Abstract
Interest in developing minimal side-effect NMDA receptor antagonists for neurological and psychiatric disorders has been energized by the recent introduction of esketamine into clinical practice for treatment-resistant depression. Structural analogs of dextromethorphan bind with low affinity to the NMDA receptor ion channel, have functional effects in vivo, and generally display a lower propensity for side-effects than that of ketamine and other higher affinity antagonists. As such, the aim of the present study was to determine whether a series of N-substituted-3-alkoxy-substituted dextromethorphan analogs produce their effects in vivo through blockade of NMDA receptors. Compounds were studied against NMDA-induced seizures in rats. Compounds were administered intracerebroventricularly in order mitigate confounds of drug metabolism that arise from systemic administration. Comparison of the anticonvulsant potencies to their affinities for NMDA, σ1, and σ2 binding sites were made in order to help define the receptor population(s) governing NMDA antagonist efficacy. The potencies to block convulsions were positively associated with their affinities to bind to the NMDA receptor ion channel ([3H]-TCP binding) (r = 0.71, p < 0.05) but not to σ1 receptors ([3H]-SKF 10047 binding) (r = −0.31, p=0.46) or to σ2 receptors ([3H]-DTG binding) (p = −0.38, p = 0.36). This is the first report demonstrating that these dextromethorphan analogs are functional NMDA receptor antagonists in vivo. Given their potential therapeutic utility and favorable side-effect profiles, such low affinity NMDA receptor antagonists could be considered for further development in neurological (e.g., anticonvulsant) and psychiatric (e.g., antidepressant) disorders.
Keywords: Dextromethorphan analogs, NMDA seizures, NMDA receptors, Sigma receptors
N-Methyl-D-Asparate (NMDA) receptors play important roles in the regulation of physiology and behavior. Their functional control in neurological disorders such as epilepsy and stroke, for example, has generated intense efforts to identify NMDA receptor antagonists that might safely modulate these disorders (Ghasemi and Schachter, 2011; Kalia et al., 2008; Wang and Reddy, 2017). Similar discovery efforts were launched for the identification of NMDA receptor antagonists that might function as novel anti-anxiety or antidepressant drugs (Ghasemi et al., 2017; Zanos et al., 2018). NMDA receptors have gained notoriety in the past decade due to their implicated role in major depressive disorder. The pioneering work of Trullas and Skolnick (1990) posited that NMDA receptor antagonism would be antidepressant based upon convergent data from a host of sources. Clinical validation of the Trullas and Skolnick hypothesis was born out ten years later by Berman and colleagues (2000) who reported rapid onset of antidepressant effects of the NMDA receptor antagonist ketamine in treatment-refractory patients. Subsequent systematic replication of this finding (Zarate et al., 2006) led to an explosion in the field of antidepressant drug discovery (Witkin, 2011; Witkin et al., 2018).
Although the efficacy of ketamine in patients for depression and pain (Hewitt, 2000) has been generally acknowledged, the use of ketamine as a therapeutic in these disease states and others such as epilepsy remains constrained by the side-effects and safety issues associated with ketamine. Ketamine produces concomitant motor-impairment and associated motor-excitation, psychotomimetic effects, abuse liability and brain lesions with prolonged use (Iadarola et al., 2015; Kim et al., 2016). Since it is known that blockade of the NMDA receptor ion pore not only drives efficacy but generates side-effects, solutions to the problem of identifying NMDA receptor antagonists without side-effects has focused on compounds binding in sites distinct from the ion channel (uncompetitive antagonists) (e.g., Willetts et al., 1990) and antagonists with low affinity for the binding domain within the ion channel. The rationale behind the later strategy is that low affinity ligands would control ion channel function in a dynamic fashion. With antagonist molecules moving on and off the NMDA receptor rapidly, it was hypothesized that NMDA receptor function could be dampened, but not completely blocked (Parsons et el., 1999a, b; Semenova et al., 1999). Therefore, low affinity channel blockers might create therapeutic advantage with reduced side effect burden. Proof of concept of this approach has been exemplified by a diversity of low affinity NMDA receptor antagonists that have NMDA receptor antagonist properties in vivo but do not fully recapitulate the side-effect profile of higher affinity drugs like phencyclidine, MK-801, or ketamine. For example, dextromethorphan does not produce full motor-impairing effects or the motor stimulation that originates with high affinity channel blockade (Ginski and Witkin, 1999; Zapata et al., 2003). Low affinity channel blockers are also predicted to lack the psychotomimetic subjective effects that can be produced by high affinity ligands (Geter-Douglass and Witkin, 1999).
