Abstract
Background & Aims:
The Fibrosis-4 Index (FIB-4) can reliably assess fibrosis risk in patients with chronic liver disease and advanced fibrosis is associated with severe liver disease (SLD) outcomes. However, CLD is under-diagnosed in primary care. We examined the association of FIB-4 risk strata and the incidence of SLD preceding a CLD diagnosis while considering incident CLD diagnoses as competing risks.
Methods:
Using primary care clinic data between 2007 and 2018, we identified patients with two FIB-4 scores and no liver disease diagnoses preceding the index FIB-4. Patients were followed from index FIB-4 until an incident SLD (a composite of cirrhosis, hepatocellular carcinoma, or liver transplantation), CLD, or were censored. Hazard ratios were computed using a Fine-Gray competing risk model.
Results:
Of 20,556 patients, there were 54.8% in the low, 34.8% in the indeterminate, 6.6% in the high, and 3.8% in the persistently high-risk FIB-4 strata. During a mean 8.2 years of follow-up, 837 (4.1%) patients experienced an SLD outcome and 11.5% of the sample received a CLD diagnosis. Of patients with an SLD event, 49% received no preceding CLD diagnosis. In the adjusted Fine-Gray model, the indeterminate (HR 1.41; 95%CI 1.17 – 1.71), high (HR 4.65; 95%CI 3.76 – 5.76), and persistently high-risk (HR 7.60; 95%CI 6.04 – 9.57) FIB-4 risk strata were associated with a higher incidence of SLD compared to the low-risk stratum.
Conclusions:
FIB-4 scores with indeterminate and high-risk values are associated with an increased incidence of SLD in primary care patients without known CLD.
Keywords: Fibrosis-4 Index, non-invasive testing, advanced fibrosis, chronic liver disease
Lay Summary
The Fibrosis-4 Index (FIB-4) provides non-invasive, accessible, and inexpensive fibrosis risk assessment in patients with known chronic liver disease. However, missed and delayed diagnosis of chronic liver disease in primary care limits FIB-4’s use in this setting. This study demonstrates that elevated FIB-4 scores are associated with severe liver disease outcomes even in patients where chronic liver disease has gone undiagnosed.
Introduction
Advanced liver fibrosis is associated with increased risk of cirrhosis, hepatocellular carcinoma, and liver-related mortality in patients with chronic liver diseases.1–7 Efforts to improve the non-invasive detection of advanced fibrosis have included the use of the Fibrosis-4 Index (FIB-4), a serologic advanced fibrosis risk assessment tool that relies upon readily available blood tests (aminotransferases and platelet count) stored within the electronic health record (EHR).8–11 FIB-4 has provided accurate fibrosis risk assessment in biopsy-proven NAFLD cohorts, patients with viral hepatitis, other chronic liver diseases, and demonstrated promise in identifying patients with elevated fibrosis risk in primary care.8,9,11–18
Beyond fibrosis assessment in patients with known chronic liver disease (CLD), high-risk FIB-4 scores have also been associated with severe liver outcomes in population-based studies.19–22 Hagstrom and colleagues first demonstrated this relationship between single and recurrent FIB-4 scores in a population-based Swedish dataset from 1985–1996.20 We reinforced this relationship using contemporary data and stratified Cox models.22 Results from these studies demonstrate an association between high-risk FIB-4 scores and severe liver disease (SLD) outcomes even in patients without known CLD diagnoses. To this point, FIB-4 calculation and interpretation has been recommended only after a CLD diagnosis is made. Unfortunately, under-diagnosis of CLD in primary care prevents the application of FIB-4 in this setting.
Despite strong and potentially practice-changing associations between high-risk FIB-4 scores and SLD outcomes in these previous analyses, limitations in the analytic approaches warrant consideration before liberalizing the use of FIB-4 for clinical decision-making in primary care. When evaluating the risk of SLD in a general population using Cox proportional hazards models, it is important to recognize that patients’ baseline risk is inherently not proportional.23 A patient has a significantly greater risk for SLD if they have a known CLD. We attempted to address this issue by developing a Cox regression model stratifying patients by known CLD and our findings confirmed the strong association of high-risk FIB-4 scores with SLD outcomes (HR 6.54; 95% CI 5.49–7.77); demonstrated significant hazard ratios for patients with known NAFLD (HR 7.71; 95% CI 3.62–16.45) and other CLD (HR 11.12; 95% CI 8.33–14.82); and still showed increased hazard of SLD outcomes in patients with high-risk FIB-4 and no previously known CLD (HR 4.04; 95% CI 3.10–5.28).22 But stratified Cox regression models also have limitations, as variable stratification can reduce estimation efficiency, complicate the ability to stratify for other predictor variables, and challenge our ability to make inferences for the stratification variable.23,24 These Cox regression methods could not fully account for the contribution of known CLD to an SLD outcome, potentially overestimating the relationship with FIB-4 in patients without a prior CLD diagnosis.22,25
To improve our understanding of FIB-4’s association with SLD for patients with undiagnosed CLD in primary care, we considered the knowledge of CLDs as competing risks for SLD outcomes. The cumulative incidence function (CIF) can illustrate the incidence of SLD while accounting for known competing CLD etiologies.23 Fine and Gray introduced the sub-distribution hazard model as a method for modeling the CIF, which considers the rate of an event (i.e. SLD) in patients who have not previously experienced the outcome or those who endured a competing event (CLD diagnosis).26,27 The Fine-Gray sub-distribution hazard model allows for the estimation of covariates’ effect on the CIF for an outcome event of interest, and allows for variables that do not satisfy the proportional hazards assumption.26,27 For clinical applications, covariates associated with increases in the sub-distribution hazard function will be associated with an increased incidence of the primary outcome.22,26,28
We conducted a retrospective cohort study of primary care patients to better characterize the relationship between combinations of repeated FIB-4 scores and the incidence of SLD in patients with undiagnosed CLD using a Fine-Gray sub-distribution hazard model. We hypothesized that even when accounting for the knowledge of CLD diagnoses, combinations of FIB-4 scores including high-risk assessments would be associated with an increased incidence of SLD outcomes in primary care patients with undiagnosed CLD.
Methods
Using retrospective data from an academic primary care clinic’s electronic health record (EHR), we identified patients with the laboratory results necessary to calculate FIB-4 scores and investigated the association of FIB-4 risk strata and SLD in undiagnosed patients while accounting for CLD diagnoses as competing risks.
