Abstract
Introduction
Femoral-femoral Veno-Venous ExtraCorporeal Life Support (V-V ECLS) has been associated with higher infections rates, vascular site bleeding complications, and restricted patient mobility. Jugular or bicaval dual lumen V-V ECLS conceptually overcomes some of these adverse factors, but experience has shown that jugular vein cannulation still limits mobility and has increased bleeding complications. Technique and outcomes of subclavian vein single-cannulation with Crescent jugular dual-lumen V-V ECLS is described.
Method
five patients with COVID-19 related acute respiratory distress syndrome (ARDS) underwent right subclavian vein V-V ECLS placement with the Crescent 32 French jugular dual-lumen V-V ECLS catheter. A standardized percutaneous technique was developed that allowed efficient insertion without need for any specialized imaging (i.e. transesophageal echocardiogram) and outcomes assessed.
Results
Mean age of the five patients was 41.2 years, all obese with an average basal mass index of 45.2 kg/m2 and mean days to decannulation of 24.2 days. Outcomes discovered included; improved patient mobility allowing physical rehabilitation, no vascular access site related complications requiring surgery or endovascular intervention, and none had evidence of superior vena cava syndrome. One patient had subclavian/axillary vein thrombosis with resolution after 3 months of direct-acting oral anticoagulants, and one patient had blood cultures positive at day 37, nearing decannulation.
Conclusion
Subclavian vein access for crescent jugular dual lumen V-V ECLS catheter appears to be safe and feasible with added benefits of decreased bleeding and increased mobility over jugular or femoral-femoral access site for long term V-V ECLS support in COVID-19 related ARDS patients.
Keywords: Extracorporeal membrane oxygenation, mechanical circulatory support, COVID 19, acute respiratory distress syndrome, alternative access
Introduction
Veno-Venous ExtraCorporeal Life Support (V-V ECLS) for COVID-19 related acute respiratory distress syndrome (ARDS) has a mortality rate of between 41.7%–54.4% in single and multi-center retrospective observational studies with a mean duration to decannulation of 14.6–29 days.1–3 Data also supports that either early ECLS cannulation prior to mechanical ventilation or less than 7 days of mechanical ventilation prior to initiation of V-V ECLS support is associated with higher survival rates.2,4 Thus, mobility becomes an important factor to consider for patients who are cannulated on high flow nasal cannula oxygen (HFNC) or bilevel positive airway pressure (BIPAP) support and those who require prolonged support to avoid intensive care unit related neuromuscular weakness. Further, femoral V-V ECLS portends an increased bleeding risk and also has higher infections rates than internal jugular vein (IJV) or subclavian vein (SV) access. While IJV is superior to femoral vein access route relative to infection, experience has shown restricted head mobility, increased bleeding with head movement and increased catheter migration ie deep diving with IJV access. Technique, outcomes and safety data for a series of five patients who underwent 32F Crescent jugular dual-lumen (MC3 Cardiopulmonary Dexter, MI, USA) V-V ECLS cannulation via right SV access is described.
Method
A single center retrospective study of five patients with COVID 19 related ARDS who underwent V-V ECLS cannulation from right SV access with the Crescent 32 French dual lumen V-V ECLS catheter, traditionally placed from IJV access site, was executed from September 2021 till March 2022. Chest X ray (CXR) for all five patients with COVID 19 related ARDS prior to V-V ECLS initiation is shown (Figure 1).
Figure 1.
(a–e). Chest X rays for all five patients with COVID-19 related acute respiratory distress syndrome prior to subclavian vein (SV) veno-venous extracorporeal life support (V-V ECLS) initiation.
While at the time these patients consented to the procedure, obtaining written informed consent from the patients whose chart will be reviewed was not required as this study met the FDA’s criteria for consent to be waived, according to its guidance document IRB Waiver or Alteration of Informed Consent for Clinical Investigations Involving No More than Minimal Risk to Human Subjects (July 2017).
Patient related factors extracted included; age, gender and body mass index (BMI). Procedure related characteristics included days Crescent dual-lumen cannula remained in place and outcomes of upper extremity deep venous thrombosis (DVT), vascular complications, bacteremia, and evidence of superior vena cava syndrome were evaluated.