Indeed, dextromethorphan is approved for the treatment of pseudobulbar affect (Nguyen et al. 2016b), has been studied in Rett syndrome (Smith-Hicks et al., 2016), in opioid use disorder (Malek et al., 2013; Lee et al., 2015), and has shown efficacy against agitation in Alzheimer’s disease (Cummings et al., 2015). Dextromethorphan has also been shown to produce antidepressant-like effects in rodent models that detect antidepressant drugs (Nguyen and Matsumoto, 2015; Nguyen et al., 2014, 2016a, 2017; Sakhaee et al., 2016) and efficacy in patients in a case report (Lauterbach et al., 2016). As an anticonvulsant, dextromethorphan has been studied in epileptic patients and shown efficacy (Wieser and Beck, 1998; Kimiskidis et al., 1999; Chien et al., 2012; see review by Nguyen et al., 2016).
A series of N-substituted-3-hydroxy and 3-alkoxy derivatives of dextromethorphan reported by Newman et al. (1992; 1996) have been characterized as binding with high affinity to σ1 receptors and as relatively low affinity at NMDA receptors, in radiolabeled binding experiments. Dextromethorphan analogs have also demonstrated efficacy against maximal electroshock-induced seizures (Newman et al., 1992, Tortella et al 1994) and against cocaine-induced seizures (Zapata et al., 2003. However, their mechanism of action in vivo has not been previously elucidated. Herein, we tested dextrorphan, dextromethorphan, and selected dextromethorphan analogs (Newman et al., 1992, 1996; Zapata et al., 2003) (Fig. 1) in a model of NMDA-induced seizures that detects NMDA receptor antagonists and for which dextromethorphan is active (Ferkany et al., 1988). Variations of this model have been used as an in vivo model to identify NMDA receptor antagonists (Leander et al., 1988). Correlational analysis of the potencies of the compounds to block seizures compared to their binding affinities to NMDA receptors, σ1, and σ2 receptors was utilized to derive insight into mechanistic processes.
Figure 1.
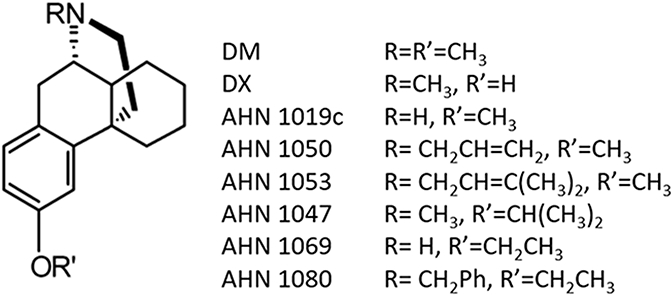
Structures of the dextromethorphan analogs studied.
Methods
Compounds.
Compounds were synthesized in house (A.H. Newman laboratory, NIDA, Baltimore, MD).
[3H]SKF 10047, [3H]TCP, and [3H]DTG Binding.
Binding affinities were established by Newman et al. (1996) per methods described therein and are used here to compare potencies to the in vivo anticonvulsant activity of these compounds. In these studies, NMDA receptor affinity was obtained by displacement of [3H]TCP binding. σ1 affinity was determined by the displacement of [3H]SKF 10047 binding in the presence of MK-801. σ2 binding affinities were obtained by displacement of [3H]DTG binding in the presence of (+)-SKF-10047.
NMDA-induced Seizures.