Patients
We evaluated all patients receiving care from the internal medicine clinic at the Medical University of South Carolina (MUSC) between 2007 and 2018. The practice conducts 32,000 patient visits yearly and delivers care to a diverse (39% non-white), adult (mean age 59 years) population. Patients with alanine (ALT) and aspartate (AST) aminotransferase results (must have included at least one pair with both values < 500 U/L) during the period were identified, and index FIB-4 scores were calculated (FIB-4=[(Age × AST)/(Platelets × √ALT)]) for the earliest available set of aminotransferase values where a platelet count was available at the time of, or in the 2 months preceding the ALT and AST results.11,22 The patient’s age at the time of the aminotransferase results was used for calculating the FIB-4. After the index FIB-4 values were determined, we searched for the next set of qualifying ALT, AST, and platelet count values and calculated a “second” FIB-4. We required the next set of values to be at least 2 months after the index FIB-4 to ensure that unique aminotransferase and platelet results were used in the calculations. All patients with two FIB-4 scores were considered for the patient sample. We excluded all patients with an International Classification of Diseases (ICD)-9/10 code for a CLD (NAFLD, viral hepatitis, hemochromatosis, Wilson’s disease, etc.) or outcome (cirrhosis, hepatocellular carcinoma, or liver transplantation) prior to the index FIB-4 score (Table S1). Patients undergoing liver transplant for acute liver failure were also excluded.22
All patient FIB-4 scores were categorized by advanced fibrosis risk: low-risk (FIB-4 < 1.3); indeterminate-risk (FIB-4 – 1.3 – 2.67); and high-risk (FIB-4 > 2.67) using the risk thresholds established for NAFLD.9,12,22,29 After categorization of each patient’s index and second FIB-4 scores, patients were categorized by combinations of FIB-4 risk scores: persistently high, high, indeterminate, and low (Figure 1). The persistently high category included patients with two high-risk assessments; the high category included patients with only one high-risk assessment; the indeterminate category included patients with at least one indeterminate score and no high scores; and the low category comprised patients with only low-risk FIB-4 assessments.
Figure 1:
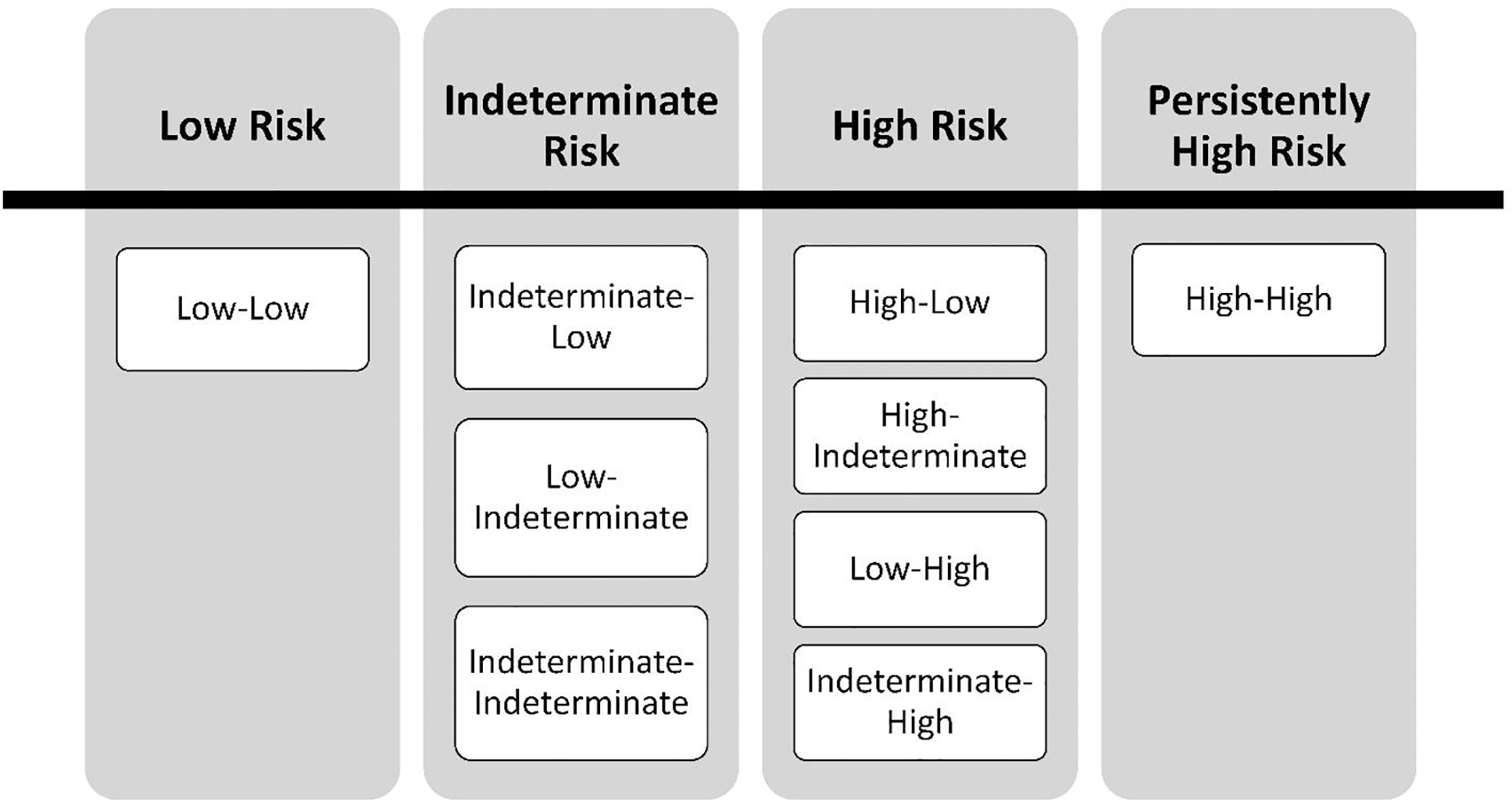
FIB-4 combination risk strata.
