Objective
Two COVID-19 outbreaks occurred in Henan province in early 2022—one was a Delta variant outbreak and the other was an Omicron variant outbreak. COVID-19 vaccines used at the time of the outbreak were inactivated, 91.8%; protein subunit, 7.5%; and adenovirus5-vectored, 0.7% vaccines. The outbreaks provided an opportunity to evaluate variant-specific breakthrough infection rates and relative protective effectiveness of homologous inactivated COVID-19 vaccine booster doses against symptomatic infection and pneumonia.
Design
Retrospective cohort study
Methods
We evaluated relative vaccine effectiveness (rVE) with a retrospective cohort study of close contacts of infected individuals using a time-dependent Cox regression model. Demographic and epidemiologic data were obtained from the local Centers for Disease Control and Prevention; clinical and laboratory data were obtained from COVID-19-designated hospitals. Vaccination histories were obtained from the national COVID-19 vaccination dataset. All data were linked by national identification number.
Results
Among 784 SARS-CoV-2 infections, 379 (48.3%) were caused by Delta and 405 (51.7%) were caused by Omicron, with breakthrough rates of 9.9% and 17.8%, respectively. Breakthrough rates among boosted individuals were 8.1% and 4.9%. Compared with subjects who received primary vaccination series ≥180 days before infection, Cox regression modelling showed that homologous inactivated booster vaccination was statistically significantly associated with protection from symptomatic infection caused by Omicron (rVE 59%; 95% CI 13% to 80%) and pneumonia caused by Delta (rVE 62%; 95% CI 34% to 77%) and Omicron (rVE 87%; 95% CI 3% to 98%).
Conclusions
COVID-19 vaccination in China provided good protection against symptomatic COVID-19 and COVID-19 pneumonia caused by Delta and Omicron variants. Protection declined 6 months after primary series vaccination but was restored by homologous inactivated booster doses given 6 months after the primary series.
Keywords: COVID-19, Epidemiology, Immunology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study was conducted in a simultaneous 2-variant outbreak in a single province in China and provided estimates of vaccine-built population immunity in an infection naïve population.
The study was conducted among close contacts of people with known SARS-CoV-2 infection who were in quarantine and tested frequently for infection, ensuring accurate outcomes assessment of the exposed population.
The study was limited by the small size of the outbreak, precluding analysis of vaccine brand-specific relative vaccine effectiveness (rVE).
Vaccine coverage in the outbreak setting was too high to have a comparable unvaccinated group, making absolute VE not possible to estimate accurately.
Clinical data on comorbidities was not available, precluding analysis of rVE by comorbidity.
Introduction
Most countries have experienced epidemic waves of COVID-19 caused by SARS-CoV-2 variants. As of 11 December 2021, five SARS-CoV-2 variants of concern (VOCs) have been identified, with Alpha (B.1.1.7) and Delta (B.1.617.2) predominant.1 Omicron emerged November 2021 and rapidly replaced Delta to be the predominant global strain, accounting for 90% of GISAID SARS-CoV-2 sequences.2–4 The WHO has listed nine COVID-19 vaccines for emergency use, including the two most commonly used vaccines in China—BBIBP-CorV and CoronaVac inactivated whole-virus vaccines. As the end of July 2022, over 3.4 billion doses of these vaccines have been used in China, and over 2 billion dosses have been procured for overseas use.5 6
Good safety and short-term efficacy against the ancestral strain have been demonstrated for these vaccines,7 and real-world evidence on protection against VOCs and protection with booster doses is available, for example, from Guangdong, Jiangsu and Henan8–11 in China and Chile and Brazil overseas.12 13 In China, the dynamic COVID-zero policy severely limits outbreak size, and very high COVID-19 primary vaccination coverage makes unvaccinated comparison groups too small and too different for conducting absolute vaccine effectiveness (VE) studies. In January 2022, two outbreaks occurred in Henan—one caused by Delta (B.1.617.2) and the other by Omicron (B.1.1.529.1)—that provided an opportunity to assess relative VE (rVE) of China-produced vaccines against COVID-19 caused by these two variants. We report results of our evaluation.