NMDA convulsions were induced in male Sprague-Dawley rats (200-250 g; Charles River) implanted with an i.c.v. cannula aimed at the right lateral ventricle under surgical anesthesia (70 mg/kg ketamine and 6 mg/kg xylazine, i.m.) Three to five days post-surgery the animals were divided into groups (n=6 per group) for testing. As described previously (Tortella et al., 1994) a suprathreshold, non-lethal dose of 12.5 nM NMDA was administered i.c.v. to induce the NMDA receptor specific clonic "popcorn" convulsions. Two indices of convulsive activity were measured during a 120 sec observation period: the latency to onset to NMDA convulsions and the number of rats per group exhibiting convulsive behavior (% responding). In all cases, NMDA was injected i.c.v. as a bolus 15 min following the i.c.v. injection of either vehicle or the respective compound. All animals were housed and used in accordance with NIH guidelines for the care and use of experimental animals. In addition, the experiments were conducted under the overview and approval of an animal care and use committee and monitored by veterinary and animal care staff.
Dose-response curves were analyzed using the methods of Litchfield and Wilcoxon (1949) (Tallarida and Murray, 1987). Potencies of the compounds at each receptor were correlated with their potencies to block NMDA-induced seizures using the Pearson correlation coefficient; statistical significance was secondarily evaluated by linear regression (ANOVA). Comparisons of latencies to produce seizures were compared to one another with post-hoc Dunnett’s test after statistically-significant ANOVA verification. All statistical evaluations were conducted with a priori value of p < 0.05 being considered significant. Data were analyzed using GraphPad Prism software (ver. 5.02).
Results
The in vitro affinities of the compounds studied are shown in Table 1. Potencies at NMDA receptors varied over two orders of magnitude from 460 nM for dextrorphan (DX) to >40 μM for AHN 1080. Many of these compounds had higher affinity for σ1 receptors than for either NMDA or σ2 receptors including dextromethorphan (8 times higher affinity for σ1 vs NMDA receptors).
Table 1.
Affinities of dextromethorphan (DM), dextrorphan (DX) and analogs at NMDA, σ1, and σ2 binding sites and their potencies to block NMDA-induced convulsions.
| Compound | NMDA Receptor Ki (nM) |
σ1 Receptor Ki (nM) |
σ2 Receptor Ki (nM) |
NMDA-Induced Seizures ED50 (95% confidence limit) (μg) |
|---|---|---|---|---|
| DX | 460 | 559 | 1128 | 5 (2-11) |
| AHN 1019c | 487 | 1034 | >10,000 | 20 (11-36) |
| AHN 1050 | 1410 | 64 | >1000 | 210 (171-258) |
| AHN 1069 | 1770 | 2078 | >10,000 | 44 (25-76) |
| DM | 3500 | 419 | 2639 | 168 (125-226) |
| AHN 1047 | 11,500 | 2181 | >1000 | >400 |
| AHN 1053 | 27,800 | 24 | 948 | 113 (72-177) |
| AHN 1080 | 40,200 | 8 | 1050 | 186 (125-277) |
Compounds are displayed in order of their NMDA receptor affinity. NMDA receptor affinity was obtained by displacement of [3H]TCP binding. σ1 receptor affinity was determined by the displacement of [3H]SKF 10047 binding in the presence of MK-801. σ2 receptor binding affinities were obtained by displacement of [3H]DTG binding in the presence of (+)-SKF-10047. Binding data are from Newman, et al. (1996).
Phencyclidine fully blocked NMDA-induced convulsions (ED50=0.7 μg; 95% confidence limits: 0.5-1.0), as did dextrorphan (DX) and dextromethorphan (DM) (Table 1; Fig. 2). The analogs of dextromethorphan were anticonvulsant in this model with the exception of AHN 1047 (Table 1; Fig. 2). Latencies to produce convulsions were significantly different from vehicle control latencies (F8,63 = 30.4, p <0.0001) for all compounds tested. However, AHN-1047 significantly differed in efficacy from dextromethorphan and the other analogues (p <0.001) except for AHN 1080 (Table 1; Fig. 2).
