Abstract
Self‐reported measures of health, in the context of developed countries, are well‐researched and commonly regarded as reliable predictors of the underlying health of the population. However, the validity of these measures is under‐researched and questionable in the context of low‐ and middle‐income countries. The authors used Longitudinal Ageing Study in India (LASI) survey data from India to compare self‐reported hypertension with biometrically‐measured hypertension. The results are reported in terms of sensitivity, specificity, and kappa as a measure of agreement. Logistic regression was undertaken to examine the characteristics of those who were unaware of their hypertensive status. Our analysis showed a low sensitivity of 56% and a high specificity of 90.5%. Agreement between self‐reported data and biometric measurement of hypertension was observed to be moderate (κ = 0.48). Large variations were observed among states and sub‐groups. The odds of false negative reporting of hypertension were lower in the individuals with higher age, high education, and greater wealth status. The authors conclude that self‐reported hypertension has important limitations and may be a source of systematic bias. It is recommended that planning and policy‐making in India be based more on an objective assessment of hypertension.
Keywords: biometric measurement, hypertension, India, self‐reported measure, sensitivity, validity
1. INTRODUCTION
In spite of being subjective in nature, self‐reported measures of health are commonly used by policy makers and planners across developed and developing countries. Unlike clinical assessment and biometric measurement, self‐reported questionnaires are cheap and easy to administer and enable gathering of information directly from the respondents. 1 However, self‐reported measures have limitations since respondents can misclassify their health status and provide inaccurate responses owing to unawareness, cognitive bias, and poor memory. 2 , 3
The issue of accuracy of self‐reported measures and the extent of their divergence from the actual measurements is well‐researched in the developed country context. 4 , 5 , 6 , 7 , 8 , 9 , 10 However, the performance of self‐reported measures in low‐ and middle‐income countries is not clear due to the limited evidence.
In recent years, a small body of literature has emerged from low‐ and middle‐income countries, which has assessed the validity of self‐reported measures. 11 , 12 , 13 However, even among these studies that have analyzed the level of divergence between self‐reported measures and biometric measurements, hardly any have attempted to explain the reasons behind the discrepancy between the two measures. Thus, significant gaps remain with respect to our understanding of issues around the validity of self‐reported measures in low‐ and middle‐income countries.
Hypertension was used in this study as it is a leading preventable cause of cardiovascular disease (CVD) and premature mortality worldwide. Notoriously known as a “silent killer”, as it tends to be asymptomatic, it likely affects a significant proportion of the population, especially in low‐ and middle‐income countries, that may not be aware of its condition and remains undetected for a prolonged period of time, contributing to avoidable morbidity and mortality. The good news is that hypertension, if detected early, can be treated with medication, diet modification, and lifestyle changes. However, if untreated, it can be a major risk factor for cardiovascular diseases, kidney failure, and dementia. 14 , 15
According to the World Health Organization, an estimated 1.28 billion adults, aged 30–79 years, have hypertension worldwide, and a majority (two‐thirds) of them live in low‐ and middle‐income countries. 16 The Global Burden of Disease study has reported that in 2019, high systolic BP accounted for 10.8 million deaths and 235.4 million disability‐adjusted life years (DALYs) across the world. 17 In the past two decades, changes in the prevalence of hypertension have not been uniform across the globe, with high‐income countries (HICs) experiencing a modest decrease and low‐ and middle‐income countries (LMICs) experiencing a significant increase. 18 In India, an estimated 207 million (25.3%) adults, aged 18+ years and above, had hypertension. 19 The number of deaths due to hypertension in India increased from 0.64 million in 1996 to 1.4 million in 2019, while the DALYs lost increased from 18.0 million in 1990 to 37.0 million in 2019. 17 The trend and projections of hypertension are a cause for concern as its prevalence among adults has risen in the last three decades and is expected to continue to rise further. 20 , 21
A recently published nationally‐representative survey, the Longitudinal Ageing Study in India (LASI), based on adults 45 years and older, 22 collected measured and self‐reported information on hypertension, allowing for us to determine the validity of the self‐reported hypertension data among Indian older adults. There is a paucity of nationally‐representative studies examining the validity of hypertension in India and exploring the reasons behind the divergence. Not only that, hardly any study has looked into the factors associated with false negative and positive reporting of hypertension among Indian older adults. Given this background, the present study aims to measure the extent of disagreement between self‐reported and objective assessment of hypertension and to examine the determinants of false negative and positive reporting of hypertension among Indian older adults. Our study attempts to contribute to the limited empirical literature on the validity of self‐assessment of hypertension in low‐ and middle‐income countries. The policy implications of reliance on self‐reported measures are discussed in the context of India and other low‐ and middle‐income countries.
2. METHODS
2.1. Data
Data were drawn from the recently released first wave of Longitudinal Ageing Study in India (LASI), conducted in 2017–2018. LASI is a full‐scale nationally‐representative survey of more than 72 000 individuals aged 45 years and above, conducted across all states and Union Territories of India (excluding Sikkim). The primary objective of the LASI survey was to scientifically investigate the health, economic, and social determinants of population aging in India. Face‐to‐face interviews were conducted in respondent households with the help of computer‐assisted personal interviews (CAPI). The LASI survey was conducted through a partnership among the International Institute for Population Sciences (IIPS), the University of Southern California (USC), and the Harvard T. H. Chan School of Public Health (HSPH). Further details on the methodology used by the LASI survey can be found in the LASI survey report. 22
2.2. Outcome variable
In the LASI questionnaire, self‐reported data on hypertension was obtained by the question: “Has any health professional ever told you that you have hypertension or high blood pressure?” The participant was identified as hypertensive if s/he answered “Yes.” As part of biomarker measurements, an electronic monitor (Omron model HEM‐7121) was used to measure the blood pressure three times (with a 1‐min gap). We took the average of the last two readings of systolic blood pressure (SBP) and diastolic blood pressure (DBP). Following the JNC‐7 guidelines, hypertension was defined as average SBP ≥ 140 mm Hg or/and DBP ≥ 90 mm Hg or as current use of any antihypertensive medication. 23
2.3. Covariates
We included various individual, household and community level characteristics in the analysis. Individual‐level characteristics were age group (45–54, 55–64, 65–74, 75+ years), sex (man, woman), educational level (no education, primary, secondary, higher), working status (never worked, currently working, not currently working), marital status (currently married, widowed, divorced/ separated/ deserted) and body mass index (underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight/obese (> 25.0 kg/m2). For analytical purposes, we combined the overweight and obesity categories. The behavioral factors were ever smoked or chewed tobacco (no, yes) and ever used alcohol (no, yes). Households factors were, religion (Hindu, Muslim, Christian, others), caste (Scheduled Caste, Scheduled Tribe, Other Backward Class (OBC), others) and monthly per capita expenditure (MPCE) (poorest, poorer, middle, richer, richest). Community level factors considered were place of residence (rural, urban) and region (North, Central, East, Northeast, West, and South).
