Abstract
Introduction
Previous research demonstrated that medical scent detection dogs have the ability to distinguish SARS-CoV-2 positive from negative samples with high diagnostic accuracy. To deploy these dogs as a reliable screening method, it is mandatory to examine if canines maintain their high diagnostic accuracy in real-life screening settings. We conducted a study to evaluate the performance of medical scent detection dogs under real-life circumstances.
Methods
Eight dogs were trained to detect SARS-CoV-2 RT-qPCR-positive samples. Four concerts with a total of 2802 participants were held to evaluate canines’ performance in screening individuals for SARS-CoV-2 infection. Sweat samples were taken from all participants and presented in a line-up setting. In addition, every participant had been tested with a SARS-CoV-2 specific rapid antigen test and a RT-qPCR and they provided information regarding age, sex, vaccination status and medical disease history. The participants’ infection status was unknown at the time of canine testing. Safety measures such as mask wearing and distance keeping were ensured.
Results
The SARS-CoV-2 detection dogs achieved a diagnostic specificity of 99.93% (95% CI 99.74% to 99.99%) and a sensitivity of 81.58% (95% CI 66.58% to 90.78%), respectively. The overall rate of concordant results was 99.68%. The majority of the study population was vaccinated with varying vaccines and vaccination schemes, while several participants had chronic diseases and were under chronic medication. This did not influence dogs’ decisions.
Conclusion
Our results demonstrate that SARS-CoV-2 scent detection dogs achieved high diagnostic accuracy in a real-life scenario. The vaccination status, previous SARS-CoV-2 infection, chronic disease and medication of the participants did not influence the performance of the dogs in detecting the acute infection. This indicates that dogs provide a fast and reliable screening option for public events in which high-throughput screening is required.
Keywords: COVID-19, diagnostics and tools, SARS
WHAT IS ALREADY KNOWN ON THIS TOPIC
Trained scent detection dogs are able to discriminate samples from SARS-CoV-2 infected individuals to samples from SARS-CoV-2 negative individuals using different body fluids as well as to samples from other viral infections.
WHAT THIS STUDY ADDS
SARS-CoV-2 scent detection dogs achieved high diagnostic accuracies in a real-life scenario and demonstrated their feasibility as a diagnostic tool for screening at public events.
The dogs’ performance was not affected by the participants’ vaccination status, disease and medication history, and previous SARS-CoV-2 infection.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Medical scent detection dogs can become an affordable rapid diagnostic tool in addition to the use of point of care-antigen and PCR-based tests.
At public events where high-throughput screening is required, SARS-CoV-2 detection dogs can provide a rapid and reliable screening option.
Introduction
The use of rapid point of care antigen-tests (PoC-antigen tests) for the diagnosis of a SARS-CoV-2 infection continues to be a key tool to manage the ongoing COVID-19 pandemic. Several studies have shown the ability of medical scent detection dogs to identify samples from SARS-CoV-2 infected individuals with high accuracy, highlighting the role such dogs could play in the management of a pandemic.1–13 Previous research showed that different body fluids, such as saliva, sweat and urine and other sample types like worn face masks are suitable for detection, which suggests that there is a general SARS-CoV-2 infection associated odour that dogs can be trained on.14–16 In addition, our group demonstrated that such dogs were able to differentiate SARS-CoV-2 infection from other acute viral respiratory tract infections.17 However, most of the current data were generated in laboratory settings, rather than in a real-world scenario or lacked the verification of SARS-CoV-2 infections by a PoC-antigen test and real-time quantitative reverse transcription-PCR (RT-qPCR). In some countries, SARS-CoV-2 detection dogs were already deployed in pilot projects in public transports, such as airports or underground transportation.3 18 In a first real-life trial in an airport setting with samples from 303 passengers, dogs identification and RT-PCR results matched for 97.7% of samples from passengers.18 Although this study clearly indicated that medical scent detection dogs can maintain their diagnostic accuracy under real-life conditions, the study cohort was too small for far-reaching conclusions. Furthermore, additional field settings should improve the general feasibility of the use of medical detection dogs to demonstrate its diagnostic use in different populations.
The WHO and the Paul Ehrlich Institute (PEI) recommend thresholds for diagnostic specificities and sensitivities for PoC-antigen tests of more than 97% and more than 80%, respectively.19 To meet the requirements of a valuable diagnostic tool, medical scent detection dogs should also meet these diagnostic performance characteristics, even under real-life circumstances when many distractions could influence their performance.
Methods
Study design
In September and October 2021, four public concerts were organised to evaluate the diagnostic performance of SARS-CoV-2 detection dogs. In total, 2802 participants took part in the study and were screened for SARS-CoV-2 infections by trained detection dogs. The number of participants per concert increased during the study period from 466 individuals at the first concert to 1018 participants at the last concert. The second concert yielded 640 participant and the third concert 678 participants. The interval between two concerts was 5–8 days.
