7.8 ± 4.4 | NR | NR | NR | NR | NR | NR | 9.4 ± 4.8 | NR | 9.4 ± 4.0 | 8.9 ± 3.7 | NR |
| MCS | 61 | 24.8 ± 7.5 | NR | 11.6 ± 5.6 | 8.2 ± 4.9 | NR | NR | NR | NR | NR | NR | 8.7 ± 4.4 | NR | 8.7 ± 3.5 | 8.7 ± 3.3 | NR | |
All data are displayed as mean ± SD. Num = number; OSDI = ocular surface disease index; FLACS = femtosecond laser-assisted cataract surgery; MCS = manual cataract surgery; and NR = not reported.
Table 4.
Post-operative changes in fluorescein staining, and tear breakup time.
| Study | Group | Num of Eyes | Fluorescein Staining | First Tear Breakup Time | Average Tear Breakup Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Day | 1 Week | 1 Month | 3 Months | Baseline | 1 Day | 1 Week | 1 Month | 3 Month | Baseline | 1 Day | 1 Week | 1 Month | 3 Months | |||
| Yu [22] | FLACS | 73 | 0.40 ± 0.52 | 1.46 ± 0.73 | 0.84 ± 0.53 | 0.59 ± 0.55 | NR | 5.5 ± 3.5 | 4.9 ± 3.4 | 4.4 ± 2.8 | 5.6 ± 3.9 | NR | 7.4 ± 4.3 | 7.2 ± 4.2 | 6.5 ± 3.3 | 7.7 ± 4.5 | NR |
| MCS | 64 | 0.36 ± 0.49 | 1.13 ± 0.70 | 0.67 ± 0.65 | 0.39 ± 0.55 | NR | 5.0 ± 2.8 | 4.7 ± 3.5 | 4.6 ± 4.0 | 4.8 ± 3.4 | NR | 6.8 ± 4.3 | 7.1 ± 4.2 | 6.3 ± 4.6 | 7.1 ± 4.6 | NR | |
| Shao [23] | FLACS | 150 | 0.46 ± 0.20 | 2.34 ± 0.31 | 1.88 ± 0.29 | 0.97 ± 0.20 | 0.51 ± 0.69 | 11.8 ± 0.8 | 8.5 ± 1.4 | 8.0 ± 1.4 | 11.7 ± 2.1 | 11.8 ± 2.8 | 12.7 ± 1.1 | 10.0 ± 0.8 | 9.0 ± 0.9 | 12.6 ± 1.7 | 12.9 ± 1.6 |
| MCS | 150 | 0.38 ± 0.22 | 1.22 ± 0.28 | 1.02 ± 0.21 | 0.48 ± 0.14 | 0.46 ± 0.35 | 11.0 ± 1.2 | 8.2 ± 0.0 | 8.1 ± 1.1 | 10.9 ± 1.6 | 11.0 ± 2.1 | 13.2 ± 1.3 | 10.1 ± 0.8 | 9.3 ± 0.9 | 13.2 ± 1.8 | 13.4 ± 1.4 | |
| Schargus [24] | FLACS | 17 | 5.14 ± 0.39 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| MCS | 17 | 5.57 ± 0.17 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ju [25] | FLACS | 38 | 0.89 ± 0.73 | 4.13 ± 1.17 | 3.21 ± 0.91 | 1.34 ± 0.71 | 1.10 ± 0.77 | 10.7 ± 1.2 | 8.1 ± 1.2 | 7.0 ± 1.7 | 10.4 ± 1.5 | 11.1 ± 2.1 | 11.6 ± 1.0 | 9.4 ± 1.0 | 8.5 ± 0.9 | 11.3 ± 0.8 | 11.3 ± 0.9 |
| Zhou [26] | FLACS | 26 | NR | NR | NR | NR | NR | 14.3 ± 2.0 | NR | 10.2 ± 2.5 | 10.7 ± 2.0 | 14.2 ± 1.9 | NR | NR | NR | NR | NR |
| MCS | 27 | NR | NR | NR | NR | NR | 14.4 ± 2.2 | NR | 8.8 ± 2.0 | 9.3 ± 1.9 | 14.3 ± 1.5 | NR | NR | NR | NR | NR | |
| Xu [27] | FLACS | 55 | 0.55 ± 0.72 | NR | 1.38 ± 0.97 | 0.93 ± 1.02 | NR | 6.2 ± 2.0 | NR | 3.6 ± 1.6 | 4.8 ± 2.1 | NR | NR | NR | NR | NR | NR |
| MCS | 61 | 0.51 ± 0.52 | NR | 1.01 ± 0.86 | 0.66 ± 0.89 | NR | 6.0 ± 1.6 | NR | 4.5 ± 2.0 | 4.8 ± 1.9 | NR | NR | NR | NR | NR | NR | |
All data are displayed as mean ± SD. Num = number; FLACS = femtosecond laser-assisted cataract surgery; MCS = manual cataract surgery; and NR = not reported.
