Abstract
Maize (Zea mays L.) is one of the most susceptible crops to pathogenic fungal infections, and in particular to the Fusarium species. Secondary metabolites of Fusarium spp.—mycotoxins are not only phytotoxic, but also harmful to humans and animals. They can cause acute or chronic diseases with various toxic effects. The European Union member states apply standards and legal regulations on the permissible levels of mycotoxins in food and feed. This review summarises the most recent knowledge on the occurrence of toxic secondary metabolites of Fusarium in maize, taking into account modified forms of mycotoxins, the progress in research related to the health effects of consuming food or feed contaminated with mycotoxins, and also the development of biological methods for limiting and/or eliminating the presence of the same in the food chain and in compound feed.
Keywords: mycotoxins, classification, Fusarium, maize, occurrence, biological methods, toxicology, detoxification, modified mycotoxins
1. Introduction
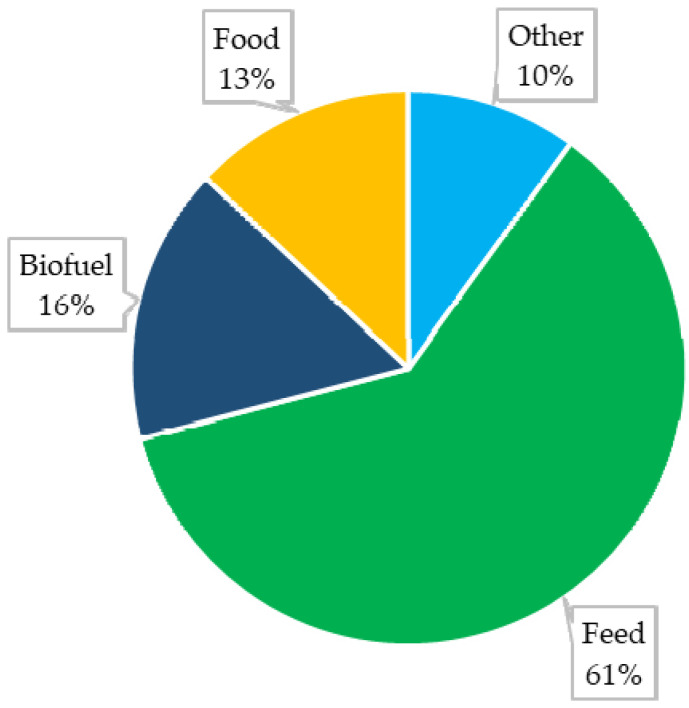
Maize (Zea mays L.) is an important—and in some countries even the most important—source of nutrition for humans and animals [1]. An increasing interest in this raw material has also been observed in various industries (Figure 1). It is estimated that as of 2022 the global production of maize will amount to approximately 1173.3 Mt from a surface area of approximately 200.4 Mha [2]. Despite a high yield potential, maize is characterised by high susceptibility to various diseases, which can lead to decreased yields and reduced kernel quality [3].
Figure 1.
Global use of maize [2].
A decade ago, the Fusarium fungi were named as one of the ten most important pathogenic fungi in the world which affect plants, in particular cereals [4]. This genus includes a large number of species that vary morphologically and phylogenetically [5]. They are epiphytic and endophytic organisms and are part of maize’s pathogenic microbiota [6]. Fusarium can damage the plant by colonising the xylem and developing mycelium in the root, thus causing vascular obstruction and hindering the normal transport of water to the aerial parts of the plant.
The Fusarium fungi are also responsible for the biosynthesis of toxic secondary metabolites referred to as mycotoxins. Those compounds can weaken the infected plant’s immune system and inhibit cell proliferation and protein synthesis [7]. There are three main causes of Fusarium infection and mycotoxin biosynthesis in maize: genetic predispositions of maize varieties, manufacturing practices, and climatic conditions; with the latter appearing to be crucial. Genetic factors contribute to the diversification of vulnerability to fungal pathogens and mycotoxin contamination between various species of crops. One example is Bt maize (genetically modified for the purpose of expression of insecticidal proteins originating from Bacillus thuringiensis), characterised by increased resistance to Fusarium infection as compared to traditional maize [7,8]. Improper agricultural and manufacturing practices increase the risk of kernel infection already during the growing season, as well as during harvest, storage, transport, and processing of the raw material [7]. However, the most crucial factors are climate conditions, since the development of toxicogenic Fusarium species can depend, to a large extent, on the geographical location of the fields, and particularly on the temperature and humidity typical to the region [9].
Climate change increasingly disturbs weather patterns associated with the distribution of precipitation and temperature fluctuations. Extreme weather and reduced annual environmental variability can cause abrupt biotic and abiotic stress in plants. It is believed that this can have a significant impact on the course of Fusarium infection in plants and on the species structure of this pathogen, the host plant, and the host–pathogen interaction [5,10].
Maize is one of the most susceptible crops to pathogenic fungal infections, and in particular to the Fusarium fungi. Mycotoxins produced by those fungi are not only phytotoxic, but also harmful to humans and animals. They can cause acute or chronic diseases and have carcinogenic, teratogenic, immunosuppressive, or estrogenic effects [11]. To ensure food and feed safety, the EU member states apply standards and legal regulations on the permissible levels of those compounds [12].
This article summarises the most recent knowledge on: (i) the incidence of toxic fungal metabolites of Fusarium in maize, (ii) developments in the identification of health-related effects of ingesting food or feed contaminated with mycotoxins, and (iii) biological methods for limiting and/or eliminating the presence of the same in the food chain and in compound feed.
2. Classification
The most common classification of mycotoxins found in cereal grains is based on the close link between these substances and the fungal species responsible for their biosynthesis and, more broadly, the diseases they cause in maize plants. The presence of Fusarium fungi can cause various types of rot, including Fusarium ear rot (FER) and Gibberella ear rot (GER) [13]. The species composition of pathogenic fungi responsible for the development of these diseases in plants can change over the years and depends on geographical factors and agrotechnical conditions [14]. However, the most common pathogens which cause FER are F. verticillioides, F. proliferatum, and occasionally F. subglutinans [15]. The dynamics of plant colonisation by fungi responsible for the development of FER is strongly linked to environmental factors. It should be taken into account that while F. verticillioides is characterised by an exceptional capability to adapt to high temperatures (approx. 30 °C), F. proliferatum and F. subglutinans require lower temperatures to grow [16]. In their recent studies, Simões et al. [17] described F. andiyazi as one of the factors responsible for FER in maize in Europe [17]. On the other hand, GER was most often caused by F. graminearum, F. culmorum, and F. avenaceum. Other species, including F. equiseti, F. poae, F. sporotrichioides, F. acuminatum, F. semitectum, F. solani, and F. temperatum were also isolated from infected maize ears [18,19]. In these cases, the conditions which promoted the development of infection were temperatures of 24–26 °C and humidity exceeding 85% [20]. In recent years, an upward trend was observed in the incidence of certain Fusarium species in maize kernels in various countries. This trend is likely associated with the change in climatic conditions and the agronomic practices used [21]. Crop rotation (wheat-maize) and the presence of after-harvest residue on the soil’s surface contribute to the preservation of fungal pathogens, which increases the risk of FER and GER [22].
It has long been known that F. verticillioides and F. proliferatum biosynthesise fumonisins, which are one of the most common Fusarium toxins found in maize kernels. They were first isolated from the F. verticillioides strain and subsequently identified in F. proliferatum cultures and in several less known Fusarium species. Those compounds belong to a small group of toxins characterised by an aliphatic structure, which have been divided into four series: A, B, C and P, of which the B series is dominant. The structure of B-series fumonisins is based on a 20-carbon backbone, with molecules of 1,2,3-tricarballylic acid bonded at C14 and C15, and an amino group bonded at C2. The location and number of hydroxyl groups within the molecule determine the type of toxin [23,24,25,26,27].
Another group of toxins most often identified in maize kernels are trichothecenes. Trichothecenes are sesquiterpenoid mycotoxins. The common structural feature of these compounds is based on the 12,13-epoxytrichothecenem skeleton. Generally, there are four recognised types of trichothecenes, i.e., A, B, C, and D, whereas C and D are not produced by Fusarium species [28]. The diversity of the trichothecene family results from the different number of hydroxyl groups located at different positions in the basic trichothecene nucleus. Type A trichothecenes include: T-2 toxin (T-2) and HT-2 toxin (HT-2), neosolaniol (NEO), diacetoxyscirpenol (DAS), monoacetoxyscirpenol (MAS), verrucarol (VER), scirpentriol (SCP), and their derivatives. T-2 and HT-2 toxins are produced primarily by F. sporotrichioides, F. acuminatum, and F. poae strains, while F. poae, F. equiseti, F. sambucinum, and F. sporotrichioides strains are responsible for the biosynthesis of DAS and MAS. NEO is particularly characteristic of F. sporotrichioides, F. poae, and F. acuminatum strains. Type B trichothecenes are represented by deoxynivalenol (DON) and its acetylated derivatives, i.e., 3-acetyldeoxynivalenol (3-AcDON) and 15-acetyldeoxynivalenol (15-AcDON), nivalenol (NIV) and fusarenone X (FUS-X). Those toxins are produced primarily by F. culmorum and F. graminearum strains [29].
Zearalenone (ZEN) contains a resorcinol moiety fused to a 14-membered macrocyclic lactone. Similar to type B trichothecenes, ZEN can be biosynthesised by F. culmorum and F. graminearum, which is why it is often detected in combination with other fusariotoxins [30]. The biosynthesis of this toxin by fungal pathogens takes place primarily during the plant’s growth, but can also occur as a result of improper crop storage. The major issue related to ZEN exposure stems from the fact that it is one of the strongest non-steroidal compounds among naturally occurring oestrogens. ZEN opens the group of five primary compounds, the biosynthesis directions of which may vary. In addition to ZEN, this family of compounds also includes α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), α-zearalanol (α-ZAL), β-zearalanol (β-ZAL), and zearalanon (ZAN) [31]. The classification of selected mycotoxins based on the pathogen is presented in Table 1.
Table 1.
Important mycotoxins and Fusarium species responsible for their biosynthesis in maize kernels.
| Mycotoxin | Abbreviation | Molar Mass [g/mol] |
Produced by: | Source |
|---|---|---|---|---|
| Fumonisin B1 | FB1 | 721.83 |
F. dlamini
F. globosum F. nygamai F. oxysporum F. proliferatum F. subglutinans F. temperatum F. thapsinum F. verticillioides |
[26,32,33,34,35,36,37] |
| Fumonisin B2 | FB2 | 705.83 | ||
| Fumonisin B3 | FB3 | 705.83 | ||
| Fumonisin B4 | FB4 | 689.83 | ||
| Fumonisin A1 | FA1 | 763.90 | ||
| Zearalenone | ZEN | 318.36 |
F. cerealis
F. culmorum F. equiseti F. graminearum F. heterosporum F. meridionale F. semitectum |
[26,32,33,36,37,38] |
| HT-2 toxin | HT-2 | 424.48 |
F. acuminatum
F. armeniacum F. langsethiae F. poae F. sporotrichioides |
[26,33,36,37] |
| T-2 toxin | T-2 | 466.52 |
F. acuminatum
F. armeniacum F. equiseti F. langsethiae F. poae F. sambucinum F. sporotrichioides |
[26,33,36,37] |
| Deoxynivalenol | DON | 296.31 |
F. boothii
F. culmorum F. graminearum F. meridionale |
[26,32,33,36,37] |
| Nivalenol | NIV | 312.32 |
F. cerealis
F. cortaderiae F. culmorum F. equiseti F. graminearum F. meridionale F. poae |
[26,32,33,36,37,39] |
| 15-acetyl-deoxynivalenol and 3-acetyldeoxynivalenol |
15- and 3-AcDON | 338.35 |
F. culmorum
F. graminearum F. meridionale |
[32,33,37] |
Previous findings concerning the incidence of mycotoxins and the processes they undergo in host organisms inspired researchers to create an informal classification of those compounds. The first group includes compounds which originate in fungal metabolic pathways and are commonly referred to as “free” mycotoxins [26,40]. A classic example of “free” secondary fungal metabolites found in maize are the compounds whose concentration in food and feed is regulated by EU legislation and standards established for the countries outside the EU [41,42,43,44]. They include fumonisins, DON, ZEN and HT-2, and T-2 toxins (the characteristics of those substances were presented above).
Mycotoxins covered by standards and legal regulations are a small group; in addition, maize kernels may be affected by other compounds produced directly by fungi, such as trichothecenes (NIV, FUS-X, 3- and 15-AcDON, MAS, DAS, NEO), beauvericin (BEA), enniatins (ENNs), ZEN derivatives, and others [45].
Secondary metabolites, produced by the Fusarium fungi, can undergo transformation in a crop. Altered structures of mycotoxins constitute a part of “modified mycotoxins”, which have been presented in detail by Rychlik et al. [40]. Modified mycotoxins are compounds that are normally not detected during routine mycotoxin analysis. They can be biosynthesized directly by fungi or generated by the defense mechanism of a plant infected with a pathogen. Sometimes these compounds can be formed during technological processes used during food production. This classification of mycotoxins is not strict, since certain modified mycotoxins can be biosynthesised both by fungi and in the plants within metabolic pathways (e.g., α- and β-ZOL from ZEN). Furthermore, recent research confirmed that ZEN-14S can be biosynthesised directly by Fusarium, concurrently with ZEN, which is likely related to the stress reaction of the pathogen to the accumulation of ZEN in the substrate [46,47]. Another interesting issue is the presence of so-called “hidden mycotoxins” in maize kernels [48]. They are part of a wider group of modified mycotoxins. It is believed that fumonisins can be physically trapped in the structure of macromolecules by means of supramolecular interactions. Various extraction solvents used during the isolation of toxins from the matrix can differ in terms of the ability to carry out the general extraction of fumonisins from the matrix. Therefore, despite the fact that there are validated methods that utilise various extraction procedures, an analysis can produce different toxin content results. There is an indirect assay method, which consists of calculating the difference between total fumonisin content after hydrolysis in a strongly alkaline environment and the “free” compounds measured using a “traditional” method [48,49]. Recently, it has been demonstrated that ZEN can also create supramolecular interactions with zein found in maize. The affinity between ZEN and zein depended on the environmental conditions. An acidic environment facilitated the production of “hidden” ZEN, while an alkaline environment caused the toxin to be released [50,51,52]. Selected “modified” toxins produced by the Fusarium fungi, which may contaminate maize kernels, are presented in Table 2.
Table 2.
Selected modified toxins produced by Fusarium, characteristic of maize kernels.
| Mycotoxin | Abbreviation | Molar Mass [g/mol] |
Type of Modification | Source |
|---|---|---|---|---|
| Deoxynivalenol 3-β-D -glucoside | DON-3G | 458.46 | plant | [26,33,37] |
| Zearalenone-14-sulphate | ZEN-14S | 415.46 | fungi | [26,37] |
| α-Zearalenol | α-ZOL | 320.39 | fungi or/and plant | [33,37] |
| β-Zearalenol | β-ZOL | 320.39 | fungi or/and plant | [33,37] |
| Hydrolysed fumonisin B1 | HFB1 | 405.61 | fungi | [26,37,48] |
In recent years, particular attention has been paid to so-called “emerging mycotoxins”. This informal term refers to mycotoxins that were identified in the past and are not routinely assayed or covered by standards; however, understanding their biosynthesis, toxicological properties, and their natural occurrence in food has become increasingly important. This group consists of a broad range of secondary metabolites of Fusarium fungi, which vary in terms of their chemical structure [26]. It includes moniliformin (MON), BEA, ENNs, and FUS-X; which often co-occur in maize kernels with free mycotoxins [53].
Their simultaneous presence in maize kernels may be associated with the growth of F. temperatum and F. subglutinans on the plants [14,54]. MON is a sodium or potassium salt of 1-hydroxycyclobut-1-ene-3,4-dione, isolated by Cole et al. [55] from maize kernels infected by F. verticilioides. BEA and ENNs belong to closely related groups of cyclodepsipeptides, produced by numerous Fusarium species (most often F. avenaceum and F. poae), which can have insecticidal, antibiotic, and cytotoxic properties [55]. ENNs is a large group of compounds, consisting of approximately 26 natural analogues [56]. FUS-X is a bicyclic sesterterpene, produced primarily by F. proliferatum, F. subglutinans, and F. temperatum [54]. As the research into mycotoxins progresses (which is firmly linked to the access to commercial references substances), new chemical compounds continue to join the group of candidates for “emerging” mycotoxins. They may include certain trichothecenes (e.g., MAS, DAS, NEO); culmorin (CUL) and its derivatives; as well as other substances, whose presence in maize has been confirmed by various authors [26,36,37]. Selected “emerging” mycotoxins, characteristic of the Fusarium species which infect maize, are presented in Table 3.
