Abstract
Continuous glucose monitoring (CGM) is rapidly becoming a vital tool in the management of type 1 diabetes. Its use has been shown to improve glycaemic management and reduce the risk of hypoglycaemic events. The cost of CGM remains a barrier to its widespread application. We aimed to identify and synthesize evidence about the cost‐effectiveness of utilizing CGM in patients with type 1 diabetes. Studies were identified from MEDLINE, Embase and Cochrane Library from January 2010 to February 2022. Those that assessed the cost‐effectiveness of CGM compared to self‐monitored blood glucose (SMBG) in patients with type 1 diabetes and reported lifetime incremental cost‐effectiveness ratio (ICER) were included. Studies on critically ill or pregnant patients were excluded. Nineteen studies were identified. Most studies compared continuous subcutaneous insulin infusion and SMBG to a sensor‐augmented pump (SAP). The estimated ICER range was [$18,734–$99,941] and the quality‐adjusted life year (QALY) gain range was [0.76–2.99]. Use in patients with suboptimal management or greater hypoglycaemic risk revealed more homogenous results and lower ICERs. Limited studies assessed CGM in the context of multiple daily injections (MDI) (n = 4), MDI and SMBG versus SAP (n = 2) and three studies included hybrid closed‐loop systems. Most studies (n = 17) concluded that CGM is a cost‐effective tool. This systematic review suggests that CGM appears to be a cost‐effective tool for individuals with type 1 diabetes. Cost‐effectiveness is driven by reducing short‐ and long‐term complications. Use in patients with suboptimal management or at risk of severe hypoglycaemia is most cost‐effective.
Keywords: continuous glucose monitoring, cost‐analysis, cost‐effectiveness, health economics, self‐monitoring blood glucose, type 1 diabetes
Continuous glucose monitoring has advanced significantly in the last few years. Multiple studies and meta‐analysis have reported the benefit of continuous glucose monitoring in improving diabetes management and hypoglycemic risk. Cost has however remained a barrier to its widespread use. This review summarises available studies assessing the cost‐effectiveness of CGM.
1. INTRODUCTION
With the exponential growth of technology, our ability to support individuals with type 1 diabetes has improved dramatically. One pivotal technology that has and will continue to help achieve this is continuous glucose monitoring (CGM). With each passing year, the clinical benefits of CGM have become more pronounced. In the long term, integrating CGM into standard diabetes care will play a crucial part in determining our future success in preventing diabetes‐related complications. Though the benefits of CGM are evident, cost remains a significant barrier to its widespread use. To expedite the use of CGM in the type 1 diabetes population, appropriate funding will be required and thus economic evaluation of CGM is vital for informed policymaking.
Diabetes‐related complications remain a significant burden for individuals with type 1 diabetes. Historically, to combat the morbidity of chronic hyperglycaemia, intensive treatment with lower HbA1c targets were utilized. Although this strategy reduced rates of chronic hyperglycaemic complications, it placed individuals with type 1 diabetes at a greater risk of hypoglycaemic events. 1 With CGM there is evidence that chronic hyperglycaemia and hypoglycaemic events can both be avoided. For example, a recent trial showed that in older adults with type 1 diabetes, CGM was able to significantly reduce time spent in hypoglycaemia. 2 Concurrently, studies have shown CGM reduces chronic hyperglycaemia with a significant reduction in HbA1c. 3 , 4 These improvements in physiological parameters have also translated into psychological benefits. With CGM use, individuals have reported reductions in diabetes‐related distress, improved hypoglycaemic confidence, and improvements in fear of hypoglycaemia (FoH) scores. 5 Whilst these clinical improvements with CGM use are important for the individual, benefits also extend to wider society due to the reduction in acute service use over time.
The importance of reducing diabetes‐related complications extends beyond the individual, as diabetes‐related complications also represent a significant economic burden on all healthcare systems. Hypoglycaemia alone is estimated to be responsible for 100,000 emergency department visits in the United States of America (USA) 6 ; with a total annual estimated cost between USD 1.8 to 5.9 billion. 7 This is on top of the cost associated with macro‐ and microvascular injury from chronic hyperglycaemia. The American Diabetes Association reported cardiovascular complications, for people with both type 1 and type 2 diabetes, were responsible for 27% of the total cost of treating diabetes in the USA. 8 This equates to USD 37.3 billion spent on diabetes associated cardiovascular disease. 8 Thus, there is hope that CGM usage costs will be offset by the reduction in diabetes‐complication related expenditure. This will be further compounded by advancements in CGM, which has already seen the cost of the technology decline. Given the chronic nature of diabetes and, the rapid evolution of CGM, timely evaluation of CGM cost‐effectiveness relies on statistical modelling rather than long‐term observation studies. Many studies have attempted to assess the lifetime cost‐effectiveness of CGM through simulation modelling. The current study aims to summarize and clarify the findings of these studies, to provide guidance in the appropriateness of implementing CGM technology. Our hypothesis is that CGM is a cost‐effective diabetes management tool through reducing complication costs and improving the quality of life for individuals with type 1 diabetes.
2. METHODS
2.1. Inclusion and exclusion criteria
In this systematic review, we included studies that assessed the cost‐effectiveness of using CGM compared to self‐monitored blood glucose (SMBG) in monitoring blood glucose levels in patients with type 1 diabetes, regardless of the mode of insulin delivery. Studies were retrospective in nature and were required to report a lifetime ICER as an outcome. They were also required to focus on modelling for an adult population. Studies were excluded if they focused on a specific cohort of patients, to allow for more generalisable results.
2.2. Search strategy
A search was conducted of the MEDLINE, Embase and the Cochrane Library databases for the period between January 2010 to February 2022, with no country limits applied. A date limit of 2010 onwards was applied due to the rapid improvements in technology, so outcomes are better matched to the technology used today. Our search strategy utilized Medical Subject Headings and text words related to “continuous glucose monitoring”, “flash glucose monitoring”, “type 1 diabetes”, “cost effectiveness” and “economic analyses” as described in Figure 1. The database searches were complimented by grey literature searches using Google's Advanced Search. To ensure literature saturation, we reviewed the reference lists of included studies or relevant reviews identified.
FIGURE 1.

Search strategy
2.3. Method of review
All studies identified were collated into an EndNote database and deduplicated before being uploaded to Covidence for screening. Through Covidence, two reviewers independently screened abstracts to identify articles potentially meeting the inclusion criteria. For those articles, full text versions were retrieved and once again independently screened by the two reviewers to determine whether they met inclusion criteria. Any disagreements about whether the inclusion criteria were met were resolved through discussion between the two reviewers. If no consensus could be reached a third reviewer was utilized to make a final decision. Review process summarized in Figure 2.