The FLACS group pooled analyses comparing the postoperative and baseline values of the six parameters are presented in Figure 2 and Figure 3. The overall SMDs showed increased values at postoperative day one in four of the six parameters (OSDI, tear meniscus height, Schirmer’s test, and fluorescein staining). The increase was statistically significant in tear meniscus height (SMD: 0.456, 95% confidence interval (CI): 0.257 to 0.655), Schirmer’s test (SMD: 0.132, 95% CI: 0.037 to 0.226) and fluorescein staining (SMD: 3.550, 95% CI: 0.354 to 6.747), but was not statistically significant in OSDI (SMD: 5.610, 95% CI: −2.191 to 13.411). Subsequently, tear meniscus height and Schirmer’s test scores decreased to a level lower than baseline, while OSDI and fluorescein staining scores remained higher than baseline. The SMDs of each parameter had a tendency toward zero over time.
Figure 2.
Overall effect of femtosecond laser-assisted cataract surgery (FLACS) on (a) ocular surface disease index (OSDI), (b) tear meniscus height, and (c) Schirmer’s test. The square represents the standardised mean difference of each study. The size of square stands for the relative weight of each study. The lozenge represents the overall standardised mean difference.
Figure 3.
Overall effect of femtosecond laser-assisted cataract surgery (FLACS) on (a) fluorescein staining, (b) first tear breakup time (fBUT), and (c) average tear breakup time (avBUT). The square represents the standardised mean difference of each study. The size of square stands for the relative weight of each study. The lozenge represents the overall standardised mean difference.
Regarding the first and average tear breakup times, both had lower values than baseline from postoperative day one to the first month. The decreased values were only significant in the first tear breakup time at postoperative week one and the first month. The SMDs of the first and average tear breakup times trended towards zero with time. Finally, at postoperative three months, the six parameters were nearly similar to their baseline values except for tear meniscus height, which was significantly lower than at baseline (SMD: −0.172, 95% CI: −0.328 to −0.015).
3.5. Outcome Assessment Comparing FLACS and MCS Group
Figure 4 and Figure 5 compare the postoperative change in six parameters between FLACS and MCS at various postoperative time points. The FLACS group had a higher reduction in tear meniscus height, Schirmer’s test, fBUT, and avBUT. In addition, it had a higher increase in OSDI and fluorescent staining than the MCS group at every postoperative time point. In addition, the FLACS group showed less tear secretion postoperatively. However, most differences between FLACS and MCS were becoming less from postoperative day one to three months. Further, the differences were only significant at the following three time points: Schirmer’s test at postoperative day one (SMD: −0.208, 95% CI: −0.397 to −0.020), one month (SMD: −0.309, 95% CI: −0.534 to −0.085), and first tear breakup time at postoperative week one (SMD: −0.685, 95% CI: −1.058 to −0.311).
Figure 4.
Comparison of (a) ocular surface disease index (OSDI), (b) tear meniscus height, and (c) Schirmer’s test between the femtosecond laser-assisted cataract surgery (FLACS) group and manual cataract surgery (MCS) group. I2 represents heterogeneity. The square represents the standardised mean difference of each study. The size of square stands for the relative weight of each study. The lozenge represents the overall standardised mean difference.
Figure 5.
