Abstract
In vertebrates and invertebrates, selenium (Se) is an essential micronutrient, and Se deficiency or excess is associated with gonadal insufficiency and gamete dysfunction in both males and females, leading to implantation failure, altered embryonic development and, ultimately, infertility. During pregnancy, Se excess or deficiency is associated with miscarriage, pre-eclampsia (hypertension of pregnancy), gestational diabetes, fetal growth restriction and preterm birth. None of this is surprising, as Se is present in high concentrations in the ovary and testes, and work in animal models has shown that addition of Se to culture media improves embryo development and survival in vitro in association with reduced reactive oxygen species and less DNA damage. Selenium also affects uterine function and conceptus growth and gene expression, again in association with its antioxidant properties. Similarly, Se improves testicular function including sperm count, morphology and motility, and fertility. In animal models, supplementation of Se in the maternal diet during early pregnancy improves fetal substrate supply and alters fetal somatic and organ growth. Supplementation of Se throughout pregnancy in cows and sheep that are receiving an inadequate or excess dietary intake affected maternal whole-body and organ growth and vascular development, and also affected expression of angiogenic factors in maternal and fetal organs. Supplemental Se throughout pregnancy also affected placental growth, which may partly explain its effects on fetal growth and development, and also affected mammary gland development, colostrum yield and composition as well as postnatal development of the offspring. In conclusion, Se supplementation in nutritionally compromised pregnancies can potentially improve fertility and pregnancy outcomes, and thereby improve postnatal growth and development. Future research efforts should examine in more detail and more species the potential benefits of Se supplementation to reproductive processes in mammals.
Keywords: selenium, supplementation, pregnancy, ovary, testis, fetus, offspring, developmental programming
Introduction
In both vertebrates and invertebrates, selenium (Se) deficiency or excess is associated with infertility (that is, the inability to conceive and establish a pregnancy), as reflected by small, poorly developed and poorly functioning gonads, primarily ovarian follicles in females and testes and spermatozoa in males. At least a portion of this problem is associated with implantation failure due to poor embryonic development and altered endometrial (uterine) function. Deficiency or excess of Se also is associated with reduced libido. Lastly, in terms of pregnancy, Se excess or deficiency is associated with spontaneous abortion (miscarriage), pre-eclampsia (hypertension of pregnancy), gestational diabetes, fetal growth restriction, and preterm birth (1–4).
Consistent with its known functions in cellular metabolism, in reproductive tissues Se appears to function primarily as a component of selenoproteins/selenoenzymes in a variety of antioxidant systems, including glutathione peroxidases (GPX), iodothyronine deiodinases (DIO), and thioredoxin reductases (TXNR). These major familes of antioxidant enzymes contribute to reductions in tissue reactive oxygen species and therefore minimize DNA damage (5).
Selenium in the female reproductive tract
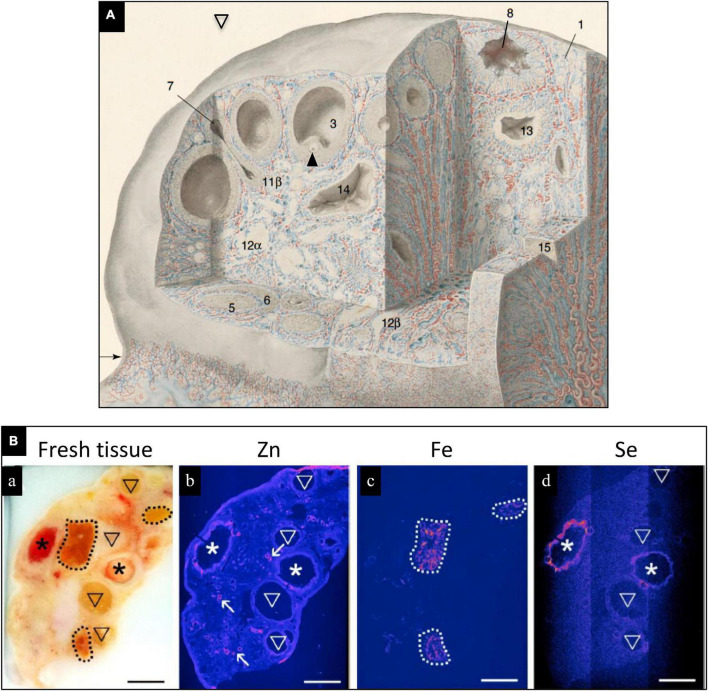
The bovine ovary contains high levels of Se (Figure 1), which are localized to healthy preovulatory follicles, but not in atretic follicles (6). Localization of Se in healthy pre-antral follicles indicates that Se is in close contact with the pre-ovulatory oocyte, which may play a preparatory role for subsequent fertilization, embryo development, and postnatal life. In humans, cases of low plasma, follicular fluid, amniotic fluid or tissue Se concentrations and(or) low tissue GPX concentrations or activity are associated with unexplained infertility, miscarriage, preterm birth, gestational diabetes mellitus, and small for gestational age (SGA) fetuses/newborns (1, 2, 7–9). Elevated serum levels of Se-binding protein 1, an autoantibody produced by the ovary, has been reported in women with unexplained intertility and premature ovarian failure (1, 10). In women with gestational diabetes mellitus, serum Se levels were low, and Se supplementation improved glycemic status and lipid profiles (11, 12).
