Abstract
Objective
Mifepristone has been used to treat endometriosis, but it can cause a constellation of endometrial alterations. Our study investigated the effects of long-term mifepristone on ovarian endometriosis.
Methods
We retrospectively analyzed the clinicopathological changes of ovarian endometriosis in 11 Chinese patients after long-term low-dose mifepristone therapy and compared these alterations with those observed in eutopic endometrium and adenomyosis side-by-side. Immunohistochemistry was applied to investigate estrogen receptor (ER), progesterone receptor (PR), and Ki67 expression in eutopic and ectopic endometrium.
Results
Nearly all patients had a pelvic mass and elevated serum CA125 levels. The ovarian lesions were grossly solid, cystic-solid, or cystic. They had a grayish–reddish appearance and a fleshy, honeycomb-like cut surface. The ovarian lesions shared morphological features with the uterine endometrium, and they were characterized by dilated, crowding endometrial glands with non-physiological changes. Immunostaining revealed consistent staining for ER and PR and a low Ki67 index in both eutopic and ectopic endometrium.
Conclusions
Our findings suggest that ovarian endometriosis can mimic an endometrioid borderline tumor after long-term mifepristone administration. Careful histological assessment and related clinical information are critical for the correct interpretation of these rare entities.
Keywords: Endometrium, endometriosis, histopathology, mifepristone, progesterone receptor modulator, adenomyosis, endometrioid borderline tumor
Introduction
Mifepristone (RU486) is a potent progesterone receptor modulator (PRM) that exerts antiprogesterone effects by competing with progesterone for endometrial progesterone receptor (PR) binding.1 It has been also reported to exhibit other biological functions (albeit to minor degrees), including estrogenic activity regulation, androgen receptor upregulation, glucocorticoid receptor antagonism, and antiproliferative effects.2,3 Thus, mifepristone and other PRMs have been applied as part of various regimens to treat several gynecological conditions. A single dose is used to induce medical abortion in early pregnancy, and it is also applied as a postcoital agent and to evacuate the uterus after intrauterine fetal death.4 At daily doses of 2 to 10 mg, mifepristone is a highly effective contraceptive as an inhibitor of ovulation and menstruation.5 In addition, previous studies reported that long-term therapy with low doses of mifepristone was effective for the management of uterine leiomyoma, adenomyosis, and endometriosis with significant shrinkages of leiomyomas, high remission of recurrent endometriosis, and reductions in symptoms (such as uterine bleeding and pain) respectively.1,6,7 Such non-surgical treatment is greatly beneficial for young women, considering the maintenance of fertility is a critical issue for this population.8
In view of the growing potential of chronic mifepristone use, it is critical to investigate its long-term safety profile, particularly its effects on the endometrium.9 A broad spectrum of endometrial histology including inactive and normal-appearing cycling endometrium or cystic changes with dense stroma (PRM-associated endometrial changes [PAECs]), but no overtly malignant lesions (atypical hyperplasia and endometrial intraepithelial neoplasia) have been reported in women who received chronic mifepristone or PRM therapy (up to 12 months).10 These studies mainly focused on tracking changes in eutopic endometrium after the cessation of different treatment regimens with various doses and intervals. However, ectopic endometrium in endometriosis is biologically different from eutopic endometrium because it can grow, attach to structures, and invade pelvic structures owing to the underlying alterations at the molecular level.11 To date, it remains unclear whether chronic mifepristone administration has any effects on ectopic endometrium such as that in ovarian endometriosis and whether ectopic endometrium following mifepristone exposure exhibits distinct biological differences from eutopic endometrium.
To address these questions, the current study retrospectively analyzed the histopathological changes in the ovarian endometriosis of Chinese patients after long-term exposure to mifepristone. In parallel, we examined the pathological changes in the uterine endometrium and adenomyosis. We aimed to identify the distinct clinicopathological features in ovarian endometriosis and their potential effects on clinical diagnosis and intervention.