Comparison of (a) fluorescein staining, (b) first tear breakup time (fBUT), and (c) average tear breakup time (avBUT) between the femtosecond laser-assisted cataract surgery (FLACS) group and manual cataract surgery (MCS) group. I2 represents heterogeneity. The square represents the standardised mean difference of each study. The size of square stands for the relative weight of each study. The lozenge represents the overall standardised mean difference.
3.6. Heterogeneity and Publication Bias
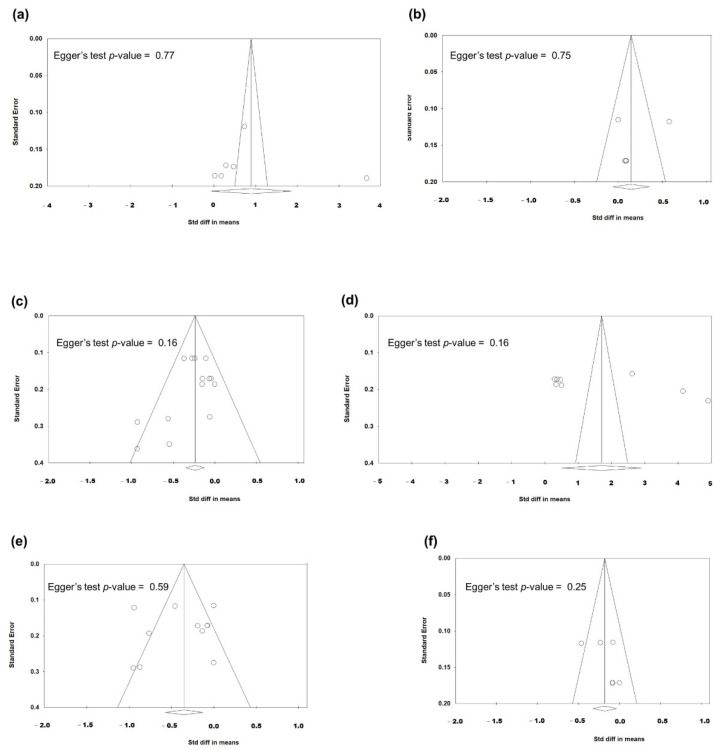
Most analyses showed high between-study heterogeneity when evaluating the SMDs of six parameters (I2 > 75%). Concerning publication bias, Figure 6 demonstrates the funnel plots of studies regarding the post-FLACS effects. Regarding OSDI, tear meniscus height and Schirmer’s test, the p-values of the Egger’s test were 0.31, 0.94, and 0.65, respectively—revealing no significant publication biases. Significant publication biases were noted regarding post-FLACS effects corresponding to fluorescent staining, first tear breakup time, and average breakup time (all Egger’s tests p < 0.01).
Figure 6.
Funnel plots evaluating the publication biases regarding post-FLACS impacts on the six dry eye parameters (a) OSDI, (b) tear meniscus height, (c) Schirmer’s test, (d) fluorescein staining, (e) fBUT, and (f) avBUT. The lozenge stands for overall standardised mean difference.
Funnel plots of the studies comparing postoperative effects between FLACS and MCS are presented in Figure 7. They exhibited no significant publication biases in all six parameters of dry eye symptoms/signs (all Egger’s tests p > 0.1).
Figure 7.
Funnel plots evaluating the publication biases regarding the comparison between FLACS and MCS on the six dry eye parameters (a) OSDI, (b) tear meniscus height, (c) Schirmer’s test, (d) fluorescein staining, (e) fBUT, and (f) avBUT. The lozenge stands for overall standardised mean difference.