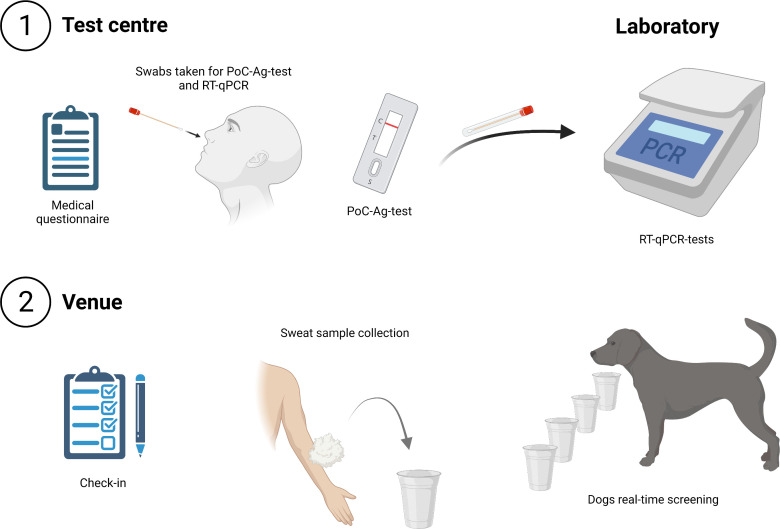
For inclusion, individuals had to book free, personalised tickets and had to be at least 18 years old. It was further ensured that individuals who provided samples for the preceding canine training phase were excluded from the study population. At the day of study, all participants visited the same certified COVID-19 test centre (figure 1). At the test centre, they had to provide a nasopharyngeal or oropharyngeal swab sample for a directly performed SARS-CoV-2 PoC-antigen test and an oropharyngeal swab sample for a RT-qPCR test. The oropharyngeal swabs (FLOQswab Minitip, 80501CS, Coban Ref. 501CS01, Mast Diagnostica) were collected in micro screw tubes with 500 µL of viral transport medium incl. Dulbecco’s Modfied Eagle’s Medium (high glucose, Gibco, 41965062, ThermoFisher) supplemented with 1% L-Glutamin (GlutaMAX, Gibco, 11574466, ThermoFisher) and antibiotics (1% penicillin/streptomycin, P433, Sigma). For PoC-antigen testing the AT079/20 COVID-19 Antigen Rapid Test, Hangzhou Clongene Biotech recommended by PEI, Langen, Germany was used.20 A positive PoC-antigen test was an exclusion criterion for entry into the concert area to minimise the infection risk. In addition, two sweat samples were collected from every participant by wiping both crooks of the arm at the test centre and transported to the venue, so that in case of exclusion of a participant the respective sample could be preserved and screened by the dogs.
Figure 1.
Schematic representation for test procedure (created with BioRender.com). PoC, point of care.
The extraction and detection of SARS-CoV-2 RNA from oropharyngeal swab samples of concert participants and samples used for training and presentation to the dogs at the concert was conducted as described previously,21 using RT-qPCR SARS-CoV-2-IP4 assay from Institute Pasteur targeting the SARS-CoV-2 RNA dependent RNA polymerase (RdRp) gene (recommended by the WHO,22 including an internal control system23). Analysis of the samples from the concert participants was performed a few days after the respective concert at the Research Centre for Emerging Infections and Zoonoses, University of Veterinary Medicine Hannover. At the test centre, written informed consent from all participants was obtained and all participants partook in a short survey. Here, information regarding sex, age, previous SARS-CoV-2 infection, presence of post-COVID-19 condition, SARS-CoV-2 vaccination status, time of vaccination, type of vaccine and medical disease history including chronic medication were obtained from all participants. Details can be found in online supplemental tables 1–4.
bmjgh-2022-010276supp001.pdf (641.4KB, pdf)
bmjgh-2022-010276supp002.pdf (12.8KB, pdf)
bmjgh-2022-010276supp003.pdf (53.5KB, pdf)
bmjgh-2022-010276supp004.pdf (57.4KB, pdf)
At the venue entrance after check-in, all participants were screened by SARS-CoV-2 detection dogs using two line-ups simultaneously (figure 1). Every line-up contained 20 mountings for sample containers. Therefore, 40 participants could be screened at once. Disposable paper cups were used as sample containers. The cups and contained samples were removed after each run and handled by the same two persons, wearing disposable gloves to prevent odour contamination. The sample containers were attached to fence elements, which served for sample presentation and as partition walls to separate the participants from the workspace of the dogs. Every participant received a cotton pad and generated a sweat sample once by wiping both arm crooks. The participants placed the sweat samples into the sample containers through an aperture in the line-up. As soon as 20 samples were placed into the line-up, the aperture was closed. Subsequently, dog handler and SARS CoV-2 detection dogs were brought to the search area and the detection dog screened all samples. Only if samples were not indicated as positive by the dog, the respective participants were allowed to enter the concert hall. If one dog indicated any of the samples, the whole line-up was double-checked by another dog. If the second dog consented and indicated the same sample, the respective participant was excluded from the concert. Each participant went through the entire screening process once. Status of PoC-antigen and RT-qPCR-tests of participants and samples were unknown to the investigators, dog handlers and the dog at the time of canine SARS-CoV-2 detection.
Dog decisions for every sample were evaluated with two possible options:
Canine test positive: two dogs indicated the respective sample as positive.
Canine test negative: the first dog did not indicate the sample and therefore it was not double checked by another dog OR the first dog indicated the sample as positive, but the second dog contradicted and did not indicate the sample OR the first dog did not indicate the sample and the second dog consented.