FIGURE 1.
(A) Drawing of human ovary with sections removed to reveal histological details of an antral (preovulatory) follicle (3) containing an oocyte (arrowhead) and a postovulatory follicle that has released its oocyte and partially collapsed (8). Blood vessels are colored red (arteries) and blue (veins); modified, with permission, from Clark, 1900. (B) Trace elements localized in bovine ovaries by synchrotron x-ray fluorescence (S-XRF). (a) Represents fresh tissue. Zinc (b, pink) localized primarily to blood vessels, Fe (c, pink) localized primarily to corpora lutea, and Se (d, pink) localized to healthy, preovulatory follicles (*) but not to atretic (regressing) antral follicles. Modified, with permission, from Ceko et al. (6).
After ovulation the oocyte moves to the oviduct, where fertilization and early embryo development take place. Oviductal fluid is secreted by the oviduct and acts as an embryotropic culture media for the oocyte and early embryo for their time in residence (13, 14). Addition of Se to in vitro fertilization cultures in animal models (cattle, dogs, pigs, yak, etc.) has resulted in positive impacts on embryo development and survival, reduced reactive oxygen species, and reduced DNA damage (15–19). Interestingly, Se-dependent mechanisms are in place to control embryo metabolic reprogramming in pro-inflammatory environments (20).
Upon deposition of semen into the reproductive tract a post-mating inflammatory response is elicited (21), and an LPS challenge of cultured bovine endometrial cells demonstrated a protective role of Se (22). In vivo effects of Se were demonstrated in cattle, where females receiving an organically bound source of Se had greater conceptus length compared with females receiving an inorganic source of Se (23). In addition, cattle receiving organic Se had differential expression of genes related to maternal recognition of pregnancy, including interferon-stimulated genes and progesterone-stimulated genes (23).
Selenium in the male reproductive tract
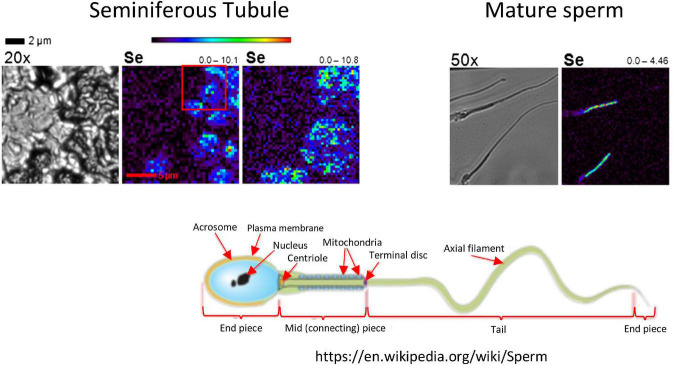
The testis contains high concentrations of Se (Figure 2), where Se has effects both in the seminiferous tubule where sperm are being produced, and in the interstitial space where testosterone production occurs and the blood supply resides (4). As sperm mature Se is localized in the mid-piece, which is also the location of sperm mitochondria (24). The action of Se is primarily as GPX4, which protects sperm from oxidative damage to their cell membranes and DNA. However, there also appears to be a specialized testes- specific isoform of TXNR (5, 25), which supports the importance of Se-containing antioxidant enzymes to testicular function and health.
