Abstract
Melioidosis is an infectious disease caused by the saprophytic gram-negative rod Burkholderia pseudomallei. The aim of this study was to establish and characterize a murine model of melioidosis to provide a basis for further investigations on the pathogenesis of the disease. After intravenous infection with B. pseudomallei, C57BL/6 mice were found to be significantly more resistant than BALB/c mice. There was a marked organotropism of B. pseudomallei for the spleen and liver in both strains of mice, with the highest bacterial load in the spleen. Electron microscopic investigations of the spleen clearly demonstrated intracellular replication within membrane-bound phagosomes. Electron micrographs of the liver provided evidence that B. pseudomallei-containing phagosomes in hepatocytes fuse with lysosomes, leading to degradation of bacteria. In both strains of mice, the course of infection was highly dependent on the infective dose and the bacterial strain used, ranging from death within a few days to death after several weeks. In comparison with BALB/c mice, the bacterial counts in C57BL/6 mice were decreased 12 h after infection, which is suggestive of an innate immune mechanism against B. pseudomallei in this early phase of infection contributing to the lower susceptibility of C57BL/6 mice. BALB/c mice developed a more pronounced lymphopenia, granulocytosis, and splenomegaly at a lower infective dose compared to C57BL/6 mice. Analysis of the antibody response against B. pseudomallei 11 days after infection revealed a significantly higher immunoglobulin G2A (IgG2a)/IgG1 ratio in C57BL/6 mice than in BALB/c mice, indicating that a T helper type 1 immune response is associated with resistance to infection with B. pseudomallei.
Melioidosis is an infectious disease of humans and animals caused by the saprophytic gram-negative rod Burkholderia pseudomallei. Southeast Asia and northern Australia are main areas where the disease is endemic, the bacterium being isolated from soil and surface waters (17, 29). There is evidence that melioidosis may also be endemic in Africa, the Indian subcontinent, and Central and South America (5), but the disease remains most likely underdiagnosed in areas of the tropics where sophisticated laboratory facilities are not available (5). The clinical manifestations of melioidosis are extremely variable, ranging from acute or chronic localized infections to fulminant septicemias (6). Severe septicemic melioidosis is typically associated with underlying diseases such as diabetes mellitus and chronic renal failure (4, 17), but it may also occur in previously healthy individuals. Epidemiological studies suggest that mild or inapparent infections which are manifested by seroconversion only are common (26). The proportion of infected but healthy individuals who may harbor viable B. pseudomallei is unknown. Long periods of latency and frequent relapses after antibiotic treatment are characteristic of melioidosis (6). In most cases, humans and animals are thought to acquire the infection by inoculation of environmental organisms into minor cuts or abrasions after contact with soil and muddy waters (7).
B. pseudomallei has an extremely broad host range. Beside humans, infections in rodents, sheep, goats, pigs, horses, and kangaroos have been reported (9, 11, 18, 32). In vitro studies have shown that B. pseudomallei can invade epithelial cells and grow intracellularly within phagocytes (16, 24). It has recently been shown that the type II O-antigenic polysaccharide moiety of the B. pseudomallei lipopolysaccharide (22) is essential for resistance against complement-mediated bacteriolysis and contributes to virulence in hamsters, guinea pigs, and infant diabetic rats (8a). Other putative virulence factors of B. pseudomallei include a siderophore (38), several relatively uncharacterized extracellular enzymes (1), and a heat-labile exotoxin (15). Recently, we identified a constitutively expressed exopolysaccharide (21, 31) and a heat-stable cytotoxic glycolipid (14), which might have implications for the pathogenesis of melioidosis.
For many bacterial infections, the mouse model has proven to be invaluable for studies of bacterial virulence factors and host-parasite interactions. The aim of this study was to establish and characterize a murine model of melioidosis for further investigations on the pathogenesis of the disease. We examined the susceptibility of four mouse inbred strains after intravenous infection. The highly susceptible BALB/c strain and the more resistant C57BL/6 strain were chosen to determine the kinetics of the bacterial colony counts in various organs over a period of several weeks and to perform electron microscopic studies to locate B. pseudomallei during infection. Changes in blood cell composition and the induction of a humoral immune response during infection were compared in both mouse strains.
MATERIALS AND METHODS
Animals.
Female 8- to 10-week-old C57BL/6, BALB/c, C3H/HeN, and DBA/2 mice were obtained from Charles River Wiga (Sulzfeld, Germany). Animals were maintained under specific-pathogen-free conditions and were provided with food and water ad libitum.
Bacteria.
The clinical l-arabinose-nonassimilating B. pseudomallei NCTC 7431 was obtained from the National Collection of Type Cultures. B. pseudomallei E8 is an l-arabinose-nonassimilating environmental strain isolated from soil in northeast Thailand (34) and was obtained from A. Simpson, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Bacteria were grown on Columbia agar at 37°C for 24 h. Colonies were then harvested using tryptic soy broth, centrifuged at 10,000 × g, suspended in tryptic soy broth containing 20% glycerol, and frozen immediately in 0.2-ml aliquots at a concentration of 109 CFU per ml at −70°C.
Infection of animals and enumeration of CFU in various organs.
For each infection experiment, an aliquot of the B. pseudomallei suspension was freshly thawed from the stock. Bacterial cells were diluted in buffer A (0.01 M potassium phosphate buffer made isotonic with saline [pH 7.5]) to obtain the appropriate concentration. By this method, commonly used in other infection models, excellent reproducibility of the B. pseudomallei infection doses was obtained. A bacterial suspension of 0.2 ml was injected into the lateral tail vein. The actual number of bacteria administered was determined for each experiment by plating 0.1 ml of serial 1:10 dilutions on Columbia agar and counting CFU after 48 h. At various time points after injection, the number of CFU present in blood and various organs was determined. Organs were aseptically removed and homogenized in 0.4 or 1 ml (depending on organ size) of sterile buffer A containing 1% (wt/vol) Tergitol TMN 10 (Fluka, Buchs, Switzerland) and 0.5% (wt/vol) BSA (bovine serum albumin; Merck, Darmstadt, Germany) in a tissue homogenizer (glass potter) (Braun, Melsungen, Germany). A 0.1-ml sample of appropriate 1:10 dilutions was plated out, and the number of CFU was determined as described above. Using this method, the detection limit was 3 to 10 CFU per organ, depending on the size of the organ. For the determination of blood CFU, an undiluted 0.1 ml sample was plated out and the number of CFU was determined as described above.