FIGURE 2.
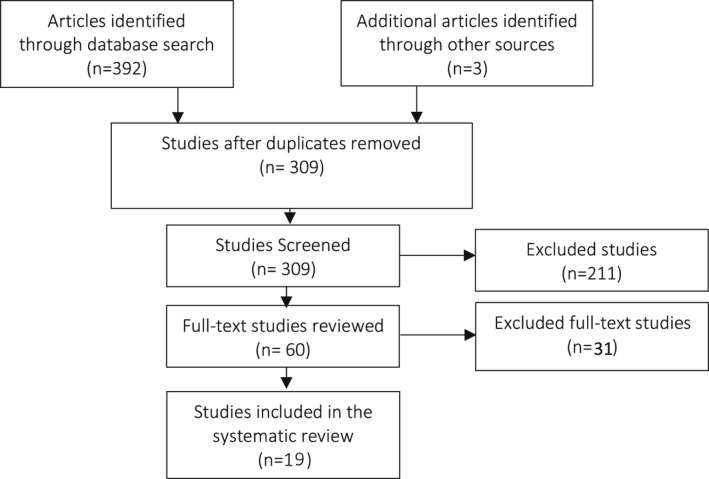
Systematic review flow diagram
2.4. Data extraction
Data extraction of relevant study information for articles meeting inclusion criteria was performed independently by two reviewers. Disagreements were resolved through discussion and if required a third reviewer's input. A data extraction template was created through Covidence to collect relevant information regarding the study design, location, intervention used, study population, calculation, source of inputs and main findings.
2.5. Statistical modelling
Multiple simulation models have been built to predict the progression of diabetes and its complications. Many of these models utilize Markov modelling to predict progression by simulating movement between different health states in a cohort of individuals. In the current context, these health states represent the development of diabetes‐related complications. Outcomes in the simulated scenarios are based on various parameters encapsulating underlying assumed probability distributions. Costs are also assigned to each complication. An ICER can be calculated by the difference in cost accumulated between two groups (control/intervention) divided by the difference in the quality‐adjusted life years (QALY). Because there are no agreed methods for pooling estimates of cost effectiveness, we did not conduct meta‐analysis of the cost‐effectiveness results. 9
2.6. Currency conversion
To make meaningful comparisons between the studies ICER were expressed using a common currency. Extracted ICERs were converted into 2021 AUD using the Campbell and Cochrane Economics Methods Group tool as recommended by Cochrane. 10 , 11 The conversion tool adjusts for estimates of costs for currency and price year. All costs depicted in this paper are in AUD unless otherwise specified.
3. RESULTS
3.1. Description of studies
The initial search identified 311 citations accepted for screening and complete abstract review. Of these initial abstracts, 98 citations were deemed to potentially meet the inclusion criteria. Additionally, three articles were identified when scanning the references of relevant published articles in the field. After review of the full text 19 studies from 11 countries were included. Common reasons for exclusion included focus on type 2 diabetes and lack of ICER as a primary or secondary outcome.
3.2. Description of study features
Study characteristics are detailed in Table 1. All 19 studies utilized Markov modelling to calculate ICER, including 12 studies which used various versions of the CORE diabetes model. These studies assessed cost‐effectiveness from the viewpoint of 11 countries. With regards to the associated treatment, seven studies compared continuous subcutaneous insulin infusion (CSII) and SMBG versus sensor‐augmented pump (SAP). 12 , 13 , 14 , 15 , 16 , 17 , 18 , 25 Four studies 19 , 20 , 21 , 22 assessed CGM versus SMBG in the context of multiple daily injections (MDI); two studies compared MDI and SMBG with SAP 23 , 24 ; three studies assessed SMBG with MDI or CSII in comparison with hybrid closed‐loop systems (HCL) 25 , 26 , 27 and three studies 28 , 29 , 30 compared CGM and SMBG regardless of the mode of insulin delivery. Fifteen of the articles were funded by medical device companies, one article was funded by the Juvenile Diabetes Research Foundation Grant and three received no funding.
TABLE 1.
Study characteristics
| Study | Study model | Treatment | Funding | |
|---|---|---|---|---|
| Roze et al. (2015) 12 | Health‐economic analysis of real‐time continuous glucose monitoring in people with Type 1 diabetes | CORE diabetes model | CSII only vs SAP | Medtronic funding |
| Roze et al. (2016) 13 | Cost‐effectiveness of sensor‐augmented pump therapy with low glucose suspend versus standard insulin pump therapy in two different patient populations with type 1 diabetes in france | CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Roze et al. (2016) 14 | Long‐term health economic benefits of sensor‐augmented pump therapy vs. continuous subcutaneous insulin infusion alone in type 1 diabetes: a U.K. perspective | CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Roze et al. (2017) 15 | Cost‐effectiveness of sensor‐augmented pump therapy versus standard insulin pump therapy in patients with type 1 diabetes in Denmark | CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Conget et al. (2018) 16 | Cost‐effectiveness analysis of sensor‐augmented pump therapy with low glucose‐suspend in patients with type 1 diabetes mellitus and high risk of hypoglycaemia in Spain | IQVIA CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Nicolucci et al. (2018) 17 | Cost‐effectiveness of sensor‐augmented pump therapy in two different patient populations with type 1 diabetes in Italy | CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Roze et al. (2019) 18 | Cost‐effectiveness of sensor‐augmented insulin pump therapy versus continuous insulin infusion in patients with type 1 diabetes in Turkey | IQVIA CORE diabetes model | CSII only vs. SAP | Medtronic funding |
| Chaugule et al. (2017) 19 | Cost‐effectiveness of G5 Mobile continuous glucose monitoring device compared to self‐monitoring of blood glucose alone for people with type 1 diabetes from the Canadian societal perspective | IMS CORE diabetes model (v.9.0) (cohort‐based bootstrap model) | MDI for both | Dexcom funding |