Materials and methods
Chinese women with ovarian endometriosis after long-term mifepristone use were consecutively enrolled from the institutional database of Women’s Hospital, School of Medicine, Zhejiang University (Hangzhou, China) between January 2005 and December 2018. The clinical data were obtained by chart review and de-identified. The archival hematoxylin and eosin-stained slides were reviewed by one experienced gynecological pathologist (SH). The hospital’s Institutional Review Board exempted the study from approval owing to its retrospective nature. Patients provided verbal consent to participate in this study. The reporting of this study conforms to the STROBE guidelines.12
Immunohistochemistry was conducted using the following diluted antibodies: estrogen receptor (ER; SP1, Thermo Fisher Scientific, Waltham, MA, USA; 1:300), PR ( SP2, Thermo Fisher Scientific; 1:500), CD10 (56C6; Leica Biosystems, Wetzlar, Germany; 1:100), cytokeratin (AE1/AE3; DAKO, Glostrup, Denmark; 1:100), inhibin (EP378; Abcam, Cambridge, UK; 1:25), Ki67 (MIB1; Thermo Fisher Scientific; 1:400), and p53 (DO-7; Thermo Fisher Scientific; 1:300). A two-step En Vision immunostaining procedure (DAKO) was performed according to the manufacturers’ protocols. The immunostaining results were individually interpreted by the researchers. ER and PR expression was scored by the percentage of positive cells (0, <5%; 1+, 5%–24%; 2+, 25%–49%; 3+, >50%–74%; 4+, ≥75%). Strong nuclear staining (>70% cells) or null staining was defined as aberrant p53 expression (mutant type), and otherwise, p53 expression was considered normal (wild-type). The Ki67 labeling index was calculated by counting cells in 10 high-power fields from the hotspots.
Results
Clinical findings
As presented in Table 1, the age of the 11 enrolled patients ranged from 36 to 50 years (median, 43 years; mean, 42.7 years). Of note, six patients underwent antecedent surgery. Among them, four patients underwent surgery to remove ovarian endometriotic cysts, one patient underwent surgery to remove the rudimentary horn of the uterus and right salpingo-oophorectomy, and one patient underwent thermomyolysis of uterine leiomyoma. One patient was treated for 2 months with a levonorgestrel intrauterine system (Mirena, Bayer, Leverkusen, Germany), for which the dissolution rate in vivo was approximately 20 µg/24 hours, 5 years before enrollment.
Table 1.
Clinical features of mifepristone-related ovarian endometriosis.
| Case # | Age | G&P | Clinical presentation | Antecedent surgery | Mifepristone(dose × treatmentduration; total dose) | Surgery |
|---|---|---|---|---|---|---|
| 1 | 39 | G0P0 | Dysmenorrhea for 14 years | Laparoscopic removal of BOEC 14 years before enrollment followed by six cycles of GnRHa; abdominal removal of BOEC and uterine adenomyosis 6 years before enrollment followed by 9 cycles of GnRHa; recurrent OC and uterine adenomyosis treated with traditional Chinese medicine 5 years before enrollment | 6.25–25 mg × 2 years; 6200 mg | TAHBSO |
| 2 | 48 | G3P1 | Dysmenorrhea for 20 years | Mirena for 2 months 5 years before enrollment | 12.5 mg × 2 years; 9100 mg | TAHBSO |
| 3 | 42 | G6P1 | Recurrent dysmenorrhea for 3 years | Laparoscopic removal of BOEC 15 years before enrollment Abdominal removal of uterine adenomyosis 4 years before enrollment followed by six cycles of GnRHa and Mirena for 1 year | 6.25–25 mg × 3 years; 17,200 mg | TAHBS; removal of BOC |
| 4 | 45 | G3P1 | Progressive dysmenorrhea for 13 years | Removal of the rudimentary horn of the uterus, RSO, and appendectomy 22 years before enrollment | 25 mg × 9 years; 27,000 mg | LSO |
| 5 | 37 | G2P1 | Dysmenorrhea for 4 years | None | 10–25 mg × 10 months; 6400 mg | TAHBS; removal of BOC |