Determination of the LD50.
Groups of six mice were infected with appropriate dilutions of bacteria as described above and observed for death for 6 weeks. The 50% lethal dose (LD50) was calculated by the method of Reed and Muench (25).
Blood cell parameters.
For differential blood counts, animals were bled through the orbital plexus, and smears were made, fixed in 100% methanol, and stained with Wright’s and Giemsa stains (Sigma). For leukocyte counts, blood was analyzed on a Coulter S Plus IV (Coulter Electronics, Krefeld, Germany).
ELISA methods.
The various antibody isotypes with specificity for B. pseudomallei antigens from mouse sera were detected in a previously described enzyme-linked immunosorbent assay (ELISA) (31), with some modifications. Briefly, single U-shaped wells of nonflexible polystyrol microtiter plates were coated for 2 h with 20 μl of B. pseudomallei cells (2 × 108 cells per ml) which had been heat treated (80°C for 1 h in buffer A) and sonicated with the microtip of a Branson Sonifier 250 (10 min; output setting of 9 and 50% duty cycle). After washing steps with buffer A and a blocking step with buffer A-BSA (buffer A containing 1% [wt/vol] BSA) for 30 min, mouse sera and a standard were diluted in buffer A-BSA and 20 μl was incubated for 2 h. For the determination of the different isotypes, plates were washed and incubated with 10 μl of either biotin-labeled rabbit anti-mouse immunoglobulin G1 (IgG1; 1:5,000 in buffer A-BSA; Zymed, San Francisco, Calif.), rabbit anti-mouse IgG2a (1:1,000; Southern Biotechnology Associates, Birmingham, Ala.), rabbit anti-mouse IgG2b (1:750; Zymed), rabbit anti-mouse IgG3 (1:1,000; Zymed), or goat anti-mouse IgM (1:1,000; Southern Biotechnology Associates) for 60 min. Microtiter plates were then developed with streptavidin coupled to β-galactosidase. 4-Methylumbelliferyl-β-d-galactopyranoside was used as substrate, and the fluorescent product was measured as relative fluorescence units (RFU). A high-titered mouse serum with detectable antibodies of all isotypes tested in this ELISA was used as a standard. For each isotype assay, a standard curve was generated from appropriate dilutions of this serum. The RFU value of the highest antibody concentration falling within the linear portion of the different standard curves was arbitrarily set as 10 ELISA units. The specific isotype concentration of each serum sample was determined from the standard regression curve constructed for each assay and expressed as ELISA units.
Electron microscopic studies.
At different time points after infection with B. pseudomallei, C57BL/6 and BALB/c mice were ether anesthetized and 100 μl of buffer A containing 3% glutaraldehyde and 5% formaldehyde were injected intravenously into the lateral tail vein. Animals were killed after injection, and the spleen and liver were removed. Organs were cut into small cubes and kept in the same fixation buffer used for injection overnight at 4°C. Tissue cubes were further fixed with 1% aqueous osmium tetroxide for 1 h at room temperature, washed with phosphate-buffered saline, and dehydrated with a graded series of acetone. Samples were infiltrated with Spurr’s epoxy resin by the method of Spurr (30). Ultrathin sections were cut with glass knives and counterstained with uranyl acetate and lead citrate. Sections were examined in a Zeiss TEM910 transmission electron microscope at an acceleration voltage of 80 kV and at calibrated magnifications.
Statistical analyses.
The statistical significance of the difference of the mean between experimental groups was determined by the two-tailed Student t test. P values of <0.05 were considered significant. Data are presented as means ± standard deviations (SD).
RESULTS
Susceptibility of different mice strains to intravenous infection with B. pseudomallei.
To examine the role of the host in B. pseudomallei infection, the well-characterized inbred mouse strains C57BL/6 (H-2b haplotype, Bcg/Ity/Lshs phenotype), C3H/HeN (H-2k; Bcg/Ity/Lshr), DBA/2 (H-2d; Bcg/Ity/Lshr), and BALB/c (H-2d; Bcg/Ity/Lshs) were infected by the intravenous route with B. pseudomallei NCTC 7431 (originally a clinical isolate), and LD50 values were determined after 6 weeks. C57BL/6 mice showed the most resistant phenotype (LD50 = 105) and were significantly more resistent compared to the most susceptible BALB/c mice (LD50 = 103). The LD50s of C3H/HeN and DBA/2 mice were 6 × 103 and 3 × 103, respectively. From the LD50 experiments, it is obvious that the Nramp1 gene (36), which is located at the Ity/Bcg/Lsh locus on mouse chromosome 1, does not confer resistance to B. pseudomallei infection. Both B. pseudomallei-resistant C57BL/6 mice and susceptible BALB/c mice are Itys.
B. pseudomallei infection in various organs of BALB/c and C57BL/6 mice.
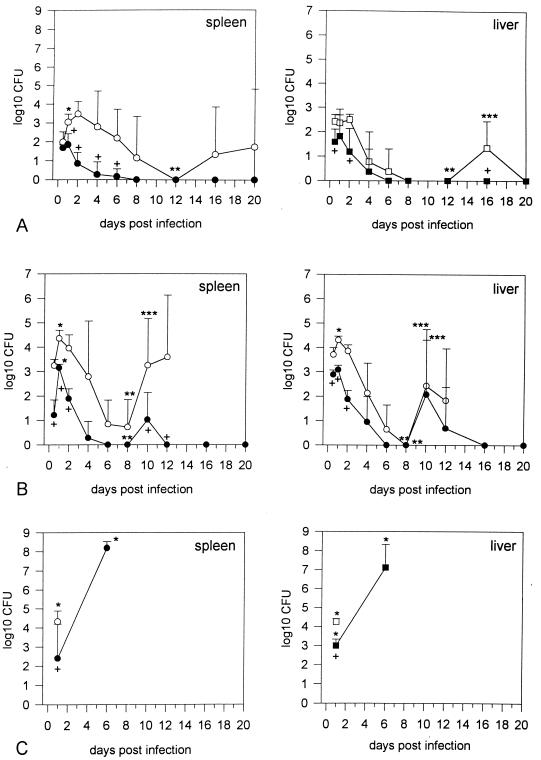
To address the question of whether the differences in susceptibility of different strains of mice correspond to bacterial burden and to examine possible tissue specificity, the kinetic of the bacillary load in various organs of C57BL/6 and BALB/c mice was determined. Both strains of mice were intravenously infected with different doses of B. pseudomallei NCTC 7431, and the numbers of bacteria in spleen, liver, lung, kidneys, and blood were determined at different time points after infection. Figure 1A shows bacterial counts in spleens and livers of the resistant and susceptible strains after infection with 2 × 102 CFU. Only very few bacteria were detected in the lungs and kidneys of BALB/c mice (data not shown). No bacteria were detected in the lungs and kidneys of C57BL/6 mice at any time point. Bacteria were not detectable in the blood in both strains of mice. In the spleen and liver, but especially in the spleen, the number of CFU at different time points was significantly higher in BALB/c mice than in C57BL/6 mice.