If a participant would have been indicated as positive by the canine test, the individual needed to provide a sample for a fast-PCR at a certified COVID-19 laboratory and had to leave the study area. The fast-PCR was proceeded with VitaPCR SARS-CoV-2 assay using a VitaPCR instrument. If these participants were tested negative via initial RT-qPCR, a second RT-qPCR-test was performed 3 days later. Latter RT-qPCR was conducted by the local health and regulatory authorities. Furthermore, to evaluate the presence of SARS-CoV-2 and other respiratory pathogens, samples from participants with a positivse canine test were sent to the Robert Koch Institute (RKI, German Federal Ministry of Health, Berlin, Germany). At the RKI, nucleic acids were extracted using the QIAamp Viral RNA Mini Kit at. To confirm absence of SARS-CoV-2, those samples were analysed by RT-PCR using the RKI/ZBS1 SARS-CoV-2 protocol targeting two different genomic regions (E-Gene and orf1ab).24 Subsequently, qualitative multiplex PCR RespiFinder 2SMART (PathoFinder) was used to determine presence of the following respiratory pathogens: influenza A and B, influenza H1N1pdm09, respiratory syncytial virus A and B, parainfluenza 1–4, human coronavirus OC43 (HCoV-OC43), HCoV-229E, HCoV-HKU1/NL63, rhinovirus/enterovirus, adenovirus, human metapneumovirus, bocavirus type 1, Mycoplasma pneumonia, Chlamydophila pneumoniae, Legionella pneumophilia, Bordetella pertussis.
Dogs
In total, eight dogs (three females and five males) were included in the current study. All dogs completed obedience training before the study, and some had a history of protection or scent detection work. Included dog breeds were Labrador Retriever (n=2), Malinois (n=3), German Shepherd (n=1), German Shepherd Mix (n=1) and Shepherd Mix (n=1). Ages ranged between 2 and 10 years (online supplemental table 5).
bmjgh-2022-010276supp005.pdf (14.5KB, pdf)
Training procedure
The dogs had participated in our previous studies8 14 17 25 and were therefore already trained for detection of SARS-CoV-2 RT-qPCR positive samples using the Detection Dog Training System (DDTS, Kynoscience UG, Hörstel). Exact training methods and operation of the DDTS are described in detail in the corresponding literature.8 14 17 After 3 days of DDTS-training, the training period was supplemented with a line-up training setting (1–2 weeks) in order to get dogs used to real-life screening conditions. To mark a sample as SARS-CoV-2-positive, the dogs showed different indication behaviour. The specific indication behaviour, like freezing or sitting, depended on the respective dog. Dogs were rewarded with food or a toy for indicating positive samples. To avoid trainer bias, the dog handler was blinded regarding presence and position of positive samples during training. After sniffing a negative sample, the dogs should not indicate it but must continue the examination. To decrease the likelihood of false positive indications due to frustration in case of low prevalence, the dogs were not only rewarded for finding a positive sample but also for not indicating negative samples in an ‘empty run’ (ie, no positive sample in the line-up). Rewards used for empty runs had lower values for the dog compared with rewards for indicating positive samples to maintain high search motivation (ie, praise instead of toy/food). During the training period, the number of empty runs gradually increased.
Training samples
For training, samples from SARS-CoV-2 infected patients were presented next to samples from healthy, non-infected individuals. Those training samples originated from and were collected during our former studies and were taken from hospitalised and non-hospitalised SARS-CoV-2 infected individuals as well as from SARS-CoV-2 RT-PCR negative healthy controls. Overall, samples from 152 individuals were used during training, 48 from SARS-CoV-2 RT-PCR positive individuals and 104 from SARS-CoV-2 negative individuals. To ensure that the location of sample collection did not bias the dogs, positive and negative samples from the test centre were also collected and used for test runs, which were carried out in the 2 weeks before the first concert and during the study. Since former studies showed dogs ability to detect SARS-CoV-2 infections using different body fluids,14 we used sweat, urine and saliva samples from infected and non-infected individuals. Samples from patients with a variety of symptom severity from asymptomatic non-hospitalised to severely diseased and hospitalised were included. Based on former results showing that beta-propiolactone (BPL) inactivation does not interfere with scent dog detection and for easier and safer handling of samples, most positive and negative samples were BPL inactivated as formerly described.8 14 As sweat samples from infected patients are not considered to pose a relevant infection risk,26 also non-inactivated sweat samples from SARS-CoV-2 positive and negative individuals were presented. These samples were collected during the training period at the cooperating test centre and used directly in training. In total, 63 non-inactivated and 89 BPL-inactivated samples were used during training. Sample collection and preparation was conducted as described in our previous study.14 The SARS-CoV-2 status of each collected sample was determined as described earlier in the ‘Study design’ section.