| 6 | 43 | G1P1 | Progressive dysmenorrhea for 1+ years | None | 5 mg × 13 months; 1900 mg | TAHLSO; removal of ROC, right fallopian tube, and masses of bilateral recto-uterine ligaments |
| 7 | 40 | G5P2 | Progressive dysmenorrhea for 2+ years | None | 7.5 mg × 2 years; 540 mg | TAHBSO |
| 8 | 46 | G2P1 | Progressive dysmenorrhea for 10 years | Thermomyolysis of uterine leiomyoma 3 years before enrollment | 12.5 mg × 8 months; 3000 mg | TAH; removal of ROC |
| 9 | 36 | G0P0 | Recurrent pelvic mass for 3 years | Removal of BOEC 4 years before enrollment | 12.5 mg × 1 years; 4500 mg | TAHLSO; removal of ROC and fallopian tube |
| 10 | 44 | G3P1 | Progressive dysmenorrhea for 5 years | Removal of LOC 3 years before enrollment | 12.5–25 mg × 22 months; 8600 mg | TAHRSO; removal of LOC and fallopian tube |
| 11 | 50 | G2P1 | Dysmenorrhea for 6 years | None | 6.25 × 50 months; 9000 mg | TAHBSO |
BOC, bilateral ovarian cysts; BOEC, bilateral ovarian endometriotic cysts; G&P, gestation & parity; LOC, left ovarian cyst; LSO, left salpingo-oophorectomy; OC, ovarian cyst; R, right; ROC, right ovarian cyst; RSO, right salpingo-oophorectomy; TAHBS, total hysterectomy with bilateral salpingectomy; TAHBSO, total hysterectomy with bilateral salpingo-oophorectomy; TAHLSO, total hysterectomy with left salpingo-oophorectomy; TAHRSO, total hysterectomy with right salpingo-oophorectomy.
The clinicopathological features of the 11 patients are summarized in Table 1. Briefly, all patients were previously diagnosed with ovarian endometriotic cysts and/or uterine adenomyosis, and all patients had an at least 1-year history of dysmenorrhea (ranging from 1 to 14 years). The patients were treated with mifepristone (5–25 mg daily) for 8 months to 9 years (median, 2 years) to alleviate their symptoms. The estimated total amount of mifepristone administered ranged from 1800 to 27,000 mg (median, 6400 mg). Serum CA125 levels were elevated in all patients (median, 383.4 U/mL; range, 50.2–1835 U/mL), and CA19-9 levels were increased in three patients (median, 746.7 U/mL; range, 73.9–1210 U/mL). All patients have had unremarkable histories to date (37–129 months).
All patients had concurrent adenomyosis and ovarian masses on sonography after being admitted to the hospital. As summarized in Table 1, eight patients presented with bilateral ovarian masses, and the remaining three patients had unilateral ovarian masses on either the left or right side. The ovarian mass diameter ranged from 3 to 17.2 cm (median, 7.4 cm). Ultrasound revealed that seven tumors were cystic, two were solid-cystic, and two were solid. Four patients displayed endometrial thickening ranging from 0.8 to 1.4 cm.
Pathological findings
On gross examination, the ovarian lesions were cystic, multiloculated, or solid. The cystic lesions had a puckered surface, and they contained altered blood contents. The cystic wall measured 0.2 to 2.0 cm (median, 0.5 cm) with occasional old blood clots present on the inner side. An exophytic (polypoid) nodule positioned toward the lumen was found in three patients, and its diameter ranged 0.5 to 5.5 cm (median, 3.5 cm). The solid areas measured 2.0 to 6.0 cm (median, 4.0 cm). The polypoid lesion, solid area, and thickened cystic wall had a grayish–reddish appearance and a fleshy, honeycomb-like cut surface with occasional dilated lumens inside. Uterine adenomyosis and leiomyoma were grossly found in nine and three patients, respectively. The endometrium in Patient 1 had a thickness of 0.6 to 0.8 cm and multiple polypoid protrusions. The cut surface was reddish, and it contained dilated cysts. Patients 5 and 6 also had polypoid formation with diameters of 0.5 to 1.5 cm, but the surrounding endometrium looked unremarkable. The fallopian tubes were grossly normal.