Table 3.
Selected “emerging” toxins produced by Fusarium fungi, identified in maize.
| Mycotoxin | Abbreviation | Molar Mass [g/mol] |
Produced by: | Source |
|---|---|---|---|---|
| Beauvericin | BEA | 783.96 |
F. acuminatum
F. anthophilum F. armeniacum F. avenaceum F. dlamini F. equiseti F. globosum F. nygamai F. oxysporum F. proliferatum F. poae F. sambucinum F. semitectum F. sporotrichioides F. subglutianans F. temperatum F. tricinctum F. verticillioides |
[26,33,36,37] |
| Culmorin | CUL | 238.37 |
F. culmorum
F. cerealis F. graminearum F. langsethiae F. poae F. sporotrichioides F. venenatum |
[26,36,37] |
| 15Hydroxyculmorin | 15h-CUL | 254.36 |
F. culmorum
F. graminearum F. poae |
[26,37] |
| 5Hydroxyculmorin | 5h-CUL | 254.36 |
F. culmorum
F. graminearum F. poae |
[26] |
| Monoacetoxyscirpenol | MAS | 324.40 |
F. acuminatum
F. equiseti F. sporotrichioides F. poae |
[26,33,37] |
| Diacetoxyscirpenol | DAS | 366.41 |
F. acuminatum
F. armeniacum F. boothii F. cerealis F. cortaderiae F. culmorum F. equiseti F. graminearum F. meridionale F. sambucinum F. semitectum F. sporotrichoides F. poae |
[26,33,36,37] |
| Neosolaniol | NEO | 382.40 |
F. acuminatum
F. poae F. sambucinum |
[26,33,37] |
| Moniliformin | MON | 120.04 |
F. acuminatum
F. avenaceum F. chlamydosporum F. culmorum F. dlamini F. equiseti F. fusariodes F. oxysporum F. proliferatum F. semitectum F. sporotrichioides F. subglutians F. temperatum F. thapsinum F. tricinctum |
[26,33,34,36,37] |
| Enniatin A | ENN A | 681.90 |
F. acuminatum
F. avenaceum F. chlamydosporum F. culmorum F. moniliforme F. nivale F. oxysporum F. poae F. proliferatum F. roseumF. solani F. sporotrichioides F. tritinctum |
[26,36,37] |
| Enniatin A1 | ENN A1 | 667.90 | ||
| Enniatin B | ENN B | 639.82 | ||
| Enniatin B1 | ENN B1 | 653.85 | ||
| Enniatin B2 | ENN B2 | 625.80 | ||
| Aurofusarin | Au-FU | 570.50 |
F. acuminatum
F. avenaceum F. cerealis F. culmorum F. graminearum F. poae F. sambucinum F. sporotrichiodes F. tricinctum |
[26,36,37] |
| Bikaverin | BKV | 382.3 |
F. antholphilum
F. bulbigenum F. dlamini F. fujikuroi F. moniliforme F. napiformi F. nygamai F. oxysporum F. proliferatum F. solani F. subglutitans F. verticillioides |
[26,37] |
| Butenolide | BUT | 156.14 |
F. cerealis
F. culmorum F. graminearum F. poae F. sporotrichioides F. verticillioides |
[26,36,37] |
| Equisetin | EQ | 373.49 |
F. equiseti
F. semitectum |
[37] |
| Fusaric acid | FA | 179.22 |
F. cerealis
F. oxysporum F. proliferatum F. solani F. sublutinans F. thapsinum F. verticillioides |
[26,36,37] |
| Siccanol | SCN | 402.58 | F. graminearum | [26,37] |
| Fusaproliferin | FUS | 444.60 |
F. fujikuroi
F. globosum F. proliferatum F. subglutinans F. temperatum |
[33,36,37] |
3. Occurrence
3.1. Free and Modified Mycotoxins
Literature data which summarises the incidence of mycotoxins and their modified forms, along with their concentrations in maize kernels, are presented in Table 4. The mycotoxins that were the most often identified in maize kernels were fumonisins, DON, ZEN, and—to a lesser degree—their modified derivatives, which co-occurred with native toxins, generally at significant concentrations. They can increase the toxicity of maize which reaches the consumers in the form of maize products, as well as constitutes a basic raw material for the manufacture of animal feed. The increase in general toxicity may occur directly or indirectly by way of transformation to the native form during digestion in the gastrointestinal system [26].
Table 4.
Natural occurrence of free and modified mycotoxins in maize.
| Mycotoxin | Positive Samples (%) | Min-Max [µg/kg] | Origin | References |
|---|---|---|---|---|
| FB1 | 121/123 (98%) | 12.6–8908 | South Africa | [26] |
| 40/79 (51%) | 68–2453 * | Egypt | [57] | |
| 9/12 (75%) | <LOQ–49 ** | China | [58] | |
| 34/45 (76%) | 59.9–9873 | Albania | [59] | |
| 79/90 (88%) | <LOD–45,145.82 | Michigan (USA) | [60] | |
| 70 (71.4%) | 1080 *** | Spain | [61] | |
| 48/55 (88%) | 101–1838 | West Africa (Togo) | [62] | |
| 55/158 (34.8%) | 60–553 * | Europe (various countries) | [63] **** | |
| FB2 | 112/123 (91%) | 7.9–3383 | South Africa | [26] |
| 14/79 (18%) | 4.7–386 * | Egypt | [57] | |
| 27/45 (60%) | 105–9218 | Albania | [59] | |
| 0/12 (0%) | <LOD | China | [58] | |
| 76/90 (84%) | <LOD–22,538.63 | Michigan (USA) | [60] | |
| 55/98 (56.1%) | 1306 *** | Spain | [61] | |
| 30/55 (55%) | 45–586.4 | West Africa (Togo) | [62] | |
| 46/158 (89.1%) | 20.4–133 * | Europe (various countries) | [63] **** | |
| FB3 | 98/123 (80%) | <LOQ–990 | South Africa | [26] |
| 6/79 (8%) | 16.8–286 * | Egypt | [57] | |
| 72/90 (80%) | <LOD–17,972.72 | Michigan (USA) | [60] | |
| 14/55 (26%) | 43–185.6 | West Africa (Togo) | [62] | |
| FB4 | 101/123 (82%) | <LOQ–1014 | South Africa | [26] |
| 29/30 (96%) | 461–2716 * | Nigeria | [64] | |
| FB1 + FB2 | 148/148 (100%) | 62.4–6 | Brasil | [65] |
| 6274 | ||||
| 34/45 (76%) | 59.9–16,970 | Albania | [59] | |
| FA1 | 66/123 (54%) | <LOQ–51.4 | South Africa | [26] |
| HFB1 ***** | 9/12 (75%) | 144–7226 ** | China | [58] |
| HFB2 ***** | 0/12 (0%) | <LOD | China | [58] |
| ZEN | 41/123 (33%) | <LOQ–146 | South Africa | [26] |
| 5/6 (83%) | <LOD–1071 | Belgium | [46] | |
| 10/79 (13%) | 3.4–184 * | Egypt | [57] | |
| 107/158 (67.7%) | 15.2–1670 * | Europe (various countries) | [63] **** | |
| 2/45 (4.4%) | 218–263 | Albania | [59] | |
| 76/90 (84%) | 0.56–4148.75 | Michigan (USA) | [60] | |
| 24/98 (24.5%) | 110.3 *** | Spain | [61] | |
| 1/55 (2%) | 79–79 | West Africa (Togo) | [62] | |
| 115/120 (96%) | <LOD–3910 | Germany | [66] | |
| 42 (73.6%) | 1.4–444.6 | Brasil | [65] | |
| 13/23 (56.52%) | <LOD–163.58 | China | [52] | |
| ZEN-14Glc | 1/6 (17%) | 274–274 | Belgium | [46] |
| ZEN-14S | 1/6 (17%) | 51–51 | Belgium | [46] |
| α-ZEL | 6/6 (100%) | 22–262 | Belgium | [46] |
| 71/120 (59%) | <LOD–423 | Germany | [66] | |
| β-ZEL | 4/6 (67%) | 12–103 | Belgium | [46] |
| 38/120 (32%) | <LOD–203 | Germany | [66] | |
| DON | 61/123 (50%) | 8.2–1380 | South Africa | [26] |
| 11/150(7.3%) | 270–1980 | Ethiopia | [67] | |
| 6/6 (100%) | 411–5245 | Belgium | [46] | |
| 6/79 (8%) | 311–807 * | Egypt | [57] | |
| 11/45 (24%) | 110–798 | Albania | [59] | |
| 120/120 (100%) | <LOQ–10,972 | Germany | [66] | |
| 107/158 (67.7%) | 303–3060 * | Europe (various countries) | [63] **** | |
| 87/90 (97%) | <LOD–20,475 | Michigan (USA) | [60] | |
| 81/81 (100%) | 44–614 | Northen Italy (Pidemont) | [68] | |
| 63/81 (78%) | <LOD–216 | Northen Italy (Lombardy) | ||
| 72/81 (89%) | <LOD–83 | Northen Italy (Vento) | ||
| 31/98 (31.6%) | 180 *** | Spain | [61] | |
| 33 (58%) | 41.6–1008 | Brasil | [65] | |
| DON-3G | 65/123 (53%) | 2.43–112 | South Africa | [26] |
| 120/120 (100%) | 95–3038 | Germany | [66] | |
| 4/79 (5%) | <LOQ–47.5 * | Egypt | [57] | |
| 8/10 (80%) | 14–121 | China | [69] | |
| 78/90 (87%) | <LOD–6266.49 | Michigan (USA) | [60] | |
| 40/158 (25.5%) | 17.1–129 * | Europe (various countries) | [63] **** | |
| 3-AcDON | 6/6 (100%) | 63–643 | Belgium | [46] |
| 29/90 (32%) | <LOD–63.04 | Michigan (USA) | [60] | |
| 5/98 (5.1%) | 63.9 *** | Spain | [61] | |
| 2/79 (3%) | <LOQ | Egypt | [57] | |
| 108/257 (42%) | 0–1046.8 * | Belgium | [70] | |
| 15-AcDON | 6/6 (100%) | 61–792 | Belgium | [46] |
| 66/90 (73.3%) | <LOD–1787.6 | Michigan (USA) | [60] | |
| 112/257 (43%) | 0–819.3 * | Belgium | [70] | |
| NIV | 14/123 (11%) | 7.7–35.7 | South Africa | [26] |
| 10/30 (33.3%) | 5.1–50.8 * | Nigeria | [64] | |
| 8/79 (10%) | 1.6–142 * | Egypt | [57] | |
| 10/30 (33.3%) | 5.1–50.8 | Nigeria | [64] | |
| T-2 | 1/123 (0.8%) | 148–148 | South Africa | [26] |
| 1/45 (2.2%) | 106–106 * | Albania | [59] | |
| 17/90 (19%) | <LOD–156.65 | Michigan (USA) | [60] | |
| 5/98 (5.1%) | 8.6 *** | Spain | [61] | |
| 6/158 (3.8%) | 2.55–4.08 * | Europe (various countries) | [63] **** | |
| HT-2 toxin | 1/123 (0.8%) | 40.2–40.2 | South Africa | [26] |
| 5/98 (5.1%) | 11.7 *** | Spain | [61] | |
| 4/90 (4%) | <LOD–276.74 | Michigan (USA) | [60] |
In accordance with literature data published in recent years, the incidence of fumonisins in maize was reported as high [26,57,58,59,60,62]. The presence of FB1 in maize kernels, which depended on the origin of the plants, was found in nearly 34% of samples (Table 4). The highest concentrations of FB1 were found in material originating from Michigan (USA) (45,145.82 µg/kg) [60]. The highest concentrations of FB2 and FB3 (22,538.63 and 17,972.72 µg/kg, respectively) were also recorded from this location [60]. In samples from other locations, fumonisin content was lower. Ekwomadu et al. [26] found significant fumonisin contamination (8908, 3383, 990, 1014 µg/kg for FB1, FB2, FB3, and FB4, respectively) in various maize varieties originating from two agricultural regions of South Africa [26]. Furthermore, Oliveira et al. [65] tested 57 samples of maize from Brazil for mycotoxins and all of them contained FB1 and FB2, with a maximum total toxin content of 66,274 µg/kg [65]. A high level of fumonisin contamination in maize is firmly linked to its growing conditions—the warmer and more humid the climate, the greater the likelihood of mycotoxin presence in the kernels. In the context of modified forms of fumonisins, there is little literature on their incidence in maize kernels. Some authors demonstrate that the majority of fumonisins can occur in maize kernels in modified form. Hu et al. [58] confirmed this hypothesis in two types of samples (raw maize kernels and maize products) [58]. That study demonstrated the presence of free fumonisins (FB1 + FB2) in 66% of all examined samples from both groups; and this increased to 86% following the alkaline hydrolysis of the samples (total fumonisin content after hydrolysis). In the case of fresh maize kernels, 75% of samples tested positive (FB1—min–max < LOQ–49 µg/kg); after taking into account free and modified FB1, those values ranged between 78 and 131 µg/kg. FB2 was not found in those samples, either during direct analysis or after hydrolysis. A similar trend was observed in maize kernel samples infected with a highly toxicogenic pathogen. The maximum FB1 and FB2 content in maize kernels was 24,890 and 5506 µg/kg, respectively, and 49,782 and 8417 µg/kg after alkaline hydrolysis of the samples [58]. The presence of modified fumonisins in maize can pose a significant problem in terms of real exposure of humans and animals to this group of compounds; however, there is very little data on the presence of total fumonisins (in free and modified form) in maize kernels. The presence of hidden, hydrolysed and partially hydrolysed fumonisins in naturally contaminated maize kernels was also confirmed in our earlier studies [71].
ZEN is a toxin which is also frequently identified in maize; its presence is determined by the location of the growing area. The maximum content of that toxin in raw maize kernels ranged between 79 and 4148.75 µg/kg [26,57,58,59,60,62,65,66], whereas the maximum content of ZEN in maize kernels was recorded in Michigan (similarly to fumonisins) [60]. The presence of ZEN in maize samples is mainly associated with the development of GER disease in maize plants. Usually, the disease is caused by F. graminearum, but also other related species such as, for example, F. culmorum. The presence of these two species may also be determined by the geographic location of the crop. GER is dominant in areas with moderate temperatures and a relatively higher level of rainfall during the growing season. Other factors influencing the spread of pathogens, and thus increasing the likelihood of contamination of the ZEN maize grain, are plant damage caused by insects and the storage of maize grain with inadequate humidity [36]. Very few scientific publications contain data on the incidence of modified forms of ZEN, i.e., ZEN-14S, ZEN-14G, α- and β-ZOL. The relationships between the occurrence of individual compounds in the maize mill are also not known. Their incidence and concentration levels are usually lower than in the basic analogues. Birr et al. [66] identified α-ZEL in 59% of maize kernel samples (maximum content was 423 µg/kg) and its content depended on the location of the growing areas in Europe [66]. β-ZEL was less common (32% of samples); its maximum content was also lower (203 µg/kg). As for ZEN-14G and ZEN-14S, their maximum concentrations were 274 and 51 µg/kg, respectively [46]. In recent years, “hidden” ZEN forms were also found, similar to the case of fumonisins. Their presence likely results from the supramolecular interactions between zein found in maize kernels and ZEN, and in some cases, it may exceed the content of the basic analogue [52]. Tan et al. [72] found that ZEN in maize kernel samples ranged from <LOD to 163.58 µg/kg, while the hidden form content (calculated as the difference between the total ZEN content after hydrolysis and the free ZEN content) ranged between 0 and 54.53 µg/kg [72].
