Abstract
Acute respiratory distress syndrome (ARDS) alveolar environment induced a pro-repair anti-inflammatory macrophage polarization. However, patients with coronavirus disease 2019 (COVID-19) ARDS frequently exhibit a huge lung inflammation and present pulmonary scars and fibrosis more frequently than patients with non-COVID-19 ARDS, suggesting that the COVID-19 ARDS alveolar environment may drives a more inflammatory or pro-fibrotic macrophage polarization. This study aimed to determine the effect of the COVID-19 ARDS alveolar environment on macrophage polarization. The main finding was that broncho-alveolar lavage fluids (BALF) from patients with early COVID-19 ARDS drove an alternative anti-inflammatory polarization in normal monocyte-derived macrophages; characterized by increased expressions of CD163 and CD16 mRNA (3.4 [2.7-7.2] and 4.7 [2.6-5.8] fold saline control, respectively – p=0.02), and a secretory pattern close to that of macrophages stimulated with IL-10, with the specificity of an increased production of IL-6. This particular alternative pattern was specific to early ARDS (compared with late ARDS) and of COVID-19 ARDS (compared with moderate COVID-19). The early COVID-19 ARDS alveolar environment drives an alternative anti-inflammatory macrophage polarization with the specificity of inducing macrophage production of IL-6.
Keywords: acute respiratory distress syndrome, COVID-19, SARS-CoV-2, macrophage, polarization
Graphical Abstract

Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- BALF
Broncho-Alveolar Lavage Fluid
- CD
Cluster of Differentiation
- COVID-19
COronaVIrus Disease 2019
- HGF
Hepatocyte Growth Factor
- ICU
Intensive Care Unit
- IFN-γ
Interferon γ
- IL-4
Interleukin 4
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- MerTK
Myeloid-epithelial-reproductive Tyrosine Kinase
- MMP
Matrix Metallo-Proteinase
- mRNA
messenger RiboNucleic Acid
- PPAR-γ
Peroxisome Proliferator-Activated Receptor-γ
- SARS-CoV-2
Severe Acute Respiratory Syndrome CoronaVirus 2
- TGF-β
Transforming Growth Factor-β
1. INTRODUCTION
Severe forms of coronavirus disease 2019 (COVID-19) are mainly marked by an acute respiratory distress syndrome (ARDS), which presents several particularities compared with ARDS caused by other factors. Among them is the delayed onset of COVID-19 ARDS beyond the usual 7-day period [1], which suggests a double-hit lung injury mechanism, in which an inappropriate and exacerbated immune response has certainly occurred. Most critically ill patients develop a hyperinflammatory phenotype [2,3], sometime called “cytokine storm”, and the survival of these patients has improved with anti-inflammatory treatments such as dexamethasone or tocilizumab (an anti-interleukin [IL]-6 receptor) [4]. Monocytes and macrophages are particularly abundant in lungs with COVID-19 ARDS [5] and are key players in inflammatory syndrome. Macrophages are plastic cells that can modify their activation profile depending on their micro-environment during the so-called “polarization” process. Along these lines, macrophages may play a pivotal role both in promoting early inflammation through a classical M1 inflammatory polarization, and in subsequent tissue repair and inflammation resolution through an alternative M2 polarization. M2 pro-fibrotic macrophages may also be involved in excessive and prolonged fibroproliferation, whose clinical expressions, such as organizing pneumonia, scarring, and pulmonary fibrosis, are observed in a higher proportion in COVID-19 than in non-COVID ARDS [6].
We aimed to determine the modulation of normal human macrophage polarization by the alveolar environment of human COVID-19 ARDS.
2. MATERIAL AND METHODS
2.1. Ethics
This study was approved by the French Anesthesiology and Critical Care Medicine ethics committee (CERAR, approval number: 00010254-2022-019) on February 18, 2022. All procedures followed the ethical standards on human experimentation of the ethical committee and the Helsinki Declaration of 1975. According to French law and due to the non-interventional design of the study, the patient's consent was waived [7].
