Abstract
Background
The risks of severe outcomes associated with SARS-CoV-2 (COVID-19) are elevated in unvaccinated individuals. It remains crucial to understand patterns of COVID-19 vaccination, particularly in younger and remote populations where coverage often lags. This study examined disparities in COVID-19 vaccine coverage in farm children and adolescents.
Methods
A cross-sectional analysis was conducted in patients of the Marshfield Clinic Health System (MCHS) in Wisconsin. The sample included children/adolescents age 5–17 years who were eligible for COVID-19 vaccine initiation for ≥ 90 days (as of September 30, 2022), stratified by those who lived vs did not live on a farm. Outcomes included COVID-19 vaccine initiation, series completion, and booster receipt. Multivariable regression was used to examine associations between COVID-19 vaccination and farm, as well as rural and non-rural, residence.
Results
There were 47,104 individuals (5% farm residents) in the sample. Overall, 33% of participants initiated and 31% completed the COVID-19 vaccine series. After adjustment, farm residence was associated with significantly lower odds of COVID-19 vaccine initiation (aOR [95% CI] = 0.68 [0.61, 0.75], p < 0.001), series completion (aOR = 0.67 [0.60, 0.75], p < 0.001), and booster receipt (aOR = 0.73 [0.61, 0.88], p = 0.001). Secondary analyses found COVID-19 vaccine coverage was lowest in young children who lived on dairy farms.
Conclusions
COVID-19 vaccine coverage is low in north-central Wisconsin children and adolescents. Those who live on farms have significantly lower likelihood of COVID-19 vaccine initiation, series completion, and booster receipt compared to non-farm counterparts. Farm families are an underserved group and require more effective public health interventions designed to prevent COVID-19.
Keywords: Rural, Farm, COVID-19, Vaccination, Children
1. Introduction
Since identification in late 2019, infection from the SARS-CoV-2 virus (COVID-19) has resulted in millions of deaths worldwide [1], [2], with a disproportionate share of the disease burden in the U.S. [3]. Within the U.S., socially disadvantaged groups have been particularly hard hit by COVID-19 [4], including those who live in rural areas [5]. Millions of children have tested positive for COVID-19 since the onset of the pandemic [6], representing approximately-one of every-five cases. COVID-19 infections in children, especially those who were unvaccinated, increased dramatically with the Omicron variant [7]. COVID-19 causes hospitalization and other severe outcomes in children, including post-COVID-19 conditions such as increased fatigue, anosmia/ageusia, neurodevelopmental impediments, and respiratory conditions [8]. In addition to these direct medical effects, children suffer substantial indirect consequences of the disease as members of affected families/households. These consequences include the loss of caregivers, financial hardships, and missed school [9], [10], [11].
Fortunately, safe and effective vaccines were rapidly developed to protect against COVID-19 and related outcomes [12]. COVID-19 vaccine uptake was rapid across the U.S. in 2021, but has now plateaued [13]. In Wisconsin, vaccine series completion rates vary widely by age, from a high of 82% in adults age ≥ 65 years, to lows of 27% in young children age 5–11 years and 2% in very young children age 0–4 years [14]. COVID‐19 vaccine completion rates are also very low in certain geographic parts of the U.S., with some rural counties in the Midwest and South lingering below 20% [15]. Relative to older adults and more urbanized settings, lower COVID-19 vaccine uptake has been observed in both children and rural residents, respectively [16]. High vaccination coverage is among the most critical strategies to minimize excess hospitalizations and deaths in underserved communities, including rural communities [17], [18].
Rural areas typically have lower average income, less available health insurance, and other access barriers to healthcare [19], as well as higher vaccine hesitancy sentiments [20]. There are also differences in COVID-19 vaccination rates within rural counties that are partially explained by socioeconomic and political factors. For example, unadjusted analyses recently found that rural counties most heavily dependent on farming- and mining-related occupations had significantly lower COVID-19 vaccination rates as compared to rural counties with more diverse economic specializations [21]. Accordingly, rural counties most heavily dependent on farming were also found to have the highest COVID-19 mortality rates [22].
It is crucial to understand community-level patterns of vaccination in rural areas of the U.S. where uptake of the COVID-19 vaccine has lagged [16]. These areas tend to have a higher concentration of farm families, for whom the burden of COVID-19 may be more severe [22], [23], [24]. In addition, rural and remote areas have limited healthcare capacity to vaccinate, manage COVID-19 surges, and provide emergency medical services and complex inpatient care for severe COVID-19 cases [25], [26], [27]. COVID-19 vaccine disparities research is still emerging, particularly in children, and no studies have yet examined disparities in COVID-19 vaccine coverage in farmers. The purpose of this study was to examine COVID-19 vaccine coverage in children and adolescents who live on farms, including comparisons between farm types and those who live in rural and non-rural areas.
2. Methods
2.1. Design and setting
A cross-sectional analysis was used with sociodemographic and clinical data from Marshfield Clinic Health System’s (MCHS) research data warehouse, which is sourced by electronic health records (EHR), as well as linked data on farm and rural residence from a surveillance system of agricultural injuries and a regional vaccine registry. The source population included children and adolescents who lived in a 20-county region of north-central Wisconsin, and who had reasonably complete capture of their medical care and vaccination status within MCHS data systems, as outlined further below.
