Abstract
Background:
Minimally invasive (MIS) calcaneal osteotomy has grown in popularity in recent years to address hindfoot deformity. A potential complication is thermal bone necrosis secondary to heat generation from the burr that may lead to osteotomy nonunion. Irrigation is commonly employed to reduce this risk. The effect of irrigation on reducing heat accumulation remains an understudied area. The purpose of this study was to evaluate the effect of cooled vs room-temperature irrigation on thermal energy dissipation during calcaneal osteotomy using a Shannon burr.
Methods:
Fourteen cadaveric limbs at room temperature (68 °F) were randomized to receive either cooled saline (7 limbs) or room-temperature (7 limbs) irrigation during MIS calcaneal osteotomy. Two thermocouple probes were inserted 5 mm away from the plane of the osteotomy, on the proximal and distal sides, respectively. A 3 × 30-mm Shannon burr was used to perform the osteotomy. The burr was run continuously with continuous irrigation using either room-temperature (68 °F) or chilled (37 °F) irrigation, until the osteotomy was completed. Temperature was recorded at 0, 15, 30, 45, and 60 seconds for the 2 groups and used as a measure of thermal energy accumulation.
Results:
Both room-temperature and chilled irrigation were effective in minimizing temperature change. On the proximal side, an overall mean increase of 2.5 °F with room-temperature irrigation and a mean decrease of 1.0 °F with chilled irrigation were observed at the 60-second interval (P = .004). On the distal side, there was a mean increase of 1.3 °F with room-temperature irrigation and a mean increase of 0.5 °F with chilled irrigation (P = .05).
Conclusion:
For the duration of an MIS calcaneal osteotomy, both continuous room-temperature and chilled irrigation can minimize temperature increases and potentially reduce the risk of an osteotomy nonunion.
Clinical Relevance:
Both room-temperature and chilled irrigation can minimize bone temperature increases during MIS calcaneal osteotomy.
Keywords: heat necrosis, calcaneal osteotomy, minimally invasive
Introduction
A calcaneal sliding osteotomy is a commonly employed technique to address hindfoot deformity to restore alignment.10,16,18 It has traditionally been performed through an open approach. Although it is a safe and reliable procedure, concerns regarding wound healing, infection, and neurovascular injury are present, with rates as high as 25% being reported.9,15 Furthermore, it is often performed in conjunction with other procedures to address pes cavus or pes planus deformities. A large open incision along the lateral heel can often compromise or affect the ability to perform concomitant procedures.1,7,15
For these reasons, a minimally invasive (MIS) approach has been adopted and increasingly employed to perform a sliding calcaneal osteotomy, using a high-torque, low-speed burr under fluoroscopic guidance.9,13 Results have demonstrated fewer wound complications, infections, and nerve injury relative to the open group.7,9,15 When using the burr to perform the osteotomy, irrigation is used to reduce the risk of heat necrosis that could potentially lead to a nonunion of the osteotomy.2-4,12 In a series of 160 patients who underwent an MIS medial displacement calcaneal osteotomy, Coleman et al4 noted a case cluster of nonunions of the osteotomy thought to be related to heat osteonecrosis from the burr. In an animal arthrodesis model, use of chilled irrigation led to a higher fusion rate and improved fusion mass strength.8 Although irrigation is commonly employed, an understudied area involves the temperature of the irrigation used when performing the osteotomy. The purpose of this study was to compare the effect of chilled vs room-temperature irrigation on thermal energy dissipation, as measured on a temperature curve, during an MIS calcaneal osteotomy using a high-torque, low-speed burr. The hypothesis was that chilled irrigation is superior to room-temperature irrigation in minimizing temperature change during the osteotomy.