FIG. 1.
(A) Course of bacterial counts in BALB/c (○) and C57BL/6 (●) mice in spleen and liver after intravenous infection with 2 × 102 CFU of B. pseudomallei NCTC 7431. Values are given as the mean ± SD for six mice. Asterisks indicate significant (P < 0.05) increase or decrease in CFU of the various organs (∗, day 0.5 versus day 1; ∗∗, day 1 versus day 12; ∗∗∗, day 12 versus day 16). Crosses indicate significant (P < 0.05) differences in organ CFU between BALB/c and C57BL/6 mice at single time points. (B) Course of bacterial counts in BALB/c (○) and C57BL/6 (●) mice in spleen and liver after intravenous infection with 5 × 103 CFU of B. pseudomallei NCTC 7431. In BALB/c mice, organ CFU were not documented beyond day 12 p.i. because of progressive death of animals. Values are given as the mean ± SD for six mice. Asterisks indicate significant (P < 0.05) increase or decrease in CFU of the various organs (∗, day 0.5 versus day 1; ∗∗, day 1 versus day 8; ∗∗∗, day 8 versus day 10). Crosses indicate significant (P < 0.05) differences in organ CFU between BALB/c and C57BL/6 mice at single time points. (C) Bacterial counts in BALB/c (○) and C57BL/6 (●) mice in spleen and liver after intravenous infection with 2 × 102 CFU of mouse-passaged B. pseudomallei NCTC 7431. BALB/c mice started to die beyond day 4 p.i.; C57BL/6 mice started to die beyond day 6. Values are given as the mean ± SD for four to six mice. Asterisks indicate significant (P < 0.05) differences from the respective values obtained with the nonpassaged strain NCTC 7431 at the same time points as shown in panel A. Crosses indicate significant (P < 0.05) differences in organ CFU between BALB/c and C57BL/6 mice.
A steep increase in the bacterial load of the spleen of BALB/c mice was detected in the first 24 h postinfection (p.i.). Interestingly, the bacterial burden in the spleens and livers of C57BL/6 mice was lower than in BALB/c mice during this early phase of infection, indicating an innate immune mechanism against B. pseudomallei contributing to the lower susceptibility of C57BL/6 mice. The course of the bacterial load in BALB/c mice was indicative of a biphasic pattern with a second increase of CFU around day 16, whereas C57BL/6 mice completely cleared the bacteria within the first few days.
After infection with 5 × 103 CFU of strain NCTC 7431, there was also a marked organotropism for the spleen and liver in both strains of mice (Fig. 1B), whereas again no significant bacterial numbers were detected in the lungs and kidneys of most animals (data not shown). It was only in BALB/c mice that very few bacteria (1 to 6 CFU per 0.1 ml) were detectable in the blood in some animals during the first 2 days p.i. At other time points no bacteria could be detected in the blood in both strains of mice. Figure 1B clearly shows a biphasic course of the bacterial load in spleens and livers in both strains. In C57BL/6, there was a peak at day 1 and no bacteria were detected between days 6 and 8. The second peak was reached at day 10, and in the following days the bacteria were finally cleared. In BALB/c mice, the first peak was also reached at day 1 and again a significant reduction was seen between days 6 and day 8. In contrast to C57BL/6 mice, BALB/c mice did not clear the bacteria after the second increase and succumbed to infection. There was a significant difference between the two mouse strains in the bacterial load of the spleen and liver in the first 12 h p.i. After infection with 5 × 103 CFU, 3 of 12 BALB/c mice developed paresis of both hind legs at days 10 and 12 p.i. Interestingly, these neurological signs were also reported for infected sheep and goats (33).
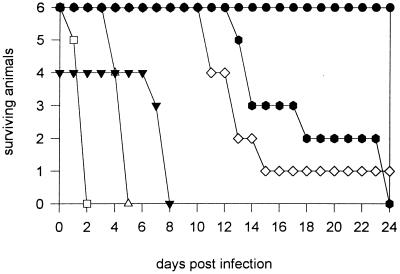
To address the question of whether the biphasic course of infection is a constitutive characteristic of strain NCTC 7431, we isolated strain NCTC 7431 from the spleen of a BALB/c mouse during the second peak at day 10 and infected BALB/c and C57BL/6 mice with the mouse-passaged isolate. Interestingly, in both strains of mice we observed a dramatic increase in virulence as well as a loss of the biphasic pattern (Fig. 1C). Infection of BALB/c mice with 2 × 102 CFU of strain NCTC 7431 after single mouse passage led to significantly higher bacterial numbers in the spleen and liver after 24 h compared to the same dose of the nonpassaged strain (Fig. 1A), and animals succumbed to infection between days 4 and 5. Infection of C57BL/6 mice with 2 × 102 CFU of passaged strain NCTC 7431 led to very high bacterial numbers at day 6, and animals died within the next 2 days. Although the course of infection changed dramatically using the mouse-passaged strain, the previously observed difference in bacterial numbers between C57BL/6 and BALB/c mice with the nonpassaged strain was also detectable in the first 24 h p.i. with the much more virulent passaged strain. Figure 2 summarizes the survival curves of BALB/c and C57BL/6 mice after infection with different infection doses of the nonpassaged and passaged strain NCTC 7431.