Technique for subclavian vein crescent jugular dual-lumen catheter cannulation
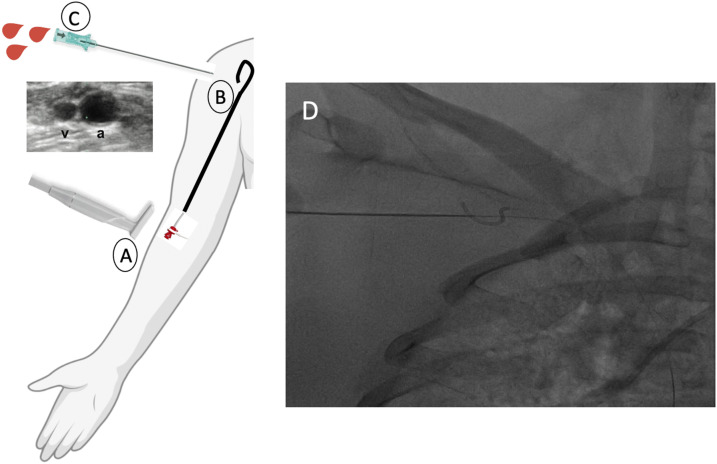
Ultrasound guided micro-puncture access is obtained in the right brachial vein and a 4–6F sheath is placed. A 4–6F omniflush or pigtail catheter is taken from the brachial vein access into the right SV and a selective SV venogram is obtained to ensure the access is not at the cephalic- basilic vein T junction and in the large axillary/subclavian vein. Under direct fluoroscopic visualization, a 7 cm. 021inch micro-puncture needle is taken into the pectoralis groove parallel to the SV and needle is targeted at the loop of the omniflush or pigtail catheter. Once SV puncture is confirmed with venous blood return, a 6F sheath is inserted into the SV. Next, a .035 inch J-guidewire is inserted into SV and into the inferior vena cava (IVC), over which a pigtail catheter is inserted and switched for a. 035 inch stiff lunderquist guidewire. Serial dilation is performed over the lunderquist guidewire and the selected crescent catheter is inserted distally into the IVC making note that the radiopaque markers are at the correct position (Figures 2 and 3). Crescent jugular dual lumen catheter cannulated at the right SV shown ex vivo in four of five cannulated patients (Figure 4).
Figure 2.
(a) Ultrasound guided micro-puncture access is obtained in the right brachial vein (BV) and a 4–6F sheath is inserted. (b). A 4–6F omniflush or pigtail catheter is taken from BV access and parked in SV, a selective venogram is obtained to avoid cephalic-basilic T junction. (c). A 7 cm .021gauge micro-puncture needle is advanced in pectoralis groove. (d). Under direct fluoroscopic guidance, the needle is targeted in the loop of the omniflush or pigtail catheter. Wire entry into subclavian vein is confirmed under fluoroscopy, and 4–6F sheath is inserted into the SV.
Figure 3.
(a–b). After following steps described in Figure 2, a pigtail catheter is advanced into the inferior venal cava (IVC) over a guidewire and exchanged for a .035 inch lunderquist guidewire. Serial dilatation is performed and the crescent jugular dual lumen catheter is inserted into IVC taking note of the radiopaque markers for proper positioning. (c–d). Radiopaque markers for proper positioning. 1. Superior vena cava drainage marker. 2. Radiopaque markers at infusion site. 3. IVC drainage maker. 4. Catheter tip marker.
Figure 4.
(a–c). Ex vivo demonstration of right SV 32F crescent jugular dual-lumen VV-ECMO catheter in four of five patients in the study.
Results
Mean age of the five patient case series was 41.2 years, all were obese with a body mass index of >30 kg/m2 (mean 45.2 kg/m2), and average cannulation duration of 24.2 days (range 5 days–47 days). None had any vascular complications that required surgical or endovascular intervention or minor complications such as hematoma requiring manual compression. One patient (patient 2) had subclavian/axillary vein thrombosis with resolution after 3 months of direct-acting oral anti-coagulant (DOAC) and one had blood cultures positive for enterococcus faecalis nearing decannulation (patient 4, day 37 post insertion). Importantly, none developed head/neck venous obstruction or superior vena cava syndrome. One patient (patient 5) did not survive due to superimposed necrotizing cavitary Enterobacter pneumonia and recurrent pneumothorax despite chest tube placement (Table 1).