2.2. Participants
Participant eligibility criteria were, as of 09/30/2022: (1) age-eligible for a U.S. Food and Drug Administration (FDA) authorized or approved COVID-19 vaccine for ≥ 90 days (i.e., age 5–11 years between November 2021 and September 2022, or age 12–15 years between May 2021 and September 2022, or age 16–17 years between December 2020 and September 2022), (2) ≥ 1 encounter with an MCHS medical provider over the previous three years, (3) a vaccine registry record (described further below), and (4) capture of medical care within MCHS data systems as evidenced by: (a) ‘medically homed’ to an MCHS medical center (i.e., ≥2 qualifying ambulatory visits over the previous three years or an assigned MCHS primary care provider), (b) member of the MCHS-affiliated health insurance plan, Security Health Plan of Wisconsin, or (c) resident of the Marshfield Epidemiologic Study Area (MESA) [28]. The requirement of having at least one medical encounter, regardless of how their medical care information is captured (as described in participant eligibility criterion 4 above), helped ensure reasonably current study information. Being age-eligible for an FDA-authorized COVID-19 vaccine for ≥ 90 days also ensured a reasonable minimum time/opportunity for participants to have considered and initiated vaccination. All study-eligible individuals were included in the analytic dataset. Study procedures were approved in advance by the MCHS Institutional Review Board, including an approval to waive documentation of informed consent and HIPAA authorization.
2.3. COVID-19 vaccination
The primary outcomes were COVID-19 vaccine primary series initiation, primary series completion, and/or booster vaccination (age ≥ 12 years). Information on all vaccination dates and vaccine products received by study participants were obtained through the Registry for Effectively Communicating Immunization Needs (RECIN) [29], a regional population-based immunization registry used by public and private immunization providers across Wisconsin. RECIN is sourced by vaccine information from MCHS and the Wisconsin Immunization Registry. We were unable to account for the very small fraction of children and adolescents contraindicated for the COVID-19 vaccine (e.g., history of severe allergic reactions). Operational definitions for each outcome were:
-
1)
Initiated – At least one mRNA COVID-19 vaccine received on or before 09/30/2022. Note that the Pfizer-BioNTech vaccine was the only vaccine authorized/approved by FDA for children and adolescents during the study timeframe.
-
2)
Completed – Two mRNA COVID-19 vaccines ≥ 17 days apart.
-
3)
Boosted – Participants aged 12–17 years with a follow-up mRNA vaccine at least five months after their second primary COVID-19 vaccine dose. Second boosters were not considered, as they were only recommended for a very small subset of adolescents who are severely immunocompromised.
2.4. Farm and rural residence
The primary exposure was farm residence, per linked data from the Wisconsin National Children’s Center for Rural and Agricultural Health and Safety surveillance system (WINS). WINS methodology is described in more detail elsewhere [30], but briefly, children and adolescents in the target population with a residential address that had evidence of agricultural production were categorized in the farm group. This included a registry of licensed dairy producers from Wisconsin’s Department of Agriculture, Trade and Consumer Protection, or a commercially-available listing of area farm producers, that were matched to MCHS patient records. Children and adolescents who did not have evidence of farm residence were categorized in the non-farm comparison group. Secondary analyses also subcategorized the farm group into dairy vs non-dairy farms, and the non-farm comparison group was subcategorized into those who lived in a rural vs non-rural residence. Rurality was based on each participant’s residential ZIP code and associated population density and commuter volume/flow to adjacent metropolitan areas [31].
2.5. Covariates
Several sociodemographic and clinical covariates were extracted from the EHR. Sociodemographic measures included age, sex, race/ethnicity, health insurance status, and residential ZIP code. Clinical data included body mass index (BMI – modeled as age/sex percentile categories for children and adolescents), number of ambulatory visits in prior three years, chronic medical condition indicator of pulmonary disease, diabetes, or cardiovascular disease (diagnostic codes available upon request), a record of prior Tdap (tetanus, diphtheria, and pertussis) vaccination for individuals 13 years and older or prior MMR (measles, mumps, and rubella [and varicella]) vaccination for individuals under age 13, prior history of COVID-19 testing and results, and number of days eligible for the COVID-19 vaccine (reported in 30-day increments). Due to skewness, the number of ambulatory visits over the prior three years was modeled as quartiles (≤2, 3–4, 5–7, and ≥ 8). Prior COVID-19 vaccine testing and results were categorized as the cross-product of: (1) having received an EHR-documented test for COVID-19 prior to the date of COVID-19 vaccine initiation (if vaccinated) or end of follow-up on 09/30/2022 (if not vaccinated), and (2) ≥ 1 COVID-19 positive test finding, all negative COVID-19 test findings, or undeterminable COVID-19 test findings. The absence of a record of prior Tdap (adolescents age ≥ 13 years) or MMR (children age < 13 years) vaccination was used as a proxy measure of vaccine hesitancy, as the state of Wisconsin requires Tdap vaccine for all students by 6th grade and two MMR vaccine doses by kindergarten [32].