2.4. Statistical methods
LASI provides information on both self‐reported and objective measures of hypertension. To assess the accuracy of self‐reported hypertension, we calculated sensitivity, specificity, and kappa (κ) coefficient. For assessing sensitivity and specificity, the biometrical data on hypertension was considered the ‘gold standard’ for diagnosing hypertension. Sensitivity was defined as the proportion of participants who had self‐reported hypertension among those with hypertension on biometric measurements. Specificity was defined as the proportion of participants who self‐reported no hypertension among those with no hypertension on biometric measurements. Kappa statistics (k) were calculated to assess the degree of agreement between self‐reported hypertension and biometrical data on hypertension. The scores were divided into four categories: (i) ≤.40 (poor agreement), (ii) .41–.60 (moderate agreement), (iii) .61–.80 (substantial or good agreement), and (iv) .81–1.0 (excellent agreement). We further assessed the different error outcomes, including false negative and false positive reporting. False negative reporting was defined as the proportion of participants who reported no hypertension out of total diagnosed with hypertension, whereas false positive reporting was defined as the proportion of participants who reported hypertension out of total diagnosed with no hypertension. Since the outcome variable has three categories: correct reporting (this includes sensitivity and specificity), false negative and false positive reporting, we used multinomial logistic regression to ascertain the role of different socio‐economic and demographic factors in explaining the correct reporting of hypertension. In multinomial logistic regression correct reporting was considered as the ‘base’ category. Stata software version 15.0 was used to calculated sensitivity, specificity, and κ coefficients, along with their 95% confidence intervals.
3. RESULTS
Table S1 provides details of the sample characteristics and the prevalence of self‐reported and measured hypertension. The overall prevalence of self‐reported, measured, and overall hypertension was 27.4%, 31.5%, and 42.3% respectively. Figure 1 present the prevalence of self‐reported and measured hypertension among the individuals aged 45 years and above. Overall, the prevalence of self‐reported and measured hypertension is seen to increase with age, but the prevalence decreases drastically at ages 49, 55, and 70 years. There is an underestimation of the prevalence of hypertension based on self‐reported response. The prevalence of hypertension is 17.6% based on respondents’ reported data and 28% based on measurement at age 45, showing an underestimation of 37.1%. The underestimating of self‐reported hypertension peaks at age 50 (53.6%) and tapers down by age 60 (37.8%).
FIGURE 1.
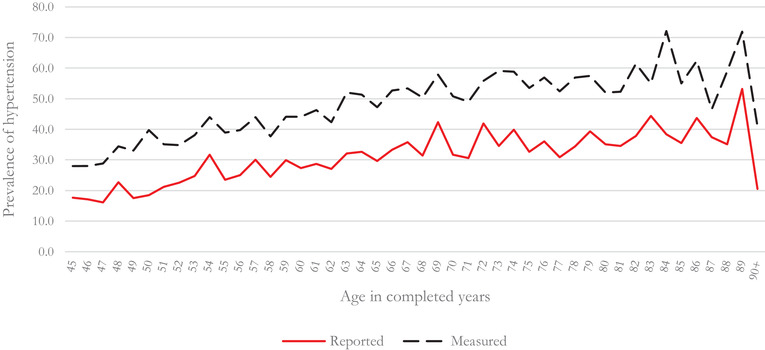
Prevalence of self‐reported hypertension and measured hypertension among individuals aged 45 years and over
Table 1 presents the sensitivity, specificity, and agreement between self‐reported and measured hypertension. The overall sensitivity of self‐reported hypertension is 56% for the age group 45 years and above (45+). It can be observed from the table that the sensitivity of self‐reported hypertension is positively correlated with increase in age, level of education, and economic status. The sensitivity is higher among females, those who are obese, those who do not consume alcohol and do not smoke or chew tobacco, and those who reside in urban areas. The sensitivity of self‐reported hypertension is comparatively higher in Northern and Southern regions and lower in Central and Northeastern regions of India.
TABLE 1.