Table 1.
Patient characteristic and clinical outcomes of 32F crescent jugular dual lumen VV-ECMO cannulation via right subclavian vein access.
| Case | Age | Sex | BMI (kg/m2) | Days in place | Upper extremity DVT | Superior vena cava syndrome | Blood culture | Vascular complication | Special indication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | 39.4 | 47 | No | No | Negative | No | |
| 2 | 36 | F | 66.1 | 8 | Yes subclavian/axillary vein | No | Negative | No | |
| 3 | 57 | M | 41.4 | 5 | No | No | Negative | No | |
| 4 | 29 | F | 45.8 | 42 | No | No | Positive Enterococcus faecalis day 37 | No | G2 P1-0-0-1 female. 76 days on femoral-femoral VV ECMO with subsequent Right IJV crescent to improve mobility and PT. Crescent dislodgment and bleeding day 41 necessitating right SV crescent cannula for remainder 39 days. |
| 5 | 50 | M | 33.1 | 19 | No | No | Negative | No | |
| ∼41.2 | ∼45.2 | ∼24.2 | |||||||
∼ Average BMI, body mass index; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; F, female; IJV, internal jugular vein; Kg, kilogram; M, male; M2, meters squared; PT, physical therapy; SV, subclavian vein.
Discussion
The crescent jugular dual lumen catheter is the first FDA approved long term V-V ECLS catheter ranging in size from 24 to 32F allowing; cannulation with one access site, accurate placement with radiopaque markers, and improved patient mobility while delivering enhanced flow dynamics.5 The first published case series of 54 patients with the crescent dual lumen IJV catheter showed a favorable safety profile with no serious life-threatening adverse events and low number of minor cannula related complications.6 The present study also showed favorable safety results with use of this catheter at the right SV access. Only one patient had an upper extremity deep venous thrombosis which resolved with short duration of anticoagulation and only one patient had line related bacteremia. Importantly, no bleeding complications or SVC syndrome occurred and improved mobility and increased sedation holidays could be achieved in young patients with immunocompromised states or co-morbidities that rendered them V-V ECLS dependent for prolonged period of time.
Advantages of subclavian vein access for long-term crescent jugular dual-lumen V-V ECLS catheter
Femoral ECLS cannulation site has been associated with an infection rate of between 7 and 20%.7–9 Obesity and malnourishment can increase femoral access site infections and frequent patient positioning changes to avoid skin pressure ulcers can increase bleeding and hematoma formation. Femoral venous catheterization is also associated with a significantly higher overall infection rate than subclavian central vein access, (19.8% vs 4.5%, p < .001) respectively, in one study of 289 randomized and concealed prospective patients.10 In another 53 patient V-V ECLS study, of which 51 cannulated via femoral-femoral access, 17% (Ref. 9) underwent surgery for bleeding complications and 13.2% (Ref. 7) had at least one bloodstream infection.11 IJV access site can limit head mobility and has an increased risk for either deep diving, dislodgment or bleeding as was seen in patient 4 (Table 1). Initial experience with single-jugular vein cannulation Avalon elite bicaval dual lumen catheter (Avalon Laboratories, Rancho Dominguez, CA) in 11 consecutive patients showed two patients (18%) had proximal cannula dislodgement requiring repositioning and one had acute cannula related thrombosis.12
Early rehabilitation in patients on ECLS support is associated with decreased rates of delirium, shorter mechanical ventilation duration, early return to independent functioning and shorter length of hospital stay.13 Survival benefit has also been shown from early cannulation with V-V ECLS in COVID-19 related ARDS patients. Kunavarapu et al. reported findings of 52 COVID-19 ARDS patients treated early with V-V ECLS cannulation and depicted a survival rate of 56% (n = 29), with further improvement in survival rate to 75% in patients placed on V-V ECLS prior to mechanical ventilation (9 of 12).4 SV access with the crescent jugular dual-lumen catheter is especially appealing in patients that are on high flow oxygen or BIPAP but not yet intubated as it allows superior patient mobility than IJV or femoral-femoral access.