FIGURE 2.
Localization of Se in mouse testis by x-ray fluorescence microscopy (XFM). In the left-hand images, a seminiferous tubule is visualized in cross section at 20×. Se (bright blue to green) localizes to elongating spermatids. In the right-hand images, Se in mature sperm localizes to the mid-piece, which is the location of sperm mitochondria (see the schematic of a mature sperm, lower right). The range below the seminiferous tubule image indicates the low and high concentrations of Se in ng/cm2. Modified, with permission, from Kehr et al. (24).
In addition, greater dietary intake of Se has been associated greater sperm concentrations in semen of men infertile men (26) and some Se supplementation studies in infertile men show improvements in testicular antioxidant activity, semen Se concentrations, sperm count, sperm morphology and motility, and fertility (1–3). Selenoproteins are abundant in the testis and epididymis, include GPX4 (testis, intracellular membranes), sperm nucleus GPX4 (snGPX4), mitochondrial GPX4 (mGPX4; sperm midpiece – see Figure 2), cytosolic GPX4 (cGPX4; testis and epididymal epithelium), secreted GPX5 (epididymal lumen), cytosolic GPX3 and GPX1 (epididymal epithelium) (3). In addition, gene knockouts of selenoproteins in male mice, including mGPX4, SELENOP, snGPX4, GPX5 and global GPX4 (mGPX4, snGPX4, and cGPX4), lead to sperm abnormalities, defects in chromatin condensation in sperm, early embryonic death, and(or) increased number of miscarriages, developmental defects and neonatal mortality (3).
In terms of our understanding of the underlying mechanisms, Se excess or deficiency affects the concentrations or activities of various selenoproteins, resulting in:
-
•
Oxidative stress/DNA damage from reactive oxygen species;
-
•
Lack of structural integrity of sperm, affecting sperm motility and fertilization capacity;
-
•
Defects in transport of Se into tissues, particularly testis and brain;
-
•
Alterations in other Se effects/functions – e.g., altered gonadal morphology/size, endocrine function (e.g., thyroid), immune function, cardiovascular function, synergism with Vit E, etc. (27).
Selenium supplementation during pregnancy
There are geographic locations and times of the year when forages grazed by livestock have insufficient Se to meet requirements. In addition, producer decisions about whether to provide supplemental mineral to grazing livestock vary widely. Therefore, our research group implemented a bovine model comparing unsupplemented beef heifers to those receiving a Se-containing mineral supplement (VTM) to understand the impacts of early gestation supplementation on maternal and fetal outcomes (28–33). An important aspect of these studies is that the control, unsupplemented heifers were receiving a basal diet that was either inadequate or in excess of requirements, both of which typically result in reduced birth weights of the offspring. Thus, they were also receiving inadequate or excess micronutrient intakes.
Evaluation of maternal and fetal samples collected on d 83 of gestation revealed heavier livers in fetuses exposed to VTM during gestation (29), and that concentrations of Se in maternal liver, fetal liver, muscle, and allantoic fluid were all greater in heifers receiving the VTM supplement. In addition, concentrations of Se in maternal liver were correlated with concentrations in fetal liver (r = 0.60), fetal muscle (r = 0.40), and allantoic fluid [r = 0.34; (33)]. Though no differential expression of selenoprotein transcripts was observed in the fetal or maternal portions of the placenta, VTM supplementation influenced genes related to amino acid activation, fat cell differentiation and metabolic processes (32). Amino acids are critical fuels for fetal growth and development (34) and our evaluation revealed that total amino acids and concentrations of 12 of 14 neutral amino acids evaluated in allantoic fluid were greater in heifers receiving VTM (28). Taken together, our results demonstrate that providing a Se-containing supplement during early gestation resulted in major alterations in substrate supply and(or) utilization in the fetus, indicating that research evaluating post-natal effects on health, growth, and metabolism is necessary.
In a series of studies we targeted feeding “supranutritional” (meaning above adequate but below toxic) levels of Se to pregnant ewes, fed as Se-enriched yeast or Se-enriched wheat (35–48). Again, the control, unsupplemented animals were receiving a basal diet that was either inadequate or in excess of requirements, both of which typically result in reduced birth weights of the offspring.
