Abstract
An association of Chlamydia pneumoniae with atherosclerosis and coronary heart disease has been determined epidemiologically and by the detection of C. pneumoniae organisms in atherosclerotic lesions in both humans and animal models of atherosclerosis. Previously, it has been shown that C. pneumoniae is capable of replicating in cell types found within atheromatous lesions, viz., endothelial cells, smooth muscle cells (SMC), and macrophages, yet the role of C. pneumoniae in the pathogenesis of atherosclerosis has not been determined. Since intimal thickening is a hallmark of atherosclerosis, we investigated whether C. pneumoniae infection of human umbilical vein endothelial cells (HUVEC) could induce the expression of a soluble factor(s) with mitogenic potential for SMC by using [3H]thymidine incorporation and direct cell counting. Conditioned medium harvested from HUVEC infected with C. pneumoniae stimulated SMC replication in a time- and dose-dependent fashion. Infection studies using various multiplicities of infection (MOIs) ranging from 0.001 to 1 demonstrated a dose-dependent production of the soluble factor(s). At an MOI of 1, SMC stimulation indices were 8.4 (P < 0.01) and 12.2 (P < 0.01) for conditioned media harvested at 24 and 48 h, respectively. To determine whether viable C. pneumoniae was required for production of the soluble factor(s), HUVEC were infected with heat-inactivated C. pneumoniae or with viable organisms in the presence of chloramphenicol. Both treatments produced stimulation indices similar to those for live C. pneumoniae in the absence of chloramphenicol (P > 0.05), indicating that the factor(s) was produced by HUVEC and not by C. pneumoniae and that signal transduction events following chlamydia endocytosis may be important in the production of a soluble factor(s). The ability of C. pneumoniae to elicit an endothelial cell-derived soluble factor(s) that stimulates SMC proliferation may be important in the pathogenesis of atherosclerosis.
Chlamydia pneumoniae is a common cause of acute respiratory conditions, such as pneumonia, sinusitis, bronchitis, and pharyngitis (11, 22). More recently, C. pneumoniae has been implicated as a possible etiologic agent of coronary artery disease and atherosclerosis (9, 23, 24). Saikku and colleagues reported the first evidence for such a relationship by demonstrating increasing titers of C. pneumoniae antibodies in men with coronary heart disease and acute myocardial infarction (35). Since then, new evidence which supports a role for C. pneumoniae in the pathogenesis of atherosclerosis has emerged. C. pneumoniae has been detected in atherosclerotic arteries by several techniques (3, 20, 21), and the organism has been isolated from both coronary (30) and carotid (16) atheromas. Recent animal models have suggested that C. pneumoniae is capable of inducing atherosclerosis in both rabbit (6, 27) and mouse (25) models of atherosclerosis. Furthermore, human clinical treatment studies which examined the use of antichlamydial macrolide antibiotics in patients with coronary atherosclerosis have been carried out. In these placebo-controlled studies, a 5 to 10% reduction in secondary adverse cardiovascular events compared to age- and sex-matched controls was observed in patients receiving either azithromycin (12) or roxithromycin (13).
Atherosclerosis is thought to occur following an inflammatory response to injury of the vessel wall (28, 32). During this process, peripheral leukocytes such as monocytes and T lymphocytes bind to upregulated receptors on activated endothelium via vascular cell adhesion molecule 1 and leukocyte function antigen 1 and transmigrate through the endothelium into the subendothelial space. Here, macrophages accumulate oxidized low-density lipoprotien (LDL), forming foam cells, and together with media-derived smooth muscle cells (SMC) form the initial fatty streak. Grade 2 and 3 lesions which contain increased numbers of foam cells surrounding a central core of lipid and cell debris then ensue, ultimately forming the end-stage fibrous plaque (28, 32).
Several groups have demonstrated the ability of C. pneumoniae to infect and replicate in cell types found within the atherosclerotic lesion, including endothelial cells, SMC, and macrophages (8, 10). Infection of these cell types has been shown to result in the production of proinflammatory cytokines which may be involved in atherogenesis. The ability of chlamydiae to persist within host cells and produce antigens in the absence of replication may provide sustained immunogenic stimulation necessary for the development of chronic inflammatory diseases such as atherosclerosis. C. pneumoniae infection of endothelial cells has been shown to upregulate the expression of endothelial adhesion molecules (18) and several inflammatory mediators, including monocyte chemoattractant protein 1 (MCP-1), interleukin 8 (IL-8) (26) and IL-1β (14). During atherosclerosis, these cytokines are also upregulated (32) and may potentiate the development of atheromatous lesions. C. pneumoniae lipopolysaccharide (LPS) has also been shown to promote the formation of macrophage foam cells (17) in vitro.