Methods
Outbreak setting
The setting was Henan province where there were two simultaneous outbreaks. Henan has a population of 99.36 million people; the three involved cities were Zhengzhou (8.6 million, whole-population primary series coverage 87.2%), Yuzhou (1.2 million, 94.7%) and Anyang (1.5 million, 80.3%). The COVID-19 vaccines used were inactivated vaccines, 91.8%; ZF2001, 7.5% and Ad5-nCoV vaccine, 0.7%. The COVID-19 prevention and control policy in the mainland of China requires that all SARS-CoV-2 infections are traced, and contacts quarantined for at least 2 weeks and tested periodically in quarantine. The COVID-19 vaccines used in the outbreak setting were two inactivated COVID-19 vaccines—BBIBP-CorV (Sinopharm, Beijing CNBG) and CoronaVac (Sinovac), accounting for 91.8% of vaccines used; a protein subunit vaccine, Zifivax (Zhifei Longcom, 7.5%); and an adenovirus5-vectored vaccine, Convidecia (Cansino, 0.7%). China’s immunisation programme records all COVID-19 vaccinations in a national vaccination database indexed by national ID number. The national COVID-19 surveillance system is also indexed by national ID.
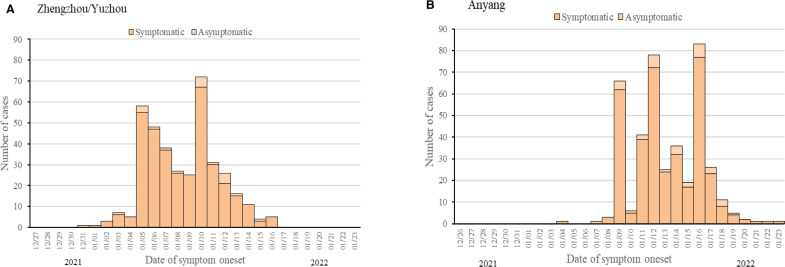
On 2 January 2022, a Yuzhou factory worker tested RT-PCR-positive in a routine presurgery screening. Contact tracing identified a Delta-variant community transmission chain that spread to Zhengzhou. On 8 January, a medical device company employee and a middle school student both tested positive for SARS-CoV-2 infection when seeking healthcare in Anyang. Investigation revealed an Omicron outbreak in a boarding school with 4103 students and teachers along with community transmission (figure 1).
Figure 1.
Epidemic curves of SARS-CoV-2 infections in Zhengzhou/Yuzhou and Anyang between 26 December 2021 and 23 January 2022. figure 1A shows the COVID-19 epidemic curve with the number of cases plotted by date of symptom onset from 26 December 2021 to 16 January 2022 in the Delta transmission chain. Confirmed and asymptomatic cases are stacked to show total daily cases by date of symptom onset. The peak onset of symptomatic infection occurred between 5 January 2020 and 10 January 2022. figure 1B shows the COVID-19 epidemic curve for confirmed cases only, with number of cases plotted by date of symptom onset of from 4 January 2022 to 23 January 2022 in the Omicron transmission chain. The confirmed cases onset of illness peaked between 9 January and 16 January.
Study design and subjects
We used a retrospective cohort design to estimate rVE. Subjects were confirmed SARS-CoV-2 infections and their close contacts, grouped by vaccination status. Outcomes were infection, pneumonia and severe illness.
A SARS-CoV-2 infection was a person with a positive RT-PCR, including asymptomatic and symptomatic infections regardless of severity; COVID-19 pneumonia was a SARS-CoV-2 infection with acute onset of fever and cough or acute onset with any three or more of the following: fever, cough, general weakness/fatigue, headache, myalgia, sore throat, coryza, dyspnoea, anorexia/nausea/vomiting, diarrhoea, altered mental status; or having chest CT imaging findings diagnostic of pneumonia. Severe illness was characterised by respiratory failure with need for mechanical ventilation or shock combined with other organ failure requiring care in an intensive care unit.14 Thus, the outcome definitions included subjective symptoms (eg, sore throat and headache), elicited signs (eg, fever, altered mental status, pneumonia on imaging) and objective healthcare actions (eg, intensive care unit admission and mechanical ventilation), with the more objective elements for the more severe outcomes.
Close contacts had exposure to confirmed SARS-CoV-2 infections up to 4 days before illness onset of symptomatic cases or the first RT-PCR-positive specimen for asymptomatic cases. Exposure included living in the same apartment, sharing a table for meals, studying, or working in the same room, or sharing a ward.
Subject data were obtained from the local Centers for Disease Control and Prevention and included the epidemiological investigation report, transmission chain, age, gender, date of first and last exposure, mode and location of exposure, and frequency and duration of exposure. For the confirmed SARS-CoV-2 infections, we reviewed medical records from designated COVID-19 management hospitals in Zhengzhou and Anyang to abstract clinical management data, laboratory testing and results, and chest imaging and results.