FIG. 2.
Survival curves of BALB/c (white symbols) and C57BL/6 mice (black symbols) after intravenous infection with nonpassaged and passaged B. pseudomallei NCTC 7431. ●, 5 × 103 CFU of NCTC 7431;  , 3 × 105 CFU of NCTC 7431; ◊, 5 × 103 CFU of NCTC 7431; □, 3 × 105 CFU of NCTC 7431; ▾, 2 × 102 CFU of mouse-passaged strain NCTC 7431; ▵, 2 × 102 CFU of mouse-passaged strain NCTC 7431.
, 3 × 105 CFU of NCTC 7431; ◊, 5 × 103 CFU of NCTC 7431; □, 3 × 105 CFU of NCTC 7431; ▾, 2 × 102 CFU of mouse-passaged strain NCTC 7431; ▵, 2 × 102 CFU of mouse-passaged strain NCTC 7431.
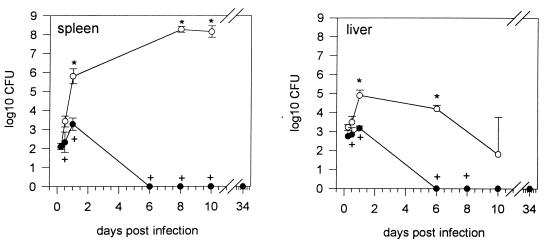
Since infections with B. pseudomallei are acquired from the environment and mammalian reservoirs are epidemiologically not important (7), we used a B. pseudomallei soil isolate (E8) to further elucidate the course of B. pseudomallei infection in our model. There was also a marked organotropism for the spleen and liver, with very few bacteria in the lung and kidney (data not shown). Figure 3 shows the results for the spleen and liver after infection of BALB/c and C57BL/6 mice with 5 × 103 CFU. In BALB/c mice, the bacterial load of the spleen and also the liver was significantly higher from 24 h p.i. onward compared to nonpassaged strain NCTC 7431 (Fig. 1B). A biphasic course of bacterial numbers was not observed, and animals succumbed to infection between days 12 and 17 p.i. There was no significant difference in the early course of infection in C57BL/6 mice compared to the course of infection of the nonpassaged strain NCTC 7431 (Fig. 1B). No bacteria were recovered from organs even after more than 1 month p.i. The previously observed difference in bacterial load between C57BL/6 and BALB/c mice detectable at very early time points (12 h p.i.) was also evident with strain E8. No bacteria were cultured from blood samples from both strains of mice. Paresis of both hind legs was observed in approximately 25% of BALB/c mice. The high virulence of nonpassaged strain E8 became also evident when four of six BALB/c mice died within 6 weeks after infection with only 10 CFU of strain E8.
FIG. 3.
Course of bacterial counts in BALB/c (○) and C57BL/6 (●) mice in spleen and liver after intravenous infection with 5 × 103 CFU of B. pseudomallei E8. In BALB/c mice, organ CFU were not documented beyond day 10 p.i. because of progressive death of animals. Values are given as the mean ± SD for five to six mice. Asterisks indicate significantly higher (P < 0.05) CFU values compared to the respective values for nonpassaged strain NCTC 7431 at the same time points as in Fig. 1B. Crosses indicate significant (P < 0.05) differences in organ CFU between BALB/c and C57BL/6 mice at single time points.
The influence of mouse passage on the virulence of strain E8 was examined in BALB/c and C57BL/6 mice infected with 2 × 102 CFU of E8. In contrast to strain NCTC 7431, there was no significant increase in bacterial numbers after mouse passage (data not shown). BALB/c mice succumbed to infection in a time range (weeks 2 to 3 p.i.) similar to that for the nonpassaged strain. In C57BL/6 mice, sporadic cases of high bacterial counts (105 to 107 CFU) were detected in the spleen at day 8, whereas most animals showed no detectable bacteria. In one experiment, C57BL/6 mice were infected with 2 × 102 CFU of double-passaged strain E8 and the observation period was extended to 3 months. Two of six animals died at days 40 and 54 p.i., respectively. These animals showed a massive splenomegaly with macroscopically visible massive abscesses and bacterial numbers in the range of 107 to 108 CFU per spleen.
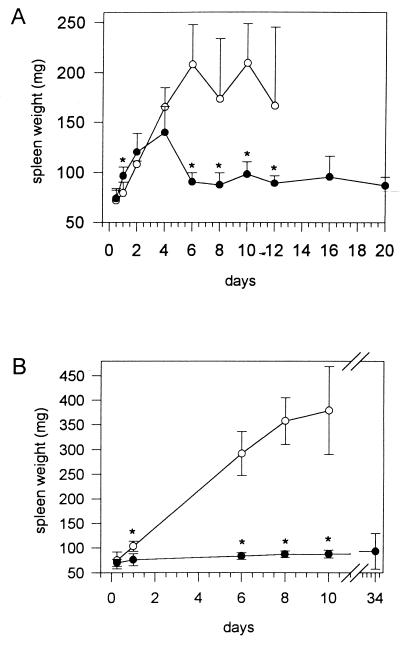
Determination of the spleen weight after infection with 5 × 103 CFU of strain NCTC 7431 (Fig. 4A) or strain E8 (Fig. 4B) revealed the development of a marked splenomegaly in BALB/c mice. However, after infection with 5 × 103 CFU of strain E8, the increase in spleen weight was significantly higher than after infection with nonpassaged NCTC 7431. After infection with strain NCTC 7431, C57BL/6 mice exhibited a steeper increase in spleen weight in the first 3 days compared to BALB/c mice. From day 4 on, the spleen weight of C57BL/6 mice decreased and remained only slightly elevated. When C57BL/6 mice were infected with 106 CFU of strain 7431 or with 2 × 102 CFU of mouse-passaged strain NCTC 7431, a significant splenomegaly developed (data not shown). No changes in spleen weight were noticed in C57BL/6 mice after infection with 5 × 103 CFU of E8 during the observation period. No significant changes in the weight of other organs were observed in the course of infection with either B. pseudomallei strain in both strains of mice.
FIG. 4.