Microscopic examination revealed PAECs in the ovarian lesions composed of endometrial glands and stroma with asymmetry of epithelial and stromal growth, resulting in a substantially high gland/stroma ratio (Figure 1a). The glands were frequently cystically dilated, and some had an irregular configuration (Figure 1a–c). In some cases, significant gland crowding superficially resembled endometrial complex hyperplasia (Figure 1d). The glands typically exhibited an inactive appearance. They were usually lined by a layer of cuboidal, low columnar, or columnar cells, and they displayed no or minimal pseudostratification (Figure 1c). Mitotic figures and apoptotic bodies were rare. The endometrial glands occasionally displayed tubal, ciliated, eosinophilic, and mucinous metaplasia (Figure 1e, f). Premalignant lesions (atypical endometrial hyperplasia/intra-epithelial neoplasia) were not detected. The endometrial stroma was compact and cellular with occasional smooth muscle differentiation, fibrosis, and edematous and myxoid changes. The endometrial stroma exhibited no pre-decidual changes. Abnormal vasculature was frequently observed, commonly taking the form of dilated thin-walled vessels, aggregates of arteriolar vessels with thickened walls containing smooth muscle cells, and anastomosing capillary networks. Fibrin thrombi were not demonstrable. Convention endometriotic cysts, such as endometrial epithelium with cuffs of densely packed endometrial stromal cells that were variably obscured by fibrosis, hemorrhage, and hemosiderin macrophages, were variably observed in seven patients.
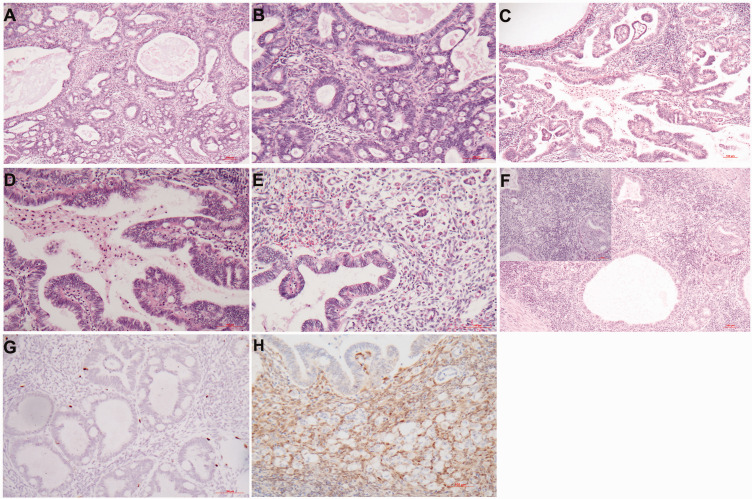
Figure 1.
Effects of long-term mifepristone on ovarian endometriosis. (a) The ovarian lesions are composed of endometrial glands and stroma with asymmetry of epithelial and stromal growth and the appearance of cystically dilated glands. (b) The dilated glands can be extensive and confluent with irregular configuration. (c) The glandular epithelium is composed of a layer of cuboidal to low columnar cells without mitotic figures. (d) Occasionally, gland crowding might superficially resemble endometrial hyperplasia and (e–f) Eosinophilic and mucinous metaplasia is occasionally present. Hematoxylin and eosin staining: a, b, and d, × 100; c, e, and f, × 200.
In most patients (8/11), the eutopic endometrium also featured PAECs that were characterized by variable, interspersed cysts frequently in the lower part of the endometrial compartment (Figure 2a). The glands had non-physiological combinations of inactivity and mild secretory changes with rare mitotic figures and apoptotic bodies (Figure 2b). Other findings on endometrial histology included atrophy (n = 1) and a middle secretory phase (n = 1). Occasionally, the endometrial stroma displayed focal fibrosis or smooth muscle differentiation. The prominent vasculature included delicate, anastomosing capillary networks and collections of thick-walled vessels outside endometrial polyps (Figure 2c). Endometrial polyps were found in three patients, and they featured prominent fibrosis and aggregates of thick-walled vessels (Figure 2d).
Figure 2.
