Abstract
Background
Left ventricular hypertrophy (LVH) is the main marker of HMOD in children and young people (CYP). We aimed to assess the prevalence of LVH and its determinants in CYP with primary hypertension (PH).
Methods
A meta-analysis of prevalence was performed. A literature search of articles reporting LVH in CYP with PH was conducted in Medline, Embase, and Cochrane databases. Studies with a primary focus on CYP (up to 21 years) with PH were included. Meta-regression was used to analyze factors explaining observed heterogeneity.
Results
The search yielded a total of 2,200 articles, 153 of those underwent full-text review, and 47 reports were included. The reports evaluated 51 study cohorts including 5,622 individuals, 73% male subjects, and a mean age of 13.6 years. LVH was defined as left ventricle mass index (LVMI) ≥ 95th percentile in 22 (47%), fixed cut-off ≥38.6 g/m2.7 in eight (17%), sex-specific fixed cut-off values in six (13%), and miscellaneously in others. The overall prevalence of LVH was 30.5% (95% CI 27.2–33.9), while heterogeneity was high (I2 = 84%). Subgroup analysis including 1,393 individuals (76% male subjects, mean age 14.7 years) from pediatric hypertension specialty clinics and LVH defined as LVMI ≥95th percentile only (19 study cohorts from 18 studies), reported prevalence of LVH at 29.9% (95% CI 23.9 to 36.3), and high heterogeneity (I2 = 84%). Two studies involving patients identified through community screening (n = 1,234) reported lower LVH prevalence (21.5%). In the meta-regression, only body mass index (BMI) z-score was significantly associated with LVH prevalence (estimate 0.23, 95% CI 0.08–0.39, p = 0.004) and accounted for 41% of observed heterogeneity, but not age, male percentage, BMI, or waist circumference z-score. The predominant LVH phenotype was eccentric LVH in patients from specialty clinics (prevalence of 22% in seven studies with 779 participants) and one community screening study reported the predominance of concentric LVH (12%).
Conclusion
Left ventricular hypertrophy is evident in at least one-fifth of children and young adults with PH and in nearly a third of those referred to specialty clinics with a predominant eccentric LVH pattern in the latter. Increased BMI is the most significant risk association for LVH in hypertensive youth.
Keywords: left ventricular hypertrophy, primary hypertension, children, adolescents, left ventricular mass index
Introduction
Arterial hypertension (HT) is considered one of the most important global health problems and represents a potentially reversible risk factor for cardiovascular disease (CVD) development (1, 2). HT affects 4–5% of children and adolescents in general, and the prevalence increases with age (3). Importantly, childhood blood pressure (BP) has been shown to track into adulthood and is associated with subsequent cardiovascular (CV) outcomes (4). The increasing prevalence of childhood HT observed over the last decades associated with the global childhood obesity epidemic is likely to have significant implications for the development of adult CVD (5).
The heart and blood vessels are the primary organs adversely affected as a result of HT as they are directly exposed to the elevated BP, with secondary involvement of the kidneys and the central nervous system. In most cases, hypertension-mediated organ damage (HMOD) occurs in several stages. The development of diagnostic techniques makes it possible to detect early changes, which are often clinically silent and potentially reversible before established CVD (6). Persistent HT results in adaptive changes to reduce wall stress in the blood vessels and heart and include increasing thickness of blood vessels and left ventricular remodeling (7). Thus, cardiomyocyte hypertrophy and thickening of the artery wall are observed. With disease progression, the deposition of the extracellular matrix develops with an increase in the stiffness of the arterial wall and impaired function of the left ventricle and arteries (8). The development of HMOD is complex and depends on several factors including the level and duration of HT, individually variable response of tissues and organs to increased BP, and accompanying metabolic abnormalities (8–11).
Left ventricular hypertrophy (LVH) is the primary marker evaluated for HMOD. Its presence is a therapeutic indication in individuals with primary hypertension (PH) (12). Numerous studies have reported that in a substantial proportion of children and adolescents with PH, LVH is present already at diagnosis, but the extent of its prevalence has not yet been evaluated systematically. Furthermore, there are few data regarding the remodeling of the left ventricle and its determinants. The aim of our study was to estimate the prevalence of LVH and its determinants in children and young people with confirmed PH by meta-analysis and meta-regression using clearly defined inclusion and exclusion criteria and focusing on studies with PH in CYP.
Materials and methods
A systematic review and meta-analysis to determine the prevalence of LVH and different remodeling patterns in children and young people with PH was performed. Meta-regression was used to analyze between-study factors explaining observed heterogeneity in reported prevalence.
This systematic review and meta-analysis were carried out and the data are presented according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13).
Eligibility criteria
The following eligibility criteria were used to select studies for the analysis:
Inclusion criteria
Original research published since 1990.
Studies that include children and young people (up to 21 years) with PH (or reports subgroup data for patients with PH).
Prevalence and definition of LVH are reported.
Exclusion criteria
Other languages than English.
Reviews, case reports, case series, and animal studies.
Studies including < 20 patients.
Studies analyzing the data from the same or overlapping cohort.
Studies primarily including patients with obesity or diabetes mellitus.
Community studies where the exclusion of secondary causes of HT was not stated.
Studies that include data from patients receiving anti-hypertensive treatment or when the treatment status of patients was not explicitly stated.
Literature search and articles selection
A literature search was performed in MEDLINE, Embase, and Cochrane Library databases on 25 April 2022 by an academic librarian (ISKS). The search was peer-reviewed by a second academic librarian. The search included relevant synonyms and MeSH/Emtree subject headings, as well as an adapted broad search filter for children from Ovid Expert Searches to best include all pediatric and young adult studies. The literature search strategy is described in detail in the Supplementary Methods. After the removal of duplicates, all articles underwent title and abstract screening by two authors (TB and JSK). Shortlisted articles that were screened as potentially meeting eligibility criteria were independently evaluated by full-text review by at least two authors (KA, KM, TB, JSK, and MDS) and selected for final analysis. In cases where repeated data were identified between two or more studies, the study with the highest sample size or most accurate cohort description (i.e., BP phenotypes reporting) was selected.
Definitions and data extraction
Definitions of HT, pre-hypertension (preHT), high-normal BP, and white-coat hypertension (WCH), both by office BP and by ambulatory BP monitoring (ABPM), that were proposed as per relevant international or national clinical practice guidelines were considered for the analyses. No restrictions on the method to estimate left ventricular mass index (LVMI), to define LVH and left ventricular remodeling patterns were applied.
All data extraction was performed independently by two authors and compared. The following data were extracted into pre-specified data extraction forms: first author; year of publication; description of study population based on recruitment: (i) community sample or (ii) specialty clinic; sample size and number of patients with different BP phenotypes; sex, ethnicity, mean age, and age range; mean body mass index (BMI) and waist circumference (WC) with their respective z-scores (BMIz and WCz, when available); and the number of obese individuals. For LVMI and definition of LVH, we analyzed the LVMI calculation method, definition of LVH, number of patients with LVH, severe LVH, concentric remodeling, concentric LVH, and eccentric LVH (if such data were reported). Data were extracted for the whole group of individuals with PH and per each BP phenotype (if such data were reported). In case of longitudinal studies, the data from the baseline visit were included.
If the study reported data on subgroups of different phenotypes of PH (e.g., WCH and preHT) and data were not reported for the overall/combined group or pooling was impossible, data were extracted and analyzed separately for each subgroup that were treated as independent (further referred as “study cohorts”). If a study compared several definitions of BP status and/or LVH, European Society of Hypertension (ESH) 2016 guidelines and LVH definition based on the 95th percentile cut-off were selected for primary analysis (12, 14). If absolute numbers were not indicated but could be determined by transforming data (percentage to absolute number) or confidently identified from graphs—these estimates were used.
Critical appraisal
All studies selected for the final analysis underwent risk of bias assessment by two authors independently (JSK, TB, KM, KA) using the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data (15). Domain regarding the sufficient coverage of the sample in the analysis (number five) was not evaluated as not being relevant based on the instrument's manual. Studies scoring low risk of bias (answer “Yes”) in more than half of the evaluated domains were considered as low risk of bias. Details on the provisional guidance to use the critical appraisal tool for the present analysis are shown in Supplementary Table S1.