Development of spleen weight after intravenous infection with 5 × 103 CFU of B. pseudomallei NCTC 7431 (A) and B. pseudomallei E8 (B) in BALB/c (○) and C57BL/6 (●) mice. Values are given as the mean ± SD for five to six mice. Asterisks indicate significant (P < 0.05) differences between the two strains of mice.
Hematoxylin-and-eosin-stained sections of the livers and spleens of BALB/c mice were investigated by light microscopy at different time points (data not shown). The formation of small abscesses was detected 12 h p.i. with strain E8 (5 × 103 CFU) in both organs. At day 3 p.i. (102 CFU), both organs showed the formation of small granulomas in addition to the presence of microabscesses. Both type of lesions were most prominent at day 10 p.i. (102 CFU). In some cases, large splenic abscesses replaced most of the spleen. The formation of abscesses and granulomas in spleen and liver was also observed in C57BL/6 mice and correlated with the bacterial burden determined at the different time points.
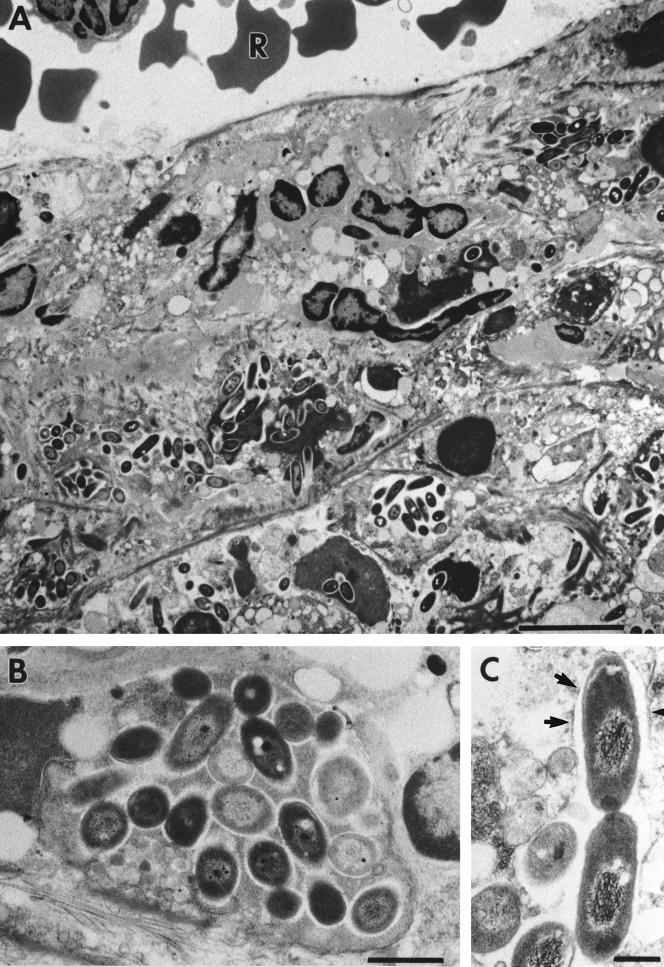
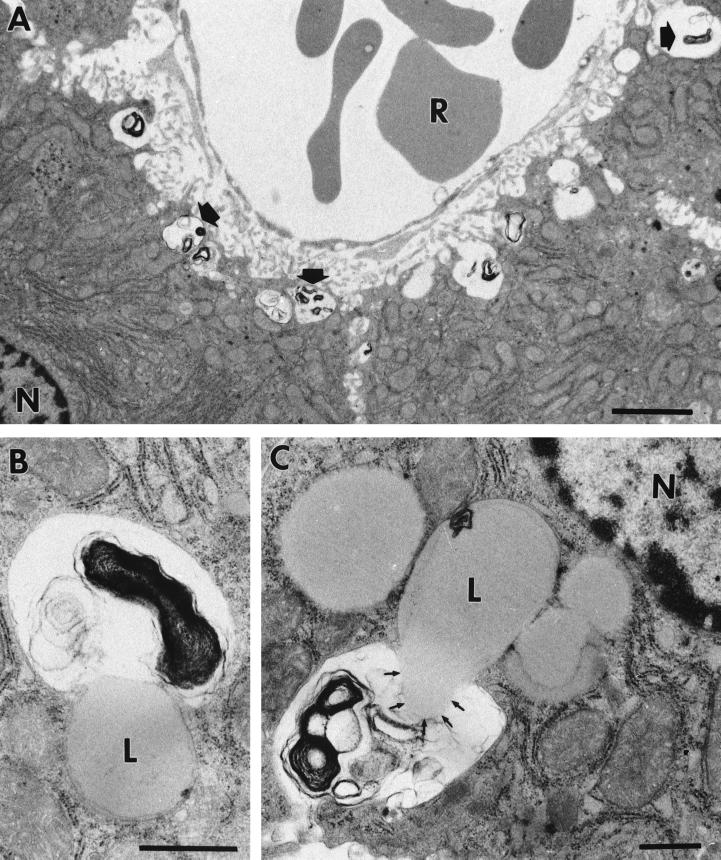
Electron microscopic investigations of lesions of the spleen of C57BL/6 mice 6 days after infection with 2 × 102 CFU of mouse-passaged strain NCTC 7431 (Fig. 5) consistently showed the presence of large numbers of intact bacterial cells, which in many cases appeared densely packed within a membrane. Intracellular replication of B. pseudomallei within phagosomes could be demonstrated. No bacteria were detected in blood-containing sinuses. Electron microscopic analysis of the liver (Fig. 6) revealed invasion of B. pseudomallei into hepatocytes adjacent to the sinus. There was evidence for a phagosome-lysosome fusion process leading to degradation of B. pseudomallei. Similar to what we found in the spleen, no bacteria were detected in sinuses of the liver.
FIG. 5.
Electron microscopic investigation of spleens of C57BL/6 mice at day 6 after infection with 2 × 102 CFU of mouse-passaged strain B. pseudomallei NCTC 7431. Ultrathin sections through lesions of the spleen are shown. Within these lesions, we consistently detected the accumulation of B. pseudomallei (A). (B) Higher magnification of panel A showing densely packed bacteria. (C) Numerous intracellularly dividing bacteria surrounded by phagosomal membranes (arrows). R, erythrocyte; Bars represent 5 μm in panel A, 1 μm in panel B and 0.5 μm in panel C.