Central to the pathology of atherosclerosis is the proliferation of SMC in the arterial intima (15, 37). Endothelial cells have been shown to secrete several soluble factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and insulin-like growth factor 1 (IGF-1) (32), which are important in SMC migration and proliferation during atherogenesis. The production of these factors during chlamydial infection, however, has not been studied. To better understand the pathophysiology of atherosclerosis and the role of C. pneumoniae in atherogenesis, we examined whether C. pneumoniae infection of endothelial cells could induce the production of soluble factors which stimulate SMC replication.
MATERIALS AND METHODS
Cell culture.
HEp2 cells (ATCC CCL-23) were maintained in minimal essential medium (MEM) (Gibco BRL, Gaithersburg, Md.) containing Earle’s salts and supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco BRL) and 2 mM l-glutamine (Gibco BRL). Cells were maintained at 37°C and 5% CO2 in 75-cm2 culture flasks and subcultured into either 25-cm2 flasks or shell vials containing glass coverslips prior to infection with C. pneumoniae. Human umbilical vein endothelial cells (HUVEC; ATCC 1730-CRL) were maintained in Ham’s F12K medium (Gibco BRL) supplemented with 10% FBS, 30 μg of endothelial cell growth supplement (Sigma, St. Louis, Mo.)/ml and 10 U of heparin (Sigma)/ml. Cells were maintained at 37°C and 5% CO2 in gelatin-coated 75-cm2 culture flasks. Prior to infection, cells were seeded into gelatin-coated 24-well plates at a density of 2 × 105 cells/well and allowed to adhere for 24 h in the absence of endothelial cell growth supplement or heparin. Primary SMC were established from bronchial explants by a method described previously (39) and were provided at passage two by G. Cox (Department of Medicine, McMaster University). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL) supplemented with 10% FBS, penicillin (50 U/ml), and streptomycin (50 μg/ml) (Gibco BRL). Cells were maintained at 37°C and 5% CO2 in 75-cm2 culture flasks and subcultured at ∼90% confluency. SMC showed typical hill and valley morphology, and the expression of α-actin was confirmed by immunofluorescence (IF). Cells were suspended in DMEM at a density of 5 × 104 cells/ml and transferred into 24-well plates (5 × 104 cells/well). SMC were used at the third and fourth passages for all experiments.
C. pneumoniae propagation.
C. pneumoniae VR-1310 (ATCC 1310-VR) was propagated in HEp2 cells as described by Roblin et al. (31) with slight modifications. C. pneumoniae was inoculated onto preformed monolayers of HEp2 cells in 25-cm2 flasks, centrifuged at 1,000 × g for 60 min at 25°C, and incubated at 37°C for 1 h. The inoculum was removed and replaced with growth medium consisting of MEM containing 1 μg of cycloheximide/ml. Infected cultures were incubated for 48 to 72 h at 37°C and 5% CO2. C. pneumoniae was harvested by disrupting HEp2 cells with glass beads followed by sonication and centrifugation at 250 × g to remove cellular debris. Supernatants containing C. pneumoniae were aliquoted and frozen at −70°C. For some experiments, supernatants containing C. pneumoniae were further centrifuged at 20,000 × g for 20 min to pellet elementary bodies (EBs). EB pellets were then suspended in sucrose-phosphate-glutamate buffer, aliquoted, and stored at −70°C. HEp2 monolayers grown in shell vials were used to monitor the course of infection by IF staining using a genus-specific fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (Kallestad, Chaska, Minn.). C. pneumoniae titrations were performed on frozen stocks and were expressed as inclusion-forming units per ml.
Preparation of HUVEC culture-conditioned medium.
HUVEC were infected in 24-well plates as described above at various multiplicities of infection (MOIs). Following centrifugation at 1,000 × g for 60 min and incubation at 37°C for 1 h, the inoculum was removed, and cells were washed twice with Hanks’ balanced salt solution and cultured in 1 ml of serum-free Ham’s F12K medium lacking growth supplements and cycloheximide. HEp2 cell lysates were prepared according to the same procedure used for C. pneumoniae isolation and used for control mock infection of HUVEC. Conditioned medium from infected or uninfected HUVEC cultures was harvested at 24 or 48 h, clarified by centrifugation at 20,000 × g for 30 min, and assessed immediately for the ability to stimulate SMC by a [3H]thymidine incorporation assay. In some experiments, chlamydiae were heat inactivated at 56°C for 30 min or cultured in the presence of chloramphenicol (80 μg/ml; Sigma). Both treatments inhibited the growth of C. pneumoniae as determined by IF staining for chlamydial inclusions.