2.6. Analyses
Descriptive characteristics were reported by farm/non-farm status, including summaries of COVID-19 vaccination coverage during the study timeframe. Multivariable logistic regression was used to examine associations between farm residence and COVID-19 vaccination in the primary analysis. Separate analyses were conducted for COVID-19 vaccine initiation, primary series completion, and booster receipt (limited to individuals aged ≥ 12 years). Univariate models were first created and multivariable models simultaneously included all a priori specified covariates. Secondary analyses used similar analytic procedures, but disaggregated the farm group by dairy and non-dairy farm residents, as well as the non-farm group by rural and non-rural residents. In addition, to better detail age-related impacts, these secondary analyses were further age-stratified by the three groups that were authorized for the COVID-19 vaccine at different times (i.e., 5–11 years, 12–15 years, 16–17 years). All analyses were conducted using SAS Version 9.4 (Cary, NC).
3. Results
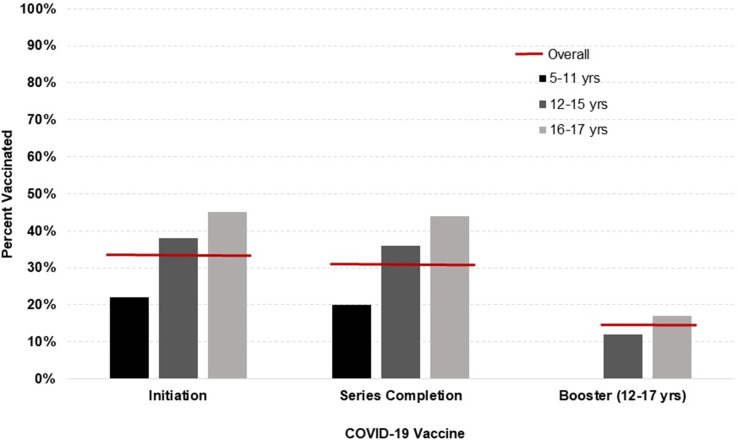
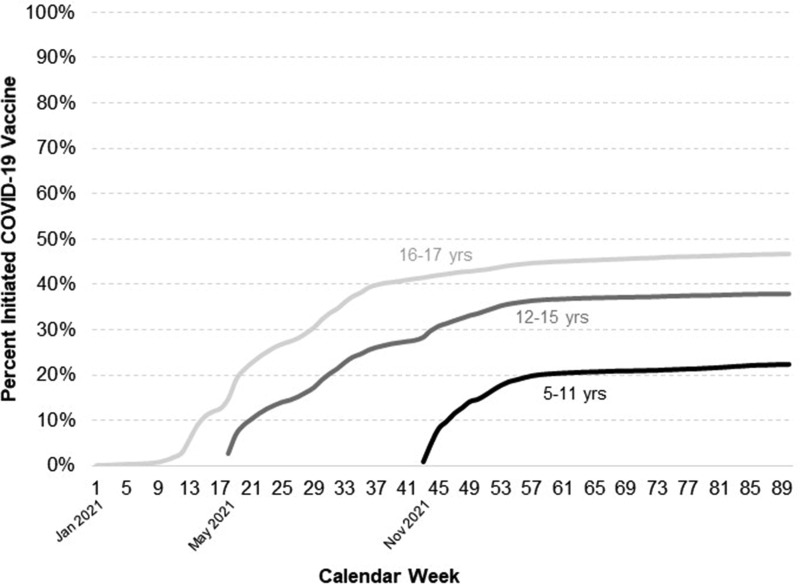
There were 47,104 children and adolescents in the sample, with 2,132 (5%) in the farm and 44,972 (95%) in the non-farm group. As outlined in Table 1 , the two groups were relatively similar across most sociodemographic characteristics, but the farm group had more White, non-Hispanic children/adolescents and fewer with public-assisted health insurance. Overall, 33% of participants initiated the COVID-19 vaccine, 31% completed the primary vaccine series, and 14% (of those aged ≥ 12 years) received a booster. These values are broken down by age in Fig. 1 , where COVID-19 vaccination coverage is clearly lower in younger age groups. A vaccine initiation curve is outlined in Fig. 2 , reflecting fairly rapid initial uptake of the COVID-19 vaccine upon release, followed by a plateau in all age groups.
Table 1.
Characteristics of north-central Wisconsin children and adolescents who do and do not live on a farm, 2022.
| Farm |
Non-farm |
|
|---|---|---|
| n = 2,132 | n = 44,972 | |
| Age (y) | 12.7 ± 4.1 | 12.1 ± 4.1 |
| Gender | ||
| Female | 993 (47%) | 22,111 (49%) |
| Male | 1,139 (53%) | 22,861 (51%) |
| Race/Ethnicity | ||
| White, non-Hispanic | 1,922 (90%) | 35,893 (80%) |
| Non-White or Hispanic | 133 (6%) | 7,088 (16%) |
| Unknown | 77 (4%) | 1,991 (4%) |
| Health insurance | ||
| Private | 1,171 (55%) | 22,249 (49%) |
| Public-assisted | 926 (43%) | 22,406 (50%) |
| None | 35 (2%) | 317 (1%) |
| Number of ambulatory visits (prior 3 years) | 7.0 ± 8.3 | 7.3 ± 8.3 |
| Body mass index (kg/m2) | 21.4 ± 5.9 | 21.4 ± 6.3 |
| Chronic medical condition | 463 (22%) | 11,092 (25%) |
| Prior Tdap (≥13 years old) or MMR (<13 years old) vaccination | ||
| Received | 2,012 (94%) | 43,053 (96%) |
| Not received | 120 (6%) | 1,919 (4%) |
| Prior COVID-19 testing and results | ||
| Tested, ≥1 positive result | 247 (12%) | 5,270 (12%) |
| Tested, only negative result(s) | 569 (27%) | 14,355 (32%) |
| Tested, unknown result(s) | 37 (2%) | 819 (2%) |
| Not tested | 1,279 (60%) | 24,528 (55%) |
| Days eligible for COVID-19 vaccine | 396.6 ± 117.0 | 384.1 ± 118.3 |
Values are reported as mean ± SD or frequency (% of total).