FIG. 6.
Electron microscopic investigation of livers of C57BL/6 mice at day 6 after infection with 2 × 102 CFU of mouse-passaged B. pseudomallei NCTC 7431. Ultrathin sections through liver hepatocytes are shown. (A) Hepatocytes showing several phagosomes with degraded bacteria (arrows). In these hepatocytes, we found evidence for a phagosome-lysosome fusion process resulting in the degradation of B. pseudomallei (B and C). (B) Earlier stage. (C) Content of a lysosome floating in a phagosome, resulting in the degradation of B. pseudomallei. L; lysosome, N; nucleus, R; erythrocyte. Bars represent 2 μm in panel A and 0.5 μm in panels B and C.
Blood cell parameters during B. pseudomallei infection.
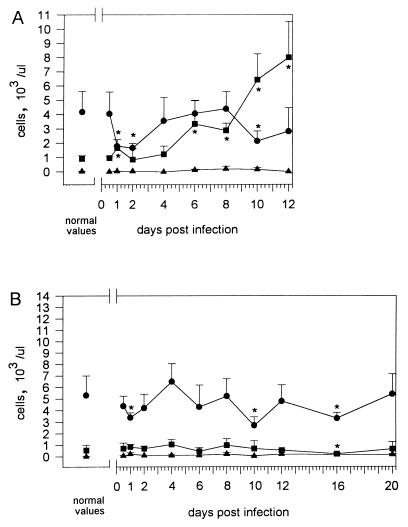
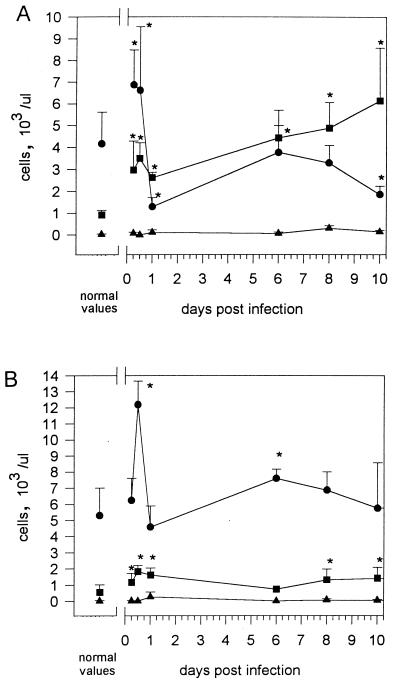
To determine changes in leukocyte count and composition during infection of C57BL/6 and BALB/c mice, blood was analyzed at various time intervals p.i. with either strain NCTC 7431 or strain E8. The results of the differential blood film after infection of BALB/c mice with 5 × 103 CFU of NCTC 7431 (Fig. 7A) revealed an initial rise in granulocytes during the first day and a subsequent slight decrease followed by a continuous increase of granulocytes until day 12. There was an initial sharp decline in lymphocytes, followed by a transient normalization and finally development of a lymphopenia. The results after infection of BALB/c mice with 5 × 103 CFU of E8 (Fig. 8A) revealed a similar pattern, although the most significant difference was a prominent lymphocytosis until 12 h p.i. and an earlier rise in granulocytes. Interestingly, the sharp decline in lymphocytes at 24 h p.i. was also followed by a transient normalization and finally by the development of a lymphopenia as observed after infection with strain NCTC 7431. The normalization of lymphocytes was not accompanied by a decrease in bacterial numbers of strain E8.
FIG. 7.
Changes in blood cell parameters after intravenous infection with 5 × 103 CFU of B. pseudomallei NCTC 7431 in BALB/c (A) and C57BL/6 (B) mice. ■, granulocytes; ●, lymphocytes; ▵, monocytes. Values are given as the mean ± SD for six mice. Asterisks indicate significant (P < 0.05) differences of the various cell types compared to normal values.
FIG. 8.
Changes in blood cell parameters after intravenous infection with 5 × 103 CFU of B. pseudomallei E8 in BALB/c (A) and C57BL/6 (B) mice. ■, granulocytes; ●, lymphocytes; ▴, monocytes. Values are given as the mean ± SD for five to six mice. Asterisks indicate significant (P < 0.05) differences of the various cell types compared to normal values.
No granulocytosis developed after infection with strain NCTC 7431 in C57BL/6 mice. However, at days 1, 10, and 16, a less pronounced but significant lyphopenia developed (Fig. 7B). When C57BL/6 mice were challenged with 106 CFU of strain NCTC 7431, there was also a dramatic lymphopenia in the early phase followed by a transient normalization and finally the development of a lymphopenia together with a granulocytosis (data not shown). After infection of C57BL/6 mice with 5 × 103 CFU of strain E8 (Fig. 8B), we observed as in BALB/c mice an initial rise of lymphocytes but no subsequent lymphopenia. There was only a slight lymphocytosis and granulocytosis at different time points p.i.
Kinetics of antibody response during infection against B. pseudomallei.
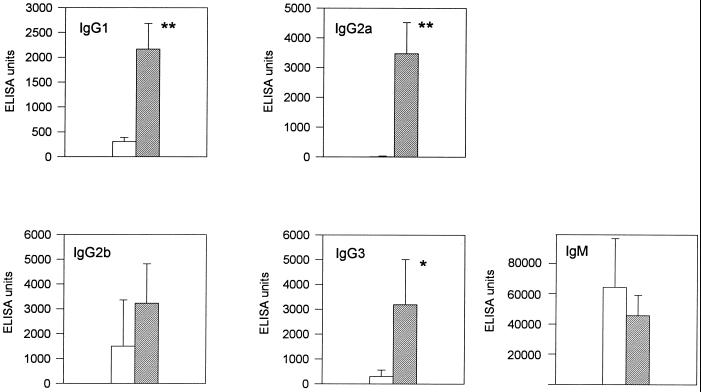
B. pseudomallei-specific IgM and IgG antibodies were determined in an ELISA using B. pseudomallei sonicate as an antigen. Sera of C57BL/6 and BALB/c mice infected with strain E8 (2 × 102 CFU) were analyzed at day 11 p.i. Figure 9 shows that C57BL/6 mice mount a significantly higher IgG1, IgG2a, and IgG3 antibody response. There was no significant difference for the IgM response between the two strains of mice. A striking finding was the fact that almost no specific IgG2a was detected in BALB/c mice. The IgG2a/IgG1 ratio calculated from the ELISA units of single animals was significantly higher in C57BL/6 mice at day 11 p.i. than in BALB/c mice (1.7 for C57BL/6 and 0.03 for BALB/c mice; P < 0.001), indicating a stronger T helper type 1 (Th1) subset activity in C57BL/6 mice during infection. In sera of BALB/c and C57BL/6 mice tested before infection, no significant differences in isotype pattern were observed.