Effects of long-term mifepristone on eutopic endometrium. (a) The endometrium contains variable, interspersed glandular cysts. (b) The glands have a non-physiological appearance displaying inactivity and mild secretory changes with rare mitotic figures. (c) The prominent vasculature includes delicate, anastomosing capillary networks and collections of thick-walled vessels and (d) Endometrial polyps with a prominent fibrosis are present in some patients. Hematoxylin and eosin staining: a and b, ×50 (inset, ×200); c, ×100; d, ×200.
Immunohistochemistry demonstrated that both eutopic and ectopic endometrial glands had diffuse ER and PR staining, wild-type p53 expression, and a low Ki67 index (<5%). The endometrial stroma was highlighted by CD10 staining.
Patient 1 displayed multifocal endometrial glands with complex patterns including glandular crowding, cribriform, and papillary formation (Figure 3a–d). There were single cells, small clusters, and micropapillae with eosinophilic cytoplasm surrounded by clear spaces in the stroma, resembling the findings in ovarian serous borderline tumors with microinvasion (Figure 3e). None of these foci measured more than 5 mm in diameter. However, the glandular cells exhibited minimal cytological atypia and virtually no mitotic figures (Figure 3b, d, and e). Focal cribriform was present in uterine adenomyosis (Figure 3f). The endometrial glands and “microinvasive” cells were positive for ER, PR, and cytokeratin, and negative for CD10 and inhibin, and they featured wild-type p53 expression, a low Ki67 index (<5%, Figure 3g), and a loss of PTEN expression (Figure 3h). Based on the presence of cribriform and stromal invasion, the final histopathological diagnosis of this patient was atypical endometrial hyperplasia with multifocal endometrioid carcinoma. The endometrium in the remaining patients displayed no evidence of endometrial carcinoma and precursor lesions (atypical endometrial hyperplasia/endometrial intraepithelial neoplasm).
Figure 3.
Atypical endometrial hyperplasia with multifocal endometrioid carcinoma (Patient 1). (a–d) Glandular crowding, cribriform, and papillary formation are consistent with atypical endometrial hyperplasia, although the epithelium has mild nuclear atypia and virtually no mitotic figures. (e) Microinvasion is suggested by the presence of single cells, small cell clusters, and micropapillae with eosinophilic cytoplasm surrounded by clear spaces in the stroma. (f) Focal cribriform pattern with microinvasion is observed in uterine adenomyosis (upper left inset: mild atypia) and (g–h) The endometrial glands and microinvasive cells have a low Ki67 index and no PTEN expression. Hematoxylin and eosin staining: a and c, ×100; b, d, and e, ×200; f, ×100 (inset, ×200). Immunohistochemistry: g & h, ×200.
Nine patients had uterine adenomyosis. Adenomyosis in eight patients shared similar morphological alterations with the eutopic endometrium. In Patient 9, adenomyosis featured a variable glandular pattern ranging from dilated cysts with a flat epithelium to small inactivating glands with a columnar epithelium and eosinophilic cytoplasm, in contrast to the middle-phase secretion in eutopic endometrium.
Discussion
Mifepristone has been proposed as an alternative treatment to improve the symptoms of endometriosis.6,7 To the best of our knowledge, the effects of long-term mifepristone therapy on ovarian endometriosis have not been specifically examined and compared with eutopic endometrium side-by-side. Thus, this study for the first time provides both a comprehensive clinicopathological description of ovarian endometriosis upon prolonged mifepristone treatment and an in-parallel comparison of the pathological changes in the uterine endometrium and adenomyosis. Overall, we observed shared distinguished histopathological alterations including large cystic glands, epithelial metaplasia, and abnormal blood vessel morphology between ectopic endometriosis and eutopic endometrium in most of the samples studied. These morphological features were consistent with previous findings on the uterine endometrium in patients treated with mifepristone and other PRMs.9,10,13–15 Mutter et al.10 termed such morphologic changes as PAECs. Meanwhile, we want to note that in eutopic endometrium, these alterations might be confused with a disordered endometrium and endometrial hyperplasia without atypia (simple hyperplasia). In addition to the shared histopathological alterations, we identified other distinct features in ovarian endometriosis including significant glandular crowding and unapparent secretory alteration. Endometriosis has been hypothesized to develop from endometrial progenitor/stem cells that are believed to reside in the basalis endometrium.16 Wu et al.17 indicated that in patients with endometriosis, the ectopic and eutopic endometrium had different gene expression profiles. The local microenvironment, particularly immune factors such as activated macrophages, is essentially involved in the pathogenesis of endometriosis.18 An in vitro study revealed the differential expression of ER and epithelial P receptor between endometriotic and endometrial cells.19 We believe that these biological and molecular factors can explain the mifepristone-related morphological difference between endometriosis and eutopic endometrium.
