Abstract
Nasal vaccine delivery is superior to oral delivery in inducing specific immunoglobulin A (IgA) and IgG antibody responses in the upper respiratory tract. Although an antibody response in the nasal passages is important in protecting against primary colonization with lung pathogens, antibodies in the lungs are usually required as well. We immunized 15 male volunteers twice nasally or orally with cholera toxin B subunit (CTB) and determined the specific antibody levels in serum, bronchoalveolar lavage (BAL) fluid, and urine before and 2 weeks after immunization. Nasal immunization induced fivefold increases in the levels of specific IgA antibodies in BAL fluid of most volunteers, whereas there were no significant specific IgA responses after oral immunization. The specific IgG antibody level increased eightfold in BAL fluid in the nasally vaccinated subjects, and the major part of IgG had most probably been transferred from serum. Since the specific IgG response in serum was lower in the individuals vaccinated orally, the IgG response in BAL fluid in this group was also lower and not significant. In conclusion, nasal immunization is also preferable to the oral route when vaccinating against lower respiratory tract infections, and a systemic immune response is considerably more important in the lower than in the upper respiratory tract. Moreover, both nasal and oral immunizations were able to stimulate 6- to 10-fold specific IgA and IgG responses in urine in about half of the individuals, which indicates that distant mucosal vaccination might be used to prevent adhesion of pathogens to the urogenital tract.
Local antibodies on mucosal surfaces play an important role in the defense against pathogens by preventing the binding of microbes and their produced toxins to the epithelium (38). A rise in mucosal antibody levels can occur either as a result of a local antibody response or via serum antibodies transferred onto the mucosal surface. Production of mucosal antibodies is most efficiently induced after uptake of antigen in the organized lymphoid tissue associated with the particular mucosa, but the concept of a common mucosal immune system also infers that activated cells are transported via the peripheral blood to distant mucosae (6, 22). Most of the immunoglobulin A (IgA) and also the IgG in the intestine and in the nasal cavities is locally produced, and serum antibodies in uninflamed tissue play a minor role in the primary defense (13, 25). However, in the urogenital tract and in the lungs, IgG transferred from serum may add to the locally produced IgG and IgA on the epithelium of these organs (9, 17, 36).
Several oral vaccines have recently been developed, and a few have been licensed for human use, one example being an oral cholera vaccine containing cholera toxin B subunit (CTB) together with a whole-cell vaccine component (13). CTB is a well-characterized nontoxic yet potent mucosal immunogen, partly because of its high-affinity binding to the receptor GM1 ganglioside, facilitating uptake at mucosal surfaces of both CTB and molecules linked to it (14). Several studies with animals have shown that CTB used as a carrier for various protein or carbohydrate antigens can enhance the mucosal immunogenicity for the linked antigens (5, 13). Conclusions drawn from experiments with CTB as an immunogen would probably also hold true for conjugate vaccines based on CTB as a carrier and possibly also for conjugate vaccines based on other mucosa-binding proteins (30).
Using CTB, we have previously shown that nasal vaccination is the method of choice for obtaining local antibodies in the nasal cavity (29) whereas oral vaccination gives rise to the greatest intestinal responses (27). It is, however, still unclear which mucosal vaccination route is optimal for evoking immune responses in the lungs and the urogenital tract. Not only is local vaccination on the mucosae of the lungs or of the urogenital tract less convenient than nasal or oral administration, but also the induction of an immune response may be less reliable because of the lack of organized lymphoid tissue such as adenoids or Peyer’s patches in the normal lungs and urogenital tract. Therefore, it is of interest to examine whether nasal and oral vaccination may give rise to an immune response in these regions. Notably, nasal immunization induces substantial antibody responses in the vagina in both animals and humans (17, 29). The aim of this study was to use the model mucosal immunogen CTB to explore whether specific local antibodies can also be obtained in the lungs and in the male urinary tract of humans as a result of nasal or oral vaccination.
MATERIALS AND METHODS
Subjects.
Fifteen healthy male Caucasian volunteers aged 19 to 33 years gave informed consent to participate in the study, which was approved by the local Human Research Ethical Committee of the Medical Faculty, Göteborg University, Göteborg, Sweden. Exclusion criteria for these studies included previous vaccination against cholera or enterotoxigenic Escherichia coli, travel in the last 2 years to a country where cholera or enterotoxigenic E. coli is prevalent, history of atopy or chronic disease, cigarette smoking, and signs of infectious respiratory disease in the week before broncoscopy. There was no difference in mean age between the groups given nasal or oral vaccination.
Vaccination.