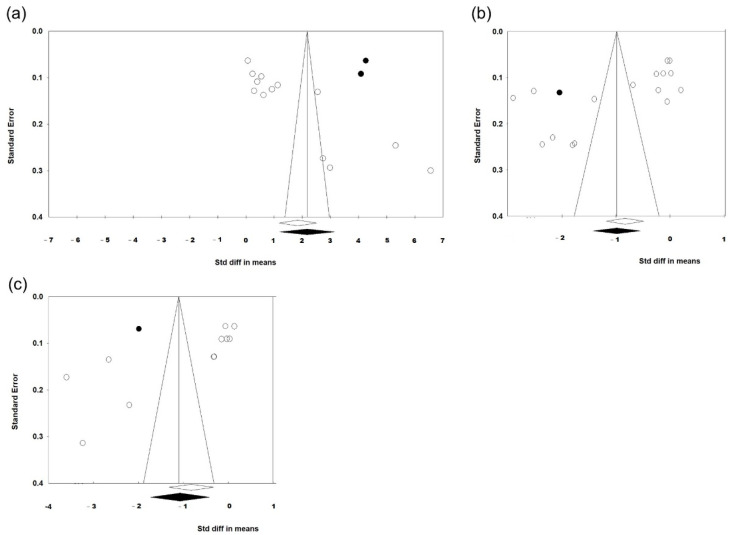
Since the publication bias is statistically significant regarding post-FLACS impacts on fluorescein staining, fBUT, and avBUT, we applied the trim-and-fill method to deal with the publication biases. After trimming the studies that caused a funnel plot’s asymmetry and filling imputed missing studies in the funnel plot based on the bias-corrected overall estimate, the funnel plots were adjusted and are presented in Figure 8. The direction and significance of SMD did not change after adjusting the publication biases. Therefore, our previous statistical analyses regarding SMD were convincible.
Figure 8.
Funnel plots after using trim-and-fill method to adjust the publication biases regarding post-FLACS impacts on the dry eye parameters (a) fluorescein staining, (b) fBUT, and (c) avBUT. The lozenge stands for overall standardised mean difference. The data points for imputed studies are highlighted in black.
4. Discussion
This meta-analysis included six studies focusing on dry eye after FLACS. Six parameters (OSDI, tear meniscus height, Schirmer’s test, fluorescein staining, first breakup time, and average breakup time) were used to evaluate dry eye symptoms/signs, which were also compared between FLACS and MCS groups. On postoperative day one, eyes receiving FLACS had transiently increased dry eye symptoms (OSDI) and tear secretion (tear meniscus height and Schirmer’s test) but then decreased. Microscopic ocular surface damage (fluorescein staining) was significantly increased on postoperative day one and week one but improved after one month. Tear film instability (first breakup time and average breakup time) lasted for one month after surgery and then returned to the baseline level. Three months after surgery, only tear meniscus height was significantly decreased, while all the other parameters were similar to baseline. Compared with MCS, FLACS had a greater tendency towards dry eye in the early postoperative stage. However, the dry eye symptoms/signs between FLACS and MCS showed no significant differences three months after surgery.
This study is the first meta-analysis to compare the impact on postoperative dry eye between FLACS and MCS, to the best of our knowledge. In our study, a transient increase in tear secretion on postoperative day one may be related to surgical-induced pain. One possible explanation for the tear film instability presenting itself immediately after surgery is inflammation. Wound epithelial cells secrete inflammatory factors that accumulate in tears. The bandage of the eye decreases the tear removal rate and aggravates the inflammatory reaction, hyperosmolarity in tears, and subjective discomfort.
Regarding microscopic ocular surface damage, multiple reasons are responsible, including preoperative instillation of povidone-iodine and local anaesthesia [28,29], intraoperative irrigation, and light exposure [30]. Dry eye symptoms improved, but signs were worse at postoperative week one, implying more cytokines were released from the wound in order to induce inflammation. In addition, our study found that FLACS had a more severe effect on dry eye than MCS. This effect may be due to the suction ring in FLACS, injuring the limbal stem cells, conjunctival epithelium, and goblet cells. It is similar to the dry eye mechanism after laser-assisted in situ keratomileusis [31]. In addition, the extra laser procedure in FLACS leads to prolonged light exposure, thereby deteriorating tear film stability.
Fortunately, in our study, the symptoms/signs of dry eye immediately following FLACS almost returned to baseline within three months postoperatively. This result might be explained by the anti-inflammatory effects of postoperative eye drops. Previous studies have revealed that neuroregeneration occurs 25 days postoperatively [32], supporting our finding that postoperative dry eye tends to improve. Furthermore, the differences in dry eye parameters between FLACS and MCS mainly have no significant difference and have a decreasing trend. However, Yu et al. have found that FLACS causes more ocular surface damage than MCS in patients with pre-existing dry eye [22]. Therefore, preoperative screening and postoperative treatment for dry eye should be performed meticulously for those receiving FLACS with a pre-existing unhealthy ocular surface.