Vaccine status
Without knowledge of whether subjects had SARS-CoV-2 infection or not, we obtained vaccination records from the national vaccination database using subjects’ national IDs. Vaccination was defined as receipt of at least one dose of COVID-19 vaccine. Partial vaccination was receipt of either one dose of an inactivated COVID-19 vaccine or two doses of an inactivated vaccine with receipt of the second dose less than 14 days before exposure. Primary vaccination was completion of two doses of inactivated vaccine 14 days or more before exposure and/or a third dose of inactivated vaccine less than 7 days of exposure. Booster vaccination was a third more than 7 days before exposure. Primary vaccination was further classified as being either <180 days before exposure or ≥180 days before exposure.
Data analysis
A breakthrough infection was an RT-PCR-confirmed SARS-CoV-2 infection at least 14 days after completion of primary vaccination. We estimated unadjusted and adjusted (gender and age group) rVE against Delta and Omicron infections using a Cox regression models. The reference group was primary vaccination ≥180 days before exposure; rVE was 1-adjusted HR (ratio of incidences of the outcome of interest) for COVID-19 symptomatic infection or pneumonia.
Ninety-five per cent of comorbidities were among subjects 50 years and older. Our age groupings were under 18 years, 18–50 years and over 50 years. Subjects in the Omicron and Delta outbreaks differed since the Omicron outbreak involved a fully vaccinated middle school with 94.7% of cases under 20 years old, with its higher force of infection. We, therefore, conducted rVE analyses with and without subjects under 20 years old.
We used SAS version 9.4 (SAS Institute) for statistical analysis and R (V.4.1.0) for Cox survival analysis of pneumonia. P values <0.05 (two tailed) were considered statistically significant.
Patient and public involvement statement
This real-world, observational study was designed without patient or public involvement. COVID-19 is managed as a level-1 infectious disease, and as such, investigations of outbreaks and public health effort to prevent illness and stop transmission are required of public health agencies. Subjects were not recruited into the study, as they were cases or close contacts managed in accordance with the National Health Commission’s Protocol for Prevention and Control. Neither patients nor the public were involved in the conduct of the study, as testing and data aggregation were conducted under the prevention and control policy. Results will be disseminated through publicly available scientific publications.
Results
Subjects
Table 1 shows the characteristics of the subjects. Between 2 January 2022 and 23 January 2022, a total of 6521 SARS-CoV-2 infections and close contacts were identified in the three-city setting, among which 784 were SARS-CoV-2 infections and 5737 were close contacts. Because of the 4103-person middle school outbreak, subjects in the Anyang (Omicron) transmission chain were younger on average that in the Zhengzhou/Yuzhou (Delta) transmission chain (43.5% vs 23.8% <20 years, p<0.05). Most (72.7%) subjects completed primary vaccination; 14.9% received 0–1 dose; and 12.4% received booster doses. In the Delta chain, the median number of days between primary vaccination and exposure was 117 days (IQR:40–134) among subjects completed primary <180 days before exposure and 196 days (IQR: 191–203) among subjects completed primary ≥180 days before exposure. Respective medians in the Omicron chain were 133 days (IQR: 121–138) and 203 days (IQR: 196–210). Boosters were completed 20 (IQR: 12–68) and 19 days (IQR: 11–64) before Delta and Omicron exposure.