| Wan et al. (2018) 20 | Cost‐effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self‐monitoring of blood glucose: The DIAMOND randomized trial | Sheffield diabetes model | MDI for both | Dexcom funding |
| Roze et al. (2020) 21 | Long‐term cost‐effectiveness of Dexcom G6 real‐time continuous glucose monitoring versus self‐monitoring of blood glucose in patients with type 1 diabetes in the UK | IQVIA CORE diabetes model | MDI for both | Dexcom funding |
| Roze et al (2021) 22 | Evaluation of the long‐term cost‐effectiveness of the Dexcom G6 continuous glucose monitor versus self‐monitoring of blood glucose in people with type 1 diabetes in Canada | IQVIA CORE diabetes model | MDI for both | Dexcom funding |
| Kamble et al. (2012) 23 | Cost‐effectiveness of sensor‐augmented pump therapy in adults with type 1 diabetes in the United States | CORE diabetes model | MDI vs. SAP | Medtronic and Duke University funding |
| Gomez et al. (2016) 24 | Clinical and economic benefits of integrated pump/CGM technology therapy in patients with type 1 diabetes in Colombia | IMS CORE Diabetes Model (CDM) version 8.5 | MDI vs. SAP | Medtronic funding |
| Jendle et al. (2019) 25 | Cost‐effectiveness analysis of the MiniMed 670G hybrid closed‐loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes | IQVIA CORE diabetes model | CSII only vs. HCL | Medtronic funding |
| Pease et al. (2020) 26 | Cost‐effectiveness analysis of a hybrid closed‐loop system versus multiple daily injections and capillary glucose testing for adults with type 1 diabetes | Study's own Markov modelling | MDI vs. HCL | Nil |
| Roze et al (2021) 27 | Cost‐effectiveness of a novel hybrid closed‐loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK | IQVIA CORE diabetes model | CSII only vs. HCL | Medtronic funding |
| Huang et al. (2010) 28 | The cost‐effectiveness of continuous glucose monitoring in type 1 diabetes | Study's own Markov modelling (Monte‐Carlo based) | (CSII 90% and MDI 10%) | Juvenile Diabetes Research Foundation grant |
| McQueen et al. (2011) 29 | Cost‐effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes | Study's own Markov Model w/input from CDC Cost‐Effectiveness Group model | CSII or MDI (varied) | Nil |
| Garcia‐Lorenzo et al. (2018) 30 | Cost‐effectiveness analysis of real‐time continuous monitoring glucose compared to self‐monitoring of blood glucose for diabetes mellitus in Spain | Study's own Markov Model | CSII and MDI | Nil |
Baseline characteristics are provided in Table 2. Six studies assessed a specific subset of patients: (1) patients with suboptimal glycaemic management at baseline; (2) those who are at high risk of hypoglycaemia episodes; and (3) those with HbA1c <7% 28 or (4) those aged >25 years. 21 Table 2. also outlines the assumed clinical benefits of CGM compared to SMBG, as well as the source of these inputs. Input relating to HbA1c reduction were sourced from nine different studies and had results that ranged from 0.23% to 1.5% reduction. In one study the source of HbA1C reduction could not be identified. 16 FoH score improvements ranged from 2.3 to 9.25‐points with use of CGM compared to SMBG. There was significant variability in the way reduction in hypoglycaemic events were reported.
TABLE 2.
Baseline characteristics and treatment effects
| Study | Age (years) | Baseline HbA1c (%) | Duration of diabetes (years) | Sex, female (%) | HbA1c % reduction | Rates of hypoglycaemia (SHE) per 100 patient‐years |
|---|---|---|---|---|---|---|
| Roze et al. (2015) 12 | 27 | 8.60 | 13 | 54.5 | SAP: 0.30 | SAP vs Control: 23% reduction |
| Roze et al. (2016) 13 | 36 ± 13.6 a | 9 ± 0.9 | 17 ± 10.8 | 47.0 | SAP: 0.88 Control: 0.48* | Nil change |
| 18.6 ± 11 | 7.5 (7.2–7.9) | 12 ± 8.9 | 50.0 | Nil effect |
CSII: 2.2 SAP: 0 |
|
| Roze et al. (2016) 14 | 27.1** | 10 | 13 | 51.5 | SAP: 1.49 Control: 0.62 |
CSII: 2.2 SAP: 0 |
| Roze et al. (2017) 15 | 27 a | 8.1 | 13.2 | 51.5 |
SAP: 0.56 Control: 0.13* |
Nil change |
| 18.6 b | 7.5 | 11 | 50.5 | Nil effect |
CSII: 2.2 SAP: 0 |
|
| Conget et al. (2018) 16 | 18.6 ± 11.1 | 7.5 (7.2–7.9) | 12 ± 8.9 | 50% | N/A | CSII: 2.2 events SAP: 0 |
| Nicolucci et al. (2018) 17 | 27 ± 15.6 a | 8.1 (1.3) | 13.2 (10.8) | 51.5 | SAP: 0.56 | Nil change |
|
SAP 19.7 ± 12.9 b CSII 17.4 ± 10.6 |
SAP 7.4 (7.2–7.6) CSII 7.6 (7.4–7.9) |
SAP 12.1 (10.0) CSII 9.8 (7.4) |
SAP 57.1 CSII 43.5 |
Nil Effect |
SAP: 0 CSII: 2.2 |
|
| Roze et al. (2019) 18 | 27 ± 15.6 a | 9.0 | 13.2 ± 10.8 | 51.5 | SAP: 1.1 Control: 0.36 | Nil effect |
| 18.6 ± 11.8 b | 7.5 | 11 ± 8.9 | 50.5 | Nil Effect |
CSII: 2.2 SAP: 0 |
|
| Chaugule et al. (2017) 19 | 46 | 8.6 ± 0.7 | 19 | 47.0 |
CGM: 1.0 (SD 0.7%) Control: 0.4 (SD 0.7%) |
50% reduction |
| Wan et al. (2018) 20 |
Cont f .: 51.4 ± 10.9 CGM: 45.7 ± 13.6 |
Cont.: 8.6 ± 0.6 CGM: 8.6 ± 0.7 |
Cont.: 23.1 ± 14.5 CGM: 19.6 ± 13.6 |
Cont: 43.0 CGM: 45.0 |
N/A |
Control: 4% CGM: 2% |
| Roze et al. (2020) 21 | 43 ± 13 d | 8.6 ± 0.6 | 20 ± 14 | 44.0 |
Control: 0.4 CGM: 1 |
Control: 12.2 CGM: 4.2 |
| 46 ± 13a,* | 9.1 ± 0.4 | 20 ± 14 | N/A | Control: 0.5 CGM: 1.3 | Control: 0 CGM: 3.8 | |
