Significance
The majority of diseases that cause irreversible vision loss result from pathologic ocular neovascularization, such as proliferative diabetic retinopathy, retinopathy of prematurity, and wet age-related macular degeneration. Further exploration of the molecular mechanisms underlying neovascularization is relevant to clinicians who need novel agents to selectively improve the clinical management of neovascular eye diseases. As shown in the present study, microRNA-29a is down-regulated during pathological ocular neovascularization in preclinical models and is identified as an antiangiogenic factor that modulates endothelial cell activities by altering platelet-derived growth factor signaling and the extracellular matrix environment. Our findings reveal the importance of microRNA-29a in vascular homeostasis.
Keywords: miR-29a, neovascular eye disease, ocular neovascularization, angiogenesis, PDGFC
Abstract
Abnormal neovascularization is an important cause of blindness in many ocular diseases, for which the etiology and pathogenic mechanisms remain incompletely understood. Recent studies have revealed the diverse roles of noncoding RNAs in various biological processes and facilitated the research and development of the clinical application of numerous RNA drugs, including microRNAs. Here, we report the antiangiogenic activity of microRNA-29a (miR-29a) in three animal models of ocular neovascularization. The miR-29a knockout (KO) mice displayed enhanced vessel pruning, resulting in a decreased vascularized area during retinal development. In contrast, miR-29a deletion in adult mice accelerated angiogenesis in preclinical disease models, including corneal neovascularization, oxygen-induced retinopathy, and choroidal neovascularization, while the administration of agomir-29a ameliorated pathological neovascularization. Furthermore, miR-29a exerted inhibitory effects on endothelial cell proliferation, migration, and tube formation capacities. RNA sequencing analysis of retinas from miR-29a KO mice and RNA interference experiments identified platelet-derived growth factor C and several extracellular matrix genes as downstream targets of miR-29a involved in regulating ocular angiogenesis. Our data suggest that miR-29a may be a promising clinical candidate for the treatment of neovascular diseases.
Abnormal angiogenesis is a common pathological feature in a spectrum of ocular disorders, such as proliferative diabetic retinopathy (PDR), retinopathy of prematurity (ROP), wet age-related macular degeneration (AMD), and corneal neovascularization (CoNV). It is one of the leading causes of irreversible blindness, as it potentially causes severe tissue edema, hemorrhage, fibrotic scarring, rhegmatogenous retinal detachment, and other pathological changes, ultimately resulting in vision loss (1, 2). A number of angiogenesis-related mediators have been identified, including members of the vascular endothelial growth factor (VEGF) family, fibroblast growth factor (FGF) family, hypoxia-inducible factors (HIFs), transforming growth factors, chemokines, interleukins, and interferons (3). The balance between angiogenic and antiangiogenic factors is disturbed by driving forces such as inflammation, hypoxia, ischemia, and oxidative stress, leading to a series of morphogenic events in endothelial cells (ECs), including sprouting, branching, lumen formation, anastomoses, remodeling, and eventually new vessel formation (4). VEGF has been shown to be a major contributor to angiogenesis, and anti-VEGF drugs, including bevacizumab, ranibizumab, and aflibercept, have been widely used for ocular neovascular disease therapy. However, not all patients respond to these treatments, and the repeated and long-term injections that are commonly needed may increase the possibility of ocular and systemic side effects, such as the deprivation of the neurotropic activity of VEGF that leads to retinal atrophy (5, 6). The identification of new, effective, and VEGF-independent factors for the treatment of pathological angiogenesis, particularly those essential for the maintenance of normal vessel quiescence or neovascularization under pathological conditions, is needed to overcome the limitations of anti-VEGF therapies for neovascular eye diseases.
MicroRNAs (miRNAs) are a group of small noncoding RNA molecules that regulate gene expression posttranscriptionally by binding to the 3′-untranslated regions (UTRs) of target mRNAs. Research has shown that miRNAs are involved in many biological processes, such as cell proliferation, differentiation, apoptosis, and migration, and they perform diverse functions in several diseases, such as cancer, autoimmune disease, fibrotic disease, and neovascularization (7–9). More than six miRNA-based drugs are being evaluated in phase I or phase II clinical trials. For example, MRG110, a locked nucleic acid–modified antisense nucleotide targeting miR-92, is being evaluated in patients with heart failure and as a treatment for wound healing; miravirsen (an miR-122 inhibitor) is being developed as a treatment for hepatitis C (10). Many miRNAs seem to affect angiogenesis by targeting angiogenic factors such as VEGF, HIF-1A, and angiopoietin-1 (11). To date, more than 1 dozen miRNAs have been reported to be associated with AMD, diabetic retinopathy, and ROP, such as miR-18a, miR-126, miR-132, miR-145, miR-146a, miR-150, and miR-155; however, the specific functions and mechanisms of these miRNAs in ocular microangiopathy remain to be explored in depth (12, 13). Based on accumulating evidence, miR-29 families (miR-29a, miR-29b, and miR-29c) play important roles in EC function and neovessel formation. Studies have shown that miR-29b simultaneously inhibits angiogenesis and tumorigenesis in breast cancer by targeting Akt (14); miR-29c modulates the proliferation and tube formation abilities of human umbilical vein endothelial cells (HUVECs) by suppressing insulin-like growth factor-1 signaling (15). Finally, miR-29a primarily inhibits tumor growth and angiogenesis (16, 17). However, researchers do not completely understand whether and how miR-29a plays a role in ocular neovascularization.
In this study, we used mouse models of CoNV, oxygen-induced retinopathy (OIR), and CNV to explore the potential role of miR-29a in regulating ocular vasculopathy. The miR-29a knockout (KO) mice exhibited increased angiogenesis after the establishment of diverse models of ocular neovascularization compared to wild-type (WT) mice, whereas agomir-29a (a chemically modified miRNA agonist) administration reduced pathological ocular angiogenesis. Through the high-throughput sequencing of miR-29a KO mice, in combination with a bioinformatics analysis, we discovered multiple angiogenic target genes of miR-29a, including solute carrier family 43 member 2 (SLC43A2), collagen type VII alpha 1 chain (COL7A1), and platelet-derived growth factor C (PDGFC), all of which were further validated by a luciferase assay and RNA interference experiments. The PDGF signaling pathway was identified to be critical for the regulatory function of miR-29a. These experiments strongly suggest that miR-29a represents a potential therapeutic target for pathological ocular neovascularization.
Results
The miR-29s are down-regulated during pathological ocular neovascularization in mice.