Positive samples included in the study
To guarantee the appearance of positive samples despite a low incidence in Germany during the study period (60–75 new infections per 100 000 inhabitants in 7 days27), samples collected previously from infected patients were presented in the line-up next to samples from participants collected at the venue. With this, an ‘artificial prevalence’ was created. Positive BPL inactivated sweat, saliva and urine samples and non-inactivated sweat samples from asymptomatic to severely diseased patients were used in the study. All samples and patients were unknown to the dogs. The position of positive samples in the line-ups was randomised and dogs, dog handlers and personnel in the dog area were blinded regarding presence and position of positive samples. Sample details can be found in online supplemental table 6.
bmjgh-2022-010276supp006.pdf (51.6KB, pdf)
Statistical analysis
The diagnostic sensitivity as well as the diagnostic specificity were calculated stratified by concert and aggregated over the entire study population. 95% CIs for sensitivity and specificity were calculated with the hybrid Wilson-Brown method.28 Positive predictive value (PPV) and negative predictive value (NPV) were calculated according to Dohoo, Martin and Stryhn, 2009, assuming a population prevalence of 0.2% as a typical outcome in Germany.29 The data of the audience were evaluated descriptively by determining the measures of location scales and the dispersion measures. Test accuracy of the canine test was additionally calculated in a logistic regression model analogous to Coughlin et al.30 Using the PCR as golden standard, sensitivity and specificity were determined depending on the concert. All calculations were performed using SAS statistical program (V.9.4 TS level 1M5, SAS Institute) and Prism V.9 software from GraphPad (La Jolla, California, USA).
Results
The study population consisted of a total of 2802 participants over four concerts. Addition of 38 samples from SARS-CoV-2 RT-qPCR positive individuals to this population led to an overall study prevalence of 1.34%. In total, 1616 females (58%) and 1058 males (38%) partook in the study (online supplemental table 1). One-hundred and twenty eight participants did not provide information on gender. The age of the study population ranged from 18 to 82 years. The median age ranged from 48 years (concert 1) to 28 years (concert 4) (online supplemental table 2). Eighty-seven per cent of all participants were fully vaccinated (ie, 2 weeks after second vaccination with Comirnaty, Spikevax or Vaxzevria or after first vaccination with Jcovden) and 5% were unvaccinated. Sixteen per cent of all subjects stated that they had a chronic illness and 15% of all participants were, according to their own statements, under chronic medication. A total of 75 different diseases and 157 drugs or drug classes were mentioned. The prevailing chronic diseases were hypertension (7%), hypothyroidism or Hashimoto’s disease (4%), asthma (2%), diabetes mellitus (1%) and rheumatic disorders (1%) (online supplemental table 3). The drugs that were listed most frequently were l-thyroxine (4%), ramipril (2%), candesartan (2%), insuline, amlodipine and formoterole (1% each) (online supplemental table 4). Three per cent of the study population had a previous SARS-CoV-2 infection and further 3% have indicated not knowing for sure if they have already had an infection with SARS-CoV-2. Three participants were diagnosed with post-COVID-19 condition according to their own statements. All information on study population regarding vaccination status, time of vaccination, type of vaccine and medical disease history are shown in detail in online supplemental tables 1, 3 and 4. None of these factors influenced the dogs’ performance. In total, only two participants had a false positive canine test. These individuals were both male, 47 and 57 years of age, respectively, and had no chronic diseases or were under chronic medication. Both were double vaccinated with Comirnaty, with the second vaccination administered 89 and 55 days, respectively, prior to the study.
Overall, the dogs achieved a sensitivity of 81.58% (95% CI 66.58% to 90.78%) and a specificity of 99.93% (95% CI 99.74% to 99.99%), both in raw as well as in the regression modelling, respectively (table 1). The overall rate of diagnostic concordant results of the SARS-CoV-2 detection dogs was 99.68%. The logistic regression model calculated sensitivity and specificity values dependent on the concert of the participants. The values deviate slightly from the directly calculated test accuracies. However, there were no significant differences between the concerts (p>0.05).
Table 1.
Diagnostic performance of the scent detection dogs stratified by concert
| Session | Detection | SARS-CoV-2 infection status | Total no of presented samples | Prevalence | Diagnostic specificity (Sp) |
Diagnostic sensitivity (SE) |
95% CI Sp | 95% CI SE | Diagnostic specificity (regression model) |
Diagnostic sensitivity (regression model) |
95% CI Sp (regression model) | 95% CI SE (regression model) | Rate of concordant results | Positive predictive value (PPV) | Negative predictive value (NPV) | |
| Positive | Negative | |||||||||||||||
| Concert 1 | Yes | 6 | 1 | 475 | 0.0189 | 0.9979 | 0.6667 | 0.9879 to 0.9999 | 0.3542 to 0.8794 | 0.9996 | 0.7562 | 0.9988 to 0.9999 | 0.5144 to 0.9009 | 0.9916 | ||
| No | 3 | 465 | ||||||||||||||
| Concert 2 | Yes | 8 | 1 | 650 | 0.0154 | 0.9984 | 0.8 | 0.9912 to 0.9999 | 0.4902 to 0.9645 | 0.9992 | 0.8510 | 0.9974 to 0.9998 | 0.6242 to 0.9515 | 0.9953 | ||
| No | 2 | 639 | ||||||||||||||
| Concert 3 | Yes | 9 | 0 | 689 | 0.0159 | 1 | 0.8182 | 0.9944 to 1 | 0.5230 to 0.9677 | 0.9995 | 0.78753 | 0.9991 to 0.9997 | 0.6716 to 0.8704 | 0.9971 | ||
| No | 2 | 678 | ||||||||||||||
| Concert 4 | Yes | 8 | 0 | 1026 | 0.0078 | 1 | 1 | 0.9962 to 1 | 0.6756 to 1 | 0.9990 | 0.87763 | 0.9913 to 0.9999 | 0.4412 to 0.9849 | 1 | ||
| No | 0 | 1018 | ||||||||||||||
| All concerts | Yes | 31 | 2 | 2840 | 0.0134 | 0.9993 | 0.8158 | 0.9974 to 0.9999 | 0.6658 to 0.9078 | 0.9993 | 0.8158 | 0.9974 to 0.9999 | 0.6658 to 0.9078 | 0.9968 | 0.7002 | 0.9996 |
| No | 7 | 2800 | ||||||||||||||
PPV and NPV were calculated according to Dohoo et al, assuming a prevalence of 0.2%29
Interestingly, two dogs identified a sample from a single individual who showed a positive SARS-CoV-2 RT-qPCR 2 days later, indicating that dogs may detect changes in the volatile organic compound (VOC) profiles before detectable virus shedding takes place. During the presentation of samples from the 2802 participants, dogs had two false positive indications and the PoC-antigen test was false positive for one participant, respectively. Due to the possible infection risk, a positive ‘canine test’ was an exclusion criterion for visiting the concert. The two individuals with positive canine test were subjected to a directly performed SARS-CoV-2 fast-PCR and a follow-up SARS-CoV-2 RT-qPCR test at an accredited laboratory 3 days after the concert. Both participants remained negative and were tested negative for other respiratory infections at the RKI (German Federal Ministry of Health, Germany). The participant who had a false positive PoC-antigen test result was tested SARS-CoV-2 negative via the canine test, RT-qPCR and fast-PCR, with a Cq value above 40.