Outcomes and Follow-Up
The first occurrence of an SLD outcome served as the primary event of interest. This outcome was defined by a composite of ICD-9/10 diagnostic codes for cirrhosis, hepatocellular carcinoma, and liver transplantation (Table S1).30,31 Hepatology clinic notes and pathology results in the EHR were reviewed to accurately assign liver transplantation dates. Diagnoses of CLDs were the secondary events, or competing risks, in this analysis. These were defined as the occurrence of ICD-9/10 codes for either NAFLD or other CLD (e.g. viral hepatitis B and C, alcohol-related liver disease, autoimmune hepatitis, etc.) prior to the incidence of an SLD outcome or the end of the study period. NAFLD was emphasized due to its emergence as the leading cause of CLD in primary care and the growing body of literature supporting FIB-4’s application for fibrosis risk stratification in patients with NAFLD.10,32,33 Beginning with the index FIB-4, patients were followed until the primary event or the end of the study period (December 31, 2018). In this analysis, subjects who did not suffer an SLD outcome by the end of the study period were considered censored. The outcome variable for the survival analysis was defined as a composite of the categorical variable event status (0=censored, 1=CLD, 2=SLD) and the time in days from index FIB-4 to the event of interest or end of the study.
Independent Variables
The primary predictor variable of interest was the FIB-4 risk stratum, categorized as low, indeterminate, high, and persistently high (Figure 1). In patients experiencing the primary liver outcome prior to their 2nd FIB-4 score, their FIB-4 risk strata were determined by the risk category of their index FIB-4 score (low, indeterminate or high) and precluded them from being categorized as persistently high-risk.
A sensitivity analysis also evaluated the association between elevated ALT and SLD outcomes. ALT values used for the first and second FIB-4 calculations were categorized as normal (ALT < 30 IU/L in M; ALT < 19 IU/L in F) and elevated (ALT > 30 in M; ALT > 19 in F). A three-level categorical predictor variable of combinations of ALT normality was created: low ALT, high ALT, and persistently high ALT (Figure S1).
Covariates
Other independent variables of interest included demographic, vital sign, and comorbidity data. Gender was coded dichotomously as Male / Female. Race was a three-level, categorical variable coded as Black, White, and Other. Body mass index (BMI, kg/m2) was coded as a continuous variable of the patient’s BMI recorded at the time of, or just prior to, the index FIB-4 score. Using the Elixhauser comorbidity index, the comorbidities of hypertension, diabetes mellitus, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease were identified by ICD-9/10 code placed at any time during the patient’s inclusion in the study.22,34
Data Sources
All data came from Medical University Hospital Authority Enterprise and EPIC© (EPIC Systems Corporation, WI) Clarity databases. Clinical, laboratory, and demographic data were obtained in the ambulatory, emergency room, and inpatient settings at MUSC during the study period.
Statistical Analysis
Patient characteristics were reported as frequency counts and proportions for categorical variables, and mean and standard deviation for continuous variables. Patient characteristics were presented for the overall sample and by the occurrence of an SLD outcome. Continuous variables were compared by the occurrence of an SLD outcome using two sample t-tests and categorical variables were compared using chi-square tests.
Cumulative incidence function (CIF) curves for SLD events were plotted by FIB-4 risk strata. The curves used time in days (but displayed as years) as the time scale from index FIB-4 to the outcome of interest or the end of the study period. We modeled the sub-distribution hazard function for SLD outcomes using Fine-Gray regression technique.27 We performed an unadjusted model with the primary predictor variable of FIB-4 risk strata, using the low-risk stratum as the reference. We then developed a Fine-Gray regression model adjusting for gender, race, marital status, smoking history, BMI, and comorbidities. The comorbidities included hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease. Covariates were selected a priori from primary care variables associated with chronic liver disease.5 Model residual assessments were performed to ensure we used the most appropriate data fit and to identify any potential outliers or influential observations. Additional model assumptions were checked via residual analysis.
As a sensitivity analysis, CIF curves were also plotted for SLD by ALT abnormality groups. The sub-distribution hazard was modeled using Fine-Gray regression methods. Unadjusted and adjusted models were developed with combinations of ALT abnormality as the primary predictor variable (low ALT was the reference). Statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Overall, 20,556 patients were included (Figure 2) and the sample had a mean age of 51 years, was 65% female, and 45% Black (Table 1). The mean BMI for the cohort was 29 kg/m2, and 67%, 30%, and 54% of the sample had diagnoses of hypertension, diabetes, and hyperlipidemia, respectively. The median time between qualifying FIB-4 scores was 384 days (IQR: 168–825). After categorizing patients by their combinations of FIB-4 risk strata, 54.8% were in the low, 34.8% were in the indeterminate, 6.6% were in the high, and 3.8% were in the persistently high FIB-4 risk strata. Of included patients, 8,885 (43.2%) had normal ALT values used for both FIB-4 calculations.
Figure 2:
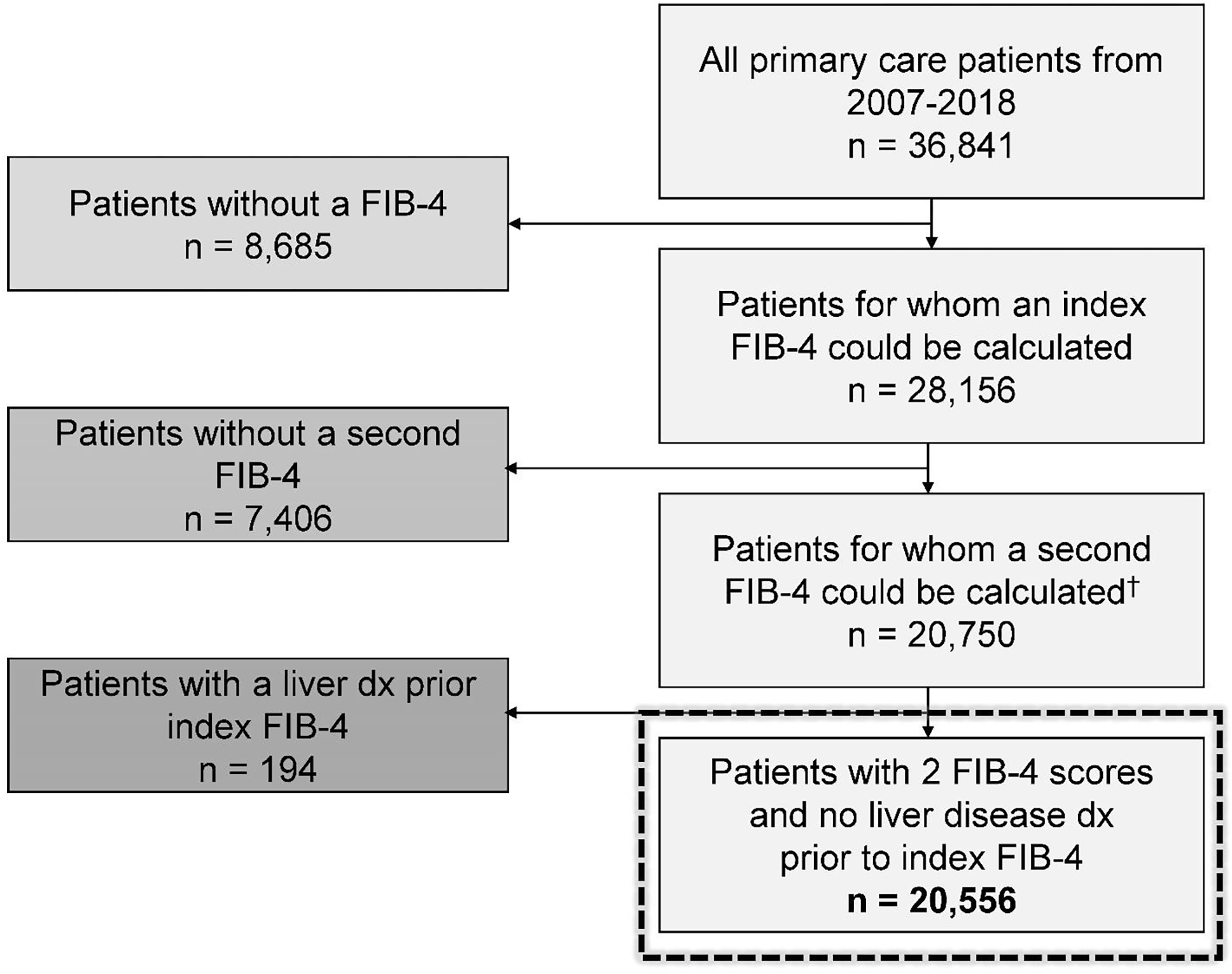
Consort diagram.