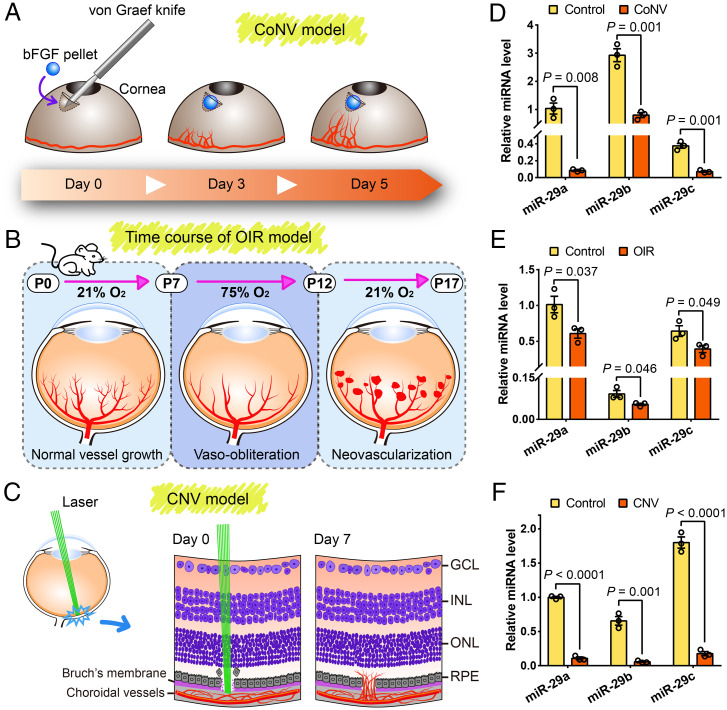
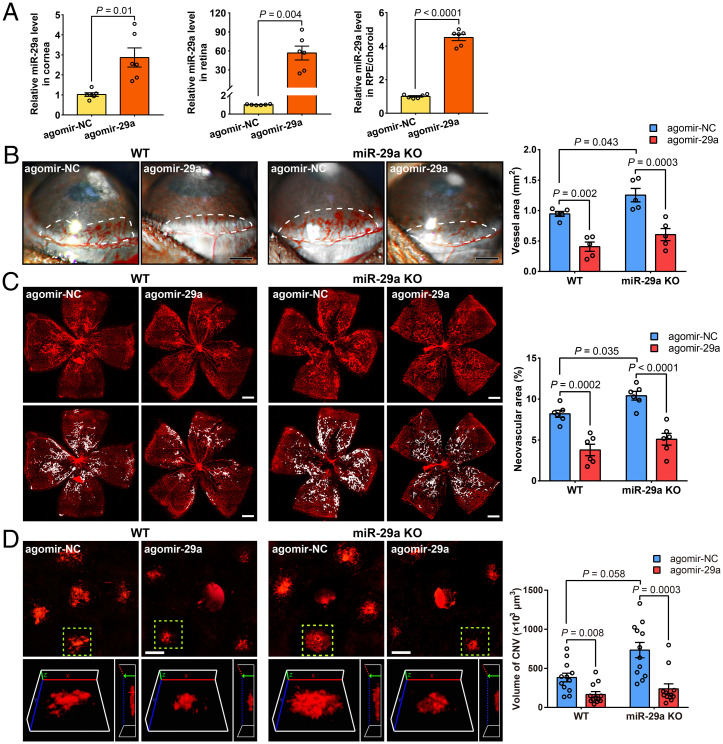
We first analyzed changes in the expression of miR-29s in several mouse models of CoNV, OIR, and CNV to obtain insights into the role of miR-29s in pathological ocular neovascularization (Fig. 1 A–C). The qRT-PCR of miR-29s revealed that the relative miR-29a, miR-29b, and miR-29c levels were decreased ∼12-, four-, and five-fold, respectively, in corneas with CoNV compared to those in control corneas at 5 d after pellet implantation (Fig. 1D). During retinal angiogenesis, miR-29 expression was reduced by ∼40% compared to the normoxic control group at P17 (Fig. 1E). Notably, the miR-29 levels in the retinal pigment epithelium (RPE)/choroids of mice with CNV (7 d after laser photocoagulation) were more than nine-fold lower than those in normal controls (Fig. 1F). Overall, these results suggest that miR-29s play a potential regulatory role in pathologic ocular neovascularization.
Fig. 1.
Changes in the expression of miR-29s during ocular neovascularization in mice. (A–C) Schematic depicting the mouse models of CoNV, OIR, and CNV. bFGF, basic fibroblast growth factor; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. (D–F) qRT-PCR analysis of miR-29 expression in ocular neovascularization models of CoNV, OIR, and CNV. The data are presented as the means ± SEM (n = 3 mice per group; Student’s 2-tailed unpaired t test).
The miR-29s regulate the function of human retinal microvascular endothelial cells.
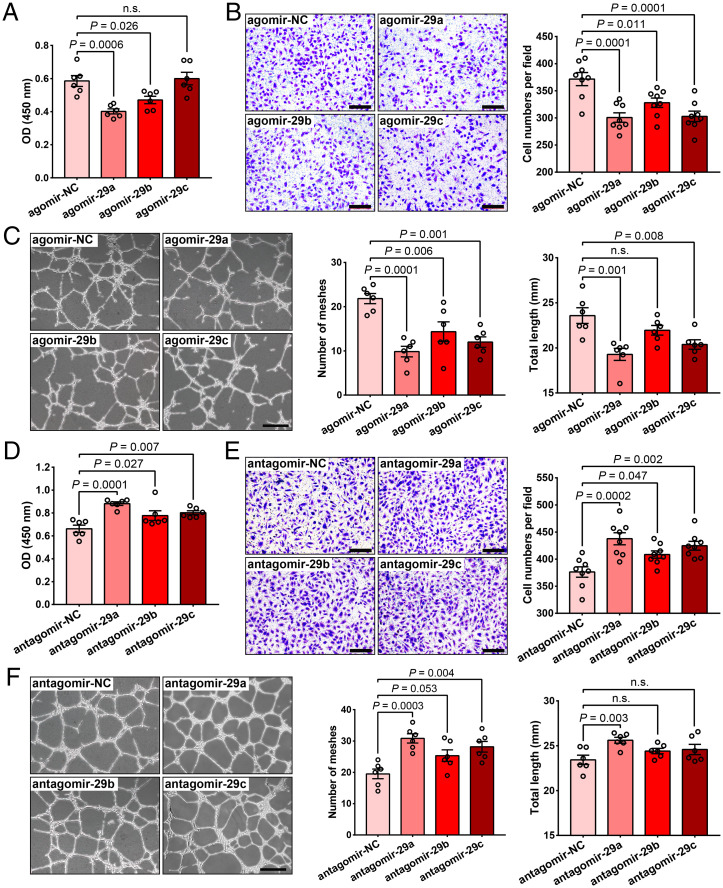
The ECs of the vascular system play an important role in responding to physiological and pathological stimuli to maintain vascular homeostasis. We conducted in vitro studies using human retinal microvascular endothelial cells (HRMECs) to further investigate the effects of miR-29s on EC function. First, we confirmed that agomir-29a, agomir-29b, or agomir-29c was successfully transfected into HRMECs with a more than 100-fold overexpression (SI Appendix, Fig. S1). We observed that overexpression of agomir-29a, agomir-29b, or agomir-29c suppressed HRMEC angiogenic functions, while antagomir-29a, antagomir-29b, or antagomir-29c (inhibitor) promoted HRMEC angiogenic functions, including proliferation (Fig. 2 A and D), migration (Fig. 2 B and E), and tube–formation capacity (Fig. 2 C and F). Among the miR-29 family members, the effect of miR-29a on EC function was more significant. Therefore, we focused on miR-29a in subsequent experiments.
Fig. 2.
MiR-29s affect HRMEC proliferation, migration, and tube formation capacities. (A) Cell proliferation assay (CCK-8 method) of HRMECs transfected with agomir-29a, agomir-29b, agomir-29c, or the negative control (agomir-NC, n = 6). (B) Representative images and quantification of the cell number in the transwell assay of HRMECs transfected with the agomirs mentioned above (n = 8) (Scale bars, 200 μm). (C) Results of the tube formation assay using HRMECs transfected with agomirs (n = 6) (Scale bars, 500 μm). (D–F) Results of the cell proliferation assay (D), transwell assay (E), and tube formation assay (F) using HRMECs transfected with antagomir-29a, antagomir-29b, antagomir-29c, or negative control (antagomir-NC). Each experiment was performed three times, and the data were analyzed using a 1-way ANOVA and Dunnett’s multiple comparisons test. n.s., not significant.
Loss of miR-29a accelerates retinal vessel pruning and deeper plexus development.