In cases of any discrepancies in the process of study selection, data extraction, or critical appraisal, discussions with a third reviewer led to the resolution and final decision.
Meta-analysis and meta-regression
Descriptive characteristics of selected studies were summarized by calculating weighted means for continuous data and weighted proportions for categorical data. A meta-analysis of proportions was performed to calculate weighted pooled prevalence using a random effects model to account for expected heterogeneity. Prevalence estimates first underwent Freeman–Tukey double arcsine transformation to stabilize variance and the data were then back-transformed to provide a pooled estimate. Heterogeneity was assessed by Higgins I2 and Cochrane Q-tests. Mixed effects meta-regression was performed to determine between-study factors leading to observed heterogeneity. Funnel plots were created and visually inspected, as well as Egger's test was used to identify potential publication bias. All statistical analyses were performed using RStudio version 1.4.1106 and packages meta and metafor.
Results
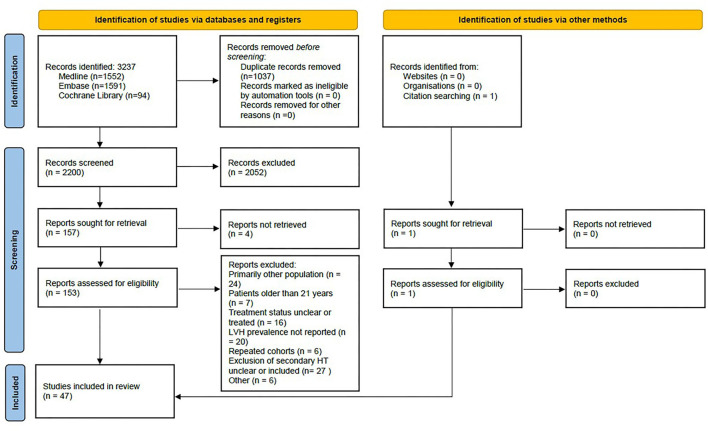
The article selection process is summarized in the PRISMA flow diagram (Figure 1). Briefly, out of 2,200 records identified through a literature search, 153 underwent full-text review (studies excluded from the analysis are shown in Supplementary Table S2) and 47 were selected for the analysis (16–62). These studies with 51 study cohorts included a total of 5,622 subjects (73% male subjects), mean age of 13.6 years (reported in 41 study cohorts), mean BMI of 25.4 kg/m2 (reported in 33 study cohorts), and BMIz 1.43 (21 study cohorts). The proportion of obese children (23 study cohorts) was 37%, while the mean WCz was 1.30 (8 study cohorts). Three were community screening-based cohorts (17, 30, 50), 41 reported data from pediatric HT specialty clinics, and four reported mixed data from both (31, 42, 45, 56). LVH was defined as LVMI above 95th percentile in 22 (47%) studies (17, 18, 21, 23, 25–28, 32–34, 37, 44, 46, 47, 50–53, 57, 60, 61), fixed cut-off ≥38.6 g/m2.7 in eight (17%) (19, 24, 36, 40, 43, 45, 49, 55), sex-specific fixed cut-offs (≥36.88 g/m2.7 and ≥39.36 g/m2.7 for female and male subjects, respectively) in six (13%) (29, 35, 38, 39, 41, 48), one used magnetic resonance imaging (MRI) (58), while the remaining were heterogeneous. Ethnicity was reported in 27 (61%) studies. Fourteen studies included subjects with WCH (28, 35, 36, 38, 45, 46, 57, 58) and/or preHT (24, 28, 37, 39, 46, 54, 57, 61). Detailed characteristics of all studies are shown in Supplementary Table S3.
Figure 1.
PRISMA flow diagram for article selection.
Prevalence of LVH
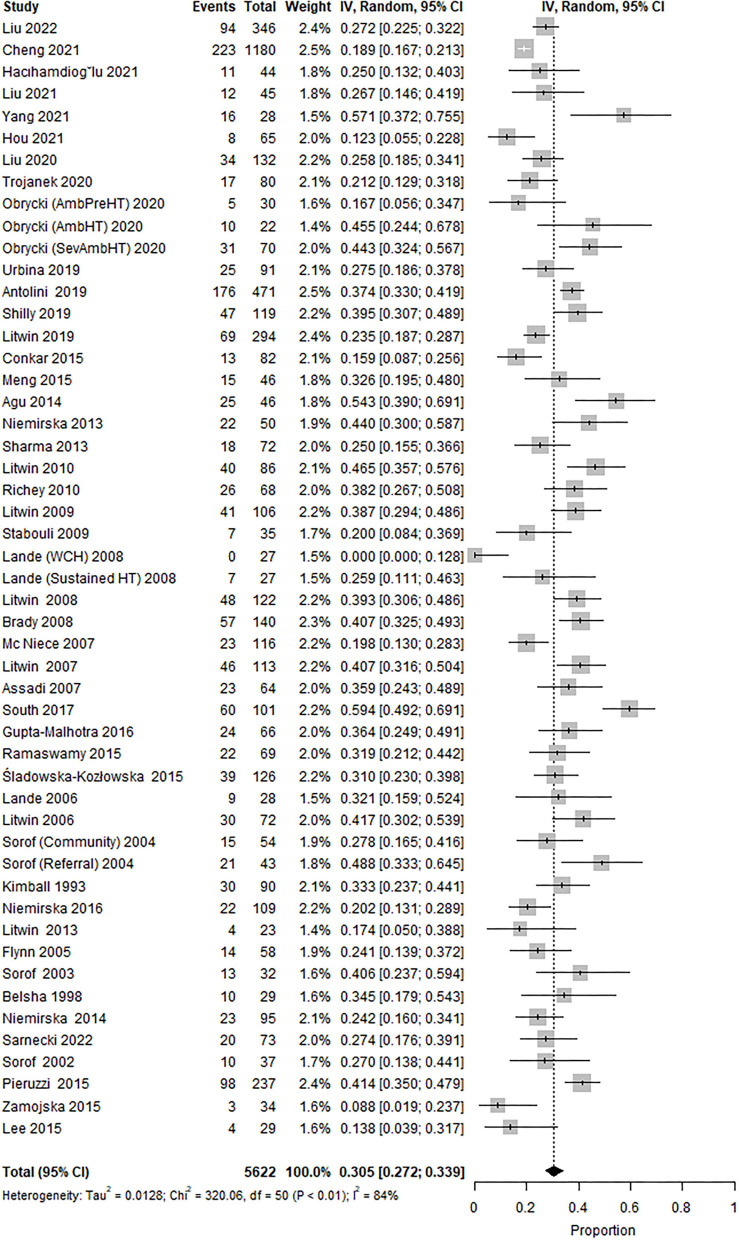
The overall prevalence of LVH was 30.5% (95% CI 27.2 to 33.9; Figure 2), while the heterogeneity was high (I2 = 84%).
Figure 2.
Forest plot showing the prevalence of LVH in all identified studies (47 studies, 51 study cohorts).