Fig. 1.
COVID-19 vaccine coverage in north-central Wisconsin children and adolescents, overall and by age groups.
Fig. 2.
Cumulative COVID-19 vaccine initiation in north-central Wisconsin children and adolescents, by age groups and calendar.
In the initial univariate models, farm status was significantly associated with COVID-19 vaccine initiation (26% farm vs 33% non-farm, p < 0.001), primary series completion (25% farm vs 31% non-farm, p < 0.001), and booster receipt (11% farm vs 15% non-farm, p = 0.002). As outlined in Table 2 , these associations were similar in the multivariable models after adjustment for age, sex, race/ethnicity, health insurance, BMI, recent ambulatory visits, chronic medical condition, prior Tdap or MMR vaccination, prior history of COVID-19 testing and results, and days eligible for the COVID-19 vaccine. Relative to the non-farm group, children and adolescents who lived on a farm had 32% lower odds of COVID-19 vaccine initiation (adjusted odds ratio [aOR] = 0.68 [95% confidence interval {CI}: 0.61, 0.75], p < 0.001), 33% lower odds of primary series completion (aOR = 0.67 [CI: 0.60, 0.75], p < 0.001), and 27% lower odds of booster receipt (aOR = 0.73 [CI: 0.61, 0.88], p = 0.001). With the exception of overweight/obesity, nearly all covariates were significant predictors of COVID-19 vaccine initiation, primary series completion, and booster receipt. None, however, appreciably shifted the univariate odds ratios for farm status (indicating little to no confounding after covariate adjustment).
Table 2.
Multivariable model of farm residence and COVID-19 vaccine initiation, series completion, and booster receipt in north-central Wisconsin children and adolescents.
| COVID-19 vaccination (yes vs no) Multivariable adjusted odds ratio (95% CI), p |
|||
|---|---|---|---|
| Initiated | Completed | Boosted (age ≥ 12 yrs) |
|
| Residence | |||
| Farm vs Non-farm | 0.68 (0.61, 0.75) p <.001 |
0.67 (0.60, 0.75) p <.001 |
0.73 (0.61, 0.88) p =.001 |
| Age (yrs) | 1.12 (1.10, 1.12) p <.001 |
1.12 (1.11, 1.13) p <.001 |
1.15 (1.13, 1.17) p <.001 |
| Sex | |||
| Female vs Male | 1.11 (1.06, 1.15) p <.001 |
1.10 (1.06, 1.15) p <.001 |
1.16 (1.08, 1.25) p <.001 |
| Race/Ethnicity | |||
| Non-White or Hispanic vs White, non-Hispanic | 2.28 (2.15, 2.41) p <.001 |
2.19 (2.06, 2.32) p <.001 |
1.62 (1.47, 1.80) p <.001 |
| Unknown vs White, non-Hispanic | 1.74 (1.57, 1.93) p <.001 |
1.74 (1.57, 1.94) p <.001 |
1.67 (1.38, 2.02) p <.001 |
| Health insurance | |||
| Private vs Public-assisted | 2.12 (2.03, 2.21) p <.001 |
2.24 (2.13, 2.25) p <.001 |
2.27 (2.10, 2.46) p <.001 |
| None vs Public-assisted | 0.96 (0.72, 1.27) p =.752 |
0.95 (0.70, 1.27) p =.705 |
0.53 (0.25, 1.48) p =.108 |
| Number of ambulatory visits (prior 3 years; quartiles) | |||
| 3–4 vs ≤ 2 | 1.24 (1.16, 1.32) p <.001 |