The main limitation of our meta-analysis is the heterogeneity among the included studies. The between-study variations may arise from differences in surgical machines, study protocols, inclusion criteria, and perioperative use of topical medication. Five of the six enrolled studies used the LenSx femtosecond laser system (Alcon Laboratories, Fort Worth, TX, USA). Only Schargus et al. used the CATALYS laser system (Johnson and Johnson, New Brunswick, NJ, USA). Different docking devices used in the laser platforms may cause different effects on the ocular surface [24,33]. Another limitation is that most included studies have a non-randomised design, increasing bias.
Moreover, the parameters used in our meta-analysis (OSDI, tear meniscus height, Schirmer’s test, fluorescein staining, and tear breakup time) are not objective enough and are prone to observers’ errors. Previous studies have suggested that tear film osmolarity and matrix metalloproteinase levels are more reliable dry eye tests and correlate well with dry eye severity [20,34,35]. In addition, meibomian gland dysfunction, lipid layer thickness, inflammatory levels, and goblet cell densities also play an important role in dry eye [36,37]. These parameters should be assessed in further studies. Still another limitation is that we cannot perform subgroup analyses according to cataract grading or phacoemulsification time, which are relevant with post-operative dry eye. We have extracted data of cataract grading from three studies (Yu, Zhou, and Xu) and phacoemulsification time from two studies (Yu and Xu). However, the information was presented as overall proportion or mean, without mentioning the individual dry eye symptoms/signs corresponding to each category of cataract grading or phacoemulsification time. The lack of details and the too few study numbers makes subgroup analyses infeasible.
The strength of our study is that our results provide an evaluation of dry eye symptoms/signs following FLACS and include comparisons with those following MCS. Therefore, we could have a better understanding of postoperative dry eye risk. More comprehensive studies will need to be conducted, thereby supplying evidence for further meta-analyses.
5. Conclusions
In conclusion, both FLACS and MCS can induce dry eye. The adverse effects of FLACS on the ocular surface are more severe in FLACS than in MCS. Fortunately, these effects are transient and are resolved within three months after surgery. Cataract surgeons should select FLACS candidates carefully and adopt preoperative evaluation and postoperative therapy for dry eye. Further studies are warranted to verify and understand the post-FLACS dry eye mechanism.
Acknowledgments
We would like to acknowledge Taichung Veterans General Hospital for their database resources.
Author Contributions
Conceptualisation, Y.-Y.C.; methodology, Y.-Y.C.; validation, W.-T.C., Y.-Y.C. and M.-C.H.; formal analysis, W.-T.C., Y.-Y.C. and M.-C.H.; investigation, W.-T.C. and Y.-Y.C.; resources, Y.-Y.C.; writing—original draft preparation, W.-T.C.; writing—review and editing, Y.-Y.C.; visualisation, Y.-Y.C. and M.-C.H.; funding acquisition, Y.-Y.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This is a meta-analysis study. The Taichung Veterans General Hospital Research Ethics Committee have confirmed that no ethical approval is required.
Informed Consent Statement
This is a meta-analysis study. The informed consent is not applicable.
Data Availability Statement
The data from this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, nor in the collection, analyses, or interpretation of data. Neither did they have a role in the writing of the manuscript, nor in the decision to publish the results.