Figure 2.
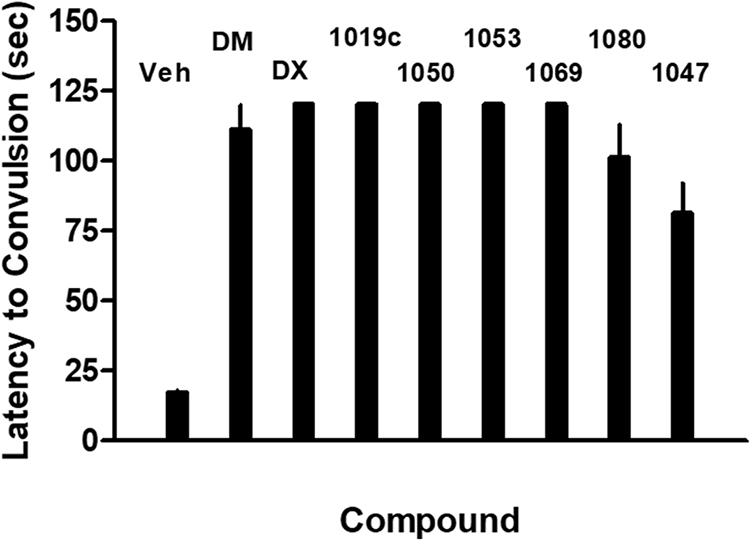
Latency to NMDA-induced convulsions in rats in the presence of vehicle or each of the dextromethorphan analogs studied. Each bar represents the mean + S.E.M. for 8 rats each. All compounds displayed higher latencies than vehicle (p<0.001). AHN 1047 differed significantly (p<0.001) from all other compounds except AHN 1080.
The potencies of these compounds to block NMDA-induced convulsions was positively associated with their affinities for the NMDA receptor ion channel ([3H]-TCP binding) (r = 0.71, p < 0.05) (Fig. 3, top panel). Linear regression analysis showed significant deviation from zero (F1,6 = 6.26, p < 0.05). Elimination of the outlier compound, ANH 1047, did not significantly alter this positive statistical association. In contrast, no significant relationship was observed between anticonvulsant potencies and affinities for σ1 receptors ([3H]-SKF 10047 binding) (r = −0.31, p > 0.05) (Fig. 3, bottom panel). Linear regression did not detect a significant deviation from zero (F1,6 = 0.63, p = 0.46). Likewise, there was not a statistically-significant relationship between anticonvulsant potencies and [3H]DTG binding (r = −0.38, p > 0.05). Linear regression confirmed this lack of relationship (F1,6 = 0.99, p = 0.36). However, this analysis for σ2 receptors is not precise as the values for binding were >1000 or > 10000 nM for one half of the compounds tested.
Figure 3.
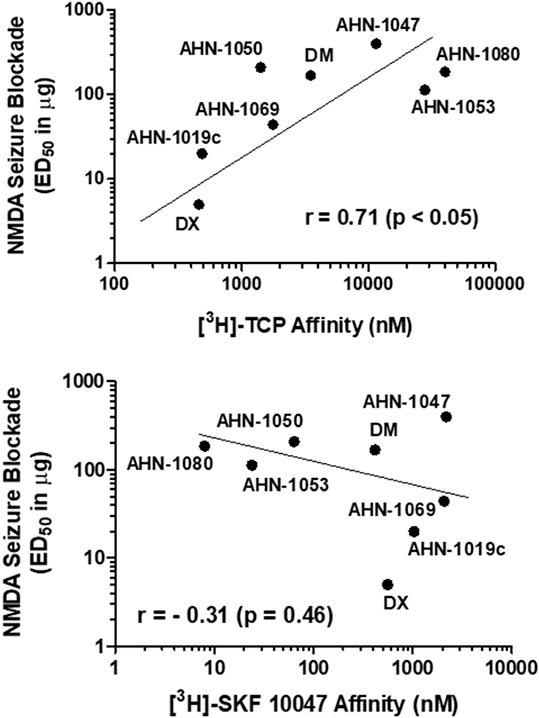
Relationship of NMDA ion channel (top panel) or σ1 receptor (bottom panel) binding affinities to anticonvulsant potency. Each point represents the mean value for each of the dextromethorphan analogs studied as shown also in Table 1. Data for σ2 binding are not plotted since half of the compounds studied had affinities designated as >1000 or > 10000 nM (Table 1).