The vaccine for nasal administration consisted of purified CTB provided by SBL Vaccine (Stockholm, Sweden) and was diluted in phosphate-buffered saline to a concentration of 0.625 mg/ml. The vaccine for oral use was the licensed oral cholera vaccine (Dukoral) produced by SBL Vaccine. This vaccine consists of 1.0 mg of CTB and 1011 heat- and formalin-killed vibrios administered in 150 ml of a sodium bicarbonate buffer solution. The CTB in both vaccines was produced and purified from a recombinant strain of Vibrio cholerae lacking the CTA gene but harboring a CTB overexpression multicopy plasmid (31). Two 250-μg doses of the nasal CTB vaccine were given, with a 2-week interval, to nine volunteers; the doses were administered as 100 μl of spray given twice in both nostrils, i.e. a total volume of 400 μl, via an atomizer (Apoteksbolaget AB). Two doses of the oral cholera vaccine (1 mg of CTB per dose) were given twice, with a 2-week interval, to six volunteers.
Fiberoptic bronchoscopy.
Bronchoscopy with bronchoalveolar lavage (BAL) was performed before and 2 weeks after nasal or oral vaccination with CTB as follows. Volunteers were premedicated with ketobemidone, 7.5 mg intramuscularly, and atropine, 0.5 mg intramuscularly, and then, as topical anaesthesia, given 4% preservative-free lidocaine sprayed with a deVilbiss nebulizer into the larynx and 2% lidocaine applied through the bronchoscope into the lower respiratory tract. All bronchoscopies were performed transorally by the same investigator (G.R.) with flexible fiberoptic bronchoscopes of two models (Olympus Corp., Lake Success, N.Y.). Blood oxygen saturation was monitored with a pulse oximeter (Ohmeda, Louisville, Ky.) throughout the procedure. All subjects were observed for 3 h after the bronchoscopy.
Collection and preparation of BAL fluid, serum, and urine samples.
All samples were collected in the morning, between 08.00 and 10.00 h. BAL was performed by seven infusions of 20 ml of warmed sterile pyrogen-free PBS into a segmental middle-lobe bronchus with the bronchoscope in a wedged position. The fluid was recovered by gentle suction, collected in a sterile container, and immediately transported on ice to the laboratory. It was filtered through a sterile 100-μm mesh to remove mucus and cell debris and centrifuged at 250 × g for 10 min at 10°C, and the supernatant was immediately frozen at −70°C. Before enzyme-linked immunosorbent assay (ELISA) analyses, the BAL fluid supernatants were concentrated 10-fold by ultrafiltration at 30 lb/in2, using a YM 10 membrane disc and a stirred cell device (Amicon Inc., Beverly, Mass.). The cell pellet from the BAL fluid was resuspended in 1 ml of phosphate-buffered saline, and 5 × 105 cells were removed for calculation of cell differentials. Since alveolar macrophages might inhibit the antibody production by plasma cells (35), we reduced the number of macrophages by allowing them to adhere to plastic prior to enzyme-linked immunospot (ELISPOT) analysis. The cells were diluted to a concentration of 1 × 106 to 2 × 106 cells per ml in Iscove’s medium (Gibco BRL, Life Technologies Ltd., Paisley, Scotland) plus 5% fetal calf serum (Sigma, St. Louis, Mo.) and transferred to 25-ml tissue culture flasks (Sarstedt Inc, Newton, N.C.) in a volume of 2.5 to 3 ml per flask. After incubation in 7.0% CO2 at 37°C for 60 min, the nonadherent cells were centrifuged at 300 × g for 5 min and used in the ELISPOT analysis.
Venous blood and urine samples were collected 2 h after the bronchoscopy, both immediately before and 2 weeks after vaccination. Serum was separated from venous blood and frozen at −70°C. The first 100-ml portion of urine (containing uretral secretions) was collected in a sterile container and centrifuged at 900 × g for 10 min. The pellet of cell debris was discarded, and the urine was immediately frozen at −70°C. The urine samples were centrifuged at 900 × g after being thawed to remove insoluble aggregates and subsequently concentrated 10-fold by ultrafiltration at 30 lb/in2, using a YM 10 membrane disc and a stirred cell device (Amicon Inc.).
BAL fluid cell differentials and albumin determination.
Cell differentials in BAL fluid samples were calculated with cytocentrifuge preparations (Cytospin 2; Shandon Southern Products Ltd., Runcorn, England), stained with May-Grünwald-Giemsa, and counted in a blinded manner (200 cells per preparation) under a light microscope (Leitz Laborlux 2). Quantitative determination of human albumin in BAL fluid was done with a radioimmunoassay kit (Pharmacia Albumin RIA; Pharmacia) and a gamma counter (COBRA, AutoGamma; Packard Instruments, Meriden, Conn.).
Detection of ASCs.