1.25 (1.17, 1.34) p <.001 |
1.18 (1.03, 1.36) p =.019 |
| 5–7 vs ≤ 2 | 1.74 (1.63, 1.86) p <.001 |
1.84 (1.72, 1.97) p <.001 |
1.89 (1.67, 2.15) p <.001 |
| ≥8 vs ≤ 2 | 2.90 (2.72, 3.09) p <.001 |
3.12 (2.92, 3.33) p <.001 |
3.55 (3.16, 3.98) p <.001 |
| Body mass index (percentile categories) | |||
| Underweight vs Normal weight | 1.25 (1.11, 1.41) p <.001 |
1.28 (1.13, 1.44) p <.001 |
1.24 (1.02, 1.51) p =.033 |
| Overweight vs Normal weight | 0.97 (0.92, 1.03) p =.359 |
0.97 (0.92, 1.03) p =.335 |
0.95 (0.86, 1.06) p =.367 |
| Obese vs Normal weight | 0.98 (0.93, 1.04) p =.531 |
0.97 (0.92, 1.02) p =.242 |
0.93 (0.85, 1.02) p =.143 |
| Unknown vs Normal weight | 1.94 (1.68, 2.25) p <.001 |
2.04 (1.76, 2.37) p <.001 |
2.45 (1.87, 3.22) p <.001 |
| Chronic medical condition | |||
| Yes vs no | 1.09 (1.04, 1.15) p <.001 |
1.09 (1.03, 1.15) p =.001 |
1.15 (1.06, 1.25) p =.001 |
| Prior Tdap (≥13 years old) or MMR (<13 years old) vaccination | |||
| Not received vs Received | 0.27 (0.23, 0.32) p <.001 |
0.26 (0.22, 0.31) p <.001 |
0.25 (0.17, 0.36) p <.001 |
| Prior COVID-19 testing and results | |||
| Tested, ≥1 positive result vs Not tested | 0.38 (0.35, 0.41) p <.001 |
0.37 (0.34, 0.39) p <.001 |
0.30 (0.26, 0.34) p <.001 |
| Tested, only negative result(s) vs Not tested | 0.84 (0.80, 0.88) p <.001 |
0.83 (0.79, 0.87) p <.001 |
0.65 (0.60, 0.71) p <.001 |
| Tested, unknown result(s) vs Not tested | 0.45 (0.37, 0.54) p <.001 |
0.41 (0.34, 0.51) p <.001 |
0.25 (0.16, 0.41) p <.001 |
| Days eligible for COVID-19 vaccine * | 1.02 (1.01, 1.03) p <.001 |
1.03 (1.02, 1.04) p <.001 |
1.01 (1.00, 1.02) p =.030 |
*The adjusted odds ratio and 95% CI were reported in 30-day (vs 1 day) increments of time eligible for the COVID-19 vaccine.
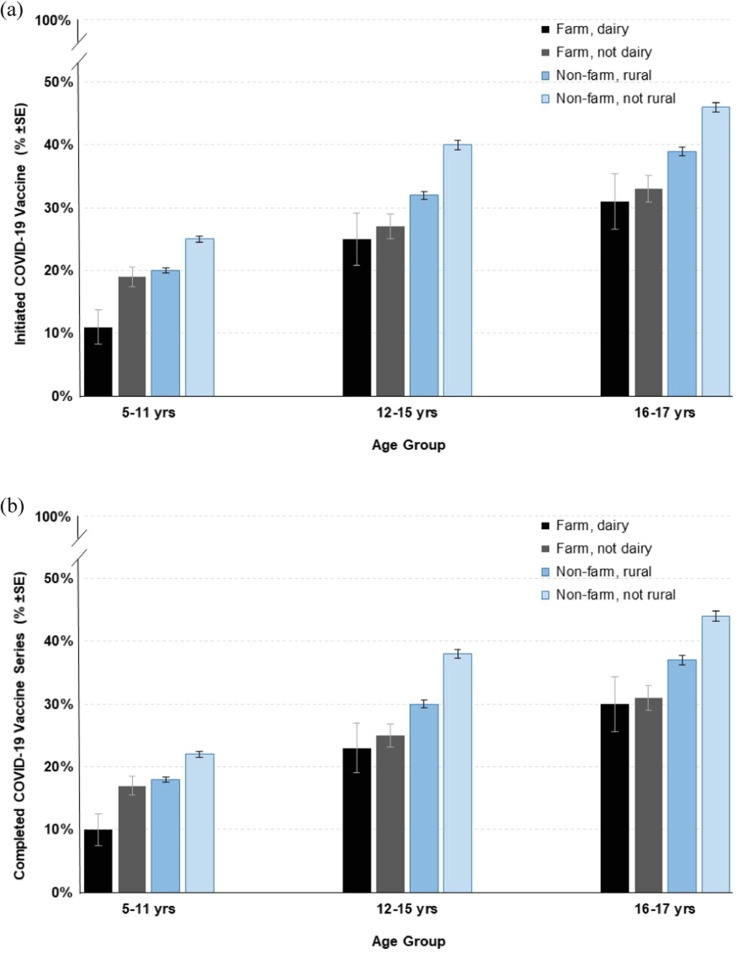
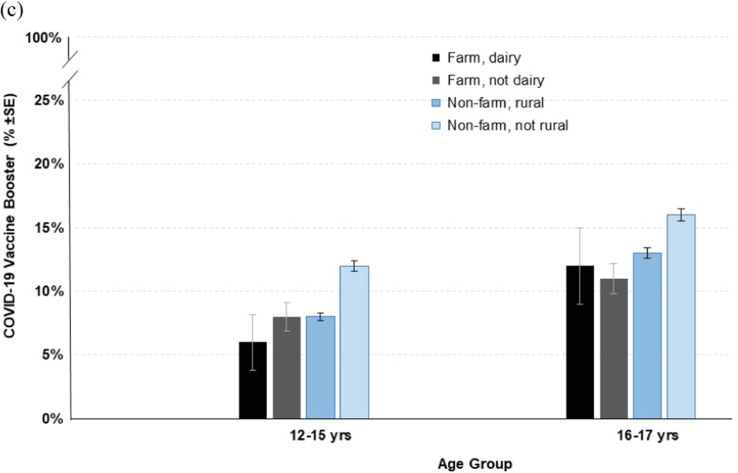
In the secondary analyses of farm type (dairy and non-dairy) and rural and non-rural residence, stratified by age groups, multivariable models indicated the farm, dairy; farm, non-dairy; and non-farm, rural groups had significantly lower odds of COVID-19 initiation, primary series completion, and booster receipt relative to the non-farm, non-rural group, across all examined age groups (full models not shown). To better illustrate the complex associations between these groups, model-based estimates of COVID-19 vaccine initiation, primary series completion, and booster receipt are summarized in Fig. 3 . In general, older adolescents who lived in non-rural areas had the highest COVID-19 vaccine coverage. The greatest differences were observed for COVID-19 vaccine initiation where, for example, an estimated 11% (SE ± 2.7%) of dairy farm children aged 5–11 years initiated the COVID-19 vaccine vs 46% (SE ± 0.8%) of non-rural adolescents aged 16–17 years.