SMC DNA synthesis.
DNA synthesis in SMC was assessed by the incorporation of [3H]thymidine as described previously (15) with slight modifications. SMC were suspended in DMEM-10% FBS and seeded into 24-well plates at 5 × 104 cells/well and cultured for 48 h to ensure logarithmic growth. At 48 h, cells were washed three times in serum-free (SF) DMEM and cultured an additional 48 h in SF-DMEM containing 0.1% bovine serum albumin (BSA) (Sigma) in order to induce cells into G0 quiescence. Quiescent SMC were washed, and conditioned assay medium from uninfected, mock-infected, or C. pneumoniae-infected HUVEC was applied to quiescent SMC cultures for 24 h. Next, 1 μCi of [3H]thymidine (methyl-3H; 6.7 Ci/mmol, 37 MBq/ml [NEN, Boston, Mass.])/ml was added after 16 h for an 8-h pulse. Each assay condition was assessed in triplicate. DMEM containing 10% FBS was used as a positive control for [3H]thymidine incorporation. Following the 24-h incubation period, medium was discarded, and cells were washed three times with ice-cold phosphate-buffered saline and then lysed in 0.25 ml of 0.2 N NaOH for 20 min at room temperature. DNA was precipitated by the addition of 1.25 ml of 10% trichloroacetic acid (TCA) (Sigma) for 1 h at 4°C. The TCA-insoluble fraction was filtered onto GF/F glass fiber disks (Whatman, Clifton, N.J.), washed, and air dried. Filters were placed into 5 ml of scintillation fluid (BCA-NA; Amersham), and radioactivity was determined by using a liquid scintillation β-counter (LS5801; Beckman Instruments) and expressed as counts per minute (CPM). Stimulation indices were calculated as follows: [3H]thymidine CPM for conditioned medium/mean [3H]thymidine CPM for uninfected medium.
SMC proliferation assay.
Proliferation of SMC was determined by cell counting. Subconfluent (5 × 104) SMC were induced into quiescence for 48 h in SF-DMEM–0.1% BSA as described above. SMC were cultured in the presence of conditioned assay medium from uninfected, mock-infected, or C. pneumoniae-infected HUVEC for 7 days. Cells were counted every other day by removing the medium, treating cells with 0.3 ml of 0.05% trypsin-EDTA, resuspending in medium, and staining cells with 0.4% trypan blue. Cell counts were done in triplicate in a Neubauer chamber and expressed as mean counts.
Statistical analysis.
Statistical comparisons were made by using one-way analysis of variance with the Dunnett’s multiple comparison post test. P values of <0.05 were considered significant.
RESULTS
Growth of C. pneumoniae in HUVEC.
An initial experiment was performed to confirm the earlier observation that HUVEC support the growth of C. pneumoniae. HUVEC supported the replication of C. pneumoniae VR-1310 as demonstrated by visualizing intracellular inclusions at 48 h postinfection. Figure 1 shows an infected HUVEC with one typical inclusion body. The number of chlamydial inclusions per high-powered field increased with increasing titers of C. pneumoniae (data not shown). Infection studies using heat-inactivated C. pneumoniae failed to give rise to productive infections in HUVEC as evidenced by the absence of any visible inclusions at either 48 or 72 h postinfection (data not shown). The chlamydial developmental cycle was also blocked when HUVEC were infected in the presence of chloramphenicol, which inhibits prokaryotic translation but does not effect eukaryotic translation at the concentrations used in these studies.
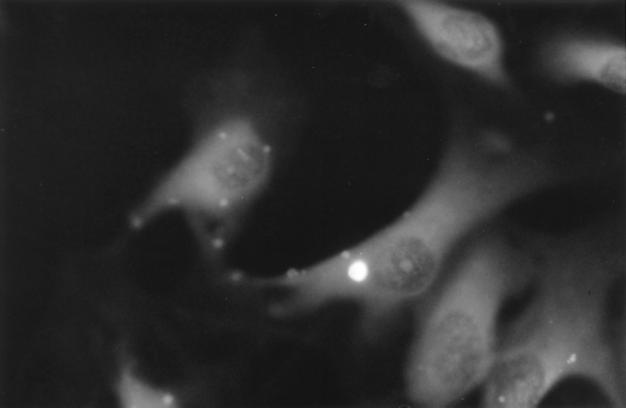
FIG. 1.