Sensitivity, specificity, and κ‐coefficients of self‐reported hypertension compared with biomedical data, LASI, 2017–2018
| 45+ Years | |||
|---|---|---|---|
| Sensitivity % (95% CI) | Specificity % (95% CI) | κ (95% CI) | |
| Overall | 56.0 (55.4, 56.6) | 90.5 (90.1, 90.8) | .48 (.47, .49) |
| Background characteristics | |||
| Individual factors | |||
| Sex | |||
| Male | 49.8 (48.9, 50.7) | 92.2 (91.7, 92.6) | .45 (.44, .46) |
| Female | 61.2 (60.4, 61.9) | 89.0 (88.5, 89.4) | .50 (.50, .51) |
| Education level | |||
| No education | 51.9 (51.0, 52.8) | 90.1 (89.6, 90.5) | .44 (.43, .45) |
| Primary | 57.3 (56.2, 58.5) | 90.7 (90.0, 91.3) | .49 (.47, .50) |
| Secondary | 59.0 (57.7, 60.3) | 91.2 (90.4, 91.9) | .55 (.54, .57) |
| Higher | 64.0 (62.3, 65.7) | 90.7 (89.6, 91.8) | .53 (.51, .55) |
| Working status | |||
| Never worked | 65.5 (64.5, 66.6) | 88.1 (87.4, 88.8) | .55 (.53, .56) |
| Currently working | 43.3 (42.4, 44.2) | 92.2 (91.8, 92.6) | .38 (.37, .39) |
| Not currently working | 62.4 (61.3, 63.4) | 89.0 (88.2, 89.7) | .52 (.51, .53) |
| Marital status | |||
| Currently married | 54.4 (53.7, 55.1) | 90.9 (90.5, 91.2) | .47 (.46,.48) |
| Widowed | 60.9 (59.8, 62.0) | 88.7 (87.9, 89.5) | .49 (.48, .51) |
| Others a | 51.4 (48.1, 54.7) | 90.0 (88.1, 91.7) | .38 (.33, .42) |
| MPCE | |||
| Poorest | 44.8 (43.4, 46.2) | 91.4 (90.7, 92.0) | .39 (.37, .41) |
| Poorer | 52.1 (50.7, 53.4) | 91.1 (90.4, 91.7) | .45 (.44, .47) |
| Middle | 55.8 (54.5, 57.1) | 90.6 (89.9, 91.3) | .46 (.45, .48) |
| Richer | 60.2 (59.0, 61.5) | 89.6 (88.8, 90.3) | .52 (.51, .54) |
| Richest | 64.5 (63.3, 65.7) | 89.5 (88.7, 90.2) | .55 (.53, .56) |
| BMI categories | |||
| Normal | 49.3 (48.4, 50.2) | 91.9 (91.5, 92.3) | .43 (.42, .44) |
| Underweight | 37.4 (35.8, 39.1) | 92.9 (92.3, 93.5) | .37 (.35, .39) |
| Overweight/obese | 64.9 (63.9, 65.8) | 90.0 (89.3, 90.7) | .51 (.50, .52) |
| Behavioral factors | |||
| Ever smoke or chew tobacco | |||
| No | 59.9 (59.2, 60.6) | 90.2 (89.7, 90.6) | .51 (.50, .52) |
| Yes | 48.0 (47.0, 49.1) | 91.1 (90.6, 91.6) | .42 (.41, .43) |
| Alcohol use | |||
| No | 58.7 (58.1, 59.4) | 90.3 (89.9, 90.6) | .50 (.49, .51) |
| Yes | 42.9 (41.6, 44.3) | 91.7 (90.9, 92.4) | .37 (.35, .38) |
| Household factors | |||
| Religion | |||
| Hindu | 55.0 (54.3, 55.7) | 90.6 (90.3, 91.0) | .47 (.46, .48) |
| Muslim | 63.7 (62.1, 65.2) | 89.7 (88.6, 90.6) | .53 (.51, .54) |
| Christian | 49.6 (47.8, 51.4) | 91.4 (90.4, 92.3) | .52 (.50, .54) |
| Others b | 62.6 (60.2, 65.0) | 87.3 (85.4, 89.0) | .49 (.46, .52) |
| Caste | |||
| Scheduled caste | 53.3 (51.8, 54.8) | 90.2 (89.4, 90.9) | .45 (.43, .47) |
| Scheduled tribe | 39.7 (38.2, 41.1) | 92.6 (91.9, 93.3) | .32 (.30, .34) |
| OBC c | 57.4 (56.4, 58.4) | 90.6 (90.1, 91.1) | .48 (.47, .50) |
| Others | |||
| Community factors | 64.3 (63.2, 65.4) | 88.8 (88.1, 89.6) | .52 (.51, .53) |
| Place of residence | |||
| Rural | 49.7 (49.0, 50.5) | 90.6 (90.2, 90.9) | .43 (.42, .43) |
| Urban | 64.8 (63.9, 65.6) | 90.2 (89.6, 90.8) | .55 (.54, .56) |
| Region | |||
| North | 62.7 (61.4, 64.0) | 86.3 (85.4, 87.1) | .48 (.47, .50) |
| Central | 43.0 (41.1, 44.8) | 91.0 (90.2, 91.8) | .39 (.37, .41) |
| East | 52.7 (51.2, 54.2) | 89.7 (89.0, 90.4) | .45 (.43, .47) |
| Northeast | 47.5 (45.8, 49.1) | 89.7 (88.8, 90.6) | .45 (.43, .47) |
| West | 57.5 (55.9, 59.0) | 93.5 (92.7, 94.2) | .48 (.46, .50) |
| South | 61.2 (60.1, 62.3) | 93.2 (92.6, 93.8) | .55 (.53, .56) |
aDivorced, separated, and deserted.
bIncludes Sikh, Buddhist/neo‐Buddhist, Jain, Parsi/Zoroastrian, and others.
cOther Backward Classes.
Abbreviations: MPCE, Monthly Per Capita Expenditure; BMI, Body Mass Index; κ, Kappa coefficient; CI, Confidence Interval.
The overall specificity of self‐reported hypertension is 90.5% for ages 45 years and above. In contrast to sensitivity, which showed a significant variation across sub‐groups, no significant differences were observed with respect to specificity. Our findings suggest that the overall k‐coefficient of self‐reported hypertension is 0.48 suggesting moderate agreement between self‐reported and measured hypertension. Significant differences are observed in k‐coefficients between sub‐groups in the study.
Table 2 provides the sensitivity, specificity, and k‐coefficients of self‐reported hypertension compared with measured hypertension in different Indian states and Union Territories. There are significant differences in sensitivities but no significant differences in specificities of self‐reported hypertension among the age groups above 45 years in the Indian states and UTs. The sensitivity of self‐reported hypertension is the highest in Jammu & Kashmir (79%) and the lowest in Nagaland (24%). Sensitivity is high in Goa (76%), Chandigarh (71%), Kerala (68%), Punjab (66%), and Puducherry (70%), and low in Chhattisgarh (31%), Dadra & Nagar Haveli (32%), Nagaland (24%), and Arunachal Pradesh (33 %). The low sensitivity in states can be viewed in the context of the shortfall in the health infrastructure in India, the details of which are presented in Table S2. At the national level, the shortfall of PHCs is 29%, while that of CHCs is 38%. It may be observed that various states with a large shortfall of PHCs, including Chhattisgarh, Jharkhand, Madhya Pradesh, Odisha, and Uttar Pradesh, had lower sensitivity of self‐reported hypertension.