2.2. Population
The remaining fluids from broncho-alveolar lavage (BAL) performed by the attending physician as routine care to confirm a clinical suspicion of pneumonia were used in this study. BAL fluids (BALF) from six patients with early COVID-19 ARDS (i.e., sampled in the first week following intensive care unit [ICU] admission and tracheal intubation), six patients with late COVID-19 ARDS (i.e., sampled after the first week following ICU admission and tracheal intubation), and six patients with early moderate COVID-19 (i.e., sampled within the first week following admission into the medical ward and oxygen delivery onset), in which all patients had no confirmed pneumonia, were used. All the patients with ARDS were hospitalized in the medical or surgical ICU of Saint-Antoine University Hospital (Paris, France) and all patients with moderate COVID-19 were hospitalized in the medical wards of Tenon University Hospital (Paris, France). Demographic data, results of arterial blood gases, and patient's outcomes were anonymously collected (Supplementary Table 1).
Peripheral blood monocytes were isolated from residual cell pellets that were available after blood cytapheresis was performed in healthy platelet donors (n=6). Apheresis residual cell pellets were obtained from the local site of the National Blood Service.
2.3. Broncho-alveolar lavage collection and treatment
BAL were performed with 5 × 20 mL of 0.9% saline solution, under fiberoptic control. The remaining BALF, which were not used for virological and/or bacteriological diagnosis, were centrifuged at 1500 rpm at 4°C for 10 min immediately after their arrival in the microbiological laboratory. Cell-free supernatants were collected and stored at −80°C until further use within the next 3 months.
2.4. Monocyte isolation, macrophage differentiation and polarization
Normal blood monocytes were isolated from residual cell pellets, which were available after blood cytapheresis was performed in healthy platelet donors, by gradient centrifugation with 15 mL of lymphocyte separation gradient (CMSMSL01, Eurobio). After recovery, peripheral blood mononuclear cells were washed three times to remove the platelets, and sorted using the negative magnetic Pan-Monocyte Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) as recommended by the manufacturer. The sorted cells were then seeded in non-coated 24-well plates (Costar, Corning Inc, New York, NY, USA) at 5 × 105 cells/well in 1 mL of RPMI 1640 GlutaMAX medium (GIBCO, Life Technologies, Carlsbad, CA, USA) for 90 minutes, before soft washing with 1x phosphate-buffered saline. Cells, which had more than 95% monocytes, were then cultured in RPMI 1640 GlutaMAX containing 2 mM of fresh glutamine, supplemented with non-essential amino-acids, 10% fetal calf serum (FCS), and 100 U/mL penicillin + 100 µg/mL streptomycin.
Monocytes were first differentiated in macrophages for 2 days with 50 ng/mL GM-CSF and 25 ng/mL M-CSF (R&D, Minneapolis, MN, USA) and subsequently polarized in RPMI 1640 GlutaMAX/5% FCS with 50 ng/mL recombinant human (rh) Interferon (IFN)-γ, 40 ng/mL rhIL-4 or 50 ng/mL rhIL-10 (R&D) for another 3 days. Non-polarized macrophages left in the culture medium alone were used as basal controls. To study the effect of the alveolar environment, we stimulated monocyte-derived macrophages for 3 days with either 25% of BALF vol/vol or 0.9% saline solution as the BALF control (saline basal control). Monocyte-derived macrophages from each healthy donor (n=6) was stimulated by two early COVID-19 ARDS BALF, two late COVID-19 ARDS BALF and two early moderate COVID-19 BALF, which were randomly selected among the six available samples. Consequently, the six BALF from the three groups were used twice on monocyte-derived macrophages from two distinct healthy donors. After 5 days of culture, the macrophages were lysed with TRIzol® and their supernatants were aliquoted in sterile tubes and stored at −80°C until further utilization.