When fed during early pregnancy (from 21 days before until 64 days after breeding – i.e., 0.44 of pregnancy), supranutritional Se increased maternal lung mass, liver mass, and total visceral organ mass, as well as cellularity, cell proliferation and vascularity of maternal small intestine. All of these effects on the maternal system would increase metabolic capacity to support the metabolic demands of pregnancy.
Supranutritional levels of Se in the maternal diet during early pregnancy also increased fetal body mass, heart mass, lung mass, spleen mass, total visceral organ mass and large intestinal mass, as well as cell density of fetal skeletal muscle. These effects of Se supplementation would potentially improve survival and growth of the fetus and offspring. In addition, the effect on fetal skeletal muscle also has important implications for postnatal growth and carcass quality, considering that the number of myocytes in skeletal muscle is “fixed” at birth (49).
When fed throughout pregnancy, supranutritional levels of Se in the maternal diet also affected maternal whole-body and organ growth and vascular development, and these effects depended on the plane of nutrition (adequate or restricted intake). For example, Se supplementation increased maternal mammary gland vascularity at 24 h postpartum, Selenium supplementation also increased fetal body weight as well as fetal heart, lung, spleen, total visceral and large intestine weights and fetal muscle DNA concentations at 0.9 of gestation. Along with the effects on vascular development, supplemental Se throughout pregnancy also increased maternal and fetal organ expression of mRNA for vascular growth (angiogenic) factors, including NOS3 and VEGF.
Supranutritional Se fed to ewes throughout gestation also increased cell density and cell proliferation in the placenta in late pregnancy as well as lamb birth weights. As the placenta is the only source of exchange of nutrients, respiratory gases and metabolic wastes between the fetal and maternal systems (50–52), the effects of Se on placental development may explain, at least in part, the effects of supranutritional Se on fetal growth and development.
Alternatively, epigenetic mechanisms within developing offspring may also explain developmental programming responses resulting from dietary Se supplementation (53). Specifically, enzymes associated with one-carbon metabolism have been shown to be affected by Se (54, 55), while others (56) reported that Se regulates microRNAs (56) and DNA methylation (55, 57). In humans experiencing Kashin-Beck disease (associated with Se deficiencies), differentially methylated genes were reported (53). Research exploring the potential role of Se induced epigenetic changes in offspring within a developmental programming paradigm are needed to further understand the mechanisms and roles of supplemental dietary Se in developmental programming events in livestock.
Lastly, supranutritional Se fed to ewes throughout gestation also increased colostrum yield, altered colostrum composition, and increased mammary gland vascular development, and resulted in increased average daily weight gain, efficiency of growth, visceral adiposity and small intestinal mass and vascular development of the lambs postnatally. These observations further suggest a role for supranutritional supplementation of Se to the dams on developmental programming of the offspring and support the need for additional research in this area.
Conclusion
As we have discussed, Se plays an important role in reproductive processes. Recent research with Se supplementation of sheep during nutritionally compromised pregnancies has suggested that “supranutritional” levels in the diet can positively impact pregnancy outcomes. However, these studies need to be replicated in other mammals as well. In addition, the effects of Se supplementation on other reproductive processes such as follicular development, oocyte and sperm development and maturation, fertilization and implantation, early embryonic development, and, especially, developmental programming of offspring, warrant further research as well (4, 58).
Importantly, when supranutritional maternal Se was fed as sodium selenate at 20 or 100×, or as Se-enriched wheat at 20×, of so-called “adequate” levels from day 50 to 134 (0.34–0.92) of pregnancy in ewes, no signs of selenosis were observed. These studies using sheep models of pregnancy therefore indicate that in addition to the role of dietary Se in other reproductive processes, supranutritional levels of Se fed to ewes during the periconceptual period or throughout pregnancy are not only non-toxic but can improve maternal and fetal pregnancy outcomes and postnatal growth and development. Taken together, these observations suggest to us that further research on adding Se to the diet during pregnancy is warranted in other mammals as well.
Author contributions
All authors contributed equally to the preparation of this manuscript.