Table 1.
Characteristics of SARS-CoV-2 infections and close contacts in Delta and Omicron transmission chain
| Characteristics | Delta transmission chain | Omicron transmission chain | ||||||||
| Non/partial vaccination (n, %) |
Primary vaccination | Booster vaccination (n, %) |
P value | Non/partial vaccination (n, %) | Primary vaccination | Booster vaccination (n, %) | P value | |||
| ≥180 days (n, %) | <180 days (n, %) | ≥180 days (n, %) | <180 days (n, %) | |||||||
| Subtotal | 49 | 97 | 182 | 51 | 12 | 65 | 319 | 9 | ||
| Gender | ||||||||||
| Female | 33 (67.3) | 53 (54.6) | 104 (57.1) | 34 (66.7) | 0.2989 | 7 (58.3) | 42 (64.6) | 176 (55.2) | 5 (55.6) | 0.5767 |
| Male | 16 (32.7) | 44 (45.4) | 78 (42.9) | 17 (33.3) | 5 (41.7) | 23 (35.4) | 143 (44.8) | 4 (44.4) | ||
| Age group (years) | ||||||||||
| <18 | 8 (16.3) | 0 (0) | 53 (29.1) | 0 (0) | <0.001 | 2 (16.7) | 0 (0) | 191 (59.9) | 0 (0) | <0.001 |
| 18–49 | 19 (38.8) | 54 (55.7) | 50 (27.5) | 31 (60.8) | 7 (58.3) | 45 (69.2) | 107 (33.5) | 7 (77.8) | ||
| ≥50 | 22 (44.9) | 43 (44.3) | 79 (43.4) | 20 (39.2) | 3 (25.0) | 20 (30.8) | 21 (6.6) | 2 (22.2) | ||
| Close contacts | ||||||||||
| Subtotal | 525 | 814 | 1619 | 575 | 386 | 524 | 1120 | 174 | ||
| Gender | ||||||||||
| Female | 270 (51.4) | 430 (52.8) | 792 (48.9) | 307 (53.4) | 0.1513 | 215 (55.7) | 310 (59.2) | 529 (47.2) | 106 (60.9) | <0.001 |
| Male | 255 (48.6) | 384 (47.2) | 827 (51.1) | 268 (46.6) | 171 (44.3) | 214 (40.8) | 591 (52.8) | 68 (39.1) | ||
| Age group (years) | ||||||||||
| <18 | 149 (28.4) | 0 (0) | 674 (41.6) | 0 (0) | <0.001 | 100 (25.9) | 0 (0) | 616 (55.0) | 0 (0) | <0.001 |
| 18–49 | 250 (47.6) | 598 (73.5) | 588 (36.3) | 421 (73.2) | 187 (48.5) | 366 (69.9) | 341 (30.5) | 123 (70.7) | ||
| ≥50 | 126 (24.0) | 216 (26.5) | 357 (22.1) | 154 (26.8) | 99 (25.6) | 158 (330.1) | 163 (14.5) | 51 (29.3) | ||
| Total | 574 | 911 | 1801 | 626 | 398 | 589 | 1439 | 183 | ||
Breakthrough infection
Breakthrough infection rates were 9.9% in the Delta chain and 17.8% in the Omicron chain (p<0.001) among subjects completing primary vaccination. By vaccination group, breakthrough rates were 10.6% (Delta) and 11.0% (Omicron) among subjects who completed primary vaccination ≥180 days (p>0.05); 10.1% (Delta) and 22.2% (Omicron) among subjects who completed primary vaccination <180 days (p<0.001); and 8.1% (Delta) and 4.9% (Omicron) among boosted subjects (p>0.05).
When subjects under 20 years old were excluded, breakthrough rates were 10.8% (Delta) and 10.6% (Omicron) among subjects who completed primary vaccination ≥180 days (p>0.05); 12.3% (Delta) and 8.7% (Omicron) among subjects who completed primary vaccination <180 days (p>0.05); and 7.9% (Delta) and 4.5% (Omicron) among boosted subjects (p>0.05).
Relative vaccine effectiveness
Table 2 shows results of the Cox regression analyses for symptomatic infection and pneumonia by Delta and Omicron variants. For both variants, univariate analysis shows that being male and booster vaccination were associated with reduced risk of symptomatic infection. Age was associated with symptomatic infection, especially among subjects ≥50 years. Cox regression adjusting for age group, gender and vaccination status were similar in magnitude for symptomatic infection risk from Delta versus Omicron. HRs among those ≥50 years differed in direction between Delta and Omicron (2.75 and 0.56), and relative protection increased from 24% to 59% in boosted subjects. After removing subjects <20 years (primarily the school outbreak students) from the Cox regression analysis, primary vaccination <180 days and booster vaccination were both associated with protection from Delta and Omicron symptomatic infections.
Table 2.