We obtained miR-29a KO mice from the Nanjing Biomedical Research Institute of Nanjing University (NBRI) to investigate the effect of miR-29a on retinal vasculogenesis during development and ocular angiogenesis in vivo. We confirmed that miR-29a was successfully knocked out (SI Appendix, Fig. S2A), and no obvious bodily or behavioral abnormalities were observed in the KO mice, including eyeball size and body weight (SI Appendix, Fig. S2 B and C). In addition, no significant morphological abnormalities were observed in the ocular sections (SI Appendix, Fig. S2D). We also performed fluorescein angiography (FFA) and electroretinogram (ERG) analyses using miR-29a KO mice to further assess the functions of the fundus. The results showed that the FFA and ERG were not significantly different between the miR-29a KO and WT groups (SI Appendix, Fig. S2 E–G).
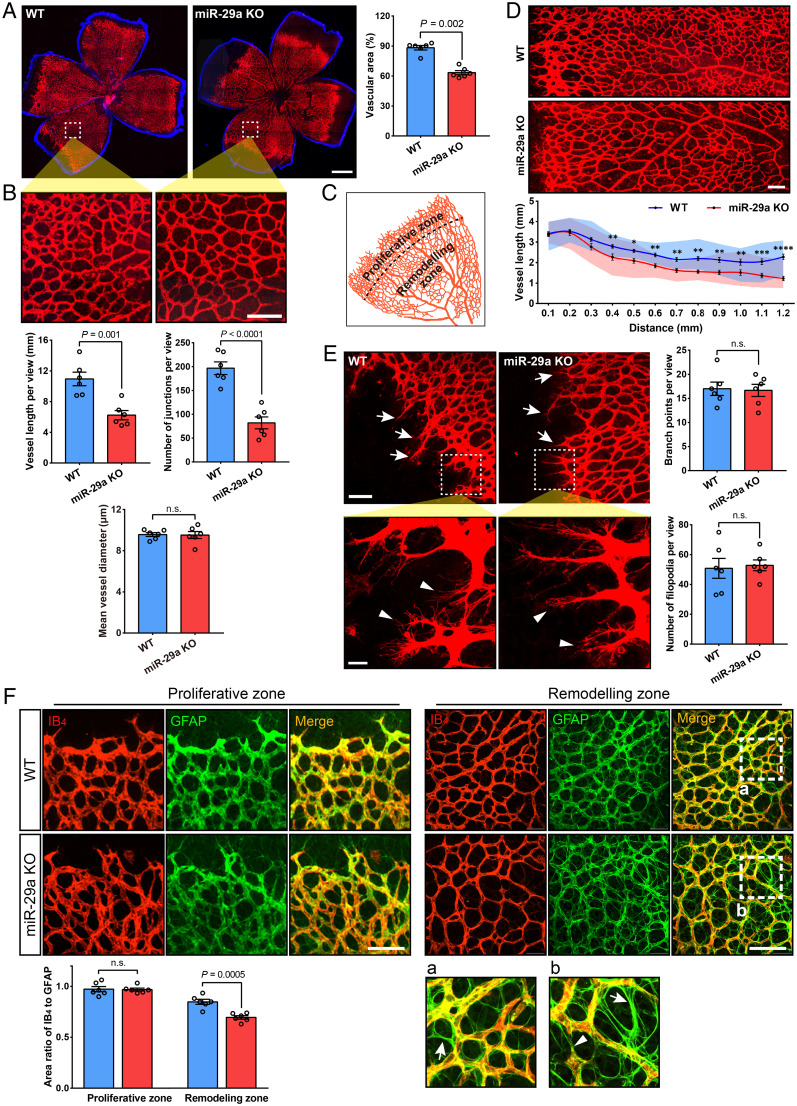
Immunolabeling with isolectin B4 (IB4) to visualize the vessels in the retina showed delayed superficial vascular plexus growth with a decreased vessel area in miR-29a KO mice during development (postnatal day 7, P7) compared to that in littermate WT controls (Fig. 3A). The retinal vasculature undergoes vascular remodeling during postnatal development. Interestingly, an increase in vessel pruning was observed in miR-29a KO mice by calculating vessel length and the number of junctions, particularly in the remodeling zone (Fig. 3 B–D). Moreover, no obvious difference was observed in endothelial sprouting toward the periphery in the primary plexus at the proliferative zone (Fig. 3E), whereas more vessel pruning was observed in the remodeling zone, as evidenced by glial fibrillary acidic protein (GFAP) positivity and IB4 negativity (Fig. 3F). In this study, we also confirmed that GFAP-positive astrocytes expanded to the periphery of the retinal surface before vessel growth occurred, suggesting that they may induce vessel outgrowth during development (SI Appendix, Fig. S3).
Fig. 3.
MiR-29a deficiency stimulates vessel pruning in the early stages of retinal vasculogenesis. (A) Representative images of the retinal vasculature (indicated by fluorescence-conjugated isolectin [IB4]) in P7 mice and comparison of vessel area between WT and miR-29a KO mice (n = 6 retinas from 6 mice). (B) High-magnification images of the vascular network in flat-mounted retinas and quantification of the vessel length, junction number, and diameter (n = 6 mice per group). (C) The primary (superficial) plexus consists of a proliferative zone and remodeling zone in the early developmental stage. (D) Variation in vessel length from the growing edge to the optic disk. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 (n = 6 mice per group). (E) Comparison of the number of vessel branch points (tip cells) and filopodia at the leading edge of the plexus. White arrows indicate typical branch points (Top), and arrowheads show typical cell filopodia (Bottom); n = 6 mice per group. (F) Representative images of the retina stained for IB4 (red) and GFAP (green) at the angiogenic front (Left) and remodeling zone (Right) and quantification of the ratio of IB4- to GFAP-positive area (n = 6 mice per group). Arrows in magnified images (a and b) show empty sleeves of the astrocyte template, which are IB4-negative and GFAP-positive, and arrowheads point to the regressing vessel (Error bars, SEM). n.s., not significant; nonparametric Mann–Whitney U test (A), Student’s t tests (B, E, and F), or 2-way ANOVA with Sidak’s multiple comparison test (D) (Scale bars, 500 μm [A] and 100 μm [B, D, E, and F]; magnified images, 20 μm [E, Bottom]).
In addition, we constructed three-dimensional (3D) images to understand the subsequent sprouting into the deep layer of retinal vessels. As shown in SI Appendix, Fig. S4, we found that the sprouting of the deeper plexus vasculature was accelerated in miR-29a KO mice at P7 and P9 (SI Appendix, Fig. S4 A and C). Immunostaining of retinal sections (P7) also showed deeper vessels in KO mice (SI Appendix, Fig. S4B). No significant differences in the characteristics of the primary (surface) and secondary (including deep and intermediate vascular plexus) vasculature were observed between miR-29a KO mice and WT littermates at P30 or P60 (SI Appendix, Fig. S5). Thus, our findings suggest that miR-29a deficiency accelerates vascular remodeling processes during the early postnatal period, reinforcing the hypothesis that miR-29a contributes to vasculogenesis during retinal development.
An miR-29a deficiency aggravates abnormal ocular angiogenesis.