C. pneumoniae infection of HUVEC. HUVEC were infected with C. pneumoniae and at 48 h postinfection, monolayers were stained with an FITC-conjugated anti-LPS specific monoclonal antibody. Shown is a HUVEC containing one typical intracellular inclusion, shown as a bright region within the cell. Neighboring uninfected cells do not contain typical inclusion bodies. Magnification, ×400 (epifluorescence microscopy).
C. pneumoniae infection of HUVEC results in the production of soluble factor(s) with mitogenic activity for SMC.
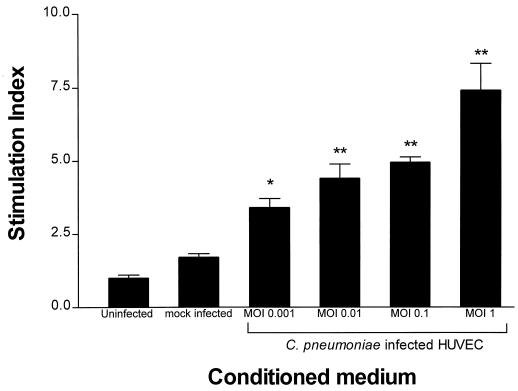
To study whether C. pneumoniae infection of HUVEC could produce soluble factors which stimulate SMC replication, conditioned medium harvested from infected cell culture was assayed for the ability to stimulate [3H]thymidine incorporation into SMC. Conditioned medium was clarified by high-speed centrifugation to remove HUVEC debris and any C. pneumoniae EBs. The clarified conditioned medium failed to give rise to chlamydial inclusions following inoculation onto HEp2 cell monolayers, verifying that C. pneumoniae EBs were absent from the medium (data not shown). Initially, the production of soluble factors by HUVEC in response to C. pneumoniae infection was determined at various MOIs ranging from 0.001 to 1. Conditioned medium from these infected cultures stimulated DNA synthesis in SMC cultures in a dose-dependent fashion. The production of HUVEC-derived soluble factor(s) was dependent on the initial dose of C. pneumoniae used to infect cultures, with the largest stimulation index (SI) (7.4 ± 0.9; P < 0.01) seen at an MOI of 1 and the smallest SI (3.4 ± 0.3; P < 0.05) seen at an MOI of 0.001, as shown in Fig. 2.
FIG. 2.
[3H]thymidine incorporation by SMC treated with conditioned medium from C. pneumoniae-infected HUVEC at various doses for 48 h. HUVEC cultures were infected with C. pneumoniae at various MOIs, and conditioned medium was harvested at 48 h and applied to SMC cultures for 24 h. [3H]thymidine was added for the last 8 h, and incorporation was determined as described in Materials and Methods. SI data are expressed as the means ± standard errors of one representative experiment performed in triplicate. ∗, P < 0.05, and ∗∗, P < 0.01 compared to uninfected controls.
The SMC mitogenic activity of conditioned medium increases with the time of HUVEC infection.