Assuming a population prevalence of 0.2% as a typical outcome in Germany in June 2022, the canine test would achieve a PPV of 70.02% and a NPV of 99.96%.
In general, a dog needed only one to two seconds to sniff one sample in a line-up. Therefore, the search of a line-up with 40 samples took a dog about 40–60s. Including sample collection, line-up loading and unloading, it took approximately 3 min to perform a line-up with 40 samples.
Discussion
In several studies, dogs demonstrated their capability to distinguish SARS-CoV-2 RT-qPCR positive samples from RT-qPCR negative samples with high diagnostic accuracy regardless of training method or sample type.1–18 31 32 However, most studies were conducted under laboratory conditions with a small number of samples and high artificial prevalences. To use medical scent detection dogs as a reliable diagnostic test, it is important to ensure that they maintain their high diagnostic accuracies in real-life scenarios while screening larger cohorts.
In this study, only two false positive canine tests were recorded, which led to a particularly high specificity of 99.93%. Dogs achieved a sensitivity of 81.58%. The overall diagnostic rate of concordance was 99.68%. These results are in accordance with findings from previous studies under laboratory conditions.8 14 The sensitivity and specificity achieved do not only meet the criteria for diagnostic tests recommended by the WHO and the PEI, but are clearly beyond the levels recommended, which opens its use even in low-level prevalence situations. At a low prevalence of 0.2%, the canine test would achieve a PPV of 70.02% and an NPV of 99.96%. In contrast, a PoC-antigen test that just meets the recommendations of WHO and PEI would only achieve a PPV of 5.07% and a NPV of 99.96%. This would result in 94.93% of positive test results being false positive, whereas the canine test would only produce 29.98% false positive results. This clearly shows that the canine test is well above the recommendations and therefore should be considered as a reliable screening tool. Our results demonstrate that medical scent detection dogs can not only detect SARS-CoV-2 infections under laboratory conditions, but also in a real-life setting. Therefore, this may be considered as a reliable diagnostic test system, especially for mass screening events.
Due to the large number of study participants in these events, individuals with various chronic conditions and chronic medications partook in the study. This did not lead to a single false positive canine test, indicating that the dogs were not influenced by chronic conditions or medications, but were trained solely on the odour of SARS-CoV-2 infection. In addition, a large proportion of the participants were vaccinated, with different COVID-19 vaccines products (Comirnaty, Jcovden, Vaxzevria, Spikevax or other) and vaccination schedules. The interval from vaccination to study participation varied from as little as 1 day to more than 8 months. Also, when vaccination was performed a few days before the study or if a high antibody titre can be assumed due to recent vaccination,33 no false positive results were obtained by the dogs. Also, prior infection with SARS-CoV-2 or diagnosis of post-COVID-19 condition did not result in positive canine test, which further confirms that antibodies against SARS-CoV-2 have not influenced canine decisions. It further confirms former studies in which it was shown that dogs did not score samples from patients with post-COVID-19 condition positive when they were presented next to ongoing actively infected individuals.25 Concerts targeting different audiences were selected in order to cover a wide range of participant ages. The age structure of the respective concert did not influence the dog’s performance.
This study used multiple test systems in comparison with canine testing. Each participant was not only screened by SARS-CoV-2 detection dogs, but RT-qPCR- and PoC-antigen testing were also performed. The main advantage of using medical scent detection dogs over that of PoC-antigen and PCR-based tests is time efficiency, apart from being more environmentally friendly and sustainable. One dog needed 40–60s to sniff 40 samples in a line-up. The performance of 1 line-up with 40 samples including sample collection, line-up loading and unloading took approximately 3 min. The most time-consuming aspects during entrance were not the examination by scent detection dogs but was the check-in procedure. In comparison, performing a PoC-antigen test involves a waiting period of approximately 15 min depending on the test manufacturer in addition to sample collection, and even a fast PCR-test usually takes at least 30 min,34 35 which support the feasibility of the method in real-life settings.