Methods
Fourteen unpaired fresh frozen below-knee cadaveric limbs were collected from a tissue bank. Specimens were stored in a freezer at –10 °C and thawed to allow specimens to come to room temperature (68 °F) prior to use. They were screened for prior surgery, injury, anatomical deficiency, and overt pathology prior to use. The limbs were randomized to receive either chilled (37 °F) saline (7 limbs) or room-temperature (7 limbs) irrigation during MIS calcaneal osteotomy. Chilled saline was created by refrigerating intravenous saline bags to 37 °F prior to use. Two thermocouple probes (EL-USB-TC-LCD; Lascar Electronics, Whiteparish, England) were inserted into 3-mm-diameter, 2-cm-depth holes that are 5 mm away from the plane of the osteotomy, on the distal (labeled T1) and proximal (labeled T2) sides, respectively (Figure 1). The skin and subcutaneous tissue were resected on the lateral aspect of the calcaneus only in the plane of the osteotomy itself for the purpose of accurately positioning the probes and also to ensure consistency via direct visualization when performing the osteotomy. A 3 × 30-mm Shannon burr (Novastep Inc, Englewood Cliffs, NJ) was used to perform the osteotomy, with the burr set at 6000 rpm using a 4:1 reducer attachment. The uniplanar osteotomy was oriented from proximal-posterior to distal-anterior in the fashion of a sliding calcaneal osteotomy. The burr was run continuously with continuous irrigation using either room-temperature (68 °F) or chilled (37 °F) irrigation, until the osteotomy was completed. Irrigation for both groups was provided via saline bags suspended on an intravenous (IV) pole connected to intravenous tubing opened fully that allowed for continuous irrigation.
Figure 1.
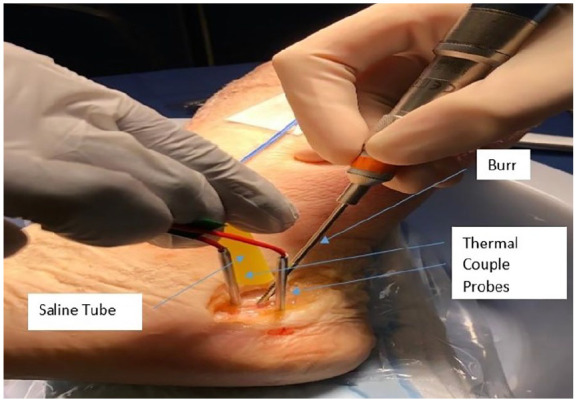
Osteotomy setup with a Shannon burr, saline tube, and 2 thermal couples.
All osteotomies were performed by 2 foot and ankle fellowship-trained orthopaedic surgeons. The burr was changed after each osteotomy to eliminate instrument wear as a potential variable. Temperature was recorded at 0, 15, 30, 45, and 60 seconds for the 2 groups and used as a measure of thermal energy accumulation.
Statistical Analysis
Descriptive data were described as mean temperature ± SD. Normality of the data was confirmed using the Shapiro Wilk test using SAS software (SAS, Cary, NC). A t test was used to analyze the temperature curves recorded at the specified time points for each probe during the osteotomy. Statistical significance was set at P ≤.05.
Results
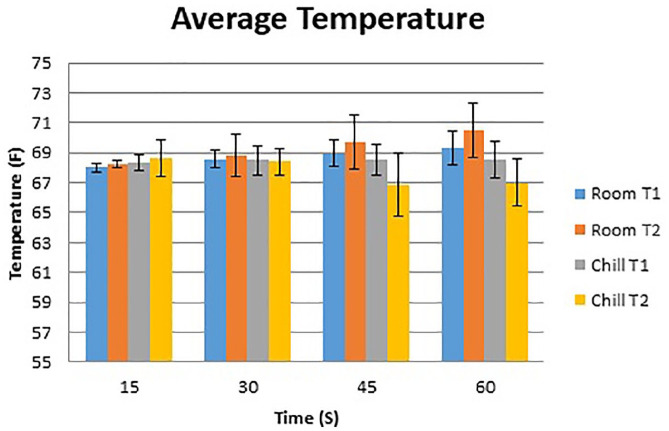
Figures 2 and 3 show the temperature curves of specimens from the room-temperature irrigation group and the chilled irrigation group. Both room-temperature and chilled irrigation were effective in minimizing temperature change for the duration of the osteotomy. On the proximal (T2) side, an overall mean increase of 2.5 °F with room-temperature irrigation and a mean decrease of 1.0 °F with chilled irrigation were observed over the course of 60 seconds (P = .004). On the distal (T1) side, there was a mean increase of 1.3 °F with room-temperature irrigation and a mean increase of 0.5 °F with chilled irrigation (P = .05) (Figure 4; Table 1). No significant differences when comparing room-temperature vs chilled irrigation were noted at the T1 probe until the 60-second interval, whereas for the T2 probe, a notable difference was noted at the 45- and 60-second intervals. There was no observable difference between the recordings of the proximal and distal probes under room-temperature irrigation, whereas there was a notable difference in the temperature recorded at the 60-second interval between the probes under chilled irrigation, with the proximal probe recording a lower temperature by 1.5 °F (Table 1).
Figure 2.