2.5. Quantitative PCR
mRNA was extracted from in-vitro polarized macrophages using the NucleoSpin RNA Mini kit (Macherey-Nagel, Dueren, Germany), and reverse transcribed using the M-MLV Reverse Transcriptase kit (Promega, Madison, WI, USA) after the determination of mRNA concentrations with the Nanodrop (Nanodrop Technologies, Wilmington, DE, USA). Finally, quantitative PCR was performed using 25 ng of cDNA, 10 μl of SYBR green master Mix (Applied Biosystems, Foster City, CA, USA) and 500 nmol/L of each primer per reaction in a real-time PCR system (Model 7300, Applied BioSystems). The primer sequences are shown in Supplementary Table 2. A probe set for hUBC was used as the normalization standard.
2.6. ELISA
Protein concentrations were measured with a commercial bead-based multiplex immunoassay (ProcartaPlex immunoassay kit, Life Technologies, ThermoFischer Scientific, Waltham, USA) using the Luminex xMAP technology (Magpix®, Luminex Corp., Austin, USA). Supernatants and BALF were assayed undiluted. BALF mediator content was removed from the corresponding culture supernatant.
2.7. Statistical analysis
Results were expressed as median [25th-75th percentile]. Results obtained on monocyte-derived macrophages from two healthy donors with the same BALF were considered duplicates, and the mean of these two results was considered as the final value in the analyses. Differences between groups were assessed by non-parametric tests using GraphPad Prism 9.3 (GraphPad Software).
3. RESULTS
3.1. Early COVID-19 ARDS alveolar environment produced an alternative anti-inflammatory macrophage polarization in-vitro
Considering the mRNA expression patterns, BALF from patients with early COVID-19 ARDS induced an alternative polarization in normal monocyte-derived macrophages, which was close to the polarization induced with rhIL-10 [M(IL-10)]. Indeed, CD163 and CD16 expressions were increased 3.4-fold [2.7-7.2] and 4.7-fold [2.6-5.8], respectively (p=0.02 vs. saline control) (Figure 1 A). In contrast, the expression of CD200R and CCL22, which were two characteristic markers of the alternative polarization induced with rhIL-4, were respectively only moderately increased (3.3 [1.4-3.6]) and strongly decreased (0.3 [0.1-0.6]) (p=0.02) (Figure 1 A). This expression pattern was similar to that induced by non-COVID ARDS BALF [8]. When observing other characteristic markers of macrophage polarization (i.e., matrix metallo-proteinase [MMP]-1, MMP-9, transforming growth factor-β [TGF-β], peroxisome proliferator-activated receptor-γ [PPAR-γ], myeloid-epithelial-reproductive tyrosine kinase [MerTK]), macrophages stimulated with early COVID-19 ARDS BALF also showed an alternative mRNA pattern; this pattern being closer to that induced by IL-10 than by IL-4 (Figure 1 B).
Figure 1.
Phenotypic characteristics of macrophages polarized with recombinant human cytokines and COVID-19 broncho-alveolar lavage fluids (BALF). (A) Relative expression of CD80, CD64, CD200R, CD163 and CD16 mRNAs in monocyte-derived macrophages polarized in-vitro with rhIFN-γ, rhIL-4, rhIL-10, early COVID-19 ARDS BALF, late COVID-19 ARDS BALF and moderate COVID-19 BALF (n=6 monocyte-derived macrophages from healthy donors; each of them was stimulated with cytokines; two BALF sampled from six different patients with early COVID-19 ARDS, six patients with late COVID-19 ARDS, and six patients with early moderate COVID-19; all patients had no concomitant bacterial pneumonia). * p<0.05; ** p<0.01; (B) Relative expression of MMP-1, MMP-9, TGF-β, PPAR-γ, and MerTK in monocyte-derived macrophages polarized in vitro with rhIFN-γ, rhIL-4, rhIL-10, and early COVID-19 ARDS BALF.