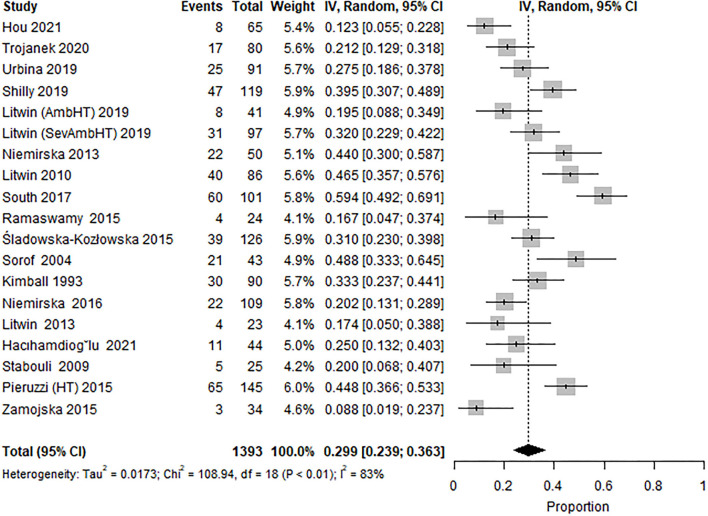
Two sub-group analysis studies were performed. (i) Subgroup 1: including studies reporting data from pediatric HT specialty clinics only (19 study cohorts from 18 studies) (18, 21, 23, 25, 27, 28, 32, 34, 37, 44, 46, 47, 50–53, 60, 61), reported prevalence of LVH at 29.9% (95% CI 23.9–36.3; Figure 3), and high heterogeneity (I2 = 84%). LVH in them was defined as LVMI ≥95th percentile; and excluded subjects with WCH and/or preHT and included 1,393 patients (76% male subjects) with a mean age of 14.7 years, mean BMI of 25.9 kg/m2 (17 study cohorts), and mean BMIz of 1.40 (14 study cohorts). Six study cohorts reported a 29% obesity rate in children, and six reported WCz (mean 1.28). The characteristics of these studies are shown in Table 1. Hypertension was defined exclusively by only office BP in six study cohorts.
Figure 3.
Forest plot showing the prevalence of LVH in the subset of studies from specialty clinics, LVH defined by ultrasound estimated LVMI ≥95th percentile and excluding subjects with WCH and/or preHT (18 studies, 19 study cohorts).
Table 1.
Characteristics of samples included in the main analysis (Subgroup 1).
| References |
Sample population (n) and PH phenotype (n, %) |
Ethnicity, n (%) |
Age (years) Male, n (%) |
BMI z-score WC z-score Obese patients, n (%) |
Patients with LVH (%) |
|---|---|---|---|---|---|
| Hacihamdioglu et al. (18) | 44 | NA | 14.0 ± 3.19 | 1.33 (0.66–1.87)c | 11 (25%) |
| NA | |||||
| NA | 34 (77.3%) | 26&& (59.1%) | |||
| Hou et al. (21) | 65 | Asian | 12.4 ± 2.3 | NA | 8 (12.3%) |
| NA | 48 (73.8%) | ||||
| Trojanek et al. (23) | 80 | NA | 15.1 ± 2 | 1.3 ± 0.74 | 17 (21.2%) |
| 1.5 ± 0.7 | |||||
| NA | 67 (83.7%) | NA | |||
| Urbina et al. (25) | 91 | White 53 (58.2%), Hispanic 15 (16.5%), Other 23 (25.3%) | 15.3 ± 1.7 | 1.094 ±−0.81 | 25 (27.5%) |
| NA | |||||
| NA | 54 (59.3%) | NA | |||
| Shilly et al. (27) | 119 | Black 35 (29.4%), Other 84 (70.6%) | 14 ± 3.3 | 1.2 ± 1.0 | 47 (39.5%) |
| NA | NA | ||||
| 84 (70.6%) | 33 (27.7%) | ||||
| Litwin et al. (AmbHT) (28) | 41 | NA | 15 ± 3 | 0.9 ± 1.1 | 8 (19.5%) |
| AmbHT (41, 100%) | 1.1 ± 1.1 | ||||
| 35 (85.4%) | NA | ||||
| Litwin et al. (SevAmbHT) (28) | 97 | NA | 15.3 ± 2.3 | 1.1 ± 0.8 | 31 (31.9%) |
| SevAmbHT (97, 100%) | 0.9 ± 0.8 | ||||
| 72 (74.2%) | NA | ||||
| South et al. (44) | 102 | White 26 (25.5%), Black 29 (28.4%), Hispanic 44 (43.1%), Other 3 (3%) | 14.9* (13.1–16.3)b | NA | 60! (59.4%) |
| HT1 (48, 47%), HT2 (14, 14%) | NA | ||||
| 76& (74.5%) | |||||
| 78 (76.5%) | |||||
| Niemirska et al. (52) | 109 | NA | 15.6 ± 1.5 | 1.35 ± 0.83 | 22 (20.2%) |
| 1.2 ± 0.9 | |||||
| HT1 (63, 58%), HT2 (46, 42%) | 90 (82.6%) | NA | |||
| Ramaswamy et al. (46) | 24 | Hispanic 7 (29.2%), Caucasian 10 (41.7%), Other 7 (29.2%) | 14.7 ± 3.3 | 1.2 ± 1.1 | 4 (16.7%) |
| NA | |||||
| 17 (70.8%) | NA | ||||
| NA | |||||
| Sladowska-Kozłowska et al. (47) | 126 | Caucasian | 15* | 1.75 ± 1.7 | 39 (30.9%) |
| AmbHT (92, 73%), SevAmbHT (34, 27%) | 95 (75.4%) | 1.62* (−2.03 to 6.12)a | |||
| NA | |||||
| Niemirska et al. (32) | 50 | NA | 15* (8.5–17)a | 2.0 ± 1.5 | 22 (44%) |
| AmbHT (21, 42%), SevAmbHT (29, 58%) | |||||
| 50 (100%) | NA | ||||
| 28 (56%) | |||||
| Litwin et al. (53) | 23 | NA | 15.0 ± 2.1 | 1.16 ± 0.8 | 4 (17.4%) |
| 0.93 ± 0.9 | |||||
| NA | 19 (82.6%) | NA | |||
| Litwin et al. (34) | 86 | Caucasian | 14.1 ± 2.4 | 1.8 ± 1.8 | 40 (46.5%) |
| AmbHT (50, 58%), SevAmbHT (36, 42%) | 1.8* (−1.1 to 6.1)a | ||||
| 66 (76.7%) | 21 (24.4%) | ||||
| Stabouli et al. (37) | 25 | Caucasian | 14.8 ± 4.2 | 1.28 ± 1.03 | 5 (20%) |
| NA | 13 (52%) | NA | |||
| NA | |||||
| Sorof et al. (50) | 43 | White 13 (30.2%), Hispanic 10 (23.25%), Black 18 (41.9%), Other 2 (4.65%) | 13.9 ± 2.0 | 1.76 ± 0.76 | 21 (48.8%) |
| NA | |||||
| NA | |||||
| 33 (76.7%) | 25∧ (58.1%) | ||||
| Kimball et al. (51) | 90 | White 46 (51.1%), Black 44 (48.9%) | 14 ± 4.0 | NA | 30 (33.3%) |
| NA$ | |||||
| NA | 54 (60%) | ||||
| Pieruzzi et al. (61) | 145 | NA | NA | NA | 65 (44.8%) |
| NA | |||||
| NA | 84 (57.9%) | 71 (48.97%) | |||
| Zamojska et al. (60) | 34 | NA | 15.3 ± 2.1 | NA | 3 (8.8%) |
| NA | |||||
| NA | 27 (79.4%) | ||||
| 0 |
Continuous data are represented as mean values with standard deviation, unless specified otherwise.
* Median.
a Data range; b Interquartile range; c 95% confidence interval.
& Overweight/obesity defined as ≥85th percentile and reported together.
&& Obese or overweight.
! n = 101.
∧ In the article, the term “Overweight” was defined as BMI ≥95th percentile.
$ Patients with obesity were excluded in this particular study.
AmbHT, Ambulatory hypertension; BMI, body mass index; n, number of patients; HT1, hypertension stage 1; HT2, hypertension stage 2 defined according to the fourth Report (ref.). LVH, left ventricular hypertrophy. LVMI, left ventricular mass index. NA, not available or not applicable. SevAmbHT, severe ambulatory hypertension. WC, waist circumference.