Cox regression analysis by vaccination status and by outcomes of SARS-CoV-2 infection
| Covariates | Delta infection for all | Omicron infection for all | Omicron infection for ≥20 years | |||||||
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Symptomatic infection | ||||||||||
| Gender | ||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | |||||
| Male | 0.80 (0.64 to 1.00) | 0.05 | 0.79 (0.63 to 0.98) | 0.04 | 0.80 (0.65 to 0.99) | 0.04 | 0.73 (0.59 to 0.90) | 0.01 | 0.52 (0.33 to 0.80) | 0.003 |
| Age group (age) | ||||||||||
| <18 | Ref | Ref | Ref | Ref | ||||||
| 18–49 | 1.17 (0.84 to 1.63) | 0.36 | 1.26 (0.87 to 1.81) | 0.22 | 0.65 (0.52 to 0.81) | <0.01 | 0.92 (0.72 to 1.18) | 0.5 | Ref (20 to 49 years) | |
| ≥50 | 2.58 (1.86 to 3.60) | <0.01 | 2.75 (1.94 to 3.91) | <0.01 | 0.40 (0.28 to 0.56) | <0.01 | 0.56 (0.39 to 0.81) | 0.01 | 1.33 (0.89 to 2.00) | 0.166 |
| Vaccination status | ||||||||||
| Primary vaccination ≥180 days | Ref | Ref | Ref | Ref | Ref | |||||
| Primary vaccination <180 days | 0.92 (0.72 to 1.18) | 0.51 | 1.04 (0.79 to 1.36) | 0.79 | 2.11 (1.60 to 2.78) | <0.01 | 1.95 (1.43 to 2.65) | <0.01 | 0.79 (0.52 to 1.20) | 0.261 |
| Booster vaccination | 0.75 (0.53 to 1.06) | 0.11 | 0.76 (0.54 to 1.07) | 0.11 | 0.41 (0.20 to 0.87) | 0.02 | 0.41 (0.20 to 0.86) | 0.02 | 0.39 (0.18 to 0.86) | 0.019 |
| Pneumonia | ||||||||||
| Gender | ||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | |||||
| Male | 0.65 (0.47 to 0.90) | 0.01 | 0.65 (0.47 to 0.90) | 0.01 | 0.96 (0.60 to 1.52) | 0.85 | 0.93 (0.58 to 1.49) | 0.77 | 0.77 (0.39 to 1.54) | 0.457 |
| Age group (years) | ||||||||||
| <18 | Ref | Ref | Ref | Ref | ||||||
| 18–49 | 8.61 (2.70 to 27.43) | <0.01 | 9.11 (2.81 to 29.5) | <0.01 | 0.88 (0.52 to 1.50) | 0.63 | 0.89 (0.48 to 1.67) | 0.73 | Ref (20 to 49 years) | |
| ≥50 | 28.00 (8.88 to 88.40) | <0.01 | 29.50 (9.26 to 94.0) | <0.01 | 1.11 (0.59 to 2.07) | 0.75 | 1.13 (0.56 to 2.27) | 0.74 | 1.70 (0.87 to 3.32) | 0.123 |
| Vaccination status | ||||||||||
| Primary vaccination ≥180 days | Ref | Ref | Ref | Ref | Ref | |||||
| Primary vaccination <180 days | 0.60 (0.43 to 0.83) | 0.01 | 0.86 (0.61 to 1.20) | 0.37 | 0.84 (0.51 to 1.37) | 0.48 | 0.83 (0.46 to 1.50) | 0.53 | 0.65 (0.32 to 1.32) | 0.231 |
| Booster vaccination | 0.38 (0.22–0.64) | <0.01 | 0.38 (0.23 to 0.66) | <0.01 | 0.13 (0.02 to 0.97) | 0.047 | 0.13 (0.02 to 0.97) | 0.047 | 0.15 (0.02 to 1.08) | 0.059 |
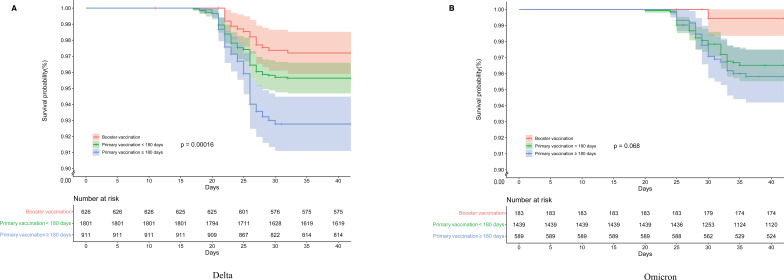
For pneumonia, the relation with age was more pronounced than was the case for symptomatic infection, and more so with Delta than Omicron. HRs for pneumonia were consistent in direction, showing greater protection associated with primary vaccination <180 days and booster dose administration, regardless of variant. After excluding subjects <20 years from the Cox regression analysis, HRs against pneumonia change little, except for an increase in protection (from 17% to 35%) among subjects who received primary vaccination within 180 days. Survival curve analysis results for pneumonia for each variant by vaccination status group are shown in figure 2. Although the three vaccination groups were statistically significantly different for Delta pneumonia, there was no statistically significant difference between the two primary vaccination groups for Omicron pneumonia, although both were different from the boosted group.
Figure 2.
Survival curves of pneumonia caused by Delta and Omicron variants. Figure 2A, B shows Cox model estimates of the survival probability of pneumonia with the Delta and Omicron variant of SARS-CoV-2 according to vaccination status, adjusting gender and age, starting from 15 December 2021. Time zero is 2 weeks before the first case.
There were few severe infections in the two transmission chains. There were 12 severe cases in the Delta chain, with 5 included in Cox analysis (2 with primary vaccination ≥180 days group, 3 cases with primary vaccination <180 days and none in the booster vaccination group. There were no severe cases in the Omicron transmission chain.