TABLE 2.
Sensitivity, specificity, and κ‐coefficients of self‐reported hypertension compared with biomedical data, by Indian states, LASI, 2017–2018
| 45+ years | |||
|---|---|---|---|
| State | Sensitivity % (95% CI) | Specificity % (95% CI) | κ (95% CI) |
| Andaman & Nicobar Islands | 60.0 (56.1, 63.8) | 87.7 (84.0, 90.7) | .44 (.39, .49) |
| Andhra Pradesh | 63.2 (60.4, 65.9) | 93.0 (91.2, 94.6) | .55 (.51, .58) |
| Arunachal Pradesh | 33.2 (28.4, 38.3) | 86.3 (83.2, 89.0) | .27 (.21, .33) |
| Assam | 56.5 (53.1, 59.9) | 87.8 (85.6, 89.7) | .47 (.43, .51) |
| Bihar | 49.1 (46.0, 52.2) | 85.2 (83.6, 86.7) | .36 (.32, .39) |
| Chandigarh | 70.7 (66.4, 74.8) | 87.9 (84.2, 91.0) | .60 (.54, .65) |
| Chhattisgarh | 30.9 (27.7, 34.2) | 94.5 (92.9, 95.9) | .27 (.23, .31) |
| Dadra & Nagar Haveli | 32.2 (27.5, 37.3) | 94.7 (92.5, 96.4) | .32 (.27, .38) |
| Daman & Diu | 60.4 (55.5, 65.1) | 92.3 (89.5, 94.6) | .51 (.45, .56) |
| Delhi | 61.6 (57.3, 65.9) | 87.2 (84.4, 89.7) | .49 (.44, .54) |
| Goa | 75.7 (72.3, 78.8) | 95.5 (93.2, 97.1) | .67 (.63, .71) |
| Gujarat | 49.3 (45.9, 52.7) | 90.4 (88.5, 92.1) | .43 (.39, .46) |
| Haryana | 64.8 (61.1, 68.3) | 77.2 (74.4, 79.8) | .41 (.36, .45) |
| Himachal Pradesh | 54.1 (50.1, 58.1) | 88.8 (86.0, 91.2) | .42 (.37, .47) |
| Jammu & Kashmir | 79.0 (75.7, 82.0) | 92.4 (90.2, 94.2) | .73 (.69, .77) |
| Jharkhand | 43.7 (40.4, 47.1) | 92.4 (90.8, 93.8) | .40 (.36, .44) |
| Karnataka | 51.3 (47.9, 54.7) | 95.0 (93.5, 96.2) | .56 (.53, .60) |
| Kerala | 67.8 (65.2, 70.4) | 93.4 (91.6, 95.0) | .56 (.53, .59) |
| Lakshadweep | 56.0 (52.2, 59.8) | 93.3 (90.1, 95.7) | .36 (.31, .40) |
| Madhya Pradesh | 46.2 (42.9, 49.6) | 90.8 (89.3, 92.2) | .39 (.35, .42) |
| Maharashtra | 58.9 (56.5, 61.2) | 94.9 (93.7, 95.9) | .50 (.48, .53) |
| Manipur | 52.7 (48.3, 57.1) | 90.2 (87.6, 92.4) | .51 (.46, .56) |
| Meghalaya | 44.9 (40.0, 49.8) | 91.4 (88.2, 93.9) | .41 (.35, .46) |
| Mizoram | 58.3 (52.9, 63.5) | 89.6 (87.0, 91.7) | .53 (.47, .58) |
| Nagaland | 24.3 (20.7, 28.1) | 95.3 (93.2, 96.8) | .20 (.17, .24) |
| Odisha | 48.2 (44.8, 51.5) | 92.1 (90.7, 93.4) | .42 (.38, .46) |
| Puducherry | 69.6 (65.8, 73.2) | 92.3 (89.8, 94.3) | .59 (.55, .63) |
| Punjab | 65.5 (62.7, 68.3) | 86.7 (84.1, 89.1) | .49 (.45, .53) |
| Rajasthan | 55.0 (51.3, 58.6) | 87.1 (85.1, 88.8) | .45 (.41, .49) |
| Tamil Nadu | 57.4 (54.8, 59.9) | 92.9 (91.6, 94.2) | .49 (.45, .52) |
| Telangana | 63.3 (60.2, 66.2) | 94.3 (92.7, 95.7) | .59 (.55, .62) |
| Tripura | 57.8 (53.1, 62.5) | 89.1 (86.2, 91.6) | .48 (.43, .54) |
| Uttar Pradesh | 48.6 (45.8, 51.5) | 89.9 (88.7, 91.0) | .42 (.38, .45) |
| Uttarakhand | 48.1 (43.8, 52.5) | 86.6 (83.8, 89.1) | .38 (.33, .43) |
| West Bengal | 63.0 (60.6, 65.5) | 91.4 (89.9, 92.7) | .55 (.52, .58) |
Abbreviations: κ, Kappa coefficient; CI, Confidence Interval.
Table 2 also presents the k‐coefficient which was used to measure the agreement between self‐reported and measured hypertension. Significant differences in k‐coefficients are observed across states. States with a high sensitivity tend to have a high degree of agreement (k‐coefficient). Self‐reported hypertension shows low sensitivity and high specificity in all the states of India. The UTs of Jammu & Kashmir, Chandigarh, and Puducherry report a high sensitivity and specificity and high k‐coefficient values for all age categories, whereas Dadra & Nagar Haveli and Lakshadweep have low values. The Southern region has a better sensitivity and specificity and higher k‐coefficient values than the other regions.