As both the number of participants and the number of dogs increased in the course of the study, 2 hours were allocated for check-in at each concert, during which all participants were examined by the dogs. One dog performed only one line-up at a time with a maximum of 40 samples, which took approximately 1 min. Subsequently, the dog had a short break (approximately 5 to 15 min) during which other dogs searched at the line-up. Consequently, our dogs worked effectively, and their performance did not deteriorate over time. In total, one dog examined up to 312 participants during a single evening. However, it can be assumed that one dog could check significantly more individuals per day if there were multiple assignments with longer intervals in between distributed throughout the day. Similarly, Guest et al stated that two dogs could screen 300 people in 30 min, which is in accordance with our findings and demonstrates the time-efficiency of scent detection dogs.1
Our results do not only show that medical scent detection dogs maintain their capability to detect SARS-CoV-2 infections in real-life scenarios but that they also achieve excellent diagnostic accuracy. In contrast, many PoC-antigen tests when performed in test stations and in private households do not always meet these criteria.36 In a previous study, the rapid antigen test used in our study achieved an overall sensitivity of 50% (Cq values 17 to 36).20 The sensitivity was 94.4% for Cq values less than 25, but only 34.8% for Cq values between 25 and 30 and 0.0% for Cq values above 30.20 Compared with this, dogs sensitivities seem to be independent of the viral load.1
Conclusion
This study demonstrated that SARS-CoV-2 detection dogs can achieve high diagnostic sensitivity and specificity in large-scale screening for SARS-CoV-2 infections at public events such as concerts. Low prevalence, vaccination status, medical history and previous SARS-CoV-2 infections of participants did not influence dogs’ olfactory accuracy in discriminating samples from SARS-CoV-2 positive and negative individuals. Our results indicate that SARS-CoV-2 detection dogs provide a fast and reliable screening option for public events in which high-throughput screening is required. These results provide further evidence that the use of medical scent detection dogs can become an affordable rapid diagnostic tool in addition to the use of PoC-antigen and PCR-based tests. Especially in areas or countries, which lack test infrastructure or limited financial means, medical scent detection dogs provide an additional opportunity to control the ongoing COVID-19 and possibly future pandemics.
Acknowledgments
We would like to thank the government and especially the Ministry of Science and Culture of Lower Saxony in Germany for their support. We are also grateful to Nico Röger, Stefan Schreckenberger ('Schreckie'), Thassilo Gehrke and the members of Hannover Concerts and ProEvent Hannover for planning and organising of the four concerts. Thanks to Annette Wiedemann and her team from AWiAS Aviation Services for support in organising the study and help in dog training. We further would like to thank our dog handlers Daniel Jannett, Joanna Mendolaro, Jessica Wollrab, Robert Zacharz, Dominik Horne, Matthias Wichow and Andy Butsch for their assistance in dog training and conducting the study. Special thanks go to our doggy noses Margo, Bellatrix, Erec Junior, Donnie, Baila, Drago, Rebell and Poldi. Further thanks to Alexandra Müller-Anders from University of Veterinary Medicine Hannover for her laboratory support and Anastasia Bröring, Moritz Hesse and Stefan Steinmann for their work at Test Centre Hannover. We would also like to thank Rouwen Stucke, Katharina Meyer, Karola Schlote, Christin Moelders, Kristin Laudeley, and Margarethe Jentzsch for their technical support in the laboratory and Karin Lübbert for her technical assistance. We are grateful to Eva Packeiser, Marios Charalambous, Verena Nerschbach, Julia Haake, Lea Henze, Marisa Wesolowski, Teresa Schmidt, Vanessa Seiffert, Enrice Hünerfauth, Anna Knebel, Jasmin Neßler, Nina Meyerhoff, Filip Kajin, Betti Länger, Michaela Rhode, Nicole Thenert, Linda ten Hagen, Merle ten Hagen, Cara Förster, Anne Schnepf, Sabrina Less, Carolin Sievert, Vera Treuner, Marie Adolph, Sandy Feye, Kevin Knurr, Katharina Dohmen, Marita Meurer, Inken Münch, Bettina Schneider, Svenja Lura, Patricia Linke, Christian Linde, Sabine Meyer, Julia Stoffregen, Betty Rehberg, Clarissa Bonzelett, Maja Strunk, Svenja Berendes, Rebekka Mach, Stephanie Klein, Katharina Heissl, Anna Frommeyer, Nicole Steffensen, Sabine Kästner, Anja Conrady, Heidrun Hartmann, Hanna Rieder, Franziska Schöttes, Sandra Brogden, Thorben Zimmermann, Sophia Schumann, Petra von Rekowski, Ute Kruse, Ulrike Kallbach, Karin Thum and Corinna Karl from University of Veterinary Medicine Hannover for the help in conducting the study. Heartfelt thanks go to all the people providing us with samples and participating in our real-life study, especially to the SARS-CoV-2-infected persons and their relatives with the sincere intention to contribute to the containment of COVID-19 and to scientific progress. We wish you lots of strength and full recovery during the current pandemic.