| Roze et al. (2021) 22 | 47.6 ± 13 | 8.6 ± 0.6 | 20 ± 14 | 44.0 |
Control: 0.4 CGM: 1 |
Control: 12.2 CGM: 4.2 |
| Kamble et al. (2012) 23 | 41.23 ± 12.19 | 8.3 ± 0.5 | 20.23 ± 11.94 | 43.2 |
SAP: 1.0 ± 0.7 MDI: 0.4 ± 0.8 |
N/A |
| Gomez et al. (2016) 24 | 34.19 ± 17.14 | 9.0 ± 2.0 | 13.96 ± 9.91 | 46.5 | 1.5% |
SMBG: 5.22 CGM + CSII: 0.37 |
| Jendle et al. (2019) 25 | 37.8 ± 16.5 | 7.4 (0.9) | 21.7 | 55.6 |
SAP: 0.5 Control: Nil Effect |
HCL: 0 CSII: SHE 1 e : 65/SHE 2: 25 |
| Pease et al. (2020) 26 | 18 | 8.5 | 10 | 53.3 | HCL: 0.3 | HCL: 0.1 g Control: 1.98 |
| Roze et al (2021) 27 | 37.8 ± 16.5 | 7.4 (0.9) | 21.7 | 55.6 |
SAP: 0.5 Control: Nil Effect |
HCL: 0 CSII: SHE 1 e : 65/SHE 2: 25 |
| Huang et al. (2010) 28 |
Cont.: 31.8 ± 17.6 a CGM: 29.4 ± 16.3.1 a |
Cont.: 6.50 ± 0.34 CGM: 6.39 ± 0.49 |
Cont.: 18.15 ± 15 CGM: 16.28 ± 15 |
Cont.: 52 CGM: 54 |
CGM: 0.53 | Nil effect |
|
Cont.: 44.7 ± 12.4 c CGM: 41.2 ± 11.2 c |
Cont.: 7.61 ± 0.50 CGM: 7.61 ± 0.49 |
Cont.: 21.83 ± 10 CGM: 23.57 ± 11 |
Cont.: 57 CGM: 60 |
Control: gained 0.3% CGM: Maintain HbA1c |
Median hypoglycaemia duration CGM: 54 min SMBG: 91 min |
|
| McQueen et al. (2011) 29 | 40 | 7.6 ± 0.5% | ~20 | N/A | 0.50% | N/A |
| Garcia‐Lorenzo et al. (2018) 30 | 26 | N/A | N/A | N/A | 0.23% | Control: 7.9% Intervention: 9.1% |
Scenario 1: Suboptimal glycaemic control (variable definitions HbA1c > 7%, HbA1c > 8.5% * and HbA1c >10% **).
Scenario 2: At risk of hypoglycaemic events.
Scenario 3: HbA1c <7% cohort.
Scenario 4: Cohort aged >25 years.
SHE 1 requiring non‐medical assistance.
Cont – control group.
Study assumed a 95% reduction in rates of SHE.
3.3. Description of study results
Table 3. details the cost‐effectiveness of CGM in the form of ICER. The seven studies 12 , 13 , 14 , 15 , 16 , 17 , 18 , 25 which compared CSII and SMBG versus SAP and reported a QALY gain within the range of [0.76–2.99] and ICER range of [$18,734–$99,941]. Studies assessing CGM versus SMBG (n = 4) in context of MDI reported QALY and ICER gain with the range of [0.54–3.35] and [$19,961– $149,634] respectively. Two studies that compared the combination of MDI and SMBG with SAP, reported QALY gain of [0.376–3.81] and ICERs of [$37,188–$386,667]. Three studies assessed HCL compared to SMBG with CSII or MDI and identified QALY and ICER gains of [1.73–3.72] and [$27,731–$37,767]. For the three studies 28 , 29 , 30 which compared SMBG with CGM regardless of insulin delivery mode, identified QALY gain and ICERs were [0.046–1.11] and [$77,269–$6,019,360] respectively.
TABLE 3.
Cost‐effectiveness study outcomes and WTP thresholds
| Study | Treatment | Location | ICER (Cost per QALY) | ICER (AUD per QALY) | QALY gained | Willingness to Pay Threshold (WTP) | WTP (AUD) a | CEAC probability |
|---|---|---|---|---|---|---|---|---|
| Roze et al. (2015) 12 | CSII only vs SAP | Sweden | SEK 545,005 | $99,942 | 0.760 | SEK 500,000 | $91,700 | N/A |
| Roze et al. (2016) 13 | CSII only vs. SAP | France | EUR 30,163 | $58,978 | 1.190 | EUR 30,000 | $58,700 |
Hyper: EUR 30,000–80% EUR 50,000–100% |
| EUR 22,005 | $43,027 | 1.440 |
Hypo: EUR 30,000–100% |
|||||
| Roze et al. (2016) 14 | CSII only vs. SAP | United Kingdom | EUR 12, 233 | $27,871 | 2.990 | GBP 20,000–30,000 | $45,600–$68,400 | GBP 20,000–100% |
| Roze et al. (2017) 15 | CSII only vs. SAP | Denmark | DKK 156,082 | $32,538 | 1.450 |
DKK 225,000 DKK 375,000 |
$46,900 –$78,200 |
Hyper: DKK 225,000–90% DKK 375,000 ‐ >99% |
| DKK 89,868 | $18,734 | 1.880 |
Hypo: DKK 225,000–100% |
|||||
| Conget et al. (2018) 16 | CSII only vs. SAP | Spain | EUR 25,394 | $59,833 | 1.880 | EUR 30,000 | $70,700 | EUR 30,000–97.50% |
| Nicolucci et al. (2018) 17 | CSII only vs. SAP | Italy | EUR 44,982 | $93,772 | 1.448 | EUR 50,000 | $104,200 |
Hyper: EUR 50,000–69.2% EUR 84,000–100% |
| EUR 33,692 | $70,237 | 1.877 |
Hypo: EUR 36,000–68.1% EUR 62,000–100% |
|||||
| Roze et al. (2019) 18 | CSII only vs. SAP | Turkey | TRY 76,971 | $65,144 | 1.400 | TRY 80,000 | $67,700 |
Hyper: TRY 80,000–64%, TRY 84,000–80% |
| TRY 69,534 | $58,850 | 1.730 |
Hypo: TRY 80,000–95% |
|||||
| Chaugule et al. (2017) 19 | MDI for both | Canada | CAD 33,78 | $43,416 | 3.350 | CAD 50,000 | $64,200 | CAD 50,000–100% |
| Wan et al. (2018) 20 | MDI for both | United States of America | USD 98,108 | $149,634 | 0.540 | USD 100,000 | $152,500 | USD 100,000–90% |
| Roze et al. (2020) 21 | MDI for both | United Kingdom | Euro 9558 | $20,130 | 1.490 | GBP 20,000 | $42,100 | Base case: GBP 20, 000 ‐ ~99% |
| Euro 9, 478 | $19,961 | 1.390 | Hyper: GBP 20,000–98% | |||||
| Roze et al. (2021) 22 | MDI for both | Canada | CAD 16,931 | $20,188 | 2.090 | CAD 50,000 | $59,600 | CAD 50,000–100% |
| Kamble et al. (2012) 23 | MDI vs. SAP | United States of America | 3‐day sensor: USD 229,675 6‐day sensor: USD 168,104 | $386,667 $283,010 | 0.376 | USD 100,000 | $168,400 | N/A |
| Gomez et al. (2016) 24 | MDI vs. SAP | Colombia | USD 24, 000 | $37,188 | 3.810 | USD 26,750 | $42,500 | USD 26,750–99% |
| Jendle et al. (2019) 25 | CSII only vs. HCL | Sweden | SEK 164,236 | $27,731 | 1.900 | SEK 300,000 | $50,700 | SEK 97.60% |
| Pease et al. (2020) 26 | MDI vs. HCL | Australia | AUD 37,767 | $37,767 | 3.724 | AUD 50,000 | $50,000 | AUD 50,000–86% |