Table 3 shows the estimated effects of the predictor variables on false negative reporting and false positive reporting of hypertension using multinomial regression model. The propensity of false negative and false positive reporting goes down as age increases. Sex of individual has a significant association with false negative but no significant association with false positive reporting. Compared to males, females have a 23% lower rate of false negative reporting. Educational level has a significant effect on false negative and false positive reporting. For example, secondary education has 38% less false positive reporting and higher education has 40% less false positive reporting of hypertension compared to no education. MPCE has a significant effect on false negative reporting but not on false positive reporting. Interesting to observe that underweight individuals have 44% higher false negative reporting, while obese individuals have 39% lower false negative reporting compared to persons of normal weight.
TABLE 3.
Multinomial logistic regression results for false negative and positive reporting (with respect to correct reporting) of hypertension among older adults in India, LASI, 2017–18
| False negative reporting | False positive reporting | |
|---|---|---|
| Background characteristics | OR (95% CI) | OR (95% CI) |
| Individual factors | ||
| Age groups (in years) | ||
| 45–54 | 1.00 | 1.00 |
| 55–64 | .63*** (.48,.83) | .45*** (.36,.56) |
| 65–74 | .54*** (.41,.71) | .30*** (.24,.39) |
| 75+ | .51*** (.39,.67) | .25*** (.18,.34) |
| Sex | ||
| Male | 1.00 | 1.00 |
| Female | .77*** (.69,.87) | 1.12 (.91,1.38) |
| Education level | ||
| No education | 1.00 | 1.00 |
| Primary | .79*** (.70,.89) | .87 (.72,1.04) |
| Secondary | .68*** (.56,.84) | .62*** (.47,.81) |
| Higher | .82 (.55,1.21) | .60*** (.45,.79) |
| Working status | ||
| Never worked | 1.00 | 1.00 |
| Currently working | 1.62*** (1.41,1.87) | 1.36** (1.12,1.65) |
| Not currently working | .91 (.80,1.04) | .94 (.77,1.13) |
| Marital Status | ||
| Currently married | 1.00 | 1.00 |
| Widowed | .91 (.80,1.04) | .71*** (.61,.84) |
| Others a | 1.29 (.95,1.74) | 1.55 (.86,2.78) |
| MPCE | ||
| Poorest | 1.00 | 1.00 |
| Poorer | .76*** (.66,.88) | .82 (.63,1.05) |
| Middle | .76*** (.66,.87) | .82 (.64,1.05) |
| Richer | .60*** (.52,.70) | .82 (.63,1.06) |
| Richest | .56*** (.49,.65) | .79 (.57,1.09) |
| BMI categories | ||
| Normal | 1.00 | 1.00 |
| Underweight | 1.44*** (1.25,1.65) | 1.88*** (1.57,2.24) |
| Overweight/obese | .61*** (.55,.68) | .49*** (.40,.60) |
| Behavioral factors | ||
| Ever smoke or chew tobacco | ||
| No | 1.00 | 1.00 |
| Yes | 1.13 (1.00,1.28) | 1.05 (.91,1.21) |
| Alcohol use | ||
| No | 1.00 | 1.00 |
| Yes | 1.18* (1.02,1.36) | .85 (.68,1.07) |
| Household factors | ||
| Religion | ||
| Hindu | 1.00 | 1.00 |
| Muslim | .83* (.72,.96) | .82 (.64,1.05) |
| Christian | .89 (.72,1.11) | .82 (.55,1.22) |
| Others b | .92 (.74,1.15) | .69* (.50,.93) |
| Caste | ||
| Scheduled Caste | 1.00 | 1.00 |
| Scheduled Tribe | 1.69*** (1.37,2.08) | .92 (.67,1.26) |
| OBC c | 1.11 (.95,1.28) | 1.23* (1.03,1.48) |
| Others | ||
| Community factors | 1.01 (.86,1.18) | 1.09 (.89,1.33) |
| Place of residence | ||
| Rural | 1.00 | 1.00 |
| Urban | .70*** (.63,.78) | .73** (.61,.89) |
| Region | ||
| North | 1.00 | 1.00 |
| Central | 1.63*** (1.36,1.96) | 1.12 (.90,1.39) |
| East | 1.11 (.94,1.30) | .89 (.72,1.10) |
| Northeast | .87 (.70,1.08) | .66** (.49,.90) |
| West | 1.37*** (1.16,1.61) | .43*** (.33,.56) |
| South | 1.18* (1.00,1.39) | .36*** (.28,.47) |
aDivorced, separated, and deserted.
bIncludes Sikh, Buddhist/neo‐Buddhist, Jain, Parsi/Zoroastrian, and others.
cOther Backward Classes.
Abbreviations: MPCE, Monthly Per Capita Expenditure; BMI, Body Mass Index; OR, Odds Ratio; CI, Confidence Intervals.
*P < .10, **P < .05, ***P < .01.
Lastly, place of residence too has a significant effect on false negative and false positive reporting. Urban residents have a 30% lower rate of false negative reporting and a 27% lower rate of false positive reporting compared to rural residents.