Table 1.
Patient characteristics by severe liver outcomes.
| Severe Liver Outcomes | ||||
|---|---|---|---|---|
|
| ||||
| Characteristics | Total | Yes | No | |
| n=20,556 | n=837 | n=19,719 | p-value | |
|
| ||||
| FIB-4 Combination Risk Strata (%) | < 0.001* | |||
| Low | 11,255 (54.8) | 231 (27.6) | 11,024 (55.9) | |
| Indeterminate | 7,159 (34.8) | 239 (28.6) | 6,920 (35.1) | |
| High | 1,356 (6.6) | 125 (14.9) | 1,231 (6.2) | |
| Persistently High | 786 (3.8) | 242 (28.9) | 544 (2.8) | |
|
| ||||
| ALT Combination Risk Strata (%) | <.0001* | |||
| Low | 8,885 (43.2) | 225 (26.9) | 8,660 (43.9) | |
| High | 5,715 (27.8) | 209 (25.0) | 5,506 (27.9) | |
| Persistently High | 5,956 (29.0) | 403 (48.1) | 5,553 (28.2) | |
|
| ||||
| Age (mean ± SD) | 51.0 ± 16.6 | 51.7 ± 13.6 | 51.0 ± 16.8 | 0.179† |
| Gender (%) | < 0.001* | |||
| Female | 64.8 | 51.3 | 65.4 | |
| Male | 35.2 | 48.7 | 34.6 | |
| Race (%) | 0.430* | |||
| Black | 45.3 | 47.1 | 45.2 | |
| Other | 2.6 | 2.9 | 2.6 | |
| White | 52.2 | 50.1 | 52.3 | |
| Marital status (%) | < 0.001* | |||
| Married | 45.5 | 34.6 | 45.9 | |
| Unmarried | 54.5 | 65.4 | 54.1 | |
| Current smoker (%) | < 0.001* | |||
| Yes | 12.5 | 23.7 | 12.0 | |
| BMI (mean ± SD) | 29.8 ± 8.2 | 28.8 ± 7.7 | 29.9 ± 8.2 | < 0.001† |
| Hypertension (%) | 67.1 | 81.0 | 66.5 | < 0.001* |
| Diabetes (%) | 29.5 | 46.1 | 28.8 | <.0001* |
| Hyperlipidemia (%) | 54.4 | 50.9 | 54.6 | 0.037* |
| CVD (%) | 26.5 | 39.1 | 26.0 | < 0.001* |
| Hypothyroid (%) | 16.0 | 17.4 | 16.0 | 0.255* |
| Kidney disease (%) | 18.4 | 42.3 | 17.4 | < 0.001* |
| Chronic liver disease (%) | ||||
| None | 18,290 | 411 (49.1) | 17,879 (90.7) | < 0.001* |
| NAFLD | 577 | 74 (8.8) | 503 (2.6) | < 0.001* |
| Other Liver Dx‡ | 1,788 | 373 (44.6) | 1,415 (7.2) | < 0.001* |
| HBV | 152 | 31 (3.7) | 121 (0.6) | < 0.001* |
| HCV | 603 | 207 (24.7) | 396 (2.0) | < 0.001* |
| ARLD | 997 | 166 (19.8) | 831 (4.2) | < 0.001* |
| Autoimmune** | 50 | 24 (2.9) | 26 (0.1) | < 0.001* |
| Metabolic | 197 | 22 (2.6) | 175 (0.9) | < 0.001* |
Chi-square tests comparing proportions by severe liver outcome occurrence
Two-sample t tests used to compare means by severe liver outcome occurrence.
1,788 unique patients had another CLD, but some patients received more than 1 CLD code.
Includes autoimmune hepatitis and PBC. FIB-4=Fibrosis-4 index. SD=standard deviation. BMI=body mass index (kg/m2). CVD=cardiovascular disease. NAFLD=nonalcoholic fatty liver disease. Dx=diagnosis. HBV=hepatitis B virus. HCV=hepatitis C virus. ARLD=alcohol-related liver disease.
During a mean 8.2 years of follow-up, 837 (4.1%) patients experienced an SLD outcome. Of the cohort, 2,365 (11.5%) of the sample received a diagnosis for a CLD, including the assignment of 577 (2.8%) NAFLD diagnoses, 603 (2.9%) HCV diagnoses, and 997 (4.9%) diagnoses of alcohol-related liver disease (ARLD). Of patients diagnosed with SLD, 411 (49%) received no preceding CLD diagnosis. In those with SLD and no preceding CLD, 190 (46%) had diabetes, 163 (40%) had a BMI > 30 kg/m2, and 254 (62%) had either diabetes or were obese (BMI > 30 kg/m2). In patients with two FIB-4’s preceding an SLD outcome, there was a mean time of 1,087 days (median 1,162 days) from the second FIB-4 until the SLD diagnosis.