(ii) Subgroup 2: additionally including study cohorts reporting data from pediatric HT specialty clinics only that defined LVH by a fixed cut-off of 38.6 g/m2.7. Twenty-nine study cohorts (25 studies) (18, 19, 21, 23–25, 27, 28, 32, 34, 36, 37, 40, 43, 44, 46, 47, 49–53, 55, 60, 61) were included with a total of 1,940 subjects. The estimated pooled prevalence of LVH was 31.4% and heterogeneity was similarly high (I2 = 79%) (Supplementary Figure S1). Mean age in this subset was 14.7 years, 75% were male subjects, mean BMI was 25.8 kg/m2 (22 study cohorts), and mean BMIz 1.38 (20 samples). The proportion of obese children and WCz was reported in 11 study cohorts and was 27% and 1.22, respectively. HT was defined by only office BP in nine study cohorts.
Severe LVH defined as LVMI >51 g/m2.7 was reported in 12 included studies (15 study cohorts) with 873 individuals (mean age 14.9 years, 79% male subjects), and the pooled prevalence of severe LVH was 11% (I2 = 72%) (Supplementary Figure S2) (20, 23, 24, 32, 34, 36, 39, 43, 48, 49, 59, 62).
Left ventricle geometry
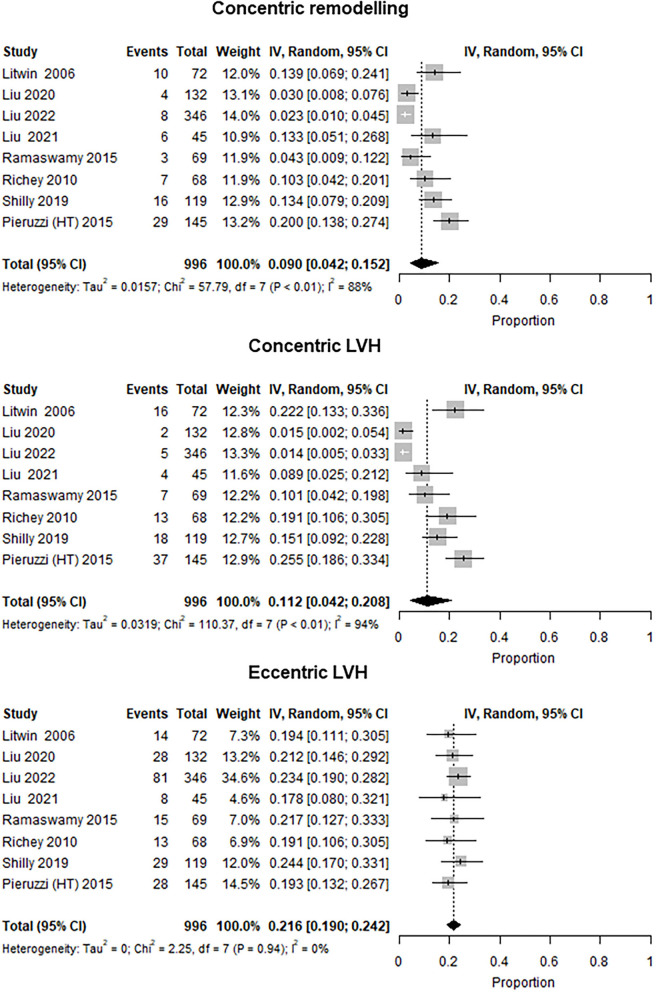
Left ventricle geometry was reported in eight studies from specialty clinics that included 996 participants (mean age 13 years, 72% male subjects) with an overall prevalence of LVH at 34.2% (I2 = 70%). The prevalence of concentric remodeling was 9.0% (I2 = 88%), concentric LVH was 11.2% (I2 = 94%), and that of eccentric LVH was 21.6% (I2 = 0%) (Figure 4) (16, 19, 22, 27, 35, 46, 49, 61). In addition, one study with an overall prevalence of LVH of 18.9% reported LV geometry data for children identified by community screening (17). In this study, the predominant LV geometry pattern was concentric LVH (12%), followed by concentric remodeling (8.7%) and eccentric LVH (6.9%).
Figure 4.
Forest plot showing the prevalence of LV geometry patterns (concentric remodeling, concentric LVH, and eccentric LVH) in studies with available data (eight studies). Three studies were excluded as being mixed [included community and specialty clinic patients: Agu et al. (31) and Gupta-Malhotra et al. (45)] or community samples (17).
Meta-regression
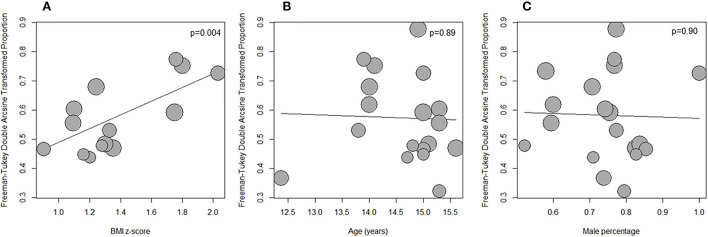
Meta-regression for LVH prevalence was performed in Subgroup 1 with the following variables: age, proportion male, BMI, BMIz, and WCz. BMIz alone was significantly associated with LVH prevalence (estimate 0.23, 95% CI 0.08–0.39, P = 0.004; Figure 5A) and accounted for 41% of the observed heterogeneity. The other variables were not associated with LVH prevalence including age and sex (Supplementary Table S4; Figures 5B,C).
Figure 5.
Bubble plot showing the relationship between LVH prevalence and BMI z-score (A), age (B), and male proportion (C) in the subset of studies from specialtyclinics, LVH defined by ultrasound estimated LVMI 95th percentile and excluding subjects with WCH and/or preHT.
Repeating the analysis in Subgroup 2 revealed similar results (BMIz as explanatory variable, estimate 0.23, 95% CI 0.09–0.36, P = 0.001; 34.7% heterogeneity accounted for).
Other subgroups
Community samples
Left ventricular hypertrophy prevalence in two community screening-based study cohorts (17, 50) was 21.5% (I2 = 60%) (Supplementary Figure S3). The studies included 1,234 participants (mean age 11.9 years, 61% male subjects).
White-coat and pre-hypertension
A total of 222 participants with WCH from four study cohorts were included (mean age 15.0 years, 80% male subjects) and the prevalence of LVH was 20.2% (I2 = 88%) (Supplementary Figure S4) (28, 36, 38, 46).
Three studies with preHT included 151 patients (mean age 15.2 years, 74% male subjects; ABPM used to define preHT in two studies) and the prevalence of LVH was 21.2% (I2 = 79%) (Supplementary Figure S5) (24, 28, 61).
Risk of bias assessment
Only 19% of the studies were evaluated as low risk of bias (Supplementary Figure S6). More than half of the studies received a high risk of bias or unclear judgment on five evaluated domains: participant sampling, sample size, subject description, methods to define LVH, and reliability of LVMI measurements. Due to a small number of studies assessed as low risk of bias, the subgroup meta-analysis based on the assessed quality of the studies was not performed.
Visual inspection of Funnel plots and Egger's test suggested the risk of publication bias in Subgroups 1 and 2 (intercept 0.827, p = 0.07 and intercept 0.803, p = 0.04, respectively) with a tendency of smaller studies to report lower LVH prevalence (Supplementary Figures 7, 8).
Discussion
The mean prevalence of LVH was estimated to be 30.5% in this systematic review and meta-analysis of 5,622 children and young people with untreated PH and 47 studies reported over the last two decades. Even with the exclusion of studies from community-based populations, studies with WCH and pre-hypertension phenotypes, and variable definitions of LVH from the analysis, the same figure of 29.9% LVH from 1,393 individuals was found. The prevalence of LVH was lower in studies at 21.5% when including children identified through community screening only.
The left ventricle is one of the primary targets for HMOD but has also been shown in hypertensive adults to be an independent risk factor for cardiovascular morbidity and mortality (63–65). Moreover, both worsening LV geometry and increasing severity of LVH are known to be associated with adverse outcomes (66–69). Although similar data associating LVH in childhood PH with subsequent CV morbidity and mortality do not exist currently, data from the Bogalusa study highlight that persistently elevated blood pressure, particularly through adolescence, is associated with a significantly higher risk for adult LVH when compared with normotensive adolescents (70). It is, therefore, widely accepted that LVH in childhood PH is an adverse surrogate marker of CV morbidity, and an important target to ameliorate future CV morbidity and mortality seen in hypertensive heart disease (12, 71).