Fig. 3.
Estimated probability of COVID-19 vaccine initiation (a),series completion (b), and booster receipt (c) in north-central.
4. Discussion
COVID-19 vaccine coverage is low in children and adolescents in this predominantly rural region of north-central Wisconsin. Just one-third of our study sample initiated the COVID-19 vaccine series, whereas 47% of those age 5–17 years initiated the COVID-19 vaccine across Wisconsin during the same timeframe [14]. Similar to the general U.S population [13], our sample showed fairly rapid initial uptake upon release of the vaccine, followed by a plateau of COVID-19 vaccine initiation, with the plateau occurring more quickly in younger age groups.
Compared to those who do not live on a farm, farm children/adolescents have ∼ 25% lower estimated likelihood of initiating the COVID-19 vaccine, completing the series, or receiving a booster. This pattern was stable under adjustment for age, time of COVID-19 vaccine availability, and prior COVID-19 infection (among other covariates), and appeared more pronounced in the secondary analysis looking at farm type and rural/non-rural comparison groups, by age. For example, over four times more adolescents (age 16–17) who lived in non-rural areas initiated the COVID-19 vaccine series relative to younger children (age 5–11) who lived on a dairy farm. This underscores the gradation of COVID-19 vaccine disparities, typified by lower vaccine coverage in younger individuals who live in more isolated areas, such as those reliant on agricultural production. This finding was consistent with previous ecological observations where rural counties in the U.S. that were heavily dependent on farming also had among the lowest COVID-19 vaccination rates [21].
Reasons for the low COVID-19 vaccine coverage in farm children are not well understood. Vaccination decisions in minors are primarily made by parents [33], and living on a farm is perhaps a surrogate marker of greater COVID-19 vaccine hesitancy in parents, particularly those on dairy farms. A recent nationwide survey found COVID-19 vaccine skepticism was indeed highest in rural residents and among those with more conservative political and religious views [34]. That study was not specific to farm families, but general sentiments are that farmers, while not monolithic, tend to be reliable conservative voters in Wisconsin [35] and across the U.S. [36], and generally harbor less trust in and alignment with the views of academic and government institutions [37], [38]. Government distrust was among the strongest predictors of COVID-19 vaccine hesitancy in underserved areas of North Carolina, including rural communities [39]. Structural barriers may also be more common in farm families, such as less flexible work schedules during certain seasons (e.g., planting, harvest) and living further away from vaccination centers.
Strengths of this study included the large sample size and complete capture of medical information and vaccination outcomes that did not rely on self-report. The main study limitation was the cross-sectional design conducted over the limited timeframe that COVID-19 vaccines have been available to children and adolescents. COVID-19 vaccine coverage may increase as safety data accrues and these analyses should be replicated in the future as eligibility for other COVID-19 vaccines change. For example, expanded analyses could soon examine differential COVID-19 vaccine coverage in children under age 5, as well as booster coverage in children age 5–11. In addition, the precision of COVID-19 vaccine coverage estimates could be improved by accounting for more specialized subgroups such as immunocompromised and contraindicated children/adolescents that require modified COVID-19 vaccine schedules. More proximal mediating factors that may explain low COVID-19 vaccine coverage trends in the farm group (e.g., hesitancy attitudes, structural barriers) were unavailable in medical records and should be considered in future prospective vaccine disparity studies. Finally, our source population was regional in scope and lacked racial/ethnic diversity, which may limit generalizability to other parts of the U.S.
5. Conclusions
Farm children/adolescents are an underserved population subgroup and likely require more intense and effective public health interventions to better control COVID-19. Although severe COVID-19 outcomes are less common in younger age groups [40] and vaccine-mediated protection against COVID-19 transmission has generally waned [41], [42], ‘pockets’ of very low COVID-19 vaccine coverage still put more children and adolescents at risk of severe COVID-19 associated conditions such as hospitalization, multisystem inflammatory syndrome in children (MIS-C), and lingering post-acute sequelae of COVID-19 (i.e., long COVID). Low vaccine coverage also amplifies strain on local healthcare resources in medically underserved rural areas during COVID-19 surges. To improve the relevance and effectiveness of COVID-19 vaccine promotions and communications, future research should investigate more proximal, causal mechanisms of COVID-19 vaccination decisions in farm parents (e.g., vaccine skepticism, fears of harm, distance to clinic). Addressing such impediments to high quality preventive healthcare in rural areas is crucial to increasing COVID-19 protection in children and adolescents over time.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the dedication and assistance of all WINS study staff.