Discussion
A series of N-substituted-3-alkoxy-analogs of dextromethorphan were studied for their ability to attenuate NMDA-induced convulsions in rats. Compounds were given by i.c.v. administration so that the effects of the compounds per se could be evaluated with minimal contamination from their metabolites. For example, dextromethorphan is metabolized to the more potent NMDA receptor antagonist, dextrorphan when given systemically (Taylor et al., 2016). All compounds blocked NMDA-induced convulsions (with AHN 1047 being an outlier) and antagonist ED50 values positively correlated with their potencies to bind to the NMDA receptor ion channel as measured by [3H]-TCP binding. ED50 values did not significantly correlate with the potencies of the compounds to bind to either σ1 or σ2 binding sites.
The pharmacology of dextromethorphan is complex and identification of mechanisms of action have not been straightforward. Work using estimated free brain concentrations of dextromethorphan led to the conclusion that NMDA receptors might be one receptor target in humans (Taylor et al., 2016). In the present study, the findings with NMDA-induced convulsions provides the first in vivo evidence that these dextromethorphan analogs act in rats through the blockade of NMDA receptors. This relationship was not fully predicted. First, Newman et al. (1992, 1996) had shown that these compounds were generally more potent at σ1 receptors than at NMDA receptors. Secondly, compound potencies to block cocaine-induced clonic convulsions in mice were negatively associated with binding to the NMDA receptor ion channel (r=−0.79, p<0.05) (Zapata et al., 2003). That lower affinity compounds were more potent than higher affinity compounds, is opposite to the present positive association of potency to affinity found here despite prediction that cocaine convulsions and NMDA convulsions would be pharmacologically isomorphic since NMDA receptor antagonists also block convulsions induced by cocaine (Witkin and Tortella, 1991; Witkin et al., 1999).
Nevertheless, the NMDA antagonist activity of the dextromethorphan analogs in vitro suggested that NMDA receptor actions of these compounds might be achieved in vivo. Blockade of NMDA receptors produces antidepressant-like effects in rodent models (Trullas and Skolnick, 1990; Skolnick et al. 2009; Zanos et al., 2018). Some of these compounds, ketamine in particular, have likewise shown rapid-acting antidepressant effects in depressed patients and in patients that have been unresponsive to standard-of-care medicines (Witkin et al., 2018). Esketamine ((S)-ketamine) has recently been approved by the U.S. FDA for treatment of major depressive disorder (February, 2019). However, ketamine and high affinity NMDA receptor ligands have side-effect and safety issues that may limit their therapeutic utility. Therefore, safer alternatives are needed.
Dextromethorphan produces antidepressant-like effects in rodent models (Nguyen and Matsumoto, 2015; Nguyen et al., 2014, 2016a, 2017; Sakhaee et al., 2016) and efficacy in patients in a case report (Lauterbach et al., 2016; see Nguyen et al., 2016 for a review). These effects have been attributed to blockade of NMDA receptors (Sakhaee et al., 2016) as well as to σ1 receptors (Nguyen et al., 2014). Based upon the functional activity of the structural analogs of dextromethorphan at NMDA receptors in vivo, it is predicted that they would also generate antidepressant-like phenotypes in rodents. Based upon the preclinical and clinical data, it is also predicted that these compounds would not produce side-effects in humans like those of higher affinity NMDA receptor antagonists like ketamine, phencyclidine, or MK-801. Preclinically, the dextromethorphan analogs did not produce complete motor-impairment or psychomotor stimulation like that of higher affinity ligands including dextrorphan (Zapata et al., 2003). Clinical data with lower affinity NMDA receptor antagonists, like memantine, have also suggested that lower affinity ligands might circumvent the undesirable side-effect profiles of high affinity ion channel blockade (Parsons et al., 1999a). Dextromethorphan has demonstrated rapid response efficacy in a treatment-resistant patient (Lauterbach, 2016) and by retrospective analysis in bipolar II patients (Kelly & Lieberman, 2014).