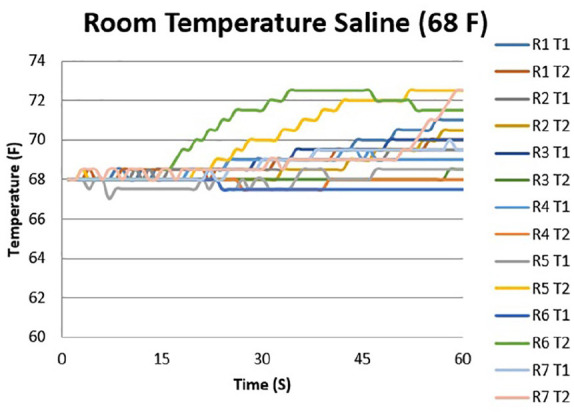
Temperature curve during minimally invasive osteotomy performed with room-temperature saline irrigation. Individual lines represent temperature probes for each specimen.
Figure 3.
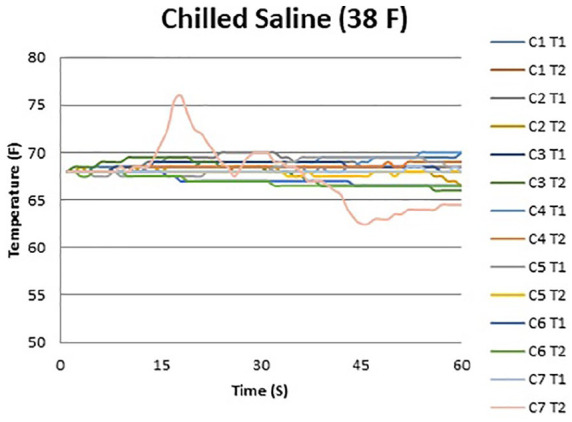
Temperature curve during minimally invasive calcaneal osteotomy performed with chilled saline irrigation. Individual lines represent temperature probes for each specimen.
Figure 4.
Average interval temperature for T1 and T2 probes under room vs chilled irrigation with SD.
Table 1.
Mean (SD) Temperature Recorded at Probes Over Duration of Osteotomy, and P Values of Statistical Analyses.a
| Average | Standard Deviation | P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Room Temp. | Chilled | Room Temp. | Chilled | Room Temp. | Chilled | T1 | T2 | |||||
| Time (s) | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 vs T2 | T1 vs T2 | Room vs Chill | Room vs Chill |
| 15 | 68.00 | 68.21 | 68.36 | 68.64 | 0.29 | 0.27 | 0.56 | 1.21 | .604 | .587 | .271 | .307 |
| 30 | 68.57 | 68.86 | 68.50 | 68.43 | 0.61 | 1.41 | 0.96 | 0.89 | .263 | .864 | .268 | .878 |
| 45 | 68.93 | 69.71 | 68.50 | 66.86 | 0.89 | 1.82 | 1.04 | 2.10 | .676 | .059 | .109 | .024 |
| 60 | 69.29 | 70.50 | 68.50 | 67.00 | 1.11 | 1.80 | 1.22 | 1.58 | .702 | .014 | .047 | .004 |
Abbreviation: Temp., temperature.
All specimens were at 68 °F at 0 seconds.
Discussion
Concerns regarding wound healing and damage to surrounding neurovascular structures with a traditional open method led to the development of the MIS calcaneal osteotomy.6,7,9 Various methods have been employed to perform the osteotomy, including the use of a Gigli saw, microsagittal saw, and more recently, the use of a low-speed, high-torque burr.4,5,9,14,15 Favorable clinical and radiographic outcomes have been in achieved in comparison to the traditional open sliding calcaneal osteotomy, with fewer complications. In particular, a low osteotomy nonunion rate has been observed when the burr is used in conjunction with sterile saline to minimize the risk of thermal necrosis.7,9 Without the use of irrigation, increases of 30 to 60 °C can be observed in human tissue with the use of burrs, drills, and K-wires, increasing the risk of nonunion, wound complications, and infection because of the heat generated from friction during the process of removing bone.11,12 Factors such as operational time of the burr, manual pressure applied by the surgeon, and speed all play a critical role in thermogenesis.12 Using a thermal imaging camera, Robinson et al12 evaluated the temperature increase using either a 2.0- or 4.3-mm MIS burr on specimens removed during total hip arthroplasty and found that the mean temperature was 28 °C (82.4 °C) with room-temperature irrigation and 43 °C (109 °F) without irrigation from a baseline temperature of 18.6 °C.12 In this particular study, the burr was used in an interval fashion of 20 seconds at an rpm of 800 to 1500 rpm. They concluded that burr temperature is dependent on several factors including the duration of burr activation time, use of irrigation, ambient temperature, and overall burr usage.12 Additionally, in the clinical setting, lowering the ambient temperature of the room and avoiding the use of a tourniquet can also reduce temperature at the osteotomy site.4,12 Despite its use, little attention has been devoted to analyzing the use of irrigation and its impact on osseous healing. In an ulnohumeral rabbit arthrodesis model, use of continuous chilled (43 °F) saline irrigation led to higher fusion rates and greater fusion mass strength compared to when no irrigation was used.8
The present study sought to compare the use of continuous chilled vs room-temperature irrigation on thermal energy generation when performing an MIS calcaneal osteotomy using a low-speed, high-torque burr. Both were effective in minimizing temperature change, with slightly lower temperatures observed with the use of chilled irrigation. It is interesting to note that an observable difference in temperature did not occur until the later stages of the osteotomy (45- and 60-second time points). This can be particularly important with osteotomies in denser bone that may take longer to perform and generate more heat, especially given the stronger quality of bone encountered in the tuberosity.17 There was a greater temperature difference observed at the proximal probe, which could be a reflection of the orientation of the osteotomy in relation to the probes as well as potential greater exposure of the proximal probe to the chilled irrigation.