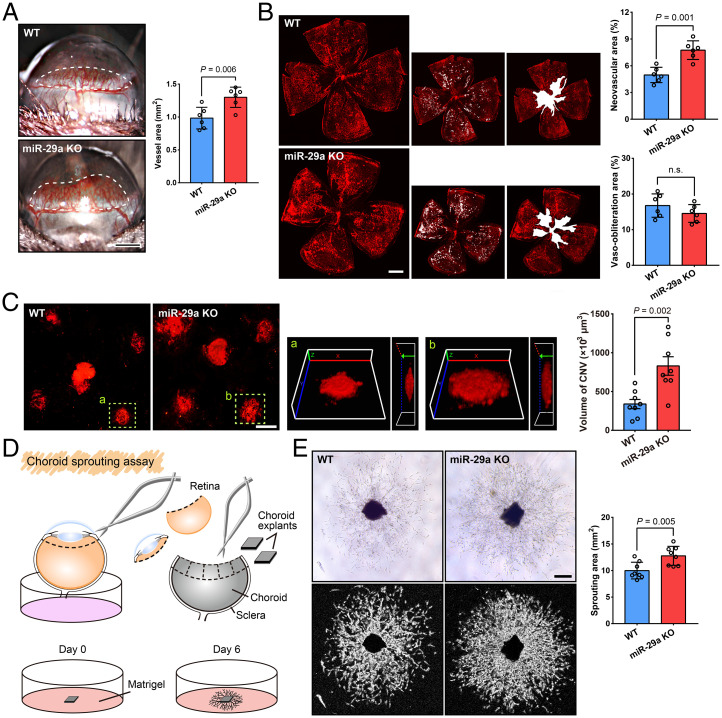
The cornea has been extensively investigated to evaluate antiangiogenic strategies because of its avascularity and easy accessibility (18). We conducted a mouse cornea micropocket angiogenesis assay, which has been frequently used, to identify the function of miR-29a in CoNV. Five days after the implantation of a basic FGF pellet, images were collected using a slit lamp and the area of CoNV was measured. The results showed an increased neovascularization area in miR-29a KO mice compared with that in WT mice (Fig. 4A). We performed experiments using the OIR model to further verify the roles of miR-29a in retinal angiogenesis. Vaso-obliteration and neovascular tufts were analyzed at P17. The miR-29a KO mice showed increased pathological retinal neovascularization (Fig. 4B), whereas no significant difference in vaso-obliteration was observed, implying that the depletion of miR-29a affects the formation of pathological neovascularization in the mouse OIR model.
Fig. 4.
An miR-29a deficiency increases abnormal ocular angiogenesis. (A) Growth factor–induced CoNV in WT and miR-29a KO mice. The neovessels (surrounded by a white dashed line) were measured using a slit lamp microscope (n = 6 mice per group). (B) Representative images of retinal whole-mounts with neovascular tufts highlighted in white (Bottom) and quantification of pathologic neovascularization in WT and miR-29a KO mice subjected to OIR (n = 6 mice per group). (C) Representative photos of IB4-labeled blood vessels on flat-mounted choroids and quantification of the neovessel volume in laser-induced CNV models (n = 8 mice per group). The yellow dashed lines delineate the CNV lesions, which are shown in magnified 3D images in a & b (Left, 3D reconstruction en face view; Right, side view). (D) Cartoon schematic of the choroid sprouting assay. (E) Choroid sprouting assay using choroid explants from WT and miR-29a KO mice. Typical images and computerized quantification (SWIFT-Choroid method, a macro for ImageJ software to calculate the area of vascular sprouts) of choroid endothelial sprouts are shown (n = 8 choroid explants from 4 mice) (Error bars, SEM; Student’s t tests; Scale bars, 500 μm [A and B], 200 μm [C], and 250 μm [E]).
Pathological choroidal neovascularization (CNV) is a common cause of vision loss in patients with a number of chorioretinal disorders, such as AMD. We also established a laser-induced CNV model using KO mice to further investigate the role of miR-29 in choroidal angiogenesis. Similar to the CoNV and OIR models, miR-29a KO mice exhibited an increased CNV volume compared to WT mice (Fig. 4C).
We used choroid explants isolated from miR-29a KO and WT mice to assess sprouting capacity ex vivo and assess the role of miR-29 in microvascular angiogenesis (Fig. 4D). Choroid explants displayed outgrowth beginning on the first day, and by day 6, the sprouting areas of explants from miR-29a KO mice were markedly greater than those of WT controls (Fig. 4E). Together, these data show that miR-29a deficiency promotes angiogenesis and that miR-29a plays an important role in regulating ocular neovascularization.
Administration of miR-29a ameliorates pathological ocular neovascularization.
We next investigated the effects of the administration of miR-29a-3p mimics (agomir-29a) on our neovascularization models using both WT and miR-29a KO mice to further determine whether miR-29a regulated ocular angiogenesis. The results confirmed that the miR-29a levels increased significantly in the eye tissue after a subconjunctival or intravitreal injection of agomir-29a (Fig. 5A). In the CoNV model established in both WT and miR-29a KO mice, agomir-29a administration markedly reduced corneal neovascularization (Fig. 5B). Similarly, overexpression of miR-29a in the retina substantially reduced the formation of pathological vascular plexuses (Fig. 5C). Moreover, agomir-29a treatment effectively reduced the lesion volume in the CNV model (Fig. 5D). Notably, increased angiogenesis resulting from miR-29a deficiency in the KO mice was reversed by agomiR-29a treatment. Consequently, our studies indicated that miR-29a administration effectively suppresses pathological ocular neovascularization in vivo.
Fig. 5.
The administration of miR-29a suppresses pathological ocular neovascularization. (A) Expression level of miR-29a in vivo 3 d after the subconjunctival or intravitreal injection of agomir-29a or scrambled agomir (agomir-NC, n = 6 mice for each group). (B) Assessment of areas (indicated by the dotted line) of basic FGF–induced CoNV in WT or miR-29a KO mice 5 d after the subconjunctival injection of agomir-29a (n = 5 mice per group). (C) Representative confocal micrographs and determination of pathological neovascular areas in the retinas of OIR mice at P17 following the intravitreal administration of agomir-29a (n = 6 mice per group). (D) Typical photos of IB4-labeled neovascular lesions in mice that underwent laser-induced CNV at 7 d after laser exposure and treatment with agomir-29a (n = 12 lesions from 3 mice for each group). The yellow dashed lines delineate the CNV lesions shown in magnified 3D images (Left, 3D reconstruction en face view; Right, side view) (Error bars, SEM; Student’s 2-tailed paired t test [A]; 2-way ANOVA and Holm–Sidak multiple comparisons test [B and C]; or Kruskal–Wallis test with 2-stage linear step-up procedure described by Benjamini, Krieger, and Yekutieli for multiple comparisons (19) [D]; Scale bars, 500 μm [B and C] and 200 μm [D]).
The miR-29a targets multiple angiogenic genes in ECs.