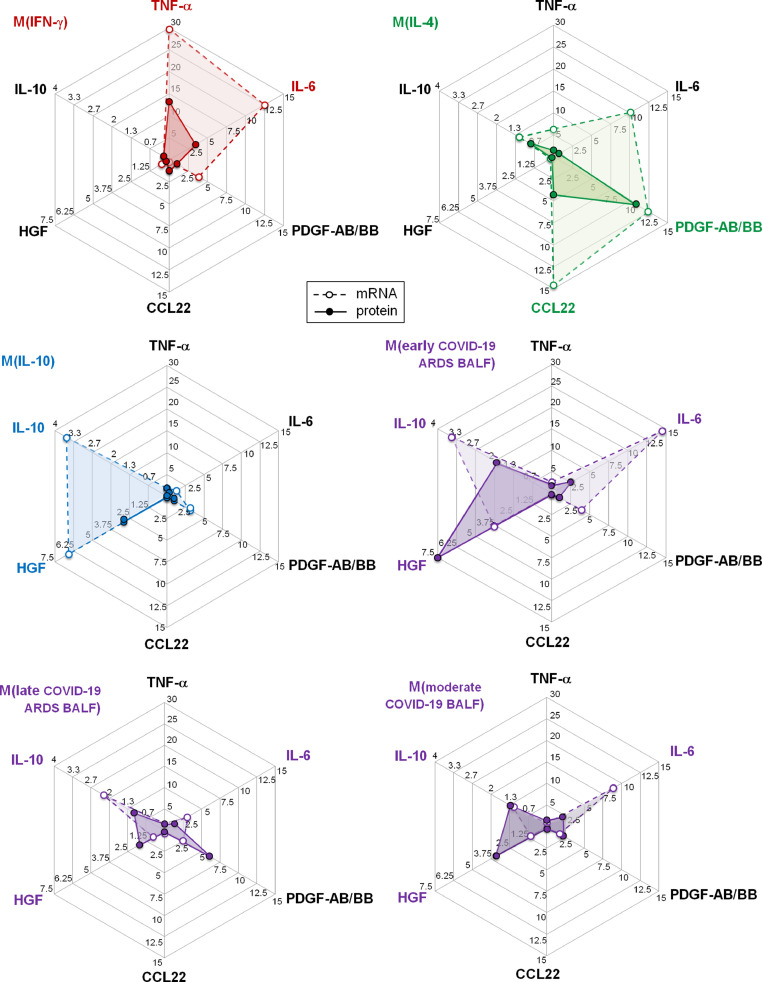
Macrophages stimulated with early COVID-19 ARDS-BALF showed a secretory pattern close to that of macrophages stimulated with IL-10, characterized by increased productions of IL-10 and HGF both at the mRNA and protein levels, and distinct from that of macrophages stimulated with IFN-γ or IL-4 (Figure 2 , and Supplementary Figures 1 and 2). This secretory pattern was similar to that previously observed after stimulation with non-COVID ARDS-BALF [8]. However, macrophages stimulated with early COVID-19 ARDS-BALF particularly showed increased expression and production of IL-6 (Figure 2 , and Supplementary Figures 1 and 2).
Figure 2.
Secretomes of macrophages polarized with recombinant human cytokines and COVID-19 broncho-alveolar lavage fluids (BALF). Radar charts represent the secretory profiles of macrophages polarized in vitro with rhIFN-γ, rhIL-4, rhIL-10, early COVID-19 ARDS BALF, late COVID-19 ARDS BALF, and moderate COVID-19 BALF, both at the mRNA (empty circles and dotted lines) and protein (filled circles and solid lines) levels (n=6). Results are expressed relatively to mRNA expression and protein concentration observed for basal or saline control macrophages. IL-10 concentration was not measured in M(IL-10) supernatants due to its interference with the rhIL-10 used for macrophage polarization.