4. DISCUSSION
Hypertension is a major public health concern globally, and especially in low‐ and middle‐income countries. For effective local and national level policy making and implementation of strategies for the prevention and control of hypertension, information on the distribution of the prevalence of hypertension in the population is an essential starting point. Although self‐reported measures of health are well‐researched and commonly regarded as reliable predictors of the underlying health of the population in the developed countries, the validity of these measures is under‐researched and questionable in the context of low‐ and middle‐income countries. Due to the high costs and time‐consuming process of the clinical diagnosis in the surveys, information on hypertension is likely to be based on self‐reported data. A number of studies have relied on the use of self‐reported information on hypertension while studying its influence on various health‐related outcomes, including quality of life, 24 depression and anxiety, 25 chronic health conditions, 26 and mortality. 27
Comparing the subjective and objective measurements of a health condition allows for assessing its precision of measurement and reliability. 28 This paper contributes to the limited but growing body of literature that assesses the validity of self‐reported hypertension and tracks inconsistent reports. Using nationally‐representative data on individuals aged 45 years and older, we investigated the accuracy of self‐reported hypertension. Our analysis shows a sensitivity of 56% and a specificity of 90.5%. Agreement between self‐reported data and measured hypertension was observed to be moderate (κ = 0.48). Large variations, particularly in sensitivity of self‐reported hypertension, were observed among states and sub‐groups. Age, sex, education, wealth status, body mass index, and place of residence were the strongest explanatory predictors of accurate self‐reported hypertension. Similar to other studies that have analyzed the validity of self‐reported hypertension, our study too found disagreement between self‐reported disease status and the corresponding clinically diagnosed measures and confirms that self‐reported hypertension underestimates the true prevalence of hypertension. 11 , 13
Previous studies examining the validity of self‐reported hypertension have found the sensitivity of self‐reported hypertension to range from 34.5% to 83.9% and the specificity from 92.0% to 98.7%. 8 , 9 , 11 , 12 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Literature suggests that self‐reported hypertension generally exerts high sensitivity and specificity and a moderate level of agreement. 38
A number of countries have reported higher sensitivity and specificity of self‐reported hypertension compared to our aggregate national‐level findings. For example, a study conducted in the United States, 8 based on the data from the Health and Retirement Study (HRS), reported a higher level of sensitivity (83.9%) and specificity (92.0%) than our study. Similarly, a study based on Korean older adults (31) aged 50 years and above, reported a higher sensitivity (73.0%) and specificity (98.5%) and higher k‐agreement (0.72) as compared to our study. Other studies to have reported a higher sensitivity and specificity of self‐reported hypertension include those from Thailand (82.4% and 70.7%), 39 South Korea (73.0% and 98.5%), 31 Brazil (77.1% and 93.4%), 40 and Russia (73.1% and 72.1%). 38 A comparative study based on Chinese older adults, which used data from the China Health and Retirement Study (CHARLS), 11 reported a sensitivity, specificity, and κ‐agreement of self‐reported hypertension of 56.3%, 96.3%, and .57, respectively, similar to our findings. Only South Africa (31.4% and 78.4%) and Ghana (13.7% and 97.3%) 38 have reported sensitivity and specificity lower than our findings for India as a whole.
Healthcare in India is a state patient and, hence, the responsibility of individual states to provide effective and efficient health care of high quality to their respective populations. However, there is a huge variation among the states of India in terms of their level of socio‐economic development, size of population, health transition, and health systems capacities with respect to physical and human resources. All these factors are likely to influence the health status experienced by a state's population. It is, therefore, not surprising that we observe large and significant variations in the sensitivity of self‐reported measures among the states of India.
As a significant proportion of the population in India is unaware of its hypertensive status, self‐reported measures tend to underestimate the true prevalence of hypertension. A number of factors contribute to this large awareness gap between the self‐reported and the true burden of hypertension. Literature suggests issues with respect to recall bias in survey questionnaires. 41 , 42 In a country like India, low levels of self‐reported hypertension indicate significant limitations of the country's health policy and health care systems. Our opinion is that the low sensitivity in India is largely due to the barriers in access to health services that result in an under‐diagnosis of hypertension. Given the low public health spending in India, with the resultant weak public health systems and a massive expansion of the private sector in the recent years, India is one of the countries with the highest out‐of‐pocket payments. Literature abounds with issues around financial barriers to access to health services and equity implications in terms of the regressive nature of health systems largely dependent on out‐of‐pocket payments. In addition to financial barriers in terms of affordability and access to health services, low levels of literacy, low health insurance coverage, and huge inequalities in financing and delivery of health care further aggravate the situation.
India spends only 3.5% of its Gross Domestic Product (GDP) on health as compared to China that spends 5.2%, with a much lower public spending on health (27% of total health expenditure), and has nine doctors per 10 000 population when compared to 20 in China. 43 Although home to 67% of India's population, rural areas have only 33% of hospital beds. 44 Chronic underinvestment in health over decades has resulted in weak public health care systems in India. For India as a whole, there is a shortfall of over 75% specialist doctors at CHCs in the rural areas. 44 In addition, there is a large shortfall of the health infrastructure itself – PHCs and CHCs – in rural India. 44
With a private sector that is unaffordable for many on the one hand, and a weak public health care system – in terms of availability of manpower, equipment, and drugs – on the other, it is not surprising that one out of two hypertensives in India are not aware of their hypertensive status. Although India launched a national‐level program for controlling NCDs, with a particular focus on diabetes, cancer, stroke, and cardiovascular diseases, in 2010, the program's outcomes were not satisfactory. 45
It is crucial to examine the disagreement between self‐reported and measured hypertension as it has potential policy implications, including serving as a misleading basis for evidence‐based policy and health service planning. 46 For example, if resources are allocated based on the self‐reported measures, it would result in a significant under‐investment in budgeting for hypertension prevention and control programs as almost half of all hypertensive individuals (44%) would remain undetected by this approach. In addition, as a result of the systematic bias, planning, programming and developing hypertension control strategies based on self‐reported measures would discriminate against the younger population, those residing in rural areas, those who are uneducated, those belonging to lower socio‐economic groups, and those who consume alcohol and are smokers, as these groups disproportionately under‐report hypertension as observed from the low sensitivity of self‐reported hypertension. Our results confirm that well‐developed states like Kerala and Goa, with high literacy rates and better access to health services, have significant advantages in terms of awareness of hypertension than the less‐developed states, where access to health care services may be a major concern. Resource allocation, based on self‐reported measure, would place more resources in the hands of the developed states at the cost of the underdeveloped states.
This study contributes to the existing literature in a number of ways. It makes an empirical contribution to the limited existing literature by providing estimates of disagreement between self‐reported hypertension status and the corresponding clinically diagnosed measure of the same individuals in a nationally‐representative sample of elderly population over 45 years in India. It also assesses the validity of self‐reported measure in terms of sensitivity, specificity, and level of agreement between the two measures of hypertension. In addition, through the use of multinomial logistic regression, this study estimates the effects of predictor variables on false negative and false positive reporting of hypertension and thus attempts to explain the reasons behind this divergence between the two measures. The strength of this paper is that it attempts to understand whether the deviation in self‐reported hypertension is a random occurrence or there are underlying factors contributing to this variation in a systematic manner.