The presence of DON in maize grain is related, as in the case of ZEN, to the presence of GER on maize plants. Harvest residues such as maize stalks and wheat straw are the main source of F. graminearum and F. culmorum. Furthermore, the optimal conditions for water activity and temperature for the development of these species differ, so that F. graminearum may dominate in one location and F. culmorum in the other. These two species are able to biosynthesize not only DON, but other type B trichothecenes (NIV, FUS-X, 3-AcDON and 15Ac-DON). Both DON and NIV chomotypes are identified within F. graminearum and F. culmorum. F. cerealis is a related species and is considered only a producer of NIV [22,33,36]. However, DON was found most often and in the highest content in maize grain. Its biosynthesis is facilitated in cooler regions of Europe and North America, but some authors suggest that this toxin can also occur in maize kernels originating from warmer growing areas [26]. The DON content in maize was strongly correlated with the content of the DON metabolite (DON-3G) [66]. The maximum content of DON in maize kernels ranged between 83 and 20,475 µg/kg [26,57,59,60,63,65,66,67,68] with the highest content in maize from Michigan (20,475 µg/kg) and Germany (10,975 µg/kg) [60,66]. In maize samples originating from regions of South Africa, the maximum reported level of DON was 1380 µg/kg [26]. Among all modified forms of DON, the majority of data is for DON-3G. The highest content of DON-3G, similar to DON, was found in maize samples from Michigan (max. 6266.49 µg/kg) [60]. Birr et al. [66] also confirmed the presence of DON-3G in all their samples. Its maximum content was 3038 µg/kg, which represented 28% of the maximum content of DON [66]. The content of 3-AcDON in maize kernels ranged between 63.04 and 1046.8 µg/kg [46,57,60,70], with the highest concentration detected in maize from Belgium [70]. In the case of 15-AcDON, another DON derivative, the highest content in maize kernels (1787.6 µg/kg) was from Michigan [60]. At 35.7–142 µg/kg, NIV in maize kernels was relatively low compared to DON [26,57,64].
T-2 and HT-2 toxins were significantly less common in maize kernels compared to other toxins produced by Fusarium (Table 4). Natural occurrence of T-2 toxin is mainly connected with the tropical and subtropical regions. T-2 toxin can be metabolized into HT-2 toxin. The most important factors that influence T-2 toxin production are weather conditions, grain defects and moisture content (13–22%) [28,36]. T-2 toxin is produced at a wide temperature range (0–32 °C), with maximum production at temperatures below 15 °C. For example, F. sporotrichioides has a low optimal temperature (6–12 °C) for T2 toxin production and can produce this mycotoxin during overwintering in the field and/or during storage. Among all grains, corn, wheat, barley, oat, and rye are most frequently contaminated with T2-toxin [46]. T-2 toxin was found in maize samples from Michigan in no more than 19% of the examined samples, at concentrations of <LOD–156.65 µg/kg. HT-2 was also rarely found in maize samples (in no more than 5% of samples) and the maximum content ranged between 40.2 and 276.74 µg/kg, depending on the sample origin [26,60].
3.2. Emerging Mycotoxins
There is limited literature data on the occurrence of “emerging” mycotoxins. However, in recent years there has been an increased interest in this topic. These compounds can be found all over the world where maize is grown and they can coexist with toxins with free as well as modified mycotoxins. The literature data indicate that some of the “emerging” mycotoxins can occur at significant concentrations (e.g., MON, BEA and FUS). Those toxins were found in nearly all maize samples. Emerging mycotoxins naturally occurring in maize kernels are presented in Table 5.
Table 5.
Natural occurrence of emerging mycotoxins in maize.
| Mycotoxin | Positive Samples (%) | Min–Max [µg/kg] | Origin | References |
|---|---|---|---|---|
| MON | 120/123 (98%) | <LOQ–1130 | South Africa | [26] |
| 26/29 (89.7%) | 15.3–1450 | Serbia (South-Backa) | [54] | |
| 21/21 (100%) | 5.06–850 | Serbia (South-Banat) | ||
| 3/6 (50%) | 34.8–405 | Serbia (West-Backa) | ||
| 12/12 (100%) | 7.18–1228 | Serbia (Middle-Banat) | ||
| 5/5 (100%) | 3.03–3856 | Serbia (Srem) | ||
| 18/79 (23%) | 1.6–142 * | Egypt | [57] | |
| 54/90 (60%) | <LOD–1160.35 | Michigan (USA) | [60] | |
| 81/81 (100%) | 93–751 | Northen Italy (Pidemont) | [68] | |
| 81/81 (100%) | 592–4800 | Northen Italy (Lombardy) | ||
| 81/81 (100%) | 8–1613 | Northen Italy (Vento) | ||
| 20/30 (66%) | 25.3–1387 * | Nigeria | [64] | |
| BEA | 107/123 (87%) | <LOQ–142 | South Africa | [26] |
| 20/21 (95.2%) | 0.41–129 | Serbia (South-Banat) | [54] | |
| 26/29 (89.7%) | 0.10–111 | Serbia (South-Backa) | ||
| 11/12 (91.7%) | 0.23–49.7 | Serbia (Middle-Banat) | ||
| 4/5 (80%) | 0.27–136 | Serbia (Srem) | ||
| 3/6 (50%) | 0.03–18.2 | Serbia (West-Backa) | ||
| 30/30 (100%) | 2.5–329 * | Nigeria | [64] | |
| 80/90 (89%) | 1.04–7446.21 | Michigan (USA) | [60] | |
| 50/79 (63%) | 0.64–72 * | Egypt | [57] | |
| FUS | 16/21 (76.2%) | 85.4–1121 | Serbia (South-Banat) | [54] |
| 11/12 (91.7%) | 450–1738 | Serbia (Middle-Banat) | ||
| 3/5 (60%) | 312–4488 | Serbia (Srem) | ||
| 22/29 (75.9%) | 91.3–4687 | Serbia (South-Backa) | ||
| 1/6 (16.7%) | 12,272–12,272 | Serbia (West-Backa) | ||
| 2/30 (7.1%) | 0.3–1.3 | Nigeria | [64] | |
| DAS | 2/123 (1.7%) | 4.4–5.0 | South Africa | [26] |
| 9/55 (17%) | 2.2–3 | West Africa (Togo) | [62] | |
| 12/30 (40%) | 5.9–6.59 * | Nigeria | [64] | |
| 22/257 (8.6%) | 0–14.9 * | Belgium | [70] | |
| MAS | 1/123 (0.8%) | 20.9–20.9 | South Africa | [26] |
| 4/158 (2.5%) | 9.91–32.9 * | Europe (various countries) | [63] ** | |
| NEO | 1/123 (0.8%) | 4.5–4.5 | South Africa | [26] |
| ENN A | 3/29 (10.3%) | 0.12–0.47 | Serbia (South-Backa) | [54] |
| 2/12 (16.7%) | 0.41–17.1 | Serbia (Middle-Banat) | ||
| 1/6 (16.7%) | 0.49 | Serbia (West-Backa) | ||
| 50/90 (56%) | <LOD–21.84 | Michigan (USA) | [60] | |
| ENN A1 | 4/90 (4%) | <LOD–27.28 | Michigan (USA) | [60] |
| 3/29 (10.3%) | 0.13–0.44 | Serbia (South-Backa) | [54] | |
| 3/12 (4.8%) | 0.11–27.4 | Serbia (Middle-Banat) | ||
| 1/21 (4.8%) | 0.59 | Serbia (South-Banat) | ||
| ENN B | 47/90 (52%) | <LOD–2.34 | Michigan (USA) | [60] |
| 1/21 (4.8%) | 7.55 | Serbia (South-Banat) | [54] | |
| 2/12 (16.7%) | 0.08–1.52 | Serbia (Middle-Banat) | ||
| 93/257 (36.2%) | 46.2–1984.9 * | Belgium | [70] | |
| ENN B1 | 6/90 (7%) | <LOD–7.94 | Michigan (USA) | [60] |
| 1/21 (4.8%) | 4.89 | Serbia (South-Banat) | [54] | |
| 1/6 (16.7%) | 0.22 | Serbia (West-Backa) | ||
| 2/12 (16.7%) | 0.20–16.3 | Serbia (Middle-Banat) | ||
| CUL | 18/123 (15%) | 13.3–465 | South Africa | [26] |
| 125/158 (79.1%) | 190–6680 | Europe (various countries) | [63] ** | |
| BUT | 35/123 (28%) | <LOQ–214 | South Africa | [26] |
| 30/158 (19%) | 28.9–583 | Europe (various countries) | [63] | |
| FA | 24/123 (20%) | 57–195 | South Africa | [26] |
| 35//158 (22.2%) | 229–4120 | Europe (various countries) | [63] | |
| Bikaverin | 82/123 (67%) | <LOQ–651 | South Africa | [26] |
| 42/158 (26.6%) | 20.3–415 | Europe (various countries) | [63] ** | |
| 15Hydroxyculmorin | 49/123 (40%) | <LOQ–2022 | South Africa | [26] |
| 84/158 (53.2%) | 229–1670 | Europe (various countries) | [63] | |
| 5Hydroxyculmorin | 18/123 (15%) | <LOQ–578 | South Africa | [26] |
| 19/158 (12%) | 571–1480 | Europe (various countries) | [63] ** | |
| Apicidin | 2/123 (1.6%) | 2.9–15.4 | South Africa | [26] |
| 79/123 (50%) | 9.49–175 | Europe (various countries) | [63] ** | |
| Aurofusarin | 89/123 (72%) | <LOQ–5470 | South Africa | [26] |
| Epiequisetin | 19/123 (15%) | <LOQ–18.9 | ||
| Equisetin | 30/123 (24%) | <LOQ–129 | ||
| Fusarinolic acid | 24/123 (20%) | <LOQ–3422 | ||
| Acuminatum B | 12/123 (9.8%) | <LOQ–219 | ||
| Acuminatum C | 7/123 (5.7%) | <LOQ–204 | ||
| Chlamydospordiol | 2/123 (1.7%) | 2.1–5.1 | ||
| Chlamydosporol | 1/123 (0.8%) | 87.0–26.9 | ||
| Chrysogin | 48/123 (30%) | <LOQ–7.7 | ||
| Siccanol | 91/123 (74%) | 34.6–252 | ||
| Fusapyron | 38/123 (31%) | <LOQ–18.0 |
Median-maximum, * no data about minimum content, ** maize silage [63].
MON was found in the majority of maize samples at relatively high concentrations as compared to other “emerging” mycotoxins. The maximum content of that toxin in maize kernels ranged between 116 and 4800 µg/kg [26,54,57,60,64,68]. Relatively high MON content was recorded in Serbia, where the maximum values ranged between 850 and 3856 µg/kg, depending on the growing season [54]. In Italy, MON concentration ranged between 751 and 4800 µg/kg, also depending on the growing area [68]. On the other hand, MON was found in as many as 98% of South African samples at a maximum concentration of 1130 µg/kg [26]. A similar incidence (87%) and a high content in maize kernels were recorded for BEA. As shown in Table 5, the maximum concentration of BEA ranged between 18.2 and 7446.21 µg/kg [26,54,57,60,64], whereas the most contaminated kernels originated in Michigan (max. 7446.21 µg/kg) [60]. Conversely, all maize samples originating from Nigeria were confirmed to contain BEA at a relatively lower maximum concentration, 329 µg/kg [64]. ENNs are a group of compounds which are structurally similar to BEA but occur in maize kernels less often. Among ENNs, ENN B was the most common (27% of samples) and occurred at the highest concentrations. Its maximum content was found in maize kernels from Belgium (1984.9 µg/kg) [70]. There has been little data published in recent years concerning the incidence of FUS in maize kernels [54,64]. Research carried out in Serbia confirmed a high incidence of FUS at an average of 64.1% across all examined regions; the highest FUS concentration ranged between 1121 and 12,272 µg/kg [54]. In Nigerian maize kernel samples, both the incidence and content of FUS were relatively low [64]. Other toxins, such as MAS, DAS or NEO, were much rarer and their concentrations were relatively low (Table 4).
There is very little literature data concerning other “emerging” mycotoxins, such as CUL, FA, fusarinolic acid, aurofusarin, 15-hydroxyculmorin and 5-hydroxyculmorin. Ekwomadu et al. [26] suggested that the content of these compounds may be significant, while Reisinger et al. [63] suggest that their concentration may be higher in maize silage [26,36]. However, more extensive research is required to more precisely estimate the concentration of those compounds in maize kernels.
4. Toxicology
4.1. Free and Modified Mycotoxins
4.1.1. Trichothecenes and Their Modified Forms
The toxicity of type B trichothecenes results from their ability to bind to ribosome subunits, thus inhibiting protein synthesis. This process is referred to as ribotoxic stress [73,74]. The EFSA Panel on Contaminants in the Food Chain has set the TDI value (Tolerable Daily Intake) for the sum of DON, 3Ac-DON, 15Ac-DON and DON-3G at 1.0 μg/kg of body weight [75]. In recent years, multiple studies evaluated the cytotoxicity of DON and its metabolites. In those studies, cell lines representing the gastrointestinal organs were used, including: HepG2 (liver), Caco-2 (small intestine), IPEC-J2 (porcine small intestine), and GES1 (stomach). The only metabolite of DON whose toxicity was comparable to its native form was 15-acetyldeoxynivalenol (15-AcDON) [75,76,77,78]. On the other hand, metabolites such as 3-AcDON, DON-3G, and deepoxy-deoxynivalenol (DOM-1) were characterised by significantly lower toxicity [76,77,79]. The acetylated derivatives of DON are linked with the ribosome by two hydrogen bonds and the native form of DON by three hydrogen bonds. The acetylated groups in DON metabolites influence the strength of the bond in the toxin-ribosome complex. The acetyl group at C-3 induces stabilising van der Waals forces, while the same group at C-15 causes the formation of an additional, stabilising hydrophobic interaction [80,81]. It is suggested that esterification at C-15 increases toxicity and acetylation at C-3 causes it to decrease [78]. In recent years, there have been reports that DON and its acetylated metabolites may induce oxidative stress in the intracellular environment. Following the exposure of GES-1 cells to 15-AcDON and DON, they were observed to have a higher concentration of reactive oxygen species, disturbed NAD+/NADH balance, and reduced ATP level [77]. Furthermore, the identification of malondialdehyde (MDA), a marker of lipid peroxidation in cells exposed to those compounds, suggests that they can induce that process [82]. The induction of oxidative stress in cells results in DNA damage, which in turn causes the formation of neoplastic lesions. Studies on cell lines demonstrated that exposure to DON and 15-AcDON caused DNA damage, which disturbed the cell cycle, and enhanced the expression of genes responsible for the antioxidant protection [78,82]. DON and 15-AcDON have proapoptotic effects [77]. The most recent studies on the GES-1 cell line and porcine hippocampus cells found that this process also involves mitogen-activated protein kinases (MAPK) p38 and JNK, as well as ERK1/2 kinases [77,83]. NIV’s structure is nearly identical to DON’s; therefore, both substances have similar toxic effects. Both compounds inhibit cell proliferation, induce the production of IL-8, activate kinases from the MAPK family and engage the κB nuclear factor in the toxicity signal transduction pathways [84]. In the majority of studies that compared NIV’s and DON’s toxicity in in vitro tests on cell lines found significantly higher cytotoxicity of NIV as compared to DON. In studies on cell lines of promyelocytic leukaemia (HL60), lymphoblastic leukaemia (MOLT-4) and rat aorta myoblasts (A-10), IC50 values (concentration which inhibits cell proliferation by 50%) for NIV were several times lower than those for DON [85,86,87,88]. However, in HepG2 cells, IC50 values for both compounds were comparable [84]. As opposed to DON, NIV did not significantly induce the secretion of anti-hematopoietic cytokines, CCL3/CCL4, in the cells [84]. Therefore, it can be assumed that for leukopenia caused by type B trichothecenes, in the case of NIV, it is the result of cytotoxicity towards leukocytes, and in the case of DON it is the result of both cytotoxicity and the inhibition of leukocyte production. The few publications describing the toxicity of NIV metabolites, usually only include fusarenone X (FUS-X) and found that this compound has similar or slightly lower toxicity compared to its native form. These observations were confirmed in GES-1 cell lines and human T cells (Jurkat Cells) [77,89]. Mouse studies demonstrated significantly greater DNA fragmentation in the thymus gland, Peyer’s patches and spleen in animals exposed to FUS-X than in those exposed to NIV [90]. It is likely that nivalenol-3-glucoside (NIV-3G) is not effectively hydrolysed to NIV in an in vivo environment, as observed in rat studies. The analysis of urine of animals exposed (using a tube) to NIV-3G showed a 30-times lower content of NIV compared to the group of animals who received equimolar doses of NIV [91].