Discussion
We used a 748-case, two-variant COVID-19 outbreak in Henan province to determine the rVE of the vaccines used in China to protect against symptomatic infection and pneumonia. Our study found an rVE of 62% against Delta pneumonia and 87% against Omicron pneumonia among homologous inactivated vaccine booster-dose recipients compared with individuals who received complete primary vaccination greater than 180 days prior to exposure to SARS-CoV-2. rVE was lower for individuals receiving primary vaccination less than 180 days prior to exposure than among boosted individuals, demonstrating an effectiveness advantage of the homologous booster dose. Results from our study support the current COVID-19 booster vaccination strategy in China in which everyone 18 years and older is recommended to receive a booster dose 6 months after their primary series.
Our study also found a higher breakthrough infection rate in the Omicron transmission chain than the Delta chain (22% vs 10%) and found no severe Omicron cases and only 12 severe Delta cases, representing 3% of the Delta outbreak cases. The low rate of severe infection provides information valuable for projection of healthcare resource needs in the future.
In licensure clinical trials for vaccines produced in China estimates of efficacy against symptomatic COVID-19 were 50%–78%.15 16 Our rVE estimates are not directly comparable, since efficacy estimates are absolute rather than relative estimates. A rVE estimate only indicates additional VE above and beyond an unmeasured absolute VE of the comparison group. There have been several real-world assessments of VE of the China-produced vaccines,8–11 but none that compare rVE against Delta and Omicron in simultaneous outbreaks, and few studies of rVE from a booster dose of China-produced vaccine.
As of 7 September 2022, 603 million confirmed cases of COVID-19 and 6.4 million COVID-19 deaths were reported worldwide to the WHO.17 To stop the virus from raging around the world, vaccines had high hopes. In less than a year after SARS-CoV-2 emergence, COVID-19 vaccines were developed via several technologies18 and have been approved by regulators and WHO for emergency use. WHO approved the two inactivated vaccines that comprised the vast majority of COVID-19 vaccines used in China and included in our study.
In the real world, several studies reported high effectiveness of mRNA-based vaccines against SARS-CoV-2 infection, with a breakthrough infection of less than 1% of symptomatic SARS-CoV-2 infections, and a ~0.1% rate of hospitalisation or death.19 20 However, rapid decrease of neutralising antibody levels in the first 3 months after the second dose was observed,21 accompanied by the significant decline of protection 6 months after completion of two-dose regimen.22 23 However, a third dose could significantly restore protection, especially protection against severe COVID-19-related outcomes. In large observational studies conducted in Israel, compared with two doses regimen completed at least 5 months previously, adding a third dose was estimated to be >90% effective in preventing severe outcomes of SARS-CoV-2 infections.24 25 Also in Israel, among residents of long-term care facilities, a relative reduction of 71% and 80% on preventing SARS-CoV-2 infection and hospitalisation were observed after receiving third doses.26 In China, inactivated vaccines were implemented widely, and a similar decline of neutralising titres was also observed. Immunogenicity of homologous booster doses of inactivated vaccine has been illustrated in clinical trials,7 27 and the likely mechanism for booster dose immunogenicity and corresponding effectiveness is activation of memory B cells induced by primary series vaccination. However, due to high primary-series COVID-19 vaccine coverage in China, it is challenging to measure absolute VE because measuring absolute VE requires an unvaccinated comparison group. This challenge is greater for estimating absolute VE against the Omicron variant since the Omicron infections were more recent than the ancestral and Delta variant infections.