The pathophysiology of LVH in PH is complex and not fully understood, and includes both modifiable and non-modifiable risk factors, including age, sex, ethnicity, genetic factors, and co-morbidities like obesity and metabolic syndrome (72). These observations are reflected in our study findings, which show that 76% of those with hypertension were male subjects, with a mean age of 14.6 years. Despite this, no significant relationship between age or male sex and the prevalence of LVH was found in individual studies.
In healthy children, changes in LV dimensions and mass associate with age, sex, and growth throughout childhood (14, 73–75). Increasing body size and adiposity have the highest correlation with LV mass, highlighting the increasing demands on LV (32, 39, 76–78). We observed that BMIz had a significant positive relationship with the prevalence of LVH. Other adiposity characteristics, such as the proportion of obese children or WCz were reported in fewer studies with no relationship with the prevalence of LVH following meta-analysis. The exclusion of studies primarily evaluating those with obesity, metabolic syndrome, and diabetes mellitus may also have contributed to our findings. Unfortunately, individual patient data were not available to investigate the complex interactions between BP and adiposity for the development of LVH further. BMI is the most important determinant of BP in childhood. Thus, our finding that BMI was the main determinant of LVH may be caused by the fact that increased BMI mediates the effects of other cardiovascular risk factors, such as birth weight, socioeconomic status, and metabolic abnormalities (79).
Overall, these issues highlight that the relationships among age, body size, and LVM are complex, especially during the process of growth as seen during adolescence. These complexities result in difficulties when defining LVH across the childhood age range for both sexes and are reflected in the diverse definitions for LVH by age and sex in Clinical Practice Guidelines from learned societies (12, 71). We observed that despite using different definitions of LVH reported in the literature, the prevalence of LVH remained at ~30%. These findings are in keeping with recent reports that have highlighted no differences in the prevalence of LVH when 2017 AAP vs. 2016 ESH clinical practice guidelines LVH criteria have been applied (26, 80).
One of the major criticisms of individual studies reporting the prevalence of LVH in PH is that they might reflect highly selected populations, derived from children referred to specialist centers. In keeping with this, we observed that there was a lower prevalence of LVH in studies representing a community-based population, with an estimated mean prevalence of LVH at 21.5% from 1,234 individuals. Although only two studies have reported data for children identified through community screening, this may reflect lesser severity of BP elevation or clinical presentation as opposed to those referred to specialty centers. The lower prevalence of LVH in this group highlights the importance of early identification of elevated BP to prevent HMOD development and may be in support of screening programs within the pediatric population.
As expected, those with lower levels of BP including preHT category and less severe hypertension phenotype, e.g., WCH, also had lower estimated prevalence of LVH at 21.2 and 20.2%, respectively. Although smaller in numbers, these findings are in keeping with similar observations in adults and the significant pathophysiological association of BP level with LVH (81–83).
In adult hypertensives, it has been suggested that LVH develops following a complex interaction of hemodynamic and non-hemodynamic variables (72). It has been a common view that LVH develops due to chronic pressure overload from hypertension and results in LV remodeling to reduce myocardial wall stress with the development of concentric LVH (84). Despite this traditional view, concentric LVH is not always the most common LV geometry in hypertensive adults and this may reflect other significant pathophysiological pathways and variables (85). These observations were confirmed in our study with sub-analyses with LVH prevalence of 34.2%, in whom concentric and eccentric LVH was seen in one- and two-thirds, respectively. This is perhaps unsurprising, given that LV remodeling in hypertensive adolescents reflects adaptations to increased demand on the ventricle as a result of an increase in body size, BP, but also interactions as a result of ventricular-vascular coupling at rest and after increased physical activity (86, 87). Eccentric hypertrophy is associated with obesity (88, 89), which was reflected in our study population with a mean of 25.4 kg/m2 and BMIz 1.43, despite the fact that these data were not reported uniformly in the studies reporting LV geometry.
In addition to cardiac remodeling, PH during childhood is associated with arterial stiffening including both structural (as assessed by carotid intima medial thickness and carotid wall cross-sectional area) and functional (as assessed by carotid-femoral pulse wave velocity) impairment of the arterial tree (24, 90). Further, although studies have previously reported LVH in the absence of other signs of arterial stiffening in childhood PH, and few investigators have performed a comprehensive evaluation of cardiac and vascular HMOD (86). We did not systematically assess arterial stiffening in subjects with LVH, but we highlight it because a better understanding of cardiac and vascular interactions during childhood in PH is required.
Based on our study findings several recommendations can be made. Despite 24-h ABPM being recommended as vital for the diagnosis of HT in this population in current guidelines (12, 71), five studies defined HT exclusively following office BP measurement. There was a large heterogeneity in the reporting of BP levels, with most studies only reporting mean values for raw data limiting the ability to analyze associations between standardized BP levels and LVH. Few studies reported ethnicity, which is a well-known predictor for BP levels (70, 77, 91, 92). Differences in the definition of LVH were common as discussed previously and can lead to significant misclassification and few studies reported LV geometry. Future studies should aim to report adjusted BP levels, BP phenotypes by ABPM, the method of indexation of LVM and its level, and the severity of LVH and LV geometry as a minimum. Future clinical practice guidelines should provide a preferred method of reporting BP levels and LVM indexation, with improved harmonization of current guidelines across ESH and AAP.
There are several limitations to this analysis, the most prominent being that none of the 47 included studies had the primary aim of reporting the prevalence of LVH in children with PH. Thus, they are unlikely to be of adequate size or optimal design to precisely estimate the prevalence of LVH in children with PH. This is in part reflected by publication bias analysis indicating lower prevalences in studies with lower sample sizes. Furthermore, other sources of biases could not have been accounted for in the original study designs. Importantly, only a few studies qualified as low risk of bias following critical appraisal, probably resulting in the observed high degree of heterogeneity between reported studies. This was mainly related to the lack of random probabilistic sampling, small sample sizes, lack of BP phenotypes descriptions, unclear duration of PH, heterogeneous LVH definitions, and lack of reporting sufficient details to confirm the reliability of LVMI measurements. In addition, different definitions of hypertension may contribute to heterogeneity in LVH prevalence, despite a recent meta-analysis finding similar predictive value between AAP and ESH guidelines (93). Finally, we were limited when evaluating by the level of BP as this was only reported in a small number of studies.
In conclusion, this systematic review and meta-analysis estimate a 30% prevalence of LVH in children and young persons with PH, highlighting the significance of childhood hypertension but also providing a treatment target for optimizing management. A large body of literature on hypertensive adults has established that LVH improves following increased physical exercise, weight loss, and anti-hypertensive therapy (94). Although similar data in hypertensive children are few and less robust, similar interventions are likely to be effective, with improved BP control following anti-hypertensive therapy and the reduction of abdominal obesity being most impactful (32, 34, 88, 95, 96). Further prospective research is required to more carefully evaluate LVH in children with PH, to understand the progression of LVH in different hypertension phenotypes and through increasing levels of BP. HMOD including cardiac and vascular assessments is needed to detect both early complications but also improve our understanding of pediatric PH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Members of the HyperChildNET Working Group 3
Michael Hecht Olsen, Katerina Chrysaidou, Asle Hirth, Giacomo Simonetti, Kjell Tullus, Rina Rus, Verónica Martínez, Empar Lurbe, Elke Wuhl, Veronica Martinez Rivera.