Acknowledgments
We would like to acknowledge the many collaborators and colleagues, graduate and undergraduate students, many of whom are cited in the Literature Cited, as well as funding sources that have supported their research efforts in this area, and without whom these efforts would not have succeeded. We also would like to thank the animal and laboratory staff who have supported their research efforts.
Funding
This study was supported by the North Dakota Agricultural Experiment Station, the North Dakota State Board of Agricultural Research and Education, the Agriculture and Food Research Initiative of the National Institute of Food and Agriculture (U.S. Department of Agriculture), and Purina Animal Nutrition LLC (Land O’Lakes, Inc., Arden Hills, MN, United States).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Mistry HD, Broughton Pipkin F, Redman CWG, Poston L. Selenium in reproductive health. Am J Ob Gyn. (2012) 206:21–30. 10.1016/j.ajog.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 2.Mirone M, Giannetta E, Isidori AM. Seleniumand reproductive function. A systematic review. J Endocrinol Invest. (2013) 36:28–36. [PubMed] [Google Scholar]
- 3.Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz MH, et al. Roel of selenium in male reproduction – A review. Anim Reprod Sci. (2014) 146:55–62. 10.1016/j.anireprosci.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Qazi IH, Angel C, Yang H, Zoidis Z, Pan B, Wu Z, et al. Role of selenium and selenoproteins in male reproductive function: A review of past and resent evidences. Antioxidants. (2019) 8:268. 10.3390/antiox8080268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reich HJ, Hondal RJ. Why nature chose selenium. ACS Chem Biol. (2016) 2016:821–41. 10.1021/acschembio.6b00031 [DOI] [PubMed] [Google Scholar]
- 6.Ceko MJ, O’Leary S, Harris HH, Hummitzsch K, Rodgers RJ. Trace elements in ovaries: measurement and physiology. Biol Reprod. (2016) 94:1–14. [DOI] [PubMed] [Google Scholar]
- 7.Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V. Maternal selenium status during early gestation and risk for preterm birth. Canad Med Assoc J. (2011) 183:549–55. 10.1503/cmaj.101095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistry HD, Kurlak LO, Young SD, Briley AL, Broughton Pipkin F, Baker PN, et al. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Maternal Child Nutr. (2014) 10:327–34. 10.1111/j.1740-8709.2012.00430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelkic KO, Sobocan M, Tac I. Low selenium levels in amniotic fluid correlate with small-for-gestational age newborns. Nutrients. (2020) 12:3046. 10.3390/nu12103046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edassery SL, Shatavl SV, Kunkel JP, Hauer C, Brucker C, Penumatsa K, et al. Autoantigens in ovarian autoimmunity associated with unexplained Infertility and premature ovarian failure. Fertil Steril. (2010) 94:2636–41. 10.1016/j.fertnstert.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amirani E, Asemi Z, Taghizadeh M. The effects of selenium plus probiotics supplementation on glycemic status and serum lipoproteins in patients with gestational diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clin Nutr ESPN. (2022) 48:56–62. 10.1016/j.clnesp.2022.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Tang Y, Ji Y, Yi H, Li Y, Piao C, et al. The association between serum selenium level and gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab Res Rev. (2022) 38:e3522. 10.1002/dmrr.3522 [DOI] [PubMed] [Google Scholar]
- 13.Coy P, Romar R, Romero-Aguirregomexcorta J. The embryo culture media in the era of epigenetics: is it time to go back to nature? Anim Reprod. (2022) 19:e20210132. 10.1590/1984-3143-AR2021-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lui S, Winuthayanon W. Oviduct: roles in fertilization and early embryo development. J. Endcinol. (2017) 232:R1–26. 10.1530/JOE-16-0302 [DOI] [PubMed] [Google Scholar]