In conclusion, we have identified an NMDA receptor antagonist mechanism of action of a series of N- and 3-alkoxy-substituted dextromethorphan analogs that may constitute a new inroad into the development of a pharmacologically safer way of transducing the medically beneficial effects of NMDA receptor antagonism. The safety and efficacy of dextromethorphan has already been exemplified by its approved use in combination with quinidine (Nuedexta) for the treatment of pseudobulbar affect in multiple sclerosis and amyotrophic lateral sclerosis (Pioro et al., 2010). Low affinity NMDA receptor antagonists, exemplified by this set of dextromethorphan analogues, may have as of yet unexploited therapeutic benefit in neurological and psychiatric disorders such as epilepsy and major depressive disorder. The ‘opioid crisis’ (Coussens et al., 2019; Stoica et al., 2019) is another area of potential impact for dextromethorphan analog-based NMDA receptor antagonists. Tolerance to the analgesic effects of opioids is one of reason for opioid dose escalation and the negative health consequences of opioid dose increases (e.g., dependence, respiratory depression; (Jaffe and Martin, 1985)). NMDA receptors provide a primary control mechanism for regulating tolerance development and dextromethorphan along with other NMDA receptor antagonists have been under consideration for this effect (Herman et al., 1995).
Acknowledgments
Primary support for this work is gratefully acknowledged from the NIDA Intramural Research Program, Baltimore, Maryland and from the Walter Reed Army Institute of Research, Washington, DC. We are also grateful to John and Nancy Peterson for their support of this research. We also thank the National Institutes of Health for support from [MH-096463] and [NS-076517] and The National Science Foundation, Division of Chemistry [CHE-1625735]. We also acknowledge UW-Milwaukee's Shimadzu Laboratory for Advanced and Applied Analytical Chemistry and support from the Milwaukee Institute of Drug Discovery and the University of Wisconsin-Milwaukee Research Foundation.
Non-standard Abbreviations
- DTG
1,3-Di-o-tolylguanidine
- DM
dextromethorphan
- DX
dextrorphan
- SKF 10047
N-allylnormetazocine
- PCP
phencyclidine
- TCP
tenocyclidine
References
- Chien Y-H, a, Lin M, Weng W-C, Du J-C, Lee W-T. Dextromethorphan in the treatment of early myoclonic encephalopathy evolving into migrating partial seizures in infancy. J. Formosan Med. Assoc (2012) 111, 290–294 [DOI] [PubMed] [Google Scholar]
- Coussens NP, Sittampalam GS, Jonson SG, Hall MD, Gorby HE, Tamiz AP, McManus OB, Felder CC, Rasmussen K, 2019. The Opioid Crisis and the Future of Addiction and Pain Therapeutics. J. Pharmacol. Exp. Ther 371, 396–408. 10.1124/jpet.119.259408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, De La Gandara JE, Agronin M, Davis CS, Nguyen U, Shin P, Tariot PN, Siffert J. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA. 2015. Sep 22-29;314(12):1242–54. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Borosky SA, Clissold DB, Pontecorvo MJ. Dextromethorphan inhibits NMDA-induced convulsions. Eur J Pharmacol. 1988. Jun 22;151(1):151–4. [DOI] [PubMed] [Google Scholar]
- Geter-Douglass B, Witkin JM. Behavioral effects and anticonvulsant efficacies of low-affinity, uncompetitive NMDA antagonists in mice. Psychopharmacology (Berl). 1999. Oct;146(3):280–9. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Phillips C, Fahimi A, McNerney MW, Salehi A. Mechanisms of action and clinical efficacy of NMDA receptor modulators in mood disorders. Neurosci Biobehav Rev. 2017. Sep;80:555–572. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Schachter SC. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 2011. Dec;22(4):6–40. [DOI] [PubMed] [Google Scholar]