| Roze et al (2021) 27 | CSII only vs. HCL | UK | GBP 20,421 | $35,942 | 1.73 | GBP 30,000 | $62,000 | GBP 30,000–99.8% |
| Huang et al. (2010) 28 | (CSII 90% and MDI 10%) | United States of America | USD 98,679 | $172,852 | 0.60 | USD 100,000 | $175,200 | N/A |
| USD 78,943 | $138,281 | 1.11 | ||||||
| McQueen et al. (2011) 29 | CSII or MDI (varied) | United States of America | USD 45,033 | $77,269 | 0.523 | USD 100,000 | $171,600 | 70% |
| Garcia‐Lorenzo et al. (2018) 30 | CSII and MDI | Spain | EUR 2554723 | $6,019,360 | 0.046 | EUR 20,000 to 25,000 | $47,100–$58,900 | ~0% |
Abbreviations: CEAC, cost effectiveness acceptability curve.
WTP threshold rounded to the nearest hundred.
Some studies focused on patients with suboptimal management or greater hypoglycaemic risk. In these studies, either benefits from improvement in glycaemic control or reduction in hypoglycaemic risk was assessed, not both. Suboptimal management studies (n = 5) 13 , 15 , 17 , 18 reported an ICER and QALY gain range of [$20,130–$93,772 per QALY] and [1.19–1.45] respectively. Studies analysing patients with greater hypoglycaemic risk (n = 5) 13 , 15 , 17 , 18 reported ICER and QALY gain in the range of [$19,961–$70,236 per QALY] and [1.44–1.88].
Seventeen studies concluded that CGM is cost effective based on willingness‐to‐pay thresholds that ranged from $42,000 to $175,000. Fifteen studies also created a cost effectiveness acceptability curve (CEAC) to help further assess cost‐effectiveness.
4. DISCUSSION
The findings of this systematic review support that the implementation of CGM in patients with type 1 diabetes is a cost‐effective strategy: especially in the setting of suboptimal glycaemic control and hypoglycaemic risk. The review also notes that there have been rapid improvements in CGM. This evolution has led to substantial reduction in usage costs and increased accuracy translating to better clinical outcomes. 2 , 14 , 31
4.1. CSII
Studies assessing CGM compared to SMBG in the context of CSII, saw narrower ranges in ICERs [$18,734–$99,942] and QALY gained [0.760–2.990] compared to other intervention groups. These studies focused on two subpopulations of type 1 diabetes individuals, those with suboptimal baseline glycaemic management and those at higher risk of hypoglycaemia. Notably, those who were at higher risk of hypoglycaemic events had greater QALY gained and lower ICER compared to those with suboptimal baseline management.
All of the five studies 19 , 20 , 22 , 24 , 26 that evaluated individuals with type 1 diabetes at high risk of hypoglycaemic events, based their treatment effects on a single randomized control trial (RCT) by Ly et al. 32 The patient population selected by Ly et al. were individuals with impaired hypoglycaemic awareness, and had a mean age of 18.6 years, suggesting a significant proportion of paediatric patients. The treatment effect based on a cohort with a paediatric skew, may raise concerns that such effects may not translate to older individuals given youth is a risk factor for hypoglycaemic events. 31 However, a recent RCT by Richard et al. would quell those concerns, showing a statistically significant reduction in hypoglycemic events with the use of CGM in adults 60 years or older. 2 It should also be noted that a “low glucose suspend” function was utilized by these SAP however this feature is not employed universally.
Sensitivity analysis from four of the five studies showed, that even if the magnitude of reduction in severe hypoglycaemic events (SHE) by CGM were more than halved, the ICER calculated remained less than the ICER of suboptimal glycaemic management counterparts. This highlights the importance of hypoglycaemic event reduction, as a driver of cost‐effectiveness in CGM.
Five studies 12 , 13 , 15 , 17 , 18 also assessed individuals with suboptimal glycaemia management at baseline. These studies had varying baseline HbA1c, ranging from 8.1% to 9%. Four of the studies used the Pickup et al. 4 meta‐analysis formula to help calculate HbA1c from baseline characteristics. The single other study 12 based its cohort characteristics on the DCCT study, and its treatment effects from an unpublished meta‐analysis. The treatment effect on HbA1c utilized by this study 12 was less than what would have been estimated by the Pickup et al. 4 formula, based on its cohort characteristics.
Of the five studies there were two notably higher ICERs, identified by Roze et al. 12 and Nicolluci et al. 17 The high ICER identified by Roze et al. 12 study can be explained by the noticeably lower QALY gained, almost half that of the other studies. The cause of the lower QALY is unclear as most utility inputs could not be sourced. The higher ICER ($93,772) reported by Nicolluci et al. 17 may largely be attributed to its high complication treatment cost, which was almost double that identified by the other studies. These five studies, discussed in the previous two paragraphs, highlights how subtle changes in the multitude of inputs required for lifetime modelling, can significantly alter outcomes. These inputs will vary from country to country, and therefore are an important consideration for policy makers when assessing the applicability of these results for their respective countries.