- 15.Wydooghe E, Heras S, Dewulf J, Piepers S, Van den Abbeel E, De Sutter P, et al. Replacing serum in culture medium with albumin and insulin, transferrin and selenium is the key to successful bovine embryo development in individual culture. Reprod Fertil Dev. (2013) 26:717–24. 10.1071/RD13043 [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Halim BR, Helmy NA. Effect of nano-selenium and nano-zinc particles during in vitro maturation on the developmental competence of bovine oocytes. Anim Prod Sci. (2017) 58:2021–8. 10.1071/AN17057 [DOI] [Google Scholar]
- 17.Xiong X, Lan D, Li J, Lin Y, Li M. Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes. Anim Sci J. (2017) 89:298–306. 10.1111/asj.12894 [DOI] [PubMed] [Google Scholar]
- 18.Jeong YW, Hossein MS, Bhandari DP, Kim YW, Kim JH, Park SW, et al. Effects of insulin–transferrin–selenium in defined and porcine follicular fluid supplemented IVM media on porcine IVF and SCNT embryo production. Anim Reprod Sci. (2008) 106:13–24. 10.1016/j.anireprosci.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez MD, Cittadini CO, Teplitz GM, De Stefano A, Lombardo DM, Salamone DF. Canine IVM With SOF Medium, Insulin-Transferrin-Selenium, and Low O2 Tension Improves Oocyte Meiotic Competence and Decreases Reactive Oxygen Species Levels. Front Cell Dev Biol. (2021) 9:694889. 10.3389/fcell.2021.694889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korwar AM, Hossain A, Lee TJ, Shay AE, Basrur V, Conlon K, et al. Selenium-dependent metabolic reprogramming during inflammation and resolution. J Biol Chem. (2021) 296:100410. 10.1016/j.jbc.2021.100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katila T. Post-mating inflammatory response of the uterus. Reprod Dom Anim. (2012) 47:31–41. 10.1111/j.1439-0531.2012.02120.x [DOI] [PubMed] [Google Scholar]
- 22.Adeniran SO, Zheng P, Feng R, Adegoke EO, Huang F, Ma M, et al. The antioxidant role of selenium via GPx1 and GPx4 in LPS-induced oxidative stress in bovine endometrial cells. Biol Trace Elem Res. (2022) 200:1140–55. 10.1007/s12011-021-02731-0 [DOI] [PubMed] [Google Scholar]
- 23.Crites BR. The effects of form of selenium on the bovine corpus luteum, uterine endometrium, and development of the conceptus., Ph.D. Dissertation. Lexington, KY: University of Kentucky; (2021). [Google Scholar]
- 24.Kehr S, Malinouski M, Finney L, Vogt S, Labunsky VM, Kasaikinam MV, et al. X-Ray fluorescence microscopy reveals the role of selenium in spermatogenesis. J Mol Biol. (2009) 389:808–18. 10.1016/j.jmb.2009.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnerì ES. Focus on mammalian thioredoxin reductases–important selenoproteins with versatile functions. Biochim Biophys Acta. (2009) 1790:495–526. 10.1016/j.bbagen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 26.Nouri M, Mehrabani S, Firoozbakht H, Vataniyan E, Abbasi H, Shirani M. The association between dietary fat and mineral intake with semen parameters: A cross-sectional study in infertile men. Int J Reprod Biomed. (2022) 20:389–99. 10.18502/ijrm.v20i5.11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Rose AH, Hoffman PR. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. (2012) 16:705–43. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menezes ACB, McCarthy KL, Kassetas CJ, Baumgaertner F, Kirsch JD, Dorsam ST, et al. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation affect concentrations of amino acids in maternal serum and allantoic fluid of beef heifers. J Anim Sci. (2021) 99:1–10. 10.1093/jas/skab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menezes ACB, McCarthy KL, Kassetas CJ, Baumgaertner F, Kirsch JD, Dorsam ST, et al. Vitamin and mineral supplementation and different rates of gain during the first trimester of gestation in beef heifers. Effects of dam hormonal and metabolic status, concentrations of glucose and fructose in fetal fluids, and fetal tissue organ mass. Animals. (2022) 12:1–18. 