Type A trichothecenes exhibit significantly greater toxicity than other types of trichothecenes. Their toxicity mechanism is based on binding with a large ribosome subunit and inhibiting translation [92]. Those compounds exhibit neurotoxicity, hepatotoxicity, hematotoxicity, and reproduction toxicity. Acute poisoning causes nausea, vomiting, diarrhoea, damage to the stomach and liver, and weakened immunity [93,94]. The EFSA Panel on Contaminants in the Food Chain determined the TDI value for the sum of T-2 and HT-2 toxins at 0.02 μg/kg of body weight [95]. Numerous in vitro studies on cell lines representing liver, intestinal and kidney cells, murine macrophages, and porcine Leydig cells demonstrated comparable toxicity of T-2 and HT-2 toxins [96,97,98]. The similar toxicity of those compounds may be the result of the effective transformation of the T-2 toxin into the HT-2 toxin in the intracellular environment. Studies on the HepG2 line showed that the effectiveness of biotransformation of the T-2 toxin to the HT-2 toxin reached 94% [96]. Toxicity similar to that of the T-2 toxin can be also found in DAS. This compound can decrease the viability of intestinal cells, liver cells, and murine macrophages to a similar degree [99].
In recent years, significant attention has been paid to the induction of anorexia by type A trichothecenes. This process involves intestinal satiety hormones, cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1), as well as neurotransmitters—substance P (SP) and serotonin, also referred to as 5-hydroxytryptamine (5-HT) [100,101]. In mink, the induction of neuropeptide YY (PYY) production occurred as early as 30 min following oral or intraperitoneal administration of DAS (a type of trichothecene) [102]. Unfortunately, the impact of the T-2/HT-2 toxins on PYY synthesis has not yet been described and there is no information on the toxicity of T-2/HT-2 glucosides in the literature. It is suggested that those compounds may be characterised by effective intestinal absorption; Broekaert et al. [103] reported that T-2 toxin-α-glucoside in the gastrointestinal tract of broiler chickens was absorbed five times faster than its aglycone [103].
4.1.2. ZEN and Its Modified Forms
The toxicity of ZEN results from its ability to cause oxidative stress, damage DNA, inhibit the cell cycle and induce apoptosis [101,104,105,106]. The EFSA Panel on Contaminants in the Food Chain determined the TDI value for ZEN at 0.25 μg/kg of body weight [107]. In vitro tests showed greater cytotoxicity of the hydroxylated ZEN metabolite, i.e., β-ZOL, as compared to the native toxin. The cytotoxicity of α-ZOL compared to ZEN is controversial—depending on the cell lines used, studies produce different results [99,108,109]. Furthermore, the data on the cytotoxicity of ZEN-14G is limited. At a concentration of 1 μM, this compound did not have a significant effect on the viability of MCF7 cells (breast cancer cells) [110]. Similarly, its cytotoxicity on small intestinal cells (Caco-2) at concentrations of 20 and 40 μM has not been demonstrated [111]. In accordance with the current knowledge, the cytotoxicity of zearalenone-14-sulfate (ZEN-14S) is unknown. In the case of ZEN and its hydroxylated metabolites (α- and β-ZOL), immunosuppressive effects have been described. Those compounds significantly inhibited the expression of pro-inflammatory cytokines, such as IL-1β, IL-8, and TNF-α [112]. In vitro tests on mononuclear porcine peripheral blood cells exposed to ZEN, α-ZOL, and β-ZOL demonstrated a decrease in neutrophil viability and inhibition of IgG antibody production [99,110]. Furthermore, α-ZOL inhibited the proliferation of T cells [113]. Unfortunately, α-ZOL and ZEN were not tested for this ability; thus, it is not known whether they have similar properties.
An important aspect of ZEN’s toxicity is its xenoestrogenic activity. With a structure similar to oestrogen, it can stimulate oestrogen receptors and disturb the metabolism of steroids and the proliferation of oestrogen-dependent cells [114,115,116]. Long-term exposure to xenoestrogens can cause numerous medical conditions, including disrupted puberty, obesity, infertility (in males), and cancers (breast, ovarian, testicular and prostate) [30,117]. In recent years, researchers have described the xenoestrogenic activity of hydroxylated ZEN metabolites. Significantly, α-ZOL was identified as a stronger xenoestrogen than ZEN [118,119]. Additionally, the affinity between ZEN-14G and oestrogen receptors is several hundred times lower than that of ZEN. However, in the cellular environment this compound is hydrolysed to xenoestrogens, such as α- and β-ZOL [110,120]. The activation of oestrogen receptors can alter gene expression, since those receptors influence the activity of numerous transcription factors [118,121]. It has been reported that α-ZOL and ZEN produce significant epigenetic changes, and cause DNA methylation and acetylation of histones in the HepG2 cell line. These changes impair the expression of many crucial metabolism genes, including the genes that regulate glucose and lipid metabolism, and those that regulate insulin secretion. It is suggested that these changes may contribute to the development of type 2 diabetes [108].
4.1.3. Fumonisins and Their Modified Forms
The main aspect of fumonisin toxicity is the inhibition of ceramide synthesis and the disruption of sphingolipid metabolism, which can cause cell apoptosis [122]. This mechanism of action stems from the similarity between the chemical structure of fumonisins and the structure of sphingosine (the primary part of sphingolipids), which is why they are recognised, and bound, by ceramide synthase [123]. The EFSA Panel on Contaminants in the Food Chain determined the TDI value for the sum of FB1, FB2, FB3, FB4 at 1.0 μg/kg of body weight [124]. The likely teratogenic activity of FB1 is by intercalating with the folic acid transporter, and thus limiting the possibility of the folic acid being captured by the embryo. Due to the crucial role of folic acid in the development of the nervous system in embryos, FB1 exposure may cause foetal neural tube defects [125]. There are numerous publications which demonstrate that fumonisins induce oxidative stress. Along with the disturbance of sphingolipid metabolism, this process is the primary mechanism of FB1 toxicity, which can lead to lipid peroxidation and DNA damage [126,127,128,129]. When exposed to FB1, rats suffer DNA damage, the extent of which depends on the dose [130]. The genotoxicity of FB1 in in vitro testing is not clear. Following exposure to FB1 no DNA damage was observed in human kidney and liver cells [131]. However, recent studies on human oesophageal epithelial cells suggest that this compound may promote carcinogenesis. FB1 significantly limited the expression of suppressor genes that inhibit the cell cycle in response to DNA damage [132].
The exposure of various animal species to fumonisins produced different symptoms: horses and mice suffered damage to the nervous system; in ruminants, the characteristic symptoms included hepatotoxicity, reproduction toxicity and weakened immunity [133]. In rodents, FB1 exposure caused liver damage, which could lead to liver cancer [134]. Existing toxicological studies suggest that this toxin can cause oesophageal cancer and bleeding, chronic cough, indigestion and weight loss in humans [125]. As a result of 48-h exposure of GES1 line cells (gastric epithelium) to 40 μM of FB1, FB2, and FB3, the following toxicity hierarchy was established: FB1 > FB2 > FB3 with cell mortality rates of 48.44%, 34.66%, and 27.37% respectively. Furthermore, a synergistic effect was found during the exposure to a mixture of fumonisins, i.e., FB1/FB2 and FB1/FB3, and the primary type of cell death was necrosis [135].
There are a very limited number of publications describing the toxicity of modified forms of fumonisins. In in vitro tests, the toxicity of HFB1 and HFB2, as compared to FB1, ranged between 0.01 and 0.9. Significantly, the absorption of HFB1 by the cells was more effective than FB1 [124]. Recently, it has been reported that HFB1 is less able to induce the expression of pro-inflammatory chemokines IL-8 and CCL20, as well as cytokine TNF-α in the IPECJ2 (porcine small intestinal cells) and PBMC (mononuclear cells of peripheral blood) cell lines [136].
4.2. Emerging Mycotoxins
Owing to the numerous reports made in recent years, the issue of toxicity of compounds classified as “emerging” mycotoxins has been increasingly common. At the same time, the multitude of compounds in that group, as well as the fact that new mycotoxins are regularly identified, means that the amount of available information concerning this topic is insufficient. Furthermore, because of the limited data on the toxicity of “emerging” mycotoxins, as well as their incidence in foodstuffs, it is impossible to suggest their permissible content in cereals. Therefore, there is currently no legislation that would regulate the content of compounds from this group in food [137,138,139].
The mechanism of toxicity of ENNs and BEA is likely related to their ionophoric properties. By penetrating cellular membranes, those compounds create cation-selective channels, causing ionic imbalance in the cells. Elevated levels of calcium ions in the cells activate caspase-3 through the release of cytochrome C and lead to apoptosis [137,140,141]. ENNs and BEA also have cytotoxic effects as a result of the induction of oxidative stress [142,143]. Following oral exposure of rats to a mixture of enniatin’s A, A1, B and B1, a change in the expression of genes responsible for the antioxidative protection, genes of suppressor proteins, as well as electron carriers (NADPH) was observed in their stomach, kidney, liver and intestinal cells. Furthermore, the abnormal activity of occludin (a tight junction protein) in the intestines suggests that these compounds may impact the permeability of the intestinal barrier [143].
Another relatively well-studied Fusarium toxin classified as an “emerging” mycotoxin is MON. Its mechanism of action is based on an inhibition of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, thus disturbing the Krebs cycle and causing ATP deficiency. The cytotoxicity of MON in in vitro tests largely depends on the cell lines used. Although not cytotoxic towards human progenitor cells of leukocytes and platelets, it significantly reduces the viability of progenitor cells of erythrocytes. In livestock, MON exposure caused muscle weakness, heart failure, and respiratory failure [144]. Data concerning FUS is much more limited. Based on a study on Artemia salina, the LD50 value for this compound was determined at 53.4 µM. Comparable LD50 values were obtained following the exposure of Artemia salina to DON, suggesting that the toxicity of those compounds is similar. Fusaproliferin may have teratogenic properties, as demonstrated in the studies on hens. Macrocephaly, cephalic dichotomy and leg deformations were observed in embryos injected with fusaproliferin [34]. In vitro tests using human small intestinal cells demonstrated that the cytotoxicity of the recently identified NX toxin, classified as a trichothecene, was similar to that of DON. A slightly smaller decrease in cell viability was recorded following exposure to 3-acetyl-NX (17.4%), as compared to 21.4% and 20.2% for NX-2 and DON, respectively. The observation of porcine jejunal explants following 4-h exposure to 10 μM of NX-2 demonstrated the presence of intraparenchymal oedema, cell vacuolation, ruptured epithelial barrier, and significant loss of intestinal villi [145]. Interesting observations have been made recently of CUL which although characterised by negligible cytotoxicity, can reduce the effectiveness of glucuronidation of DON by liver cells. This process is the main pathway of DON detoxification in humans; therefore, CUL and DON are likely characterised by synergistic toxicity [146]. Recently, there have been reports of the possible induction of oxidative stress by aurofusarin and fusaric acid [147,148]. However, the available literature data concerning this topic is limited, and further research should be conducted.
The vast majority of studies into the toxicity of secondary metabolites of Fusarium fungi take into account only the native toxins. Significantly less attention is paid to the metabolites of toxins which co-occur with the native forms in the environment and can be characterised by comparable toxicity (e.g., 15-AcDON, α-ZOL). Many plant metabolites of toxins produced by Fusarium fungi discovered in recent years have not undergone a cytotoxicity evaluation (DON-14S, T-2-Glc, HT-2-Glc). The issue of mycotoxin interactions, which may occur in the in vivo environment and cause antagonistic, additive or synergistic effects, is relatively rarely discussed in the literature. The toxicity evaluation based on in vitro experiments should take into account the properties of the examined compounds in the in vivo environment. Important factors include the potential degradation of the gastrointestinal tract, intestinal absorption, rate of elimination, volume of distribution, and maximum concentration.
5. Progress in Detoxification
Currently, the reduction of mycotoxin contamination in various agricultural commodities is a major issue in many nations [149] as they are heat stable compounds. To avoid these concerns, several preventive measures have been implemented, including pre-harvesting procedures aimed at inhibiting the growth of toxigenic fungus and mycotoxins production, as well as post-harvesting strategies aimed at detoxifying food once mycotoxins have been synthesized. Unfortunately, none of these measures guarantee the complete absence of mycotoxins in food or feed. However, it is critical to keep the prevalence of mycotoxins below the threshold of economic impact, which is why so many studies aim to tackle these challenges. Different approaches for inhibiting fungal growth and minimizing mycotoxin production in maize have been explored including prevention and decontamination mechanisms. Fungal growth and mycotoxin prevention includes strategies related to good agricultural practices (GAPs), good manufacturing practices (GMPs), and good hygienic practices (GHPs), while decontamination mostly include chemical, physical and biological techniques [150]. Agricultural practices can include crop rotation, tillage, use of chemicals as well as breaking the fungal disease cycle by adapting the sowing period or using resistant hosts; physical techniques (cleaning, sorting, irradiation, thermal or ultrasound treatment, temperature and humidity control); chemical approaches (acids and bases such as ammonia, hydrogen peroxide, or antifungal agents); and biological control methods made of the use of microorganisms (bacterial, yeast and fungi) and plant products (plant essential oils and plant extracts etc.) [151].
Although these approaches can reduce fungal infection and/or mycotoxins biosynthesis, there are various limitations, problems, and concerns with regards to physical and chemical detoxification. Physical approaches typically lack specificity and efficacy, whereas chemical approaches sometimes necessitate either specialized equipment and expensive chemicals or extreme treatment conditions, which may have an unacceptable negative impact on the environment and non-targeted organisms. Even more significantly, they may pose health risks to humans and animals, and some methods are commonly regarded as impossible, costly, ineffective, laborious, or time-consuming [151,152]. In addition, fungicide-based approaches represent a possible health, safety, and environmental danger because many antifungal chemical compounds are not biodegradable or have a long degradation time, can contaminate soil and water, and have a negative impact on food quality and human health. Prolonged chemical treatment of grains can also result in fungal strains developing resistance, necessitating higher concentrations, and an increase in hazardous residues in food crops [153]. However, driven by health and environmental concerns, various laws addressing the usage of chemical control measures are being established as consumers become more aware of the potential dangers of chemicals in the food supply [154], and there is a societal and ecological drive movement toward safe and natural food that is free of chemical treatments and/or preservatives. Thus, biological approaches have gained more attention due to their benefits such as target specificity, being economically feasible, and being environmentally benign [155]. In this section, a comprehensive overview of the recent biological detoxification of maize is reviewed.
5.1. Biological Detoxification of Maize
Currently, different pre-harvest and post-harvest biological control systems have been developed for maize against Fusarium spp. and their respective mycotoxins. These have used a variety of potential Biological Control Agents (BCAs), including fungal and bacterial strains or toxigenic fungal strains, botanicals (essential oils, crude plant extracts or phenolic acids). However, most of the reported BCAs are still limited to the in vitro lab scale and are not typically commercialized, and this field is still in its infancy [156]. Many aspects must still be investigated to enable effective integration, and understanding the processes that govern the interaction of BCAs and pathogens throughout time is especially important. Four common modes of actions of BCAs that have been identified and described by various researchers: antibiosis, competition for niche or nutrients, mycoparasitism, and stimulation or enhancement of plant defence [157,158,159]. BCAs typically rely on more than one mode of action to combat the pathogen, and the existence of one dominant mode of action does not preclude the presence of the others [157] and modes may depend on the parameters and kind of BCAs under consideration.
Although many BCAs have been developed and their successful application to fight against mycotoxigenic fungi have been reported, the following paragraphs will only discuss a selection because of their importance in sustainability protection of maize against Fusarium spp.
5.1.1. Trichoderma as Biological Detoxifying Organisms
Trichoderma are soil-dwelling, free-living filamentous fungi that include rhizosphere-competent strains connected with root ecosystems. As potential antagonistic microbes, the genus Trichoderma has been widely studied for their capabilities against plant pathogenic fungi, and their biological control mechanisms mainly include faster growth speed and antibiotic production to compete for nutrients and living space with pathogens, mycoparasitism mediated by producing cell wall degrading enzymes, and the ability to induce plant’s defence systems [157,159,160]. The general mechanisms of Trichoderma spp. biocontrol can be separated into direct and indirect impacts. Competition for nutrients or space, synthesis of volatile and non-volatile antibiotics and lytic enzymes, inactivation of pathogen enzymes, and parasitism are all direct consequences. Indirect impacts include morphological and metabolic changes in the host plants, such as stress tolerance, inorganic nutrient solubilization or sequestration, and induction of fungal phytopathogen-caused disease resistance [161].