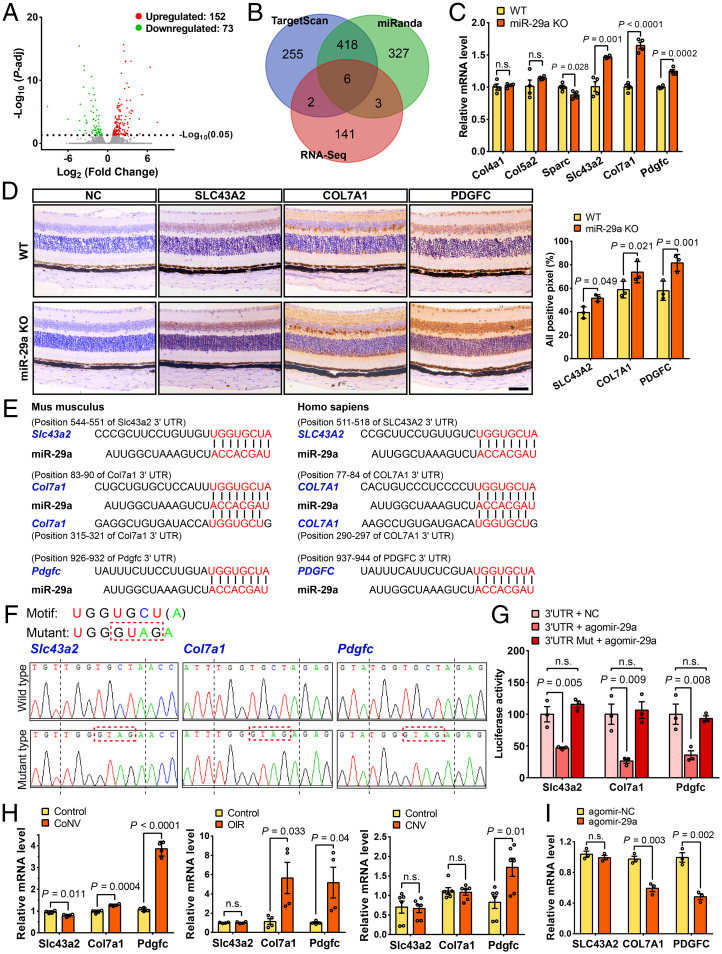
Next, we investigated the expression of target genes by performing high-throughput RNA sequencing (RNA-seq) of samples from miR-29a KO mice (GEO accession no. GSE186369) to elucidate the underlying molecular mechanisms regulating pathological ocular neovascularization. The high-throughput RNA-seq analysis of the retinas of miR-29a KO and WT littermates revealed altered expression levels of 225 genes, including 152 up-regulated genes and 73 down-regulated genes (Fig. 6A). The differentially expressed genes were enriched in several pathways and functional processes, including the degradation of the extracellular matrix, cell surface interactions at the vascular wall, regulation of angiogenesis, and chemokine activity, according to signal pathway and gene ontology enrichment analyses (SI Appendix, Fig. S6). Then, we performed a bioinformatics analysis of the target genes of miR-29a using the TargetScan and miRanda databases and selected six genes based on Venn diagram intersections of the 152 up-regulated genes (Fig. 6B). The quantitative analysis verified that Slc43a2, Col7a1, and Pdgfc expression levels were significantly up-regulated in miR-29a KO mice (Fig. 6C), consistent with the RNA-seq results. Furthermore, we also confirmed that three genes were expressed in the retina and exhibited increased expression in miR-29a KO mice (Fig. 6D).
Fig. 6.
Slc43a2, Col7a1, and Pdgfc are direct targets of miR-29a. (A) Volcano plot analyzing differentially expressed mRNAs in miR-29a KO retinas. P-adj, adjusted P value. (B) Venn diagram for the analysis of genes that were up-regulated in miR-29a KO mice and predicted as miR-29a targets by TargetScan and miRanda software. (C) Validation of the expression levels of candidate target genes using qRT-PCR (n = 4). (D) Representative images of immunohistochemical staining and relative levels of SLC43A2, COL7A1, and PDGFC in sagittal sections of the retinas from WT or miR-29a KO mice (n = 3) (Scale bars, 50 μm). (E) The consensus binding sequences (highlighted in red font) of miR-29a and its target genes in mice and humans. (F) Sequencing results of pmirGLO-3′UTR (pmirGLO vector containing 3′UTR sequence of miR-29a target genes) recombinant plasmids with normal or mutant binding motifs (only the sequences near the binding site are shown). The mutated bases among the binding motifs are marked with a red dashed box. (G) Luciferase activity of HEK293 cells cotransfected with pmirGLO-3′UTR or 3′UTR-Mut and agomir-29a or agomir-NC (n = 3). Firefly luciferase activity was used to monitor miRNA regulation, and Renilla luciferase activity was measured to normalize the transfection efficiency. (H) Pdgfc expression, but not Slc43a2 or Col7a1 expression, was consistently up-regulated in CoNV, OIR, and CNV mouse models, as revealed by qRT-PCR (n = 4–6). Mouse corneas (5 d after pellet implantation), retinas (P17), and RPE/choroids (7 d after laser photocoagulation) were collected for an analysis of RNA expression in CoNV, OIR, and CNV models. (I) The mRNA levels of COL7A1 and PDGFC were significantly reduced in HRMECs at 24 h after transfection with agomir-29a (n = 3). 3′UTR, 3′ untranslated region; Mut, mutant; HEK293, human embryonic kidney 293 cells. COL4A1, collagen type IV alpha 1 chain; COL5A2, collagen type V alpha 2 chain; SPARC, secreted protein acidic and cysteine rich; SLC43A2, solute carrier family 43 member 2; COL7A1, collagen type VII alpha 1 chain; PDGFC, platelet-derived growth factor C (Error bars, mean ± SEM; Student’s t test [C, D, H, and I] or 1-way ANOVA and Dunnett’s multiple comparisons test [G]).
The seed sequence of miR-29a is conserved between mice and humans and shows complementarity at the 3′UTR with three observed target gene mRNAs (Fig. 6E). We next conducted a luciferase reporter assay against the genes Slc43a2, Col7a1, and Pdgfc to verify whether they were direct target genes of miR-29a. We observed a significant repression of their luciferase activities upon miR-29a overexpression only when the genes were not mutated (Fig. 6 F and G), implying that these angiogenic genes were target genes of miR-29a.
In addition, we further investigated the expression profiles of the three target genes during the process of neovascularization in vivo. The Pdgfc level was substantially increased in corneal, retinal, and choroidal neovascularization models, and the Col7a1 expression level was significantly up-regulated in the CoNV and OIR models (Fig. 6H). We verified the results by overexpressing agomir-29a in HRMECs and observed a reduced expression of COL7A1 and PDGFC (Fig. 6I).
Effects of candidate targets of miR-29a on ocular neovascularization.
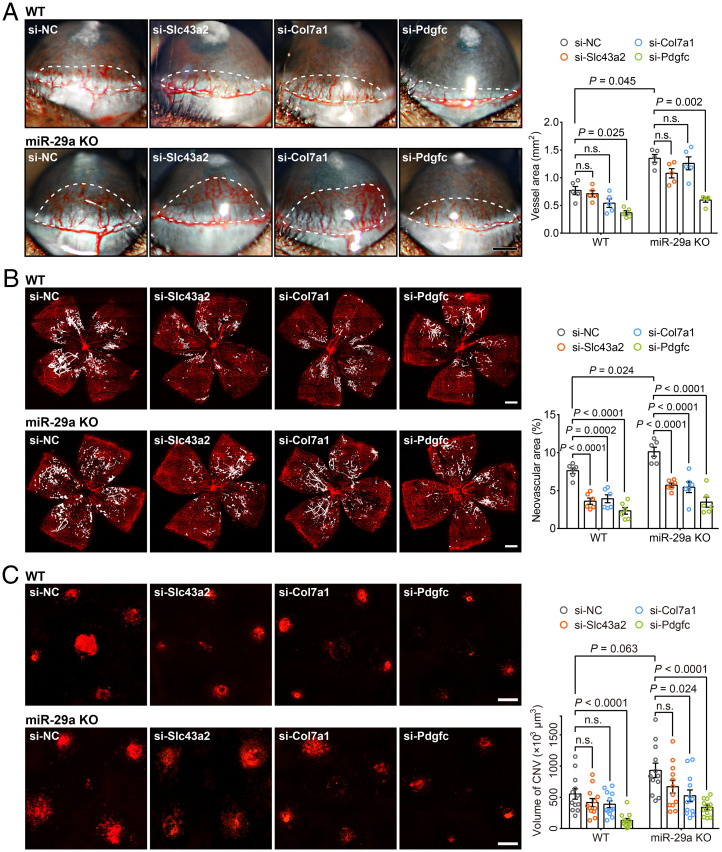
We performed RNA interference experiments to explore the functions of the candidate target genes of miR-29a in our in vivo ocular neovascularization models. We designed siRNAs for the target genes and confirmed that the siRNA pools efficiently reduced both the mRNA and protein levels of the corresponding genes in vivo (SI Appendix, Fig. S7 A–F) and in vitro (SI Appendix, Fig. S7 G–H). Inhibition of Slc43a2, Col7a1 and Pdgfc effectively suppressed neovascularization in the OIR model in both WT and miR-29a KO mice (Fig. 7B). Moreover, Pdgfc silencing also significantly mitigated neovascularization in the CoNV and CNV models (Fig. 7 A and C). Thus, Pdgfc is a direct target of miR-29a and functions to effectively inhibit pathological angiogenesis.