5. CONCLUSIONS
Our findings are consistent with other studies in the literature, in that self‐reported measures of hypertension can grossly underestimate the true prevalence of the condition, especially in low‐ and middle‐income countries. Almost half of all patients identified with biometric hypertension would not be identified by self‐reported measures, with significant variations across the states of India. This is more so for some sub‐groups and the less developed states, where the use of self‐reported measures would miss seven out of 10 hypertensives. The large awareness gap between self‐reports and the true burden of disease indicates major deficiencies in India's health care system, including issues relating to access to health services as a result of financial affordability. Self‐reported hypertension has important limitations and may be a source of systematic bias, under‐representing individuals who are illiterate, belong to lower socio‐economic groups, reside in rural areas, and are from the less‐developed states. Therefore, it is recommended that in countries like India, planning, budgeting, and program prevention and control activities be based on an objective assessment of hypertension.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Mrigesh Bhatia and Priyanka Dixit conceptualized this study. Manish Kumar and Laxmi Kant Dwivedi were involved in data analysis. Funding for this study was acquired by Mrigesh Bhatia as a part of the LSE Covid Impact Fund for Research and Knowledge Exchange. All the authors contributed to the writing of the original draft, the subsequent review, and to the editing. All the authors have seen and approved the final version of the manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENT
This research has received funding from the LSE Covid Impact Fund for Research and Knowledge Exchange.
Bhatia M, Dixit P, Kumar M, Dwivedi LK. Validity of self‐reported hypertension in India: Evidence from nationally representative survey of adult population over 45 years. J Clin Hypertens. 2022;24:1506–1515. 10.1111/jch.14542
REFERENCES
- 1. Paulhus DL, Vazire S. The self‐report method. Handb Res Meth Personal Psychol. 2007; 1(2007): 224‐239. [Google Scholar]
- 2. McDonald JD. Measuring personality constructs: the advantages and disadvantages of self‐reports, informant reports and behavioural assessments. Enquire. 2008; 1(1): 1‐19. [Google Scholar]
- 3. El Fakiri F, Bruijnzeels MA, Hoes AW. No evidence for marked ethnic differences in accuracy of self‐reported diabetes, hypertension, and hypercholesterolemia. J Clin Epidemiol. 2007; 60(12): 1271‐1279. [DOI] [PubMed] [Google Scholar]
- 4. Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: evidence from hypertension for the income/health gradient. J Health Econ. 2009; 28(3): 540‐552. [DOI] [PubMed] [Google Scholar]
- 5. Lindeboom M, Van Doorslaer E. Cut‐point shift and index shift in self‐reported health. J Health Econ. 2004; 23(6): 1083‐1099. [DOI] [PubMed] [Google Scholar]
- 6. Jones F, Partridge C, Reid F. The Stroke Self‐Efficacy Questionnaire: measuring individual confidence in functional performance after stroke. J Clin Nurs. 2008; 17(7b): 244‐252. [DOI] [PubMed] [Google Scholar]
- 7. Atwood KM, Robitaille CJ, Reimer K, Dai S, Johansen HL, Smith MJ. Comparison of diagnosed, self‐reported, and physically‐measured hypertension in Canada. Can J Cardiol. 2013; 29(5): 606‐612. [DOI] [PubMed] [Google Scholar]
- 8. White K, Avendaño M, Capistrant BD, Moon JR, Liu SY, Glymour MM. Self‐reported and measured hypertension among older US‐and foreign‐born adults. J Immigr Minor Heal. 2012; 14(4): 721‐726. [DOI] [PubMed] [Google Scholar]
- 9. Taylor A, Dal Grande E, Gill T, Pickering S, Grant J, Adams R, et al. Comparing self‐reported and measured high blood pressure and high cholesterol status using data from a large representative cohort study. Aust N Z J Public Health. 2010; 34(4): 394‐400. [DOI] [PubMed] [Google Scholar]
- 10. Peterson KL, Jacobs JP, Allender S, Alston LV, Nichols M. Characterising the extent of misreporting of high blood pressure, high cholesterol, and diabetes using the Australian Health Survey. BMC Public Health. 2016; 16: 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ning M, Zhang Q, Yang M. Comparison of self‐reported and biomedical data on hypertension and diabetes: findings from the China Health and Retirement Longitudinal Study (CHARLS). BMJ Open. 2016; 6(1): e009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie D, Wang J. Comparison of self‐reports and biomedical measurements on hypertension and diabetes among older adults in China. BMC Public Health. 2020; 20: 1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onur I, Velamuri M. The gap between self‐reported and objective measures of disease status in India. PLoS One. 2018; 13(8): e0202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanno A, Kikuya M, Ohkubo T, Hashimoto T, Satoh M, Hirose T, et al. Pre‐hypertension as a significant predictor of chronic kidney disease in a general population: the Ohasama Study. Nephrol Dial Transplant. 2012; 27(8): 3218‐3223. [DOI] [PubMed] [Google Scholar]
- 15. Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, et al. Association of midlife to late‐life blood pressure patterns with incident dementia. Jama. 2019; 322(6): 535‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHOa) . Fact sheet on Hypertension [Internet]. 2021 [cited 2021 Aug 21]. Available from: https://www.who.int/news‐room/fact‐sheets/detail/hypertension. Accessed March 26, 2022.
- 17. GBD Results Tool . Global health data exchange [Internet]. 2019 [cited 2021 Aug 31]. Available from: http://ghdx.healthdata.org/gbd‐results‐tool. Accessed March 21, 2022.