Funding Statement
This work was supported by the Taichung Veterans General Hospital (grant number: TCVGH-1116901B).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zamora M.G., Caballero E.F., Maldonado M.J. Short-term changes in ocular surface signs and symptoms after phacoemulsification. Eur. J. Ophthalmol. 2020;30:1301–1307. doi: 10.1177/1120672119896427. [DOI] [PubMed] [Google Scholar]
- 2.Lu Q., Lu Y., Zhu X. Dry eye and phacoemulsification cataract surgery: A systematic review and meta-analysis. Front. Med. 2021;8:649030. doi: 10.3389/fmed.2021.649030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasetsuwan N., Satitpitakul V., Changul T., Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS ONE. 2013;8:e78657. doi: 10.1371/journal.pone.0078657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X.M., Hu L., Hu J., Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26:S16–S20. doi: 10.1097/ICO.0b013e31812f67ca. [DOI] [PubMed] [Google Scholar]
- 5.Khanal S., Tomlinson A., Esakowitz L., Bhatt P., Jones D., Nabili S., Mukerji S. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2008;28:127–134. doi: 10.1111/j.1475-1313.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- 6.Oh T., Jung Y., Chang D., Kim J., Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn. J. Ophthalmol. 2012;56:113–118. doi: 10.1007/s10384-012-0117-8. [DOI] [PubMed] [Google Scholar]
- 7.Park D.H., Chung J.K., Seo D.R., Lee S.J. Clinical effects and safety of 3% diquafosol ophthalmic solution for patients with dry eye after cataract surgery: A randomized controlled trial. Am. J. Ophthalmol. 2016;163:122–131.e122. doi: 10.1016/j.ajo.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi T., Takahashi H., Kobayashi M., Kunishige T., Arima T., Fujimoto C., Suzuki H., Okuda T., Takahashi H. Changes in tear osmolarity after cataract surgery. J. Nippon. Med. Sch. Nippon. Ika Daigaku Zasshi. 2021;88:204–208. doi: 10.1272/jnms.JNMS.2021_88-405. [DOI] [PubMed] [Google Scholar]
- 9.Kolb C.M., Shajari M., Mathys L., Herrmann E., Petermann K., Mayer W.J., Priglinger S., Kohnen T. Comparison of femtosecond laser-assisted cataract surgery and conventional cataract surgery: A meta-analysis and systematic review. J. Cataract. Refract. Surg. 2020;46:1075–1085. doi: 10.1097/j.jcrs.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 10.Naderi K., Gormley J., O’Brart D. Cataract surgery and dry eye disease: A review. Eur. J. Ophthalmol. 2020;30:840–855. doi: 10.1177/1120672120929958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chee S.P., Yang Y., Wong M.H.Y. Randomized controlled trial comparing femtosecond laser-assisted with conventional phacoemulsification on dense cataracts. Am. J. Ophthalmol. 2021;229:1–7. doi: 10.1016/j.ajo.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Day A.C., Burr J.M., Bennett K., Bunce C., Doré C.J., Rubin G.S., Nanavaty M.A., Balaggan K.S., Wilkins M.R. Femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery (fact): A randomized noninferiority trial. Ophthalmology. 2020;127:1012–1019. doi: 10.1016/j.ophtha.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanojcic N., Roberts H.W., Wagh V.K., Li J.O., Naderi K., O’Brart D.P. A randomised controlled trial comparing femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery: 12-month results. Br. J. Ophthalmol. 2021;105:631–638. doi: 10.1136/bjophthalmol-2020-316311. [DOI] [PubMed] [Google Scholar]
- 14.Popovic M., Campos-Möller X., Schlenker M.B., Ahmed I.I.K. Efficacy and safety of femtosecond laser-assisted cataract surgery compared with manual cataract surgery: A meta-analysis of 14,567 eyes. Ophthalmology. 2016;123:2113–2126. doi: 10.1016/j.ophtha.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Ang R.E.T., Quinto M.M.S., Cruz E.M., Rivera M.C.R., Martinez G.H.A. Comparison of clinical outcomes between femtosecond laser-assisted versus conventional phacoemulsification. Eye Vis. 2018;5:8. doi: 10.1186/s40662-018-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira T.B., Ribeiro F.J., Pinheiro J., Ribeiro P., O’Neill J.G. Comparison of surgically induced astigmatism and morphologic features resulting from femtosecond laser and manual clear corneal incisions for cataract surgery. J. Refract. Surg. 2018;34:322–329. doi: 10.3928/1081597X-20180301-01. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., Reis B.L. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 18.Wei A., Le Q., Hong J., Wang W., Wang F., Xu J. Assessment of lower tear meniscus. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2016;93:1420–1425. doi: 10.1097/OPX.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 19.Stevens S. Schirmer’s test. Community Eye Health. 2011;24:45. [PMC free article] [PubMed] [Google Scholar]