10.3390/ani12141757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amat S, Holman DB, Schmidt K, McCarthy KL, Dorsam ST, Ward AK, et al. Characterization of the microbiota associated with 12-week-old bovine fetuses exposed to divergent in utero nutrition. Front Microbiol. (2022) 12:771832. 10.3389/fmicb.2021.771832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crouse MS, McCarthy KL, Menezes ACB, Kassetas CJ, Baumgaertner F, Kirsch JD, et al. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation in beef heifers alters the fetal liver amino acid, carbohydrate, and energy profile at day 83 of gestation. Metabolites. (2022) 12:696. 10.3390/metabo12080696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diniz WJS, Reynolds LP, Ward AK, Borowicz PP, Sedivec KK, McCarthy KL, et al. Untangling the placentome gene network of beef heifers in early gestation. Genomics. (2022) 114:110274. 10.1016/j.ygeno.2022.110274 [DOI] [PubMed] [Google Scholar]
- 33.McCarthy KL, Menezes ACB, Kassetas CJ, Baumgaertner F, Kirsch JD, Dorsam ST, et al. Vitamin and mineral supplementation and rate of gain in beef heifers: effects on concentration of trace minerals in maternal liver and fetal liver, muscle, allantoic, and amniotic fluids at day 83 of gestation. Animals. (2022) 12:1925. 10.3390/ani12151925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowden A. Growth and metabolism. In: Harding R, Bocking A. editors. Fetal growth and development. Cambridge, MA: Cambridge University Press; (2001). p. 44–69. [Google Scholar]
- 35.Borowicz P, Ward N, Caton J, Soto-Navarro SA, Redmer D, Taylor J, et al. Effects of supernutritional levels of Selenium (Se) on vascular density and cell proliferation in the sheep placenta. J Anim Sci. (2005) 83:104. [Google Scholar]
- 36.Reed JJ, Ward MA, Vonnahme KA, Neville TL, Julius SL, Borowicz PP, et al. Effects of selenium supply and dietary restriction on maternal and fetal body weight, visceral organ mass and cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J Anim Sci. (2007) 85:2721–33. 10.2527/jas.2006-785 [DOI] [PubMed] [Google Scholar]
- 37.Neville TL, Ward MA, Reed JJ, Soto-Navarro SA, Julius SL, Borowicz PP, et al. Effects of level and source of dietary selenium on maternal and fetal body weight, visceral organ mass, cellularity estimates, and jejunal vascularity in pregnant ewe lambs. Anim Reprod Sci. (2008) 141:131–41. 10.1016/j.anireprosci.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 38.Swanson TJ, Hammer CJ, Luther JS, Carlson DB, Taylor JB, Redmer DA, et al. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J Anim Sci. (2008) 86:2415–23. 10.2527/jas.2008-0996 [DOI] [PubMed] [Google Scholar]
- 39.Ward MA, Neville TL, Reed JJ, Taylor JB, Hallford DM, Soto-Navarro SA, et al. Effects of selenium supply and dietary restriction on maternal and fetal metabolic hormones in pregnant ewe lambs. J Animal Sci. (2008) 86:1254–62. 10.2527/jas.2007-0509 [DOI] [PubMed] [Google Scholar]
- 40.Hammer CJ, Thorson JF, Meyer AM, Redmer DA, Luther JS, Neville TL, et al. Effects of maternal selenium supply and plane of nutrition during gestation on passive transfer of immunity and health in neonatal lambs. J Anim Sci. (2011) 89:3690–8. 10.2527/jas.2010-3724 [DOI] [PubMed] [Google Scholar]
- 41.Meyer AM, Reed JJ, Neville TL, Thorson JF, Maddock-Carlin KR, Taylor JB, et al. Nutritional plane and selenium supply during gestation affect yield and nutrient composition of colostrum and milk in primiparous ewes. J Anim Sci. (2011) 89:1627–39. 10.2527/jas.2010-3394 [DOI] [PubMed] [Google Scholar]
- 42.Meyer AM, Reed JJ, Neville TL, Taylor JB, Reynolds LP, Redmer DA, et al. Effects of nutritional plane and selenium supply during gestation on visceral organ mass and indices of intestinal growth and vascularity in primiparous ewes at parturition and during early lactation. J Anim Sci. (2012) 90:2733–49. 10.2527/jas.2011-4524 [DOI] [PubMed] [Google Scholar]
- 43.Meyer AM, Neville TL, Reed JJ, Taylor JB, Reynolds LP, Redmer DA, et al. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J Anim Sci. (2013) 91:2628–39. 10.2527/jas.2012-5953 [DOI] [PubMed] [Google Scholar]