BAL fluid lymphocytes from five volunteers having sufficient numbers of cells were analyzed for numbers of total and specific IgA and IgG antibody-secreting cells (ASCs) by the ELISPOT assay (8) with slight modifications as previously described (15). Briefly, nitrocellulose-bottom wells (Millipore Corp., Bedford, Mass.) were coated with GM1 ganglioside (Sigma), with affinity-purified goat anti-human IgG F(ab)2 (Jackson ImmunoResearch Laboratories, West Grove, Pa.) for total IgA and IgG determination, and with bovine serum albumin for control purposes. After the GM1-coated wells were blocked and CTB (SBL Vaccine) was added, the mononuclear cells in BAL fluid were incubated in the wells for 3 to 4 h in numbers ranging from 105 to 106 cells per well. The spots were visualized by incubation with horseradish peroxidase (HRP)-conjugated goat anti-human IgG or IgA (Southern Biotechnology Associates, Birmingham, Ala.) and the enzyme chromogen substrate. The ASCs were enumerated in duplicate or triplicate wells, and the results were transformed to numbers of spot-forming cells per 106 mononuclear cells.
Determination of total Ig and specific antibodies.
The total IgA and IgG antibody contents in BAL fluid, nasal secretions and urine were determined by ELISA as described previously (4). Briefly, the plates were coated with goat anti-human IgA, α-chain specific (Jackson), and goat-anti-human IgG (Fab)2 (Jackson). Thereafter, the samples and standards (polyclonal human plasma IgA and IgG; Calbiochem Corp., La Jolla, Calif.) were added in duplicate and serially diluted. The BAL fluid samples were centrifuged at 10,000 × g for 10 min immediately before analysis. Bound total IgA and IgG antibody levels were determined by using HRP-conjugated goat anti-human serum IgA, α-chain specific, and HRP-conjugated goat anti-human IgG, Fcγ specific, followed by o-phenylenediamine and H2O2 as the enzyme substrate. The end-point titers were determined as the reciprocal dilution giving an absorbance at 450 nm of 0.4 above background. The concentrations of total IgA or IgG antibody in the secretion samples were then calculated by using the standards.
CTB-specific antibodies were measured by a modified GM1 ELISA (34) as previously described (4). Briefly, plates were coated first with GM1 ganglioside (Sigma) and then with CTB. The samples and a positive serum reference were added in duplicate and serially diluted. Bound CTB-specific IgA and IgG antibodies were determined with HRP-conjugated rabbit anti-human serum IgA, α-chain specific, and HRP-conjugated goat anti-human IgG, Fcγ specific, and developed as described above.
The ELISA was repeated if the end-point titers determined in duplicate for the reference on the plate varied more than twofold. Samples with titers below the detection limit were assigned a titer of half the lowest dilution. The specific antibody content in secretions was expressed as arbitrary units per milliliter. The CTB-specific IgA and IgG antibody contents were divided by the total IgA and IgG concentrations (micrograms per milliliter), respectively, in the BAL fluid and urine samples to adjust for variations in the Ig content in secretion sample eluates collected from different volunteers and on different days. The fold increases were calculated by dividing the adjusted postvaccination value by the adjusted prevaccination value from each individual. On the basis of calculations of the methodological error for the different ELISAs used, a greater than twofold increase was chosen to define the volunteers who responded to the vaccine.
Statistical methods.
Before the calculations were performed, all the specific antibody titers in BAL fluid and urine were adjusted for variations in total Ig content and all values were log10 transformed. Analyses of the significance of the titer differences between the prevaccination values and the maximal postvaccination values were performed by a paired Student’s t test, and all volunteers were included in the calculations. Differences were considered statistically significant at P < 0.05.
RESULTS
Study group characteristics.
BAL was performed before and 2 weeks after the second nasal or oral vaccination. The recovery of BAL fluid was 67 ± 9.7% (mean ± standard deviation [SD]), and the total number of cells was 141 × 103 ± 71 × 103 per ml of fluid, with no difference in numbers before and after vaccination. The mean percentages of the different BAL fluid cells in prevaccination and postvaccination samples are shown in Table 1. Cell differentials in the BAL fluid samples did not change after either nasal or oral vaccination with CTB, and the levels are in agreement with those found in other studies of normal individuals (2, 10). Additional data supporting the finding that no inflammation was induced in the lower respiratory tract by the vaccination was that total Ig and albumin levels were similar in BAL fluid before and after vaccination (47 ± 22 and 51 ± 16 mg of albumin per ml, respectively).
TABLE 1.
Differential cell content in BAL fluid samples before and after nasal or oral vaccination
| Cell type | Differential cell content (%)
|
|||
|---|---|---|---|---|
| Prevaccination
|
Postvaccination
|
|||
| Mean ± SD | Range | Mean ± SD | Range | |
| Macrophages | 84.2 ± 9.1 | 58–96 | 85.6 ± 4.8 | 73–94 |
| Lymphocytes | 12.6 ± 7.6 | 2.5–34 | 11.7 ± 3.9 | 3.5–21 |
| Monocytes | 2.2 ± 1.9 | 0.5–8 | 1.4 ± 0.8 | 0.5–3 |
| Eosinophils | 1.1 ± 0.5 | 0–2 | 1.4 ± 1.0 | 0–3.5 |
| Neutrophils | 0.03 ± 0.13 | 0–0.5 | 0.03 ± 0.13 | 0–0.5 |
Adverse reactions.