Our study also demonstrated the distinct clinicopathological features in ovarian endometriosis following long-term mifepristone administration. The patients had elevated serum CA125 levels and occasionally displayed elevated CA19-9 levels, and large ovarian masses were present. On casual examination in the absence of information on mifepristone administration, the unusual microscopic features might lead to an initial impression of endometrioid adenofibroma or a borderline endometrioid tumor. However, the distinguishing features of these lesions from an endometrioid tumor are as follows: (1) a clinical history of long-term mifepristone therapy in the presence of endometriosis; (2) the presence of endometrial stroma, highlighted by CD10 staining, surrounded the endometriotic glands rather than a dense fibrotic stroma in endometrioid tumors; (3) the glands, particularly the cystically dilated glands, are lined by cuboid or flatten epithelium with minimal pseudostratification; and (4) the glands exhibit features of inactivation, mild secretory alterations, rare mitotic figures and apoptosis, and a low Ki67 index.
Similarly as eutopic endometrium, endometriotic glands can undergo metaplasia, unusual hormone changes, endometrial hyperplasia, and atypical hyperplasia. These alterations are sometimes related to an endogenous or exogenous hormone stimulus or drugs. Tamoxifen-related polypoid endometriosis frequently exhibited glandular hyperplasia.20,21 The lesion can be confused with a low-grade or well-differentiated neoplasm by pathologists who lack recognition of this variant of endometriosis.20,21 We believe like that the endometrial glands in mifepristone-related cases do not represent endometrial hyperplasia without atypia microscopically. Mifepristone-related tumor-like endometriosis can best characterize our cases. However, from this study, we cannot determine whether there is a defined succession of endometriotic changes over time and the relationships of the sequence and composition of these changes with administration schedules. More importantly, we cannot judge whether these changes may remain, resolve, or progress to atypical hyperplasia or carcinoma, although no overt premalignant lesions of atypical hyperplasia have been identified in ovarian endometriosis to date. Follow-up data will be needed to determine the long-term outcomes of these changes.
Lastly, Patient 1 had distinct histopathological alterations in the endometrium and adenomyosis. There were multifocal endometrial glands with cribriform and papillary formation and stromal “microinvasion.” However, these microinvasive foci displayed mild cytological atypia, a loss of PTEN expression, the absence of mitotic figures, and a low Ki67 index. Considering mifepristone therapy has proven safe in clinical practice and no premalignant lesions or cancers have been reported to date,9,10,14 we tend to interpret that this special case does not represent a causative link between endometrial carcinoma risk and mifepristone administration, although the initial endometrial biopsy before treatment was unavailable. Most likely, the perplexing morphology might reflect the suppressive effects of mifepristone on preexisting atypical endometrial hyperplasia or low-grade endometrioid carcinoma in the endometrium and adenomyosis.
Conclusions
We have identified distinct histopathological alterations in ovarian endometriosis after long-term exposure to mifepristone in addition to the shared pathological features observed in the eutopic endometrium and adenomyosis. This novel observation highlights the necessity to monitor the effect of long-term mifepristone treatment on both eutopic and ectopic endometrium and examine the correlations of these changes with clinical information in the treatment of ovarian endometriosis. Furthermore, careful histological assessment and clinical information are critical for making the right diagnosis because significant endometriotic glandular crowding may initially lead to a misdiagnosis in patients with borderline or benign endometrioid tumors.
Acknowledgement
We thank Ms. Minghua Yu for her excellent technical support.