Fig. 7.
SLC43A2, COL7A1, and PDGFC have varying degrees of influence on corneal, retinal, and choroidal neovascularization. (A) Representative images and quantification of corneal neovascularization in WT or miR-29a KO mice with decreased expression of Slc43a2, Col7a1, or Pdgfc (n = 5 mice per group). The white dashed lines delineate the neovascular area. A subconjunctival injection of siRNAs was administered as soon as the model was established, and the cornea with neovessels sprouting from the limbus was observed with a slit lamp after five days. (B) Intravitreal administration of siRNAs against the three target genes to WT or miR-29a KO mice distinctly reduced neovascular tuft formation at P17 of OIR compared to the NC group (n = 6 mice per group). (C) The effect of Slc43a2, Col7a1, or Pdgfc knockdown on laser-induced CNV (n = 12 lesions from 3 mice per group). The CNV model was established, followed by an intravitreal injection of siRNAs on the same day. The IB4-labeled CNV volume was assessed on day 7 (Error bars, SEM; Kruskal–Wallis test with 2-stage linear step-up procedure described by Benjamini, Krieger, and Yekutieli for multiple comparisons (19) [A and C] or 2-way ANOVA and Holm–Sidak multiple comparisons test [B]; Scale bars, 500 μm [A and B] and 200 μm [C]).
The miR-29a/PDGF axis inhibits ocular angiogenesis.
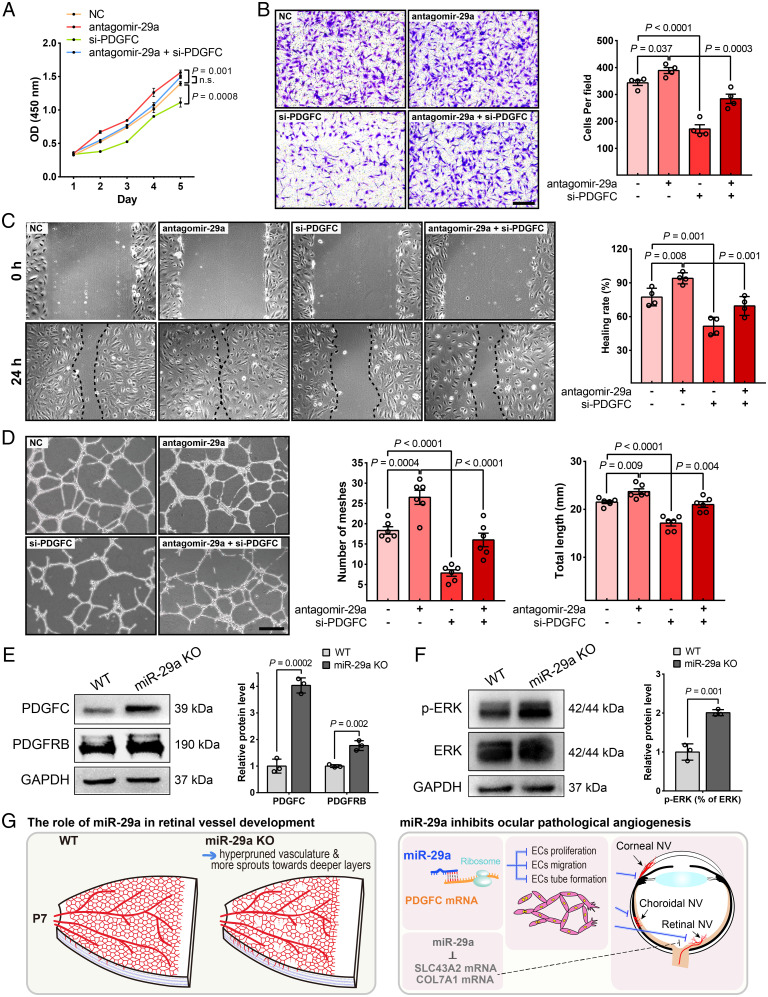
We conducted experiments in HRMECs cotransfected with the PDGFC siRNA and antagomir-29a to validate that PDGFC is the mechanistic target by which miR-29 regulates blood vessel formation. We showed that PDGFC suppression significantly inhibited cell proliferation, migration, and tube formation and reversed EC functions that were enhanced by antagomir-29a treatment (Fig. 8 A–D). Moreover, the immunoblot analysis also verified the high expression of PDGFC in miR-29a KO mouse retinas (Fig. 8E). Interestingly, the expression of its receptor PDGFRB was also up-regulated in the retinas of miR-29a KO mice (Fig. 8E). In addition, we detected increased phosphorylation of ERK (Fig. 8F). In conclusion, miR-29a regulates ocular vasculogenesis and angiogenesis by targeting genes such as PDGFC and may be a therapeutic target for the treatment of neovascularization (Fig. 8G).
Fig. 8.
PDGFC is an important functional target of miR-29a that modulates HRMEC proliferation, migration, and tube formation ability. (A) PDGFC knockdown markedly inhibited HRMEC proliferation and partially reversed the effect of antagomir-29a on increasing cell viability (n = 3). (B) PDGFC knockdown exerted an inhibitory effect on the migratory behavior of HRMECs, as evaluated using the Transwell assay (n = 4). (C) Down-regulation of PDGFC expression in HRMECs slowed wound closure compared to that in the NC or antagomir-29a group (n = 4). (D) The tube formation ability of HRMECs decreased after transfection with siRNAs against PDGFC (n = 6). (E and F) The protein levels of molecules related to the miR-29a-PDGFC axis in miR-29a KO and WT retinas were detected using Western blot assays (n = 3). (G) Schematic diagram illustrating the mechanism by which miR-29a contributes to ocular developmental and pathological neovascularization (Error bars, mean ± SEM; 2-way ANOVA with Dunnett’s multiple comparisons test [A], 1-way ANOVA with Holm–Sidak multiple comparisons test [B–D], or Student’s t test [E and F]; Scale bars, 200 μm [B] and 500 μm [D]).
Discussion
Ocular neovascularization is a common feature of the pathological processes of PDR, AMD, ROP, and corneal chemical damage, which leads to vision impairment and even blindness. VEGF-targeted therapy has become the first-line treatment for ocular angiogenic diseases (20). However, the long-term use of anti-VEGF drugs has limitations, as a considerable number of patients do not respond to anti-VEGF treatment, develop resistance, or experience various degrees of retinal atrophy (5, 6, 21). Therefore, in-depth studies on the pathogenesis of ocular neovascularization are needed to search for novel therapeutic targets. RNA-based therapies have been widely developed worldwide, as evidenced by ongoing clinical trials and the approval of several RNA-based drugs, including short-interfering RNAs (siRNAs), antisense oligonucleotides, and miRNAs (22). Studies have shown that miRNAs bind to their targets in the 3′-UTRs of messenger RNAs to posttranscriptionally regulate gene expression. Because pathological angiogenesis is a complex process involving numerous molecules, an advantage of miRNAs is that they target multiple processes with minor or minimal side effects.