The side effects of the nasal vaccination were of the same character and frequency as reported in our previous study (29). Four of nine nasally vaccinated volunteers experienced sneezing or increased nasal secretions lasting a maximum of 24 h. No systemic or severe local side effects were observed.
Antibody responses in serum, BAL fluid, and urine.
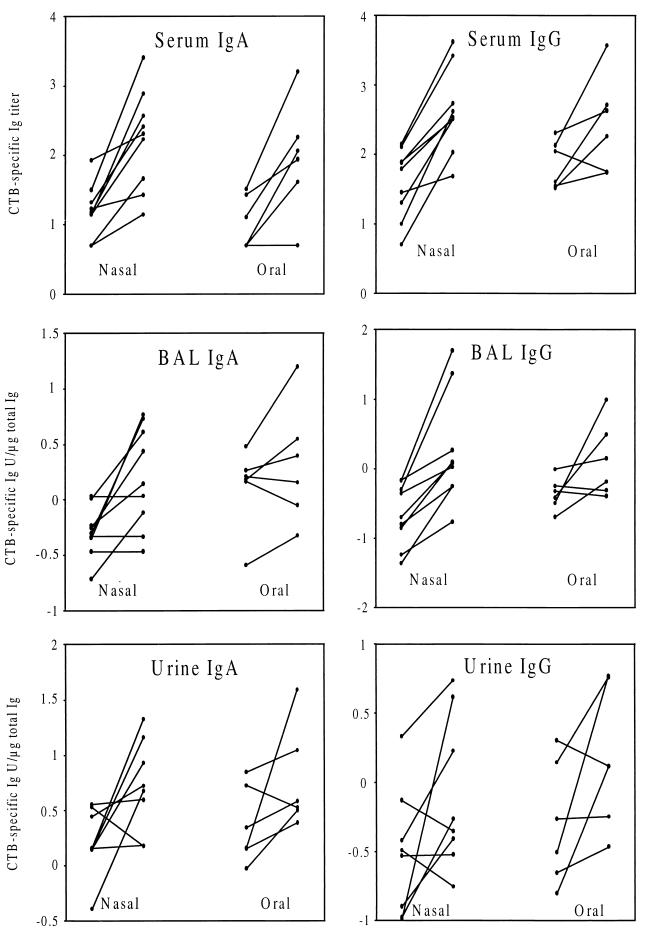
We immunized the volunteers twice nasally or orally with CTB, and 2 weeks after the last dose we collected serum, BAL fluid, and urine for analysis of the specific antibody responses. The results are expressed as individual prevaccination and postvaccination values (Fig. 1) and geometric mean fold increases (Tables 2 and 3). Most subjects responded with significant increases of CTB-specific IgA and IgG levels in serum to the nasal vaccination, and the fold increases in antibody titers as a result of vaccination were of similar magnitudes for both isotypes (Fig. 1; Table 2). Specific IgG anti-CTB antibody levels increased significantly in BAL fluid of all individuals given nasal vaccinations, and in the majority of cases we also found an increase in specific IgA levels (Fig. 1). The fold increases of IgG antibody levels in BAL fluid were higher than the increases of IgA antibody levels (Table 2).
FIG. 1.
CTB-specific increases in IgA and IgG levels in serum, BAL fluid, and urine after two nasal or two oral vaccinations. The individual titers, or titers adjusted to the total Ig content, from the time points before and 2 weeks after vaccination are shown.
TABLE 2.
Fold increases in CTB-specific IgA and IgG responses in serum, BAL fluid, and urine after nasal vaccination
| Isotype | Sample | Response frequency (no. of responders/ total no.)a | Geometric mean fold increaseb for:
|
P for all subjectsc | |
|---|---|---|---|---|---|
| All subjects | Responders | ||||
| IgA | Serum | 8/9 | 10.6 | 13.4 | 0.0009 |
| BAL fluid | 6/9 | 3.1 | 5.5 | 0.009 | |
| Urine | 4/8d | 3.1 | 10.2 | 0.04 | |
| IgG | Serum | 8/9 | 11.0 | 13.9 | 0.0001 |
| BAL fluid | 9/9 | 8.2 | 8.2 | 0.001 | |
| Urine | 5/8 | 2.6 | 5.9 | 0.09 | |
Responders were defined as having a greater than twofold increase in titer.
The fold increases for all samples except serum are calculated from the titers adjusted for the total Ig content in the sample.
Statistical significance of titer increases obtained by using the paired Student t test.
One urine sample was inadvertently destroyed.