- Ginski MJ, Witkin JM. Sensitive and rapid behavioral differentiation of N-methyl-D-aspartate receptor antagonists. Psychopharmacology (Berl). 1994. May;114(4):573–82. [DOI] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology. 1995. Dec;13(4):269–93. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ. The use of NMDA-receptor antagonists in the treatment of chronic pain. Clin J Pain. 2000. Jun;16(2 Suppl):S73–9. [DOI] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA Jr., 2015. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis 6 (3), 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Martin WR, 1985. Opioid analgesics and antagonists, in: Gilman AG, Goodman LS, Rall TW, Murad F (Eds.), Goodman and Gilman's The pharmacological basis of therapeutics. Macmillan Publishing Company, New York, pp. 491–531 [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008. Aug;7(8):742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Cho S, Lee JH, 2016. Aug. The effects of long-term ketamine treatment on cognitive function in complex regional pain syndrome: a preliminary study. Pain Med. 17 (8), 1447–1451. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Mirtsou-Fidani V, Papaioannidou PG, Niopas I, Georgiadis G, Constadinidis TC, Kazis AD. A phase I clinical trial of dextromethorphan in intractable partial epilepsy. Methods Find Exp Clin Pharmacol. 1999. Dec;21(10):673–8. doi: 10.1358/mf.1999.21.10.795765. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. Treatment Resistant Depression with Loss of Antidepressant Response: Rapid-Acting Antidepressant Action of Dextromethorphan, A Possible Treatment Bridging Molecule. Psychopharmacol Bull. 2016. Aug 15;46(2):53–58. [PMC free article] [PubMed] [Google Scholar]
- Leander JD, Lawson RR, Ornstein PL, Zimmerman DM (1988) N-methyl-D-aspartic acid-induced lethality in mice: selective antagonism by phencyclidine-like drugs. Brain Res 448:115–120 [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chu CH, Chen SH, Chen PS, Huang SY, Tzeng NS, Wang LJ, Lee IH, Wang TY, Chen KC, Yang YK, Hong JS, Lu RB. A placebo-controlled trial of dextromethorphan as an adjunct in opioid-dependent patients undergoing methadone maintenance treatment. Int J Neuropsychopharmacol. 2015. Feb 25;18(7): pyv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 95: 99–113 [PubMed] [Google Scholar]
- Malek A, Amiri S, Habibi Asl B. The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial. ISRN Psychiatry. 2013. Jun 20;2013:546030. doi: 10.1155/2013/546030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Bevan K, Bowery N, Tortella FC. Synthesis and Evaluation of 3-substituted 17-methylmorphinan analogs as potential anticonvulsant agents. J Med Chem 1992; 35: 4135–4142. [DOI] [PubMed] [Google Scholar]
- Newman AH, Shah JS, Izenwasser S, Heller B, Mattson M, Tortella FC. Highly selective sigma1 ligands based on dextromethorphan. Med Chem Res 1996; 6:102–17. [Google Scholar]
- Nguyen L, Matsumoto RR. Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behav Brain Res. 2015. Dec 15;295:26–34 [DOI] [PubMed] [Google Scholar]
- Nguyen L, Lucke-Wold BP, Logsdon AF, Scandinaro AL, Huber JD, Matsumoto RR. Behavioral and biochemical effects of ketamine and dextromethorphan relative to its antidepressant-like effects in Swiss Webster mice. Neuroreport. 2016a. Sep 28;27(14):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One. 2014. Feb 28;9(2):e89985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Scandinaro AL, Matsumoto RR. Deuterated (d6)-dextromethorphan elicits antidepressant-like effects in mice. Pharmacol Biochem Behav. 2017. Oct;161:30–37. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther. 2016b. Mar;159:1–22 [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999a. Jun;38(6):735–67 [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Bartmann A, Spielmanns P, Frankiewicz T, Hesselink M, Eilbacher B, Quack G. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: in vitro and in vivo characterization. Neuropharmacology. 1999b. Jan;38(1):85–108. [DOI] [PubMed] [Google Scholar]