Author contributions
JS-K, TB, KM, MS, KA, AJ, FF-A, and ML contributed to conception and design of the study. IS performed literature search. JS-K and TB performed title and abstract screening. JS-K, TB, KM, MS, and KA performed full-text reviews, risk of bias assessment and data extraction. JS-K, KM, and TB performed data checking. KA conducted statistical analysis. MS and KA prepared the first draft of the manuscript. MS, KA, ML, TB, and JS-K wrote sections of the manuscript. JS-K, TB, KM, MS, KA, IS, AJ, BB, LO, MP, and ML contributed to critical manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This publication is based on the work of the COST Action HyperChildNET (CA19115), with the support of COST (European Cooperation in Science and Technology) and the Horizon 2020 Framework Program of the European Union.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Contributor Information
HyperChildNet Working Group:
Michael Hecht Olsen, Katerina Chrysaidou, Asle Hirth, Giacomo Simonetti, Kjell Tullus, Rina Rus, Verónica Martínez, Empar Lurbe, Elke Wuhl, and Veronica Martinez Rivera
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.993513/full#supplementary-material
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardiovascular disease chronic kidney disease and and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment . Lancet Diab Endocrinol. (2014) 2:634–47. 10.1016/S2213-8587(14)70102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. (2019) 173:1154. 10.1001/jamapediatrics.2019.3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azegami T, Uchida K, Tokumura M, Mori M. Blood pressure tracking from childhood to adulthood. Front Pediatr. (2021) 9:785356. 10.3389/fped.2021.785356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan SR, Myers L, Berenson GS. Changes in metabolic syndrome variables since childhood in prehypertensive and hypertensive subjects: the Bogalusa Heart Study. Hypertension. (2006) 48:33–9. 10.1161/01.HYP.0000226410.11198.f4 [DOI] [PubMed] [Google Scholar]
- 6.Daniels SR. Hypertension-induced cardiac damage in children and adolescents. Blood Press Monit. (1999) 4:165–70. 10.1097/00126097-199906000-00011 [DOI] [PubMed] [Google Scholar]
- 7.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. (2000) 102:470–9. 10.1161/01.CIR.102.4.470 [DOI] [PubMed] [Google Scholar]
- 8.Weber KT, Brilla CG. Structural basis for pathologic left ventricular hypertrophy. Clin Cardiol. (1993) 16:II10–14. 10.1002/clc.4960161404 [DOI] [PubMed] [Google Scholar]
- 9.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. (1995) 91:2400–6. 10.1161/01.CIR.91.9.2400 [DOI] [PubMed] [Google Scholar]
- 10.Malmqvist K, Ohman KP, Lind L, Nyström F, Kahan T. Relationships between left ventricular mass and the renin-angiotensin system, catecholamines, insulin and leptin. J Intern Med. (2002) 252:430–9. 10.1046/j.1365-2796.2002.01053.x [DOI] [PubMed] [Google Scholar]
- 11.Litwin M, Michałkiewicz J, Niemirska A, Gackowska L, Kubiszewska I, Wierzbicka A, et al. Inflammatory activation in children with primary hypertension. Pediatr Nephrol. (2010) 25:1711–8. 10.1007/s00467-010-1548-4 [DOI] [PubMed] [Google Scholar]
- 12.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. 10.1097/HJH.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 2021:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. (2009) 22:709–14. 10.1016/j.echo.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. (2014) 3:123–8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Shi L, Lin Y, Zhang M, Chen F, Li A, et al. Relationship between serum 25-hydroxyvitamin D and target organ damage in children with essential hypertension. J Hum Hypertens. (2022). 10.1038/s41371-021-00622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H, Xi B, Liu J, Yan Y, Mi J. Performance of different adiposity measures for predicting left ventricular remodeling in Chinese hypertensive youth. Sci Rep. (2021) 11:21943. 10.1038/s41598-021-00978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacihamdioglu DO, Kocak G, Dogan BN, Koyuncu E. Challenges in choosing the appropriate guidelines for use in children and adolescents with hypertension. Arch Pediatr. (2021) 28:451–8. 10.1016/j.arcped.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Hou C, Hou M, Xu QQ, Wang H, Gu PP, et al. Ultrasonography to detect cardiovascular damage in children with essential hypertension. Cardiovasc Ultrasound. (2021) 19:26. 10.1186/s12947-021-00257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Huang Y, Qin Y, Pang Y. Clinical characteristics and factors associated with hypertension in 205 hospitalized children: a single-center study in Southwest China. Front Pediatr. (2021) 9:620158. 10.3389/fped.2021.620158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou M, Cao L, Ding Y, Chen Y, Wang B, Shen J, et al. Neutrophil to lymphocyte ratio is increased and associated with left ventricular diastolic function in newly diagnosed essential hypertension children. Front Pediatr. (2021) 9:576005. 10.3389/fped.2021.576005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Lin Y, Zhang MM Li XH, Liu YY, Zhao J, Shi L. The relationship of plasma renin, angiotensin, and aldosterone levels to blood pressure variability and target organ damage in children with essential hypertension. BMC Cardiovasc Disord. (2020) 20:296. 10.1186/s12872-020-01579-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trojanek JB, Niemirska A, Grzywa R, Wierzbicka A, Obrycki L, Kulaga Z, et al. Leukocyte matrix metalloproteinase and tissue inhibitor gene expression patterns in children with primary hypertension. J Hum Hypertens. (2020) 34:355–63. 10.1038/s41371-019-0197-8 [DOI] [PubMed] [Google Scholar]
- 24.Obrycki L, Feber J, Derezinski T, Lewandowska W, Kulaga Z, Litwin M. Hemodynamic patterns and target organ damage in adolescents with ambulatory prehypertension. Hypertension. (2020) 75:826–34. 10.1161/HYPERTENSIONAHA.119.14149 [DOI] [PubMed] [Google Scholar]
- 25.Urbina EM, Mendizabal B, Becker RC, Daniels SR, Falkner BE, Hamdani G, et al. Association of blood pressure level with left ventricular mass in adolescents. Hypertension. (2019) 74:590–6. 10.1161/HYPERTENSIONAHA.119.13027 [DOI] [PubMed] [Google Scholar]
- 26.Antolini L, Giussani M, Orlando A, Nava E, Valsecchi MG, Parati G, et al. Nomograms to identify elevated blood pressure values and left ventricular hypertrophy in a paediatric population: American Academy of Pediatrics Clinical Practice vs. Fourth Report/European Society of Hypertension Guidelines. J Hypertens. (2019) 37:1213–22. 10.1097/HJH.0000000000002069 [DOI] [PubMed] [Google Scholar]
- 27.Shilly S, Merchant K, Singer P, Frank R, Gurusinghe S, Infante L, et al. Left ventricular cardiac geometry and ambulatory blood pressure in children. J Clin Hypertens. (2019) 21:566–71. 10.1111/jch.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litwin M, Obrycki L, Niemirska A, Sarnecki J, Kulaga Z. Central systolic blood pressure and central pulse pressure predict left ventricular hypertrophy in hypertensive children. Pediatric Nephrology. (2019) 34:703–12. 10.1007/s00467-018-4136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conkar S, Yilmaz E, Hacikara S, Bozabali S, Mir S. Is daytime systolic load an important risk factor for target organ damage in pediatric hypertension? J Clin Hypertens. (2015) 17:760–6. 10.1111/jch.12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng L, Hou D, Zhao X, Hu Y, Liang Y, Liu J, et al. Cardiovascular target organ damage could have been detected in sustained pediatric hypertension. Blood Press. (2015) 24:284–92. 10.3109/08037051.2015.1049424 [DOI] [PubMed] [Google Scholar]