3.2. Macrophage polarization induced by COVID-19 ARDS alveolar environment is specific to early COVID-19-related lung injury
In comparison to the early COVID-19 ARDS BALF, BALF from patients with late COVID-19 ARDS had not induced any significant polarization (1.5 [0.7-2.2] and 2.2 [0.8-3.1] for CD163 and CD16 relative mRNA expressions, respectively – Figure 1 A). The characteristic HGF, IL-10, and IL-6 up-regulations, which were induced by early COVID-19 ARDS BALF at the mRNA and protein levels, were significantly reduced in normal monocyte-derived macrophages stimulated with late COVID-19 ARDS BALF (Figure 2).
3.3. Macrophage polarization induced by COVID-19 ARDS alveolar environment is dependent on the level of lung injury
In comparison to the early COVID-19 ARDS BALF, early BALF from patients with moderate COVID-19 who were hospitalized in the wards had not induced any significant polarization (0.9 [0.8-2.4] and 1.3 [0.7-2.2] for CD163 and CD16 relative mRNA expressions, respectively –Figure 1 A). The characteristic HGF, IL-10, and IL-6 up-regulations, which were induced by early COVID-19 ARDS BALF at the mRNA and protein levels, were significantly reduced in normal monocyte-derived macrophages stimulated with early moderate COVID-19 BALF (Figure 2).
4. Discussion
Stimulation of normal human monocyte-derived macrophages with COVID-19 ARDS BALF induced an alternative macrophage polarization. Previous studies based on single-cell analyses have shown that several macrophage sub-populations co-exist in COVID-19-injured lungs [5,9,10]. These studies have reported that critically ill patients suffering from SARS-CoV-2-induced ARDS exhibit an enriched fraction of inflammatory macrophages compared with patients with mild COVID-19 or healthy controls [5]. However, transcriptomic analyses have revealed that an inflammatory pattern has mainly been identified in recently recruited monocytes and newly differentiated macrophages, whereas macrophages present at the injured site have progressively shifted toward an alternative polarization [9,11]. Thus, macrophages pass gradually through several phenotypes, first characterized by high CD163 and legumain expressions (i.e., the so-called CD163/LGMN macrophages by Wendish et al.); and then acquired more mature phenotypes characterized by the expression of genes related to TGF-β signaling (notably TGF-β), scavenger receptors and molecules associated with apoptotic cell uptake (notably CD163 and MerTK) and extracellular matrix components or molecules involved in its breakdown (such as MMP-9) [9]. Interestingly, in this study, we found that macrophages stimulated with COVID-19 ARDS BALF expressed all these markers, such as macrophages stimulated with IL-4 or IL-10, whereas macrophages stimulated with IFN-γ had almost no expression. However, this expression pattern was closer to that observed after stimulation with IL-10 than with IL-4. This finding may appear, at first glance, in contrast with the results reporting an alternative pro-fibrotic polarization following in vitro macrophage infection with SARS-CoV-2 [9,11]. However, in these studies, normal monocyte-derived macrophages were only infected with SARS-CoV-2, apart from the co-stimulation with all other components of the alveolar environment. In addition, the alternative polarization close to that induced by IL-10 reported in this study could also appear in contrast with ex vivo results reporting an alternative polarization close to that exhibited by lung macrophages from idiopathic pulmonary fibrosis (IPF) [9,10]. However, in these studies, the macrophages were isolated from autopsy samples [9,10], and BAL were performed in the most critically ill patients requiring extra-corporeal membranous oxygenation [9]. These factors may constitute a selection bias leading to reports of macrophage polarization in patients with defective alveolar repair, who consequently exhibited aberrant lung fibroproliferation and IPF-like macrophage polarization.