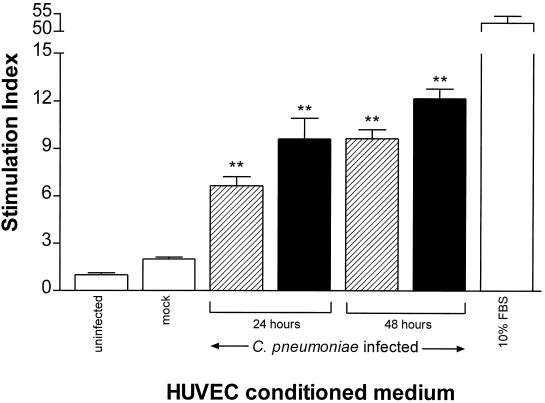
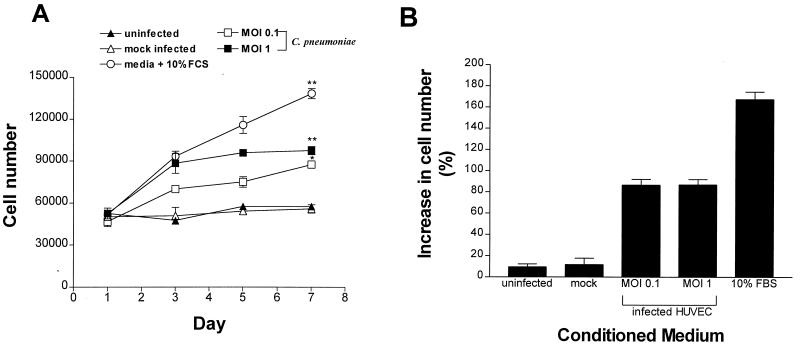
The kinetics of the production of soluble factor(s) from HUVEC infected with C. pneumoniae was investigated by using various MOIs and by comparing the abilities of 24- and 48-h-postinfection conditioned media to stimulate DNA synthesis. As shown in Fig. 3, by 24 h postinfection (MOI, 0.1), the SI was 6.65 ± 0.57 (P < 0.01), and it increased to 9.64 ± 0.56 by 48 h (P < 0.01) compared to uninfected controls. In contrast, mock infection of HUVEC with HEp-2 cell lysates (SI, 1.99 ± 0.13) gave an SI similar to that of uninfected cell controls (SI, 1.0 ± 0.13; P > 0.05) at 24 and 48 h (48-h results are shown). At an MOI of 1, the SI reached 9.62 ± 1.3 (P < 0.01) at 24 h postinfection and increased to 12.2 ± 0.61 (P < 0.01) at 48 h postinfection. Figure 4 shows the results of a separate experiment performed to confirm the effect of soluble factor(s) on SMC proliferation by using direct cell counting. Conditioned medium from infected HUVEC stimulated the proliferation of SMC during a 7-day observation period, whereas medium from mock-infected or uninfected HUVEC cultures did not result in an increase in cell numbers. By day 7, cell numbers had increased by 86.6% (P < 0.05) for medium from HUVEC infected with C. pneumoniae at an MOI of 0.1 and 87.5% (P < 0.01) for HUVEC infected at an MOI of 1; these increases in cell numbers were significantly higher than those of mock-infected HUVEC (11.7%) and uninfected HUVEC (9.5%) (Fig. 4B). During the same observation period, control SMC cultures overlaid with uninfected HUVEC medium but containing 10% FBS showed an increase in cell number of 168% at day 7.
FIG. 3.
[3H]thymidine uptake by SMC in response to conditioned medium from HUVEC cultures infected with C. pneumoniae for 24 and 48 h. HUVEC cultures were either uninfected, mock infected, or infected with C. pneumoniae at an MOI of 0.1 (striped bars) or 1 (solid bars). Conditioned medium from these cultures was harvested at either 24 or 48 h and applied to SMC cultures for 24 h as described in the legend for Fig. 2. Data are the means ± standard errors of triplicate determinations of one representative experiment. ∗∗, P < 0.01, compared to uninfected controls.
FIG. 4.
SMC replication in response to conditioned medium from C. pneumoniae-infected HUVEC cultures. SMC were grown in 24-well plates and overlaid with the indicated conditioned medium from either uninfected or mock-infected HUVEC or HUVEC that were infected with C. pneumoniae for 48 h. On days 1, 3, 5, and 7, cells were harvested and counted as described in Materials and Methods. (A) Increases in SMC numbers as measured by direct cell counting and (B) increases in SMC cell numbers on day 7 expressed as percentages of cell numbers on day 1. The data are means ± standard errors of one experiment performed in triplicate. ∗, P < 0.05, and ∗∗, P < 0.01 compared to uninfected controls.
C. pneumoniae endocytosis followed by signal transduction events appears to be important in the induction of mitogenic activity.