Footnotes
The authors declare that there is no conflict of interest.
Funding: None.
ORCID iD
Yongmiao Pan https://orcid.org/0000-0003-3741-4242
References
- 1.Islam MS, Afrin S, Jones SI, et al. Selective progesterone receptor modulators-mechanisms and therapeutic utility. Endocr Rev 2020; 41: bnaa012. doi: 10.1210/endrev/bnaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narvekar N, Cameron S, Critchley HO, et al. Low-dose mifepristone inhibits endometrial proliferation and up-regulates androgen receptor. J Clin Endocrinol Metab 2004; 89: 2491–2497. doi: 10.1210/jc.2003-031945. [DOI] [PubMed] [Google Scholar]
- 3.Spitz IM, Bardin CW. Mifepristone (RU 486)–a modulator of progestin and glucocorticoid action. N Engl J Med 1993; 329: 404–412. doi: 10.1056/NEJM199308053290607. [DOI] [PubMed] [Google Scholar]
- 4.Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet 2020; 396: 770–778. doi: 10.1016/S0140-6736(20)31788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A, Cheng L, Lin S, et al. Daily low-dose mifepristone has contraceptive potential by suppressing ovulation and menstruation: a double-blind randomized control trial of 2 and 5 mg per day for 120 days. J Clin Endocrinol Metab 2002; 87: 63–70. doi: 10.1210/jcem.87.1.8140. [DOI] [PubMed] [Google Scholar]
- 6.Kettel LM, Murphy AA, Morales AJ, et al. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum Reprod 1994; 9: 116–120. doi: 10.1093/humrep/9.suppl_1.116. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Liu M, Shen F, et al. Use of mifepristone to treat endometriosis: a review of clinical trials and trial-like studies conducted in China. Womens Health (Lond) 2011; 7: 51–70. doi: 10.2217/whe.10.79. [DOI] [PubMed] [Google Scholar]
- 8.De Ziegler D Borghese B, andChapron C.. Endometriosis and infertility: pathophysiology and management. Lancet 2010; 376: 730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 9.Baird DT, Brown A, Critchley HO, et al. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod 2003; 18: 61–68. doi: 10.1093/humrep/deg022. [DOI] [PubMed] [Google Scholar]
- 10.Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008; 21: 591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 11.Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10: 261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Williams AR, Bergeron C, Barlow DH, et al. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012; 31: 556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 14.Fiscella J, Bonfiglio T, Winters P, et al. Distinguishing features of endometrial pathology after exposure to the progesterone receptor modulator mifepristone. Hum Pathol 2011; 42: 947–953. doi: 10.1016/j.humpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioffe OB Zaino RJ, andMutter GL.. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol 2009; 22: 450–459. doi: 10.1038/modpathol.2008.204. [DOI] [PubMed] [Google Scholar]
- 16.Leyendecker G, Herbertz M, Kunz G, et al. Endometriosis results from the dislocation of basal endometrium. Hum Reprod 2002; 17: 2725–2736. doi: 10.1093/humrep/17.10.2725. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Strawn E, Basir Z, et al. Genomic alterations in ectopic and eutopic endometria of women with endometriosis. Gynecol Obstet Invest 2006; 62: 148–159. doi: 10.1159/000093130. [DOI] [PubMed] [Google Scholar]
- 18.Berbic M, Schulke L, Markham R, et al. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod 2009; 24: 325–332. doi: 10.1093/humrep/den393. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Wu RF, Wang ZH, et al. Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertil Steril 2002; 77: 995–1000. doi: 10.1016/s0015-0282(02)03081-9. [DOI] [PubMed] [Google Scholar]
- 20.Parker RL, Dadmanesh F, Young RH, et al. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol 2004; 28: 285–297. doi: 10.1097/00000478-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Schlesinger C, Silverberg SG. Tamoxifen-associated polyps (basalomas) arising in multiple endometriotic foci: a case report and review of the literature. Gynecol Oncol 1999; 73: 305–311. doi: 10.1006/gyno.1998.5305. [DOI] [PubMed] [Google Scholar]