The remaining study by Roze et al., 14 which reported the greatest gains in QALYs and lowest ICERs, combined the treatment effects of HbA1c and hypoglycaemic event reduction. Roze et al. 14 utilized a baseline HbA1c of 10% with a treatment effect of 0.9% HbA1c reduction, as well as a reduction in SHE from 2.2 to 0.0 per patient month. Given an inverse relationship between HbA1c and SHE, it would be prudent to question whether a SHE risk‐reduction of this magnitude would occur in a population with a baseline HbA1c of 10%, noting that the original study had a baseline HbA1c of 7.6%. This study also highlights the paucity of data surrounding real‐world effects of CGM, which is required to accurately evaluate the cost‐effectiveness.
4.2. MDI
Currently, four studies 19 , 20 , 21 , 22 assessed CGM with MDI. Assessment of CGM with MDI remains important, given >65% of patients with type 1 diabetes still use MDI rather than an insulin pump. 33 Wan et al. 20 study reported a significantly higher ICER ($149,634) than the other studies despite deeming CGM to be cost‐effective. This was correlated with a noticeably lower QALY gain of 0.54. Chaugule et al. 19 identified the greatest QALY gain at 3.35, whilst Roze et al. 21 reported 1.49 and 1.39 QALY gained for its general and suboptimal glycaemic management populations respectively, as well as a QALY gain of 2.09 in the Canadian study. 22 The variability in the identified ICERs is likely explained by the cost difference of CGM, cost of complications and number of QALY gained.
Additionally, the four studies were based on three different CGM models; Wan et al. 20 utilized the Dexcom G4, Chaugule et al. 19 used Dexcom G5 and the two Roze et al. 21 , 22 used Dexcom G6. The main impacts of the advancing models include, the introduction of mobile devices being used as receivers from the Dexcom 5 onward, and reduced calibration requirements as well as a longer sensor usage duration with Dexcom 6. As a result, the Chaugule et al. 19 and Roze et al. 21 , 22 studies assumed no or 0.13% of users would require a receiver respectively. This significantly reduced the lifetime costs of CGM, as the original receiver quoted in the Wan et al. study was priced at $737 (USD 482) per year. The cost difference of CGM between the Roze et al. 21 , 22 studies and Chaugule et al. 19 studies also extend from the reduction in transmitter cost and sensor use duration. The cost of transmitters for the Dexcom 5 compared to Dexcom 6 model, as quoted in the studies was approximately $500 and $422 respectively. Additionally, advancement in sensor technology meant the Dexcom 6 sensor used by Roze et al. 21 , 22 lasted longer at 10 days whilst, Dexcom 5 (Chaugule et al. 19 ) sensors were only used for 7‐days. This resulted in a reduction of 16 sensors required by patients each year, another significant cost reduction. These four studies conducted short periods (2017–2021) apart have already utilized three different model evolutions, highlighting the rapid advancements in CGM technology. The evolving CGM models have also already rapidly cut usage costs, foreshadowing ongoing reductions in CGM ICERs.
4.3. HCL
HCL represents a crucial steppingstone to creating a full‐automated artificial pancreas. Of the identified studies only three assessed HCL. All three studies reported low ICERs driven by high QALYs gained. The studies by Jendle et al., Pease et al. and Roze et al. combined the benefits of reduction in HbA1c and reduction in severe hypoglycaemic events. This combined effect reflects evidence that HCL is better able to maintain time within target range and further reduce the risk of hypoglycaemic events compared to SAP. 34 , 35
4.4. Cost effectiveness assessment
With the rapid discoveries of new treatment and technologies, there has been significant increase in healthcare expenditure. As a result, cost‐effectiveness analysis has become a useful tool for healthcare decision‐makers to help identify treatments that offer larger health gains with less impact on the healthcare budget. Evaluation of therapies and health‐technology are also important for the individual patient, to prevent exposure to “financial toxicity”. 36 Rapid development of treatments means assessment of cost‐effectiveness are increasingly reliant on outcomes, such as ICER, that are derived from lifetime modelling.
To establish if an intervention is “cost‐effective”, ICERs are compared to a willingness‐to‐pay (WTP) threshold. WTP threshold is defined as “an estimate of what a consumer of health care might be prepared to pay for the health benefit”. 37
There is no consensus on how best to determine a WTP threshold, which also will vary based on many societal factors. Many countries with a centralized systems of healthcare have WTP threshold to help guide policymakers. Other approaches to identifying WTP thresholds include: (1) per capita income based thresholds, and (2) previous treatment thresholds. 38 The World Health Organization (WHO) considers an intervention to be cost‐effective if it is three times less than the national annual gross domestic product (GDP) per capita. 39 Those less than one time the national GDP per capita are considered highly cost‐effective. As for previous treatment thresholds, in the USA, a WTP threshold of AUD ~ $70,000 (USD 50,000) is often quoted. 38 This was based on the cost of treating end‐stage renal disease when it became enrolled onto Medicare. In this study, we found that limited studies discussed WTP threshold decisions. Two studies utilized the WHO recommended threshold based on GDP per capita, and one study utilized the previous threshold of AUD ~ $70,000. There are additional tools that can be used such as the cost‐effectiveness acceptability curve (CEAC) to support decision makers. The CEAC helps to evaluate the cost‐effectiveness of an intervention by showing the probability that an intervention is considered cost‐effective for a range of monetary values. Thirteen of the reviewed studies also created a CEAC to help further assess cost‐effectiveness.
4.5. Outcomes
Two studies (Kamble et al. 23 and Garcia‐Lorenzo et al. 30 ) concurred that CGM was not cost‐effective. These two studies had noticeably higher ICERs at $386,667 (3‐day sensor) and $6,019,360 respectively. There are several areas of contention in these studies. The Kamble et al. study 23 was performed in 2012 and utilized dated technology. The study itself suggested with further advancements the technology would become more economically attractive Garcia‐Lorenzo et al. 30 evaluated CGM irrespective of the insulin delivery mode, which likely confounded its assessment, and failed to include the impact of hypoglycaemic events. Additionally, the study created its own Markov Modelling system, which the authors themselves identified as lacking the sophistication of more established models such as the CORE diabetes model. In the model by Garcia‐Lorenzo et al., 30 individuals were limited to three concomitant complications and risk factors such as age, other comorbidities and duration of diabetes were not adjusted for. The Garcia‐Lorenzo et al. study highlights the need for robust statistical models to accurately assess cost effectiveness.
Overall, there was acceptance of CGM as a cost‐effective strategy. The majority of ICERs for CGM with MDI was significantly less than three times the GDP per capita of most first world countries. Notably, many were less than one time the GDP per capita of the USA and Australia (as based on data from the World Bank) suggesting CGM is a highly cost‐effective intervention.