Left SV access for V-V ECLS has been described with bicaval Avalon Elite cannula, however it is described with use of fluoroscopy and access guided by pulmonary artery catheter from IJV along with a transesophageal echocardiogram.14 Risks associated with SV access can include; dislodgment during ambulation or physical therapy, deep vein thrombosis of the SV or axillary vein, upper extremity edema, SVC syndrome, and requirement of different image modalities for cannulation or venous injury.15 Though only one upper extremity deep vein thrombosis was noted in current case series, there was no incidence of catheter dislodgement or vascular site bleeding complications or SVC syndrome and only one blood stream infection occurred at day 37 (patient 4). Further, our novel percutaneous technique for cannulation mitigated use of any additional imaging modalities for successful cannulation.
Limitations
While alternative access route for Crescent jugular dual-lumen V-V ECLS catheter via the right SV was safe, allowed improved mobility and had low rate of vascular complications in these 5 case series of patients, further prospective investigation with larger patient sample size would strengthen the preference for this route versus jugular. Additionally, all five patients were obese with a BMI >30 kg/m2, clinical outcomes utilizing this technique in non-obese patients is also necessary. Finally, while the focus was on safety, outcomes and feasibility of this technique, a comparative study with traditional femoral-femoral V-V ECLS or Crescent V-V ECLS via jugular route and differences in mechanical ventilator settings prior to cannulation and calculations of re-circulation after cannulation would be a relevant factor to explore further. Although this technique was only applied to COVID-19 related ARDS patients, outcomes in non-COVID patients should also be undertaken.
Conclusion
Subclavian vein V-V ECLS cannulation with the Crescent jugular dual-lumen catheter is technically feasible and has a favorable safety profile with low rates of vascular complications, low infection rates and is practical for patients requiring long term V-V ECLS support allowing higher degree of patient mobility than jugular or femoral access. Larger randomized studies to validate these findings is warranted.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Sehrish Memon https://orcid.org/0000-0003-1087-4011
References
- 1.Tabatabai A, Ghneim MH, Kaczorowski DJ, et al. Mortality risk assessment in COVID-19 venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2021; 112(6): 1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Supady A, Taccone FS, Lepper PM, et al. Survival after extracorporeal membrane oxygenation in severe COVID-19 ARDS: results from an international multicenter registry. Crit Care 2021; 25: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biancari F, Mariscalco G, Dalén M, et al. Six-month survival after extracorporeal membrane oxygenation for severe COVID-19. J Cardiothorac Vasc Anesth 2021; 35(7): 1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunavarapu C, Yeramaneni S, Melo J, et al. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int J Artif Organs 2021; 44(11): 861–867. [DOI] [PubMed] [Google Scholar]
- 5.Medtronic . ECLS Catheters - Crescent Jugular Dual Lumen Catheter. Medtronic, https://www.medtronic.com/us-en/healthcare-professionals/products/cardiovascular/extracorporeal-life-support/crescent-jugular-dual-lumen-catheter.html [Google Scholar]
- 6.Fleet D, Morris I, Faulkner G, et al. Experience with the crescent® cannula for adult respiratory VV ECMO: a case series. Perfusion 2021; 13: 10.1177/02676591211031462 [DOI] [PubMed] [Google Scholar]
- 7.Lamb KM, DiMuzio PJ, Johnson A, et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J Vasc Surg 2017; 65: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 8.Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg 2013; 28: 190–192. [DOI] [PubMed] [Google Scholar]
- 9.Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011; 92: 626–631. [DOI] [PubMed] [Google Scholar]
- 10.Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically Ill patients: a randomized controlled trial. JAMA 2001; 286(6): 700–707. [DOI] [PubMed] [Google Scholar]
- 11.Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care 2013; 17: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermudez CA, Rocha RV, Sappington PL, et al. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg 2010; 90(3): 991–995. [DOI] [PubMed] [Google Scholar]
- 13.Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18(1): R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafii AE, McCurry KR. Subclavian insertion of the bicaval dual lumen cannula for venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2012; 94(2): 663–665. [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, MacHannaford JC, Chamogeorgakis T, et al. Ambulatory extracorporeal membrane oxygenation with subclavian venoarterial cannulation to increase mobility and recovery in a patient awaiting cardiac transplantation. Proc (Bayl Univ Med Cent) 2017; 30(2): 224–225. [DOI] [PMC free article] [PubMed] [Google Scholar]