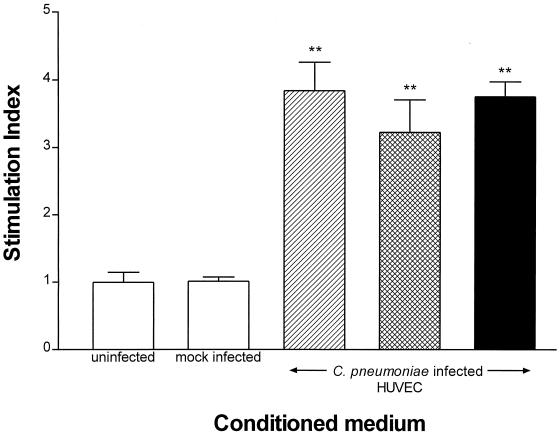
To determine whether viable C. pneumoniae was required for the induction of mitogenic factor(s) from HUVEC, experiments were carried out by using heat-inactivated C. pneumoniae or viable organisms in the presence of chloramphenicol. Figure 5 demonstrates that both heat-inactivated C. pneumoniae and C. pneumoniae grown in the presence of chloramphenicol were able to induce the synthesis of soluble factor(s) that stimulated SMC replication. Although the SIs obtained from HUVEC infected with either viable, heat-inactivated, or chloramphenicol-treated C. pneumoniae were significantly different from those of uninfected or mock-infected control groups (P < 0.01), there was no significant difference between the infected groups (P > 0.05), indicating that heat-treated EBs and EBs grown in the presence of chloramphenicol were similar to viable organisms in their ability to induce HUVEC-derived factors. These results suggest that chlamydial endocytosis or cell surface contact alone is sufficient for the production of soluble factor(s).
FIG. 5.
Effect of heat treatment and chloramphenicol on the production of soluble factor(s) by HUVEC cultures infected with C. pneumoniae. HUVEC cultures were infected with viable (striped bar) or heat-treated (hatched bar) C. pneumoniae or with viable C. pneumoniae in the presence of chloramphenicol (solid bar). Conditioned medium was harvested at 48 h and applied to SMC for 24 h as described in the legend for Fig. 2. Data are the means ± standard errors of triplicate determinations of one representative experiment. ∗∗, P < 0.01 compared to uninfected controls.
DISCUSSION
Our results demonstrate that HUVEC infected with C. pneumoniae produce soluble factor(s) that is mitogenic for SMC. Induction of this SMC growth factor activity by HUVEC was dependent on the time of infection, as media harvested at 48 h postinfection had greater mitogenic activity than that of medium harvested at 24 h. In addition, the HUVEC response to C. pneumoniae was dose dependent, as HUVEC infected with C. pneumoniae at higher MOIs produced conditioned medium with greater mitogenic potential for SMC than that obtained with lower MOIs. This is presumably due to the increased proportion of HUVEC infected at higher MOIs and more cells producing the soluble factor. Interestingly, HUVEC infected with extremely low doses of C. pneumoniae (MOI, 10−3) were also able to generate SMC stimulatory activity, indicating that the HUVEC response to C. pneumoniae is sensitive and may involve the production of soluble cytokines or chemokines by a signal transduction phenomenon and/or an autocrine feedback mechanism, where cytokines released from HUVEC could stimulate the production of mitogenic factors and thereby amplify the response at low C. pneumoniae doses.
Experiments using chloramphenicol and heat-inactivated C. pneumoniae were performed to investigate whether newly synthesized chlamydial cell components were required for the production of SMC growth factor(s). In the presence of chloramphenicol, chlamydiae can bind to host cells and become phagocytosed, but their developmental cycle is blocked. Likewise, heat-inactivated chlamydiae can bind to host cells, albeit at a lower efficiency than native, viable organisms (2), and become endocytosed. From a signal transduction perspective, the vesicular trafficking events following uptake are similar in both cases. Heat-inactivated organisms as well as chloramphenicol-treated organisms do not have the ability to actively prevent phagolysosomal fusion, since this event in C. trachomatis has been shown to be dependent on early de novo chlamydial protein synthesis (36). The ability of heat-inactivated and chloramphenicol-treated organisms to activate HUVEC in a manner similar to that of viable ones suggests that the SMC growth factor(s) is a bona fide HUVEC product, since both types of treated organisms do not have the potential to synthesize bacterial proteins. Furthermore, our results demonstrate that viable C. pneumoniae is not required for HUVEC stimulation, suggesting that cell surface binding and/or phagosomal entry events are important in transducing the signals involved in upregulating cytokine or chemokine gene expression in the HUVEC. Such early events capable of inducing host cell signal transduction pathways via host protein tyrosine phosphorylation have been described for C. trachomatis (4, 5) and are activated also by heat- or UV-treated C. trachomatis. A common signal transduction pathway may be activated in C. pneumoniae-infected HUVEC. Preliminary experiments with C. trachomatis have indicated that this species also stimulates the production of SMC growth factors in a fashion similar to that of C. pneumoniae; however, the existence of C. trachomatis in atherosclerotic lesions has not been demonstrated, making this observation less relevant to atherogenesis.