- 31.Agu NC, McNiece Redwine K, Bell C, Garcia KM, Martin DS, Poffenbarger TS, et al. Detection of early diastolic alterations by tissue Doppler imaging in untreated childhood-onset essential hypertension. J Am Soc Hypertens. (2014) 8:303–11. 10.1016/j.jash.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemirska A, Litwin M, Feber J, Jurkiewicz E. Blood pressure rhythmicity and visceral fat in children with hypertension. Hypertension. (2013) 62:782–8. 10.1161/HYPERTENSIONAHA.113.01292 [DOI] [PubMed] [Google Scholar]
- 33.Sharma AP, Mohammed J, Thomas B, Lansdell N, Norozi K, Filler G. Nighttime blood pressure, systolic blood pressure variability, and left ventricular mass index in children with hypertension. Pediatr Nephrol. (2013) 28:1275–82. 10.1007/s00467-013-2468-x [DOI] [PubMed] [Google Scholar]
- 34.Litwin M, Niemirska A, Sladowska-Kozlowska J, Wierzbicka A, Janas R, Wawer ZT, et al. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr Nephrol. (2010) 25:2489–99. 10.1007/s00467-010-1626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richey PA, Disessa TG, Somes GW, Alpert BS, Jones DP. Left ventricular geometry in children and adolescents with primary hypertension. Am J Hypertens. (2010) 23:24–9. 10.1038/ajh.2009.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litwin M, Niemirska A, Ruzicka M, Feber J. White coat hypertension in children: not rare and not benign? J Am Soc Hypertens. (2009) 3:416–23. 10.1016/j.jash.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 37.Stabouli S, Kotsis V, Rizos Z, Toumanidis S, Karagianni C, Constantopoulos A, et al. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. (2009) 24:1545–51. 10.1007/s00467-009-1165-2 [DOI] [PubMed] [Google Scholar]
- 38.Lande MB, Meagher CC, Fisher SG, Belani P, Wang H, Rashid M. Left ventricular mass index in children with white coat hypertension. J Pediatr. (2008) 153:50–4. 10.1016/j.jpeds.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litwin M, Sladowska J, Syczewska M, Niemirska A, Daszkowska J, Antoniewicz J, et al. Different BMI cardiovascular risk thresholds as markers of organ damage and metabolic syndrome in primary hypertension. Pediatr Nephrol. (2008) 23:787–96. 10.1007/s00467-007-0739-0 [DOI] [PubMed] [Google Scholar]
- 40.Assadi F. Relation of left ventricular hypertrophy to microalbuminuria and C-reactive protein in children and adolescents with essential hypertension. Pediatr Cardiol. (2008) 29:580–4. 10.1007/s00246-007-9153-4 [DOI] [PubMed] [Google Scholar]
- 41.Brady TM, Fivush B, Flynn JT, Parekh R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. (2008) 152:73–78.e1. 10.1016/j.jpeds.2007.05.053 [DOI] [PubMed] [Google Scholar]
- 42.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, et al. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension. (2007) 50:392–5. 10.1161/HYPERTENSIONAHA.107.092197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litwin M, Sladowska J, Antoniewicz J, Niemirska A, Wierzbicka A, Daszkowska J, et al. Metabolic abnormalities, insulin resistance, and metabolic syndrome in children with primary hypertension. Am J Hypertens. (2007) 20:875–82. 10.1016/j.amjhyper.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 44.South AM, Arguelles L, Finer G, Langman CB. Race, obesity, and the renin-angiotensin-aldosterone system: treatment response in children with primary hypertension. Pediatr Nephrol. (2017) 32:1585–94. 10.1007/s00467-017-3665-9 [DOI] [PubMed] [Google Scholar]
- 45.Gupta-Malhotra M, Hamzeh RK, Poffenbarger T, McNiece-Redwine K, Hashmi SS. Myocardial performance index in childhood onset essential hypertension and white coat hypertension. Am J Hypertens. (2016) 29:379–87. 10.1093/ajh/hpv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaswamy P, Chikkabyrappa S, Donda K, Osmolovsky M, Rojas M, Rafii D. Relationship of ambulatory blood pressure and body mass index to left ventricular mass index in pediatric patients with casual hypertension. J Am Soc Hypertens. (2016) 10:108–14. 10.1016/j.jash.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 47.Sladowska-Kozlowska J, Litwin M, Niemirska A, Wierzbicka A, Roszczynko M, Szperl M. Associations of the eNOS G894T gene polymorphism with target organ damage in children with newly diagnosed primary hypertension. Pediatr Nephrol. (2015) 30:2189–97. 10.1007/s00467-015-3164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. (2006) 48:40–4. 10.1161/01.HYP.0000227029.10536.e8 [DOI] [PubMed] [Google Scholar]
- 49.Litwin M, Niemirska A, Sladowska J, Antoniewicz J, Daszkowska J, Wierzbicka A, et al. Left ventricular hypertrophy and arterial wall thickening in children with essential hypertension. Pediatr Nephrol. (2006) 21:811–9. 10.1007/s00467-006-0068-8 [DOI] [PubMed] [Google Scholar]
- 50.Sorof JM, Turner J, Martin DS, Garcia K, Garami Z, Alexandrov AV, et al. Cardiovascular risk factors and sequelae in hypertensive children identified by referral versus school-based screening. Hypertension. (2004) 43:214–8. 10.1161/01.HYP.0000114696.96318.4e [DOI] [PubMed] [Google Scholar]
- 51.Kimball TR, Daniels SR, Loggie JM, Khoury P, Meyer RA. Relation of left ventricular mass, preload, afterload and contractility in pediatric patients with essential hypertension. J Am Coll Cardiol. (1993) 21:997–1001. 10.1016/0735-1097(93)90359-9 [DOI] [PubMed] [Google Scholar]
- 52.Niemirska A, Litwin M, Trojanek J, Gackowska L, Kubiszewska I, Wierzbicka A, et al. Altered matrix metalloproteinase 9 and tissue inhibitor of metalloproteinases 1 levels in children with primary hypertension. J Hypertens. (2016) 34:1815–22. 10.1097/HJH.0000000000001024 [DOI] [PubMed] [Google Scholar]
- 53.Litwin M, Michalkiewicz J, Trojanek J, Niemirska A, Wierzbicka A, Szalecki M. Altered genes profile of renin-angiotensin system, immune system, and adipokines receptors in leukocytes of children with primary hypertension. Hypertension. (2013) 61:431–6. 10.1161/HYPERTENSIONAHA.111.00181 [DOI] [PubMed] [Google Scholar]
- 54.Flynn JT, Alderman MH. Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol. (2005) 20:961–6. 10.1007/s00467-005-1855-3 [DOI] [PubMed] [Google Scholar]
- 55.Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. (2003) 111:61–6. 10.1542/peds.111.1.61 [DOI] [PubMed] [Google Scholar]
- 56.Belsha CW, Wells TG, McNiece KL, Seib PM, Plummer JK, Berry PL. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. (1998) 11:410–7. 10.1016/S0895-7061(98)00014-4 [DOI] [PubMed] [Google Scholar]
- 57.Niemirska A, Obrycki L, Litwin M. Utility of pulse wave velocity and pulse wave analysis in assessment of hypertensive target organ damage in children with primary hypertension. Nadcisnienie Tetnicze. (2014) 18:194–203. [Google Scholar]
- 58.Sarnecki J, Obrycki L, Feber J, Chelstowska S, Jurkiewicz E, Litwin M. Isolated systolic hypertension is associated with increased left ventricular mass index and aortic stiffness in adolescents: a cardiac magnetic resonance study. J Hypertens. (2022) 21:21. 10.1097/HJH.0000000000003101 [DOI] [PubMed] [Google Scholar]
- 59.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. (2002) 39:903–8. 10.1161/01.HYP.0000013266.40320.3B [DOI] [PubMed] [Google Scholar]
- 60.Zamojska J, Niewiadomska-Jarosik K, Wosiak A, Lipiec P, Stańczyk J. Myocardial dysfunction measured by tissue Doppler echocardiography in children with primary arterial hypertension. Kardiol Pol. (2015) 73:194–200. 10.5603/KP.a2014.0189 [DOI] [PubMed] [Google Scholar]
- 61.Pieruzzi F, Antolini L, Salerno FR, Giussani M, Brambilla P, Galbiati S, et al. The role of blood pressure, body weight and fat distribution on left ventricular mass, diastolic function and cardiac geometry in children. J Hypertens. (2015) 33:1182–92. 10.1097/HJH.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 62.Lee H, Kong Y-H, Kim K-H, Huh J, Kang I-S, Song J. Left ventricular hypertrophy and diastolic function in children and adolescents with essential hypertension. Clin Hypertens. (2015) 21:21. 10.1186/s40885-015-0031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. (2000) 140:848–56. 10.1067/mhj.2000.111112 [DOI] [PubMed] [Google Scholar]