Trichoderma species are efficient bio-controllers of phytopathogenic Fusarium [158,162] with the ability to combine numerous benefits in one product, including the control of various plant diseases, the stimulation of plant growth, and the creation of a clean environment for the benefit of sustainable agriculture [157]. In a screening experiment, the abilities of twenty-four isolates belonging to ten different Trichoderma species were tested against the mycelial growth and mycotoxin production by five Fusarium strains [158]. All the selected strains were capable of affecting the mycelial growth of at least four of all five Fusarium species on the fourth day after co-inoculation, when there was the first apparent physical contact between antagonist and pathogen. However, T. atroviride AN240 was found to be the most efficient (69–100% toxin reduction) suppressor of mycotoxin (DON, 3-AcDON, 15-AcDON, NIV, ZEN, BEA, MON) production by all five Fusarium species (F. avenaceum, F. cerealis, F. culmorum, F. graminearum and F. temperatum) on solid substrates [158]. Recently, there has been increased interest in managing DON production with Trichoderma strains as a biological control-based technique. Eight selected Trichoderma strains effectively inhibited F. graminearum mycelial growth and mycotoxin DON synthesis. Furthermore, the modified mycotoxin deoxynivalenol-3-glucoside (DON-3G), which was once regarded as a detoxification product of DON in plant defense, was detected when Trichoderma interacted with F. graminearum [163].
Further, the inhibitory effects of a biocontrol agent based on the Trichoderma asperellum isolate GDFS1009 on the management of stalk rot in maize caused by F. graminearum, as well as the effect on the maize plant growth were reported with a 60% inhibition rate against F. graminearum [164]. Another research group explored the effect of cellulase from T. harzianum Th22 on triggering the biosynthesis of 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) and defence-related gene expressions in maize roots against DON producing F. graminearum and it was found that DIMBOA inhibited the pathogenicity and mycotoxin related proteins in pathogen F. graminearum [165]. The efficacy of seven T. asperellum strains obtained from fields in Southern China was evaluated against F. graminearum. The T. asperellum ZJSX5003 strain enhanced the antagonist activity against F. graminearum and reduced disease prevalence by 71% in inoculated maize plants compared to negative control [166].
Other mycotoxins are also reduced by Trichoderma strains. Tian et al. [167] discovered three Trichoderma isolates that could successfully limit the mycelia spread and mycotoxin synthesis of ZEN-producing F. graminearum [167]. Furthermore, ZEN-treated experiments revealed that although these Trichoderma isolates could not detoxify ZEN by glycosylation, they could convert ZEN to its reduced (α-ZOL and β-ZOL) and sulfated metabolites (ZEN14S and ZOL14S), providing more detail on how Trichoderma isolates and ZEN-producing fungus interact. In addition, Trichoderma harzianum strains have received special attention. In controlled and uncontrolled environments, seeding maize with the T. harzianum T22 and Th-8 strains lowered F. verticillioides kernel colonization and FBs contamination, as well as generated systemic resistance in maize against these pathogens [158]; also T. harzianum T16 and T23 strains have been found to be effective antagonists towards F. verticillioides and FBs production in maize kernels in liquid as well as agar medium.
5.1.2. Lactic Acid Bacteria as Biological Detoxifying Agent
Lactic acid bacteria (LAB) are a group of related bacteria (e.g., Streptococcus spp., Lactobacillus spp., Lactococcus spp., and Leuconostoc spp.) that produce lactic acid as a result of carbohydrate fermentation [168]. LAB occur naturally in foods and are either considered to be harmless or possibly supportive of human health in their capacity as probiotics. LAB is one of the most studied microorganisms for mycotoxin degradation due to their high safety profile in food applications. They produce various bioactive compounds such as organic acids (acetic and lactic acid), hydrogen peroxide, proteinaceous compounds, reuterin, hydroxyl fatty acids, and phenolic compounds that can inhibit fungal development and prevent the generation of mycotoxins in food [169]. The detoxification ability of LAB can be attributed to mycotoxin adsorption by the bacterial cell structure or degradation via its metabolism [170].
The ability of LAB to inhibit fungal development and remove several mycotoxins, as well as its general safety and probiotic potential, make it a promising candidate for the biological control of fungi such as F. graminearum or F. verticillioides in maize and during food production and processing. The most prevalent bacterial isolates associated with anti-Fusarium action are Lactobacillus spp. with L. plantarum appearing to be particularly efficient [169]. Li et al. [170] investigated the effect of lactic acid bacteria on the fermentation quality and mycotoxin concentrations of corn silage infested with mycotoxigenic fungi [170]. They found all inoculants decreasing the DON and FB1 concentrations, while only L. buchneri and L. plantarum reduced the ZEN. Researchers investigating whether LAB fermentation (using indigenous microflora) reduced FB1 and ZEN concentrations in fermented maize meal found a significant decrease in the concentration of the two mycotoxins with a 68 to 75% for ZEN and 56–67% for FB1 [171]. Similarly, Franco et al. [172] reported that LAB inhibits F. graminearum and DON detoxification [172]. The application of the LAB as detoxifying agent is still limited in maize and maize products intended for human consumption. Although its application in silages and feed maize continues to develop [170,173,174], further research is still needed.
5.1.3. Plant Secondary Metabolites as Biocontrol Detoxifying Agent
Essential oils, spices, herbs, and crude extracts from plants are excellent candidates for the development of bio-fungicides and nutraceuticals to treat mycotoxicosis and associated infections. In general, botanicals are widely recognized as environmentally friendly and safer sources of bioagent for the prevention of fungal growth and mycotoxin biosynthesis in food and feed. They are less expensive than other materials used for the same purpose, provide a synergistic approach as protectants against fungal/mycotoxin contamination, and trigger pathways that elicit natural defence systems in plant tissues.
Essential Oils
Essential oils (EOs) are natural compounds extracted from different plant parts including the roots, fruits, flowers, and seeds. They are characterised by their odour that result from the aromatic compounds, terpenes and other substances synthesised by plants as secondary metabolites [175]. The production of EOs and their constituents is influenced by intrinsic and extrinsic factors, including environmental conditions such as climate, rainy and sunny periods, seasonality, and others; and these factors have a direct impact on the majority of compounds, which in turn act on a variety of microorganisms [176]. Essential oils suppress fungi growth and mycotoxin synthesis through a variety of mechanisms, including altered fungal growth rate and lag phase, disruption of cell permeability, disruption of the electron transport chain, and manipulation of gene expression patterns and metabolic processes [177].
Recently, various types of essential oils; such as cinnamon, verbena, palmarosa, orange, and spearmint, Litsea cubeba; have been reported to have inhibitory effects on Fusarium growth and mycotoxins detoxification [178,179]. Perczak et al. [179] investigated the effects of cinnamon, palmarosa, orange, and spearmint EOs on the growth of F. graminearum and F. culmorum and the biosynthesis of mycotoxins in maize seeds and found that these EOs have a significant ability to prevent Fusarium fungal growth (F. graminearum and F. culmorum) and in maize seed reduced mycotoxin concentrations of ZEN (99.10–99.92%) and DON (90.69–100%) [179]. However, the efficiency of three cinnamon, verbena, and palmarosa in decreasing ZEN and DON in maize grains is concentration dependant [179]. Additionally, essential oils with certain constituents extracted from aromatic plants (Aloysia polystachya, Origanum vulgane, Mentha piperita, and Aloysia triphylla) suppressed the growth of F. verticillioides and fumonisin production in maize grain [180]. Castro et al. [175] evaluated the antifungal and anti-mycotoxigenic activity of essential oils (EOs) from Zingiber officinale, Cinnamomum zeylanicum, and Cymbopogon martinii against F. verticillioides and fumonisins biosynthesis and found that all tested EOs had inhibitory effects on F. verticillioides by promoting structural damage to the fungal cell wall, decreasing conidia size, and mycelial reduction [175]. Similarly, in vitro examination of the antifungal effects of Litsea cubeba essential oil against F. verticillioides revealed that the investigated EOs significantly decreased FB1 and FB2 synthesis as well as the mycelial development of F. verticillioides. The minimum inhibitory concentration of the EOs was 125 µg/mL and the inhibitory effect was dose dependent [181]. Moreover, multiple essential oils (cedarwood, cinnamon leaf, cinnamon bark, white grapefruit, pink grapefruit, lemon, eucalyptus, palmarosa, mint, thymic, and rosemary) on ZEN reduction under various in vitro conditions, including the influence of temperature, pH, incubation time and mycotoxin and essential oil concentrations have also been explored [182]. In another study, cinnamon oil was effective in reducing FB1 from 15.03 to 0.89 μg/mL (94.06%) [183]. Generally, essential oils from plants offer hope in the prevention and detoxification of Fusarium mycotoxins present in cereals.
Plant Extracts
Plants contain antimutagens, antimicrobials, antioxidants, and anticarcinogens that can mitigate the harmful and genotoxic effects of mycotoxins. Several substances derived from plant extracts have been shown to decrease the growth and toxin generation of Fusarium spp. Plant extracts contain a variety of chemicals, including polyphenols, phenolic acids, and flavonoids, which may serve as the biological basis for their antimicrobial activities. Furthermore, plant extracts having antimicrobial capabilities have been utilized to control mycotoxigenic fungus in foods and feeds, potentially eliminating or reducing the use of synthetic chemicals [184]. Equisetum arvense and Stevia rebaudiana extracts significantly reduced the growth of F. verticillioides [185]. Seepe et al. [186], in in vivo experiments of the antifungal effectiveness of various plant extracts against Fusarium pathogens on maize seeds, found that Melia azedarach acetone extract had strong antifungal activity (97% inhibition) against F. proliferatum, while combined acetone extracts from Combretum erythrophyllum and Quercus acutissima had 96, 67, and 56% inhibition against F. verticilloides, F. proliferatum, and F. solani, respectively [186]. Furthermore, natural phenolic acids (caffeic, ferulic, p-coumaric, and chlorogenic) inhibit Fusarium growth and mycotoxin production in culture medium and in maize kernels [187]. Currently, the application of plant extracts to inhibit Fusarium growth and mycotoxins biosynthesis in maize grains is still limited. However, their experimental use gives hope for their application in near future. Recently, the in vitro efficacy of sixteen extracts derived from eight natural sources using subcritical water extraction at two temperatures was assessed against fungal growth and type B trichothecene (TCTB) production by F. graminearum. Maritime pine sawdust extract was shown to be exceptionally efficient, leading to a significant inhibition of up to 89% of the fungal growth and a reduction of up to 65% of the mycotoxin production by F. graminearum [188]. Furthermore, a study by Uwineza et al. [24] reported the antifungal and mycotoxins reduction of lemon balm extracts against F. proliferatum and F. culmorum [24].
5.2. Edible Mushrooms Source of Biological Detoxifying Agent
Edible mushrooms are macro fungus with a specific fruiting body, which can be either a Basidiomycete or an Ascomycete, aerial or underground, and large enough to be seen with naked eye and to be collected by hand [189]. Mushroom oyster or white-rot fungus (Pleurotus ostreatus) is one of the most well-known edible mushrooms due to its economic (edible), ecological (bioremediation agents), and medicinal value (antioxidant activity and bio-compounds source) [190,191]. Its use as a biocontrol organism for mycotoxin detoxification in cereals especially maize has grown in popularity in recent years [192,193,194] owing to its bioactive compounds (phenolic compounds and proteins) [188,194], and its highly efficient enzymatic systems (manganese peroxide and laccases) for degrading mycotoxins [191,195,196].
Furthermore, other forms of mushrooms have been employed to inhibit Fusarium growth and their mycotoxins biosynthesis, since its employment as a natural detoxifying agent has been predicted for the future. Merel et al. [197] studied the in vitro effect of crude extracts (CEs) from A. subrufescens, L. edodes, and P. ostreatus fruiting bodies on the production of biomass and mycotoxins by two strains of F. verticillioides mostly contaminating the maize [197].
6. Conclusions
Accelerating climate change and the cosmopolitan nature of Fusarium fungi significantly affect the profile of these microorganisms present in maize crops, and thus the quality and quantity of mycotoxins. Additionally, the presence of modified forms of toxins and the lack of legal regulations in this regard exacerbates the risk of an increase in their concentration in both raw materials and cereal products. Various biological methods are very promising and safe for the environment and the consumer, and may significantly limit the growth of Fusarium and thus the biosynthesis of mycotoxins.