Existing evidence linking C. pneumoniae to atherosclerosis is expanding (20, 23, 24, 35), and different pathophysiological mechanisms have been suggested (17, 34). Implicit in the progression of atherosclerotic lesions is the crucial role played by SMC. Intimal SMC proliferation has been described as a hallmark of disease progression (33) and is under complex regulation in the artery (32) by way of growth factors and cytokine agonists and antagonists. For example, several growth factors have been detected in atherosclerotic lesions, such as PDGF, fibroblast growth factor, EGF, and IGF-1. It is thought that these factors induce not only proliferation of vascular SMC but also, in some cases, the migration of SMC into the intima. The dual chemotactic and proliferative nature of these growth factors is thought to be largely responsible for the focal accumulation of media-derived SMC in advanced lesions. As a result of these growth factors, SMC growth antagonists are also at work in the artery. For example, IL-1, tumor necrosis factor alpha, and transforming growth factor β are all factors that can inhibit cell proliferation and attenuate the action of growth factors (32) as a regulatory mechanism to control the response of SMC to growth factors. In addition to these traditional SMC growth factors, evidence suggesting that other chemokines may be more promiscuous in their actions than previously thought is emerging. For example, the β-chemokine interferon-inducible protein 10 (IP-10), originally described as a chemokine for activated T lymphocytes, may include SMC chemotactic and mitogenic capabilities in its repertoire (38). Interestingly, monocyte chemotactic protein 1 (MCP-1), which has been found to be upregulated in the atherosclerotic artery and during C. pneumoniae infection of HUVEC (26), has been shown to be mitogenic for rat vascular SMC (29).
Pathological mechanisms resulting in excessive SMC proliferation in the artery have been described, including the ability of mildly oxidized LDL to activate SMC mitogen-activated protein kinase, and transduce S-phase entry signals for vascular SMC (1). Since arterial hyperlipidemia is an established risk factor for atherosclerosis, this phenomenon may play a significant role in SMC growth regulation in vivo. Our results are consistent with a role of C. pneumoniae in atherogenesis where C. pneumoniae-infected vascular endothelial cells release soluble growth factors that stimulate SMC replication and contribute to intimal thickening and fibrous plaque formation. Of interest is the identification of the endothelial factor(s) which stimulates SMC and the bacterial component which triggers the signal transduction pathways, resulting in production of the growth factor(s). There are several candidate factors that could be produced by HUVEC in response to C. pneumoniae infection which are mitogenic for SMC. For example, many classical SMC growth factors are endothelial cell derived, such as PDGF and FGF, and may play a role in SMC proliferation during the progression of atherosclerosis due to the regional proximity of endothelial cells and intimal SMC. Other factors may also play a role in this scenario. Endothelial cells produce both IP-10 and MCP-1, and evidence suggests that these inflammatory mediators may have proliferative actions on SMC. Experiments using neutralizing monoclonal antibodies to specific cytokines or growth factors to identify the factor(s) generated by HUVEC in response to C. pneumoniae infection and to characterize the molecular mechanism responsible for activation of the host cells are under way. Possible candidates responsible for the induction of these factors in HUVEC include chlamydial LPS, components of the bacterial outer membrane complex, and heat shock protein 60 (HSP-60), which are known to be highly immunogenic. HSP-60 has recently been shown to induce cytokine and adhesion molecule expression in HUVEC (7) and has been found to colocalize with human HSP-60 in lesions of atherosclerosis (19). The induction of SMC growth factor(s) by C. pneumoniae-infected endothelial cells represents a novel mechanism by which this bacterium may contribute to the immunopathogenesis of atherosclerosis.
ACKNOWLEDGMENT
This work was supported in part by a studentship to B.K.C. from the Father Sean O’Sullivan Research Centre, St. Joseph’s Hospital.