In the univariate analysis, higher proportions of patients experiencing an SLD event were in the high and persistently high FIB-4 risk strata compared to those without an SLD event. Also, a higher proportion of patients suffering an SLD outcome were male (49% vs. 35%, p<0.001), unmarried (65% vs. 54%, p<0.001), and current smokers (24% vs. 12%), p<0.001). Patients with SLD diagnoses had lower mean BMIs (28.8 vs. 29.9, p=0.001) and higher burdens of hypertension (81% vs. 67%, p<0.001), diabetes (46% vs. 29%, p<0.001), cardiovascular disease (39% vs. 26%, p<0.001), chronic kidney disease (42% vs. 17%, p<0.001), and CLD (51% vs. 9%, p<0.001).
Figure 3 demonstrates the adjusted cumulative incidence function curves by FIB-4 risk strata. In the unadjusted Fine-Gray model, the indeterminate (HR 1.54; 95%CI 1.28 – 1.84), high (HR 6.25; 95%CI 5.16 – 7.57), and persistently high (10.83; 95%CI 8.86 – 13.23) FIB-4 risk strata were all associated with a higher incidence of SLD outcomes, compared to the low-risk stratum (Table 2). After adjusting for demographic and clinical covariates in the Fine-Gray model, the indeterminate (HR 1.41; 95%CI 1.17 – 1.71), high (HR 4.65; 95%CI 3.76 – 5.76), and persistently high (HR 7.60; 95%CI 6.04 – 9.57) FIB-4 risk strata continued to associate with a higher incidence of SLD events, compared to the low risk stratum. When FIB-4 risk strata were compared pairwise in the unadjusted and adjusted models, each successive risk category was associated with an increased incidence of SLD compared to the preceding risk stratum (Figure 4).
Figure 3.
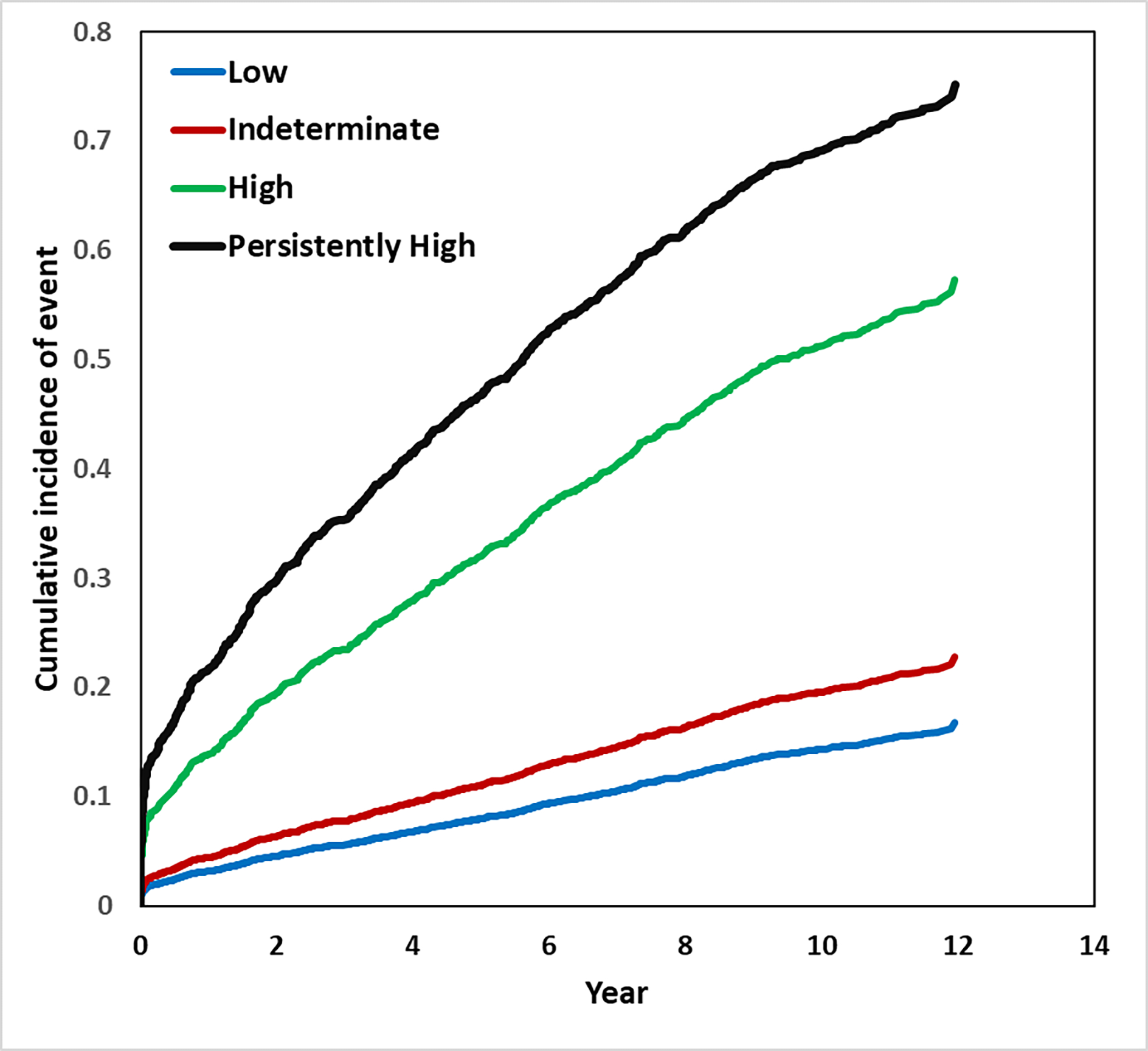
Adjusted cumulative incidence function curves for the outcome of severe liver disease by combined FIB-4 risk strata.*
*Full adjusted model controls for gender, race, marital status, smoking history, body mass index, and the presence of hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease.
Table 2.