In addition to this alternative anti-inflammatory polarization, we observed a specific increased production of IL-6, which has not been formerly observed in macrophages polarized with BALF from patients suffering from non-COVID-induced ARDS [8]. Lung compartmentalization of inflammation, which had already been reported in non-COVID-19 ARDS, is now also established during SARS-CoV-2-induced ARDS. Indeed, alveolar IL-6 concentrations are approximately 300-times higher than those in serum of patients with critical COVID-19 [12,13]. Several lung cell types, including alveolar macrophages, can produce IL-6 during ARDS. Our results confirmed that the COVID-19 ARDS alveolar environment had the particularity of increasing macrophage IL-6 production. We found that this feature was specific to early and critically injured alveolar environment, which is consistent with a previous report showing that IL-6 and HGF concentrations, two of the mediators whose macrophage production is particularly increased following COVID-19 ARDS BALF stimulation, are increased in patients with the most severe SARS-CoV-2-induced lung injury [14]. Given that we did not perform single-cell transcriptional analyses, we could not exclude the possibility that several variations of macrophage polarization may exist in different macrophages, some of them being responsible for IL-6 production, whereas the others fully adopted an anti-inflammatory pattern. In any case, alveolar macrophages may contribute to the high alveolar IL-6 concentrations observed during critical COVID-19. This finding may be due to the direct infection of macrophages by SARS-CoV-2 [11] and due to macrophage stimulation by elevated concentrations of IFN-γ [15]. Notably, IL-6, which is classically considered a typical pro-inflammatory cytokine, may have mild anti-inflammatory properties on macrophages [16] and may enhance the induction of an alternative polarization when combined with other cytokines, such as IL-4 and IL-13 [17]. This effect is partially mediated by STAT-3 activation [17], which is also the key transcription factor of IL-10-induced polarization. Thus, this deserves further investigations to determine whether COVID-19-induced IL-6 up-regulation in macrophages maintains an inflammatory component within the injured alveoli or whether it contributes to increasing the pro-repair and anti-inflammatory properties of alternatively polarized macrophages.
Author contributions
M.G conceived the study, supervised the experiments, analyzed the data and drafted the original version of the manuscript and revisions.
F.B performed the experiments and analyzed the data.
L.M-J provided the BALF samples and corrected the manuscript.
C.Q, A.M, and B.C corrected the manuscript for important intellectual content.
Financial support
This work has been funded by a research grant from the French Society of Anesthesiology and Critical Care Medicine (SFAR), by a grant from the ANR (FIBROCO AAP RA-COVID-19 V12) and by the “projet INFLAMEX” (ANR-10-LABX-17).
Declaration of Competing Interest
None.
Acknowledgments
The authors want to thank all staff members of the Trinité site of the “Etablissement Français du Sang” and of the Saint-Antoine hospital and Trousseau hospital virological laboratories for their helpful willingness.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imlet.2022.11.003.
Appendix. Supplementary materials
References
- 1.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavillegrand J.-R., Garnier M., Spaeth A., Mario N., Hariri G., Pilon A., Berti E., Fieux F., Thietart S., Urbina T., Turpin M., Darrivière L., Fartoukh M., Verdonk F., Dumas G., Tedgui A., Guidet B., Maury E., Chantran Y., Voiriot G., Ait-Oufella H. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann. Intensive Care. 2021;11:9. doi: 10.1186/s13613-020-00798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Veerdonk F.L., Giamarellos-Bourboulis E., Pickkers P., Derde L., Leavis H., van Crevel R., Engel J.J., Wiersinga W.J., Vlaar A.P.J., Shankar-Hari M., van der Poll T., Bonten M., Angus D.C., van der Meer J.W.M., Netea M.G. A guide to immunotherapy for COVID-19. Nat. Med. 2022;28:39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 5.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri L., Moss S., Khan F.A., Chi W., Xia J., Robinson K., Smyth A.R., Jenkins G., Stewart I. Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-218275. thoraxjnl-2021-218275. [DOI] [PubMed] [Google Scholar]