Based on accumulating evidence, the miR-29 family, consisting of miR-29a, miR-29b-1, miR-29b-2, and miR-29c, is a key regulator of a number of biological processes. The abnormal expression of miR-29s contributes to the etiology of numerous diseases, such as osteoarthritis, osteoporosis, immune disease, and cardiovascular disease (23, 24). Therapy with an miR-29 mimic (Remlarsen/MRG-201) is currently being evaluated in a clinical trial for cutaneous fibrosis, reinforcing the feasibility of its future application. Research has shown that miR-29a is mainly involved in glucose homeostasis (25), fibrosis (26), allergic inflammation (27), macrophage polarization (28), tumorigenesis (29), and angiogenesis (16). Overexpression of miR-29a attenuates insulin sensitivity, hence decreasing glucose uptake (25, 30). In animal models of kidney or liver fibrosis, miR-29a expression is down-regulated and exerts antifibrotic effects (26, 31). Furthermore, miR-29a is frequently down-regulated in several cancers, such as acute myeloid leukemia (32) and gastric carcinoma (29, 33), accompanied by reduced angiogenesis, a pivotal process occurring during cancer development (16, 17). In previous studies, miR-29a exerted inhibitory effects on the proliferation, migration, and tube formation capacities of HUVECs and aggravated diabetes-induced retinal pericyte degeneration (16, 17, 34). Here, we directly showed that miR-29a is a critical regulator of both pathological and physiological ocular neovascularization. By performing a comprehensive analysis focused on neovascular eye diseases, we confirmed that miR-29a levels show an obvious decreasing trend during ocular neovascularization using three different models (Fig. 1). Furthermore, we identified an important role for miR-29a in angiogenesis in vivo, as miR-29a deletion increased abnormal ocular angiogenesis in the cornea, retina, and choroid (Fig. 3). In contrast, miR-29a overexpression markedly suppressed pathological angiogenesis in the cornea, retina, and choroid (Fig. 4). Moreover, miR-29a exerted strong inhibitory effects on the proliferation, migration, and tube formation abilities of retinal vascular endothelial cells (Fig. 5). These results suggest that miR-29a has broad effects on different ocular neovascular diseases and is a strong potential candidate for therapeutic intervention.
In view of the regulatory effect of miR-29a on EC activity and the formation of pathological neovascularization, we inferred that miR-29a is essential for vascular homeostasis. The miR-29a KO mice showed normal physiological and eye development (SI Appendix, Fig. S2), unlike mice deficient in miR-29a/b1, which presented with serious developmental defects, including growth retardation and increased intraocular pressure, leading to eye degeneration, blindness, and premature death (35). Deletion of miR-29a promoted vessel pruning and sprouting into the deeper retinal layers at the early stages of retinal vasculogenesis (Fig. 2 and SI Appendix, Fig. S4). This result implies that the members of the miR-29 family play different roles or have various degrees of involvement in development. In addition, miR-29a KO did not affect the final maturation of the retinal vascular network (SI Appendix, Fig. S5). Considering the modulatory effects of miR-29b and miR-29c on the activity of vascular ECs (14, 15), we speculate that miR-29 family members exert compensatory effects to some extent on vessel development, which requires further verification.
At the early developmental stages in the mouse, the retinal vasculature undergoes multiple steps in the process of vascular maturation, including sprouting, branching, remodeling, and maturation, to form a hierarchical vascular network (36). Vascular expansion of the superficial layer follows an astrocyte template between postnatal days 1 and 8 (P1–P8) that spreads radially from the optic nerve (37). Astrocytes play important roles in constraining retinal vessels to the retina and maintaining their integrity (38). In our study, a network of astrocytes preceded the expanding vascular front (SI Appendix, Fig. S3). The lack of miR-29a had a minimal effect on horizontal sprouting and branching in the proliferative zone of the retinal superficial vascular plexus. Nevertheless, miR-29a deficiency stimulated the vascular remodeling process, including vessel pruning and regression (Fig. 2), resulting in a phenotype similar to that of endothelial-specific semaphorin 3G KO mice (39). To date, multiple signaling pathways that control vessel pruning and regression, i.e., VEGF/VEGF receptor 2 (VEGFR2) signaling, wingless-type MMTV integration site family (WNT) signaling, blood flow–induced signaling, delta like canonical Notch ligand 4 (DLL4)/Notch signaling, and angiogenin (ANG)/tyrosine kinase with immunoglobulin-like and epidermal growth factor homology (TIE) signaling, have been identified (40). More research is needed to elucidate which pathway miR-29a modulates to alter the pruning of blood vessels. Retinal astrocytes have been reported to be limited to the primary plexus and do not influence the deeper vascular network in the retina. However, our results revealed that while GFAP-labeled astrocytes synchronously penetrated the deep retinal layers coupled with vascular sprouting, they were not in a leading position, unlike in the superficial network (SI Appendix, Fig. S4). We observed that the loss of miR-29a promoted the vertical sprouting process of the superficial vascular plexus as well as that of astrocyte networks.
According to previous studies, miR-29 family members have various target genes that are involved in vasculogenesis and angiogenesis, including VEGF and extracellular matrix proteins such as collagen, fibronectin, and matrix metalloproteinases. In this study, we also discovered target angiogenic genes—SLC43A2, COL7A1, and PDGFC—by performing an integrative analysis of high-throughput sequencing data from miR-29a KO retinas and bioinformatic predictions. We also confirmed the interaction between miR-29a and the three targets by performing a dual–luciferase reporter assay (Fig. 6). Furthermore, we verified that these target genes are involved in ocular angiogenesis using RNA interference approaches (Fig. 7). COL7A1 is a member of the collagen superfamily and a major structural component of anchoring fibrils (41). SLC43A2, a member of the solute carrier family, is responsible for the transmembrane transport of neutral amino acids, including leucine, isoleucine, valine, phenylalanine, and methionine (42). PDGFC is a member of the PDGF family and is a known angiogenic gene (43). Among these genes, PDGFC showed the most consistent changes in expression and regulatory effects on different models of neovascularization. In addition, PDGFC knockdown partially reversed EC functions that were accelerated by antagomir-29a treatment (Fig. 8). Signaling pathway and gene ontology analyses (analysis of enriched terms for differentially expressed genes in miR-29a KO mice) revealed that miR-29a is mainly implicated in the pathways of extracellular matrix organization, signaling by PDGF, cell surface interactions at the vascular wall, and chemokine-related pathways and participates in the biological processes of the regulation of angiogenesis and chemokine activity (SI Appendix, Fig. S6). Rosano et al. similarly showed that miR-29a expression was down-regulated during sprouting angiogenesis in EC spheroids and was associated with 25 genes, mostly related to extracellular matrix remodeling (44). Notably, PDGFC has been suggested to be a VEGF-independent stimulator (45) of angiogenesis and a proinflammatory cytokine that regulates the expression of the chemokines C-C motif chemokine ligand 2 (CCL2) and CCL5 (46). PDGFC is also involved in remodeling the extracellular matrix (47). Taken together, these findings indicate that PDGFC represents an important functional target of miR-29a in the regulation of pathological neovascularization. As miRNAs have multiple targets, are highly conserved, and are easy to design and synthesize, miR-29a has promising therapeutic potential in neovascular eye diseases. Based on the discovery of target genes of miR-29a through RNA-seq using neural retina tissues from KO mice, we considered that the roles of three targets (Pdgfc, Slc43a2, and Col7a1) in OIR models were significant but were not sufficient (Slc43a2 and Col7a1) in CoNV and CNV models. More experiments may be needed to verify more-significant target genes of miR-29a in the cornea and RPE/choroid tissues. In addition, the analysis of different time points after injury or treatments may show more significant changes in the expression of these target genes. Further determination of the roles of SLC43A2 and COL7A1 may be needed at different time points in future studies.
In summary, our study extensively clarified the anti-angiogenic effect of miR-29a on regulating ocular neovascularization and the underlying mechanisms. We suggest that miR-29a is a promising therapeutic target for the improved clinical management of neovascular diseases.
Materials and Methods
Animals.