Limitations of the study include its biomechanical nature. There were several deviations made from clinical practice when performing the MIS osteotomy in order to isolate the effect of irrigation on temperature changes. First, the burr was operated continuously until the osteotomy was completed, whereas in the clinical setting the burr is often operated in an interval fashion to allow for heat dissipation. The frequency and duration of pauses are operator-dependent and a source of variability that can affect heat generation.12 Second, the skin and subcutaneous tissue was resected at the plane of the osteotomy in order to ensure accuracy when placing the temperature probes 5 mm away from the osteotomy. This minimized the potential dampening effect that different skin and subcutaneous thicknesses can have on the irrigation, and it also maintained consistency of the osteotomy in spatial relation to the probes. An additional limitation is the placement of the probes. We chose 5 mm as we felt it was as close as possible to place the probe without risking damage to it or breaching the hole containing the probe. We used a 3-mm drill bit and a 3-mm burr, which left a 2-mm bridge between the burr and the probe once the osteotomy was complete. Robinson et al12 noted that the heat generated from burr use is primarily concentrated within 3 mm of the osteotomy, and the placement of our probes fall within this range. Furthermore, although there was no significant difference in temperature recording at the proximal and distal probes with room-temperature irrigation, there was a significant difference observed when chilled irrigation was used, with the proximal probe recording a lower average temperature than the distal probe at the 60-second time point, which could reflect a greater amount of irrigation reaching the proximal probe. A strength of the study was the fact that the osteotomies were performed by 2 separate surgeons, which improves the overall generalizability of the findings.
Conclusion
For the duration of an MIS calcaneal osteotomy, both continuous room-temperature and chilled irrigation can minimize bone temperature increases and potentially reduce the risk of osteotomy nonunion.
Footnotes
Ethical Approval: Ethical approval for this study was waived by the INOVA Fairfax Hospital Institutional Review Board as it is a cadaveric biomechanical study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Sudheer C. Reddy reports all support from Novastep, Inc, for the present manuscript (eg, funding, provision of study materials, medical writing, article processing charges, etc) and receipt of equipment, materials, drugs, medical writing, gifts, or other services. Oliver N. Schipper reports all support from Novastep, Inc, for the present manuscript (eg, funding, provision of study materials, medical writing, article processing charges, etc), funding for study, royalties or licenses, and consulting fees from Novastep, Inc. ICMJE forms for all authors are available online.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Novastep Inc
ORCID iDs: Sudheer C. Reddy, MD,  https://orcid.org/0000-0002-8222-6956
https://orcid.org/0000-0002-8222-6956
Oliver N. Schipper, MD,  https://orcid.org/0000-0003-1248-640X
https://orcid.org/0000-0003-1248-640X
References
- 1. Abbasian A, Zaidi R, Guha A, Goldberg A, Cullen N, Singh D. Comparison of three different fixation methods of calcaneal osteotomies. Foot Ankle Int. 2013;34(3):420-425. [DOI] [PubMed] [Google Scholar]
- 2. Augustin G, Davila S, Udiljak T, Vedrina D, Bagatin D. Determination of spatial distribution of increase in bone temperature during drilling by infrared thermography: preliminary report. Arch Orthop Trauma Surg. 2009;129:703-709. [DOI] [PubMed] [Google Scholar]