Footnotes
Handling editor: Seye Abimbola
Twitter: @andreaspuyskens, @NeurologyVet
Contributors: NAtH participated in the planning of the study, carried out the main practical work, data analyses and drafted and wrote the manuscript. HAV was the guarantor of the study. FT, SM and HAV designed and coordinated the study, drafted the manuscript and conducted and coordinated (FT) the sample acquisition. LW supported dog training and helped carrying out the main practical work. MvK-B, CSchu and AO participated in the planning of the laboratory part of the study and were in charge for the legal permission for sample processing. CSchu, VB, VP, FKK, MGH carried out the laboratory work at the Research Centre for Emerging Infections and Zoonoses including sample preparation and RT-qPCR. CSchu and VP conducted virus inactivation. HE was involved in planning and coordinating the study, programmed the DDTS software and were responsible for dog training. CScho and LK gave statistical support and helped with the planning of the results presentation. IP, ND, TW, MPM and TI were in charge for the ethical approval, patient recruitment and sample collection (IP, ND) at Hannover Medical School and for use of samples from Hannover Unified Biobank (TI). AP and AN coordinated the testing on other respiratory tract infections at the Robert Koch Institute. A-LB, KB and VB coordinated PoC-antigen testing and sample collection at Test Centre Hannover and helped with planning of the study (KB). JH helped plan the study and obtain approval by the local health and regulatory authorities. CE was responsible for the special research proposal of the German Armed Forces as project manager on the part of the German Armed Forces. ME coordinated the cooperation with the University of Veterinary Medicine Hannover. ES was responsible for the dog training. ME and ES were also involved in designing and coordinating the study. All authors have read and approved the final manuscript.
Funding: The project was funded as a research project of the Ministry of Science and Culture of Lower Saxony in Germany. This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 "Open Access Publication Funding" and the University of Veterinary Medicine Hannover, Foundation.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are majorly included in the manuscript or uploaded as online supplemental information. Any additional data are available on request from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was carried out in accordance with the ethical requirements established by the Declaration of Helsinki. The study obtained ethical approval by the local Ethics Committee of Hannover Medical School (MHH) (ethic consent number 9042_BO_K_2020 and 9940_BO_S_2021). The concerts and the study were officially approved by the local health and regulatory authorities. Written informed consent from all participants was obtained before sample collection. Animal work was approved by the German Armed Forces.
References
- 1.Guest C, Dewhirst SY, Lindsay SW, et al. Using trained dogs and organic semi-conducting sensors to identify asymptomatic and mild SARS-CoV-2 infections: an observational study. J Travel Med 2022;29:taac043. 10.1093/jtm/taac043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskandari E, Ahmadi Marzaleh M, Roudgari H, et al. Sniffer dogs as a screening/diagnostic tool for COVID-19: a proof of concept study. BMC Infect Dis 2021;21:243. 10.1186/s12879-021-05939-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vesga O, Agudelo M, Valencia-Jaramillo AF, et al. Highly sensitive scent-detection of COVID-19 patients in vivo by trained dogs. PLoS One 2021;16:e0257474. 10.1371/journal.pone.0257474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandjean D, Sarkis R, Lecoq-Julien C, et al. Can the detection dog alert on COVID-19 positive persons by sniffing axillary sweat samples? A proof-of-concept study. PLoS One 2020;15:e0243122. 10.1371/journal.pone.0243122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maia RdeCC, Alves LC, da Silva JES, et al. Canine olfactory detection of SARS-COV2-Infected patients: a one health approach. Front Public Health 2021;9:647903. 10.3389/fpubh.2021.647903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkis R, Lichaa A, Mjaess G, et al. New method of screening for COVID-19 disease using sniffer dogs and scents from axillary sweat samples. J Public Health 2022;44:e36–41. 10.1093/pubmed/fdab215 [DOI] [PubMed] [Google Scholar]
- 7.Angeletti S, Travaglino F, Spoto S, et al. COVID-19 sniffer dog experimental training: which protocol and which implications for reliable sidentification? J Med Virol 2021;93:5924–30. 10.1002/jmv.27147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jendrny P, Schulz C, Twele F, et al. Scent dog identification of samples from COVID-19 patients - a pilot study. BMC Infect Dis 2020;20:536. 10.1186/s12879-020-05281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaber A, Hazel S, Matthews B. Evaluation of canine detection of COVID‐19 infected individuals under controlled settings. Transbound Emerg Dis 2022:1–8. 10.1111/tbed.14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hag-Ali M, AlShamsi AS, Boeijen L, et al. The detection dogs test is more sensitive than real-time PCR in screening for SARS-CoV-2. Commun Biol 2021;4:686. 10.1038/s42003-021-02232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendel J, Frank K, Edlin L, et al. Preliminary accuracy of COVID-19 odor detection by canines and HS-SPME-GC-MS using exhaled breath samples. Forensic Sci Int Synerg 2021;3:100155. 10.1016/j.fsisyn.2021.100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandjean D, Slama D, Gallet C. Screening for SARS-CoV-2 persistence in long COVID patients using sniffer dogs and scents from axillary sweats samples. J Clin Trials 2022;12:2. [Google Scholar]
- 13.Grandjean D, Al MDH, Lecoq-Julien C. Use of canine olfactory detection for COVID-19 testing study on U.A.E. trained detection dog sensitivity. bioRxiv 2021. [Google Scholar]
- 14.Jendrny P, Twele F, Meller S, et al. Scent dog identification of SARS-CoV-2 infections in different body fluids. BMC Infect Dis 2021;21:707. 10.1186/s12879-021-06411-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essler JL, Kane SA, Nolan P, et al. Discrimination of SARS-CoV-2 infected patient samples by detection dogs: a proof of concept study. PLoS One 2021;16:e0250158. 10.1371/journal.pone.0250158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devillier P, Gallet C, Salvator H, et al. Biomedical detection dogs for the identification of SARS-CoV-2 infections from axillary sweat and breath samples*. J Breath Res 2022;16:037101. 10.1088/1752-7163/ac5d8c [DOI] [PubMed] [Google Scholar]
- 17.Ten Hagen NA, Twele F, Meller S, et al. Discrimination of SARS-CoV-2 infections from other viral respiratory infections by scent detection dogs. Front Med 2021;8:749588. 10.3389/fmed.2021.749588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantele A, Paajanen J, Turunen S, et al. Scent dogs in detection of COVID-19: triple-blinded randomised trial and operational real-life screening in Airport setting. BMJ Glob Health 2022;7:e008024. 10.1136/bmjgh-2021-008024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . SARS-CoV-2 antigen-detecting rapid diagnostic tests: an implementation guide, 2020. Available: https://www.who.int/publications/i/item/9789240017740 [Accessed 30 Jul 2022].