FIG. 9.
Analysis of IgG and IgM responses against B. pseudomallei antigens of BALB/c (open bars) and C57BL/6 (solid bars) mice at day 11 after intravenous infection with 2 × 102 CFU of B. pseudomallei E8. Values are given as the mean ± SD for five to six mice. Asterisks indicate significant (∗, P < 0.05; ∗∗, P < 0.001) differences between the two strains of mice.
DISCUSSION
At present, there are very few studies describing the pathogenesis of melioidosis. It was demonstrated some decades ago that various laboratory animals can be infected with B. pseudomallei, and LD50 values were determined. The hamster proved to be the most susceptible animal (18). Guinea pigs were found to be moderately susceptible with a high degree of variation, whereas rabbits, rats, and monkeys were found to be relatively resistant (18). Recently, experimental infection in rats made diabetic by the injection of streptozocin was reported (37). The determination of LD50s revealed that animals treated this way were much more susceptible to infection than controls. It was reported some time ago that mice can be infected with B. pseudomallei (19) and recently determination of LD50s after intraperitoneal infection of mice was used to differentiate virulent B. pseudomallei from a nonvirulent l-arabinose-assimilating biotype (28). It was found in a small study that outbred mice were more susceptible to intraperitoneal infection after administration of gamma interferon neutralizing antibodies (27). However, only mortality rates were recorded, and neither the kinetic of the infection nor the bacterial load in various organs or any immunological parameters were determined (27).
The comparison of the susceptibility of different genetically defined inbred mouse strains to the intravenous route of infection in this study provided the first step in the characterization of a murine model of melioidosis. The intravenous route of infection was chosen to mimic systemic melioidosis, which is the most common clinical presentation in Thailand (4). The observation that C57BL/6 mice were much more resistant to infection than BALB/c mice demonstrates that susceptibility to B. pseudomallei infection is not linked to the Ity locus, which has been shown to determine the susceptibility of mice to pathogens such as Leishmania donovani, Salmonella typhimurium, and Mycobacterium bovis (36), since both C57BL/6 and BALB/c mice are Itys. Resistance to gram-negative bacterial infections is not an inherent characteristic of C57BL/6 mice compared to BALB/c mice. For example C57BL/6 mice exhibit more severe inflammation after Helicobacter felis infection (19) and are more susceptible to Pseudomonas aeruginosa infection (20) than BALB/c mice. Interestingly, the susceptibility pattern of BALB/c and C57BL/6 mice determined in this study has also been observed after intravenous infection of mice with the gram-negative bacterium Yersinia enterocolitica (13). However, there are several features of the B. pseudomallei infection in BALB/c and C57BL/6 mice which differ clearly from those observed in the yersiniosis model. The organotropism of B. pseudomallei for spleen and liver, especially marked in BALB/c mice in this study, is less pronounced in systemic infection with Y. enterocolitica (2), where BALB/c mice exhibit high bacterial numbers in the lung in addition to spleen and liver. Interestingly, the resistant and susceptible phenotype in B. pseudomallei infection is expressed 12 h p.i., indicating that some innate resistance mechanism(s) against B. pseudomallei must be present at this early time point. This is in contrast to the literature on the murine Yersinia model, where differences in the bacterial load of various organs between BALB/c and C57BL/6 are found only 4 days p.i. and are attributed to specific T-cell responses (2, 13).
An interesting observation in this study is the fact that strain NCTC 7431 no longer exhibited a biphasic course of infection after one mouse passage but had a significantly increased rate of replication and led to death of animals within a few days. It seems likely that the highly virulent organisms selected during the first passage were now capable to resist the primary attack of host defense mechanisms. In contrast, the environmental strain E8, which did not show a biphasic course of infection, was not significantly more virulent after mouse passages.
The development of histopathological lesions in the spleen and liver, most prevalent in BALB/c mice, consisting of abscesses together with granulomatous lesions, is in good accordance with lesions found in human melioidosis (3, 23, 35). Future studies will need to elucidate the cellular basis of the observation that BALB/c mice develop a pronounced splenomegaly compared to C57BL/6. Splenomegaly is also observed in acute systemic melioidosis of humans (12). This study provides in vivo evidence that B. pseudomallei is capable of intracellular replication within phagosomes. This observation is in good accordance with previous in vitro studies demonstrating intracellular replication in phagocytes (16, 24). The determination of viable bacteria during the course of infection in all experiments revealed a higher capacity of the liver to restrict growth of B. pseudomallei compared to the spleen. The finding that hepatocytes are capable to kill B. pseudomallei in phagosomes by fusion with lysosomes describes a mechanism for how the liver can restrict growth of B. pseudomallei. Future studies will have to elucidate the distribution of B. pseudomallei between hepatocytes and Kupffer cells during infection and their roles in host defense.
In the mouse, IgG2a isotype switching is promoted by gamma interferon, a Th1 cell-derived cytokine, whereas IgG1 isotype switching is induced by interleukin-4 released from Th2 cells (10). The significantly higher IgG2a/IgG1 ratio in C57BL/6 than in BALB/c mice is suggestive of a Th1-type immune response in the more resistant mice. However, additional studies are needed to characterize the cellular type of immune response in the two mouse strains more precisely. The experimental system described in this study should help to clarify mechanisms of host resistance in melioidosis and should also be useful in future studies on the virulence of B. pseudomallei and vaccine development.
ACKNOWLEDGMENT
We thank D. Bitter-Suermann for continuous encouragement.