- 44.Vonnahme KA, Wienhold CM, Borowicz PP, Neville TL, Redmer DA, Reynolds LP, et al. Supranutritional selenium increases mammary gland vascularity in postpartum ewe lambs. J Dairy Sci. (2011) 94:2850–8. 10.3168/jds.2010-3832 [DOI] [PubMed] [Google Scholar]
- 45.Camacho LE, Meyer AM, Neville TL, Hammer CJ, Redmer DA, Reynolds LP, et al. Neonatal hormone changes and growth in lambs born to dams receiving differing nutritional intakes and selenium supplementation during gestation. Reproduction. (2012) 144:23–35. 10.1530/REP-11-0302 [DOI] [PubMed] [Google Scholar]
- 46.Neville TL, Meyer AM, Reyaz A, Borowicz PB, Redmer DA, Reynolds LP, et al. Mammary gland growth and vascularity at parturition and during lactation in primiparous ewes fed differing levels of selenium and nutritional plane during gestation. J Anim Sci Biotechnol. (2013) 4:6. 10.1186/2049-1891-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemley CO, Meyer AM, Neville TL, Hallford DM, Camacho LE, Maddock-Carlin KR, et al. Dietary selenium and nutritional plane alter specific aspects of maternal endocrine status during pregnancy and lactation. Domest Anim Endocrinol. (2014) 46:1–11. 10.1016/j.domaniend.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 48.Caton JS, Neville TL, Reynolds LP, Hammer CJ, Vonnahme KA, Meyer AM, et al. Biofortification of maternal diets with selenium: Postnatal growth outcomes. In: Banuelos GS, Lin Z-Q, Yin X. editors. Selenium in the Environment and Human Health. London: CRC Press; (2014). p. 159–61. [Google Scholar]
- 49.Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, et al. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. (2010) 88:E51–60. 10.2527/jas.2009-2311 [DOI] [PubMed] [Google Scholar]
- 50.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, et al. Utero-placental vascular development and placental function: An update. Invited review. Internat J Develop Biol. (2010) 54:355–66. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds LP, Borowicz PP, Caton JS, Crouse MS, Dahlen CR, Ward AK. Developmental programming of fetal growth and development. invited review. Vet Clin North Am Food Anim Pract. (2019) 35:229–47. 10.1016/j.cvfa.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 52.Reynolds LP, McLean KJ, McCarthy KL, Diniz WJS, Menezes ACB, Forcherio JC, et al. Nutritional regulation of embryonic survival, growth and development. Invited Review. G. Wu (ed.), Recent Advances in Animal Nutrition and Metabolism. Adv Exp Med Biol. (2022) 1354:63–76. 10.1007/978-3-030-85686-1_4 [DOI] [PubMed] [Google Scholar]
- 53.Lei XG, Combs GF, Jr., Sunder RA, Caton JS, Arthington JD, Vatamaniuk MZ. Dietary selenium across species. Annu Rev Nutr. (2022) 42:1–18. 10.1146/annurev-nutr-062320-121834 [DOI] [PubMed] [Google Scholar]
- 54.Speckmann B, Grune T. Epigenetic effects of selenium and their implications for health. Epigenetics. (2015) 10:179–90. 10.1080/15592294.2015.1013792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speckmann B, Schulz S, Hiller F, Hesse D, Schumacher F, Kleuser B, et al. Selenium increases DNA methylation and modulates one-carbon metabolism in the liver of mice. J Nutr Biochem. (2017) 48:112–9. 10.1016/j.jnutbio.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 56.Huang X, Dong Y-L, Li T, Xiong W, Zhang X, Wang PJ, et al. Dietary selenium regulates microRNAs in metabolic disease: Recent progress. Nutrients. (2021) 13:1527. 10.3390/nu13051527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wischhusen P, Saito T, Heraud C, Kaushik SJ, Fauconneau B, Prabhu PAJ, et al. Parental selenium nutrition affects the one-carbon metabolism and the hepatic DNA methylation pattern of rainbow trout (Oncorhynchus mykiss) in the progeny. Life. (2020) 10:121. 10.3390/life10080121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qazi IH, Angel C, Yang H, Zoidis Z, Pan B, Zoldis E, et al. Selenium, selenoproteins, and female reproduction: A review. Molecules. (2018) 23:3053. [DOI] [PMC free article] [PubMed] [Google Scholar]