Author contributions
JV was involved in the study design, data analysis and interpretation, and manuscript preparation. GA was involved in the literature review, data analysis and interpretation, and manuscript preparation. BW was involved in the data interpretation and manuscript review. RB was involved in the data interpretation and manuscript review. MS was involved in the literature review, study design, data analysis and interpretation, and manuscript review.
Disclosures
The authors have no conflicting financial interests to report.
Funding
This work was supported in part by the National Children’s Center for Rural and Agricultural Health and Safety through the National Institute for Occupational Safety & Health Cooperative Agreement U54 OH009568, as well as donors to the Marshfield Clinic Research Institute’s Summer Student Research Internship Program.
Data availability
The authors do not have permission to share data.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. [Accessed 08/10/2022].
- 2.World Health Organization. Global excess deaths associated with COVID-19, January 2020 - December 2021. www.who.int/data/stories/global-excess-deaths-associated-with-covid-19-january-2020-december-2021. [Accessed 08/05/2022].
- 3.The New York Times. U.S. Has Far Higher Covid Death Rate Than Other Wealthy Countries. www.nytimes.com/interactive/2022/02/01/science/covid-deaths-united-states.html. [Accessed 06/06/2022].
- 4.Kaiser Family Foundation. COVID-19 Cases and Deaths by Race/Ethnicity: Current Data and Changes Over Time. www.kff.org/coronavirus-covid-19/issue-brief/covid-19-cases-and-deaths-by-race-ethnicity-current-data-and-changes-over-time/. [Accessed 07/22/2022].
- 5.Itzhak N., Shahar T., Moskovich R., Shahar Y. The Impact of US county-level factors on COVID-19 morbidity and mortality. J Urban Health. 2022:1–9. doi: 10.1007/s11524-021-00601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics. Children and COVID-19: State-Level Data Report. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. [Accessed 08/15/2022].
- 7.Marks K.J., Whitaker M., Anglin O., et al. Hospitalizations of Children and Adolescents with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271–278. doi: 10.15585/mmwr.mm7107e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. Post-COVID-19 Conditions in Children and Adolescents. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/post-covid-19-conditions-in-children-and-adolescents/. [Accessed 08/15/2022].
- 9.Unwin H.J.T., Hillis S., Cluver L., et al. Global, regional, and national minimum estimates of children affected by COVID-19-associated orphanhood and caregiver death, by age and family circumstance up to Oct 31, 2021: an updated modelling study. Lancet Child Adolesc Health. 2022;6:249–259. doi: 10.1016/S2352-4642(22)00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Zalewski M., Lengua L., Gunnar M.R., Giuliani N., Fisher P.A. Material hardship level and unpredictability in relation to U.S. households' family interactions and emotional well-being: Insights from the COVID-19 pandemic. Soc Sci Med. 2022;307 doi: 10.1016/j.socscimed.2022.115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engzell P, Frey A, Verhagen MD. Learning loss due to school closures during the COVID-19 pandemic. Proc Natl Acad Sci USA 2021;118:e2022376118. [DOI] [PMC free article] [PubMed]
- 12.Wang A.Y.L. Modified mRNA-Based Vaccines Against Coronavirus Disease 2019. Cell Transplant. 2022 doi: 10.1177/09636897221090259. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser Family Foundation. KFF COVID-19 Vaccine Monitor. www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/. [Accessed 08/12/2022].
- 14.Wisconsin Department of Health Services. COVID-19: Vaccine Data. Available at: www.dhs.wisconsin.gov/covid-19/vaccine-data.htm. Accessed on 10/27/2022.
- 15.New York Times. See How Vaccinations Are Going in Your County and State. www.nytimes.com/interactive/2020/us/covid-19-vaccine-doses.html. [Accessed on 08/02/2022].
- 16.Saelee R., Zell E., Murthy B.P., Castro-Roman P., et al. Disparities in COVID-19 Vaccination Coverage Between Urban and Rural Counties - United States, December 14, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:335–340. doi: 10.15585/mmwr.mm7109a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown School of Public Health. COVID-19 Vaccine-preventable Deaths Analysis. https://globalepidemics.org/vaccinations/. [Accessed 07/19/2022].
- 18.Tan S.T., Park H.J., Rodríguez-Barraquer I., Rutherford G.W., Bibbins-Domingo K., Schechter R., et al. COVID-19 Vaccination and estimated public health impact in California. JAMA Netw Open. 2022;5:e228526. doi: 10.1001/jamanetworkopen.2022.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putzer G.J., Koro-Ljungberg M., Duncan R.P. Critical challenges and impediments affecting rural physicians during a public health emergency. Disaster Med Public Health Prep. 2012;6:342–348. doi: 10.1001/dmp.2012.59. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser Family Foundation. KFF COVID-19 Vaccine Monitor- Rural America. www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-rural-america/. [Accessed 07/19/2022].