- 20.Wolffsohn J.S., Arita R., Chalmers R., Djalilian A., Dogru M., Dumbleton K., Gupta P.K., Karpecki P., Lazreg S., Pult H., et al. Tfos dews ii diagnostic methodology report. Ocul. Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Hong J., Sun X., Wei A., Cui X., Li Y., Qian T., Wang W., Xu J. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32:716–721. doi: 10.1097/ICO.0b013e3182714425. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y., Hua H., Wu M., Yu Y., Yu W., Lai K., Yao K. Evaluation of dry eye after femtosecond laser-assisted cataract surgery. J. Cataract. Refract. Surg. 2015;41:2614–2623. doi: 10.1016/j.jcrs.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Shao D., Zhu X., Sun W., Cheng P., Chen W., Wang H. Effects of femtosecond laser-assisted cataract surgery on dry eye. Exp. Ther. Med. 2018;16:5073–5078. doi: 10.3892/etm.2018.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schargus M., Ivanova S., Stute G., Dick H.B., Joachim S.C. Comparable effects on tear film parameters after femtosecond laser-assisted and conventional cataract surgery. Int. Ophthalmol. 2020;40:3097–3104. doi: 10.1007/s10792-020-01532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju R.H., Chen Y., Chen H.S., Zhou W.J., Yang W., Lin Z.D., Wu Z.M. Changes in ocular surface status and dry eye symptoms following femtosecond laser-assisted cataract surgery. Int. J. Ophthalmol. 2019;12:1122–1126. doi: 10.18240/ijo.2019.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Zhang H. Changes of tear film and corneal sensativity after femtosecond laser-assisted cataract extraction surgery. Chin. J. Exp. Ophthalmol. 2018;36:222–226. [Google Scholar]
- 27.Xu R., Zhao S. The effect comparison of femtosecond laser-assisted phacoemusification and microincision phacoemusification on ocualr surface. Chin. J. Exp. Ophthalmol. 2018;37:907–913. [Google Scholar]
- 28.Yanai R., Yamada N., Ueda K., Tajiri M., Matsumoto T., Kido K., Nakamura S., Saito F., Nishida T. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: Antimicrobial activity and cytotoxicity for corneal epithelial cells. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2006;29:85–91. doi: 10.1016/j.clae.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Rosenwasser G.O. Complications of topical ocular anesthetics. Int. Ophthalmol. Clin. 1989;29:153–158. doi: 10.1097/00004397-198902930-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hwang H.B., Kim H.S. Phototoxic effects of an operating microscope on the ocular surface and tear film. Cornea. 2014;33:82–90. doi: 10.1097/ICO.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 31.Salomão M.Q., Ambrósio R., Jr., Wilson S.E. Dry eye associated with laser in situ keratomileusis: Mechanical microkeratome versus femtosecond laser. J. Cataract. Refract. Surg. 2009;35:1756–1760. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaheen B.S., Bakir M., Jain S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu B.M., Williams G.P., Tan A., Mehta J.S. A comparison of different operating systems for femtosecond lasers in cataract surgery. J. Ophthalmol. 2015;2015:616478. doi: 10.1155/2015/616478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson A., Khanal S., Ramaesh K., Diaper C., McFadyen A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 35.Chotikavanich S., de Paiva C.S., Li D.Q., Chen J.J., Bian F., Farley W.J., Pflugfelder S.C. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Investig. Ophthalmol. Vis. Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usuba F.S., de Medeiros-Ribeiro A.C., Novaes P., Aikawa N.E., Bonfiglioli K., Santo R.M., Bonfá E., Alves M.R. Dry eye in rheumatoid arthritis patients under tnf-inhibitors: Conjunctival goblet cell as an early ocular biomarker. Sci. Rep. 2020;10:14054. doi: 10.1038/s41598-020-70944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim W.J., Ahn Y.J., Kim M.H., Kim H.S., Kim M.S., Kim E.C. Lipid layer thickness decrease due to meibomian gland dysfunction leads to tear film instability and reflex tear secretion. Ann. Med. 2022;54:893–899. doi: 10.1080/07853890.2022.2056238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are available on request from the corresponding author.