The number of studies of COVID-19 VE against Omicron is growing. VEs of mRNA vaccines and adenovirus vector vaccines against Omicron were significantly lower than against Delta. At the end of 2021, a real-world study on VE against Omicron pneumonia in South Africa showed that VE of Pfizer mRNA vaccine was 70%.2 VE against infection was limited and VE against hospitalisation decreased significantly with time since vaccination (82% within 14 days, 52% between 15 and 179 days, and 38% over 180 days). However, after a homologous booster dose, VE against hospitalisation caused by Delta and Omicron increased to 94% and 90%. Although our study measured rVE, the booster dose impact appears consistent, showing that booster vaccination improves protective effectiveness of the vaccines.3 28
We found a higher breakthrough infection rate for Omicron than Delta exposure. A likely explanation is that most Omicron transmission was in a large boarding middle school. The crowded student dormitories, classrooms and canteens may greatly increase pathogen exposure, leading to reduced protection.29 After receiving boosters, we observed significantly lower breakthrough rates for Delta (22% vs 8%) and Omicron exposure (10% vs 5%). Immunity elicited by COVID-19 vaccines wanes over time,30 and giving booster doses is necessary for restoration.4 31 32
Consistent with what is well known about COVID-19, older age was risk factor for pneumonia in our study. The HR among people over 50 years old was higher than in people under 18 years old for Delta pneumonia. Hyporesponsiveness among the elderly has been reported from clinical studies of COVID-19 vaccines.19 33 34 Due to immunosenescence, elderly have a lower ability to fight respiratory infections and are hyporesponsive to vaccination.35 36
There are several limitations in our study. First, the age distributions in the Delta and Omicron transmission chains were different. Delta transmission occurred in a community, while Omicron transmission was concentrated in a middle school. The imbalance in age did not appear to influence the rVE of booster doses against Delta and Omicron in age-based sensitivity analysis. Second, we did not have data on comorbidities of most subjects. We; therefore, could not use comorbidities in the Cox regression model. Comorbidity data were available in a small subgroup, however, and these data indicated that more than 80% of comorbidity occurred in people ≥50 years. We therefore used 50 years of age as a cut-off for age group aggregation. Third, because coverage was very high at 90%, there were too few unvaccinated people to serve as control to measure absolute VE. The small number of unvaccinated individuals are likely to be significantly different than vaccinated people, making them an unreliable control group. We; therefore, excluded subjects who did not complete primary vaccination, and instead used the numerous subjects who completed the primary vaccination ≥180 days as reference to measure the rVE throughout the analysis. Finally, the outbreak was too small and the subjects too few to assess rVE for the different vaccines. Since almost all of the vaccine used in Henan (and China) are the two inactivated vaccines in our study, we believe it is reasonable to conclude that we assessed rVE for China’s inactivated vaccines, especially the homologous booster dose, and that our results can generalise to the rest of mainland China. However, our study results cannot generalise to other countries.
Conclusion
COVID-19 vaccination in China provided good protection against symptomatic COVID-19 and COVID-19 pneumonia caused by Delta and Omicron variants. Protection declined 6 months after primary series vaccination but was restored by homologous inactivated booster doses given 6 months after the primary series.
bmjopen-2022-063919supp001.pdf (254.4KB, pdf)
Supplementary Material
Acknowledgments
We are very grateful for the data collection efforts by local CDC and infectious hospital staff of Zhengzhou, Yuzhou, and Anyang cities in Henan province. We are very grateful to Yaowen Zhang, Yang Liu (Beijing Institute of Biological Products, SINOPHARM), and Xiaobin Cao and Yupeng Liang (Sinovac Research & Development) for help with data extraction and running SAS code for data analysis.
Footnotes
LT and YZ contributed equally.
Contributors: LT and YZ contributed equally to this work as joint first authors. ZY is the guarantor of the work. ZY, Z-YW, X-YW, X-YL and Y-MS acquired funding and contributed to the study’s conception and design. A-BW, RZ, Z-HQ, FW, CH, ZA, HW and YY conceptualised the study and prepared the original study protocol, which was subsequently reviewed by LR. DW, LT and CH developed the statistical methods. DW, CH and X-YW wrote and tested the SAS code for the data analysis and drafted the manuscript. YY, HW, YZ, JP, Y-FL, ML, CW and Y-TM collected data from local CDCs and abstracted the data. DW, LT, LR, ZA, ZY and Y-MS interpreted the results and revised the manuscript and critically reviewed the manuscript. All authors approved the final submitted version.
Funding: This work was supported by the Operation of Public Health Emergency Response Mechanism of Chinese Center for Disease Control and Prevention (131031001000150001) and the National Key Research and Development Program of China(2021YFC2301600).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but COVID-19 is a class A notifiable infectious disease according to the Law on the Prevention and Control of Infectious Diseases (SCSNPC, 1989) in China; case reporting and investigation are mandatory. This study used data from COVID-19 surveillance and control and COVID-19 vaccination records available to public health officials; individual informed consent is not required or possible for routine surveillance data collection for a class A notifiable infectious disease. Individual identifying data were not avilable to the investigators. The study was approved and exempted by the Institutional Review Board of the Chinese Center for Disease Control and Prevention. Approval Notice:202101-01. Investigation of COVID-19 outbreaks is mandatory public health work. Our COVID-19 VE studies are exempted by IRB Approval Notice:202101-01. The study used public health disease surveillance data that are routinely collected for all COVID-19 cases, and public health vaccination data that are routinely collected for all vaccinations administered. Consent for use of the routine public health data was not obrained and not possible to obtain from individual subjects.