Author Contributions
Conceptualization, A.W. and M.B.; writing—original draft preparation, A.P., A.Z., E.K.-W., M.M., P.A.U., A.W. and M.B.; writing—review and editing, supervision, A.W. and M.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The part of the research was funded by Polish National Science Centre, grant No. 2018/31/B/NZ9/03485.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soto-Gómez D., Pérez-Rodríguez P. Sustainable agriculture through perennial grains: Wheat, rice, maize, and other species. A review. Agric. Ecosyst. Environ. 2022;325:107747. doi: 10.1016/j.agee.2021.107747. [DOI] [Google Scholar]
- 2.OECD-FAO . Agricultural Outlook 2022–2031. OECD-FAO; Rome, Italy: Paris, France: 2022. [DOI] [Google Scholar]
- 3.Liliane T., Mutengwa C. Factors Affecting Yield of Crops, Agronomy—Climate Change & Food Security. IntechOpen; Amanullah, Afghanistan: 2020. [(accessed on 14 September 2022)]. Available online: https://www.intechopen.com/books/agronomy-climate-change-food-security/factors-affecting-yield-of-crops. [Google Scholar]
- 4.Dean R., Van Kan J.A., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Foster G.D. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmusk S., Nevo E., Ayele F., Noe S., Niinemets Ü. Fighting Fusarium pathogens in the era of climate change: A Conceptual approach. Pathogens. 2020;9:419. doi: 10.3390/pathogens9060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina Á., González-Jartín J.M., Sainz M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017;18:76–81. doi: 10.1016/j.cofs.2017.11.009. [DOI] [Google Scholar]
- 7.Chukwudi U.P., Kutu F.R., Mavengahama S. Mycotoxins in maize and implications on food security: A Review. Agric. Rev. 2021;140:42–49. doi: 10.18805/ag.R-140. [DOI] [Google Scholar]
- 8.Bouwer G.A. Framework for effective Bt maize IRM Programs: Incorporation of lessons learned from Busseola fusca resistance development. Front. Bioeng. Biotechnol. 2020;8:717. doi: 10.3389/fbioe.2020.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De La Campa R., Hooker D.C., Miller J.D., Schaafsma A.W., Hammond B.G. Modeling effects of environment, insect damage, and Bt genotypes on fumonisin accumulation in maize in Argentina and Philippines. Mycopathologia. 2005;159:539–552. doi: 10.1007/s11046-005-2150-3. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan M., Backhouse D., Del Ponte E.M. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016;9:685–700. doi: 10.3920/WMJ2016.2053. [DOI] [Google Scholar]
- 11.Carbas B., Simões D., Soares A., Freitas A., Ferreira B., Carvalho A.R.F., Silva A.S., Pinto T., Diogo E., Andrade E., et al. Occurrence of Fusarium spp. in maize grain harvested in Portugal and accumulation of related mycotoxins during storage. Foods. 2021;10:375. doi: 10.3390/foods10020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commission of the European Communities Commission Regulation EC (1881/2006) Off. J. Eur. Union. 2006;49:5–24. [Google Scholar]
- 13.Miedaner T., Boeven A.L.G.C., Gaikpa D.S., Kistner M.B., Grote C.P. Genomics-assisted breeding for quantitative disease resistances in small-grain cereals and maize. Int. J. Mol. Sci. 2020;21:9717. doi: 10.3390/ijms21249717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stępień Ł., Gromadzka K., Chełkowski J., Basińska-Barczak A., Lalak-Kańczugowska J. Diversity and mycotoxin production by Fusarium temperatum and Fusarium subglutinans as causal agents of pre-harvest Fusarium maize ear rot in Poland. J. Appl. Genet. 2019;60:113–121. doi: 10.1007/s13353-018-0478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfordt A., Ramos Romero L., Schiwek S., Karlovsky P., von Tiedemann A. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear-and stalk rot in maize. Pathogens. 2020;9:236. doi: 10.3390/pathogens9030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanubile A., Maschietto V., De Leonardis S., Battilani P., Paciolla C., Marocco A. Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant Microbe Interact. 2015;28:546–557. doi: 10.1094/MPMI-09-14-0269-R. [DOI] [PubMed] [Google Scholar]
- 17.Simões D., Diogo E., de Andrade E. First report of Fusarium andiyazi presence in Portuguese maize kernels. Agriculture. 2022;12:336. doi: 10.3390/agriculture12030336. [DOI] [Google Scholar]
- 18.Tillmann M., von Tiedemann A., Winter M. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J. Plant Dis. Prot. 2017;124:121–130. doi: 10.1007/s41348-016-0064-6. [DOI] [Google Scholar]
- 19.Galiano-Carneiro A.L., Kessel B., Presterl T., Gaikpa D.S., Kistner M.B., Miedaner T. Multi-parent QTL mapping reveals stable QTL conferring resistance to Gibberella Ear Rot in maize. Euphytica. 2021;217:2. doi: 10.1007/s10681-020-02748-x. [DOI] [Google Scholar]
- 20.Rossi V., Scandolara A., Battilani P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009;123:159–169. doi: 10.1007/s10658-008-9351-9. [DOI] [Google Scholar]
- 21.Castañares E., Martínez M., Cristos D., Rojas D., Lara B., Stenglein S., Dinolfo M.I. Fusarium species and mycotoxin contamination in maize in Buenos Aires province, Argentina. Eur. J. Plant Pathol. 2019;155:1265–1275. doi: 10.1007/s10658-019-01853-5. [DOI] [Google Scholar]
- 22.Czembor E., Stępień Ł., Waśkiewicz A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE. 2015;10:e0133644. doi: 10.1371/journal.pone.0133644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mshelia P.L., Selamat J., Samsudin I.P.N., Rafii M.Y., Mutalib N.A., Nordin N., Berthiller F. Effect of temperature, water activity and carbon dioxide on fungal growth and mycotoxin production of acclimatised isolates of Fusarium verticillioides and F. graminearum. Toxins. 2020;12:478. doi: 10.3390/toxins12080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uwineza P.A., Urbaniak M., Bryła M., Stepien Ł., Modrzewska M., Waśkiewicz A. In vitro effects of lemon balm extracts in reducing the growth and mycotoxins biosynthesis of Fusarium culmorum and F. proliferatum. Toxins. 2022;14:355. doi: 10.3390/toxins14050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waśkiewicz A., Beszterda M., Golinski P. Occurrence of fumonisins in food—An interdisciplinary approach to the problem. Food Control. 2012;26:491–499. doi: 10.1016/j.foodcont.2012.02.007. [DOI] [Google Scholar]
- 26.Ekwomadu T.I., Dada T.A., Nleya N., Gopane R., Sulyok M., Mwanza M. Variation of Fusarium free, masked, and emerging mycotoxin metabolites in maize from agriculture regions of South Africa. Toxins. 2020;12:149. doi: 10.3390/toxins12030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Abdelraheem A., Zhu Y., Elkins-Arce H., Dever J., Whitelock D., Wheeler T.A. Studies of evaluation methods for resistance to Fusarium wilt race 4 (Fusarium oxysporum f. sp. vasinfectum) in cotton: Effects of cultivar, planting date, and inoculum density on disease progression. Front. Plant Sci. 2022;13:900131. doi: 10.3389/fpls.2022.900131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shank R.A., Foroud N.A., Hazendonk P., Eudes F., Blackwell B.A. Current and future experimental strategies for structural analysis of trichothecene mycotoxins—A prospectus. Toxins. 2011;3:1518–1553. doi: 10.3390/toxins3121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piec J., Pallez M., Beyer M., Vogelgsang S., Hoffmann L., Pasquali M. The Luxembourg database of trichothecene type B F. graminearum and F. culmorum producers. Bioinformation. 2016;12:1–3. doi: 10.6026/97320630012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ropejko K., Twarużek M. Zearalenone and its metabolites—General overview, occurrence, and toxicity. Toxins. 2021;13:35. doi: 10.3390/toxins13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao K., Suryoprabowo S., Song S., Liu L., Kuang H. Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food Agric. Immunol. 2018;29:498–510. doi: 10.1080/09540105.2017.1406461. [DOI] [Google Scholar]
- 32.Zhou D., Wang X., Chen G., Sun S., Yang Y., Zhu Z., Duan C. The major Fusarium species causing maize ear and kernel rot and their toxigenicity in Chongqing, China. Toxins. 2018;10:90. doi: 10.3390/toxins10020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logrieco A., Mule G., Moretti A., Bottalico A. Toxigenic Fusarium Species and Mycotoxins Associated with Maize Ear Rot in Europe. Mycotoxins in Plant Disease. Springer; Dordrecht, The Netherlands: 2002. pp. 597–609. [Google Scholar]
- 34.Jestoi M. Emerging Fusarium—Mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- 35.Marin S., Magan N., Ramos A.J., Sanchis V. Fumonisin-producing strains of Fusarium: A review of their ecophysiology. J. Food Prot. 2004;67:1792–1805. doi: 10.4315/0362-028X-67.8.1792. [DOI] [PubMed] [Google Scholar]
- 36.Munkvold G.P., Arias S., Taschl I., Gruber-Dorninger C. Mycotoxins in corn: Occurrence, impacts, and management. AACC Int. Press. 2019:235–287. doi: 10.1016/B978-0-12-811971-6.00009-7. [DOI] [Google Scholar]
- 37.Streit E., Schwab C., Sulyok M., Naehrer K., Krska R., Schatzmayr G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins. 2013;5:504–523. doi: 10.3390/toxins5030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinedine A., Soriano J.M., Molto J.C., Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Pronk M.E.J., Schothorst R.C., van Egmond H.P. Toxicology and Occurrence of Nivalenol, Fusarenon X, Diacetoxyscirpenol, Neosolaniol and 3- and 15-Acetylnivalenol: A Review of Six Trichothecenes. RIVM; Utrecht, The Netherlands: 2002. p. 75. RIVM Report. [Google Scholar]
- 40.Rychlik M., Humpf H.U., Marko D., Dänicke S., Mally A., Berthiller F., Lorenz N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014;30:197–205. doi: 10.1007/s12550-014-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA . Guidance for Industry. Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed (June 29, 2010) U.S. Food and Drug Administration; Silver Spring, MD, USA: 2010. [(accessed on 29 August 2018)]. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/default.htm. [Google Scholar]
- 42.CFIA . RG-8 Regulatory Guidance. Contaminants in Feed (for- Merly RG-1, Chapter 7), Section 1: Mycotoxins in Livestock Feed. Canadian Food Inspection Agency (CFIA); Alberta, BC, USA: 2017. [(accessed on 1 September 2022)]. Available online: http://www.inspection.gc.ca/animals/feeds/regulatory-guidance/rg-8/eng/1347383943203/1347384015909. [Google Scholar]
- 43.FSANZ . Australia New Zealand Food Standards Code, 2017. Schedule 19, Maximum Levels of Contaminants and Natural Toxicants. Prepared by Food Standards Australia New Zealand (FSANZ) on 13 April 2017. Australian Government, Federal Register of Legislation; Canberra, ACT, Canada: 2017. [(accessed on 1 September 2022)]. Available online: https://www.legislation.gov.au/Details/F2017C00333. [Google Scholar]
- 44.FAMIC . Regulation Value of Pesticides, Heavy Metals and Myco-Toxins (Administrative Guideline) (Revised December 18, 2015) Food and Agricultural Materials Inspection Centre (FAMIC); Tokyo, Japan: 2015. [(accessed on 1 September 2022)]. Available online: http://www.famic.go.jp/ffis/feed/r_safety/r_feeds_%20safety22.html. [Google Scholar]
- 45.Asam S., Habler K., Rychlik M. Chemical Contaminants and Residues in Food. Woodhead Publishing; Amsterdam, The Netherlands: 2017. Fusarium mycotoxins in Food. [Google Scholar]
- 46.De Boevre M., Di Mavungu J.D., Maene P., Audenaert K., Deforce D., Haesaert G., De Saeger S. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. 2012;29:819–835. doi: 10.1080/19440049.2012.656707. [DOI] [PubMed] [Google Scholar]
- 47.Ksieniewicz-Woźniak E., Bryła M., Michałowska D., Waśkiewicz A., Yoshinari T. Transformation of selected Fusarium toxins and their masked forms during malting of various cultivars of wheat. Toxins. 2021;13:866. doi: 10.3390/toxins13120866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryła M., Roszko M., Szymczyk K., Jędrzejczak R., Obiedziński M.W. Fumonisins and their masked forms in maize products. Food Control. 2016;59:619–627. doi: 10.1016/j.foodcont.2015.06.032. [DOI] [Google Scholar]
- 49.Dall’Asta C., Galaverna G., Mangia M., Sforza S., Dossena A., Marchelli R. Free and bound fumonisins in gluten-free food products. Mol. Nutr. Food Res. 2009;53:492–499. doi: 10.1002/mnfr.200800088. [DOI] [PubMed] [Google Scholar]
- 50.Tan H., Hongyuan Z., Ting G., Jiaxin L., Chi Z., Shuo W., Yuhao Z., Liang M. Zein structure and its hidden zearalenone: Effect of zein extraction methods. Food Chem. 2022;374:131563. doi: 10.1016/j.foodchem.2021.131563. [DOI] [PubMed] [Google Scholar]
- 51.Tan H., Li Y., Zhou H., Guo T., Zhou Y., Zhang Y., Ma L. Temperature and pH levels: Key factors effecting hidden/free zearalenone during maize processing. Int. Food Res. J. 2022;160:111721. doi: 10.1016/j.foodres.2022.111721. [DOI] [PubMed] [Google Scholar]
- 52.Tan H., Zhou H., Guo T., Zhang Y., Li J., Zhang C., Ma L. Effect of temperature and pH on the conversion between free and hidden zearalenone in zein. Food Chem. 2021;360:130001. doi: 10.1016/j.foodchem.2021.130001. [DOI] [PubMed] [Google Scholar]
- 53.Kovalsky P., Kos G., Nahrer K., Schwab C., Jenkins T., Schatzmayr G., Sulyok M., Krska R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize-an extensive survey. Toxins. 2016;8:363. doi: 10.3390/toxins8120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jajić I., Dudaš T., Krstović S., Krska R., Sulyok M., Bagi F., Savić Z., Guljaš D., Stankov A. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in Serbian maize. Toxins. 2019;11:357. doi: 10.3390/toxins11060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole R.J., Kirksey J.W., Cutler H.G., Doupnik B.L., Peckham J.C. Toxin from Fusarium moniliforme: Effects on plants and animals. Science. 1973;179:1324–1326. doi: 10.1126/science.179.4080.1324. [DOI] [PubMed] [Google Scholar]
- 56.Orlando B., Grignon G., Vitry C., Kashefifard K., Valade R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019;35:369–380. doi: 10.1007/s12550-019-00363-x. [DOI] [PubMed] [Google Scholar]
- 57.Abdallah M.F., Girgin G., Baydar T., Krska R., Sulyok M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. J. Sci. Food Agric. 2017;97:4419–4428. doi: 10.1002/jsfa.8293. [DOI] [PubMed] [Google Scholar]
- 58.Hu L., Liu H., Yang J., Wang C., Wang Y., Yang Y., Chen X. Free and hidden fumonisins in raw maize and maize-based products from China. Food Addit. Contam. 2019;12:90–96. doi: 10.1080/19393210.2018.1564371. [DOI] [PubMed] [Google Scholar]
- 59.Topi D., Babič J., Pavšič-Vrtač K., Tavčar-Kalcher G., Jakovac-Strajn B. Incidence of Fusarium mycotoxins in wheat and maize from Albania. Molecules. 2021;26:172. doi: 10.3390/molecules26010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fusilier K., Chilvers M.I., Limay-Rios V., Singh M.P. Mycotoxin co-occurrence in Michigan harvested maize grain. Toxins. 2022;14:431. doi: 10.3390/toxins14070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarazona A., Gómez J.V., Jiménez M., Mateo F., Romera D., Mateo E.M. Study on mycotoxin contamination of maize kernels in Spain. Food Control. 2020;118:107370. doi: 10.1016/j.foodcont.2020.107370. [DOI] [Google Scholar]
- 62.Hanvi D.M., Lawson-Evi P., De Boevre M., Goto C.E., De Saeger S., Eklu-Gadegbeku K. Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Res. 2019;35:321–327. doi: 10.1007/s12550-019-00351-1. [DOI] [PubMed] [Google Scholar]
- 63.Reisinger N., Schürer-Waldheim S., Mayer E., Debevere S., Antonissen G., Sulyok M., Nagl V. Mycotoxin Occurrence in Maize Silage-A Neglected Risk for Bovine Gut Health? Toxins. 2019;11:577. doi: 10.3390/toxins11100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anjorin S.T., Fapohunda S., Sulyok M., Krska R. Natural co-occurrence of emerging and minor mycotoxins on maize grains from Abuja, Nigeria. Ann. Agric. Environ. Sci. 2016;1:21–29. [Google Scholar]
- 65.Oliveira M.S., Rocha A., Sulyok M., Krska R., Mallmann C.A. Natural mycotoxin contamination of maize (Zea mays L.) in the South region of Brazil. Food Control. 2017;73:127–132. doi: 10.1016/j.foodcont.2016.07.033. [DOI] [Google Scholar]
- 66.Birr T., Jensen T., Preußke N., Sönnichsen F.D., De Boevre M., De Saeger S., Hasler M., Verreet J.A., Klink H. Occurrence of Fusarium mycotoxins and their modified forms in forage maize cultivars. Toxins. 2021;13:110. doi: 10.3390/toxins13020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Worku A.F., Merkuz A., Kalsa K.K., Subramanyam B., Habtu N.G. Occurrence of mycotoxins in stored maize in Ethiopia. Ethiop. J. Agric. Sci. 2019;29:31–43. [Google Scholar]