- 20.Scheiblauer H, Filomena A, Nitsche A, et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Euro Surveill 2021;26:44. 10.2807/1560-7917.ES.2021.26.44.2100441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciurkiewicz M, Armando F, Schreiner T, et al. Ferrets are valuable models for SARS-CoV-2 research. Vet Pathol 2022;59:661–72. 10.1177/03009858211071012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institut Pasteur, Paris . Protocol: real-time RT-PCR assays for the detection of SARS-CoV-2. Available: https://www.who.int/docs/default-%0Asource/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=662fcb6_2%0A [Accessed 27 Jul 2022].
- 23.Hoffmann B, Depner K, Schirrmeier H, et al. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J Virol Methods 2006;136:200–9. 10.1016/j.jviromet.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 24.Michel J, Neumann M, Krause E, et al. Resource-efficient internally controlled in-house real-time PCR detection of SARS-CoV-2. Virol J 2021;18:110. 10.1186/s12985-021-01559-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twele F, ten Hagen NA, Meller S, et al. Detection of post COVID-19 patients using medical scent detection dogs – a pilot study. Front Med 2022;9:877259. 10.3389/fmed.2022.877259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arslan B, Bercin S, Aydogan S, et al. SARS-CoV-2 is not found in the sweat of COVID-19 positive patients. Ir J Med Sci 2022;191:27–9. 10.1007/s11845-021-02537-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert Koch Institute . Archive of the situation reports of the Robert Koch Institute on COVID-19. Available: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Sept_2021/Archiv_September_2021.html [Accessed 28 Jul 2022].
- 28.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statist Sci 2001;16:101–33. 10.1214/ss/1009213286 [DOI] [Google Scholar]
- 29.Dohoo I, Martin W, Stryhn H. Veterinary epidemiologic research. 2nd edn. Charlottetown, Canada: VER Inc, 2010: 10. [Google Scholar]
- 30.Coughlin SS, Trock B, Criqui MH, et al. The logistic modeling of sensitivity, specificity, and predictive value of a diagnostic test. J Clin Epidemiol 1992;45:1–7. 10.1016/0895-4356(92)90180-U [DOI] [PubMed] [Google Scholar]
- 31.Grandjean D, Gallet C, Julien C, et al. Identifying SARS-COV-2 infected patients through canine olfactive detection on axillary sweat samples; study of observed sensitivities and specificities within a group of trained dogs. PLoS One 2022;17:e0262631. 10.1371/journal.pone.0262631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancilla-Tapia JM, Lozano-Esparza V, Orduña A, et al. Dogs detecting COVID-19 from sweat and saliva of positive people: a field experience in Mexico. Front Med 2022;9:837053. 10.3389/fmed.2022.837053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2022;10:64. 10.3390/vaccines10010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Commission - Directorate-General for Health and Food Safety EU . Common list of COVID-19 antigen tests, 2022. Available: https://health.ec.europa.eu/system/files/2022-07/covid-19_rat_common-list_en.pdf [Accessed 28 Jul 2022].
- 35.Liu W, Zhou Z, Zhang L, et al. Establishment and evaluation of a 30-minute detection method for SARS-CoV-2 nucleic acid using a novel ultra-fast real-time PCR instrument. J Thorac Dis 2021;13:6866–75. 10.21037/jtd-21-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2021;3:CD013705. 10.1002/14651858.CD013705.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-010276supp001.pdf (641.4KB, pdf)
bmjgh-2022-010276supp002.pdf (12.8KB, pdf)
bmjgh-2022-010276supp003.pdf (53.5KB, pdf)
bmjgh-2022-010276supp004.pdf (57.4KB, pdf)
bmjgh-2022-010276supp005.pdf (14.5KB, pdf)
bmjgh-2022-010276supp006.pdf (51.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are majorly included in the manuscript or uploaded as online supplemental information. Any additional data are available on request from the corresponding author on reasonable request.