- 7.Toulouse E., Lafont B., Granier S., Mcgurk G., Bazin J.-E. French legal approach to patient consent in clinical research. Anaesth. Crit. Care Pain Med. 2020;39:883–885. doi: 10.1016/j.accpm.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Garnier M., Gibelin A., Mailleux A.A., Leçon V., Hurtado-Nedelec M., Laschet J., Trebbia G., Neuville M., Tanaka S., Crestani B., Dehoux M., Quesnel C. Macrophage Polarization Favors Epithelial Repair During Acute Respiratory Distress Syndrome. Crit. Care Med. 2018;46:e692–e701. doi: 10.1097/CCM.0000000000003150. [DOI] [PubMed] [Google Scholar]
- 9.Wendisch D., Dietrich O. T. Mari, S. von Stillfried, I.L. Ibarra, M. Mittermaier, C. Mache, R.L. Chua, R. Knoll, S. Timm, S. Brumhard, T. Krammer, H. Zauber, A.L. Hiller, A. Pascual-Reguant, R. Mothes, R.D. Bülow, J. Schulze, A.M. Leipold, S. Djudjaj, F. Erhard, R. Geffers, F. Pott, J. Kazmierski, J. Radke, P. Pergantis, K. Baßler, C. Conrad, A.C. Aschenbrenner, B. Sawitzki, M. Landthaler, E. Wyler, D. Horst, Deutsche COVID-19 OMICS Initiative (DeCOI), S. Hippenstiel, A. Hocke, F.L. Heppner, A. Uhrig, C. Garcia, F. Machleidt, S. Herold, S. Elezkurtaj, C. Thibeault, M. Witzenrath, C. Cochain, N. Suttorp, C. Drosten, C. Goffinet, F. Kurth, J.L. Schultze, H. Radbruch, M. Ochs, R. Eils, H. Müller-Redetzky, A.E. Hauser, M.D. Luecken, F.J. Theis, C. Conrad, T. Wolff, P. Boor, M. Selbach, A.-E. Saliba, L.E. Sander, SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184:6243–6261. doi: 10.1016/j.cell.2021.11.033. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A., Querrey M., Markov N.S., Kim S., Kurihara C., Garza-Castillon R., Manerikar A., Shilatifard A., Tomic R., Politanska Y., Abdala-Valencia H., Yeldandi A.V., Lomasney J.W., Misharin A.V., Budinger G.R.S. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020;12:eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boumaza A., Gay L., Mezouar S., Bestion E., Diallo A.B., Michel M., Desnues B., Raoult D., Scola B.La, Halfon P., Vitte J., Olive D., Mege J.-L. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. J. Infect. Dis. 2021;224:395–406. doi: 10.1093/infdis/jiab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendib I., Beldi-Ferchiou A., Schlemmer F., Surenaud M., Maitre B., Plonquet A., Carteaux G., Razazi K., Godot V., Hüe S., Mekontso Dessap A., de Prost N. Alveolar compartmentalization of inflammatory and immune cell biomarkers in pneumonia-related ARDS. Crit. Care Lond. Engl. 2021;25:23. doi: 10.1186/s13054-020-03427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouan Y., Baranek T., Si-Tahar M., Paget C., Guillon A. Lung compartmentalization of inflammatory biomarkers in COVID-19-related ARDS. Crit. Care Lond. Engl. 2021;25:120. doi: 10.1186/s13054-021-03513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quartuccio L., Fabris M., Sonaglia A., Peghin M., Domenis R., Cifù A., Curcio F., Tascini C. Interleukin 6, soluble interleukin 2 receptor alpha (CD25), monocyte colony-stimulating factor, and hepatocyte growth factor linked with systemic hyperinflammation, innate immunity hyperactivation, and organ damage in COVID-19 pneumonia. Cytokine. 2021;140 doi: 10.1016/j.cyto.2021.155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemand C., Nimmesgern A., Haan S., Fischer P., Schaper F., Rossaint R., Heinrich P.C., Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. Baltim. Md. 1950;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. 2003. [DOI] [PubMed] [Google Scholar]
- 17.Fernando M.R., Reyes J.L., Iannuzzi J., Leung G., McKay D.M. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PloS One. 2014;9:e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.