TABLE 3.
Fold increases in CTB-specific IgA and IgG responses in serum, BAL fluid, and urine after oral vaccination
| Isotype | Sample | Response frequency (no. of responders/ total no.)a | Geometric mean fold increaseb for:
|
P for all subjectsc | |
|---|---|---|---|---|---|
| All subjects | Responders | ||||
| IgA | Serum | 4/6 | 8.6 | 13.2 | 0.01 |
| BAL fluid | 2/6 | 1.6 | 3.5 | 0.2 | |
| Urine | 2/6 | 2.5 | 9.4 | 0.1 | |
| IgG | Serum | 4/6 | 3.8 | 8.0 | 0.08 |
| BAL fluid | 3/6 | 3.0 | 9.2 | 0.1 | |
| Urine | 3/6 | 3.0 | 8.6 | 0.09 | |
Responders were defined as having a greater than twofold increase in titer.
The fold increases for all samples except serum are calculated from the titers adjusted for the total Ig content in the sample.
Statistical significance of titer increases obtained by using the paired Student t test.
Although the orally vaccinated individuals responded with similar levels of CTB-specific IgA antibodies in serum to those found in the nasally vaccinated individuals, the former group contained a lower proportion of subjects who responded with specific IgA antibodies in BAL fluid and the responders showed a lower fold increase in antibody level than did the nasally vaccinated group (Table 3). The fold increases in levels of specific IgG antibodies serum in the subjects vaccinated orally were considerably lower than in those vaccinated nasally, and only half of the individuals in the orally vaccinated group showed BAL fluid responses with specific IgG, whereas all those given nasal vaccination responded (Fig. 1; Table 3). Neither IgA nor IgG titers increased significantly in BAL fluid when all the subjects given oral vaccinations were included in the statistical calculations (Table 3). The fact that the orally vaccinated group happened to have higher mean prevaccination levels of specific IgG antibodies could not explain the lower responses in this group, since we found no relationship between prevaccination antibody levels and fold increases in IgG antibody levels in response to nasal or oral vaccination in previous studies (4, 29).
In about half of the nasally vaccinated individuals and half of the orally vaccinated individuals, there was a CTB-specific IgA and IgG response in the urogenital tract (Fig. 1; Tables 2 and 3). The reason for the relatively low frequency of responders is probably that the natural-lavage method is a less sensitive method for sampling urogenital secretions than are tampon methods. However, the increases in IgA and IgG antibody levels in those who responded were similar in magnitude to the serum responses (Tables 2 and 3). When calculated for all vaccinated subjects, the only significant titer increases were in CTB-specific IgA after nasal vaccination.
Table 4 shows the geometric means of all unadjusted CTB-specific titers before and after vaccination. These data demonstrate that the titer increases are not due to differences in total Ig level but are clearly present also when the absolute titers are shown. Many of the prevaccination samples of BAL fluid and urine had titers below the cutoff and were therefore assigned a titer of half the lowest dilution tested, i.e., a titer of 1. The true mean prevaccination levels in these samples are probably lower than those shown in Table 4, and the fold increases are consequently underestimated.
TABLE 4.
Unadjusted specific IgA and IgG titers in BAL fluid and urine before and after nasal or oral vaccination with CTB
| Vaccination | Isotype | Unadjusted mean anti-CTB titer in:
|
|||
|---|---|---|---|---|---|
| BAL
|
Urine
|
||||
| Prea | Postb | Pre | Post | ||
| Nasal | IgA | 1.3 | 3.9 | 1.0 | 4.0 |
| IgG | 1.6 | 14.6 | 1.1 | 2.7 | |
| Oral | IgA | 1.6 | 3.6 | 1.0 | 2.0 |
| IgG | 1.9 | 8.4 | 1.0 | 2.5 | |
Samples taken before the first vaccination.
Samples taken 2 weeks after the second vaccination.
Antibody-producing cells in BAL fluid.
It is difficult to evaluate whether the specific antibodies present in BAL fluid are produced locally or have been transferred from serum. Although the proportion of B cells in the BAL fluid lymphocytes is only about 3% (data not shown), we were able to detect antibody-producing cells in BAL fluid by using the ELISPOT assay. The number of total IgA ASCs per 106 mononuclear cells was 65 ± 27 (mean ± SD), and the number of total IgG ASCs was 33 ± 17. The low proportion of antibody-producing cells among the BAL fluid lymphocytes precluded the detection of CTB-specific ASCs, since the CTB-specific cells maximally amount to 1 to 2% of the total ASCs (in analyses of circulating mononuclear cells after nasal or oral vaccination).