All animal experiments were performed strictly according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Care and Use Committee of Wenzhou Medical University (approval number: wydw2020-0043). C57BL/6J mice were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Heterozygous miR-29a KO mice (T001311 B6/J-Mir29aem1Cd401/Nju) on a C57BL/6J background were obtained from the NBRI (Nanjing, China). MiR-29a KO mice were generated by breeding the heterozygotes to produce homozygotes and WT littermates for subsequent experiments. Animals were maintained in an air-conditioned room at 22 °C on a controlled 12-h light–dark cycle. All mice were provided standard laboratory chow and water ad libitum.
For information on animal models of ocular neovascularization; qRT-PCR; cell culture, transfection, cell proliferation, migration, and tube formation assays; immunohistochemistry; choroidal sprouting assays; FFA; ERG; high-throughput sequencing and data analysis; luciferase reporter assays; Western blot; and statistical analyses please refer to SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank I. Pang and J. Chen for critically reading the manuscript drafts, participating in scientific discussions, and providing comments on the manuscript. This research was supported by the National Natural Science Foundation of China under grant nos. 32000433 and 81770918, the Zhejiang Provincial Natural Science Foundation of China under grant no. LZ22H120001, and departmental funds from Wenzhou Medical University (KYQD20200801 and 89214018).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2204795119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. The raw data (48) can be downloaded from https://figshare.com/s/b505dec0a183d4872f07. The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus database under accession number GSE186369 (49).
References
- 1.Campochiaro P. A., Ocular neovascularization. J. Mol. Med. (Berl.) 91, 311–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajappa M., Saxena P., Kaur J., Ocular angiogenesis: Mechanisms and recent advances in therapy. Adv. Clin. Chem. 50, 103–121 (2010). [PubMed] [Google Scholar]
- 3.Siemerink M. J., Augustin A. J., Schlingemann R. O., Mechanisms of ocular angiogenesis and its molecular mediators. Dev. Ophthalmol. 46, 4–20 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Potente M., Gerhardt H., Carmeliet P., Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Falavarjani K. G., Nguyen Q. D., Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye (Lond.) 27, 787–794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire M. G., et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group, Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology 123, 1751–1761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L., Hannon G. J., MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Xu S., microRNA expression in the eyes and their significance in relation to functions. Prog. Retin. Eye Res. 28, 87–116 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Lui P. Y., Jin D. Y., Stevenson N. J., MicroRNA: Master controllers of intracellular signaling pathways. Cell. Mol. Life Sci. 72, 3531–3542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna J., Hossain G. S., Kocerha J., The potential for microRNA therapeutics and clinical research. Front. Genet. 10, 478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung J., Miscianinov V., Sluimer J. C., Newby D. E., Baker A. H., Targeting non-coding RNA in vascular biology and disease. Front. Physiol. 9, 1655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C. H., Huang S., Britton W. R., Chen J., MicroRNAs in vascular eye diseases. Int. J. Mol. Sci. 21, 649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan J. T., et al. , MicroRNA-18a-5p administration suppresses retinal neovascularization by targeting FGF1 and HIF1A. Front. Pharmacol. 11, 276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., et al. , MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 397, 111–119 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Hu Y., et al. , Evaluation of miR-29c inhibits endotheliocyte migration and angiogenesis of human endothelial cells by suppressing the insulin like growth factor 1. Am. J. Transl. Res. 7, 866–877 (2015). [PMC free article] [PubMed] [Google Scholar]
- 16.Purvis I. J., et al. , Role of MYC-miR-29-B7-H3 in medulloblastoma growth and angiogenesis. J. Clin. Med. 8, 1158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S., et al. , IGF1 3'UTR functions as a ceRNA in promoting angiogenesis by sponging miR-29 family in osteosarcoma. J. Mol. Histol. 47, 135–143 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Bock F., et al. , Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 34, 89–124 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y., Krieger A. M., Yekutieli D., Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507 (2006). [Google Scholar]
- 20.Amadio M., Govoni S., Pascale A., Targeting VEGF in eye neovascularization: What’s new? A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 103, 253–269 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Sene A., Chin-Yee D., Apte R. S., Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 21, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts T. C., Langer R., Wood M. J. A., Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horita M., Farquharson C., Stephen L. A., The role of miR-29 family in disease. J. Cell. Biochem. 122, 696–715 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M. N., Luo G., Gao W. J., Yang S. J., Zhou H., miR-29 family: A potential therapeutic target for cardiovascular disease. Pharmacol. Res. 166, 105510 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Li J., et al. , Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J. Extracell. Vesicles 10, e12055 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto Y., et al. , MiR-29a assists in preventing the activation of human stellate cells and promotes recovery from liver fibrosis in mice. Mol. Ther. 24, 1848–1859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi A., Matsumoto K., Matsuda A., MicroRNA-29s suppressed both soluble ST2 release and IFNAR1 expression in human bronchial epithelial cells. Allergy 76, 2264–2267 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., et al. , Hypoxia-reoxygenation induces macrophage polarization and causes the release of exosomal miR-29a to mediate cardiomyocyte pyroptosis. In Vitro Cell. Dev. Biol. Anim. 57, 30–41 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Wang J. Y., et al. , MiR-29a: A potential therapeutic target and promising biomarker in tumors. Biosci. Rep. 38, BSR20171265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massart J., et al. , Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes 66, 1807–1818 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Shi S., et al. , Knockdown of LncRNA-H19 ameliorates kidney fibrosis in diabetic mice by suppressing miR-29a-mediated EndMT. Front. Pharmacol. 11, 586895 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J., Song Y., Xu J., Li H. H., Zheng J. F., LncRNA PVT1 promotes the malignant progression of acute myeloid leukaemia via sponging miR-29 family to increase WAVE1 expression. Pathology 53, 613–622 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., et al. , Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 375, 331–339 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q., et al. , Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction. J. Clin. Invest. 130, 3833–3847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caravia X. M., et al. , The microRNA-29/PGC1α regulatory axis is critical for metabolic control of cardiac function. PLoS Biol. 16, e2006247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fruttiger M., Development of the retinal vasculature. Angiogenesis 10, 77–88 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Stone J., Dreher Z., Relationship between astrocytes, ganglion cells and vasculature of the retina. J. Comp. Neurol. 255, 35–49 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Stone J., Role of astrocytes in the control of developing retinal vessels. Invest. Ophthalmol. Vis. Sci. 38, 1653–1666 (1997). [PubMed] [Google Scholar]
- 39.Chen D. Y., et al. , Endothelium-derived semaphorin 3G attenuates ischemic retinopathy by coordinating β-catenin-dependent vascular remodeling. J. Clin. Invest. 131, e135296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korn C., Augustin H. G., Mechanisms of vessel pruning and regression. Dev. Cell 34, 5–17 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Sakai L. Y., Keene D. R., Morris N. P., Burgeson R. E., Type VII collagen is a major structural component of anchoring fibrils. J. Cell Biol. 103, 1577–1586 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodoy S., et al. , Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 280, 12002–12011 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Anderberg C., et al. , Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 69, 369–378 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosano S., et al. , A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis. eLife 9, e48095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford Y., et al. , PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15, 21–34 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Eitner F., et al. , PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 19, 281–289 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiradjaja F., Cottle D. L., Jones L., Smyth I., Regulation of PDGFC signalling and extracellular matrix composition by FREM1 in mice. Dis. Model. Mech. 6, 1426–1433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng D., Lan C., Chi Z., Anti-angiogenic properties of microRNA-29a in preclinical ocular models (raw dataset). Figshare. https://figshare.com/s/b505dec0a183d4872f07. Deposited 20 October 2022. [DOI] [PMC free article] [PubMed]
- 49.Chi Z., Peng D., Lan C., Changes in mRNA transcripts in retina of miR-29a knockout mice. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186369. Deposited 21 October 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. The raw data (48) can be downloaded from https://figshare.com/s/b505dec0a183d4872f07. The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus database under accession number GSE186369 (49).