- Pioro EP, Brooks BR, Cummings J, Schiffer R, Thisted RA, Wynn D, Hepner A, Kaye R; Safety, Tolerability, and Efficacy Results Trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010. Nov;68(5):693–702. doi: 10.1002/ana.22093. [DOI] [PubMed] [Google Scholar]
- Sakhaee E, Ostadhadi S, Khan MI, Yousefi F, Norouzi-Javidan A, Akbarian R, Chamanara M, Zolfaghari S, Dehpour AR. The role of NMDA receptor and nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effect of dextromethorphan in mice forced swimming test and tail suspension test. Biomed Pharmacother. 2017. Jan;85:627–634. [DOI] [PubMed] [Google Scholar]
- Semenova S, Danysz W, Bespalov A. Low-affinity NMDA receptor channel blockers inhibit acquisition of intravenous morphine self-administration in naive mice. Eur J Pharmacol. 1999. Jul 28;378(1):1–8. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009. Nov;30(11):563–9. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks CL, Gupta S, Ewen JB, Hong M, Kratz L, Kelley R, Tierney E, Vaurio R, Bibat G, Sanyal A, Yenokyan G, Brereton N, Johnston MV, Naidu S. Randomized open-label trial of dextromethorphan in Rett syndrome. Neurology. 2017. Oct 17;89(16):1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoicea N, Costa A, Periel L, Uribe A, Weaver T, Bergese SD, 2019. Current perspectives on the opioid crisis in the US healthcare system: A comprehensive literature review. Medicine (Baltimore) 98, e15425. 10.1097/MD.0000000000015425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ and Murray RB. Manual of pharmacologic calculations with computer programs. Second edition. By Springer-Verlag: New York. 1987 [Google Scholar]
- Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol Ther. 2016. Aug;164:170–82 [DOI] [PubMed] [Google Scholar]
- Tortella FC, Robles L, Witkin JM, Newman AH. Novel anticonvulsant analogs of dextromethorphan: improved efficacy, potency, duration and side-effect profile. J Pharmacol Exp Ther. 1994. Feb;268(2):727–33. [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990. Aug 21;185(1):1–10. [DOI] [PubMed] [Google Scholar]
- Wang R, Reddy PH. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J Alzheimers Dis. 2017;57(4):1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG and Beck H. Improvement of Medically Refractory Temporal Lobe Epilepsy with Dextromethorphan. J Epilepsy 1992;5:246–247 [Google Scholar]
- Willetts J, Balster RL, Leander JD. The behavioral pharmacology of NMDA receptor antagonists. Trends Pharmacol Sci. 1990. Oct;11(10):423–8. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tortella FC. Modulators of N-methyl-D-aspartate protect against diazepam- or phenobarbital-resistant cocaine convulsions. Life Sci. 1991;48(11):PL51–6. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Gasior M, Heifets B, Tortella FC. Anticonvulsant efficacy of N-methyl-D-aspartate antagonists against convulsions induced by cocaine. J Pharmacol Exp Ther. 1999. May;289(2):703–11. [PubMed] [Google Scholar]
- Witkin JM, Knutson DE, Rodriguez GJ, Shi S. Rapid-Acting Antidepressants. Curr Pharm Des. 2018;24(22):2556–2563. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gasior M, Geter-Douglass B, Tortella FC, Newman AH, Witkin JM. Attenuation of the stimulant and convulsant effects of cocaine by 17-substituted-3-hydroxy and 3-alkoxy derivatives of dextromethorphan. Pharmacol Biochem Behav. 2003. Jan;74(2):313–23. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018. Jul;70(3):621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]