4.6. Australian context
A recent consensus statement released by the ADS/ADEA/APEG/ADIPS working group highlighted concerns regarding access to diabetes technology. 40 It identified that high acquisition cost resulted in underutilisation of diabetes management technology, with only 21% of individuals with type 1 diabetes accessing insulin pumps over their lifetime. 41 Additionally, most of those who accessed this technology did so via private health insurance and were of high‐socioeconomic background. Like insulin pumps, CGM funding remains limited to a select population of individuals. Funding was initially only provided to individuals under 21 years of age, but later expanded to include those intending to conceive or are pregnant, concession cardholders and Aboriginal and Torres Strait Islanders. The limited funding has two main implications. Firstly, most individuals with type 1 diabetes will not qualify for these criteria, thus will not have ready access to a technology that significantly improves clinical outcomes. Resulting in preventable complications and cost being placed on these individuals and society. Secondly, it stands to cause significant distress in individuals who move out of the qualifying categories, putting at risk the progress that was previously made.
Given the similarities between the centralized healthcare systems of Australia and the UK (Medicare and National Health Service), if Australia were to follow in the footsteps of the UK where flash glucose monitoring is funded for all individuals with type 1 diabetes. The benefits experienced in the UK, such as significant reductions in paramedic call‐outs and hospital admissions due to hypoglycaemia and hyperglycaemia, would likely translate. 42
4.7. Limitations
Whilst current cost‐effectiveness studies provide a good basis in guiding healthcare policymakers, limitations remain. As CGM technology improves at rapid rates, there is often a delay in supporting research becoming available. One key example of this is the paucity of studies assessing HCL. It is expected that HCL will be the mainstay version of CGM, yet only three studies were identified in this review. As such, we acknowledge that current on the market CGM devices may be more cost‐effective than what is reported. Additionally, further high qualities studies that minimize confounding factors are needed, with variation in method of treatment being a key confounding factor to be avoided.
We have utilized broad search terms during the review of the available literature to capture greater number or articles, however we acknowledge that there are limitations that come with this. As there are a multitude of different names given to devices under the umbrella term of “continuous glucose monitoring”, if articles had not utilized the umbrella term they may have been missed during the search. The broad nature of CGM also means not all forms (e.g. SAP vs. HCL) under this title are suitable to be compared directly with each other. This is due to the different outcomes and costs associated with each. We have separated the different ways CGM is used in the above discussion to reflect this. Finally, there is also a multitude of different brands of CGM devices which were not all included in this review.
As with any new technology, there is cost associated with widespread implementation. Notably, this includes time spent to help set up and provide support to patients using CGM. Discussion and cost implications surrounding this were absent in all studies. Additionally, CGM provides large volumes of complex data that will require additional time and expertise for a healthcare professional to utilize. Factoring appropriate remuneration for this service was also noticeably absent. These important cost considerations for the successful widespread implementation of CGM will also need to be addressed by healthcare policymakers.
5. CONCLUSION
This systematic review provides evidence that CGM appears to be a cost‐effective intervention for individuals with type 1 diabetes. Key drivers of CGM cost‐effectiveness include reduction of chronic complications through improvement in glycaemic management, and reduction in frequency and duration of hypoglycaemic episodes. These studies also highlight the rapidly evolving nature of CGM which has driven down usage costs and may continue to do so with further advances.
AUTHOR CONTRIBUTIONS
Yuxin Jiao: Conceptualization (equal); investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal). Rose Lin: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – review and editing (equal). Xinyang Hua: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Leonid Churilov: Data curation (equal); formal analysis (equal); validation (equal). Michele J Gaca: Conceptualization (equal); data curation (equal); writing – review and editing (equal). Steven James: Conceptualization (equal); data curation (equal); resources (equal); validation (equal); writing – review and editing (equal). Philip M Clarke: Formal analysis (equal); validation (equal). David Norman O'Neal: Formal analysis (equal); supervision (equal); writing – review and editing (equal). Elif I Ekinci: Conceptualization (equal); project administration (equal); supervision (equal); writing – review and editing (equal).
FUNDING INFORMATION
No funding was obtained for this article.
CONFLICT OF INTEREST
EIE is the principal investigators in the NHMRC CTCS funded trial (APP1182464). EIE's institution receives research funding for unrelated research from EliLilly, Boehringer, AstraZeneca, Gilead.
Jiao Y, Lin R, Hua X, et al. A systematic review: Cost‐effectiveness of continuous glucose monitoring compared to self‐monitoring of blood glucose in type 1 diabetes. Endocrinol Diab Metab. 2022;5:e369. doi: 10.1002/edm2.369
DATA AVAILABILITY STATEMENT
No further data are available
REFERENCES
- 1. Nathan DM, for the DERG . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow‐up from the COMISAIR study. Diabetes Care. 2020;43(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 4. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta‐analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polonsky WH, Hessler D, Ruedy KJ, Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736‐741. [DOI] [PubMed] [Google Scholar]
- 6. Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174(5):678‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vigersky RA. The benefits, limitations, and cost‐effectiveness of advanced technologies in the management of patients with diabetes mellitus. J Diabetes Sci Technol. 2015;9(2):320‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shields GE, Elvidge J. Challenges in synthesising cost‐effectiveness estimates. Syst Rev. 2020;9(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morciano ISJTM. A web‐based tool for adjusting costs to a specific target currency and price year. Evidence & Policy A Journal of Research Debate and Practice. 2010;6:51‐60. [Google Scholar]