REFERENCES
- 1.Auge N, Escargueil-Blanc I, Lajoie-Mazenc I, Suc I, Andrieu-Abadie N, Pieraggi M T, Chatelut M, Thiers J C, Jaffrezou J P, Laurent G, Levade T, Negre-Salvayre A, Salvayre R. Potential role for ceramide in mitogen-activated protein kinase activation and proliferation of vascular smooth muscle cells induced by oxidized low density lipoprotein. J Biol Chem. 1998;273:12893–12900. doi: 10.1074/jbc.273.21.12893. [DOI] [PubMed] [Google Scholar]
- 2.Byrne G I, Moulder J W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978;19:598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell L A, O’Brien E R, Cappuccio A L, Kuo C C, Wang S P, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 4.Fawaz F, Mutka S, Engel J. Abstracts of the 93rd General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1993. Chlamydia-host cell interaction, abstr. D-150; p. 121. [Google Scholar]
- 5.Fawaz F S, van Ooij C, Homola E, Mutka S C, Engel J N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galdiero M, Cipolloaro de L’Ero G, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs R G J, Carey N, Davies H. Chlamydia pneumoniae and vascular disease. Br J Surg. 1998;85:1191–1197. doi: 10.1046/j.1365-2168.1998.00861.x. [DOI] [PubMed] [Google Scholar]
- 10.Godzik K L, O’Brien E R, Wang S K, Kuo C C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayston J T, Wang S P, Kuo C C, Campbell L A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989;8:191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Leatham E W, Carrington D, Mendall M A, Kaski J C, Camm J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 13.Gurfinkel E, Bozovich G, Daroca A, Beck E, Mautner B ROXIS Study Group. Randomised trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS pilot study. Lancet. 1997;350:404–407. doi: 10.1016/s0140-6736(97)07201-2. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida A, Sasaguri T, Kosaka C, Nojima H, Ogata J. Induction of the cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) by nitric oxide-generating vasodilator in vascular smooth muscle cells. J Biol Chem. 1997;272:10050–10057. doi: 10.1074/jbc.272.15.10050. [DOI] [PubMed] [Google Scholar]
- 16.Jackson L A, Campbell L A, Kuo C C, Rodriguez D I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 17.Kalayoglu M V, Byrne G I. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun. 1998;66:5067–5072. doi: 10.1128/iai.66.11.5067-5072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaukoranta-Tolvanen S S, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 19.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumour necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C-C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 21.Kuo C-C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 22.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linnanmaki E, Leinonen M, Matilla K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae-specific circulating immune complexes in patients with chronic coronary heat disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 24.Melnick S, Shahar E, Folsom A R, Grayston J T, Sorlie P D, Wang S P. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Atherosclerosis Risk in Communities (ARIC) investigators. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 25.Moazed T C, Kuo C-C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 26.Molestina R E, Dean D, Miller R D, Ramirez J A, Summersgill J T. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect Immun. 1998;66:1370–1376. doi: 10.1128/iai.66.4.1370-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhlestein J B, Anderson J L, Hammond E H, Zhao L, Trehan S, Schwobe E P, Carlquist J F. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 28.Navab M, Fogelman A M, Berliner J A, Territo M C, Demer L L, Frank J S, Watson A D, Edwards P A, Lusis A J. Pathogenesis of atherosclerosis. Am J Cardiol. 1995;76:18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- 29.Porreca E, DiFebbo C, Reale M, Castellani M L, Baccante G, Barbacane R, Conti P, Cuccurullo F, Poggi A. Monocyte chemotactic protein 1 is a mitogen for cultured rat vascular smooth muscle cells. J Vasc Res. 1997;34:58–65. doi: 10.1159/000159202. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez J, Ahkee S, Summersgill J, Ganzer B, Ogden L, Quinn T, Gaydos C, Bobo L, Hammerschlag M, Roblow P, Lebar W, Grayston J, Campbell L, Patton D, Dean D, Schachter J. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Roblin P M, Dunornay W, Hammerschlag M R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990’s. Nature. 1993;362:801–808. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Glomset J A. Atherosclerosis and the arterial smooth muscle cell. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 34.Saikku P. Chlamydia pneumoniae infection as a risk factor in acute myocardial infarction. Eur Heart J. 1993;14(Suppl. K):62–65. [PubMed] [Google Scholar]
- 35.Saikku P, Mattila K, Nieminen M S, Huttunen J K, Leinonen M, Ekman M R, Makela P H, Valtonen V. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–985. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 36.Scidmore M A, Rockey D D, Fischer E R, Heinzen R A, Hackstadt T. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect Immun. 1996;64:5366–5372. doi: 10.1128/iai.64.12.5366-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stary H C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(Suppl. E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Yue T L, Ohlstein E H, Sung C P, Feuerstein G Z. Interferon inducible protein-10 involves vascular smooth muscle cell migration, proliferation and inflammatory response. J Biol Chem. 1996;271:24286–24293. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z-D, Cox G. MTT assay overestimates human airway smooth muscle cell number in culture. Biochem Mol Biol Int. 1996;38:431–436. [PubMed] [Google Scholar]