- 68.Bertuzzi T., Giorni P., Rastelli S., Vaccino P., Lanzanova C., Locatelli S. Co-occurrence of moniliformin and regulated Fusarium toxins in maize and wheat grown in Italy. Molecules. 2020;25:2440. doi: 10.3390/molecules25102440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Z., Rao Q., Song S., Liu N., Han Z., Hou J., Wu A. Simultaneous determination of major type B trichothecenes and deoxynivalenol-3-glucoside in animal feed and raw materials using improved DSPE combined with LC-MS/MS. J. Chromatogr. B. 2014;963:75–82. doi: 10.1016/j.jchromb.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 70.Vandicke J., De Visschere K., Croubels S., De Saeger S., Audenaert K., Haesaert G. Mycotoxins in Flanders’ Fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms. 2019;7:571. doi: 10.3390/microorganisms7110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bryła M., Waśkiewicz A., Szymczyk K., Jędrzejczak R. Effects of pH and temperature on the stability of fumonisins in maize products. Toxins. 2017;9:88. doi: 10.3390/toxins9030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan H., Zhou H., Guo T., Zhang Y., Ma L. Zein-bound zearalenone: A hidden mycotoxin found in maize and maize-products. Food Control. 2021;124:107903. doi: 10.1016/j.foodcont.2021.107903. [DOI] [Google Scholar]
- 73.Wang W., Zhu Y., Abraham N., Li X.Z., Kimber M., Zhou T. The ribosome-binding mode of trichothecene mycotoxins rationalizes their structure—Activity relationships. Int. J. Mol. Sci. 2021;22:1604. doi: 10.3390/ijms22041604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aupanun S., Poapolathep S., Giorgi M., Imsilp K., Poapolathep A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J. Vet. Med. Sci. 2017;79:6–13. doi: 10.1292/jvms.16-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.European Food Safety Authority (EFSA) Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017;15:4718. doi: 10.2903/j.efsa.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broekaert N., Devreese M., Demeyere K., Berthiller F., Michlmayr H., Varga E., Adam G., Meyer E., Croubels S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016;95:103–109. doi: 10.1016/j.fct.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y., Yu S., Tan Y., Liu N., Wu A. Individual and combined cytotoxic effects of co-occurring deoxynivalenol family mycotoxins on human gastric epithelial cells. Toxins. 2017;9:96. doi: 10.3390/toxins9030096. [DOI] [Google Scholar]
- 78.He Y., Yin X., Dong J., Yang Q., Wu Y., Gong Z. Transcriptome analysis of Caco-2 cells upon the exposure of mycotoxin deoxynivalenol and its acetylated derivatives. Toxins. 2021;13:167. doi: 10.3390/toxins13020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Springler A., Hessenberger S., Reisinger N., Kern C., Nagl V., Schatzmayr G., Mayer E. Deoxynivalenol and its metabolite deepoxy-deoxynivalenol: Multi-parameter analysis for the evaluation of cytotoxicity and cellular effects. Mycotoxin Res. 2017;33:25–37. doi: 10.1007/s12550-016-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khaneghah A.M., Martins L.M., von Hertwig A.M., Bertoldo R., Sant’Ana A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018;71:13–24. doi: 10.1016/j.tifs.2017.10.012. [DOI] [Google Scholar]
- 81.Payros D., Alassane-Kpembi I., Pierron A., Loiseau N., Pinton P., Oswald I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016;90:2931–2957. doi: 10.1007/s00204-016-1826-4. [DOI] [PubMed] [Google Scholar]
- 82.Juan-García A., Juan C., Tolosa J., Ruiz M.J. Effects of deoxynivalenol, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol on parameters associated with oxidative stress in HepG2 cells. Mycotoxin Res. 2019;35:197–205. doi: 10.1007/s12550-019-00344-0. [DOI] [PubMed] [Google Scholar]
- 83.Wang X., Fan M., Chu X., Zhang Y., Rahman S.U., Jiang Y., Chen X., Zhu D., Feng S., Li Y. Deoxynivalenol induces toxicity and apoptosis in piglet hippocampal nerve cells via the MAPK signaling pathway. Toxicon. 2018;155:1–8. doi: 10.1016/j.toxicon.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Nagashima H. Deoxynivalenol and nivalenol toxicities in cultured cells: A review of comparative studies. Food Saf. 2018;6:51–57. doi: 10.14252/foodsafetyfscj.2017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagashima H., Nakagawa H., Iwashita K. Cytotoxic effects of nivalenol on HL60 cells. Mycotoxins. 2006;56:65–70. doi: 10.2520/myco.56.65. [DOI] [Google Scholar]
- 86.Nagashima H., Kushiro M., Nakagawa H., Iwashita K. Comparison of antiproliferative effects of trichothecene mycotoxins, nivalenol and deoxynivalenol, in cultured cells. Rep. Natl. Food Res. Inst. 2012;76:29–32. doi: 10.24514/00002888. [DOI] [Google Scholar]
- 87.Thuvander A., Wikman C., Gadhasson I. In vitro exposure of human lymphocytes to trichothecenes: Individual variation in sensitivity and effects of combined exposure on lymphocyte function. Food Chem. Toxicol. 1999;37:639–648. doi: 10.1016/S0278-6915(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 88.Minervini F., Fornelli F., Flynn K.M. Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicol. Vitr. 2004;18:21–28. doi: 10.1016/S0887-2333(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 89.Aupanun S., Phuektes P., Poapolathep S., Alassane-Kpembi I., Oswald I.P., Poapolathep A. Individual and combined cytotoxicity of major trichothecenes type B, deoxynivalenol, nivalenol, and fusarenon-X on Jurkat human T cells. Toxicon. 2019;160:29–37. doi: 10.1016/j.toxicon.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Aupanun S., Poapolathep S., Phuektes P., Giorgi M., Zhang M., Oswald I.P., Poapolathep A. Individual and combined mycotoxins deoxynivalenol, nivalenol, and fusarenon-X induced apoptosis in lymphoid tissues of mice after oral exposure. Toxicon. 2019;165:83–94. doi: 10.1016/j.toxicon.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz-Zimmermann H.E., Binder S.B., Hametner C., Miró-Abella E., Schwarz C., Michlmayr H., Berthiller F. Metabolism of nivalenol and nivalenol-3-glucoside in rats. Toxicol. Lett. 2019;306:43–52. doi: 10.1016/j.toxlet.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Janik E., Niemcewicz M., Podogrocki M., Ceremuga M., Stela M., Bijak M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules. 2021;26:6868. doi: 10.3390/molecules26226868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji F., He D., Olaniran A.O., Mokoena M.P., Xu J., Shi J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019;1:6. doi: 10.1186/s43014-019-0007-2. [DOI] [Google Scholar]
- 94.Taroncher M., Rodríguez-Carrasco Y., Ruiz M.J. Interactions between T-2 toxin and its metabolites in HepG2 cells and in silico approach. Food Chem. Toxicol. 2021;148:111942. doi: 10.1016/j.fct.2020.111942. [DOI] [PubMed] [Google Scholar]
- 95.Arcella D., Gergelova P., Innocenti M.L., Steinkellner H. Human and animal dietary exposure to T-2 and HT-2. EFSA J. 2017;15:4972. doi: 10.2903/j.efsa.2017.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taroncher M., Rodríguez-Carrasco Y., Ruiz M.-J. T-2 toxin and its metabolites: Characterization, cytotoxic mechanisms and adaptive cellular response in human hepatocarcinoma (HepG2) cells. Food Chem. Toxicol. 2020;145:111654. doi: 10.1016/j.fct.2020.111654. [DOI] [PubMed] [Google Scholar]
- 97.Ling A., Sun L.W., Guo W.B., Sun S.Y., Yang J.H., Zhao Z.H. Individual and combined cytotoxic effects of T-2 toxin and its four metabolites on porcine Leydig cells. Food Chem. Toxicol. 2020;139:111277. doi: 10.1016/j.fct.2020.111277. [DOI] [PubMed] [Google Scholar]
- 98.Tran V.N., Viktorova J., Augustynkova K., Jelenova N., Dobiasova S., Rehorova K., Fenclova M., Stranska-Zachariasova M., Vitek L., Hajslova J. In silico and in vitro studies of mycotoxins and their cocktails; Their toxicity and its mitigation by silibinin pre-treatment. Toxins. 2020;12:148. doi: 10.3390/toxins12030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marin D.E., Taranu I., Burlacu R., Tudor D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon. 2010;56:956–963. doi: 10.1016/j.toxicon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J., Sheng K., Wu W., Zhang H. Anorectic responses to T-2 toxin, HT-2 toxin, diacetoxyscirpenol and neosolaniol correspond to plasma elevations of neurotransmitters 5-hydroxytryptamine and substance P. Ecotoxicol. Environ. Saf. 2018;161:451–458. doi: 10.1016/j.ecoenv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Zhang G.L., Sun X.F., Feng Y.Z., Li B., Li Y.P., Yang F., Nyachoti C.M., Shen W., Sun S.D., Li L. Zearalenone exposure impairs ovarian primordial follicle formation via down-regulation of Lhx8 expression in vitro. Toxicol. Appl. Pharmacol. 2017;317:33–40. doi: 10.1016/j.taap.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Wu Q., Kuca K., Nepovimova E., Wu W. Type A trichothecene diacetoxyscirpenol-induced emesis corresponds to secretion of peptide YY and serotonin in mink. Toxins. 2020;12:419. doi: 10.3390/toxins12060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Broekaert N., Devreese M., De Boevre M., De Saeger S., Croubels S. T-2 toxin-3α-glucoside in broiler chickens: Toxicokinetics, absolute oral bioavailability, and in vivo hydrolysis. J. Agric. Food Chem. 2017;65:4797–4803. doi: 10.1021/acs.jafc.7b00698. [DOI] [PubMed] [Google Scholar]
- 104.Tatay E., Espín S., García-Fernández A.J., Ruiz M.J. Oxidative damage and disturbance of antioxidant capacity by zearalenone and its metabolites in human cells. Toxicol. Vitr. 2017;45:334–339. doi: 10.1016/j.tiv.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 105.Salah-Abbès J.B., Belgacem H., Ezzdini K., Abdel-Wahhab M.A., Abbès S. Zearalenone nephrotoxicity: DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. Toxicon. 2020;175:28–35. doi: 10.1016/j.toxicon.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 106.Pierzgalski A., Bryła M., Kanabus J., Modrzewska M., Podolska G. Updated review of the toxicity of selected Fusarium toxins and their modified forms. Toxins. 2021;13:768. doi: 10.3390/toxins13110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.EFSA Panel on Contaminants in the Food Chain (CONTAM) Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016;14:e04425. doi: 10.2903/j.efsa.2017.4851. [DOI] [Google Scholar]
- 108.Karaman E.F., Zeybel M., Ozden S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and α-zearalenol. Toxicol. Lett. 2020;326:52–60. doi: 10.1016/j.toxlet.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Marin D.E., Taranu I., Burlacu R., Manda G., Motiu M., Neagoe I., Dragomir C., Stancu M., Calin L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. Vitr. 2011;25:1981–1988. doi: 10.1016/j.tiv.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 110.Dellafiora L., Perotti A., Galaverna G., Buschini A., Dall’Asta C. On the masked mycotoxin zearalenone-14-glucoside. Does the mask truly hide? Toxicon. 2016;111:139–142. doi: 10.1016/j.toxicon.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 111.Cirlini M., Barilli A., Galaverna G., Michlmayr H., Adam G., Berthiller F., Dall’Asta C. Study on the uptake and deglycosylation of the masked forms of zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2016;98:232–239. doi: 10.1016/j.fct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Marin D.E., Pistol G.C., Bulgaru C.V., Taranu I. Cytotoxic and inflammatory effects of individual and combined exposure of HepG2 cells to zearalenone and its metabolites. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392:937–947. doi: 10.1007/s00210-019-01644-z. [DOI] [PubMed] [Google Scholar]
- 113.Luongo D., Severino L., Bergamo P., De Luna R., Lucisano A., Rossi M. Interactive effects of fumonisin B1 and α-zearalenol on proliferation and cytokine expression in Jurkat T cells. Toxicol. Vitr. 2006;20:1403–1410. doi: 10.1016/j.tiv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 114.Ben Salem I., Boussabbeh M., Da Silva J.P., Guilbert A., Bacha H., Abid-Essefi S., Lemaire C. SIRT1 protects cardiac cells against apoptosis induced by zearalenone or its metabolites α- and β-zearalenol through an autophagy-dependent pathway. Toxicol. Appl. Pharmacol. 2017;314:82–90. doi: 10.1016/j.taap.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 115.Steinkellner H., Binaglia M., Dall’Asta C., Gutleb A.C., Metzler M., Oswald I.P., Parent-Massin D., Alexander J. Combined hazard assessment of mycotoxins and their modified forms applying relative potency factors: Zearalenone and t2/ht2 toxin. Food Chem. Toxicol. 2019;131:110599. doi: 10.1016/j.fct.2019.110599. [DOI] [PubMed] [Google Scholar]
- 116.Liu L., Xie M., Wei D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022;23:1064. doi: 10.3390/ijms23031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bittner G.D., Denison M.S., Yang C.Z., Stoner M.A., He G. Chemicals having estrogenic activity can be released from some bisphenol a-free, hard and clear, thermoplastic resins. Environ. Health. 2014;13:103. doi: 10.1186/1476-069X-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tatay E., Espín S., García-Fernández A.-J., Ruiz M.-J. Estrogenic activity of zearalenone, α-zearalenol and β-zearalenol assessed using the E-screen assay in MCF-7 cells. Toxicol. Mech. Methods. 2018;28:239–242. doi: 10.1080/15376516.2017.1395501. [DOI] [PubMed] [Google Scholar]
- 119.Eze U.A., Huntriss J., Routledge M.N., Gong Y.Y., Connolly L. The effect of individual and mixtures of mycotoxins and persistent organochloride pesticides on oestrogen receptor transcriptional activation using in vitro reporter gene assays. Food Chem. Toxicol. 2019;130:68–78. doi: 10.1016/j.fct.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 120.Dellafiora L., Ruotolo R., Perotti A., Cirlini M., Galaverna G., Cozzini P., Buschini A., Dall’Asta C. Molecular insights on xenoestrogenic potential of zearalenone-14-glucoside through a mixed in vitro/in silico approach. Food Chem. Toxicol. 2017;108:257–266. doi: 10.1016/j.fct.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 121.Foryst-Ludwig A., Clemenz M., Hohmann S., Hartge M., Sprang C., Frost N., Krikov M., Bhanot S., Barros R., Morani A., et al. Metabolic actions of estrogen receptor beta (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qu L., Wang L., Ji H., Fang Y., Lei P., Zhang X., Jin L., Sun D., Dong H. Toxic mechanism and biological detoxification of fumonisins. Toxins. 2022;14:182. doi: 10.3390/toxins14030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith G.W. Fumonisins. In: Gupta R.C., editor. Veterinary Toxicology. Elsevier Inc.; Amsterdam, The Netherlands: 2018. pp. 1003–1018. [Google Scholar]
- 124.EFSA Panel on Contaminants in the Food Chain (CONTAM) Knutsen H., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018;16:5172. doi: 10.2903/j.efsa.2018.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wangia-Dixon R.N., Nishimwe K. Molecular toxicology and carcinogenesis of fumonisins: A review. J. Environ. Sci. Health Part C. 2021;39:44–67. doi: 10.1080/26896583.2020.1867449. [DOI] [PubMed] [Google Scholar]
- 126.Riley R.T., Merrill A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019;60:1183–1189. doi: 10.1194/jlr.S093815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Singh M.P., Kang S.C. Endoplasmic reticulum stress-mediated autophagy activation attenuates fumonisin b1 induced hepatotoxicity in vitro and in vivo. Food Chem. Toxicol. 2017;110:371–382. doi: 10.1016/j.fct.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 128.Arumugam T., Pillay Y., Ghazi T., Nagiah S., Abdul N.S., Chuturgoon A.A. Fumonisin B1-induced oxidative stress triggers Nrf2-mediated antioxidant response in human hepatocellular carcinoma (HepG2) cells. Mycotoxin Res. 2019;35:99–109. doi: 10.1007/s12550-018-0335-0. [DOI] [PubMed] [Google Scholar]
- 129.Sheik Abdul N., Marnewick J.L. Fumonisin B1-induced mitochondrial toxicity and hepatoprotective potential of rooibos: An update. J. Appl. Toxicol. 2020;40:1602–1613. doi: 10.1002/jat.4036. [DOI] [PubMed] [Google Scholar]
- 130.Molina-Pintor I.B., Rojas-Garcia A.E., Medina-Dias I.M., Barrón-Vivanco B.S., Bernal-Hernández Y.Y., Ortega-Cervantes L., Ramos A.J., Herrera-Moreno J.F., Gonzáles-Ariaz C.A. An update of geneotoxic and epigenetic studies of fumonisin B1. World Mycotoxin J. 2021;15:57–72. doi: 10.3920/WMJ2021.2720. [DOI] [Google Scholar]
- 131.Pinhão M., Tavares A.M., Loureiro S., Louro H., Alvito P., Silva M.J. Combined cytotoxic and genotoxic effects of ochratoxin A and fumonisin B1 in human kidney and liver cell models. Toxicol. Vitr. 2020;68:104949. doi: 10.1016/j.tiv.2020.104949. [DOI] [PubMed] [Google Scholar]
- 132.Yu S., Jia B., Liu N., Yu D., Zhang S., Wu A. Fumonisin B1 triggers carcinogenesis via HDAC/PI3K/Akt signalling pathway in human esophageal epithelial cells. Sci. Total Environ. 2021;787:147405. doi: 10.1016/j.scitotenv.2021.147405. [DOI] [PubMed] [Google Scholar]