REFERENCES
- 1.Ashdown L, Koehler J M. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J Clin Microbiol. 1990;28:2331–2334. doi: 10.1128/jcm.28.10.2331-2334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2597. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunlage W G, Thuss C J, Walden D C. Four fatal cases of melioidosis in U.S. soldiers in Vietnam. Am J Trop Med Hyg. 1968;17:183–191. doi: 10.4269/ajtmh.1968.17.183. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community acquired septicemia in Northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 5.Dance D A B. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dance D A B. Melioidosis. Rev Med Microbiol. 1990;1:143–150. [Google Scholar]
- 7.Dance D A B. Pseudomonas pseudomallei: danger in the paddy fields. Trans R Soc Trop Med Hyg. 1991;85:1–3. doi: 10.1016/0035-9203(91)90134-k. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg A M, Jr, Scott E M. Melioidosis: pathogenesis and immunity in mice and hamsters. J Exp Med. 1958;107:153–164. doi: 10.1084/jem.107.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.De Shazar D, Brett P J, Woods D E. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistence and virulence. Mol Microbiol. 1998;30:1081–1100. doi: 10.1046/j.1365-2958.1998.01139.x. [DOI] [PubMed] [Google Scholar]
- 9.Egerton J R. Melioidosis in a tree-climbing kangeroo. Aust Vet J. 1963;39:243–244. [Google Scholar]
- 10.Finkelmann F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 11.Galimand M, Dodin A. Le point sur la melioidosis dans le monde. Bull Soc Pathol Exot Fil. 1982;75:373. [PubMed] [Google Scholar]
- 12.Greenawald K A, Nash G, Foley F D. Acute systemic melioidosis. Am J Clin Pathol. 1969;52:188–198. doi: 10.1093/ajcp/52.2.188. [DOI] [PubMed] [Google Scholar]
- 13.Hancock G E, Schaedler R W, MacDonald T T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986;53:26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häußler S, Nimtz M, Domke T, Wray V, Steinmetz I. Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect Immun. 1998;66:1588–1593. doi: 10.1128/iai.66.4.1588-1593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckly R J. Differentiation of exotoxin and other biologically active substances in Pseudomonas pseudomallei filtrates. J Bacteriol. 1964;88:1730–1736. doi: 10.1128/jb.88.6.1730-1736.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leelarasmee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 18.Miller W R, Pannell L, Cravitz L, Tanner W A, Rosebury T. Studies on certain biological characteristics of Malleomyces mallei and Malleomyces pseudomallei. II. Virulence and infectivity for animals. J Bacteriol. 1948;55:127–135. [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–243. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morissette C, Skamene E, Gervais F. Endobronchial inflammation following Pseudomonas aeruginosa infection. Infect Immun. 1995;63:1718–1724. doi: 10.1128/iai.63.5.1718-1724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimtz M, Wray V, Domke T, Brenneke B, Häußler S, Steinmetz I. Structure of an acidic exopolysaccharide of Burkholderia pseudomallei. Eur J Biochem. 1997;250:608–616. doi: 10.1111/j.1432-1033.1997.0608a.x. [DOI] [PubMed] [Google Scholar]
- 22.Perry M B, MacLean L L, Schollaardt T, Bryan L E, Ho M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect Immun. 1995;63:3348–3352. doi: 10.1128/iai.63.9.3348-3352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pigott J A, Hochholzer L. Human melioidosis: A histopathologic study of acute and chronic melioidosis. Arch Pathol. 1970;90:101–111. [PubMed] [Google Scholar]
- 24.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 25.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 26.Sanford J P. Pseudomonas species (including melioidosis and glanders) In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious disease. 4th ed. New York, N.Y: John Wiley & Sons; 1995. pp. 2003–2009. [Google Scholar]
- 27.Santanirand P, Harley V S, Dance D A B, Raynes J G, Drasar B S, Bancroft G J. Interferon-γ mediates host resistance in a murine model of melioidosis. Biochem Soc Trans. 1997;25:287S. doi: 10.1042/bst025287s. [DOI] [PubMed] [Google Scholar]
- 28.Smith M D, Angus B J, Wuthiekanun V, White N J. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect Immun. 1997;65:4319–4321. doi: 10.1128/iai.65.10.4319-4321.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith M D, Wuthiekanum V, Walsh A L, White N J. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans R Soc Trop Med Hyg. 1995;89:488–490. doi: 10.1016/0035-9203(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 30.Spurr R A. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz I, Rhode M, Brenneke B. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect Immun. 1995;63:3959–3965. doi: 10.1128/iai.63.10.3959-3965.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutmolle P, Kraneveld F C, van der Schaaf A. Melioidosis (Pseudomalleus) in sheep, goats, and pigs on Aruba (Netherlands Antilles) J Am Vet Med Assoc. 1957;130:415–417. [PubMed] [Google Scholar]
- 33.Thomas A D, Forbes-Faulkner J C, Norton J H, Trueman K F. Clinical and pathological observations on goats experimentally infected with Pseudomonas pseudomallei. Aust Vet J. 1988;65:43–46. doi: 10.1111/j.1751-0813.1988.tb07350.x. [DOI] [PubMed] [Google Scholar]
- 34.Trakulsomboon S, Dance D A B, Smith M D, White N J, Pitt T L. Ribotype differences between clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1997;46:565–570. doi: 10.1099/00222615-46-7-565. [DOI] [PubMed] [Google Scholar]
- 35.Vateharapreechasakul T, Suputtamongkol Y, Dance D A B, Chaowagul W, White N J. Pseudomonas pseudomallei liver abscesses: a clinical, laboratory, and ultrasonographic study. Clin Infect Dis. 1992;14:412–417. doi: 10.1093/clinids/14.2.412. [DOI] [PubMed] [Google Scholar]
- 36.Vidal S, Tremblay M, Govani G, Gauthier S, Sebastini G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods D E, Jones A L, Hill P J. Interaction of insulin with Pseudomonas pseudomallei. Infect Immun. 1993;61:4045–4050. doi: 10.1128/iai.61.10.4045-4050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Chaowagul W, Sokol P. Siderophore production by Pseudomonas pseudomallei. Infect Immun. 1991;59:776–780. doi: 10.1128/iai.59.3.776-780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]