- 21.Sun Y., Monnat S.M. Rural-urban and within-rural differences in COVID-19 vaccination rates. J Rural Health. 2022 doi: 10.1111/jrh.12625. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y., Cheng K.J., Monnat S. Rural-Urban and Within-Rural Differences in COVID-19 Mortality Rates. J Rural Soc Sci. 2022;37: Article 3 [Google Scholar]
- 23.Lewnard JA, Mora AM, Nkwocha O, et al. Prevalence and Clinical Profile of Severe Acute Respiratory Syndrome Coronavirus 2 Infection among Farmworkers, California, USA, June-November 2020. Emerg Infect Dis 202;27:1330-1342. [DOI] [PMC free article] [PubMed]
- 24.Chen Y.H., Glymour M., Riley A., Balmes J., Duchowny K., Harrison R., et al. Excess mortality associated with the COVID-19 pandemic among Californians 18–65 years of age, by occupational sector and occupation: March through November 2020. PLoS ONE. 2021;16:e0252454. doi: 10.1371/journal.pone.0252454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mofleh D., Almohamad M., Osaghae I., et al. Spatial patterns of COVID-19 vaccination coverage by social vulnerability index and designated COVID-19 vaccine sites in Texas. Vaccines. 2022;10:574. doi: 10.3390/vaccines10040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King N, Pigman M, Huling S, Hanson B. EMS Services in Rural America: Challenges and Opportunities. www.ruralhealth.us/NRHA/media/Emerge_NRHA/Advocacy/Policy%20documents/2019-NRHA-Policy-Document-EMS-Services-in-Rural-America-Challenges-and-Opportunities.pdf. [Accessed on 08/15/2022].
- 27.Miller K.E.M., James H.J., Holmes G.M., Van Houtven C.H. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55:288–300. doi: 10.1111/1475-6773.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeStefano F., Eaker E.D., Broste S.K., et al. Epidemiologic research in an integrated regional medical care system: the Marshfield Epidemiologic Study Area. J Clin Epidemiol. 1996;49:643–652. doi: 10.1016/0895-4356(96)00008-x. [DOI] [PubMed] [Google Scholar]
- 29.Irving S.A., Donahue J.G., Shay D.K., Ellis-Coyle T.L., Belongia E.A. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine. 2009;14:6546–6549. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 30.VanWormer JJ, Berg RL, Burke RR, Barnes KL, Weichelt BP. Regional surveillance of medically-attended agricultural injuries in children and adolescents. Front Public Health 2022;In press. [DOI] [PMC free article] [PubMed]
- 31.WWAMI Rural Health Research Center. Rural-Urban Commuting Area Codes. http://depts.washington.edu/uwruca/index.php. [Accessed 07/26/2022].
- 32.Wisconsin Department of Health Services. Student Immunization Law Age/Grade Requirements. https://www.dhs.wisconsin.gov/publications/p44021.pdf. [Accessed 08/12/2022].
- 33.Gowda C., Schaffer S.E., Dombkowski K.J., Dempsey A.F. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health. 2012;12:509–519. doi: 10.1186/1471-2458-12-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin J., Bradshaw M. Determinants of COVID-19 skepticism and SARS-CoV-2 vaccine hesitancy: findings from a national population survey of U.S. adults. BMC Public Health. 2022;22:1047. doi: 10.1186/s12889-022-13477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christian Science Monitor. Why Midwest farmers are sticking with Trump. https://www.csmonitor.com/Business/2020/1030/Why-Midwest-farmers-are-sticking-with-Trump. [Accessed 08/15/2022].
- 36.Business Insider. American farmers are facing a political paradox because of Republicans' hard line on immigration. https://www.businessinsider.com/conservative-farmers-dont-fit-neatly-into-republican-party-undividing-america-2017-7. [Accessed 08/11/2022].
- 37.Rust N.A., Stankovics P., Jarvis R.M., et al. Have farmers had enough of experts? Environ Manage. 2022;69:31–44. doi: 10.1007/s00267-021-01546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergtold J.S., Caldas M.M., Ramsey S.M., Sanderson M.R., Granco G., Mather M.E. The gap between experts, farmers and non-farmers on perceived environmental vulnerability and the influence of values and beliefs. J Environ Manage. 2022;316 doi: 10.1016/j.jenvman.2022.115186. [DOI] [PubMed] [Google Scholar]
- 39.Doherty IA, Pilkington W, Brown L, et al. COVID-19 Vaccine Hesitancy in Underserved Communities of North Carolina. medRxiv 2022;In press. [DOI] [PMC free article] [PubMed]
- 40.Butt AA, Dargham SR, Loka S, et al. COVID-19 Disease Severity in Children Infected with the Omicron Variant. Clin Infect Dis 2022;In press. [DOI] [PMC free article] [PubMed]
- 41.Tan S.H.X., Cook A.R., Heng D., Ong B., Lye D.C., Tan K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N Engl J Med. 2022;387:525–532. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eyre D.W., Taylor D., Purver M., et al. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med. 2022;386:744–756. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.