DISCUSSION
We have previously shown that nasal immunization of humans is the method of choice for induction of antigen-specific antibodies in the upper respiratory tract (29). Others have demonstrated that nasal vaccination with a streptococcal vaccine not only can protect against clinical illness but also can reduce colonization, which results in decreased transmission of the disease (24). Moreover, an attenuated influenza virus vaccine given nasally to children was recently shown to be protective against disease with an efficacy of 93% (3). For protection against many pulmonary pathogens, it is important that the lower respiratory tract also contain antigen-specific antibodies in case the colonization of the pathogens is not completely inhibited in the upper respiratory tract or the microbes are inhaled. In this paper we show that nasal vaccination indeed resulted in specific IgA and IgG responses in human lungs, even though the optimal time point for sampling secretions from the lower respiratory tract after nasal vaccination is probably several weeks later (29). While nasal vaccination seemed to be more efficient than oral vaccination in inducing specific IgA responses in the lungs, the specific IgG responses in BAL fluid originated from serum, and thus all vaccination routes resulting in a specific serum antibody response should be efficient in protecting the lungs. As demonstrated in this study, oral vaccination is less reliable in giving rise to serum antibody responses than is nasal vaccination, but local intestinal responses are also found in most orally vaccinated individuals who do not respond in serum (20). Consequently, to obtain both specific IgA and IgG antibodies on the epithelial surface of the lower respiratory tract, nasal vaccination has the dual advantage of resulting in local production of specific IgA antibodies in the airways as well as in higher levels of IgG antibodies in serum.
The proportion of IgG to IgA in secretions is known to increase gradually as the respiratory tract is descended (9). There are several indications that IgG antibodies can be transferred from serum through the lung epithelial lining even in the absence of any inflammation. For example, passive administration of serum antibodies protects the lungs but not the nasal passages of animals from infection by respiratory viruses (25). In this study, an argument for the transfer of serum IgG into the bronchiolar and alveolar lumen is that the ratio of IgG to IgA in lavage fluid was 4:1 whereas the ratio of IgG-producing cells to IgA-producing cells was 1:2. Moreover, it is evident that there is a relationship between the magnitude of the fold increases in IgG levels serum and BAL fluid, while the fold increases in IgA levels in these fluids differ considerably. These results suggest that all vaccination routes resulting in a specific IgG response in serum are equally effective in inducing a protective antibody response in the lungs. However, specific IgA in BAL fluid may also play a role in immune system exclusion of pathogens in the airways, and both IgA and IgG can be locally produced from cells either in the lamina propria or in the airway lumen (28). Our present results clearly show that lymphocytes in BAL fluid are antibody producers, and the dominance of IgA-producing cells indicates that these cells are of mucosal origin.
Antibody responses have previously been detected in BAL fluid after aerosol vaccination with inactivated influenza virus inhaled into the lungs (36), but to our knowledge this is the first study examining the antibody responses in BAL fluid after nasal or oral vaccination of humans. In a more recent study of healthy volunteers, the experimental protein keyhole limpet hemocyanin was instilled directly into a lung lobe, resulting in local inflammation and a significantly higher specific antibody content in the immunized lobe than in the contralateral lobe (32). We did not find any changes in lymphocyte counts or total Ig or albumin levels after either oral or nasal vaccination, indicating that neither nasal nor oral immunization with CTB induced an inflammation in the lungs. This is not surprising, since probably neither nasally nor orally administered CTB comes into direct contact with the lower respiratory tract. An argument for using the nasal route is that the nasal mucosa contains organized inductive lymphoid sites, while the presence of such sites in the lower respiratory tract is less certain, at least in older children and adults (12). However, airway antigen uptake, processing, and transport from the epithelium to the draining lymph nodes is efficiently done by dendritic cells, and therefore a specialized lymphoid tissue might not be needed for a local immune response in these regions (21). In accordance with this, studies with animals suggest that activation of naive lymphocytes induced by inhaled antigens occurs in regional and central lymph organs rather than in the lungs and that once activated, the lymphocytes are recruited back to the lungs (18).
Oral vaccination did not induce a specific IgA response in BAL fluid, and the specific IgG antibodies in BAL fluid after oral vaccination are very probably of serum origin. Thus, the findings from this study do not support the notion that oral vaccination results in activated B cells being transported to the respiratory tract. In the literature there is some disagreement to what extent antigen-specific cells are transported to the respiratory tract after oral immunization. Pierce and Cray showed that in rats immunized with CT, neither colonic nor duodenal immunization resulted in any antigen-specific cells in the trachea and that intestinal immunization also did not prime for a tracheal response to a local booster challenge. In contrast, tracheal immunization resulted in very large numbers of such cells (23). On the other hand, Weisz-Carrington showed that low but significant levels of antigen-specific IgA but not IgG were found in the bronchial mucosa after transfer of mesenteric lymph node cells from orally immunized mice and that local intrabronchial challenge boosted this response (37). Moreover, in animal models of acute respiratory infection with P. aeruginosa, oral priming followed by intratracheal boosting was as efficient as intratracheal immunization alone in protecting against infection (7). In conclusion, the results from our study and from other groups suggest that antibody-producing cells do not disseminate to the lungs after oral immunization alone, or that they do so only poorly, but that oral priming followed by respiratory boosting might result in an immune response in the respiratory tract. This agrees with the fact that lung and gut lymphocytes migrate differently (16), the molecular basis of which is probably the differential expression of homing receptors and addressins. Thus, lymphocytes in lung lymph express much lower levels of the gut-specific homing receptor α4β7-integrin than do lymphocytes in gut lymph, and the intestinal addressin MAdCAM-1 is not expressed by lung endothelial cells (1). It is probable that similar specific addressins and homing receptors operate in the upper and lower respiratory tracts, although such tissue-specific molecules remain to be identified.