- 64.Kannel WB. Left Ventricular Hypertrophy by Electrocardiogram: Prevalence, Incidence, and Mortality in the Framingham Study. Ann Intern Med. (1969) 71:89. 10.7326/0003-4819-71-1-89 [DOI] [PubMed] [Google Scholar]
- 65.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. (2001) 141:334–41. 10.1067/mhj.2001.113218 [DOI] [PubMed] [Google Scholar]
- 66.Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. (2004) 43:731–8. 10.1161/01.HYP.0000121223.44837.de [DOI] [PubMed] [Google Scholar]
- 67.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. (2000) 35:580–6. 10.1161/01.HYP.35.2.580 [DOI] [PubMed] [Google Scholar]
- 68.Koren MJ. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. (1991) 114:345. 10.7326/0003-4819-114-5-345 [DOI] [PubMed] [Google Scholar]
- 69.Jung JY, Park SK, Oh C-M, Kang JG, Choi J-M, Ryoo J-H, et al. The influence of prehypertension, controlled and uncontrolled hypertension on left ventricular diastolic function and structure in the general Korean population. Hypertens Res. (2017) 40:606–12. 10.1038/hr.2016.191 [DOI] [PubMed] [Google Scholar]
- 70.Zhang T, Li S, Bazzano L, He J, Whelton P, Chen W. Trajectories of childhood blood pressure and adult left ventricular hypertrophy: the Bogalusa Heart Study. Hypertension. (2018) 72:93–101. 10.1161/HYPERTENSIONAHA.118.10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140:e20171904. 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 72.Yildiz M, Oktay AA, Stewart MH, Milani RV, Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. (2020) 63:10–21. 10.1016/j.pcad.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 73.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. (1998) 97:1907–11. 10.1161/01.CIR.97.19.1907 [DOI] [PubMed] [Google Scholar]
- 74.Henry WL, Ware J, Gardin JM, Hepner SI, McKay J, Weiner M. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation. (1978) 57:278–85. 10.1161/01.CIR.57.2.278 [DOI] [PubMed] [Google Scholar]
- 75.Desimone G, Kizer J, Chinali M, Roman M, Bella J, Best L, et al. Normalization for body size and population-attributable risk of left ventricular hypertrophyThe Strong Heart Study. Am J Hypertens. (2005) 18:191–6. 10.1016/j.amjhyper.2004.08.032 [DOI] [PubMed] [Google Scholar]
- 76.Marciniak M, van Deutekom AW, Toemen L, Lewandowski AJ, Gaillard R, Young AA, et al. A three-dimensional atlas of child's cardiac anatomy and the unique morphological alterations associated with obesity. Eur Heart J Cardiovasc Imag. (2021) 2021:jeab271. 10.1093/ehjci/jeab271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pruette CS, Fivush BA, Flynn JT, Brady TM. Effects of obesity and race on left ventricular geometry in hypertensive children. Pediatr Nephrol. (2013) 28:2015–22. 10.1007/s00467-013-2507-7 [DOI] [PubMed] [Google Scholar]
- 78.Brady TM, Appel LJ, Holmes KW, Fivush B, Miller ER, 3rd. Association between adiposity and left ventricular mass in children with hypertension. J Clin Hypertens. (2016) 18:625–33. 10.1111/jch.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Kelishadi R, Khadilkar A, Mi Hong Y, Nawarycz T, Krzywińska-Wiewiorowska M, et al. Body mass index percentiles and elevated blood pressure among children and adolescents. J Hum Hypertens. (2020) 34:319–25. 10.1038/s41371-019-0215-x [DOI] [PubMed] [Google Scholar]
- 80.Di Bonito P, Valerio G, Pacifico L, Chiesa C, Invitti C, Morandi A, et al. Impact of the 2017 Blood pressure guidelines by the american academy of pediatrics in overweight/obese youth. J Hypertens. (2019) 37:732–8. 10.1097/HJH.0000000000001954 [DOI] [PubMed] [Google Scholar]
- 81.Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, et al. Blood pressure variability and organ damage in a general population: results from the PAMELA study. Hypertension. (2002) 39:710–4. 10.1161/hy0202.104376 [DOI] [PubMed] [Google Scholar]
- 82.Cuspidi C, Sala C, Tadic M, Gherbesi E, Grassi G, Mancia G. Pre-hypertension and subclinical cardiac damage: a meta-analysis of echocardiographic studies. Int J Cardiol. (2018) 270:302–8. 10.1016/j.ijcard.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 83.Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G, Mancia G. White-coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta-analysis. J Hypertens. (2015) 33:24–32. 10.1097/HJH.0000000000000416 [DOI] [PubMed] [Google Scholar]
- 84.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure. JACC: Heart Failure. (2017) 5:543–51. 10.1016/j.jchf.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 85.Cuspidi C Sala C Negri F Mancia G Morganti A on on behalf of the Italian Society of Hypertension . Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. (2012) 26:343–9. 10.1038/jhh.2011.104 [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Gu H, Sinha MD, Chowienczyk P. Hemodynamic characterization of primary hypertension in children and adolescents. J Am Heart Assoc. (2020) 9:e015097. 10.1161/JAHA.119.015097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu H, Singh C, Li Y, Simpson J, Chowienczyk P, Sinha MD. Early ventricular contraction in children with primary hypertension relates to left ventricular mass. J Hypertens. (2021) 39:711–7. 10.1097/HJH.0000000000002699 [DOI] [PubMed] [Google Scholar]
- 88.Sladowska-Kozlowska J, Litwin M, Niemirska A, Wierzbicka A, Wawer ZT, Janas R. Change in left ventricular geometry during antihypertensive treatment in children with primary hypertension. Pediatr Nephrol. (2011) 26:2201–9. 10.1007/s00467-011-1916-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Messerli F. Cardiovascular effects of obesity and hypertension. Lancet. (1982) 319:1165–8. 10.1016/S0140-6736(82)92234-6 [DOI] [PubMed] [Google Scholar]
- 90.Azukaitis K, Sinha MD, Obrycki Ł, Pac M, Bjelakovic B, Jankauskiene A, et al. HyperChildNet Working Group 3. Disparities between determinants of impaired vascular structure and function in young people with primary hypertension: a systematic review. J Hypertens. (2022) 40:1369–79. 10.1097/HJH.0000000000003155 [DOI] [PubMed] [Google Scholar]
- 91.Mendizábal B, Khoury P, Woo JG, Urbina EM. Racial differences in the influence of risk factors in childhood on left ventricular mass in young adulthood. J Pediatr. (2020) 217:152–7. 10.1016/j.jpeds.2019.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brady TM, Fivush B, Parekh RS, Flynn JT. Racial differences among children with primary hypertension. Pediatrics. (2010) 126:931–7. 10.1542/peds.2009-2972 [DOI] [PubMed] [Google Scholar]
- 93.Goulas I, Farmakis I, Doundoulakis I, Antza C, Kollios K, Economou M, et al. Comparison of the 2017 American Academy of Pediatrics with the fourth report and the 2016 European Society of Hypertension guidelines for the diagnosis of hypertension and the detection of left ventricular hypertrophy in children and adolescents: a systematic review and meta-analysis. J Hypertens. (2022) 40:197–204. 10.1097/HJH.0000000000003005 [DOI] [PubMed] [Google Scholar]
- 94.Bourdillon MT, Vasan RS. A contemporary approach to hypertensive cardiomyopathy: reversing left ventricular hypertrophy. Curr Hypertens Rep. (2020) 22:85. 10.1007/s11906-020-01092-8 [DOI] [PubMed] [Google Scholar]
- 95.Assadi F. Effect of microalbuminuria lowering on regression of left ventricular hypertrophy in children and adolescents with essential hypertension. Pediatr Cardiol. (2007) 28:27–33. 10.1007/s00246-006-1390-4 [DOI] [PubMed] [Google Scholar]
- 96.Seeman T, Gilik J, Vondrak K, Simkova E, Flogelova H, Hladikova M, et al. Regression of left-ventricular hypertrophy in children and adolescents with hypertension during ramipril monotherapy. Am J Hypertens. (2007) 20:990–6. 10.1016/j.amjhyper.2007.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.