Estimated Hazard Ratio and 95% Confidence Intervals using Fine-Gray proportional hazard regression models for the association between combined FIB-4 risk strata and severe liver disease.†
| Fine and Gray Regression Models | ||||
|---|---|---|---|---|
| Unadjusted Model | Adjusted Model† | |||
| Combined FIB-4 Risk Strata | HR | 95% CI | HR | 95% CI |
| Low (ref.) | ||||
| Indeterminate | 1.54 | 1.28 – 1.84 | 1.41 | 1.17 – 1.71 |
| High | 6.25 | 5.16 – 7.57 | 4.65 | 3.76 – 5.76 |
| Persistently High | 10.83 | 8.86 – 13.23 | 7.60 | 6.04 – 9.57 |
| Pairwise FIB-4 Risk Strata Comparison | ||||
| Indeterminate vs. Low | 1.54 | 1.28 – 1.84 | 1.41 | 1.17 – 1.71 |
| High vs. Indeterminate | 4.07 | 3.37 – 4.93 | 3.30 | 2.71 – 4.01 |
| Persistently High vs. Indeterminate | 7.06 | 5.79 – 8.61 | 5.39 | 4.38 – 6.63 |
| Persistently High vs. High | 1.73 | 1.40 – 2.14 | 1.63 | 1.32 – 2.03 |
Full adjusted model is in the Appendix. All models use days from the index FIB-4 score as the time scale. The adjusted model controls for gender, race, marital status, smoking history, body mass index, and the presence of hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease (Full model in the appendix). FIB-4=Fibrosis-4 index. HR=hazard ratio. CI=confidence interval.
Figure 4.
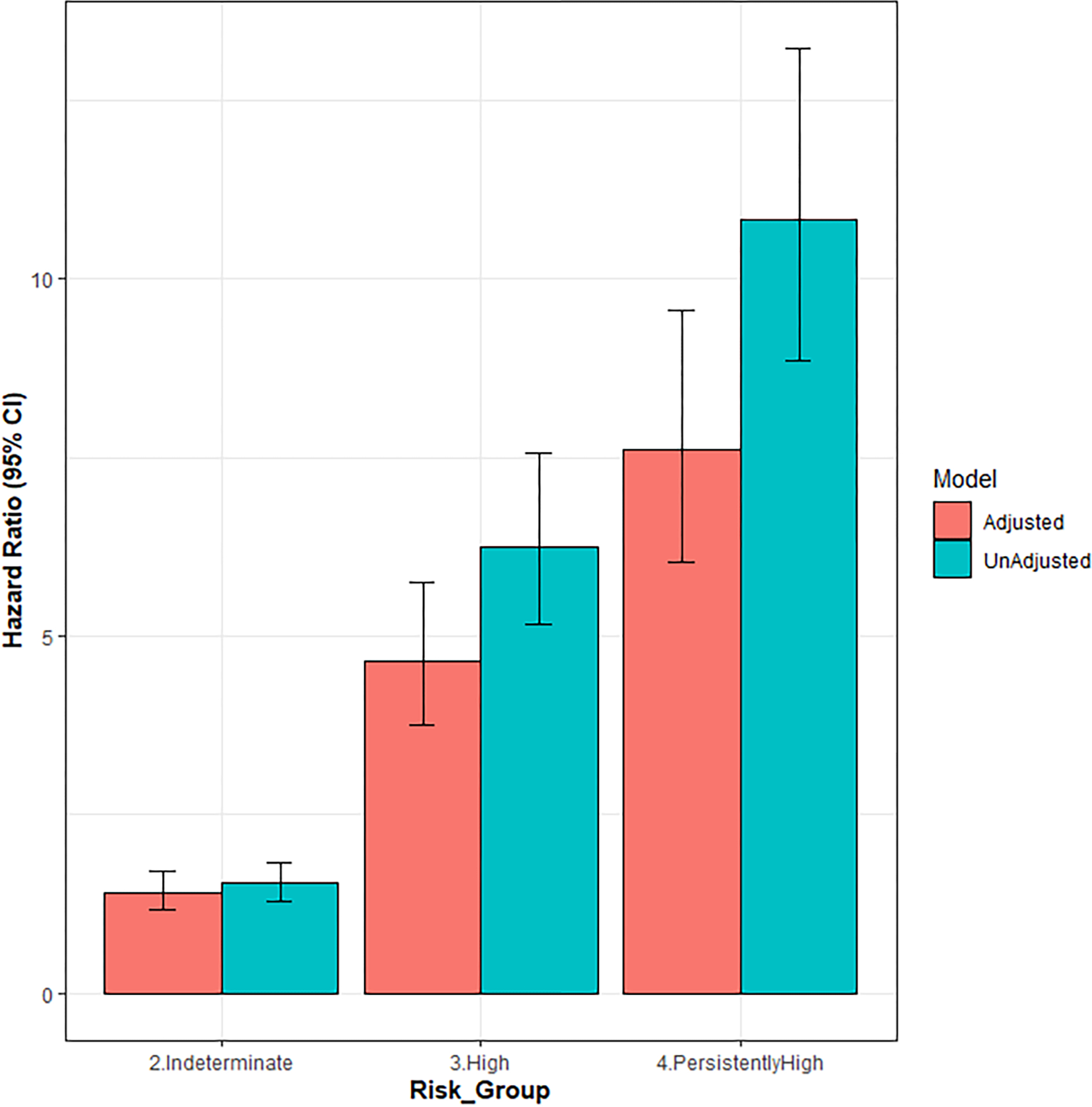
Unadjusted and adjusted Fine-Gray estimated hazard ratios by FIB-4 combination risk strata for the outcome of severe liver disease.
The unadjusted Fine-Gray model using the combinations of abnormal ALT variable demonstrates an association between high ALT (HR 1.79; 95%CI 1.50–2.13) and persistently high ALT (HR 2.19; 95%CI 1.85–2.59) with the incidence of SLD compared to the low ALT group (Table 3, CIF curves Figure S2). The adjusted models also show the association of high (HR 2.01; 95%CI 1.69–2.40) and persistently high (HR 2.89; 95%CI 2.43–3.44)) ALT with SLD outcomes.
Table 3.
Estimated Hazard Ratio and 95% Confidence Intervals using Fine-Gray proportional hazard regression models for the association between combined ALT abnormality strata and severe liver disease.†
| Fine and Gray Regression Models | ||||
|---|---|---|---|---|
| Unadjusted Model | Adjusted Model† | |||
| Combined ALT Strata | HR | 95% CI | HR | 95% CI |
| Low ALT (ref.) | ||||
| High ALT | 1.79 | 1.50 – 2.13 | 2.01 | 1.69 – 2.40 |
| Persistently High ALT | 2.19 | 1.85 – 2.59 | 2.89 | 2.43 – 3.44 |
| Pairwise ALT Strata Comparison | ||||
| Persistently High vs. High | 1.22 | 1.04 – 1.44 | 1.44 | 1.22 – 1.69 |
The adjusted model controls for gender, race, marital status, smoking history, body mass index, and the presence of hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease.