- 11. Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews Of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 12. Roze S, Saunders R, Brandt AS, de Portu S, Papo NL, Jendle J. Health‐economic analysis of real‐time continuous glucose monitoring in people with Type 1 diabetes. Diabet Med. 2015;32(5):618‐626. [DOI] [PubMed] [Google Scholar]
- 13. Roze S, Smith‐Palmer J, Valentine W, et al. Cost‐effectiveness of sensor‐augmented pump therapy with low glucose suspend versus standard insulin pump therapy in two different patient populations with type 1 diabetes in france. Diabetes Technol Ther. 2016;18(2):75‐84. [DOI] [PubMed] [Google Scholar]
- 14. Roze S, Smith‐Palmer J, Valentine WJ, et al. Long‐term health economic benefits of sensor‐augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: a U.K. perspective. J Med Econ. 2016;19(3):236‐242. [DOI] [PubMed] [Google Scholar]
- 15. Roze S, de Portu S, Smith‐Palmer J, Delbaere A, Valentine W, Ridderstråle M. Cost‐effectiveness of sensor‐augmented pump therapy versus standard insulin pump therapy in patients with type 1 diabetes in Denmark. Diabetes Res Clin Pract. 2017;128:6‐14. [DOI] [PubMed] [Google Scholar]
- 16. Conget I, Martin‐Vaquero P, Roze S, et al. Cost‐effectiveness analysis of sensor‐augmented pump therapy with low glucose‐suspend in patients with type 1 diabetes mellitus and high risk of hypoglycemia in Spain. Endocrinologia Diabetes y Nutricion. 2018;65(7):380‐386. [DOI] [PubMed] [Google Scholar]
- 17. Nicolucci A, Rossi MC, D'Ostilio D, Delbaere A, de Portu S, Roze S. Cost‐effectiveness of sensor‐augmented pump therapy in two different patient populations with type 1 diabetes in Italy. Nutr Metab Cardiovasc Dis. 2018;28(7):707‐715. [DOI] [PubMed] [Google Scholar]
- 18. Roze S, Smith‐Palmer J, de Portu S, Ozdemir Saltik AZ, Akgul T, Deyneli O. Cost‐effectiveness of sensor‐augmented insulin pump therapy versus continuous insulin infusion in patients with type 1 diabetes in Turkey. Diabetes Technol Ther. 2019;21(12):727‐735. [DOI] [PubMed] [Google Scholar]
- 19. Chaugule S, Graham C. Cost‐effectiveness of G5 Mobile continuous glucose monitoring device compared to self‐monitoring of blood glucose alone for people with type 1 diabetes from the Canadian societal perspective. J Med Econ. 2017;20(11):1128‐1135. [DOI] [PubMed] [Google Scholar]
- 20. Wan W, Skandari MR, Minc A, et al. Cost‐effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self‐monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. 2018;41(6):1227‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roze S, Isitt J, Smith‐Palmer J, Javanbakht M, Lynch P. Long‐term cost‐effectiveness of dexcom G6 real‐time continuous glucose monitoring versus self‐monitoring of blood glucose in patients with type 1 diabetes in the U.K. Diabetes Care. 2020;43(10):2411‐2417. [DOI] [PubMed] [Google Scholar]
- 22. Roze S, Isitt JJ, Smith‐Palmer J, Lynch P. Evaluation of the long‐term cost‐effectiveness of the Dexcom G6 continuous glucose monitor versus self‐monitoring of blood glucose in people with type 1 diabetes in Canada. Clinicoecon Outcomes Res. 2021;13:717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamble S, Schulman KA, Reed SD. Cost‐effectiveness of sensor‐augmented pump therapy in adults with type 1 diabetes in the United States. Value Health. 2012;15(5):632‐638. [DOI] [PubMed] [Google Scholar]
- 24. Gomez AM, Alfonso‐Cristancho R, Orozco JJ, et al. Clinical and economic benefits of integrated pump/CGM technology therapy in patients with type 1 diabetes in Colombia. Endocrinologia y Nutricion. 2016;63(9):466‐474. [DOI] [PubMed] [Google Scholar]
- 25. Jendle J, Pohlmann J, de Portu S, Smith‐Palmer J, Roze S. Cost‐effectiveness analysis of the MiniMed 670G hybrid closed‐loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther. 2019;21(3):110‐118. [DOI] [PubMed] [Google Scholar]
- 26. Pease A, Zomer E, Liew D, et al. Cost‐effectiveness analysis of a hybrid closed‐loop system versus multiple daily injections and capillary glucose testing for adults with type 1 diabetes. Diabetes Technol Ther. 2020;22(11):812‐821. [DOI] [PubMed] [Google Scholar]
- 27. Roze S, Buompensiere MI, Ozdemir Z, de Portu S, Cohen O. Cost‐effectiveness of a novel hybrid closed‐loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK. J Med Econ. 2021;24(1):883‐890. [DOI] [PubMed] [Google Scholar]
- 28. Huang ES, O'Grady M, Basu A, et al. The cost‐effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33(6):1269‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McQueen RB, Ellis SL, Campbell JD, Nair KV, Sullivan PW. Cost‐effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Effect Resource Allocat. 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Lorenzo B, Rivero‐Santana A, Vallejo‐Torres L, et al. Cost‐effectiveness analysis of real‐time continuous monitoring glucose compared to self‐monitoring of blood glucose for diabetes mellitus in Spain. J Eval Clin Pract. 2018;24(4):772‐781. [DOI] [PubMed] [Google Scholar]
- 31. Lehecka KE, Renukuntla VS, Heptulla RA. Insight into hypoglycemia in pediatric type 1 diabetes mellitus. Int J Pediatr Endocrinol. 2012;2012(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor‐augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240‐1247. [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association . 7. Approaches to Glycemic Treatment. Diabetes Care. 2015;38(Suppl 1):S41‐S48. [DOI] [PubMed] [Google Scholar]
- 34. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed‐loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tauschmann M, Allen JM, Wilinska ME, et al. Day‐and‐night hybrid closed‐loop insulin delivery in adolescents with type 1 diabetes: a free‐living. Randomized Clinical Trial Diabetes Care. 2016;39(7):1168‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost‐effectiveness analysis in the United States. Ann Intern Med. 2020;174(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 37. Bertram MY, Lauer JA, De Joncheere K, et al. Cost‐effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDougall JA, Furnback WE, Wang BCM, Mahlich J. Understanding the global measurement of willingness to pay in health. Journal of market access & health policy. 2020;8(1):1717030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. WHO , Baltussen RMPM, Adam T, et al. Making choices in health: WHO guide to cost‐effectiveness analysis. Tan‐Torres Edejer T., et al. World Health Organization; 2003. [Google Scholar]
- 40. Pease AJ, Andrikopoulos S, Abraham MB, et al. Utilisation, access and recommendations regarding technologies for people living with type 1 diabetes: consensus statement of the ADS/ADEA/APEG/ADIPS Working Group. Med J Aust. 2021;215(10):473‐478. [DOI] [PubMed] [Google Scholar]
- 41. Australian Institue of Health and Welfare A . Insulin pump use in Australia.
- 42. Deshmukh H, Wilmot EG, Gregory R, et al. Effect of flash glucose monitoring on glycemic control, hypoglycemia, diabetes‐related distress, and resource utilization in the Association of British Clinical Diabetologists (ABCD) nationwide audit. Diabetes Care. 2020;43(9):2153‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No further data are available