- 133.Ahangarkani F., Rouhi S., Gholamour Azizi I. A review on incidence and toxicity of fumonisins. Toxin. Rev. 2014;33:95–100. doi: 10.3109/15569543.2013.871563. [DOI] [Google Scholar]
- 134.Cao C., Xian R., Lin F., Li X., Li X., Qiang F., Li X. Fumonisin B1 induces hepatotoxicity in mice through the activation of oxidative stress, apoptosis and fibrosis. Chemosphere. 2022;296:133910. doi: 10.1016/j.chemosphere.2022.133910. [DOI] [PubMed] [Google Scholar]
- 135.Yu S., Jia B., Liu N., Yu D., Wu A. Evaluation of the Individual and combined toxicity of fumonisin mycotoxins in human gastric epithelial cells. Int. J. Mol. Sci. 2020;21:5917. doi: 10.3390/ijms21165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gu M.J., Han S.E., Hwang K., Mayer E., Reisinger N., Schatzmayr D., Park B.-C., Han S.H., Yun C.-H. Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019;305:110–116. doi: 10.1016/j.toxlet.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Fraeyman S., Croubels S., Devreese M., Antonissen G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins. 2017;9:228. doi: 10.3390/toxins9070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gruber-Dorninger C., Novak B., Nagl V., Berthiller F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017;65:7052–7070. doi: 10.1021/acs.jafc.6b03413. [DOI] [PubMed] [Google Scholar]
- 139.De Sá S.V.M., Monteiro C., Fernandes J.O., Pinto E., Faria M.A., Cunha S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.1967282. [DOI] [PubMed] [Google Scholar]
- 140.Mallebrera B., Prosperini A., Font G., Ruiz M.J. In vitro mechanisms of beauvericin toxicity: A review. Food Chem. Toxicol. 2018;111:537–545. doi: 10.1016/j.fct.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 141.Wu Q., Patocka J., Kuca K. Beauvericin, a Fusarium mycotoxin: Anticancer activity, mechanisms, and human exposure risk assessment. Mini Rev. Med. Chem. 2019;19:206–214. doi: 10.2174/1389557518666180928161808. [DOI] [PubMed] [Google Scholar]
- 142.Agahi F., Álvarez-Ortega N., Font G., Juan-García A., Juan C. Oxidative stress, glutathione, and gene expression as key indicators in SH-SY5Y cells exposed to zearalenone metabolites and beauvericin. Toxicol. Lett. 2020;334:44–52. doi: 10.1016/j.toxlet.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 143.Cimbalo A., Alonso-Garrido M., Font G., Frangiamone M., Manyes L. Transcriptional changes after enniatins A, A1, B and B1 ingestion in rat stomach, liver, kidney and lower intestine. Foods. 2021;10:1630. doi: 10.3390/foods10071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fremy J.M., Alassane-Kpembi I., Oswald I.P., Cottrill B., Van Egmond H.P. A review on combined effects of moniliformin and co-occurring Fusarium toxins in farm animals. World Mycotoxin J. 2019;12:281–291. doi: 10.3920/WMJ2018.2405. [DOI] [Google Scholar]
- 145.Pierron A., Neves M., Puel S., Lippi Y., Soler L., Miller J.D., Oswald I.P. Intestinal toxicity of the new type A trichothecenes, NX and 3ANX. Chemosphere. 2021;288:132415. doi: 10.1016/j.chemosphere.2021.132415. [DOI] [PubMed] [Google Scholar]
- 146.Woelflingseder L., Warth B., Vierheilig I., Schwartz-Zimmermann H., Hametner C., Nagl V., Novak B., Šarkanj B., Berthiller F., Adam G. The Fusarium metabolite culmorin suppresses the in vitro glucuronidation of deoxynivalenol. Arch. Toxicol. 2019;93:1729–1743. doi: 10.1007/s00204-019-02459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jarolim K., Wolters K., Woelflingseder L., Pahlke G., Beisl J., Puntscher H., Braun D., Sulyok M., Warth B., Marko D. The secondary Fusarium metabolite aurofusarin induces oxidative stress, cytotoxicity and genotoxicity in human colon cells. Toxicol. Lett. 2018;284:170–183. doi: 10.1016/j.toxlet.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 148.Arumugam T., Ghazi T., Abdul N.S., Chuturgoon A.A. A review on the oxidative effects of the fusariotoxins: Fumonisin B1 and fusaric acid. Toxicology. 2021;19:181–190. doi: 10.1016/B978-0-12-819092-0.00019-4. [DOI] [Google Scholar]
- 149.Agriopoulou S., Stamatelopoulou E., Varzakas T. Control strategies: Prevention and detoxification in foods. Foods. 2020;86:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sujayasree O.J., Chaitanya A.K., Bhoite R., Pandiselvam R., Kothakota A., Gavahian M., Mousavi Khaneghah A. Ozone: An advanced oxidation technology to enhance sustainable food consumption through mycotoxin degradation. Ozone Sci. Eng. 2022;44:17–37. doi: 10.1080/01919512.2021.1948388. [DOI] [Google Scholar]
- 151.Wielogorska E., Ahmed Y., Meneely J., Graham W.G., Elliott C.T., Gilmore B.F. A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 2019;301:125281. doi: 10.1016/j.foodchem.2019.125281. [DOI] [PubMed] [Google Scholar]
- 152.Alabouvette C., Olivain C., Migheli Q., Steinberg C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009;184:529–544. doi: 10.1111/j.1469-8137.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 153.da Cruz Cabral L., Fernández Pinto V., Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 154.Liu J., Sui Y., Wisniewski M., Droby S., Liu Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013;167:153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 155.Guan S., Zhou T., Yin Y., Xie M., Ruan Z., Young J. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011;4:413–424. doi: 10.3920/WMJ2011.1290. [DOI] [Google Scholar]
- 156.Abdallah M.F., De Boevre M., Landschoot S., De Saeger S., Haesaert G., Audenaert K. Fungal endophytes control Fusarium graminearum and reduce trichothecenes and zearalenone in maize. Toxins. 2018;10:493. doi: 10.3390/toxins10120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abdallah M.F., Ameye M., De Saeger S., Audenaert K., Haesaert G. Biological Control of Mycotoxigenic Fungi and Their Toxins: An Update for the Pre-Harvest Approach. Mycotoxins-Impact and Management Strategies. IntechOpen; London, UK: 2018. [DOI] [Google Scholar]
- 158.Błaszczyk L., Basińska-Barczak A., Ćwiek-Kupczyńska H., Gromadzka K., Popiel D., Stępień Ł. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Pol. J. Microbiol. 2017;66:85. doi: 10.5604/17331331.1234997. [DOI] [PubMed] [Google Scholar]
- 159.Pellan L., Ahmeth C., Dieye T., Durand N., Fontana A., Strub C., Schorr-Galindo S. Biocontrol agents: Toolbox for the screening of weapons against mycotoxigenic Fusarium. J. Fungi. 2021;7:446. doi: 10.3390/jof7060446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Soler S., Rey M., Ait-lahsen H., Monte E., Llobell A. Biocontrol fungus Trichoderma harzianum. Society. 2001;67:5833–5839. doi: 10.1128/AEM.67.12.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren X., Branà M.T., Haidukowski M., Gallo A., Zhang Q., Logrieco A.F., Li P., Zhao S., Altomare C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins. 2022;14:86. doi: 10.3390/toxins14020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zin N.A., Badaluddin N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020;65:168–178. doi: 10.1016/j.aoas.2020.09.003. [DOI] [Google Scholar]
- 163.Tian Y., Tan Y., Liu N., Yan Z., Liao Y., Chen J., De Saeger S., Yang H., Zhang Q., Wu A. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of Trichoderma when confronted with Fusarium graminearum. Toxins. 2016;8:335. doi: 10.3390/toxins8110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He A.L., Jia L.I.U., Wang X.H., Zhang Q.G., Wei S., Jie C. Soil application of Trichoderma asperellum GDFS1009 granules promotes growth and resistance to Fusarium graminearum in maize. J. Integr. Agric. 2019;18:599–606. doi: 10.1016/S2095-3119(18)62089-1. [DOI] [Google Scholar]
- 165.Saravanakumar K., Dou K., Lu Z., Wang X., Li Y., Chen J. Enhanced biocontrol activity of cellulase from Trichoderma harzianum against Fusarium graminearum through activation of defense-related genes in maize. Physiol. Mol. Plant Pathol. 2018;103:130–136. doi: 10.1016/j.pmpp.2018.05.004. [DOI] [Google Scholar]
- 166.Li Y., Sun R., Yu J., Saravanakumar K., Chen J. Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 2016;56:318–327. doi: 10.1007/s12088-016-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tian Y., Tan Y., Yan Z., Liao Y., Chen J., De Boevre M., De Saeger S., Wu A. Antagonistic and detoxification potentials of Trichoderma isolates for control of zearalenone (ZEN) producing Fusarium graminearum. Front. Microbiol. 2018;8:2710. doi: 10.3389/fmicb.2017.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Agriopoulou S., Stamatelopoulou E., Sachadyn-Król M., Varzakas T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms. 2020;8:952. doi: 10.3390/microorganisms8060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smaoui S., Agriopoulou S., D’Amore T., Tavares L., Khaneghah A.M. The control of Fusarium growth and decontamination of produced mycotoxins by lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2022 doi: 10.1080/10408398.2022.2087594. [DOI] [PubMed] [Google Scholar]
- 170.Li J., Wang W., Chen S., Shao T., Tao X., Yuan X. Effect of lactic acid bacteria on the fermentation quality and mycotoxins concentrations of corn silage infested with mycotoxigenic fungi. Toxins. 2021;13:699. doi: 10.3390/toxins13100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mokoena M.P., Chelule P.K., Gqaleni N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005;68:2095–2099. doi: 10.4315/0362-028X-68.10.2095. [DOI] [PubMed] [Google Scholar]
- 172.Franco T.S., Garcia S., Hirooka E.Y., Ono Y.S., dos Santos J.S. Lactic acid bacteria in the inhibition of Fusarium graminearum and deoxynivalenol detoxification. J. Appl. Microbiol. 2011;111:739–748. doi: 10.1111/j.1365-2672.2011.05074.x. [DOI] [PubMed] [Google Scholar]
- 173.Niderkorn V., Morgavi D.P., Aboab B., Lemaire M., Boudra H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009;106:977–985. doi: 10.1111/j.1365-2672.2008.04065.x. [DOI] [PubMed] [Google Scholar]
- 174.Niderkorn V., Morgavi D.P., Pujos E., Tissandier A., Boudra H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in an in vitro simulated corn silage model. Food Addit. Contam. 2007;24:406–415. doi: 10.1080/02652030601101110. [DOI] [PubMed] [Google Scholar]
- 175.Castro J.C., Pante G.C., Centenaro B.M., Almeida R.T.R.D., Pilau E.J., Dias Filho B.P., Machinski Junior M. Antifungal and antimycotoxigenic effects of Zingiber officinale, Cinnamomum zeylanicum and Cymbopogon martinii essential oils against Fusarium verticillioides. Food Addit. Contam. Part A. 2020;37:1531–1541. doi: 10.1080/19440049.2020.1778183. [DOI] [PubMed] [Google Scholar]
- 176.Castro J.C., Endo E.H., de Souza M.R., Zanqueta E.B., Polonio J.C., Pamphile J.A., Ueda-Nakamura T., Nakamura C.V., Filho B.P.D., de Abreu Filho B.A. Bioactivity of essential oils in the control of Alternaria alternata in dragon fruit (Hylocereus undatus Haw.) Ind. Crops Prod. 2017;97:101–109. doi: 10.1016/j.indcrop.2016.12.007. [DOI] [Google Scholar]
- 177.Mirza Alizadeh A., Golzan S.A., Mahdavi A., Dakhili S., Torki Z., Hosseini H. Recent advances on the efficacy of essential oils on mycotoxin secretion and their mode of action. Crit. Rev. Food Sci. Nutr. 2022;62:4726–4751. doi: 10.1080/10408398.2021.1878102. [DOI] [PubMed] [Google Scholar]
- 178.Gwiazdowska D., Marchwińska K., Juś K., Uwineza P.A., Gwiazdowski R., Waśkiewicz A., Kierzek R. The concentration-dependent effects of essential oils on the growth of Fusarium graminearum and mycotoxins biosynthesis in wheat and maize grain. Appl. Sci. 2022;12:473. doi: 10.3390/app12010473. [DOI] [Google Scholar]
- 179.Perczak A., Gwiazdowska D., Gwiazdowski R., Juś K., Marchwińska K., Waśkiewicz A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens. 2020;9:23. doi: 10.3390/pathogens9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.López A.G., Theumer M.G., Zygadlo J.A., Rubinstein H.R. Aromatic plants essential oils activity on Fusarium verticillioides fumonisin B1 production in corn grain. Mycopathologia. 2004;158:343–349. doi: 10.1007/s11046-005-3969-3. [DOI] [PubMed] [Google Scholar]
- 181.Pante G.C., Castro J.C., Lini R.S., Romoli J.C.Z., Almeida R.T.R.D., Garcia F.P., Machinski M. Litsea cubeba essential oil: Chemical profile, antioxidant activity, cytotoxicity, effect against Fusarium verticillioides and fumonisins production. J. Environ. Sci. Health A. 2021;56:387–395. doi: 10.1080/03601234.2021.1890519. [DOI] [PubMed] [Google Scholar]
- 182.Perczak A., Juś K., Marchwińska K., Gwiazdowska D., Waśkiewicz A., Goliński P. Degradation of zearalenone by essential oils under in vitro conditions. Front. Microbiol. 2016;7:1224. doi: 10.3389/fmicb.2016.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xing F., Hua H., Selvaraj J.N., Yuan Y., Zhao Y., Zhou L., Liu Y. Degradation of fumonisin B1 by cinnamon essential oil. Food Control. 2014;38:37–40. doi: 10.1016/j.foodcont.2013.09.045. [DOI] [Google Scholar]
- 184.Makhuvele R., Naidu K., Gbashi S., Thipe V.C., Adebo O.A., Njobeh P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon. 2020;6:e05291. doi: 10.1016/j.heliyon.2020.e05291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garcia D., Ramos A.J., Sanchis V., Marín S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int. J. Food Microbiol. 2012;153:21–27. doi: 10.1016/j.ijfoodmicro.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 186.Seepe H.A., Lodama K.E., Sutherland R., Nxumalo W., Amoo S.O. In vivo antifungal activity of South African medicinal plant extracts against Fusarium pathogens and their phytotoxicity evaluation. Plants. 2020;9:1668. doi: 10.3390/plants9121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferruz E., Loran S., Herrera M., Gimenez I., Bervis N., Barcena C., Carramiñana J.J., Juan T., Herrera A., Ariño A. Inhibition of Fusarium growth and mycotoxin production in culture medium and in maize kernels by natural phenolic acids. J. Food Prot. 2016;79:1753–1758. doi: 10.4315/0362-028X.JFP-15-563. [DOI] [PubMed] [Google Scholar]
- 188.Montibus M., Vitrac X., Coma V., Loron A., Pinson-Gadais L., Ferrer N., Verdal-Bonnin M.N., Gabaston J., Waffo-Téguo P., Richard-Forget F., et al. Screening of wood/forest and vine by-products as sources of new drugs for sustainable strategies to control Fusarium graminearum and the production of mycotoxins. Molecules. 2021;26:405. doi: 10.3390/molecules26020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Savoie J.M., Mata G., Atanasova Penichon V., Foulonge-Oriol M. Using mushroom-forming fungi in preventing and reducing mycotoxins in cereal products. Sci. Fungorum. 2019;49:e1256. doi: 10.33885/sf.2019.49.1256. [DOI] [Google Scholar]
- 190.El-Ramady H., Abdalla N., Fawzy Z., Badgar K., Llanaj X., Törős G., Hajdú P., Eid Y., Prokisch J. Green biotechnology of oyster mushroom (Pleurotus ostreatus L.): A sustainable strategy for myco-remediation and bio-fermentation. Sustainability. 2022;14:3667. doi: 10.3390/su14063667. [DOI] [Google Scholar]
- 191.Mihai R.A., Melo Heras E.J., Florescu L.I., Catana R.D. The edible gray oyster fungi Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm a potent waste consumer, a biofriendly species with antioxidant activity depending on the growth substrate. J. Fungi. 2022;8:274. doi: 10.3390/jof8030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Branà M.T., Cimmarusti M.T., Haidukowski M., Logrieco A.F., Altomare C. Bioremediation of aflatoxin B1-contaminated maize by king oyster mushroom (Pleurotus eryngii) PLoS ONE. 2017;12:e0182574. doi: 10.1371/journal.pone.0182574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jackson L.W., Pryor B.M. Degradation of aflatoxin B1 from naturally contaminated maize using the edible fungus Pleurotus ostreatus. AMB Express. 2017;7:110. doi: 10.1186/s13568-017-0415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Motomura M., Toyomasu T., Mizuno K., Shinozawa T. Purification and characterization of an aflatoxin degradation enzyme from Pleurotus ostreatus. Microbiol. Res. 2003;158:237–242. doi: 10.1078/0944-5013-00199. [DOI] [PubMed] [Google Scholar]
- 195.Alberts J.F., Gelderblom W.C.A., Botha A., van Zyl W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009;135:47–52. doi: 10.1016/j.ijfoodmicro.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 196.Yehia R.S. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014;45:127–133. doi: 10.1590/S1517-83822014005000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Merel D., Savoie J.M., Mata G., Salmones D., Ortega C., Atanasova V., Chéreau S., Monribot-Villanueva J.L., Guerrero-Analco J.A. Methanolic extracts from cultivated mushrooms affect the production of fumonisins b and fusaric acid by Fusarium verticillioides. Toxins. 2020;12:366. doi: 10.3390/toxins12060366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.