Discussion
In a sample of primary care patients with two unique FIB-4 scores, combinations of FIB-4 risk categories including indeterminate- and high-risk FIB-4 values were associated with an increased incidence of SLD outcomes compared to consecutive low-risk FIB-4 assessments. Additionally, when comparing FIB-4 risk strata in ascending order of risk, each successive risk stratum was associated with a higher incidence of SLD events (Figure 4): indeterminate vs. low (HR 1.41; 95%CI 1.17–1.71), high vs. indeterminate (HR 3.30; 95%CI 2.71–4.01), and persistently high vs. high (HR 1.63; 95% CI 1.32–2.03).
By using a Fine-Gray sub-distribution hazard model, this work demonstrates a strong relationship between high-risk FIB-4 scores and SLD outcomes in patients with undiagnosed liver disease while accounting for known CLD diagnoses as competing risks. These findings are important because though FIB-4 can accurately predict advanced fibrosis in patients with biopsy-proven CLD, we do not know how well FIB-4 performs in a primary care population where the prevalence of advanced fibrosis is expected to be lower and only a subset of patients have received CLD diagnoses.11,35 Our results demonstrate that the occurrence of indeterminate, high, and repeatedly high-risk FIB-4 scores are associated with an increased incidence of SLD events even in situations where CLD is undiagnosed. Our use of a competing risk model in this work focuses on diagnostic information. Classically, competing risk models consider survival data where included subjects can only experience one of several different types of events (e.g. cardiovascular death vs. death from other causes).23 From a pathophysiology perspective, we would expect most patients to have a CLD prior to an SLD event. However, only some of those patients experiencing the ultimate outcome carried a prior CLD diagnosis (426 [50.9%]). We wanted to know if FIB-4 as a “signal” was associated with SLD in patients with undiagnosed CLD. In our model, knowledge of a CLD diagnosis was the competing risk, since receiving a CLD diagnosis is mutually exclusive of having undiagnosed liver disease and suffering an SLD outcome. Ultimately, improving the diagnosis of CLD in primary care would have the biggest impact on optimizing care delivery to patients and predicting the risk for future SLD events. As work continues to address under-diagnosis, developing methods to identify patients at risk for SLD with incomplete diagnostic information remains important. The baseline hazard of SLD is not proportional for all primary care patients and our currently limited administrative diagnostic information incompletely addresses this variability. Competing risk analyses, like Fine-Gray, can refine our understanding of the relationship between FIB-4 and SLD outcomes in patients with undiagnosed liver disease using known CLD diagnoses as competing risks to provide more conservative hazard ratio estimates than stratified Cox regression and Cox proportional hazards models.
Our findings may highlight the importance of high-risk FIB-4 scores, or demonstrate the value of repeated low-risk FIB-4 assessments in ruling out advanced fibrosis. FIB-4 assessments < 1.3 (low-risk) have been found to have high negative predictive values (90% and up) for advanced fibrosis in previous studies, and we would expect patients with repeated low-risk fibrosis assessments to also have a low risk for future SLD outcomes.8,9 Low-risk FIB-4s help identify patients with CLD, specifically NAFLD, who can remain in the primary care domain.10,12,18,36 But, the pairwise comparisons of risk strata emphasize the potential value in indeterminate and high-risk FIB-4 scores for signaling a hazard of future SLD (Table 2). The hazard ratio was significantly higher in patients with two high-risk FIB-4s compared to patients with one high-risk score, patients with one high-risk score were at greater hazard than those with only indeterminate scores, and patients with indeterminate scores had a higher incidence of SLD outcomes compared to those with only low-risk FIB-4 values. High-risk FIB-4 scores, and to some extent indeterminate-risk values, signal future risk for SLD events, and may do so better than ALT elevations alone. Since FIB-4 can be easily calculated, often without incurring additional cost, we should consider routinely performing this test and interpreting the results in primary care. All high-risk scores (FIB-4 > 2.67), and arguably indeterminate-risk scores, could prompt primary care physicians to look for CLD, since nearly half of patients progressing to SLD in our study had no prior CLD diagnosis. Based on the low prevalence of NAFLD diagnoses (2.8%) compared to the expected NAFLD prevalence in the U.S. population (≈25%), we believe a significant proportion of patients with an SLD outcome and no preceding CLD diagnosis likely had undiagnosed NAFLD.33,37 This hypothesis was supported by the high proportion (62%) of these previously undiagnosed CLD patients with either diabetes or obesity (BMI>30kg/m2). In situations where CLD is identified, or is already known, FIB-4 scores would signal to PCPs those patients needing specialty care, intensified therapies, or heightened surveillance.
One of FIB-4’s advantages in primary care is the availability of the necessary inputs for calculation. Aminotransferases (as part of complete metabolic and liver function test panels) and platelet counts (as part of complete blood counts) are among some of the most frequently ordered tests in medicine and, along with patient age, exist in a structured format in most EHRs.38 The frequency with which new values are available for calculation would likely result in many FIB-4 values being available for interpretation. This study only focuses on the first two qualifying FIB-4 scores during the study period, simplifies each score by risk category, and combines the risk categories into strata. While this approach costs us information, we hoped to study a signal that might be manageable to perform in an already busy primary care practice. Further, we used a FIB-4 risk threshold established for NAFLD (high-risk > 2.67), as this is likely to the be the CLD most often encountered by PCPs in the future, and this threshold is the one currently endorsed for escalating advanced fibrosis risk assessment.9,39 Our results suggest that a provider recognizing a high-risk (and to some extent, an indeterminate-risk) score in a patient’s last two FIB-4 calculations should consider diagnostic action, or escalation of surveillance and management in situations where liver disease is known. Investigation of the relationship of repeated FIB-4 scores over time with future SLD outcomes is warranted.
We recognize limitations in this work. First, these results require cautious interpretation since FIB-4 was originally designed and studied for predicting risk for advanced fibrosis in patients with viral hepatitis C. FIB-4 performance has been studied extensively in patients with known CLD, but its performance in a general population is not known. Also, sub-distribution hazard ratios can provide overlapping information, but we think the total hazard is clinically meaningful here, particularly in the setting of under-diagnosis. Thus, we chose Fine-Gray over a cause specific hazard model for analyzing SLD outcomes with competing risks. Other limitations include the reliance on a composite of diagnostic codes (ICD-9/10) for CLD and our outcome of interest.37,40 To address this concern, we utilized previously validated techniques to capture CLD and cirrhosis diagnostic codes and performed a chart review on patients with a liver transplantation outcome.30,31,41 Also, data on patient deaths in our EHR are suboptimal. Patients dying in the hospital are accurately recorded, but those dying out of the hospital are incompletely ascertained and documented. On account of this, we were unable to use death as a right censoring event, resulting in the attribution of prolonged survival periods. Thus, our results overestimate our sample’s survival time, and this leads to a conservative underestimation of hazard ratios. Alcohol use is also inadequately recorded and available within the EHR, and is a critical variable in understanding the risk of future severe liver events.42 Additionally, FIB-4 calculations come from lab results during the study period, but we did not determine why labs were obtained. We restricted qualifying FIB-4 calculations by degree of AST and ALT elevation (<500 IU/L) and the timing of the platelet counts (within 2 months) to limit the number and frequency with which these data derived from acute liver diseases. Lastly, these data come from a single center and only include patients with laboratory inputs for 2 FIB-4 values which could threaten generalizability. However, the primary care focus, the distribution of comorbid conditions, and the number of included patients with normal ALT values on both sets of included liver chemistries (n=8,885, 43%) reflect patients in the general population.