- 3. Brisman DL. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int J Oral Maxillofac Implants. 1996;11:35-37. [PubMed] [Google Scholar]
- 4. Coleman MM, Abousayed MM, Thompson JM, Bean BA, Guyton GP. Risk factors for complications associated with minimally invasive medial displacement calcaneal osteotomy. Foot Ankle Int. 2021;42(2):121-131. [DOI] [PubMed] [Google Scholar]
- 5. Didomenico LA, Anain J, Wargo-Dorsey M. Assessment of medial and lateral neurovascular structures after percutaneous posterior calcaneal displacement osteotomy: a cadaver study. J Foot Ankle Surg. 2011;50(6):668-671. [DOI] [PubMed] [Google Scholar]
- 6. Durston A, Bahoo R, Kadambande S, Hariharan K, Mason L. Minimally invasive calcaneal osteotomy: does the Shannon burr endanger the neurovascular structures? A cadaveric study. J Foot Ankle Surg. 2015;54(6):1062-1066. [DOI] [PubMed] [Google Scholar]
- 7. Guttek N, Zeh A, Wohlrab D, Delank K. Comparative results of percutaneous calcaneal osteotomy in correction of hindfoot deformities. Foot Ankle Int. 2019;40(3):276-281. [DOI] [PubMed] [Google Scholar]
- 8. Haddad SL, Hsu AR, Templin CR, et al. Effect of continuous irrigation during burring on thermal necrosis and fusion strength in a rabbit arthrodesis model. Foot Ankle Int. 2014;35(8):796-801. [DOI] [PubMed] [Google Scholar]
- 9. Kendal AR, Khalid A, Ball T, Rogers M, Cooke P, Sharp R. Complications of minimally invasive calcaneal osteotomy versus open osteotomy. Foot Ankle Int. 2015;36(6):685-690. [DOI] [PubMed] [Google Scholar]
- 10. Myerson MS, Badekas A, Schon LC. Treatment of stage II posterior tibial tendon deficiency with flexor digitorum longus tendon transfer and calcaneal osteotomy. Foot Ankle Int. 2004;25(7):445-450. [DOI] [PubMed] [Google Scholar]
- 11. Palmisano AC, Tai BL, Belmont B, Irwin TA, Shih A, Holmes JR. Comparison of cortical bone drilling induced heat production among common drilling tools. J Orthop Trauma. 2015;29:e188–e193. [DOI] [PubMed] [Google Scholar]
- 12. Robinson D, Heller E, Yassin M. Comparing the temperature effect of dedicated minimally invasive motor system to the discontinuous use of rotatory burrs in the correction of hallux valgus. Foot Ankle Spec. 2020;13(6):478-487. [DOI] [PubMed] [Google Scholar]
- 13. Talusan PT, Ezequiel C, Tan EW, Parks BG, Guyton GP. Safe zone for neural structures in medial displacement calcaneal osteotomy: a cadaveric and radiographic investigation. Foot Ankle Int. 2015;36(12):1493-1498. [DOI] [PubMed] [Google Scholar]
- 14. Tennant J, Veljkovic A, Phisitkul P. Technique tip: percutaneous endoscopically-assisted calcaneal slide osteotomy. Iowa Orthop J. 2013;33:191-195. [PMC free article] [PubMed] [Google Scholar]
- 15. Veljkovic A, Symes M, Younger A, et al. Neurovascular and clinical outcomes of the percutaneous endoscopically assisted calcaneal osteotomy (PECO) technique to correct hindfoot alignment. Foot Ankle Int. 2019;40(2):178-184. [DOI] [PubMed] [Google Scholar]
- 16. Williamson ERC, Chan JY, Burket JC, Deland JT, Ellis SJ. New radiographic parameter assessing hindfoot alignment in stage II adult-acquired flatfoot deformity. Foot Ankle Int. 2015;36(4):417-423. [DOI] [PubMed] [Google Scholar]
- 17. Yamada M, Ito M, Hayashi K, Nakamura T. Calcaneus as a site for assessment of bone mineral density: evaluation in cadavers and healthy volunteers. Am J Roentgenol. 1993;161:621-627. [DOI] [PubMed] [Google Scholar]
- 18. Zanolli DH, Glisson RR, Nunley JA, II, Easley ME. Biomechanical assessment of flexible flatfoot correction: comparison of techniques in a cadaver model. J Bone Joint Surg. 2014;96:e45. [DOI] [PubMed] [Google Scholar]