In about half of the volunteers, both nasal and oral vaccination resulted in a considerable antibody response in the male urogenital tract. The differences observed here between nasal and oral vaccination are too small for us to state that the two routes differ in their capacity to evoke an antibody response in the male urogenital tract. Mattsby-Baltzer et al. demonstrated protection against urinary tract infection after oral immunization in rodent models (19), and placebo-controlled clinical trials with patients with recurrent urinary tract infection have shown that membrane proteins of gram-negative bacteria given orally are effective in decreasing the incidence of infectious episodes (11). The mechanism is supposed to be production of antibodies on the epithelium of the urinary tract inhibiting the binding of the bacteria to the cells (33). Specific antibodies and effector T cells could be produced locally in the urogenital tract since the male uretral lamina propria contains numerous IgA- and IgG-producing cells as well as T cells (26). Such a local immune response in the male urogenital tract might efficiently inhibit the adherence and the proliferation of sexually transmitted pathogens. It is possible, although it has not been conclusively shown, that lymphocytes induced in the upper respiratory tract or in the intestine will repopulate the urogenital mucosa. The proportion of individuals showing a response in the urogenital tract to nasal and oral vaccination was lower for men than in our previous study of women (29). In that study, we analyzed the antibody content in vaginal secretions accumulated for 2 h by using a tampon, whereas in this study the natural urination lavage method was used. The difference in the sensitivity of the sampling method may account for the lower proportion of male responders.
In this study, we have shown that nasal vaccination is more efficient than oral vaccination in inducing specific IgA responses in the human lungs but that serum IgG induced by both nasal and oral vaccination is transferred from blood to the lungs. This suggests that there is a connection between the mucosal immune systems in the upper and lower respiratory tracts, probably because lymphocytes activated in the former may also repopulate the latter. However, although systemic antibodies do not protect against colonization of the upper respiratory tract by pathogens, such antibodies are probably sufficient to protect the lungs. The advantage of nasal immunization is that it not only evokes antibody responses in the upper respiratory tract but often also results in strong serum antibody responses. We have also shown that the nasal and oral routes are equally potent in inducing urogenital antibody responses in men and that these routes may therefore also be considered for vaccinating men against sexually transmitted diseases. It remains to be shown whether local urogenital vaccination might induce even stronger immune responses in men and women.
ACKNOWLEDGMENTS
This work was supported by Maxim Pharmaceuticals, the Swedish Medical Research Council (grant 16x-3382), the Swedish Society of Medicine, the Göteborg Medical Society, SIDA-SAREC Sweden Special Program for AIDS and Related Diseases, Stiftelsen Ragnhild och Einar Lundströms minne, and Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond.
We gratefully acknowledge all volunteers who participated in the study, Marie Karlsson and Maja Berg for excellent technical assistance, and SBL Vaccine for providing us with the CTB vaccine preparation.