References
- 1.World Health Organization . Weekly epidemiological update on COVID-19[Accessed 22 Feb 2022].
- 2.Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022;386:494–6. 10.1056/NEJMc2119270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen CH, Schelde AB, Moustsen-Helm IR. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv 2021:2021:21267966. 10.1101/2021.12.20.21267966 [DOI] [Google Scholar]
- 4.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139–45. 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TGAfVa I. COVAX global supply forecast, 2021. Available: https://www.gavi.org/sites/default/files/covid/covax/COVAX-Supply-Forecast.pdf
- 6.World Health Organization . Covid-19 vaccines. Available: https://www.who.int/teams/regulation-prequalification/eul/covid-19
- 7.Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis 2022;22:483-495. 10.1016/S1473-3099(21)00681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Tao B, Li Z, et al. Effectiveness of inactivated COVID-19 vaccines against severe illness in B.1.617.2 (Delta) variant-infected patients in Jiangsu, China. Int J Infect Dis 2022;116:204–9. 10.1016/j.ijid.2022.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Zhang Y, Tang L, et al. Effectiveness of Inactivated COVID-19 Vaccines Against Symptomatic, Pneumonia, and Severe Disease Caused by the Delta Variant: Real World Study and Evidence - China, 2021. China CDC Wkly 2022;4:57–65. 10.46234/ccdcw2022.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang M, Yi Y, Li Y, et al. Effectiveness of Inactivated COVID-19 Vaccines Against Illness Caused by the B.1.617.2 (Delta) Variant During an Outbreak in Guangdong, China : A Cohort Study. Ann Intern Med 2022;175:533–40. 10.7326/M21-3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X-N, Huang Y, Wang W, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect 2021;10:1751–9. 10.1080/22221751.2021.1969291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385:875–84. 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am 2021;1:100025. 10.1016/j.lana.2021.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A TGOoNHCNT. Infectious Diseases & Immunity. In: Diagnosis and treatment protocol for COVID-19 patients. 1. 8 th, 2021: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021;326:35–45. 10.1001/jama.2021.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RaB P, Paula A, González E, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study https://ssrn.com/abstract=3822780 10.2139/ssrn.3822780 [DOI]
- 17.Organization WH . Who coronavirus (COVID-19) Dashboard, 2022. Available: https://covid19.who.int/ [Accessed 02 Mar 2022].
- 18.Krammer F. SARS-CoV-2 vaccines in development. Nature 2020;586:516–27. 10.1038/s41586-020-2798-3 [DOI] [PubMed] [Google Scholar]
- 19.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med Overseas Ed 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet 2021;397:1819–29. 10.1016/S0140-6736(21)00947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021;385:e85. 10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385:e83. 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093–100. 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021;385:2413–20. 10.1056/NEJMoa2115624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhsen K, Maimon N, Mizrahi A, et al. Effects of BNT162b2 Covid-19 vaccine booster in long-term care facilities in Israel. N Engl J Med 2022;386:399–401. 10.1056/NEJMc2117385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Yang Y, Qiao N, et al. Early assessment of the safety and immunogenicity of a third dose (booster) of COVID-19 immunization in Chinese adults. Front Med 2022;16:93–101. 10.1007/s11684-021-0914-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med 2021;27:2136–43. 10.1038/s41591-021-01583-4 [DOI] [PubMed] [Google Scholar]
- 29.Langwig KE, Gomes MGM, Clark MD, et al. Limited available evidence supports theoretical predictions of reduced vaccine efficacy at higher exposure dose. Sci Rep 2019;9:3203. 10.1038/s41598-019-39698-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 Omicron–reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol 2022;7. 10.1126/sciimmunol.abo2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science 2021;374:1561–2. 10.1126/science.abl8487 [DOI] [PubMed] [Google Scholar]
- 32.Petersen M, Schwab J, Havlir DV. SARS-CoV-2 vaccine boosters: the time to act is now. PLoS Med 2021;18:e1003882. 10.1371/journal.pmed.1003882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med Overseas Ed 2022;386:340–50. 10.1056/NEJMoa2115481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crooke SN, Ovsyannikova IG, Poland GA, et al. Immunosenescence and human vaccine immune responses. Immun Ageing 2019;16:25. 10.1186/s12979-019-0164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh S-J, Lee JK, Shin OS. Aging and the immune system: the impact of Immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw 2019;19:e37. 10.4110/in.2019.19.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063919supp001.pdf (254.4KB, pdf)
Data Availability Statement
No data are available. Not applicable.