- 18. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016; 134(6): 441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta R, Gaur K, Ram CVS. Emerging trends in hypertension epidemiology in India. J Hum Hypertens. 2019; 33(8): 575‐587. [DOI] [PubMed] [Google Scholar]
- 20. Gupta R, Gupta S. Hypertension in India: trends in prevalence, awareness, treatment and control. RUHS J Heal Sci. 2017; 2(1): 40‐46. [Google Scholar]
- 21. Gupta R, Gupta VP, Prakash H, Agrawal A, Sharma KK, Deedwania PC. 25‐Year trends in hypertension prevalence, awareness, treatment, and control in an Indian urban population. Jaipur Heart Watch Indian Heart J. 2018; 70(6): 802‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International Institute for Population Sciences (IIPS) . Longitudinal Ageing Study in India (LASI) Wave 1 [Internet]. Mumbai, India; 2020. Available from: http://iipsindia.org/research_lasi.htm. Accessed March 18, 2022
- 23. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003; 42(6): 1206‐1252. [DOI] [PubMed] [Google Scholar]
- 24. Yao Q, Liu C, Zhang Y, Xu L. Health‐related quality of life of people with self‐reported hypertension: a national cross‐sectional survey in China. Int J Environ Res Public Health. 2019; 16(10): 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grimsrud A, Stein DJ, Seedat S, Williams D, Myer L. The association between hypertension and depression and anxiety disorders: results from a nationally‐representative sample of South African adults. PLoS One. 2009; 4(5): e5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tetteh J, Entsua‐Mensah K, Doku A, Mohammed S, Swaray SM, Ayanore MA, et al. Self‐reported hypertension as a predictor of chronic health conditions among older adults in Ghana: analysis of the WHO Study on global Ageing and adult health (SAGE) Wave 2. Pan Afr Med J. 2020; 36: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aune D, Huang W, Nie J, Wang Y. Hypertension and the risk of all‐cause and cause‐specific mortality: an outcome‐wide association study of 67 causes of death in the national health interview survey. Biomed Res Int. 2021; 2021: 9376134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004; 1(2): 182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Najafi F, Pasdar Y, Shakiba E, Hamzeh B, Darbandi M, Moradinazar M, et al. Validity of self‐reported hypertension and factors related to discordance between self‐reported and objectively measured hypertension: evidence from a cohort study in Iran. J Prev Med Public Heal. 2019; 52(2): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torres Oliveira EC, Nobre de M. . Validity and concordance of hypertension self‐reported among elderly people. Int Arch Med. 2016; 9: 95. [Google Scholar]
- 31. Chun H, Kim I‐H, Min K‐D. Accuracy of self‐reported hypertension, diabetes, and hypercholesterolemia: analysis of a representative sample of Korean older adults. Osong public Heal Res Perspect. 2016; 7(2): 108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molenaar EA, Van AmeijdenEJC, Grobbee DE, Numans ME. Comparison of routine care self‐reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health. 2007; 17(2): 199‐205. [DOI] [PubMed] [Google Scholar]
- 33. Tsai AC‐H, Chang T‐L. Quality issues of self‐report of hypertension: analysis of a population representative sample of older adults in Taiwan. Arch Gerontol Geriatr. 2012; 55(2): 338‐342. [DOI] [PubMed] [Google Scholar]
- 34. Tompkins G, Forrest LF, Adams J. Socio‐economic differences in the association between self‐reported and clinically present diabetes and hypertension: secondary analysis of a population‐based cross‐sectional study. PLoS One. 2015; 10(10): e0139928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldman N, Lin I‐F, Weinstein M, Lin Y‐H. Evaluating the quality of self‐reports of hypertension and diabetes. J Clin Epidemiol. 2003; 56(2): 148‐154. [DOI] [PubMed] [Google Scholar]
- 36. Huerta JM, Tormo MJ, Egea‐Caparrós JM, Ortolá‐Devesa JB, Navarro C. Accuracy of self‐reported diabetes, hypertension, and hyperlipidemia in the adult Spanish population. DINO study findings. Rev Española Cardiol (English Ed). 2009; 62(2): 143‐152. [DOI] [PubMed] [Google Scholar]
- 37. Mentz G, Schulz AJ, Mukherjee B, Ragunathan TE, Perkins DW, Israel BA. Hypertension: development of a prediction model to adjust self‐reported hypertension prevalence at the community level. BMC Health Serv Res. 2012; 12: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tenkorang EY, Sedziafa P, Sano Y, Kuuire V, Banchani E. Validity of self‐report data in hypertension research: findings from the Study on Global Ageing and Adult Health. J Clin Hypertens. 2015; 17(12): 977‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thawornchaisit P, De Looze F, Reid CM, Seubsman S, Sleigh A, Team TCS. Validity of self‐reported hypertension: findings from the Thai Cohort Study compared to physician telephointerview. Glob J Health Sci. 2013; 6(2): 1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Menezes TN, Oliveira ECT, de Sousa Fischer MAT. Validity and concordance between self‐reported and clinical diagnosis of hypertension among elderly residents in northeastern Brazil. Am J Hypertens. 2014; 27(2): 215‐221. [DOI] [PubMed] [Google Scholar]
- 41. Mohan S, Campbell N, Chockalingam A. Time to effectively address hypertension in India. Indian J Med Res. 2013; 137(4): 627. [PMC free article] [PubMed] [Google Scholar]
- 42. Feng XL, Pang M, Beard J. Health system strengthening and hypertension awareness, treatment and control: data from the China health and retirement longitudinal study. Bull World Health Organ. 2013; 92: 29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Bank . World Development Indicators [Internet]. 2020 [cited 2021 Aug 28]. Available from: http://data.worldbank.org/indicator/. Accessed March 20, 2022.
- 44. Government of India (GoI) . Rural health statistics 2019–20 [Internet]. 2020. Available from: https://hmis.nhp.gov.in/downloadfile?filepath=publications/Rural‐Health‐Statistics/RHS2019–20.pdf. Accessed March 18, 2022.
- 45. Mohan V, Shah SN, Joshi SR, Seshiah V, Sahay BK, Banerjee S, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014; 18(3): 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al Shamsi HS, Almutairi AG, Al Mashrafi SS. Implications of disagreement between self‐reporting and objective measures: a scoping review. Glob J Health Sci. 2018; 10(6): 199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