REFERENCES
- 1.Abitorabi M A, Mackay C R, Jerome E H, Osorio O, Butcher E C, Erle D J. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol. 1996;156:3111–3117. [PubMed] [Google Scholar]
- 2.Banks D E, Morgan J E, Deshazo R D, Weissman D, Rodriguez F H, Barkman H W, Salvaggio J E. Reliability of cell counts and protein determinations in serial bronchoalveolar lavage procedures performed on healthy volunteers. Am J Med Sci. 1990;300:275–282. doi: 10.1097/00000441-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R B, Mendelman P M, Treanor J, King J, Gruber W C, Piedra P, Bernstein D I, Hayden F G, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccinatino of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist C, Lagergård T, Lindblad M, Holmgren J. Local and systemic responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig S W, Cebra J J. Rabbit Peyer’s patches, appendix, and popliteal lymph node B lymphocytes: a comparative analysis of their membrane immunoglobulin components and plasma cell precursor potential. J Immunol. 1975;114:492–502. [PubMed] [Google Scholar]
- 7.Cripps A W, Dunkley M L, Clancy R L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun. 1994;62:1427–1436. doi: 10.1128/iai.62.4.1427-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerkinsky C, Nilsson L Å, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 9.Daniele R P. Immunoglobulin secretion in the airways. Annu Rev Physiol. 1990;52:177–195. doi: 10.1146/annurev.ph.52.030190.001141. [DOI] [PubMed] [Google Scholar]
- 10.Garlepp M J, Rose A H, Bowman R V, Mavaddat N, Dench J, Holt B J, Baron-Hay M, Holt P G, Robinson B W S. A clonal analysis of lung T cells derived by bronchoalveolar lavage of healthy individuals. Immunology. 1992;77:31–37. [PMC free article] [PubMed] [Google Scholar]
- 11.Hachen H J. Oral immunotherapy in paraplegic patients with chronic urinary tract infections: a double-blind, placebocontrolled trial. J Urol. 1992;143:759–763. doi: 10.1016/s0022-5347(17)40084-x. [DOI] [PubMed] [Google Scholar]
- 12.Hiller A S, Tschernig T, Kleemann W J, Pabst R. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found in different frequencies in children, adolescents and adults. Scand J Immunol. 1998;47:159–162. doi: 10.1046/j.1365-3083.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A-M. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen carrier and adjuvant. Am J Trop Med Hyg. 1994;50(Suppl.):42–54. [PubMed] [Google Scholar]
- 14.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 15.Jertborn M, Svennerholm A-M, Holmgren J. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078–1081. doi: 10.1016/0264-410x(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 16.Joel D D, Chanana A D. Comparison of pulmonary and intestinal lymphocyte migration patterns in sheep. Ann N Y Acad Sci. 1985;459:56–66. doi: 10.1111/j.1749-6632.1985.tb20815.x. [DOI] [PubMed] [Google Scholar]
- 17.Johansson E-L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal and intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaltreider H B, Byrd P K, Daughety T W, Shalaby M R. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after intratracheal immunization with sheep erythrocytes. Am Rev Respir Dis. 1983;127:316–321. doi: 10.1164/arrd.1983.127.3.316. [DOI] [PubMed] [Google Scholar]
- 19.Mattsby-Baltzer I, Hanson L-A, Olling S, Kaijser B. Experimental Escherichia coli ascending pyelonephritis in rats: active peroral immunization with live Escherichia coli. Infect Immun. 1982;35:647–653. doi: 10.1128/iai.35.2.647-653.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson A, Lönroth H, Quiding-Järbrink M, Svennerholm A-M. Induction of B-cell responses in the stomach of Helicobacter pylori-infected subjects after oral cholera vaccination. J Clin Investig. 1998;102:51–56. doi: 10.1172/JCI22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWilliam A S, Marsh A M, Holt P G. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: involvement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 23.Pierce N F, Cray W C., Jr Cellular dissemination of priming for a mucosal immune response to cholera toxin in rats. J Immunol. 1981;127:2461–2464. [PubMed] [Google Scholar]
- 24.Polly S M, Waldman R H, High P, Wittner M K, Dorfman A, Fox E N. Protective studies with a group A streptococcal M protein vaccine. II. Challenge of volunteers after local immunization in the upper respiratory tract. J Infect Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 25.Prince G A, Horswood R L, Chanock R M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pudney J, Anderson D J. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 27.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin J A, Naefel G P, Schrader C E, Matthay R A, Reynolds H Y. Air-space immunoglobulin production and levels in bronchoalveolar lavage fluid of normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1983;127:442–448. doi: 10.1164/arrd.1983.127.4.442. [DOI] [PubMed] [Google Scholar]
- 29.Rudin A, Johansson E-L, Bergquist C, Holmgren J. Differential kinetics and distribution of antibodies in serum, and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell M W, Moldoveanu Z, White P L, Silbert G J, Mestecky J, Michalek S. Salivary, nasal, genital and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–486. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuyler M, Lyons C R, Masten B, Bice D. Immunoglobulin response to intrapulmonary immunization of asthmatics. Immunology. 1997;91:167–175. doi: 10.1046/j.1365-2567.1997.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svanborg-Eden C, Svennerholm A-M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm A-M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 35.Thepen T, McMenamin C, Oliver J, Kraal G, Holt P G. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs immunity. Eur J Immunol. 1991;21:2845–2850. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- 36.Waldman R H, Jurgensen P F, Olsen G N, Ganguly R, Johnson J E. Immune response of the human respiratory tract. I. Immunoglobulin levels and influenza virus vaccine antibody response. J Immunol. 1973;111:38–41. [PubMed] [Google Scholar]
- 37.Weisz-Carrington P, Grimes S R, Lamm M E. Gut-associated lymphoid tissue as source of an IgA immune response in the respiratory tract after oral immunization and intrabronchial challenge. Cell Immunol. 1987;106:132–138. doi: 10.1016/0008-8749(87)90156-0. [DOI] [PubMed] [Google Scholar]
- 38.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]