Conclusion
Combinations of consecutive FIB-4 scores with indeterminate and high-risk values are associated with an increased incidence of SLD outcomes in primary care patients without known CLD. FIB-4 calculation and application in primary care may serve as a signal to pursue a liver diagnosis and provide prognostic information in the management of patients with known chronic liver diseases.
Supplementary Material
Acknowledgments
Funding
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of any of the following funding agencies supporting the investigators. Support comes from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK K23DK118200 PI: Schreiner). This project is also supported in part by the Southern Society for Clinical Investigation (SSCI) Research Scholar Award. This project was also supported by the South Carolina Clinical & Translational Research Institute with an academic home at the Medical University of South Carolina CTSA National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under UL1 TR001450.
Financial Support:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Southern Society for Clinical Investigation (SSCI).
Footnotes
Conflicts of Interest
All authors report no conflicts of interest with this study.
Ethics
The Institutional Review Board at the Medical University of South Carolina approved this study.
Data Sharing Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology (Baltimore, Md). 2017;65(5):1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. [DOI] [PubMed] [Google Scholar]
- 3.Simon TG, Roelstraete B, Sharma R, Khalili H, Hagstrom H, Ludvigsson JF. Cancer Risk in Patients With Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology (Baltimore, Md). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65(3):589–600. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. [DOI] [PubMed] [Google Scholar]
- 8.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. [DOI] [PubMed] [Google Scholar]
- 9.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(10):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–378. [DOI] [PubMed] [Google Scholar]
- 11.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology (Baltimore, Md). 2007;46(1):32–36. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the L, List of panel m, Berzigotti A, et al. Easl Clinical Practice Guidelines (Cpgs) On Non-Invasive Tests For Evaluation Of Liver Disease Severity And Prognosis- 2020 Update. J Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 13.Adler M, Gulbis B, Moreno C, et al. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology (Baltimore, Md). 2008;47(2):762–763; author reply 763. [DOI] [PubMed] [Google Scholar]
- 14.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver international : official journal of the International Association for the Study of the Liver. 2010;30(4):546–553. [DOI] [PubMed] [Google Scholar]
- 15.Mallet V, Dhalluin-Venier V, Roussin C, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2009;29(4):409–415. [DOI] [PubMed] [Google Scholar]
- 16.Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology (Baltimore, Md). 2008;48(5):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naveau S, Gaude G, Asnacios A, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology (Baltimore, Md). 2009;49(1):97–105. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen DN, Thiele M, Johansen S, et al. Prognostic performance of 7 biomarkers compared to liver biopsy in early alcohol-related liver disease. J Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Ability of Noninvasive Scoring Systems to Identify Individuals in the Population at Risk for Severe Liver Disease. Gastroenterology. 2020;158(1):200–214. [DOI] [PubMed] [Google Scholar]
- 20.Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73(5):1023–1029. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Cho YK, Cho J, et al. Alcoholic and Nonalcoholic Fatty Liver Disease and Liver-Related Mortality: A Cohort Study. Am J Gastroenterol. 2019;114(4):620–629. [DOI] [PubMed] [Google Scholar]
- 22.Schreiner AD, Moran WP, Zhang J, et al. The Association of Fibrosis-4 Index Scores with Severe Liver Outcomes in Primary Care. J Gen Intern Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinbaum DG KM. Survival Analysis: A Self-Learning Text, Third Edition. Springer Science. [Google Scholar]
- 24.Mehrotra DV, Su SC, Li X. An efficient alternative to the stratified Cox model analysis. Stat Med. 2012;31(17):1849–1856. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Qadir H, Fang J, Lee DS, et al. Importance of Considering Competing Risks in Time-to-Event Analyses: Application to Stroke Risk in a Retrospective Cohort Study of Elderly Patients With Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2018;11(7):e004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine JP GR. A Proportional Hazards Model for the Subdistribution Function of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 28.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterling RKD-R A; Patel K; Asrani SK; Alsawas M; Dranoff J; Fiel MI; Murad MH; Leung DH; Levine D; Taddei TH; Taoli B; Rockey DC; AASLD Guidelines Committee. Executive Summary: Non-invasive Liver Disease Assessments (NILDA) of hepatic fibrosis, steatosis, and portal hypertension: 2021 Practice Guidelines and Guidance from teh American Association for the Study of Liver Diseases. The Liver Meeting; 2021; Anaheim, CA. [Google Scholar]
- 30.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(10):1677–1678. [DOI] [PubMed] [Google Scholar]
- 31.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47(5):e50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019;3(10):1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 35.Usher-Smith JA, Sharp SJ, Griffin SJ. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ. 2016;353:i3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3(7):509–517. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen EM, Anderson KP, Marsden J, Zhang J, Schreiner AD. Nonalcoholic Fatty Liver Disease Underdiagnosis in Primary Care: What Are We Missing? J Gen Intern Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton S, Fleming KA, Kuti M, et al. The Top 25 Laboratory Tests by Volume and Revenue in Five Different Countries. Am J Clin Pathol. 2019;151(5):446–451. [DOI] [PubMed] [Google Scholar]
- 39.Kanwal F, Shubrook JH, Adams LA, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(5):1657–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagström H, Adams LA, Allen AM, et al. Administrative Coding in Electronic Health Care Record-Based Research of NAFLD: An Expert Panel Consensus Statement. Hepatology (Baltimore, Md). 2021;74(1):474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer A, Kosowan L, Loewen S, Spitoff S, Greiver M, Lynch J. Who is asked about alcohol consumption? A retrospective cohort study using a national repository of Electronic Medical Records. Prev Med Rep. 2021;22:101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


